- 1Department of Gastrointestinal Medical Oncology, Fudan University Shanghai Cancer Center, Shanghai, China
- 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
- 3Department of Head & Neck Tumors and Neuroendocrine Tumors, Fudan University Shanghai Cancer Center, Shanghai, China
- 4Department of Pathology, Fudan University Shanghai Cancer Center, Shanghai, China
Background: An inflammatory myofibroblastic tumor (IMT) is a mesenchymal tumor with a prevalence ranging from 0.04% to 0.7% worldwide, in which the lung is the most common predilection site, accounting for 33% of cases, followed by the abdomen, pelvis, mesentery, and uterus. Approximately 50% of uterine IMTs present as anaplastic lymphoma kinase (ALK) positive along with ALK gene fusion, which lays a solid foundation for the development of ALK-based target therapy to optimize treatment strategies.
Case presentation: Herein we describe a 57-year-old woman who presented with a slow-growing mass in the uterus for over 10 years and then received surgical resection because of significant progressive enlargement of the mass during follow-up. She was diagnosed with uterine leiomyosarcoma (LMS) with no further interventions until recurrence. We revised the diagnosis to uterine IMT based on diffuse ALK expression, ALK-IGFBP5 gene fusion, and the morphologic features of the tumors by pathology consultation. Based on these, we recommended an ALK tyrosine kinase inhibitor (TKI) treatment, crizotinib (250 mg bid), and she achieved a complete response (CR) with at least 18 months of progression-free survival (PFS). We monitored the dynamics of target lesions and peripheral blood cells at regular intervals through CT scans and routine blood tests during the treatment process. We present patient responses to ALK inhibitor-based targeted therapy with uterine IMT harboring ALK-IGFBP5 fusion, and the neutrophil-to-lymphocyte ratio (NLR) may be an effective indicator to predict prognosis.
Introduction
An inflammatory myofibroblastic tumor (IMT) is a distinctive mesenchymal tumor with a prevalence ranging from 0.04% to 0.7% worldwide, in which the lung is the most common predilection site, accounting for 33% of cases, followed by the abdomen, pelvis, mesentery, and uterus (1). Symptoms and signs vary depending on the site of the tumor, while patients with uterine IMT commonly present with pain, tenderness, and abnormal vaginal bleeding (2). The recurrence rate of uterine IMT is approximately 25%, even though surgery has long been recognized as the preferred treatment (3), which lays a solid foundation for the development of chemotherapy and target therapy to optimize treatment strategies.
While considered rare, uterine IMT is diagnosed pathologically according to the criteria established by the World Health Organization (WHO) (4). Approximately 50% of uterine IMTs are driven by the rearrangements of the anaplastic lymphoma kinase (ALK) locus on chromosome 2p23 (5), which then results in the dysregulation of ALK expression. Structural rearrangements commonly lead to the expression and activation of ALK, which then create considerable opportunities for chimeric fusion. ALK has now been accepted as a specific diagnostic marker and driver gene for IMT of the uterus (6), and several ALK fusion partners have been identified retrospectively, for instance, FN1, DES, DCTN1, TNS1, IGFBP5, TIMP3, TNC, TPM3, THBS1, and SEC31 (3, 7–10). ALK fusion has been confirmed as a therapeutic target for patients with ALK-positive tumors, such as non-small cell lung cancer (NSCLC) (11). Previous studies uncovered patients with uterine IMT harboring DCTN1-ALK fusion who achieved an ongoing partial response (PR) with an ALK inhibitor (crizotinib/Xalkori®) and a multikinase VEGF inhibitor (pazopanib/Votrient®) treatment by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria (8). However, new therapeutic approaches based on other ALK fusion partners are rarely reported and need further exploration.
Here, we describe the case of a 57-year-old woman diagnosed with ALK-positive uterine IMT. Next-generation sequencing (NGS) identified the presence of an ALK-IGFBP5 fusion in her tumor. She, therefore, received an ALK tyrosine kinase inhibitor (TKI) treatment, crizotinib (250 mg bid), with significant symptomatic improvement and a rapid decrease in her tumor burden. This case reports for the first time that TKI is the preferred treatment option for patients with uterine IMT harboring ALK-IGFBP5 fusion. Our data not only offer insight into the diagnosis and treatment of uterine IMT but also provide a rationale for patient management.
Methods
Patient and treatment
One female patient with uterine IMT was admitted to the Fudan University Shanghai Cancer Center and was treated with an ALK inhibitor, crizotinib, 250 mg bid orally, for a 30-day cycle at Fudan University Shanghai Cancer Center. She provided signed informed consent prior to treatment.
Histological analysis
Surgical resection specimens of uterine IMT were fixed in 10% formalin, embedded in paraffin, and cut into 5-μm-thick sections. Sectioned tissues were then performed for hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC) staining with ALK (Cat. # 3633S, Cell Signaling Technology, Danvers, MA, USA), AE1/3 (Cat. # 67306S, Cell Signaling Technology), PR (Cat. # 8757S, Cell Signaling Technology), and caldesmon (Cat. # ab183339, Abcam, Cambridge, UK).
