- 1Department of Tumor Radiotherapy, The Second Hospital of Jilin University, Changchun, China
- 2School of Life Sciences, Department of Biology, Southern University of Science and Technology, Shenzhen, China
Biomolecular modifications play an important role in the development of life, and previous studies have investigated the role of DNA and proteins. In the last decade, with the development of sequencing technology, the veil of epitranscriptomics has been gradually lifted. Transcriptomics focuses on RNA modifications that affect gene expression at the transcriptional level. With further research, scientists have found that changes in RNA modification proteins are closely linked to cancer tumorigenesis, progression, metastasis, and drug resistance. Cancer stem cells (CSCs) are considered powerful drivers of tumorigenesis and key factors for therapeutic resistance. In this article, we focus on describing RNA modifications associated with CSCs and summarize the associated research progress. The aim of this review is to identify new directions for cancer diagnosis and targeted therapy.
1 Introduction
Epigenetic modifications are heritable phenotypic changes that occur without altering the nucleic acid sequence (1–3), and they include DNA methylation, histone modification, chromatin remodeling, genomic imprinting, maternal effects, gene silencing, and RNA editing (4–7). The function of epigenetic mechanisms in tumors has been extensively studied in recent decades. In contrast, epitranscriptomics has received relatively limited attention in the study of tumors. Approximately 70 years ago, scientists discovered the first RNA modification in yeast tRNA, namely, pseudouridine (Ψ) (8), which was mainly distributed on noncoding RNAs (ncRNAs), such as tRNA and rRNA. At that time, the diversity of RNA modification sites was unknown, and the occurrence of RNA modifications on almost all RNA species had not been identified. In recent years, with advances in mass spectrometry and high-throughput sequencing techniques, more RNA modifications have been discovered. For example, Li et al. (9) combined the carbodiimide metho-p-toluenesulfonate (CMCT) reaction with the biotin pull-down method to map over 1000 Ψ sites in mouse and human mRNAs and ncRNAs. Two groups independently developed similar methods, MeRIP-seq (methylated RNA immunoprecipitation followed by sequencing) and m6A-seq (10) (11). To briefly explain the methods, RNA is fragmented into ∼100 nt long segments and is immunoprecipitated with anti-m6A antibody, which results in selective enrichment of methylated RNA fragments. Eluted RNAs and input control samples are deep sequenced, and the reads are mapped to the genome. Using peak-calling algorithms, regions enriched in the immunoprecipitate relative to input samples are identified as “m6A peaks.” These methods allowed genome-wide mapping of m6A modification with a resolution of ∼200 nt, detected over 12,000 m6A peaks in transcripts of >7,000 genes in human cells and mouse tissues. Huang et al. (12) reported transcriptome-wide loci for m5C using an improved bisulfite sequencing method and a novel computational approach. Pandolfini et al. (13) and Zhang et al. (14) used chemical processing combined with a reverse transcription labeling method and antibody immunoprecipitation to identify internal 7-methylguanosine (m7G). To date, over 170 posttranscriptional RNA modifications have been identified, and they include abundant RNA methylation modifications and other complex modifications (15, 16). However, these experimental methods also have certain disadvantages, such as time-consuming and expensive, and they are not able to keep pace with the explosive increase of RNA sequences revealed by the rapid development of sequencing technology. Instead, computational methods can be able to provide a faster and more cost-effective way for RNA modifications site identification, which contributed a lot to the development of RNA epitranscriptome study. So far, several computational methods for predicting RNA modifications site have been reported, such as the most popular databases RMBase, Modomics, m6A2target, m6A-Atlas,m5C-Atlas (17–21). In addition, some functional tools like m6ASNP, ConsRM can be used to boost further functional studies investigating genetic variants (22, 23).
Transcriptome diversity induced by RNA modifications is considered an important mechanism that drives proteome diversity (24). Recent findings suggest that various RNA modifications, including attenuation, translocation, splicing, and translation, can affect transcriptome metabolism in a tissue-specific manner (25), and these findings have contributed to the emergence of the epitranscriptomic domain. Similar to DNA methylation and histone modification, RNA modifications represent another layer of gene expression regulation. Such modifications are observed for various forms of RNA and are involved in various life processes of cellular organisms, where RNA-modifying enzymes play a decisive role (26). Moreover, abnormal RNA modifications can affect the normal life activities of organisms and lead to the development of diseases, such as cancer, neurological disorders (27), and immune disorders (28). Continued research on RNA modifications will reveal new possibilities for the rapid alteration of gene expression following specific environmental changes.
