
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 05 May 2023
Sec. Thoracic Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1148786
This article is part of the Research TopicUpdates on Immune Checkpoint Inhibitors in Lung CancerView all 7 articles
 Menglu Dai
Menglu Dai Wei Wu*
Wei Wu*Background: There are numerous articles investigating whether C-reactive protein to albumin ratio (CAR) is significant for predicting prognosis of cancer cases receiving immune checkpoint inhibitors (ICIs), whereas the results were inconsistent. We thus retrieved the literature and conducted the present meta-analysis for clarifying relation of CAR with survival outcomes among ICI-treated cancer patients.
Methods: Through search against the Web of Science, PubMed, Cochrane Library, and Embase databases was carried out. The search was updated on 11 December 2022. This work later determined the combined hazard ratios (HRs) together with 95% confidence intervals (CIs) for estimating CAR for its prognostic efficiency for overall survival (OS) and progression-free survival (PFS) in cancer patients receiving ICIs.
Results: A total of 11 studies consisting of 1,321 cases were enrolled into the present meta-analysis. As revealed by combined data, the increased CAR level markedly predicted dismal OS (HR = 2.79, 95% CI = 1.66–4.67, p < 0.001) together with shortened PFS (HR = 1.95, 95% CI = 1.25–3.03, p = 0.003) among carcinoma cases using ICIs. The prognostic effect of CAR was not influenced by clinical stage or study center. Our result reliability was suggested by sensitivity analysis and publication bias test.
Conclusions: High CAR expression showed marked relation to worse survival outcomes among ICI-treated cancer cases. CAR is easily available and cost effective, which can be the potential biomarker for selecting cancer cases benefiting from ICIs.
Cancer refers to one of the most lethal diseases with high morbidity, mortality, and economic burden around the world (1). In recent years, immunotherapy has played pivotal roles in cancer treatment (2). Immune checkpoint inhibitors (ICIs) including antibody drugs that target programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1) can provide a durable clinical response among diverse cancers in the case of effective treatment (3). ICIs such as nivolumab (anti-PD-1), atezolizumab (anti-PD-L1), or ipilimumab [anti-cytotoxic T lymphocyte–associated antigen 4 (CTLA-4)] have shown prolonged survival against cancer (4–6). It is important to note, however, that only a small proportion of patients can benefit from ICIs, and others do not respond to immunotherapy, thus limiting their use in the clinic (7). Consequently, identifying effective prognostic biomarkers for predicting the survival of cancer patients undergoing ICIs is urgently needed.
Cancer metabolism draws much attention from scientific community in the era of cancer immunotherapy (8–10). Many laboratory-derived parameters have been investigated as promising biomarkers in prognosis of cancer patients undergoing ICIs, such as systemic immune-inflammation index (SII) (11), prognostic nutritional index (PNI) (12), modified Glasgow Prognostic Score (mGPS) (13), C-reactive protein to albumin ratio (CAR) (14), along with neutrophil-to-lymphocyte ratio (NLR) (15). According to albumin and C-reactive protein (CRP) levels in the serum, CAR is determined to be CRP-to-albumin ratio. CAR is calculated as the following formula: CAR = CRP (mg/liter)/albumin (g/liter) (16). It is reported that the median value and normal range of CAR in healthy individuals are as follows: 0.21 (0.05–1.08) (17). Previous studies have analyzed relation of CAR with patient survival using ICIs, whereas the results were controversial (14, 18–27). For example, some researchers identified CAR to be the efficient biomarker for predicting prognosis of cancer patients receiving ICIs (18, 23–25). However, some other scholars reported that relation of CAR with prognosis among carcinoma cases using ICIs was nonsignificant (21). Therefore, we collected the most recent and comprehensive literature and carried this meta-analysis for precisely identifying CAR’s effect on predicting prognosis of carcinoma cases receiving ICIs treatment.
The present meta-analysis was performed following guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (28).
Through search of Web of Science, PubMed, Cochrane Library, and Embase databases was carried out. The search strategy was shown below: (“C-reactive protein to albumin ratio” or “C-reactive protein albumin ratio” or “CRP/albumin ratio” or “C-reactive protein/albumin ratio”) and (“immune check point inhibitor” or “PD-1” or “PD-L1” or “immunotherapy” or “nivolumab” or “CTLA-4” or “pembrolizumab” or “avelumab”). The search was updated on 11 December 2022. The publication language was restricted to English. Reference lists in enrolled articles were manually searched for identifying possible missing articles.
Studies conforming to criteria below were included: (1) studies recruiting carcinoma patients receiving ICIs treatment, (2) CAR data before treatment were measured, (3) studies reported relation of CAR with survival outcomes among cancer cases undergoing ICIs, (4) the hazard ratios (HRs) together with 95% confidence intervals (CIs) were available or calculable by provided information, (5) identification of a threshold for stratifying high/low CAR, and (6) English articles. Studies conforming to conditions below were excluded: (1) case reports, reviews, letters, comments, conference abstracts, and editorials; (2) duplicates; (3) studies with not enough data to analyze survival; and (4) animal studies. Overall survival (OS) and progression-free survival (PFS) were treated as primary and secondary outcomes, separately.
