
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 04 April 2023
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1149628
This article is part of the Research Topic Tumor Accommodation: The Importance of the Niche in Neurological Tumors View all 5 articles
 Lea Baumgart1,2*
Lea Baumgart1,2* Amir Kaywan Aftahy1
Amir Kaywan Aftahy1 Aida Anetsberger3,4
Aida Anetsberger3,4 Dennis Thunstedt5
Dennis Thunstedt5 Benedikt Wiestler6
Benedikt Wiestler6 Denise Bernhardt7,8
Denise Bernhardt7,8 Stephanie E. Combs7,9,8
Stephanie E. Combs7,9,8 Bernhard Meyer1
Bernhard Meyer1 Hanno S. Meyer1,2
Hanno S. Meyer1,2 Jens Gempt1,2
Jens Gempt1,2Background: Due to demographic changes and an increased incidence of cancer with age, the number of patients with brain metastases (BMs) constantly increases, especially among the elderly. Novel systemic therapies, such as immunotherapy, have led to improved survival in recent years, but intracranial tumor progression may occur independently of a systemically effective therapy. Despite the growing number of geriatric patients, they are often overlooked in clinical trials, and there is no consensus on the impact of BM resection on survival.
Objectives: The aim of this study was to analyze the impact of resection and residual tumor volume on clinical outcome and overall survival (OS) in elderly patients suffering from BM.
Methods: Patients ≥ 75 years who had surgery for BM between April 2007 and January 2020 were retrospectively included. Residual tumor burden (RTB) was determined by segmentation of early postoperative brain MRI (72 h). Contrast-enhancing tumor subvolumes were segmented manually. “Postoperative tumor volume” refers to the targeted BMs. Impact of preoperative Karnofsky performance status scale (KPSS), age, sex and RTB on OS was analyzed. Survival analyses were performed using Kaplan-Meier estimates for the univariate analysis and the Cox regression proportional hazards model for the multivariate analysis.
Results: One hundred and one patients were included. Median age at surgery was 78 years (IQR 76-81). Sixty-two patients (61%) had a single BM; 16 patients (16%) had two BMs; 13 patients (13%) had three BMs; and 10 patients (10%) had more than three BMs. Median preoperative tumor burden was 10.3 cm3 (IQR 5–25 cm3), and postoperative tumor burden was 0 cm3 (IQR 0–1.1 cm3). Complete cytoreduction (RTB = 0) was achieved in 52 patients (52%). Complete resection of the targeted metastases was achieved in 78 patients (78%). Median OS was 7 months (IQR 2–11). In univariate analysis, high preoperative KPSS (HR 0.986, 95% CI 0.973–0.998, p = 0.026) and small postoperative tumor burden (HR 1.025, 95% CI 1.002–1.047, p = 0.029) were significantly associated with prolonged OS. Patients with RTB = 0 survived significantly longer than those with residual tumor did (12 [IQR 5–19] vs. 5 [IQR 3–7] months, p = 0.007). Furthermore, prolongation of survival was significantly associated with surgery in patients with favorable KPSS, with an adjusted HR of 0.986 (p = 0.026). However, there were no significances regarding age.
Conclusions: RTB is a strong predictor for prolonged OS, regardless of age or cancer type. Postoperative MRI should confirm the extent of resection, as intraoperative estimates do not warrant a complete resection. It is crucial to aim for maximal cytoreduction to achieve the best long-term outcomes for these patients, despite the fact the patients are advanced in age.
Brain metastases (BMs) occur in 20–30% of patients with systemic cancer and are the most common brain tumor, with high recurrence rates of 40–60% (1–5). In addition, the incidence of newly diagnosed BMs is 3–10 times that of newly diagnosed malignant primary brain tumors (3). In light of an aging population and an increased incidence of cancer with age, the number of patients with BMs is growing, especially elderly patients (2, 6–8). Even though there has been tremendous progress in the field of systemic therapy, particularly in immunotherapy in recent years, allowing patients to survive much longer, intracranial tumor progression may occur despite a systematically effective therapy. However, as advanced age and decreased functional independence are associated with poor outcome in patients with BMs, age and functional status have become key criteria of established classification systems for selecting patients for surgical and adjuvant treatment (1, 9–12). This is critical because elderly patients are a vulnerable group, which is often overlooked in numerous studies. Therefore, the development and provision of optimal treatment options have not been satisfactorily defined to date and remain unclear (6, 7, 10, 12–14). Furthermore, studies demonstrated that surgical resections of BMs have a favorable impact on the pre- and postoperative Karnofsky performance status scale (KPSS), which may also apply to elderly patients (9). For this reason, the impact of surgical treatment on geriatric patients with BM, focusing on postoperative clinical outcome and overall survival, was analyzed in this study.