Most malignant tumors are incurable. Over the past two decades, numerous studies have shown that tumorigenesis is centered on cancer stem cells (CSCs), which represent a small subset of cancer cells with tumor initiation capacity (29). CSCs are a population of malignant tumor cells in tumor tissue capable of self-renewal, rapid proliferation, and multidirectional differentiation potential, and they have the ability to initiate and reconstitute the tumor tissue phenotype. They can self-renew through cell division to form identical daughter cells and also differentiate into various types of daughter cells (30). CSCs are involved in cancer metastasis and recurrence and promote tumor vascularization, chemotherapy and radiotherapy resistance, and immune cell surveillance evasion. According to recent therapeutic studies, specifically targeting CSCs represents a promising therapeutic strategy. RNA modifications have been extensively studied in relation to many key features of cancer, such as evading the immune system, sustaining proliferation, avoiding growth suppression, escaping apoptosis, achieving replicative immortality, acquiring metastatic potential, promoting angiogenesis, and reprogramming metabolism (25, 31–34). However, the role of RNA modifications in CSCs has not been thoroughly studied.
In this review, we present several mechanisms of RNA modifications and a brief overview of CSCs and summarize the latest progress in understanding how these RNA modifications regulate CSCs. Moreover, we explore questions that remain to be addressed in this field and offer insights and suggestions for further research.
2 Regulatory machinery of the epitranscriptome: Writers, erasers, and readers
Many types of RNA modifications have been identified, such as modifications with 5−methylcytosine (m5C), N6−methyladenosine (m6A), Ψ, N1−methyladenosine (m1A), 2´-O-methylation (Nm), N6,2´-O-dimethyladenosine (m6Am), and internal 7-methylguanosine (m7G), uridylation, and adenosine-to-inosine (A-to-I) editing (31, 35, 36). These modifications are also present in various types of RNA, including ribosomal RNA (rRNA), transfer RNA (tRNA), messenger RNA (mRNA), and other noncoding RNA (ncRNA), and they are mediated by RNA modification proteins (RMPs), which can be divided into dynamic modification and irreversible modification proteins. RMPs are divided into enzymes that deposit RNA chemical tags, enzymes that remove these tags, and enzymes that recognize these tags, which are referred to as “writers,” “erasers,” and “readers,” respectively.
2.1 N6-Methyladenosine (m6A)
As the most abundant modification in eukaryotic mRNAs, m6A plays an important role in regulating the processing of mRNAs (37). It accounts for more than 80% of RNA base modifications and is commonly found in different species (11, 38–40). The development of RNA immunoprecipitation sequencing (RIP-Seq) technology has led to increasing attention to m6A modifications in recent years (10). The m6A modification presents a dynamic and reversible process within cells (Figure 1).
2.1.1 m6A methyltransferases—writers
The m6A modification is catalyzed by the methyltransferase complex, which consists of the methyltransferases METTL3 and METTL14 and its cofactors WTAP, RBM15, RBM15B, HAKAI, VIRMA (KIAA1429), and ZC3H13 (41–45). METTL3 selectively induces the methylation of the RNA motifs GAC and AAC, while METTL14 selectively induces the methylation of the RNA motif GAC. METTL3 and METTL14 are both required for the induction of m6A methylation (41, 46). However, the other components of the complex lack RNA methyltransferase activity. WTAP promotes m6A methylation by recruiting METTL3 and METTL14 to nuclear patches (47). RBM15 and RBM15B can bind to METTL3 and WTAP, and they can be localized to specific RNA sites for m6A modification. VIRMA preferentially mediates mRNA methylation near the 3′-untranslated region (UTR) and the stop codon (44). ZC3H13 and WTAP synergistically regulate m6A methylation in the nucleus (43, 45).