Two independent reviewers (M.D. and W.W.) analyzed the included articles and extracted the data. All disagreements were resolved by discussion until consensus. Data collected included first author, country, publication year, study period, sample size, age, sex, cancer type, cancer stage, treatment, ICIs antibody type, threshold CAR, follow-up, study center, HR analysis type, and HRs together with 95% CIs. Newcastle–Ottawa Scale (NOS) was adopted for assessing enrolled study methodological quality (29). Typically, NOS assesses study quality from three perspectives, including comparability (0–2 points), selection (0–4 points), and outcomes (0–3 points). Total NOS score was 0–9, while articles of NOS score ≥ 6 were defined to be high-quality ones.
This work determined combined HRs together with 95% CIs for estimating whether CAR was efficient in predicting prognosis of OS and PFS for cancer cases receiving ICIs. Inter-study heterogeneities were assessed by Higgins I² statistics and Cochran’s Q test. I2 > 50% or P < 0.10 in heterogeneity indicates obvious heterogeneity, so the random-effects model was employed; or else, the fixed-effects model is used. Pooled odds ratios (ORs) and 95% CIs were calculated to estimate the impact of CAR on objective response rate (ORR). Subgroup analyses according to different factors were carried out for detecting potential heterogeneity source. We performed sensitivity analyses to examine the effects of omitting studies potentially contributing to data heterogeneity. Begg’s test and funnel plot were carried out for examining publication bias. Stata software version 12.0 (Stata Corporation, College Station, TX) was adopted for statistical analysis. p < 0.05 (two sided) stood for statistical significance.
Based on already published studies, ethical approval was waived for this meta-analysis.
Upon primary database search, altogether 282 records were obtained, among which 134 studies were remained following removal of duplicates. Subsequently, through title and abstract screening, 83 articles were excluded, while the rest 51 were assessed through reading the full texts. Then, we discarded 40 articles due to no analysis of CAR (n = 38), no ICIs treatment used (n = 1), and duplicate patients involved (n = 1). Finally, the present meta-analysis included altogether 11 studies involving 1,321 cases (14, 18–27) (Figure 1; Table 1).
Table 1 displays basic features of enrolled articles. All articles were published from 2018 to 2022, indicating the recent literature on the research topic. There were 10 articles carried out in Japan (14, 18–26), and one study was carried out in China (27). All articles were published in the English language and of retrospective design (14, 18–27), with the median sample size of 93 (range, 34-304). Five articles recruited non-small cell lung cancer (NSCLC) cases (14, 18, 20–22), two studies enrolled head and neck squamous cell carcinoma (HNSCC) cases (23, 26), two studies enrolled patients with esophageal squamous cell carcinoma (ESCC) (24, 25), while one each study included patients with melanoma (19) and intrahepatic cholangiocarcinoma (ICC) (27). Nine studies used anti-PD-1 treatment (14, 18–20, 23–27), and two studies applied anti-PD-1/PD-L1 strategy (21, 22). Thresholds of CAR were 0.057–0.83 in included studies. The median value of CAR cutoff values was 0.3, and the mean value was 0.324. Six articles were multicenter researches (14, 18, 20, 22–24), and five studies were conducted in single center (19, 21, 25–27). Nine studies reported whether CAR was important for predicting the prognosis of OS in cancer cases receiving ICIs treatment (14, 18, 21–27), and six articles investigated relation of CAR with PFS (14, 19–23). Multivariate regression from six articles (14, 20, 23–26), while univariate regression from five articles (18, 19, 21, 22, 27), reported HRs together with 95%CIs. For those enrolled articles, their NOS scores were 6–9 (median, 8), suggesting that high quality of those eligible articles.
Nine studies with 1,069 patients (14, 18, 21–27) provided the data on relation of CAR with OS among cancer cases receiving ICIs treatment. Combined HR = 2.79, 95% CI = 1.66–4.67, p < 0.001 were obtained, which indicated that a higher CAR level significantly predicted poor OS in carcinoma patients using ICIs (Figure 2 and Table 2). This work adopted a random-effects model because of the obvious heterogeneity (I2 = 89.7%, Ph < 0.001). Subgroup analysis was utilized stratified by country, sample size, cancer type, clinical stage, treatment, CAR cutoff value, HR type, and study center. As shown in Table 2, CAR’s role in predicting OS remained unchanged by country, sample size, clinical stage, or study center (all p < 0.05).