The local ethics committee approved the study (no. 5626:12). The study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki. The ethics committee waived written informed consent.
A retrospective chart of elderly patients (defined as age 75 and older) with BMs was developed. Between April 2007 and January 2020, the department surgically treated 119 patients (≥ 75 years) with newly diagnosed single or multiple BMs. Eighteen patients (15%) had biopsies only and did not receive postoperative MRIs. One hundred and one patients (85%) met the inclusion criteria (i.e., histopathological diagnosis of a BM; availability of preoperative MRI and early postoperative MRI (within 72 hours after surgery), and tumor resection beyond biopsy). Key demographic variables, including age at diagnosis, sex, smoking status, number of BMs, tumor localization, pre- and postoperative KPSS, and pre- and postoperative tumor volumes related to the targeted BMs, were evaluated. Furthermore, postoperative treatment structure (radiotherapy), and date of death or date of last contact were reviewed. Progression free survival (PFS), and overall survival (OS) were analyzed.
Surgery was performed with the goal of maximum tumor resection while sparing eloquent regions of the brain. Intraoperative neuronavigation was routinely used. If necessary, neuromonitoring and preoperative mapping were also conducted. The indication for surgical treatment was based on the decision of an interdisciplinary neuro-oncology tumor board. The decision was usually based on the following parameters: (1) symptomatic “target” lesion, (2) mass effect, (3) intratumoral hemorrhage, (4) unclear diagnosis, and (5) large posterior fossa tumors with consecutive risk of herniation and hydrocephalus. Intraoperative frozen sections were obtained in all patients.
Within 72 hours after surgery, all postoperative MRIs were analyzed, and postoperative tumor volume was obtained. T1-weighted MRI sequences with gadolinium contrast media were investigated. Any remaining contrast-enhancing lesions were classified as residual tumor, including those that measured less than 10 mm in at least one dimension of imaging. “Postoperative tumor volume” refers to the targeted BMs. Moreover, the term of complete resection also refers to the targeted BMs, whereas complete cytoreduction (RTB=0) addresses all BMs. An experienced neuroradiologist (BW, 10 years of experience) and neurosurgeon (KA, 6 years of experience) performed volumetric measurements. Volumes of the contrast-enhancing tumor parts were manually segmented using the Origin®software (Origin®, Brainlab, version 3.1, Brainlab AG, Munich, Germany).
KPSS was used to classify and quantify the patient’s pre- and postoperative functional status. The performance status was rated on a numerical scale ranging from 0 to 100, representing the patient’s ability to conduct normal activity, to undertake active work, and need for assistance, with 100 representing full activity and 0 representing death. Adjuvant therapy was selected on an individual basis for each patient after histological diagnosis by an interdisciplinary tumor board. Adjuvant radiation recommendations were based on a variety of factors, including the number of brain lesions, extent of resection (EOR), and the KPSS. Follow-up data was gathered from the institutional outpatient clinic’s electronic patient files, as well as paper-based correspondence from the treating oncologists.
Data that have a normal distribution are expressed as mean ± standard deviation (SD) and non-normally distributed data as median and interquartile range (IQR). Logistic regression analyses were performed to identify possible risk factors for outcome changes. Survival analyses were performed using Kaplan-Meier estimates for the univariate analysis and Cox regression proportional hazards model for the multivariate analysis. To determine the optimal cutoff for differences in survival curves, the maximally selected log-rank statistic was found, followed by a comparison of the survival curves separated by the resulting cutoff. A two-tailed significance level of p < 0.05 was defined as statistically significant.