2.1.2 m6A demethylases—erasers
The m6A modification is reversible. The removal of the methyl group from m6A can be achieved via active demethylation by the demethylase FTO and the FTO homologue ALKBH5. To date, only these two proteins (FTO and ALKBH5) have been identified as having demethylase activity (48, 49), and they both belong to the ALKB family of Fe(II)/α-ketoglutarate-dependent dioxygenases. FTO is the first enzyme identified to induce RNA demethylation (50), and it is mainly concentrated in the brain and adipose tissue (51). Compared with the tissue-based expression of FTO, ALKBH5 is highly expressed in the testis (49). These findings suggest that different demethylation enzymes may be involved in different biological processes. Consistent with the specificity of expression of these enzymes in different tissues in vivo, FTO knockout mice exhibit phenotypes that include increased postnatal mortality and reduced body weight (52), whereas ALKBH5 knockout mice showed impaired male fertility [43]. In addition, these proteins interact with different protein ligands to catalyze substrates of different tissues (53).
2.1.3 m6A recognition proteins—readers
m6A modifications perform biological functions by binding to m6A “readers” containing the YTH structural domain, and these readers mainly include YTHDC1/YTHDC2, YTHDF1/YTHDF2, YTHDF3, and insulin-like growth factor (IGF)2BP1/IGF2BP2/IGF2BP3. YTHDC1 can bind to m6A-modified pre-mRNAs and promotes exon packaging, splicing, and mRNA export from the nucleus to the cytoplasm by recruiting the splicing factor SRSF3 and preventing SRSF10 from entering the nuclear spot (54–56). YTHDC2 selectively binds m6A modifications of specific motifs, thereby increasing the translation efficiency of mRNAs and decreasing the abundance of target mRNAs (57, 58). YTHDF2 and YTHDF3 can promote mRNA degradation by recognizing and binding to the m6A site of mRNA. In particular, the C-terminal YTH domain of YTHDF2 and YTHDF3 can bind to the m6A site via the conserved G(m6A)C core motif, whereas the N-terminal domain is responsible for localizing to the RNA decay site and promoting the formation of protein−mRNA complexes. Knockdown of YTHDF2 and YTHDF3 leads to a significant increase in mRNAs with m6A modification in cells (59, 60). At the level of mRNA translation, recognition and binding of m6A by YTHDF1 and YTHDF3 promote protein synthesis. YTHDF1 enhances the translation efficiency of m6A-modified mRNAs mainly by interacting with the translation initiation factors eIF3 and eIF4A3, whereas YTHDF3 promotes the translation of m6A-modified mRNAs by binding to YTHDF1 and eIF4A3 (60, 61). The m6A modification in 5′-UTRs increases in response to cellular stress, and m6A residues in 5′-UTRs promote mRNA translation by binding directly to eIF3. This process does not depend on YTHDF1 (62). IGF2BP1/2/3 differs from YTH domain-containing proteins. It recognizes the GG(m6A)C sequence through the K homology domain and enhances the stability of its target mRNA in an m6A-dependent manner under normal and stressful conditions (63).
YBX1 is a multifunctional RNA-binding protein that can act as a “reader” for m5C, which is crucial in regulating the survival of myeloid leukemia cells and the occurrence and development of AML. Recently, YBX1 was found to act synergistically with IGF2BPs to stabilize m6A-tagged mRNAs, which in turn regulate MYC and BCL2 expression levels in AML cells. This may provide a theoretical basis for therapy targeting YBX1 in myeloid leukemia (64) and could demonstrate its role in m6A modification.
2.2 N6,2´-O-dimethyladenosine (m6Am)
In addition to m6A, another reversible modification is observed in higher eukaryotes called N6,2’-O-dimethyladenosine (m6Am). The 5’ end of eukaryotic mRNA usually consists of a 7-methylguanosine (m7G) cap. The first nucleotide after the m7G cap can be methylated on the ribose. If this first nucleotide is 2-O-methyladenosine (Am), then it can be further methylated at its N6 position to produce m6Am. Reports have indicated that the initial adenosine is modified as m6Am in 50–80% of mammalian mRNA. Moreover, this modification plays a crucial role in RNA splicing (65), small nuclear RNA biogenesis (66), mRNA stability (67), and cap-dependent translation (68).