Next-generation sequencing
An NGS, i.e., massively parallel sequencing, of 630 genes was performed to detect the gene expression profile of the tumor sample, in addition to ALK rearrangement and gene fusion type within the tumor. NGS was performed to an average depth of >5,000× on the NovaSeq 6000 platform (Illumina, San Diego, CA, USA).
Positron emission tomography/computed tomography and contrast-enhanced CT
Positron emission tomography/computed tomography (PET/CT) was applied to identify the target lesions of distant metastasis, while contrast-enhanced CT was performed to assess the efficacy of ALK inhibitor-based targeted therapy in the patient.
Results
A 57-year-old woman presented with a 10-year history of uterine myomas by physical examination without significant discomfort and noticed progressive enlargement of the mass during a follow-up in November 2020. She then received surgical resection in December 2020 at an outside institution, and the excised mass measured 7 × 5 × 4 cm. The postoperative pathology of the mass suggested uterine leiomyosarcoma (LMS) by IHC staining: SMA(+), AE1/AE3 (−), Vimentin (+), Desmin (partial+), CD117 (−), ER (partial+), S-100 (−), PR (partial+), CD10 (partial+), CyclinD1 (partial +), P53 (partial+), CD34 (−), and Ki-67(30%+). She was followed up with routine postoperative clinic visits every 3 months and was diagnosed with progressive disease (PD) by contrast-enhanced CT in February 2021.
Immediately afterward, the patient was evaluated at the Fudan University Shanghai Cancer Center for therapy recommendations. A whole-body PET/CT scan revealed multiple metastases with increased 18F-fluoro-2-deoxyglucose (18F-FDG) uptake in the vaginal stump, left-side vulva, abdominal pelvic