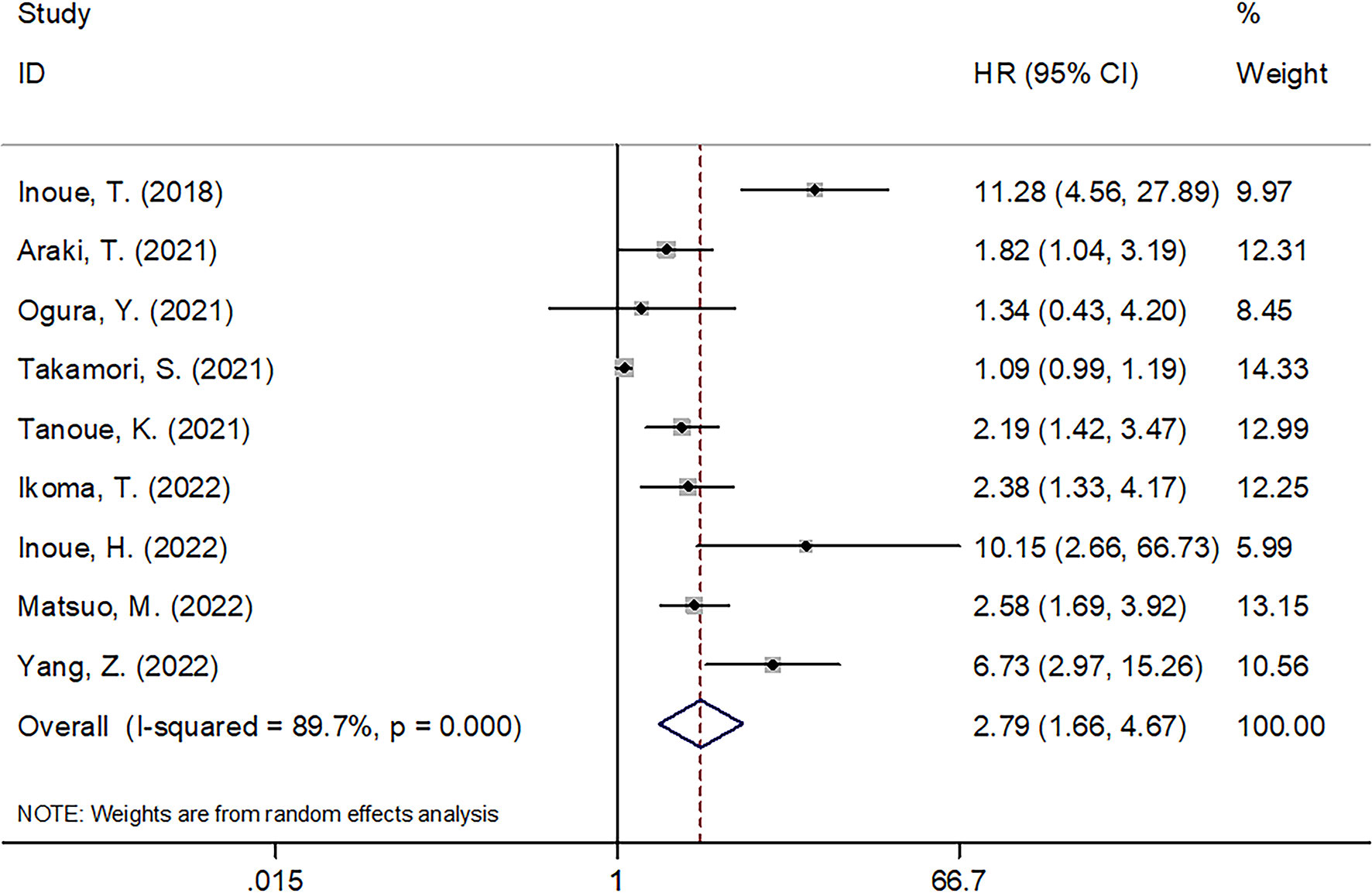
Figure 2 Forest plots of studies evaluating the association between CAR level and OS in cancer patients receiving ICIs.
There were six articles involving 749 cases (14, 19–23) analyzing whether CAR was significant in predicting PFS among cancer patients receiving ICIs. According to Table 3 and Figure 3, combined results included HR = 1.95, 95% CI = 1.25–3.03, p = 0.003, indicating a significant relation of CAR with worse PFS among carcinoma cases receiving ICIs treatment. According to subgroup analysis, CAR’s role in predicting PFS remained significant irrespective of cancer type, clinical stage, ICIs treatment, or study center (Table 3).
