
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 12 July 2023
Sec. Radiation Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1146754
This article is part of the Research Topic Advances in Treatment Planning, Optimization and Delivery for Radiotherapy of Breast Cancer View all 14 articles
Purpose: To report outcomes on a subset of patients with triple negative breast cancer (TNBC) treated on prospective trials with post-lumpectomy partial breast irradiation and concurrent chemotherapy (PBICC) and compare them to a retrospectively assessed similar cohort treated with whole breast irradiation after adjuvant chemotherapy (WBIaC).
Methods and materials: Women with T1-2, N0-1 invasive breast cancer with ≥ 2mm lumpectomy margins were offered therapy on one of two PBICC trials. PBI consisted of 40.5 Gy in 15 daily 2.7 Gy fractions delivered concurrently with the first 2 cycles of adjuvant chemotherapy. The comparison cohort received WBI to a median dose of 60.7 Gy, (including boost, range 42.5 – 66 Gy), after completion of non-concurrent, adjuvant chemotherapy. We evaluated disease-free survival (DFS), and local/loco-regional/distant recurrence-free survival (RFS). We compared survival rates using Kaplan-Meier curves and log-rank test of statistical significance.
Results: Nineteen patients with TNBC were treated with PBICC on prospective protocol, and 49 received WBIaC. At a median follow-up of 35.5 months (range 4.8-71.9), we observed no deaths in the PBICC cohort and 2 deaths in the WBIaC cohort (one from disease recurrence). With a median time of 23.4 (range 4.8 to 47) months, there were 7 recurrences (1 nodal, 4 local, 4 distant), all in the WBIaC group. At 5 years, there was a trend towards increased local RFS (100% vs. 85.4%, p=0.17) and loco-regional RFS (100% vs. 83.5, p=0.13) favoring the PBICC cohort. There was no significant difference in distant RFS between the two groups (100% vs. 94.4%, p=0.36). Five-year DFS was 100% with PBICC vs.78.9% (95% CI: 63.2 to 94.6%, p=0.08) with WBIaC.
Conclusion: This study suggests that PBICC may offer similar and possibly better outcomes in patients with TNBC compared to a retrospective cohort treated with WBIaC. This observation is hypothesis-generating for prospective trials.
Triple negative breast cancer (TNBC) is characterized by lack of expression of estrogen (ER) and progesterone (PR) receptors and lack of overexpression of the human epidermal growth factor receptor 2 (HER2). Women with TNBC are reported to have inferior overall survival, disease free survival, and local control than their non-TNBC counterparts when treated with whole breast irradiation (WBI) (1–3).
Routine management of stage I and II TNBC usually includes mastectomy or breast conserving surgery (BCS) followed by sequential administration of chemotherapy and 3 to 6 weeks of daily WBI (with length of course predicated on nodal coverage, fractionation scheme, and use of boost) (4). In this regard, concurrent chemotherapy and radiation offers potential logistic benefits. While shortening the overall duration of therapy, both adjuvant treatments are completed sooner after surgery. Concurrency can also take advantage of potential oncologic synergy between the two modalities in improving tumor control. Concurrent chemoradiation is used in most other adenocarcinoma-based disease sites, including lung, gastrointestinal, and bladder cancers (5–9), albeit often in the definitive or pre-operative setting. However, concerns of prohibitive toxicity with concurrent administration of anthracycline-based chemotherapy regimens and others along with whole breast radiotherapy have made this approach unpopular (10). The smaller fields employed during partial breast irradiation potentially allow for mitigation of this concurrent toxicity and acceleration of the radiotherapy schedule.
We previously reported results of the first of two prospective phase I/II trials of PBI and concurrent chemotherapy (PBICC) in women with early stage breast cancer (11). Given reports of inferior oncologic outcomes in patients with TNBC and the potential of improved local control with concurrent chemotherapy and irradiation, we hypothesized that patients with TNBC treated with our novel PBICC approach will have similar or improved clinical outcomes as TNBC patients treated more traditionally with WBI after chemotherapy (WBIaC). In this report, we describe the outcome of the subset of TNBC patients enrolled in these PBICC trials. To provide an internal contemporary reference, we also retrospectively describe the outcomes of a series of patients with TNBC patients treated with WBIaC during the same time period.
We evaluated a subgroup of 19 TNBC patients treated on two prospective trials of PBICC that enrolled women with T1-2, N0-1 invasive breast cancer and ≥ 2mm lumpectomy margins between 2004-2009. Both trials were approved by the Institutional Review Board. We also retrospectively identified 51 similar patients with TNBC (T1-2, N0-1 invasive breast cancer with ≥ 2mm lumpectomy margins), treated with standard WBIaC followed by standard chemotherapy at Johns Hopkins University between 2004 and 2009 by using an Institutional Review Board-approved database and chart review. Full details on study designs and participants can be found in the original publication (11).
All patients underwent three-dimensional conformal or intensity-modulated radiation treatment planning, using five to seven non-coplanar photon beams.