Several studies have shown that cap-terminated m6Am is catalyzed by phosphorylated CTD interacting factor 1 (also known as cap-specific adenosine methyltransferase, CAPAM) (68–71). Chen et al. (65)demonstrated that METTL4 can mediate the formation of m6Am within U2 small nuclear RNA. The demethylase FTO was shown to be an “eraser” of the m6Am modification. For example, when Mauer et al. (72) tested whether m6Am was a substrate for FTO, they found that FTO did target m6Am and had very high catalytic efficiency. They later confirmed that the catalytic activity of FTO for m6Am was nearly 100 times higher than that for m6A (72). Other studies have confirmed that mRNA-decamping enzyme 2 is the reader of m6Am. m6Am can stabilize mRNA by preventing decamping enzyme 2-mediated decapping and microRNA-mediated mRNA degradation (67). Mauer et al. (67) suggested that m6Am makes mRNA less susceptible to decapsidation and significantly contributes to mRNA stability. Thus, m6Am is an RNA modification that is important for mRNA stability.
2.3 5-methylcytosine (m5C)
Although cytosine methylation has been described as a major epigenetic marker and often occurs in the CpG region of eukaryotic DNA (73), previous studies have reported that m5C is related to the nucleotide export (74) and translation efficiency (75) of certain target RNAs. However, the general regulatory function of m5C on gene expression and its precise mechanism need to be further studied. The deposition pattern of m5C on RNA is enriched at CG dinucleotides near the start site of mRNA transcription (73). In eukaryotes, the functions of two key writers, DNMT2 and NSUN2, in relation to m5C have been investigated. DNMT2 was initially identified as a DNA m5C methyltransferase in eukaryotic cells, although later studies showed that it functions mainly as an RNA m5C methyltransferase, which mainly affects the stability and biogenesis of tRNA (76) In contrast, NSUN2 has a wider range of target specificity, including long noncoding RNA (lncRNA), mRNA, and other small regulatory RNAs (such as dome RNA, 7SK, and Y-RNA) (73), and it does not overlap with DNMT2. Three recent studies published in bioRxiv almost simultaneously found that NSUN6 is a new type of mRNA m5C methyltransferase. NSUN6 and NSUN2 play a role in the nonoverlapping m5C site of mRNA, and they participate in almost all m5C modifications of mRNA (77, 78).
Recently, m5C recognition proteins have also been identified, including ALYREF and YBX1 (74–79). ALYREF is a kind of mRNA export factor that can recognize mRNA modified by m5C and promote its nuclear export (80). YBX1 (Y-box binding protein) is a reader of m5C in the cytoplasm and can regulate the stability of its target by recruiting ELAVL1 (mRNA stability maintenance protein). In human bladder urothelial carcinoma, YBX1 targets the m5C site on the 3’-UTR of the oncogene HDGF to stabilize its mRNA and provide a carcinogenic function (79). In zebrafish embryogenesis, YBX1 maintains the stability of m5C by recruiting Pabpc1a to C-modified maternal mRNA to regulate the transition from mother to zygote (81).
2.4 Pseudouridine (ψ)
Pseudouridine (also called ψ) is the most abundant modification in RNA and was also the earliest modification identified (82). With updated detection methods and techniques, researchers found that ψ modified almost all RNA (83). The isomerization of uridine to ribonucleic acid can improve the base accumulation of RNA by forming additional hydrogen bonds, thus affecting the secondary structure and changing the stability of RNA (84). Pseudouracil usually has a strong effect on different aspects of the cellular process. This base modification is catalyzed by pseudouracil synthase (PUS), which acts on the substrate through two different mechanisms. One of these mechanisms is to guide RNA-dependent Ψ acidification, in which H/ACA box snoRNAs interact with the target RNA through a specific sequence (85). Furthermore, pseudouracil can also catalyze the specific target RNA directly through an independent PUS (86). Each enzyme has a unique specificity for its target RNA and modifies uridine in a common sequence.
2.5 N1-methyladenosine (m1A)
The nucleotide modified by methylation at the N1 position of adenosine is m1A, which was initially found only in tRNA and rRNA (87). Its deposition in tRNA occurs in all processes of life. Recently, it was also found to be present in mRNA (88). However, different studies have come to different conclusions about the overall amount of m1A among mRNA modifications (31). In humans, m1A has been detected at positions 9 and 58 of tRNA in the cytoplasm and mitochondria and at position 1322 in the 28S subunit of rRNA (89, 90). The modification of m1A at position 58 in tRNA is dynamically reversible. Its methylation transferase complex is composed of TRMT6 (tRNA methyltransferase noncatalytic subunit 6) and TRMT61 (tRNA methyltransferase catalytic subunit 61) (91). ALKBH1 and ALKBH3 can act as demethylases to remove the m1A modification (92, 93). Moreover, YTHDF2 has been shown to bind to m1A with low affinity. Therefore, it is considered a potential m1A reader in cells (94). At present, studies on m1A are still very limited, which is mainly because of the low abundance of m1A (87) and technical limitations.