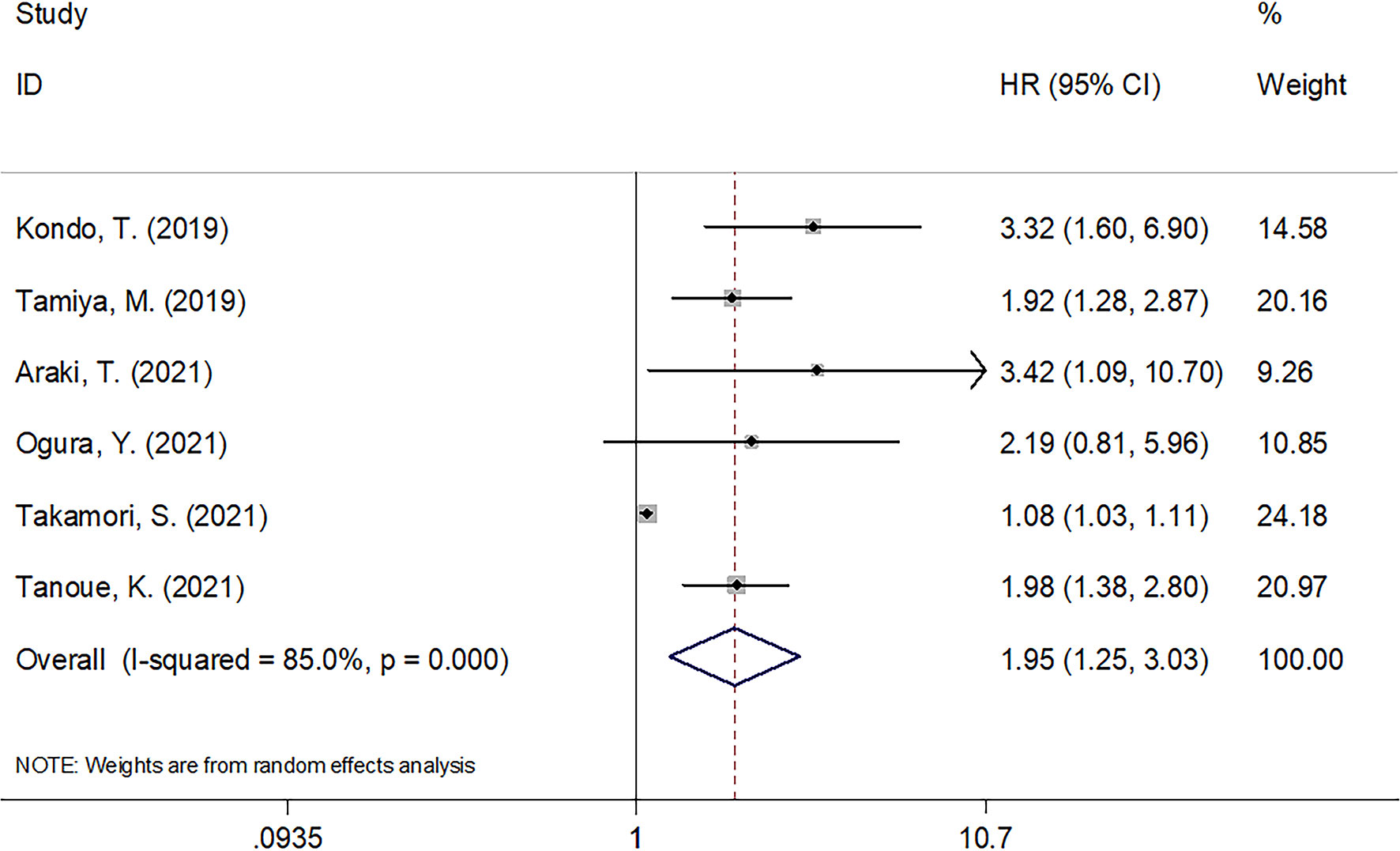
Figure 3 Forest plots of studies evaluating the association between CAR level and PFS in cancer patients treated with ICIs.
A total of three studies with 180 cases (23–25) reported the impact of CAR on ORR in cancer patients undergoing ICIs. Due to significant heterogeneity (I2 = 78.5%, Ph = 0.010), a random-effects model was used. The pooled results were as follows: OR = 0.23, 95% CI = 0.04–1.19, p = 0.079, suggesting that there was no significant association between CAR and ORR (Figure 4).