cavity, and mesentery and iliac paravascular and retroperitoneal lymph nodes (Figures 1A, B). We, therefore, performed pathology consultation on the surgical resection specimens, and H&E showed myofibroblastic and fibroblastic spindle cells accompanied by the infiltration of plasma cells, lymphocytes, and eosinophils within the tumor (Figure 2A). IHC showed strong positive ALK [D5F3 (+)] staining, along with AE1/AE3 (partial+), MyoD1(−), caldesmon (−), BCOR (1+), HMB45 (−), CD10 (−), and PR (−) (Figure 2B). NGS was performed to detect the ALK rearrangement, and the result showed that there was an ALK-IGFBP5 fusion in the tumor (Figures 3A, B). As we all know, approximately 50% of uterine IMTs are ALK-positive with ALK gene fusion (5). We, therefore, revised the diagnosis as uterine IMT according to diffuse ALK expression, ALK-IGFBP5 gene fusion, and the morphologic features of the tumors by pathology consultation.
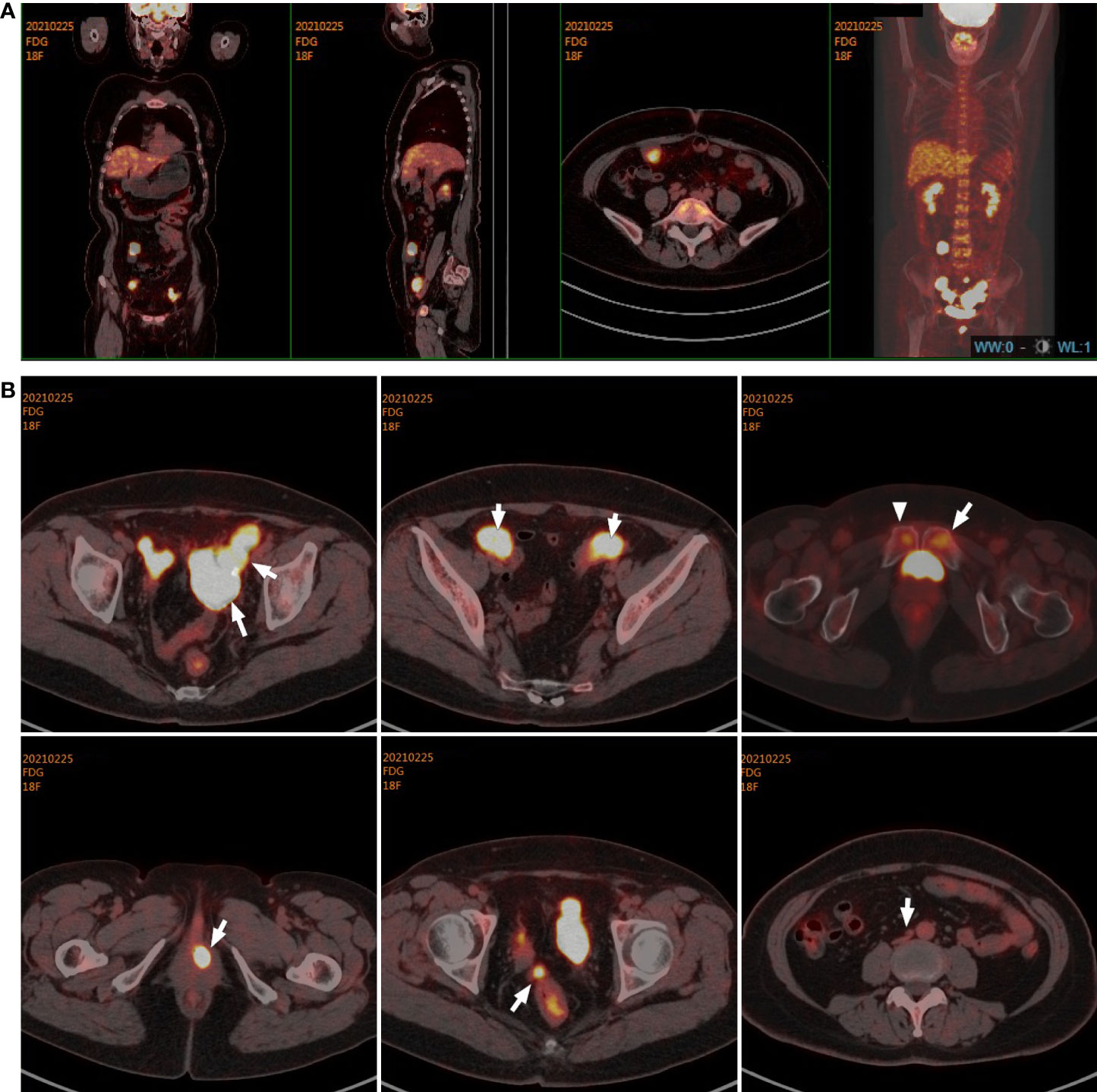
Figure 1 Target lesions of the patient with uterine IMT by PET/CT scans. (A, B) PET/CT scans indicated multiple metastases with increased 18F-fluoro-2-deoxyglucose (18F-FDG) uptake in the vaginal stump, left-side vulva, abdominal pelvic cavity, mesentery, and iliac paravascular and retroperitoneal lymph nodes in the abdomen and pelvic cavity. IMT, inflammatory myofibroblastic tumor.
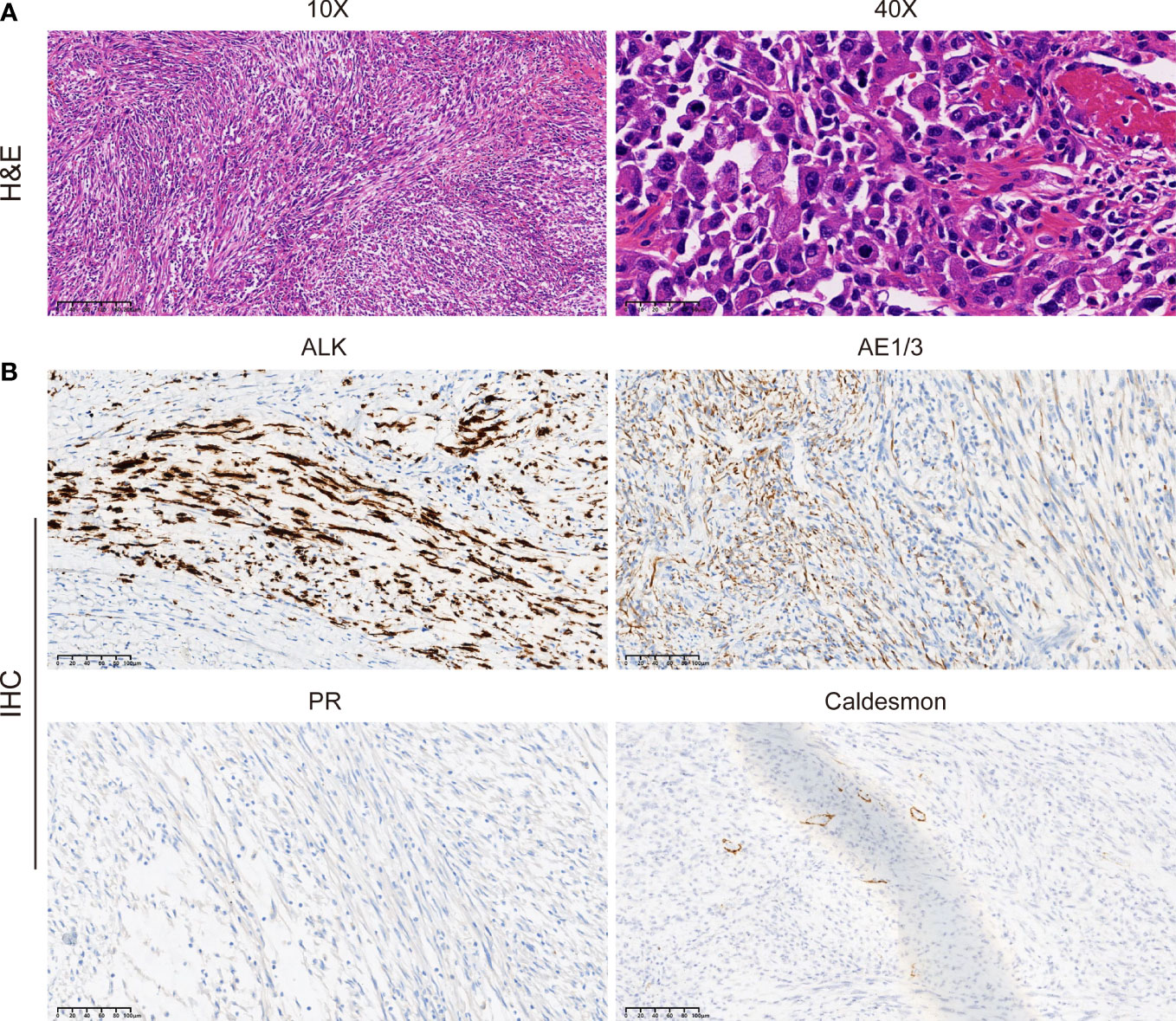
Figure 2 Morphologic features and IHC staining of the uterine IMT samples. Representative pictures of H&E staining (A) and IHC analysis (B) of ALK, AE1/3, PR, and caldesmon expression levels in uterine IMT. Scale bars are illustrated in the pictures. IHC, immunohistochemistry; IMT, inflammatory myofibroblastic tumor; H&E, hematoxylin, and eosin.