The median dose of WBI (including boost) in the triple-negative comparison cohort was 59.89 Gy (range 42.56 – 66.60 Gy). Whole breast radiotherapy was delivered in 180-270 cGy fractions. Nodal regions were treated in 20% of the WBIaC patients.
PBI consisted of 40.5 Gy in 15 daily 2.7 Gy fractions delivered concurrently with the first 2 of 4 cycles of chemotherapy. For the PBI trials, the clinical target volume (CTV) was defined by a uniform expansion on the lumpectomy cavity, as delineated on computed tomography (CT), by 1.5cm in all directions then cropped to 5mm from skin surface and the chest wall/lung interface. The planning target volume (PTV) was created by uniformly expanding the CTV by 5 mm. Nodal regions did not receive directed radiotherapeutic treatment.
WBIaC and PBICC patients received cyclophosphamide +doxorubicin +/- paclitaxel (AC+T) or cyclophosphamide + docetaxel (TC), at the discretion of the treating medical oncologist.
In all WBIaC cases, radiotherapy was delivered after adjuvant chemotherapy. Decisions about additional systemic chemotherapy after completion of PBICC were made independently by the medical oncologist and the patient.
Two patients who were lost to follow-up within 12 months of lumpectomy were excluded from the retrospective cohort, therefore 49 patients were considered evaluable. Primary endpoints were disease-free survival (DFS), local recurrence-free survival (RFS), locoregional RFS, and distant RFS which were measured from the date of lumpectomy to time of any recurrence, local failure, locoregional failure, or distant failure, respectively. Local failure was defined as a biopsy-proven recurrence in the ipsilateral breast. Locoregional failure was defined as recurrence either in the ipsilateral breast or regional nodes, including the axilla, internal mammary nodes, or supraclavicular nodes. Distant failure was defined as the development of metastatic foci other than regional lymph nodes. Only distant recurrences that occurred as a first recurrence were considered in the estimation of distant disease-free survival. Progression free survival (PFS) curves comparing treatment modalities were analyzed using the Kaplan-Meier method, and comparisons were made using log-rank χ2 testing.
Fisher’s exact and χ2 tests were used to compare proportions between two or more groups. Nonparametric data testing consisted of the Mann-Whitney U test and the Kruskal-Wallis nonparametric analysis of variance test for comparison of two and three different groups. All statistics were calculated with SSPS (19.0 for Windows; SSPS, Inc., Chicago, Illinois, USA) and GraphPad Prism (5.0 for Windows; GraphPad Software Inc) software. A two-tailed P value of < 0.05 was considered statistically significant for all analyses.
Patient characteristics are summarized in Table 1. The median follow-up was 33.9 (range 4.8 to 71.9) and 41.9 (range 17 to 68.4) months for the WBIaC and PBICC groups respectively. Median follow up time for all patients was 35.5 months (range 4.8 to 71.9).
There was no statistically significant difference between the WBIaC and PBICC groups with respect to clinical T stage, clinical N stage, median age, race, menopausal status, type of chemotherapy used, or pathologic features.
Overall, seven of 49 (14.3%) of TNBC patients treated with WBIaC had disease recurrence at a median of 23.4 (range 4.8 to 47) months. Sites of recurrence included one nodal, four local, and two distant. Two WBIaC patients died (one of disease recurrence). There were no deaths or recurrences in the PBICC cohort. Patterns of treatment are summarized in Table 2.
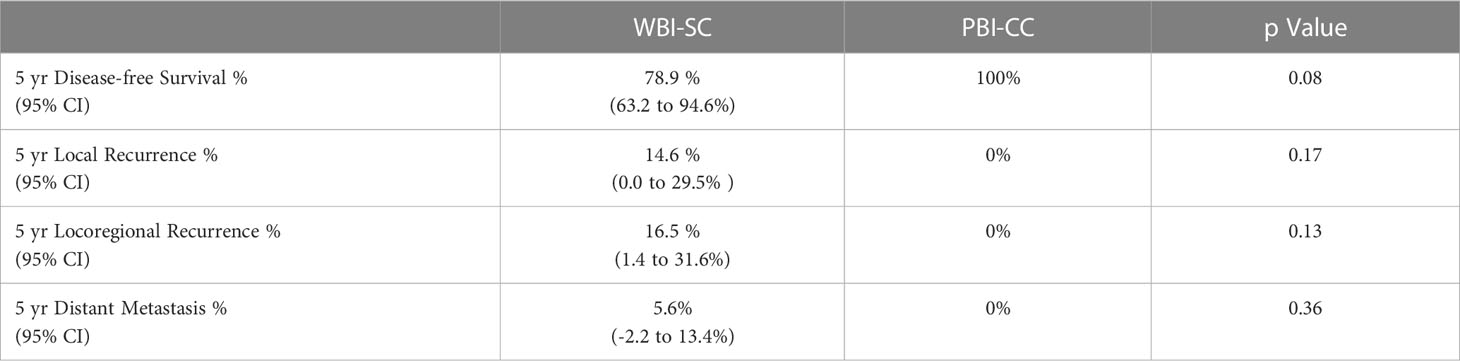
Table 2 Patterns of treatment Failure in Breast Cancer Patients with Triple Negative Receptor Status According to Treatment Modality.