One hundred and one patients (85%) met the inclusion criteria (see Methods, Figure 1); 56% were male. Median age at surgery was 78 years (IQR 76-81 years). Elderly BM patients presented with a median preoperative KPSS of 80% (IQR 60–90). Median postoperative KPSS was 70% (IQR 60–90). Sixty-two patients (61%) had a single BM; 16 patients (16%) had two BMs; 13 patients (13%) had 3 BMs; and 10 patients (10%) had three or more BMs. BMs most commonly originated from cancers of the lung (23%), melanoma (22%), and breast (11%). Median preoperative tumor burden of the targeted lesions was 10.3 cm3 (IQR 5–25 cm3) and postoperative tumor burden 0 cm3 (IQR 0–1.1 cm3). Seventy-four patients (73%) underwent postoperative radiotherapy; about half of those (46%) had HSRS; 38% had whole brain radiation therapy (WBRT); and 3% had SRS (Table 1). For 13% of patients, information on radiation modality was unavailable. Twenty-three patients (23%) underwent postoperative chemotherapy, and 14 patients (14%) had immunotherapy.
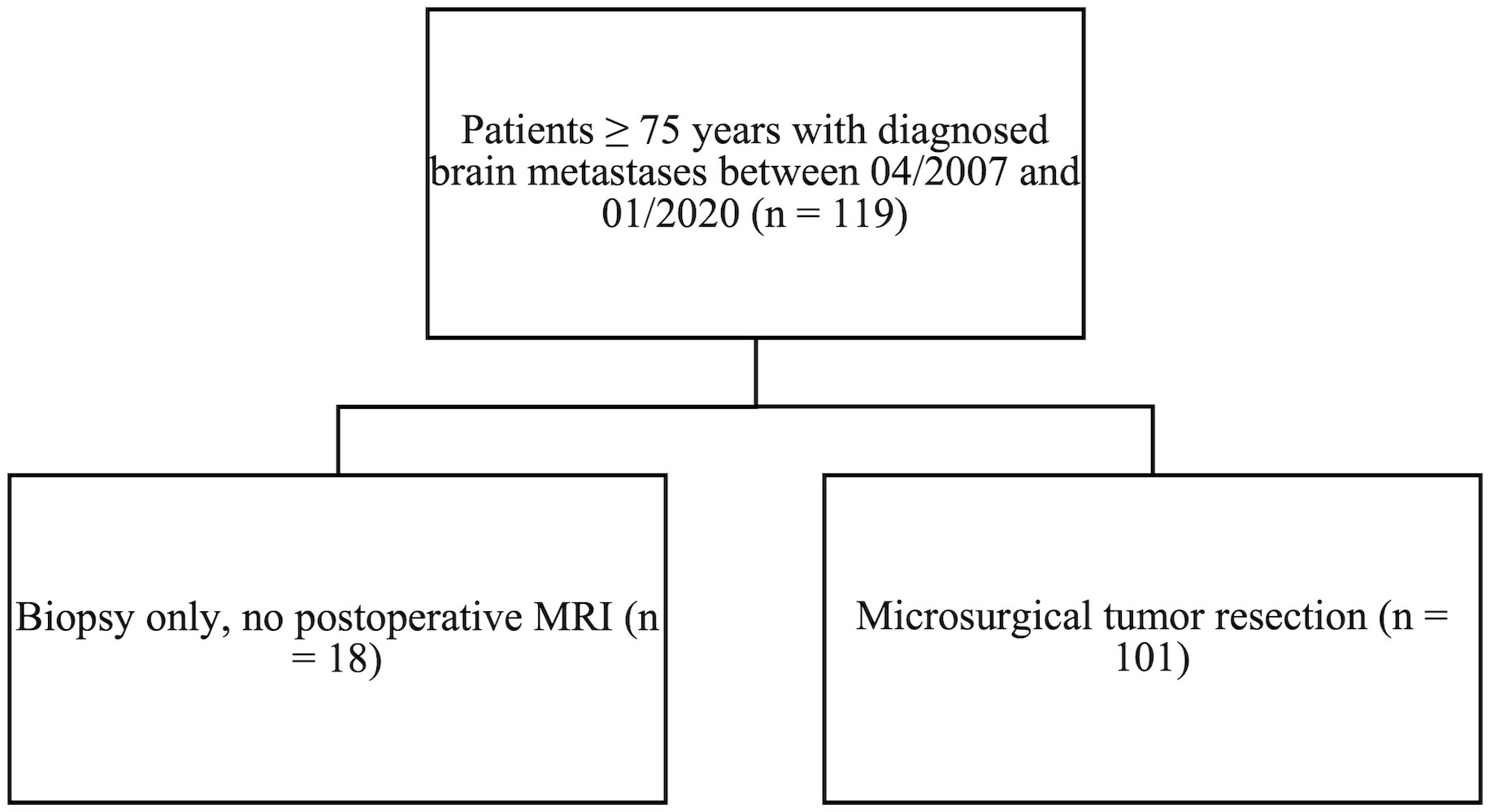
Figure 1 All patients ≥ 75 years with diagnosed brain metastases between April 2007 and January 2020. Patients who met the inclusion criteria of tumor resection beyond biopsy.