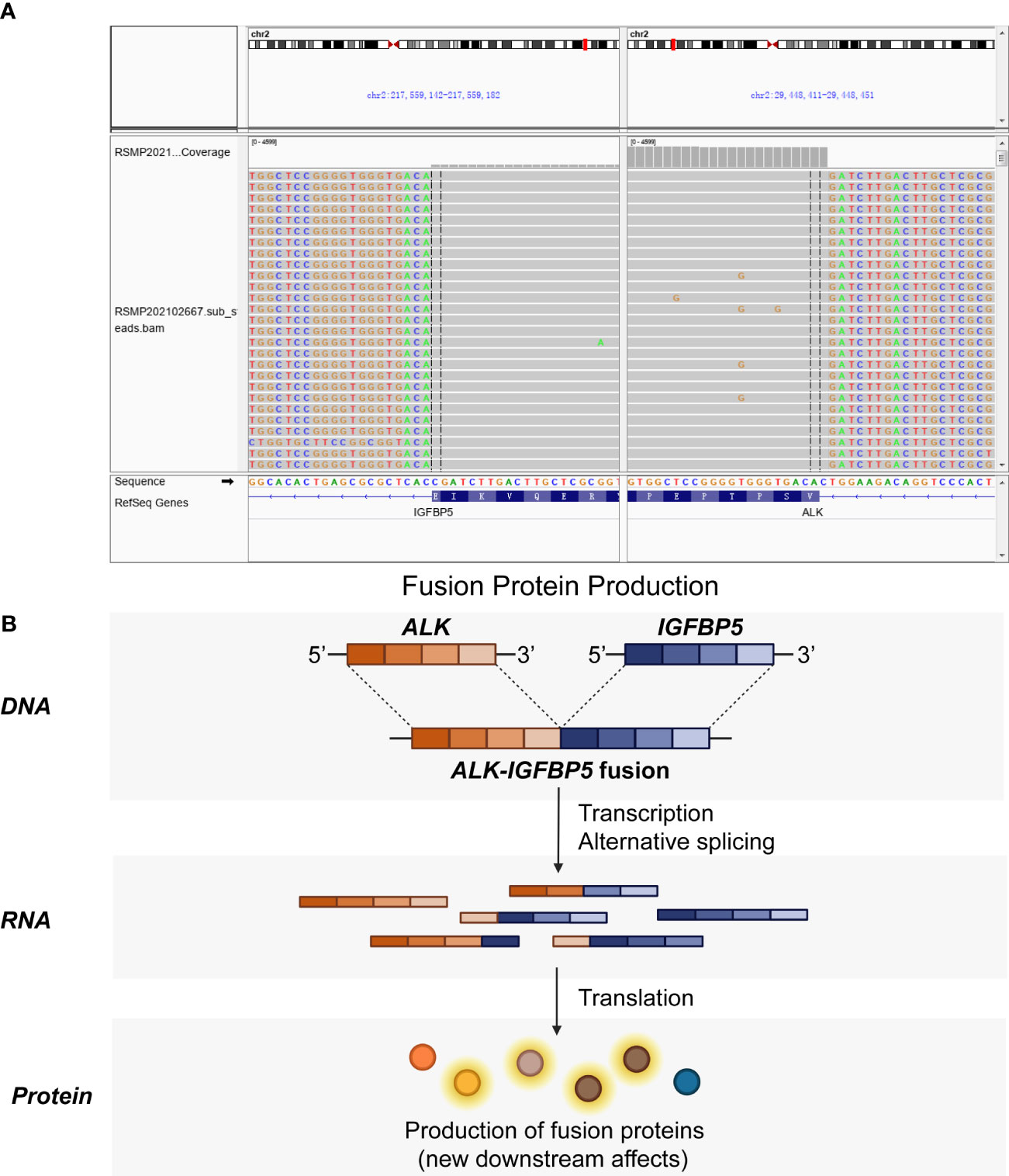
Figure 3 ALK-IGFBP5 fusion within the uterine IMT samples. The ALK-IGFBP5 gene fusion was identified by next-generation sequencing (A) and the working model (B) of the fusion. IMT, inflammatory myofibroblastic tumor.