At 5 years, there was a numeric trend towards decreased local recurrence (0% vs. 14.6%, p=0.17) in the PBICC cohort compared to the WBIaC cohort. The 3 year rates of local recurrence were 0% and 7.9% for PIBCC and WBIaC cohorts, respectively. Figure 1 demonstrates Kaplan-Meier analysis comparing both groups with respect to local recurrence-free survival. The median time to initial-site local recurrence was 25.9 months (range 4.8 to 47).
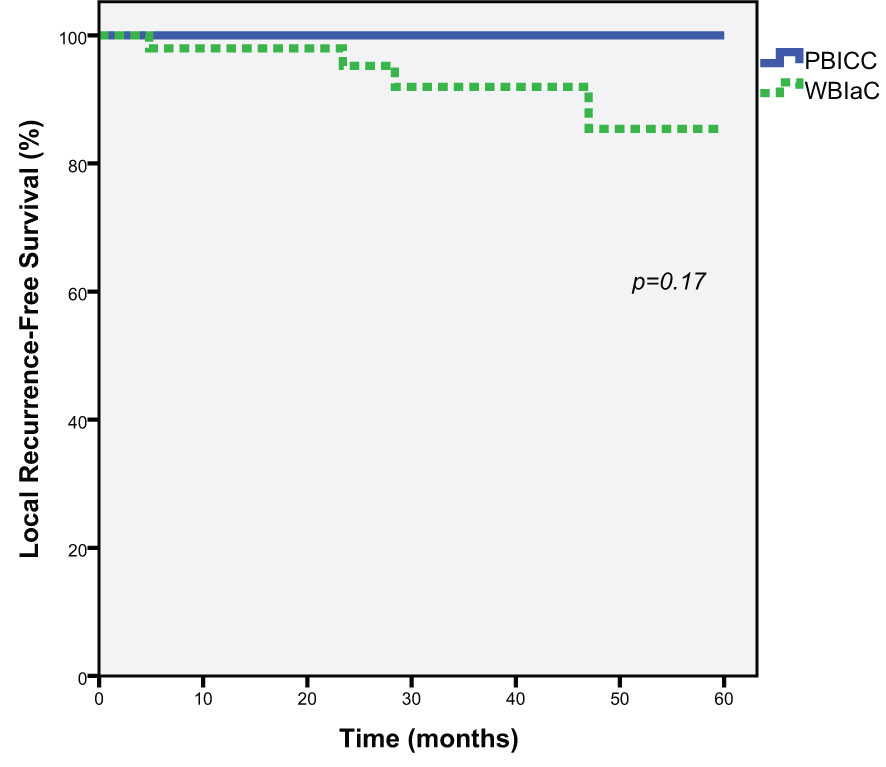
Figure 1 Kaplan-Meier estimates of Local Progression-Free Survival for Triple Negative Receptor Patients treated with PBICC and WBIaC.
At 5 years, there was a trend towards decreased loco-regional recurrence (0% vs. 17.8%, p=0.13) in the PBICC cohort compared to the WBIaC cohort. The 3 year locoregional recurrence rates were 0% and 13.2% for the PBICC and WBIaC cohorts, respectively. Figure 2 demonstrates Kaplan-Meier analysis comparing both groups with respect to locoregional recurrence-free survival. The time to recurrence in the single patient with initial-site nodal recurrence was 18.5 months. The median time to any locoregional recurrence was 23.4 months (range 4.8 to 47 months).
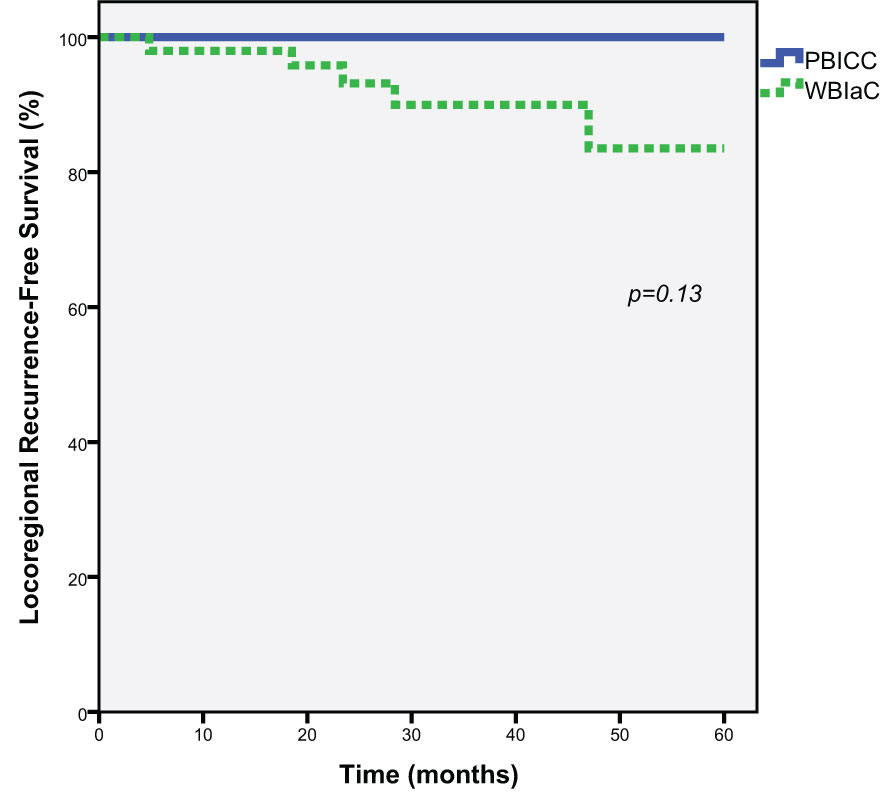
Figure 2 Kaplan-Meier estimates of Locoregional Progression-Free Survival for Triple Negative Receptor Patients treated with PBICC and WBIaC.