2.6 Adenosine-to-inosine editing
A-to-I is a type of RNA editing that is commonly observed in repetitive sequences in mammals (95). Its editing enzymes mainly include ADAR1 (adenosine deaminase acting on dsRNA 1), ADAR2, and ADAD3, and its editing activity is mainly exerted by ADAR1 and ADAR2. A-to-I is present on mRNA, tRNA, and miRNA (96), and the modification sites are also relatively abundant and include coding and noncoding regions (95). Adenosine is usually associated with uridine base pairs, while inosine has a similar structure as guanosine. Therefore, inosine produced by the A-to-I modification is misinterpreted as guanosine and, therefore, with cytosine base pairs. This may lead to alterations in the translated protein product. Since A-to-I modifications are widely present in postnatal to mature organisms and play a critical biological role in humans, it is important to map the location of editing sites to better understand the implications of this editing.
3 Brief overview of CSCs
The concept of CSCs was introduced in the 1970s (97). The first article demonstrating the existence of CSCs was reported in 1994 (98). CSCs usually account for a small fraction of cells in a malignant tumor, and they have the properties of stem cells. They can survive and renew themselves in their niche (99). The CSC hypothesis suggests that a lump of cancer has a hierarchical structure similar to that of normal tissue and that CSCs are located at the apex of this hierarchical tissue (100). Initially, CSCs were mainly observed in hematologic malignancies (98). With further research, CSCs were discovered in solid cancers. CSC populations have been detected in breast cancer (101), glioblastoma (GBM) (102), lung cancer (103), colorectal cancer (CC) (104), prostate cancer (105), and ovarian cancer (106).
CSCs are a population of cancer cells with the ability to self-renew and differentiate, and they are often hidden among cancers and not easily identified (107). Since CSCs are mainly derived from stem cells of the corresponding organs or tissues, stem cell markers vary from tissue to tissue (108). For example, CD44 is mainly found in CSCs of organs such as the breast, ovary, and pancreas. However, unlike normal stem cells, some markers in CSCs are associated with tumorigenic ability. CSCs have high plasticity, and the non-CSC cells surrounding CSCs also present plasticity (108). CSCs can differentiate into non-CSCs, and non-CSC cells can be dedifferentiated into CSCs and become supplemental cells. Therefore, cancer may be cured only by eliminating both types of cells.
The signals involved in the development of CSCs are numerous and include the same signaling pathways as in normal stem cells, such as the Wnt, Hedgehog, Notch, and Hippo signaling pathways (109). Other important signaling pathways in the life course that play important roles in CSCs are PI3K/Akt, MAPK, JAK/Stat, and TGF-β pathway, autophagy, and ferroptosis (110, 111). Research on small molecule drugs targeting these pathways is also ongoing. For example, clonidine, a drug approved by the FDA as an anthelmintic, is an inhibitor of the Wnt/β-catenin pathway that has also been found to inhibit CSCs in ovarian, breast, and prostate cancers and GBM (112–114). Because of the important role of CSCs in cancer development, targeting CSCs is a key strategy for cancer treatment. Research is ongoing on small-molecule drugs targeting CSCs, although these drugs have not yet been approved for use in the clinic.
CSCs have the same genetically driven mutations as most cancer cells. However, CSCs have developmental features that differ from those of non-stem cells, including epigenetic modifications and differences in gene expression profiles (30). Studies have shown that epigenetic modifications contribute to the functional heterogeneity of stem cells and maintain the hierarchy of normal tissue stem cells (115). With the development of epitranscriptomics over the last ten years, studies have shown that RNA modifications also play a role in maintaining the stemness of CSCs. Therefore, studying the role of epitranscriptomics in CSCs can help reveal small molecule drugs that be used to target RNA modifications and likely represent a therapeutic modality for eradicating tumors.
4 Epitranscriptomics in CSC development
RNA modification plays a variety of roles in mechanisms such as genesis of CSCs in several ways. In this section, we show the signaling pathways in which RNA modifications play a role in CSCs (Table 1).