No further interventions were administered until recurrence after surgery, and the patient received an ALK inhibitor, crizotinib, at a dose of 250 mg bid orally for a 30-day cycle initiated on 25 April 2021. Tumor evaluations were performed with contrast-enhanced CT every two cycles thereafter. The patient achieved significant improvement in vaginal discharge, pelvic pressure sensation, and abdominal circumference after one cycle of crizotinib treatment. She tolerated the therapy well and presented with mild lower-extremity edema, which was controlled with spironolactone 40 mg tid for 5 days. After six cycles of treatment, the patient achieved a greater than 60% reduction in the sum of the longest diameter (SLD) of the target lesions according to RECIST 1.1 (Figures 4A, B), demonstrating a PR to therapy. She thereafter continued to receive crizotinib treatment with routine follow-ups to date. We observed the radiographic disappearance of all target lesions through contrast-enhanced CT scans of the abdomen and pelvis in November 2022, indicating a complete response (CR) with at least 18 months of progression-free survival (PFS) (Figures 4A, B).
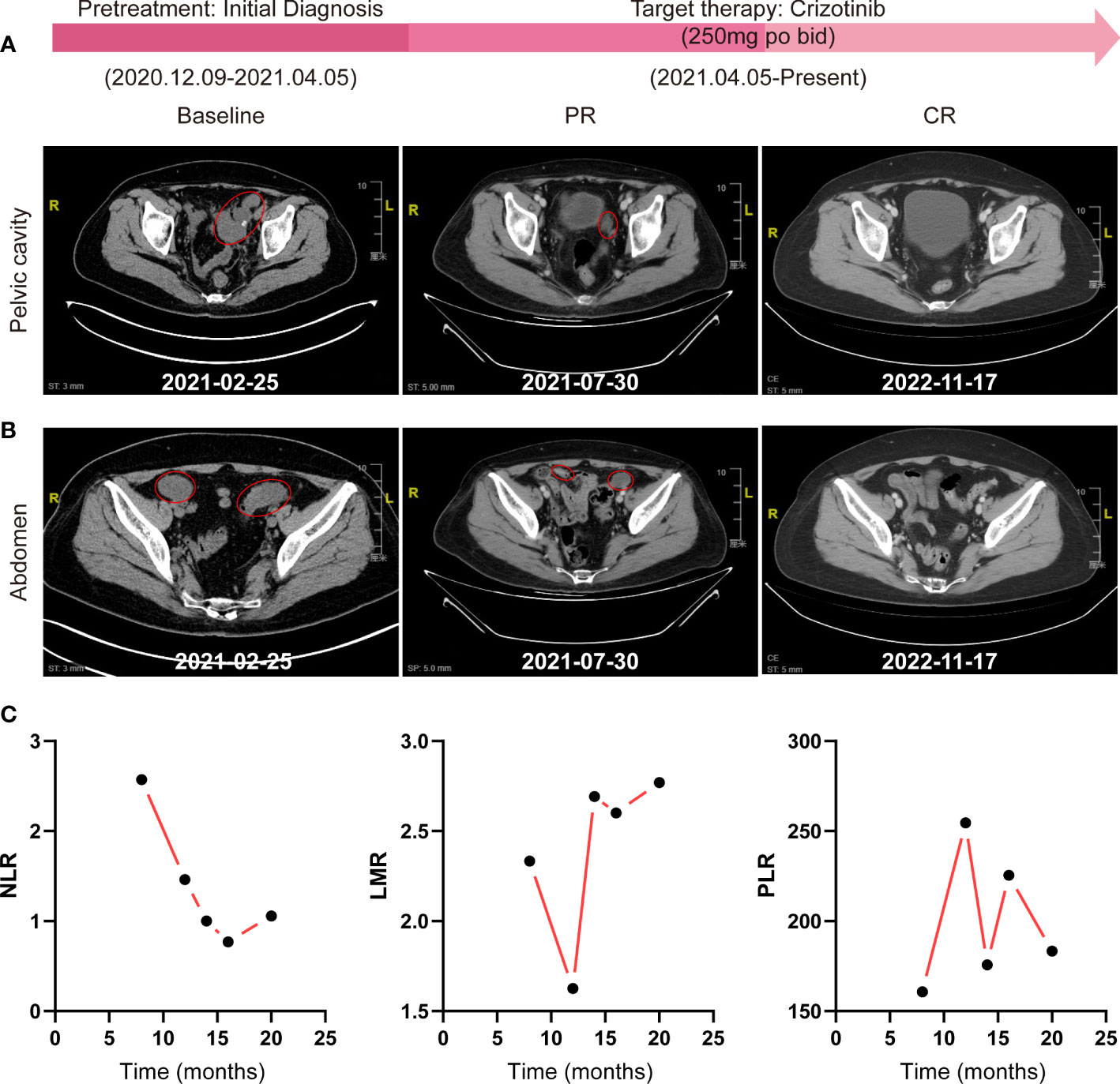
Figure 4 Effective evaluation by contrast-enhanced abdomen/pelvis CT scans and routine blood tests. (A, B) Radiographic assessments of target lesions by contrast-enhanced CT of the abdomen and pelvic cavity during the whole treatment process. (C) The change trends of NLR, LMR, and PLR were detected by routine blood tests. NLR, neutrophil-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; PLR, platelet-to-lymphocyte ratio.