At 3 and 5 years, there was a no significant difference in the rate of distant metastasis (0% vs. 5.6%, p=0.36) between the PBICC and WBIaC cohorts. Figure 3 demonstrates Kaplan-Meier analysis comparing both groups with respect to distant recurrence-free survival. The median time to initial-site distant recurrence was 21.4 months (range 13.9 to 28.9).
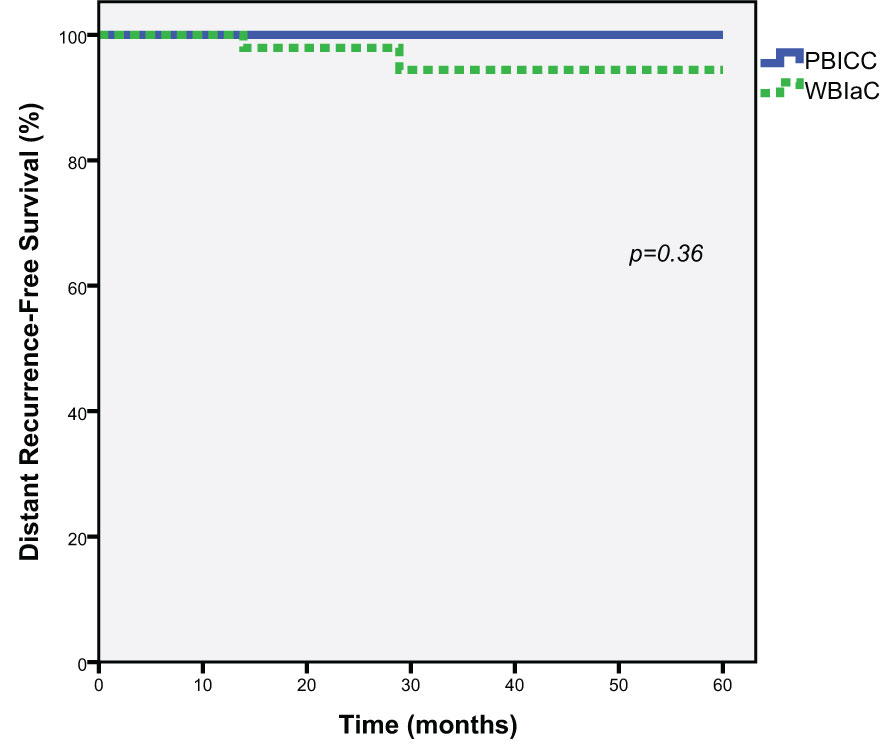
Figure 3 Kaplan-Meier estimates of Distant Progression-Free Survival for Triple Negative Receptor Patients treated with PBICC and WBIaC.
Five-year DFS estimates were 78.9% (95% CI: 63.2 to 94.6%) vs. 100% in the WBIaC vs. PBICC group respectively by Kaplan-Meier survival analysis, p=0.08 (Figure 4). The 3 year DFS for the groups was 83.6% in the WBIaC group and 100% for the PBICC group. The hazard ratio for disease-free survival was 0.24, numerically in favor of the PBICC group at 5 years (95% CI: 0.05 to 1.12).