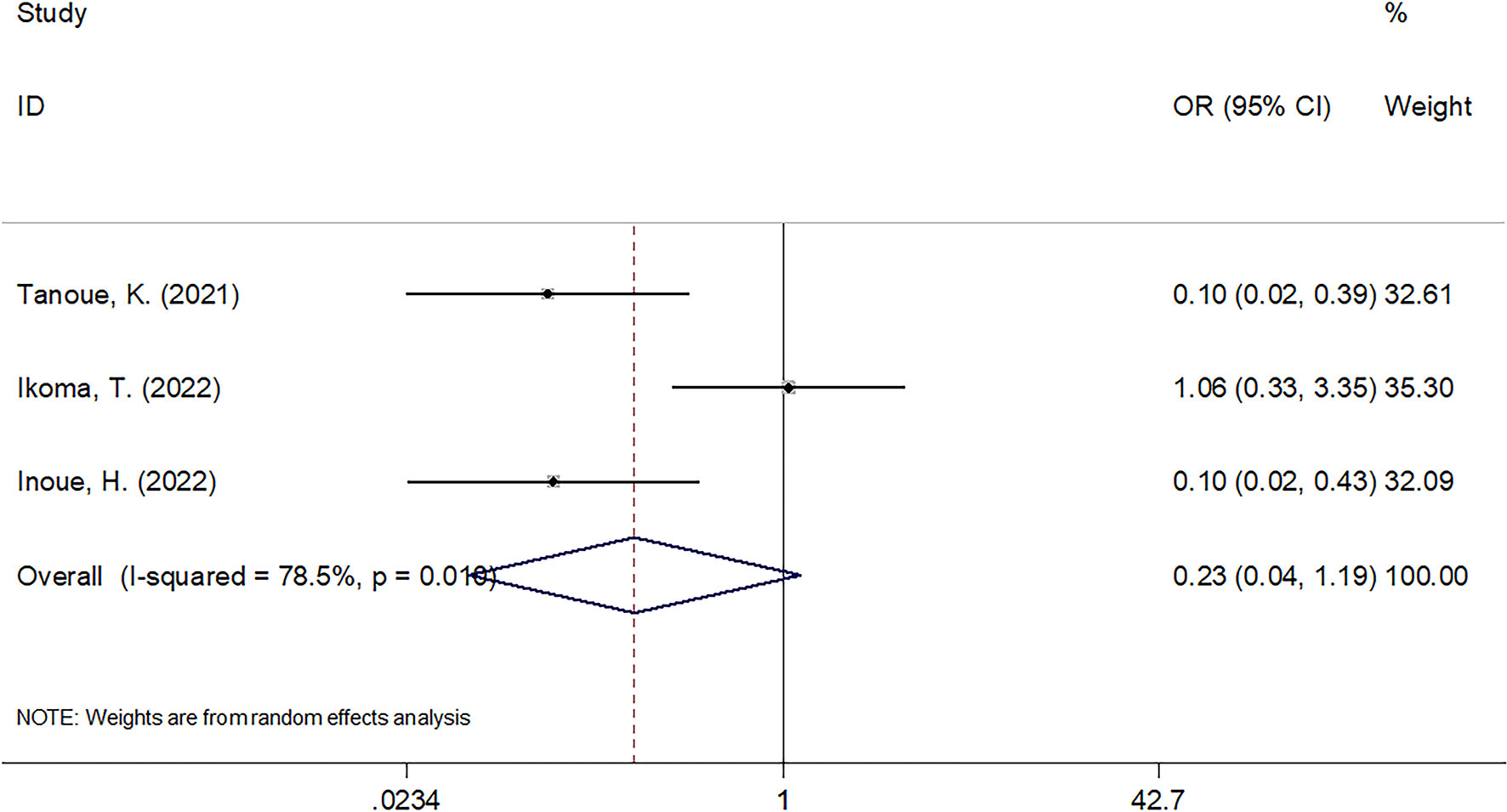
Figure 4 The forest plots of studies evaluating the association between CAR level and ORR in cancer patients treated with ICIs.
This work conducted sensitivity analysis through omitting a work each time for testing our result robustness. According to Figure 5, OS and PFS HR estimates did not significantly change, suggesting that our meta-analysis was credible.
Funnel plots and Begg’s test were performed to test possible publication bias. According to Figure 6, the funnel plots were symmetrical and Begg’s test revealed no evidence of obvious publication bias for OS (p = 0.466) or PFS (p = 0.851).
CAR’s effect on prognosis prediction for cancer cases receiving ICIs treatment remains controversial in prior works. According to this current meta-analysis, data from 11 articles involving 1,321 cases were synthesized (14, 18–27) to accurately identify the association of CAR with cancer cases receiving ICIs. According to this meta-analysis, the high CAR level markedly predicted the poor OS and PFS among ICI-treated carcinoma cases. Moreover, the prognostic effect of CAR was not influenced by clinical stage and study center for OS or PFS. Publication bias test and sensitivity analysis suggested that our results were reliable. Collectively, in the present meta-analysis, elevated CAR was a reliable and cost-effective marker for poor survival in cancer patients undergoing ICIs. Monitoring CAR level could aid in identifying high-risk ICIs treated patients and therefore help tailor treatment strategy. As far as we know, the present meta-analysis was the first to explore CAR for its value in prognosis prediction for cancer cases receiving ICIs.
CAR was computed by CRP to albumin ratio; consequently, an increased CAR is attributed to the increased CRP level and/or the decreased serum albumin level. The mechanisms of CAR for prognosis of ICI-treated cancer patients are not comprehensively analyzed hitherto; however, it is interpreted as follows. First, CRP is synthesized in the liver and plays a role in acute inflammation (30). CRP is a pro-inflammatory protein regulated via numerous pro-inflammatory factors, containing IL-1 and IL-6 (31). As a result of elevated serum CRP levels, vascular endothelial growth factor levels often increase as well, contributing to the progression and formation of cancers (32). Second, serum albumin accounts for approximately 60% of the total protein; therefore, albumin level can be usually recognized to be the biological indicator of nutritional status (33). In many cancers, there is a significant correlation between serum albumin levels and systemic inflammatory response, body nutritional status, as well as clinical survival (34). As a result of cancer-related inflammation or infection, serum albumin escapes into the interstitium through increased capillary permeability, which also leads to hypoalbuminemia (35). Therefore, the CAR combines serum CRP and albumin levels concurrently, can more accurately reflect the host’s inflammatory status, and can participate in prediction of survival outcomes in ICI-treated cancer patients. Notably, CAR is derived from blood test. These parameters are easily available and cost effective. No additional cost was added for patients, because blood test is a routine test in clinics. Therefore, CAR is cost effective for patients and clinicians.