Interestingly, we also observed a significant dynamic reduction of the neutrophil-to-lymphocyte ratio (NLR) by routine blood tests during the treatment process, while the lymphocyte-to-monocyte ratio (LMR) and platelet-to-lymphocyte ratio (PLR) showed no regularity (Figure 4C).
Discussion
IMT is a group of mesenchymal tumors with a prevalence of 0.04% to 0.7% worldwide (1), which always develop in children or young adults but also can occur at any age. IMT usually begins in the lung but can also start in the larynx, stomach, liver, bladder, mesentery, and uterus (12), which makes uterine IMT a rare type of tumor. Accurate diagnostics is therefore particularly important for precision therapy because of the rarity of uterine IMT. In this case, we report a 57-year-old woman with recurrent uterine IMT who achieved a CR with at least 18 months of PFS after being administered conventional treatment with crizotinib without any adverse events. Our findings not only provide insight into the diagnosis and clinical therapy of uterine IMT but also offer experience in treatment monitoring and patient management.
In this case, the patient was initially diagnosed with uterine LMS by surgical pathology at an outside hospital. She then went to Fudan University Shanghai Cancer Center for therapy recommendations, and we updated the diagnosis to uterine IMT for subsequent treatment through pathology consultation. Accurate, specialized pathological diagnosis is fundamental to the clinical therapy of rare cancers. During the diagnostic process, uterine IMT is difficult to differentiate from LMS and uterine leiomyoma (UL) based on morphology alone (9). Due to the rarity of uterine IMT, most patients experience misdiagnosis or delayed diagnosis. From a morphological point of view, uterine IMT presented with myofibroblastic and fibroblastic spindle cells, accompanied by the infiltration of immune cells such as plasma cells, lymphocytes, and eosinophils (8). An immunohistochemical study is a gold standard for the diagnosis, as ALK positivity is specific to uterine IMT with a prevalence of more than 50% (13). ALK has now been acknowledged as a key driver in the progress of uterine IMT (6), as well as in NSCLC and other solid tumors (14). ALK positivity creates optimal opportunities for the generation of ALK gene fusion within a tumor, which has not been thus far reported in UL (15). Gene fusions drive the progression of 16.5% of cancers and serve as the sole driver in more than 1% of them (16, 17). Presently, there are nearly 30 different ALK fusion partners that have been identified across different types of tumors, while FN1, DES, DCTN1, TNS1, IGFBP5, TIMP3, TNC, TPM3, THBS1, and SEC31 have been proven to exist in uterine IMT (7–10, 18). Among the 10 fusion partners, IGFBP5 and FN1 are both situated on the same chromosome as ALK, while IGFBP5 and THBS1 are the most frequent fusion types in uterine IMT (7).
In reality, different oncogenic ALK fusion partners always exhibit differential stability, leading to deregulated ALK activity along with differential sensitivity to ALK TKI. Wild-type ALK activates multiple signaling pathways such as PI3K-AKT, JAK-STAT, CRKL-C3G, MAPK, and MEKK2/3-MEK5-ERK5 pathways, to regulate cell growth and apoptosis (19). When fused with another gene, the protein conformation, stability localization, and function of ALK were changed, depending on the properties of the fusion partner (20). IGFBP5 has been reported to regulate cell survival, apoptosis, migration, and metastasis by IGF-dependent or IGF-independent mechanisms (21), and over-expression of IGFBP5 can confer resistance to PI3K inhibitors in breast cancer (22). Once fused with IGFBP5, ALK may function synergistically with IGFBP5 to trigger different signaling outputs that drive the initiation and progression of uterine IMT. However, the definite role and specific molecular mechanisms of how ALK-IGFBP5 fusion drives uterine IMT progression still remain unclear and need further exploration.
Although recognized as an accomplice to carcinogenesis, ALK-positive tumors exhibit better overall survival (OS), providing possibilities for targeted therapy (23). Crizotinib, a first-generation TKI, was first approved to treat patients with ALK-positive NSCLC according to strong clinical response data (24). A previous study indicated that a patient with uterine IMT harboring DCTN1-ALK fusion suffered an ongoing PR after 6 months of therapy with crizotinib/Xalkori and pazopanib/Votrient (8). In another case, a patient with metastatic lung IMT with TPM3-ALK rearrangement obviously responded to ceritinib after progression on crizotinib (25). Further, a 66-year-old man with pulmonary IMT presenting with GCC2-ALK fusion was administered ensartinib therapy and achieved a PR (26), which provided a reference for patients who were crizotinib-resistant.
In addition, we also observed a significant decrease in NLR during the treatment process in addition to CT scans, which may provide a new reference index for efficacy evaluation. A high NLR has been acknowledged to be associated with unfavorable overall survival (OS) in several solid tumors (27), and to predict the prognosis of patients receiving immunotherapy (28, 29). However, no studies have validated the prognostic role of NLR in IMT-administered TKI target therapy. The detailed mechanisms underlying the correlation between high NLR and poor OS of patients with cancer may be due to high NLR, indicating an inflammatory and immunosuppressive status of patients (27). Further studies that involve more patients are required to determine whether NLR is an effective evaluation criterion. LMR and PLR also played a prognostic role in predicting the clinical outcome of patients with cancer (30, 31), although no correlation was observed in our case.