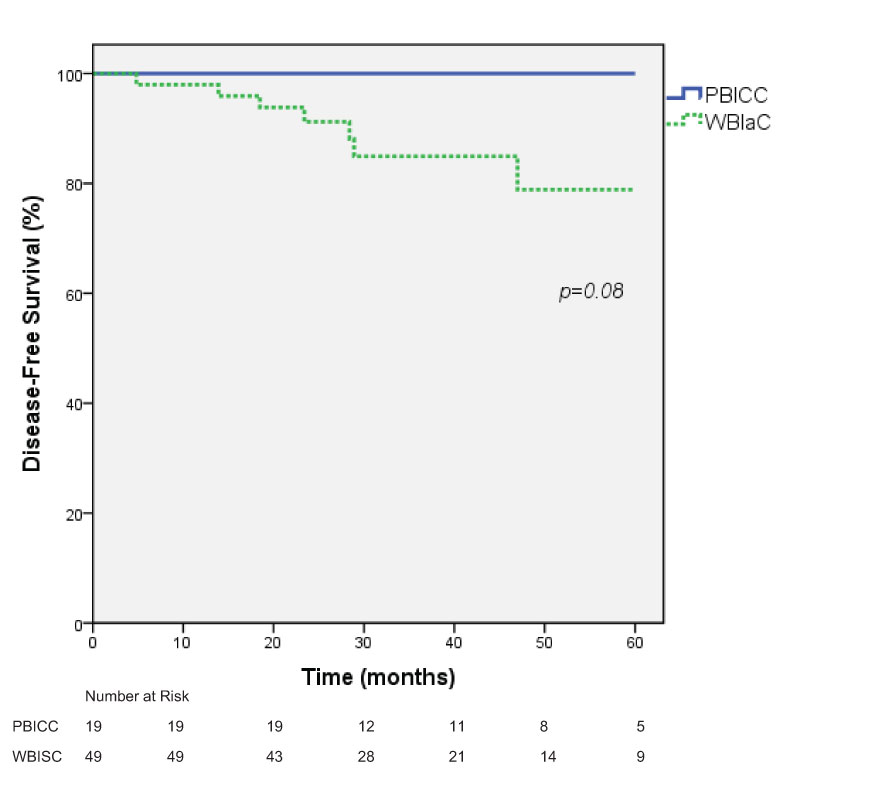
Figure 4 Kaplan-Meier estimates of Disease-Free survival for Triple Negative Receptor Patients treated with PBICC and WBIaC.
Patients with TNBC are at increased risk of breast cancer recurrence. Radiation with concurrent chemotherapy is known to improve local control via the radiation sensitizing effects of chemotherapy in many other disease sites (5–9). We chose to retrospectively review TNBC patients treated on 2 prospective phase I/II trials of PBI and concurrent chemotherapy, and compare their outcomes to retrospectively reviewed TNBC patients treated with WBI after adjuvant chemotherapy during the same period. The results of this study showed a trend towards improved local and loco-regional recurrence-free survival and overall disease-free survival with PBI and concurrent chemotherapy. As previously reported from the entire phase I/II cohort, this approach also has a favorable safety profile (11) in contrast with some other reports of concurrent chemoradiation for breast cancer (10). Specifically, patients in the entire cohort had an 84% rate of grade 1 dermatitis and 0% rate of grade 2+ skin toxicity. There were no incidences of pneumonitis (0%) in that report.
Increased local recurrences in women with TNBC treated with lumpectomy and whole breast irradiation are noted in several reports. In a paper by Arvold et al. (12), 1434 patients treated with breast-conserving therapy were divided into standard breast cancer sub-types, 171 of whom had TNBC. With a median follow-up of 84 months, the authors reported that the TNBC sub-type was independently associated with increased local recurrence on multivariate analysis (MVA). Zaky et al. (13) reviewed 193 and 160 women with TNBC and non-TNBC respectively, all treated with BCS and WBI. With a median follow-up of 3.4 yrs, the authors reported a 12% and 4% rate of local recurrence respectively (p=0.01). On MVA, TNBC was again independently associated with local recurrence. This elevated rate of local recurrence has also been reported in patients treated with PBI. Pashtan et al. (14) recently reported a 5 year actuarial local recurrence rate of 32.5% in 9 TNBC patients treated with 3D-Accelerated partial breast irradiation. When compared to HR positive/Her 2 negative patients, TNBC patients treated with PBI had a local recurrence hazard ratio of 15.2 (95% CI, 2.5-91). However, this increased rate of local recurrence after breast conserving therapy in TNBC patients is not a universal finding. A study by Wilkinson et al. (15), which included 20 TNBC patients and 182 receptor positive patients (almost half of whom were treated with 3D-APBI), reported a 0% actuarial rate of ipsilateral breast recurrence, nodal recurrence and distant metastases at 5 years in the TNBC cohort, which was not statistically distinct from the receptor positive patients.
There is also a suggestion of worse outcomes in patients with TNBC with regards to regional control, distant metastasis, and survival. For example, Haffty et al. (16) reported a statistically significant inferior nodal relapse-free rate (94 vs 99%), distant metastasis-free rate (68% vs. 83%) and cause-specific survival (72 vs. 85%) at 5 years with conventional breast-conserving therapy for TNBC patients compared to others. Wilder et al. (17) also demonstrated significantly inferior non-local relapse (81 vs 100%) and cause-specific survival (89 vs 100%) at 3 years for TNBC patients compared to others, when treated with PBI. Taken together with the previous discussion of local recurrences, TNBC patients are at higher risk for both local and distant recurrences.