Many recent articles report that CAR can be used to predict prognosis of different cancers based on meta-analysis (36–39). Wu et al. showed that the large CAR before treatment effectively predicted dismal outcome of urinary cancer cases based on a meta-analysis including 2,941 cases (36). Our previous work reported that the high CAR showed marked relation with dismal OS together with decreased disease-free survival (DFS) or recurrence-free survival among bile duct cancer cases through the meta-analysis comprising fourteen articles (38). According to one meta-analysis involving 771 cases, the large CAR negatively predicted the prognosis of metastatic colorectal cancer cases (40). Luan et al. demonstrated that patients with head and neck cancer have a poorer prognosis when their pretreatment CAR is elevated by a meta-analysis containing 7,080 participants (41). Xie et al. carried out the meta-analysis involving 2,271 subjects, according to their results, pancreatic cancer patients with elevated CAR had inferior OS, PFS, and DFS (42). In this meta-analysis, high CAR was recognized to be the significant prognostic biomarker for carcinoma patients treated with ICIs, which was in line with findings of previous studies on diverse cancers.
Certain limitations should be pointed out in the present meta-analysis. Initially, all enrolled articles are from east Asia, especially Japan (10/11). There was no restriction on geographical region of enrolled articles. Second, cutoff values of CAR were not consistent among eligible studies. The cutoff values are various across included studies from 0.057 to 0.83. The cutoff values can be influenced by the recruiting subjects and the determination methods. We adopted the CAR = 0.3 for subgroup analysis, because the median value of CAR cutoff was 0.3 of included studies. Third, the enrolled articles were of retrospective nature, possibly leading to inherent selection bias. Fourth, the units of CAR have not been standardized across studies. Some studies used the formula: CAR = CRP (mg/liter)/albumin (g/liter) (16). Whereas some other used CAR = CRP (mg/dl)/albumin (g/dl) (43). Although the CAR ratio was not influenced by the units: (mg/liter)/(g/liter) or (mg/dl)/(g/dl). Due to these limitations, large, prospective clinical studies are still warranted for validating our results.
In summary, the high CAR level before treatment significantly predicted inferior OS and PFS among cancer cases undergoing ICIs. The prognostic effect of CAR was not influenced by clinical stage or study center for ICI-treated cancer cases. CAR is easily available and cost effective and serves as the potential biomarker for selecting cancer cases benefiting from ICIs.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
MD and WW conceived the study concept and design. MD and WW were responsible for statistical analysis. MD and WW helped the data analysis and consultation for manuscript preparation. MD wrote the first draft. WW revised the manuscript. All authors made the critical revision of the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CAR, C-reactive protein to albumin ratio; ICIs, immune checkpoint inhibitors; HR, hazard ratio; CI, confidence interval; OS, overall survival; PFS, progression-free survival; PD-1, programmed death-1; PD-L1, programmed death ligand-1; CTLA-4, cytotoxic T lymphocyte–associated antigen 4; SII, systemic immune-inflammation index; PNI, prognostic nutritional index; Mgps, modified Glasgow Prognostic Score; NLR, neutrophil-to-lymphocyte ratio; CRP, C-reactive protein; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; NOS, Newcastle–Ottawa Scale; NSCLC, non-small cell lung cancer; HNSCC, head and neck squamous cell carcinoma; ESCC, esophageal squamous cell carcinoma; ICC, intrahepatic cholangiocarcinoma.