According to the univariate analysis, a favorable preoperative KPSS was significantly associated with prolonged survival in this population, with an adjusted HR of 0.986 (p = 0.026). However, the analysis showed age to be a negligible parameter regarding OS (HR 1.024, p = 0.570).
Median OS of all patients was 7 months (IQR 2–11; Figure 2). However, 20% of patients with BMs survived longer than 12 months, and 10% survived longer than 24 months.
Patients with RTB = 0 had significantly higher survival rates (p = 0.007) and longer median survival: 12 (IQR 5–19) versus 5 (IQR 3–7) months, as shown by Kaplan-Meier estimates (Figure 3).

Figure 3 Kaplan-Meier curves for OS of elderly patients with BMs, stratified according to RTB = 0, RTB >0.
A multivariate Cox regression analysis was conducted, including the following risk factors for mortality: sex (female), age, KPSS at admission, preoperative tumor volume, and RTB > 0 (Table 2). Cox regression multivariate analysis identified RTB > 0 to be a significant risk factor for shorter OS (HR 1.898, IQR 1.162–3.099, p = 0.010).
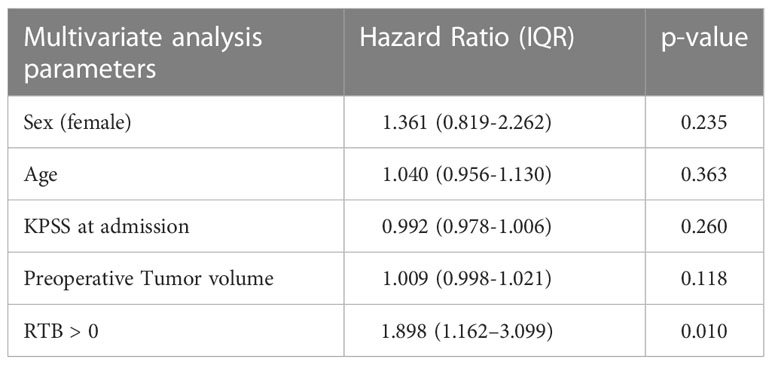
Table 2 Multivariate Cox regression analysis including the following risk factors for mortality: sex (female), age, KPSS at admission, preoperative tumor volume, and RTB > 0.
In a subgroup analysis, patients who underwent complete resection of the targeted metastasis, but had intracranial tumor burden at other locations (N = 78), still had significantly higher survival rates than patients with incomplete resection of the targeted BM (p = 0.042) did. In addition, these patients had longer median OS: 8 (IQR 3–13) versus 5 (IQR 2–8) months.
Patients who underwent postoperative radiotherapy did not have significantly longer OS than the patients who did not receive postoperative radiotherapy.
Considering only patients with a single BM (n = 62), 48 patients (77%) had complete resection. Cox regression multivariate analysis including the above-mentioned risk factors showed incomplete resection a significant risk factor for mortality (HR = 2.398, IQR 1.198-4.779, p = 0.011). Therefore, patients with complete resection have a significantly higher OS (Figure 4).

Figure 4 Kaplan-Meier curves for OS of elderly patients with a single brain metastasis (singular, solitary), stratified for complete vs. incomplete resection.
The results clearly indicate that, for elderly patients with BMs, surgical resection of BMs significantly improves PFS and OS, as RTB is a strong predictor for OS, regardless of age or cancer type. Preoperative high KPSS (HR 0.986, 95% CI 0.973–0.998, p = 0.026) and low RTB (HR 1.025, 95% CI 1.002–1.047, p = 0.029) were significant protective factors for longer OS. Hereby, RTB = 0 was significantly associated with prolonged median OS compared to RTB > 0 (12 months vs. 5 months, p = 0.007). Furthermore, prolongation of survival was significantly associated with surgery in patients who had a favorable KPSS (adjusted HR of 0.986, p = 0.026). However, there were no significances regarding age.