These local and distant recurrence issues may have different solutions. One implication of the increased recurrences seen with TNBC is a relative radioresistance of this phenotype. Concurrent chemotherapy has been shown to improve response rates and overcome radioresistance to certain degrees in multiple tumor types (9). The trend towards improved outcomes with PBICC compared to WBIaC in our study may be due to the radiation sensitizing effects of concurrent chemotherapy but may also be due to the temporal proximity of radiation to surgery. Most commonly, chemotherapy is delivered after surgery and before radiation. Consequently, chemotherapy delays the start of radiation therapy. The importance of RT timing following surgery to reduce the risk of local recurrence is controversial. Bellon et al. (4) randomized 244 women to receive 12 weeks of cyclophosphamide, doxorubicin, methotrexate, fluorouracil and prednisone (CAMFP) before or after RT. At a median follow-up of 135 months, there were no significant differences between the chemotherapy-first and radiotherapy-first arms in time to any event, distant metastasis or death. Conversely, a systematic review by Huang et al. (18) of 11 studies involving 1,927 breast cancer patients demonstrated an increase in the 5-year locoregional recurrence from 6% in the RT-first group to 16% in the chemotherapy-first group (HR 2.28, 95% CI, 1.45 to 3.57). Additional evidence may possibly be seen in the study of PBI by Pashtan et al. above, in which all TNBC PBI recurrences occurred in patients who had their radiation delayed secondary to chemotherapy. How this timing is affected by the increasing use of hypofractionated (19, 20) and ultrahypofractionated (21, 22) radiation therapy is unknown. By definition, the PBICC strategy described here has a shorter interval between surgery and radiation, as the concurrent chemoradiation starts after the patient is sufficiently healed from surgery whereas the conventional standard is to complete all of adjuvant chemotherapy (several months of therapy) prior to radiation.
Our study suggests that combining concurrent chemotherapy with radiation may improve outcomes in TNBC. Concurrency in breast cancer has traditionally been avoided due to previous reports of increased toxicity (10). A recent Phase I prospective trial of concurrent carboplatin with whole breast standard fractionation radiation therapy, and found favorable safety profiles, with planned Phase II study opening thereafter (23). The patients in these trials had multiagent regimens consistent with standard recommendations for TNBC such as AC+T or TC.
We posit that the use of PBI with concurrent chemotherapy would mitigate these toxicities, and that has been supported by previous reports of these trials (11). Nonetheless, there is enough uncertainty about their propensity to recur more often, that the ASTRO guidelines for PBI stress caution in patients who are hormone-receptor-negative (24, 25). Part of the rationale in the discussion for those guidelines was a relative paucity of TNBC patients on APBI clinical trials, rather than specifically citing the recurrence propensity. For example, NSABP B39 and Florence trials had 19% and 1-2.3% of triple negative patients, respectively (22, 26). RAPID and IMPORT LOW studies had only 9-11% and 5% of ER negative patients, respectively (27, 28). One study has suggested ER negativity as a predictor of local recurrence in brachytherapy APBI (29). In contrast, Goulding et al. (30) analyzed patients on two prospective APBI trials that were treated with external beam RT, and when specifically looking at TNBC and other “high risk” patients compared with “suitable” patients, TNBC was not associated with higher in-breast recurrence risk, with no local recurrences occurring in this cohort.
There are limitations to this analysis. Although the patients treated with PBICC were participants of two prospective clinical trials, the trials were not originally designed to address this question. Thus our reported analyses of both PBICC and WBIaC cohorts are truly retrospective in nature. As a retrospective study, it is subject to limitations common with this type of analysis. Specifically there may be patient and treatment differences as well as unknown factors that may have influenced the results. We attempted to mitigate these limitations by choosing comparative cohort (WBIaC) patients with comparable stages and treated during the same time period. For instance, the doses of radiation used in the WBIaC patients were more variable and with a higher range than the PBICC group. While this is an imbalance, it does further support the trend toward control in triple negative patients with concurrent chemotherapy even at lower overall radiation doses in the concurrent cohort. An additional limitation is that our study cohorts are relatively small, likely explaining the lack of statistical significance in our findings. Nonetheless, the local recurrence rate in the WBIaC cohort is comparable to other published studies. For example, Dent et al. (3) reported a 13% rate of local recurrence in 180 TNBC patients with clinically localized disease treated with WBIaC, with a mean time to local recurrence of 2.8 years. Conversely, the lack of local recurrences in the PBICC cohort is unexpected. As the risk of recurrence in TNBC rapidly declines after the first 3 years, we believe that the median follow-up of the TNBC patients in our study is likely to be adequate to capture a majority of recurrence events. While small, the PBICC cohort is, to the best of our knowledge, the first report of breast cancer outcomes using this approach in TNBC from prospectively collected data. Given these limitations, we consider our results hypothesis-generating.