1. Hulvat MC. Cancer incidence and trends. Surg Clin North Am (2020) 100(3):469–81. doi: 10.1016/j.suc.2020.01.002
2. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. (2021) 16:223–49. doi: 10.1146/annurev-pathol-042020-042741
3. He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res (2020) 30(8):660–9. doi: 10.1038/s41422-020-0343-4
4. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-Small-Cell lung cancer. N Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
5. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-Positive non-Small-Cell lung cancer. N Engl J Med (2016) 375(19):1823–33. doi: 10.1056/NEJMoa1606774
6. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med (2015) 373(1):23–34. doi: 10.1056/NEJMoa1504030
7. Zhang Q, Gong X, Sun L, Miao L, Zhou Y. The predictive value of pretreatment lactate dehydrogenase and derived neutrophil-to-Lymphocyte ratio in advanced non-small cell lung cancer patients treated with PD-1/PD-L1 inhibitors: a meta-analysis. Front Oncol (2022) 12:791496. doi: 10.3389/fonc.2022.791496
8. Leone RD, Powell JD. Metabolism of immune cells in cancer. Nat Rev Cancer (2020) 20(9):516–31. doi: 10.1038/s41568-020-0273-y
9. Bader JE, Voss K, Rathmell JC. Targeting metabolism to improve the tumor microenvironment for cancer immunotherapy. Mol Cell (2020) 78(6):1019–33. doi: 10.1016/j.molcel.2020.05.034
10. Stine ZE, Schug ZT, Salvino JM, Dang CV. Targeting cancer metabolism in the era of precision oncology. Nat Rev Drug Discovery (2022) 21(2):141–62. doi: 10.1038/s41573-021-00339-6
11. Tian BW, Yang YF, Yang CC, Yan LJ, Ding ZN, Liu H, et al. Systemic immune-inflammation index predicts prognosis of cancer immunotherapy: systemic review and meta-analysis. Immunotherapy (2022) 14(18):1481–96. doi: 10.2217/imt-2022-0133
12. Shao Y, Cao W, Gao X, Tang M, Zhu D, Liu W. Pretreatment "prognostic nutritional index" as an indicator of outcome in lung cancer patients receiving ICI-based treatment: systematic review and meta-analysis. Med (Baltimore) (2022) 101(43):e31113. doi: 10.1097/md.0000000000031113
13. Zhang Y, Chen S, Chen H, Li W. A comprehensive analysis of Glasgow prognostic score (GPS)/the modified Glasgow prognostic score (mGPS) on immune checkpoint inhibitor efficacy among patients with advanced cancer. Cancer Med (2022) 12(1):38–48. doi: 10.1002/cam4.4940
14. Araki T, Tateishi K, Sonehara K, Hirota S, Komatsu M, Yamamoto M, et al. Clinical utility of the c-reactive protein: albumin ratio in non-small cell lung cancer patients treated with nivolumab. Thorac Cancer (2021) 12(5):603–12. doi: 10.1111/1759-7714.13788
15. Xie X, Liu J, Yang H, Chen H, Zhou S, Lin H, et al. Prognostic value of baseline neutrophil-to-Lymphocyte ratio in outcome of immune checkpoint inhibitors. Cancer Invest (2019) 37(6):265–74. doi: 10.1080/07357907.2019.1639057
16. Jiang Y, Gu H, Zheng X, Pan B, Liu P, Zheng M. Pretreatment c-reactive Protein/Albumin ratio is associated with poor survival in patients with 2018 FIGO stage IB-IIA HPV-positive cervical cancer. Pathol Oncol Res POR (2021) 27:1609946. doi: 10.3389/pore.2021.1609946
17. Çelikkol A, Güzel E, Doğan M, Erdal B, Yilmaz A. C-reactive protein-to-Albumin ratio as a prognostic inflammatory marker in COVID-19. J Lab Physicians (2022) 14(1):74–83. doi: 10.1055/s-0041-1741439
18. Inoue T, Tamiya M, Tamiya A, Nakahama K, Taniguchi Y, Shiroyama T, et al. Analysis of early death in Japanese patients with advanced non-small-cell lung cancer treated with nivolumab. Clin Lung Cancer (2018) 19(2):e171–e6. doi: 10.1016/j.cllc.2017.09.002
19. Kondo T, Nomura M, Otsuka A, Nonomura Y, Kaku Y, Matsumoto S, et al. Predicting marker for early progression in unresectable melanoma treated with nivolumab. Int J Clin Oncol (2019) 24(3):323–7. doi: 10.1007/s10147-018-1345-9
20. Tamiya M, Tamiya A, Hosoya K, Taniguchi Y, Yokoyama T, Fukuda Y, et al. Efficacy and safety of pembrolizumab as first-line therapy in advanced non-small cell lung cancer with at least 50% PD-L1 positivity: a multicenter retrospective cohort study (HOPE-001). Investigational New Drugs (2019) 37(6):1266–73. doi: 10.1007/s10637-019-00843-y
21. Ogura Y, Kataoka N, Kunimatsu Y, Tachibana Y, Sugimoto T, Tani N, et al. Predictors of survival among Japanese patients receiving first-line chemoimmunotherapy for advanced non-small cell lung cancer. Thorac Cancer (2021) 12(1):97–105. doi: 10.1111/1759-7714.13720
22. Takamori S, Takada K, Shimokawa M, Matsubara T, Fujishita T, Ito K, et al. Clinical utility of pretreatment Glasgow prognostic score in non-small-cell lung cancer patients treated with immune checkpoint inhibitors. Lung Cancer (Amsterdam Netherlands) (2021) 152:27–33. doi: 10.1016/j.lungcan.2020.11.026