Several studies have found that lower age is a protective factor for prolonged overall survival in patients receiving surgical BM resections (15, 16). This study’s data does not support this, as younger age did not correlate with lower postoperative complication rates or better outcomes. High preoperative KPSS was found to be a significant parameter for prolonged OS. KPSS is a critical parameter when choosing adjuvant therapy (5, 17–20). The reason for the statistical results regarding age in this study is the highly age-selected patient population, as only patients ≥ 75 years were included in the study. Thus, biological age (KPSS) plays a more decisive role, than calendar age does, in patients who have exceeded a certain age.
Therefore, a crucial outcome can be stated, which is beneficial for future treatment of elderly patients with BMs. High KPSS is the more important protective prognostic factor, for better neurological outcomes and longer OS. Thus, when looking at the postoperative treatment structure, patients with a high KPSS and good functional status are more likely to receive adjuvant therapy, which is critical for a long PFS and OS (5, 17, 18, 21).
As the cut-off age of ≤ 65 years in recent publications often overlooks elderly patients, the findings are even more meaningful (6, 7, 9, 12, 14). Advanced age was often determined to be a poor prognostic factor (15). Therefore, surgery for patients in this age group was only performed with caution.
This study showed that advanced age was a negligible risk factor in the selected cohort.
EOR has been a focus of research on the treatment of older glioma patients, and cytoreduction has been shown to improve overall survival in the elderly (20, 22). This study shows that maximal EOR is critical for good long-term outcomes in older patients with BMs, as RTB = 0 significantly improves OS and local tumor control regardless of the cancer type. This finding is in accordance with recent investigations regarding the impact of RTB in younger patients (≤ 65 years) with brain metastases, which have proven low RTB to be a significant predictor for prolonged OS (23). Furthermore, it has been argued that maximal cytoreduction should be achieved, independent of the postoperative radiotherapy type (23). Postoperative MRI should confirm EOR, as intraoperative estimates can misjudge complete resection. Furthermore, previous analyses identified EOR as a strong prognostic factor for long-term local tumor control (13).
Radiotherapy and systemic therapy are important components in oncological therapy of BMs. Data from prospective randomized trials demonstrate that radiotherapy has significant impact on local tumor control and OS (24–26). However, in this research, postoperative radiotherapy did not have a significant impact on prolonged OS. This result can be interpreted by the assumption that, in the selected cohort, only a small proportion of patients did not receive postoperative radiotherapy. To identify the effect of radiotherapy more clearly, the number of patients in the investigated cohort would need to be greater. Furthermore, due to the highly selected patient population in this investigation, there may be a slight bias regarding these results.
Nevertheless, in this study and as shown by Sivansaker et al., the EOR was significantly more decisive for local tumor control and patients’ prolonged OS (13). Thus, detailed analysis focusing on postoperative treatment options should be considered in future interdisciplinary studies.
This study offers a single-center experience with a reasonable number of patients, as well as a homogeneous diagnostic and treatment strategy that allows for comparison. However, it has some limitations.
A limitation of this trial is the retrospective study design, as it introduces an unavoidable selection bias. Furthermore, new histopathological and molecular pathological findings have been discovered, and therapeutic options have been dynamically expanded in recent years, resulting in heterogeneity in the current population. This study cannot reflect the most recent innovations and improvements in systematic chemotherapy for the different cancer entities.
In conclusion, the results indicate that, in elderly patients with BMs, surgical resection of BMs improves PFS and OS, as RTB is a strong predictor for OS, regardless of age or cancer type. Tumor remnant in an early postoperative MRI is the only risk factor for local in-brain recurrence. Therefore, a postoperative MRI should be used to confirm the EOR. Although more patients with metastasized cancer have been treated aggressively in recent years, some studies have argued that elderly patients with brain metastases should not be resected because there might be a higher prevalence of treatment-related toxicity, leading to a poorer prognosis (27). This paradigm must be reconsidered: the data suggests that these patients also benefit significantly from maximal surgical cytoreduction in the presence of intracranial metastases, despite advanced age. The results indicate that, in the elderly, the prognostic value of age becomes less important for outcome and OS, especially among patients ≥75 years. Instead, the patient’s functional status is the most important prognostic factor for long-term outcome and OS. Additionally, surgical resection can enable adjuvant therapy that would otherwise have been impossible by improving the functional outcome.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The local ethics committee approved the study (no. 5626:12). The study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki. The ethics committee waived written informed consent.