The finding of extremely low recurrence rates in patients with TNBC treated with PBICC differs both from the comparison cohort of retrospectively reviewed contemporary patients treated with WBIaC, and from earlier reports of a high rate of local recurrence in TNBC patients treated with PBI. This data generates a hypothesis that the PBICC approach is associated with improved clinical outcomes, potentially due to shorter intervals from surgery to radiotherapy and/or to a synergy between radiotherapy and chemotherapy. The ongoing randomized Phase II trial (PBI 3.0, NCT01928589) currently accruing patients that will provide additional information on outcomes using PBICC.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Johns Hopkins Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
RR: Manuscript creation, data interpretation and analysis. JW: Data interpretation and analysis, review/editing manuscript. LD: Data interpretation and analysis, review/editing manuscript. VS: Patient enrollment, review/editing manuscript, data interpretation. AW: Patient enrollment, review/editing manuscript, data interpretation. RZ: Trial design and execution, data collection, and review/editing manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the Breast Cancer Research Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rodriguez-Pinilla SM, Sarrio D, Honrado E, Hardisson D, Calero F, Benitez J, et al. Prognostic significance of basal-like phenotype and fascin expression in node-negative invasive breast carcinomas. Clin Cancer Res (2006) 12(5):1533–9. doi: 10.1158/1078-0432.CCR-05-2281
2. Banerjee S, Reis-Filho JS, Ashley S, Ashworth A, Lakhani SR, Smith E. Basal-like breast carcinomas: clinical outcome and response to chemotherapy. J Clin Pathol (2006) 59(7):729–35. doi: 10.1136/jcp.2005.033043
3. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res (2007) 13(15 Pt 1):4429–34. doi: 10.1158/1078-0432.CCR-06-3045
4. Bellon JR, Come SE, Gelman RS, Henderson IC, Shulman LN, Silver BJ, et al. Sequencing of chemotherapy and radiation therapy in early-stage breast cancer: updated results of a prospective randomized trial. J Clin Oncol (2005) 23(9):1934–40. doi: 10.1200/JCO.2005.04.032
5. Choy H, Gerber DE, Bradley JD, Iyengar P, Monberg M, Treat J, et al. Concurrent pemetrexed and radiation therapy in the treatment of patients with inoperable stage III non-small cell lung cancer: a systematic review of completed and ongoing studies. Lung Cancer (2015) 87(3):232–40. doi: 10.1016/j.lungcan.2014.12.003
6. Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al-Sarraf M, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). radiation therapy oncology group. JAMA (1999) 281(17):1623–7. doi: 10.1001/jama.281.17.1623
7. Gerard JP, Conroy T, Bonnetain F, Bouche O, Chapet O, Colson-Dejardin M-T, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol (2006) 24(28):4620–5. doi: 10.1200/JCO.2006.06.7629
8. Moertel CG, Childs DS Jr, Reitemeier RJ, Colby Jr MY, Holbrook MA. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet (1969) 2(7626):865–7. doi: 10.1016/S0140-6736(69)92326-5
9. Seiwert TY, Salama JK, Vokes EE. The concurrent chemoradiation paradigm–general principles. Nat Clin Pract Oncol (2007) 4(2):86–100. doi: 10.1038/ncponc0714
10. Fiets WE, van Helvoirt RP, Nortier JW, van der Tweel I, Struikmans H, et al. Acute toxicity of concurrent adjuvant radiotherapy and chemotherapy (CMF or AC) in breast cancer patients. a prospective, comparative, non-randomised study. Eur J Cancer (2003) 39(8):1081–8. doi: 10.1016/S0959-8049(03)00178-3
11. Zellars RC, Stearns V, Frassica D, Asrari F, Tsangaris T, Myers L, et al. Feasibility trial of partial breast irradiation with concurrent dose-dense doxorubicin and cyclophosphamide in early-stage breast cancer. J Clin Oncol (2009) 27(17):2816–22. doi: 10.1200/JCO.2008.20.0139
12. Arvold ND, Taghian AG, Niemierko A, Abi Raad RF, Sreedhara M, Nguyen PL, et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol (2011) 29(29):3885–91. doi: 10.1200/JCO.2011.36.1105
13. Zaky SS, Lund M, May KA, Godette KD, Beitler JJ, Holmes LR, et al. The negative effect of triple-negative breast cancer on outcome after breast-conserving therapy. Ann Surg Oncol (2011) 18(10):2858–65. doi: 10.1245/s10434-011-1669-4
14. Pashtan IM, Recht A, Ancukiewicz M, Brachtel E, Abi-Raad RF, D'Alessandro HA, et al. External beam accelerated partial-breast irradiation using 32 gy in 8 twice-daily fractions: 5-year results of a prospective study. Int J Radiat Oncol Biol Phys (2012) 84(3):e271–7. doi: 10.1016/j.ijrobp.2012.04.019