4.1 m6A in CSCs
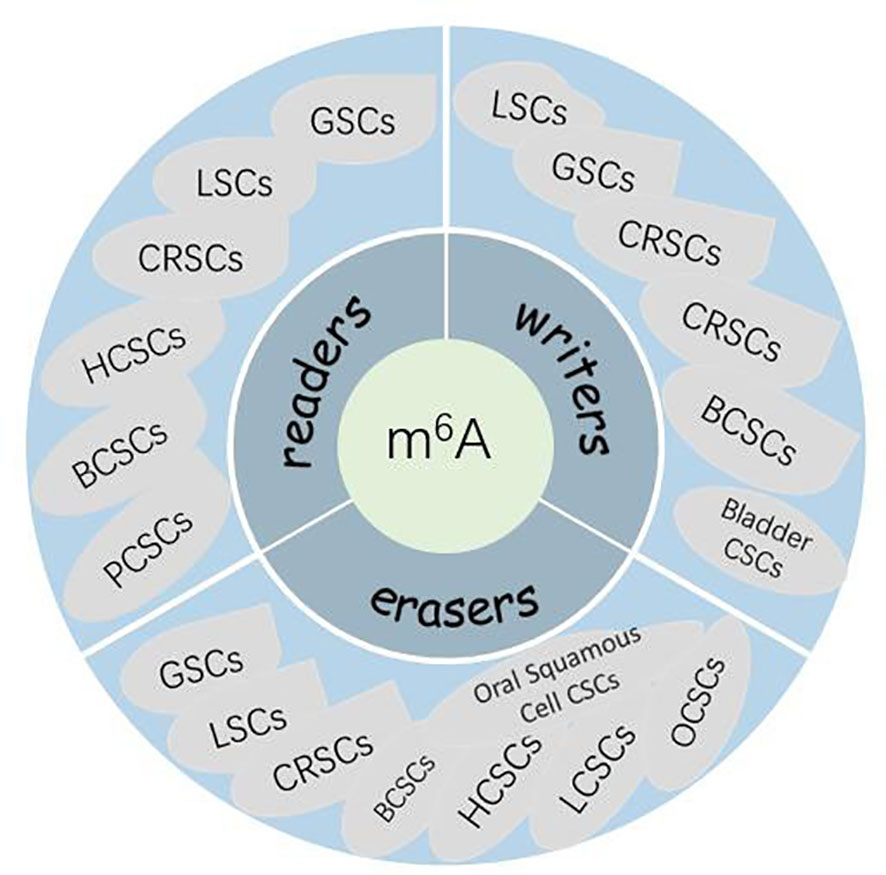
Given the important role of m6A modifications of RNA in regulating gene expression and various biological processes, whether aberrant m6A modifications also play a role in human carcinogenesis should be investigated. It has been shown that m6A modifications are associated with cancer cell proliferation and differentiation, tumorigenesis, invasion, and metastasis and play an oncogenic or anticancer role in malignant tumors (116, 117). The relationship between m6A modifications and malignancies has been extensively reported. However, knowledge about the mechanism between m6A and CSC genesis is limited. CSCs are present in many myeloid leukemias and solid tumors, including GBM, breast cancer, rectal cancer, and squamous cell carcinoma of the skin. The CSC theory suggests that carcinoma initiation and growth are driven by a small number of malignant cells called CSCs. This small population undergoes continuous self-renewal to regenerate itself and differentiate into heterogeneous malignant cells, thereby initiating and maintaining tumorigenesis (118–120). A growing body of evidence suggests that CSCs may be a major cause of carcinoma resistance to conventional chemotherapy and radiotherapy (120–123). Therefore, a better understanding of the link between these stem cell-like malignant tumor cells and m6A modifications is necessary for developing new effective therapeutic approaches and designing new small molecules targeting CSCs. Currently, the link between RNA modifications and CSCs has also been addressed in leukemia stem cells (LSCs), breast CSCs (BCSCs), glioblastoma stem cells (GSCs), and colorectal CSCs (CRSCs). In this article, we present a comprehensive summary of studies related to m6A and various CSCs (Figure 2).
4.1.1 m6A in hematopoietic/leukemic stem cells
Leukemia is a group of hematologic malignancies characterized by the monoclonal expansion of abnormally differentiated hematopoietic cells that can infiltrate the bone marrow and invade the blood and other extramedullary tissues. In general, leukemias can be classified as acute and chronic or gonadal and myeloid according to their progression and type of cells affected. Thus, we can identify the following subtypes: acute lymphocytic leukemia (ALL), chronic lymphocytic leukemia (CLL), acute myeloid leukemia (AML), and chronic myeloid leukemia (CML). Regarding the association between m6A modification and cancers of the hematopoietic system, AML is the most studied by far.