Conclusion
We presented the case of a 57-year-old woman initially diagnosed with LMS, which was then corrected to uterine IMT based on morphology and ALK-IGFBP5 fusion within the tumor. The patient was treated with crizotinib 250 mg initiated on 25 April 2021 and currently achieved CR with at least 18 months of PFS. This case may provide some insights for the precision diagnosis, target therapy, and post-intervention assessment of patients with uterine IMT harboring ALK-IGFBP5 fusion.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
TZ, XZ, and ZL conceived the study, interpreted the data, and wrote the manuscript. XL, MR, YC, and JW collected the clinical data. XZ and ZL participated in data interpretation and discussions. All the authors read and approved the final manuscript.
Funding
This work was supported by the Shanghai Anticancer Association Program (grant number: SACA-AX202206 to ZL).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ALK, anaplastic lymphoma kinase; CR, complete response; IHC, immunohistochemistry; IMT, inflammatory myofibroblastic tumor; LMR, lymphocyte-to-monocyte ratio; NGS, next-generation sequencing; NLR, neutrophil-to-lymphocyte ratio; NSCLC, non-small cell lung cancer; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PLR, platelet-to-lymphocyte ratio; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; TKI, tyrosine kinase inhibitor; UL, uterine leiomyosarcoma.
References
1. Lovly CM, Gupta A, Lipson D, Otto G, Brennan T, Chung CT, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discovery (2014) 4:889–95. doi: 10.1158/2159-8290.CD-14-0377
2. Shukla PS, Mittal K. Inflammatory myofibroblastic tumor in female genital tract. Arch Pathol Lab Med (2019) 143:122–9. doi: 10.5858/arpa.2017-0575-RA
3. Kuisma H, Jokinen V, Pasanen A, Heikinheimo O, Karhu A, Valimaki N, et al. Histopathologic and molecular characterization of uterine leiomyoma-like inflammatory myofibroblastic tumor: Comparison to molecular subtypes of uterine leiomyoma. Am J Surg Pathol (2022) 46:1126–36. doi: 10.1097/PAS.0000000000001904
4. Anderson WJ, Doyle LA. Updates from the 2020 world health organization classification of soft tissue and bone tumours. Histopathology (2021) 78:644–57. doi: 10.1111/his.14265
5. Butrynski JE, D'Adamo DR, Hornick JL, Dal Cin P, Antonescu CR, Jhanwar SC, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med (2010) 363:1727–33. doi: 10.1056/NEJMoa1007056
6. Mohammad N, Haimes JD, Mishkin S, Kudlow BA, Leong MY, Chew SH, et al. ALK is a specific diagnostic marker for inflammatory myofibroblastic tumor of the uterus. Am J Surg Pathol (2018) 42:1353–9. doi: 10.1097/PAS.0000000000001120
7. Haimes JD, Stewart CJR, Kudlow BA, Culver BP, Meng B, Koay E, et al. Uterine inflammatory myofibroblastic tumors frequently harbor ALK fusions with IGFBP5 and THBS1. Am J Surg Pathol (2017) 41:773–80. doi: 10.1097/PAS.0000000000000801
8. Subbiah V, McMahon C, Patel S, Zinner R, Silva EG, Elvin JA, et al. STUMP un"stumped": anti-tumor response to anaplastic lymphoma kinase (ALK) inhibitor based targeted therapy in uterine inflammatory myofibroblastic tumor with myxoid features harboring DCTN1-ALK fusion. J Hematol Oncol (2015) 8:66. doi: 10.1186/s13045-015-0160-2
9. Collins K, Ramalingam P, Euscher ED, Reques Llanos A, Garcia A, Malpica A. Uterine inflammatory myofibroblastic neoplasms with aggressive behavior, including an epithelioid inflammatory myofibroblastic sarcoma: A clinicopathologic study of 9 cases. Am J Surg Pathol (2022) 46:105–17. doi: 10.1097/PAS.0000000000001756
10. Zarei S, Abdul-Karim FW, Chase DM, Astbury C, Policarpio-Nicolas MLC. Uterine inflammatory myofibroblastic tumor showing an atypical ALK signal pattern by FISH and DES-ALK fusion by RNA sequencing: A case report. Int J Gynecol Pathol (2020) 39:152–6. doi: 10.1097/PGP.0000000000000588
11. Camidge DR, Kim HR, Ahn MJ, Yang JC, Han JY, Lee JS, et al. Brigatinib versus crizotinib in ALK-positive non-Small-Cell lung cancer. N Engl J Med (2018) 379:2027–39. doi: 10.1056/NEJMoa1810171
12. Casanova M, Brennan B, Alaggio R, Kelsey A, Orbach D, van Noesel MM, et al. Inflammatory myofibroblastic tumor: The experience of the European pediatric soft tissue sarcoma study group (EpSSG). Eur J Cancer (2020) 127:123–9. doi: 10.1016/j.ejca.2019.12.021