Conceptualization, LB and JG. Methodology, LB. Software, LB. Formal analysis, AA. Investigation, LB, AA, BW, AKA. Writing—original draft preparation, LB. Writing—review and editing, LB, AKA, AA, DT, BW, DB, SC, BM, HM, JG. Visualization, AA. Supervision, JG, BM, HM, BW, DB, SC. Project administration, BM and JG. All authors contributed to the article and approved the submitted version.
BM works as consultants for Brainlab Brainlab AG, Feldkirchen. In addition, BM works as a consultant for Medtronic, Spineart, Icotec, Relievant and Depuy/Synthes, as a member of their advisory boards. Furthermore, BM reports financial relationships with Medtronic, Ulrich Medical, Brainlab, Spineart, Icotec, Relievant and Depuy/Synthes. He received personal fees and research grants for clinical studies from Medtronic, Ulrich Medical, Brainlab, Icotec and Relievant. All of this happened independently of the submitted work. BM receives royalties from and holds a patent with Spineart. All of the named potential conflicts of interest are unrelated to this study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Munoz-Bendix C, Rapp M, Mijderwijk HJ, von Sass C, Dibué-Adjei M, Cornelius JF, et al. Risk factors for in-brain local progression in elderly patients after resection of cerebral metastases. Sci Rep (2019) 9(1):1–9. doi: 10.1038/s41598-019-43942-9
2. Van Eeghen EE, Bakker SD, van Bochove A, Loffeld RJLF. Impact of age and comorbidity on survival in colorectal cancer. J Gastrointest Oncol (2015) 6(6):605–12. doi: 10.3978/j.issn.2078-6891.2015.070
3. 3Soffietti R, Abacioglu U, Baumert B, Combs SE, Kinhult S, Kros JM, et al. Diagnosis and treatment of brain metastases from solid tumors: Guidelines from the European association of neuro-oncology (EANO). Neuro Oncol (2017) 19(2):162–74. doi: 10.1093/neuonc/now241
4. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. New English J Med (1990) 323(16):1120–3. doi: 10.1056/NEJM199002223220802
5. Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, et al. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol (2015) 16(6):e270–8. doi: 10.1016/S1470-2045(15)70057-4
6. Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med (1999) 341(27):2061–7. doi: 10.1056/NEJM199912303412706
7. Schmidt E, Balardy L, Geeraerts T, Costa N, Bowers CA, Hamilton M. Geriatric neurosurgery: the unfolding of a new subspecialty. Neurosurg Focus. (2020) 49(4):1–5. doi: 10.3171/2020.7.FOCUS20509
8. Rogne SG, Konglund A, Meling TR, Scheie D, Johannesen TB, Rønning P, et al. Intracranial tumor surgery in patients >70 years of age: Is clinical practice worthwhile or futile? Acta Neurol Scand (2009) 120(5):288–94. doi: 10.1111/j.1600-0404.2009.01157.x
9. Proescholdt M, Jünger ST, Schödel P, Schebesch KM, Doenitz C, Pukrop T, et al. Brain metastases in elderly patients–the role of surgery in the context of systemic treatment. Brain Sci (2021) 11(1):1–10. doi: 10.3390/brainsci11010123
10. Heimann M, Schäfer N, Bode C, Borger V, Eichhorn L, Giordano FA, et al. Outcome of elderly patients with surgically treated brain metastases. Front Oncol (2021) 11:713965. doi: 10.3389/fonc.2021.713965
11. Lagerwaard FJ, Levendag PC, Nowak PJCM, Eijkenboom WMH, Hanssens PEJ, Schmitz PIM. Identification of prognostic factors in patients with brain metastases: A review of 1292 patients. Int J Radiat Oncol Biol Phys (1999) 43(4):795–803. doi: 10.1016/S0360-3016(98)00442-8
12. Lutterbach J, Bartelt S, Momm F, Becker G, Frommhold H, Ostertag C. Is older age associated with a worse prognosis due to different patterns of care? a long-term study of 1346 patients with glioblastomas or brain metastases. Cancer. (2005) 103(6):1234–44. doi: 10.1002/cncr.20895