15. Wilkinson JB, Reid RE, Shaitelman SF, Chen PY, Mitchell CK, Wallace MF, et al. Outcomes of breast cancer patients with triple negative receptor status treated with accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys (2011) 81(3):e159–64. doi: 10.1016/j.ijrobp.2010.12.031
16. Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol (2006) 24(36):5652–7. doi: 10.1200/JCO.2006.06.5664
17. Wilder RB, Curcio LD, Khanijou RK, Eisner ME, Kakkis JL, Chittenden L, et al. Results with accelerated partial breast irradiation in terms of estrogen receptor, progesterone receptor, and human growth factor receptor 2 status. Int J Radiat Oncol Biol Phys (2010) 78(3):799–803. doi: 10.1016/j.ijrobp.2009.08.081
18. Huang J, Barbera L, Brouwers M, Browman G, Mackillop WJ. Does delay in starting treatment affect the outcomes of radiotherapy? a systematic review. J Clin Oncol (2003) 21(3):555–63. doi: 10.1200/JCO.2003.04.171
19. Haviland JS, Owen JR, Dewar JA, Agrawal RK, Barrett J, Barrett-Lee PJ, et al. The UK standardisation of breast radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol (2013) 14(11):1086–94. doi: 10.1016/S1470-2045(13)70386-3
20. Whelan TJ, Pignol J-P, Levine MN, Julian JA, MacKenzie R, Parpia S, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med (2010) 362(6):513–20. doi: 10.1056/NEJMoa0906260
21. Brunt AM, Haviland JS, Sydenham M, Agrawal RK, Algurafi H, Alhasso A, et al. Ten-year results of FAST: a randomized controlled trial of 5-fraction whole-breast radiotherapy for early breast cancer. J Clin Oncol (2020) 38(28):3261–72. doi: 10.1200/JCO.19.02750
22. Meattini I, Marrazzo L, Saieva C, Desideri I, Scotti V, Simontacchi G, et al. Accelerated partial-breast irradiation compared with whole-breast irradiation for early breast cancer: long-term results of the randomized phase III APBI-IMRT-Florence trial. J Clin Oncol (2020) 38(35):4175–83. doi: 10.1200/JCO.20.00650
23. Bellon JR, Chen Y-H, Rees R, Taghian AG, Wong JS, Punglia RS, et al. A phase 1 dose-escalation trial of radiation therapy and concurrent cisplatin for stage II and III triple-negative breast cancer. Int J Radiat Oncol Biol Phys (2021) 111(1):45–52. doi: 10.1016/j.ijrobp.2021.03.002
24. Smith BD, Arthur DW, Buchholz TA, Haffty BG, Hahn CA, Hardenbergh PH, et al. Accelerated partial breast irradiation consensus statement from the American society for radiation oncology (ASTRO). Int J Radiat Oncol Biol Phys (2009) 74(4):987–1001. doi: 10.1016/j.ijrobp.2009.02.031
25. Correa C, Harris EE, Leonardi MC, Smith BD, Taghian AG, Thompson AM, et al. Accelerated partial breast irradiation: executive summary for the update of an ASTRO evidence-based consensus statement. Pract Radiat Oncol (2017) 7(2):73–9. doi: 10.1016/j.prro.2016.09.007
26. Vicini FA, Wilkinson JB, Lyden M, Beitsch P, Vicini FA. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, phase 3, equivalence trial. Lancet (2019) 394(10215):2155–64. doi: 10.1016/S0140-6736(19)32514-0
27. Whelan TJ, Asmar L, Wang Y, Tole S, Barke L, Widner J, et al. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): a randomised controlled trial. Lancet (2019) 394(10215):2165–72. doi: 10.1016/S0140-6736(19)32515-2
28. Coles CE, Cecchini RS, White JR, Arthur DW, Julian TB, Rabinovitch RA, et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet (2017) 390(10099):1048–60. doi: 10.1016/S0140-6736(17)31145-5
29. Shah C, Julian JA, Berrang TS, Kim D-H, Germain I, Nichol AM, et al. Predictors of local recurrence following accelerated partial breast irradiation: a pooled analysis. Int J Radiat Oncol Biol Phys (2012) 82(5):e825–30. doi: 10.1016/j.ijrobp.2011.11.042
30. Goulding A, Haviland JS, Kirby AM, Griffin CL, Sydenham MA, Titley JC, et al. Outcomes after accelerated partial breast irradiation in women with triple negative subtype and other "High risk" variables categorized as cautionary in the ASTRO guidelines. Front Oncol (2021) 11:617439. doi: 10.3389/fonc.2021.617439
Keywords: partial breast irradiation, triple negative breast cancer, concurrent chemoradiation, breast cancer, clinical trial
Citation: Rhome R, Wright J, De Souza Lawrence L, Stearns V, Wolff A and Zellars R (2023) Concurrent chemotherapy with partial breast irradiation in triple negative breast cancer patients may improve disease control compared with sequential therapy. Front. Oncol. 13:1146754. doi: 10.3389/fonc.2023.1146754
Received: 17 January 2023; Accepted: 06 June 2023;
Published: 12 July 2023.
Edited by:
Vishruta Dumane, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Hidekazu Tanaka, Yamaguchi University Graduate School of Medicine, JapanCopyright © 2023 Rhome, Wright, De Souza Lawrence, Stearns, Wolff and Zellars. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ryan Rhome, cnJob21lQGl1aGVhbHRoLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.