13. Parra-Herran C. ALK immunohistochemistry and molecular analysis in uterine inflammatory myofibroblastic tumor: Proceedings of the ISGyP companion society session at the 2020 USCAP annual meeting. Int J Gynecol Pathol (2021) 40:28–31. doi: 10.1097/PGP.0000000000000704
14. Hallberg B, Palmer RH. The role of the ALK receptor in cancer biology. Ann Oncol (2016) 27 Suppl 3:iii4–iii15. doi: 10.1093/annonc/mdw301
15. Berta DG, Kuisma H, Valimaki N, Raisanen M, Jantti M, Pasanen A, et al. Deficient H2A.Z deposition is associated with genesis of uterine leiomyoma. Nature (2021) 596:398–403. doi: 10.1038/s41586-021-03747-1
16. Gao Q, Liang WW, Foltz SM, Mutharasu G, Jayasinghe RG, Cao S, et al. Driver fusions and their implications in the development and treatment of human cancers. Cell Rep (2018) 23:227–238 e223. doi: 10.1016/j.celrep.2018.03.050
17. Dorney R, Dhungel BP, Rasko JEJ, Hebbard L, Schmitz U. Recent advances in cancer fusion transcript detection. Brief Bioinform (2022) 24(1):1–12. doi: 10.1093/bib/bbac519
18. Makhdoum S, Nardi V, Devereaux KA, Kunder CA, Nielsen GP, Oliva E, et al. Inflammatory myofibroblastic tumors associated with the placenta: a series of 9 cases. Hum Pathol (2020) 106:62–73. doi: 10.1016/j.humpath.2020.09.005
19. Huang H. Anaplastic lymphoma kinase (ALK) receptor tyrosine kinase: A catalytic receptor with many faces. Int J Mol Sci (2018) 19(11):3448. doi: 10.3390/ijms19113448
20. Armstrong F, Duplantier MM, Trempat P, Hieblot C, Lamant L, Espinos E, et al. Differential effects of X-ALK fusion proteins on proliferation, transformation, and invasion properties of NIH3T3 cells. Oncogene (2004) 23:6071–82. doi: 10.1038/sj.onc.1207813
21. Ding M, Bruick RK, Yu Y. Secreted IGFBP5 mediates mTORC1-dependent feedback inhibition of IGF-1 signalling. Nat Cell Biol (2016) 18:319–27. doi: 10.1038/ncb3311
22. Wang W, Lim KG, Feng M, Bao Y, Lee PL, Cai Y, et al. KDM6B counteracts EZH2-mediated suppression of IGFBP5 to confer resistance to PI3K/AKT inhibitor treatment in breast cancer. Mol Cancer Ther (2018) 17:1973–83. doi: 10.1158/1535-7163.MCT-17-0802
23. Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: A review. JAMA (2019) 322:764–74. doi: 10.1001/jama.2019.11058
24. Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med (2010) 363:1693–703. doi: 10.1056/NEJMoa1006448
25. Mansfield AS, Murphy SJ, Harris FR, Robinson SI, Marks RS, Johnson SH, et al. Chromoplectic TPM3-ALK rearrangement in a patient with inflammatory myofibroblastic tumor who responded to ceritinib after progression on crizotinib. Ann Oncol (2016) 27:2111–7. doi: 10.1093/annonc/mdw405
26. He W, Ji X, Song C, Song S, Liu L. Case report: Efficacy of ensartinib treatment in pulmonary inflammatory myofibroblastic tumor with a rare GCC2-ALK fusion. Front Oncol (2022) 12:934887. doi: 10.3389/fonc.2022.934887
27. Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst (2014) 106:dju124. doi: 10.1093/jnci/dju124
28. Capone M, Giannarelli D, Mallardo D, Madonna G, Festino L, Grimaldi AM, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer (2018) 6:74. doi: 10.1186/s40425-018-0383-1
29. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-Lymphocyte ratio (NLR) and platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer (2017) 111:176–81. doi: 10.1016/j.lungcan.2017.07.024
30. Tan D, Fu Y, Tong W, Li F. Prognostic significance of lymphocyte to monocyte ratio in colorectal cancer: A meta-analysis. Int J Surg (2018) 55:128–38. doi: 10.1016/j.ijsu.2018.05.030
Keywords: uterine inflammatory myofibroblastic, inflammatory myofibroblastic tumor, ALK-IGFBP5 fusion, crizotinib, neutrophil-to-lymphocyte ratio (NLR)
Citation: Zhao T, Zhang X, Liu X, Ren M, Cheng Y, Wang J and Luo Z (2023) Case Report: Clinical response to anaplastic lymphoma kinase inhibitor-based targeted therapy in uterine inflammatory myofibroblastic tumor harboring ALK-IGFBP5 fusion. Front. Oncol. 13:1147974. doi: 10.3389/fonc.2023.1147974
Received: 20 January 2023; Accepted: 08 March 2023;
Published: 23 March 2023.
Edited by:
Matiullah Khan, AIMST University, MalaysiaReviewed by:
Elise Nassif, University of Texas MD Anderson Cancer Center, United StatesKenji Nakano, Cancer Institute Hospital of the Japanese Foundation for Cancer Research, Japan
Copyright © 2023 Zhao, Zhang, Liu, Ren, Cheng, Wang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiguo Luo, bHVvemhpZ3VvODhAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Ting Zhao
Ting Zhao Xiaowei Zhang1,2†
Xiaowei Zhang1,2† Xin Liu
Xin Liu Min Ren
Min Ren Zhiguo Luo
Zhiguo Luo