13. Sivansaker M, Madhugiri VS, Moiyadi AV, Shetty P, Subi TS. Surgery for brain metastases: An analysis of outcomes and factors affecting survival. Clin Neurol Neurosurg (2018) 168:153–62. doi: 10.1016/j.clineuro.2018.03.011
14. Halani SH, Babu R, Adamson DC. Management of glioblastoma multiforme in the elderly: a review of the literature sameer. World Neurosurg (2017) 105:53–62. doi: 10.1016/j.wneu.2017.04.153.This
15. Kavouridis VK, Harary M, Hulsbergen AFC, Lo YT, Reardon DA, Aizer AA, et al. Survival and prognostic factors in surgically treated brain metastases. J Neurooncol. (2019) 143(2):359–67. doi: 10.1007/s11060-019-03171-6
16. Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: Data from the glioma outcomes project. J Neurosurg (2003) 99(3):467–73. doi: 10.3171/jns.2003.99.3.0467
17. Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. J Am Med Assoc (2006) 295(21):2483–91. doi: 10.1001/jama.295.21.2483
18. Barz M, Bette S, Janssen I, Aftahy AK, Huber T, Liesche-Starnecker F, et al. Age-adjusted charlson comorbidity index in recurrent glioblastoma: a new prognostic factor? BMC Neurol (2022) 22(1):4–11. doi: 10.1186/s12883-021-02532-x
19. Sacko A, Michael MH. Evolution of the Karnosky Performance Status throughout life in glioblastoma patients. J Neurooncol. (2015) 122(3)567–73. doi: 10.1007/s11060-015-1749-6.
20. Grabowski MM, Recinos PF, Nowacki AS, Schroeder JL, Angelov L, Barnett GH, et al. Residual tumor volume versus extent of resection: Predictors of survival after surgery for glioblastoma. J Neurosurg (2014) 121(5):1115–23. doi: 10.3171/2014.7.JNS132449
21. Noordijk EM, Vecht CJ, Haaxma-Reiche H, Padberg GW, Voormolen JH, Hoekstra FH, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys (1994) 29(4):711–7. doi: 10.1016/0360-3016(94)90558-4
22. Álvarez de Eulate-Beramendi S, Álvarez-Vega MA, Balbin M, Sanchez-Pitiot A, Vallina-Alvarez A, Martino-González J. Prognostic factors and survival study in high-grade glioma in the elderly. Br J Neurosurg (2016) 30(3):330–6. doi: 10.3109/02688697.2016.1139049
23. Aftahy AK, Barz M, Lange N, Baumgart L, Thunstedt C, Eller MA, et al. The impact of postoperative tumor burden on patients with brain metastases. Front Oncol (2022) 12:869764. doi: 10.3389/fonc.2022.869764
24. Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, Meyers C, et al. Memantine for the prevention of cognitive. Neuro Oncol (2013) 15(10):1429–37. doi: 10.1093/neuonc/not114
25. Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol (2017) 18(8):1049–60. doi: 10.1016/S1470-2045(17)30441-2
26. Li J, Brown PD. The diminishing role of whole-brain radiation therapy in the treatment of brain metastases. JAMA Oncol (2017) 3(8):1023–4. doi: 10.1001/jamaoncol.2016.5411
Keywords: brain metastasis, elderly, overall survival, extent of resection, postoperative MRI, postoperative tumor volume
Citation: Baumgart L, Aftahy AK, Anetsberger A, Thunstedt D, Wiestler B, Bernhardt D, Combs SE, Meyer B, Meyer HS and Gempt J (2023) Brain metastases in the elderly – Impact of residual tumor volume on overall survival. Front. Oncol. 13:1149628. doi: 10.3389/fonc.2023.1149628
Received: 22 January 2023; Accepted: 21 March 2023;
Published: 04 April 2023.
Edited by:
Leonora Balaj, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Atsuto Katano, The University of Tokyo Hospital, JapanCopyright © 2023 Baumgart, Aftahy, Anetsberger, Thunstedt, Wiestler, Bernhardt, Combs, Meyer, Meyer and Gempt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lea Baumgart, bGVhYmF1bWdhcnQxOTk2QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.