23. Tanoue K, Tamura S, Kusaba H, Shinohara Y, Ito M, Tsuchihashi K, et al. Predictive impact of c-reactive protein to albumin ratio for recurrent or metastatic head and neck squamous cell carcinoma receiving nivolumab. Sci Rep (2021) 11(1):2741. doi: 10.1038/s41598-021-82448-1
24. Ikoma T, Shimokawa M, Matsumoto T, Boku S, Yasuda T, Shibata N, et al. Inflammatory prognostic factors in advanced or recurrent esophageal squamous cell carcinoma treated with nivolumab. Cancer Immunol Immunother CII (2023) 72(2):427–35. doi: 10.1007/s00262-022-03265-7
25. Inoue H, Shiozaki A, Fujiwara H, Konishi H, Kiuchi J, Ohashi T, et al. Absolute lymphocyte count and c-reactive protein-albumin ratio can predict prognosis and adverse events in patients with recurrent esophageal cancer treated with nivolumab therapy. Oncol Lett (2022) 24(2):257. doi: 10.3892/ol.2022.13377
26. Matsuo M, Yasumatsu R, Masuda M, Toh S, Wakasaki T, Hashimoto K, et al. Inflammation-based prognostic score as a prognostic biomarker in patients with recurrent and/or metastatic head and neck squamous cell carcinoma treated with nivolumab therapy. In Vivo (2022) 36(2):907–17. doi: 10.21873/invivo.12780
27. Yang Z, Zhang D, Zeng H, Fu Y, Hu Z, Pan Y, et al. Inflammation-based scores predict responses to PD-1 inhibitor treatment in intrahepatic cholangiocarcinoma. J Inflammation Res (2022) 15:5721–31. doi: 10.2147/jir.S385921
28. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol (2009) 62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005
29. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
30. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest (2003) 111(12):1805–12. doi: 10.1172/jci18921
31. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis (2009) 30(7):1073–81. doi: 10.1093/carcin/bgp127
32. Xavier P, Belo L, Beires J, Rebelo I, Martinez-de-Oliveira J, Lunet N, et al. Serum levels of VEGF and TNF-alpha and their association with c-reactive protein in patients with endometriosis. Arch Gynecol Obstet (2006) 273(4):227–31. doi: 10.1007/s00404-005-0080-4
33. Caraceni P, Tufoni M, Bonavita ME. Clinical use of albumin. Blood transfusion = Trasfusione del sangue (2013) 11 Suppl 4(Suppl 4):s18–25. doi: 10.2450/2013.005s
34. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J (2010) 9:69. doi: 10.1186/1475-2891-9-69
35. Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J parenteral enteral Nutr (2019) 43(2):181–93. doi: 10.1002/jpen.1451
36. Wu M, Zhou Y, Chen Q, Yu Z, Gu H, Lin P, et al. Prognostic role of pretreatment c-reactive protein to albumin ratio in urological cancers: a systematic review and meta-analysis. Front Oncol (2022) 12:879803. doi: 10.3389/fonc.2022.879803
37. Lu H, Huang MN, Li CY, Tang SJ. The effect of c-reactive protein/albumin ratio on overall survival in patients with oral squamous cell carcinoma: a meta-analysis. Asian J Surg (2023) 46(4):1728–30. doi: 10.1016/j.asjsur.2022.09.144
38. Dai M, Zhao X, Yu A, Zhao L, Kang Q, Yan S, et al. Prognostic and clinicopathological significance of c-reactive protein to albumin ratio in patients with bile duct cancer: a meta-analysis. Nutr Cancer (2022), 1–13. doi: 10.1080/01635581.2022.2104876
39. Yu Q, Li KZ, Fu YJ, Tang Y, Liang XQ, Liang ZQ, et al. Clinical significance and prognostic value of c-reactive protein/albumin ratio in gastric cancer. Ann Surg Treat Res (2021) 100(6):338–46. doi: 10.4174/astr.2021.100.6.338
40. Pan Y, Lou Y, Wang L. Prognostic value of c-reactive protein to albumin ratio in metastatic colorectal cancer: a systematic review and meta-analysis. Med (Baltimore) (2021) 100(46):e27783. doi: 10.1097/md.0000000000027783
41. Luan CW, Yang HY, Tsai YT, Hsieh MC, Chou HH, Chen KS. Prognostic value of c-reactive protein-to-Albumin ratio in head and neck cancer: a meta-analysis. Diagnostics (Basel Switzerland) (2021) 11(3):403. doi: 10.3390/diagnostics11030403
42. Xie Q, Wang L, Zheng S. Prognostic and clinicopathological significance of c-reactive protein to albumin ratio in patients with pancreatic cancer: a meta-analysis. Dose-response Publ Int Hormesis Soc (2020) 18(2):1559325820931290. doi: 10.1177/1559325820931290
Keywords: CAR, immune checkpoint inhibitors, meta-analysis, prognosis, clinical management
Citation: Dai M and Wu W (2023) Prognostic role of C-reactive protein to albumin ratio in cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Front. Oncol. 13:1148786. doi: 10.3389/fonc.2023.1148786
Received: 20 January 2023; Accepted: 18 April 2023;
Published: 05 May 2023.
Edited by:
Liyun Shi, Nanjing University of Chinese Medicine, ChinaCopyright © 2023 Dai and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Wu, d3V3ZWk1MDEzQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.