AML is an aggressive mutated disease of hematopoietic stem cells (HSCs) and hematopoietic stem progenitor cells (HSPCs), in which HSPCs present with such mutations that their myeloid differentiation is blocked, resulting in self-renewing LSCs (124). LSCs trigger leukemia. Because they are treatment-resistant, they are often considered a major cause of leukemia relapse. Therefore, there is a current clinical need to be able to identify specific therapeutic targets for the elimination of LSCs. AML is one of the malignancies with the highest expression levels of METTL3 and METTL14 (data from The Cancer Genome Atlas). Moreover, METTL3 and METTL14 were highly expressed in AML cells compared to that in normal hematopoietic progenitor cells (125–128). Consistent with the oncogenic role of METTL3 and METTL14 in AML, overexpression of these two genes enhances cancer cell proliferation in AML cells and progenitor cells while their downregulation strongly induces the onset of apoptosis (125, 126). In addition, the METTL3/METTL14 complex was found to promote the development of AML and maintenance of LSCs in transplanted mouse models (126, 127). In AML cells, METTL3 and METTL14 bind mainly to transcriptional start sites, although METTL3 does not always bind with METTL14. Early m6A cotranscriptional deposition results showed that m6A methylation promoted the translation of mRNAs of AML proliferation-related genes, such as c-MYC, BCL2, PTEN, SP1, and MYB (125–127). The transcription factor CEBPZ has an important role in hematopoietic differentiation and was shown to recruit METTL3 to the gene promoter (126).
In AML, METTL3 was shown to be localized in the cytoplasm and associated with ribosome translation. Similar results have been found in lung cancer. Furthermore, higher levels of cytoplasmic METTL3 can also lead to increased WTAP protein expression (128, 129). Since WTAP mRNA is not highly expressed in AML, WTAP protein levels may be associated with a concomitant elevation of the METTL3/METTL14 nuclear complex. This phenomenon is associated with carcinogenesis in AML.
Furthermore, METTL3 and METTL14 are also highly expressed in mouse and human HSCs. However, their expression is reduced when myeloid cells are differentiated. Silencing of METTL3 and METTL14 reduces stem cell proliferation and promotes bone marrow differentiation in human and mouse HSCs. Furthermore, overexpression of METTL3 or METTL14 in HSCs promoted cell proliferation and inhibited myeloid differentiation. This implies that increased m6A levels may alter the normal differentiation pathway in HSCs and lead to the accumulation of progenitor cells (125–128). These results suggest that METTL3 and METTL14 play a crucial role in the development of leukemia and the maintenance of LSCs.
As the first identified m6A demethylase, FTO has been shown to promote leukemia oncogene-mediated cell transformation as well as leukemogenesis (130). Moreover, FTO expression is significantly elevated in AML subtypes carrying MLL-AF9, PML-RARA, and FTL3-ITD translocations (131). Downregulation of FTO expression reduces the proliferative capacity of these subtypes in cell models carrying these fusion products. In vitro experiment results showed that FTO can promote cell proliferation/transformation, inhibit cell apoptosis, and significantly promote the occurrence of leukemia in vivo. Su et al. (132) found that FTO was significantly overexpressed in LSCs and showed that targeting FTO inhibited the self-renewal of LSCs. Recent studies have found that ALKBH5 is aberrantly overexpressed in AML and is essential for LSC self-renewal but dispensable for normal hematopoietic production (133). Shen et al. (133) identified its target gene, TACC3, in AML through sequencing and experimental verification. TACC3 is strongly associated with poor prognosis in several malignancies. Thus, the ALKBH5/m6A/TACC3 axis is a very promising target for therapy. Another study identified a signaling pathway involved in the critical role of ALKBH5 in AML. KDM4C (a histone demethylase) is significantly elevated in LSCs and increases the expression of ALKBH5 by increasing the chromatin accessibility of ALKBH5 loci (134). This signaling pathway can also be used as a potential therapeutic target for LSCs.
YTHDF2 is responsible for the decay of m6A-modified mRNA transcripts in cells (127), which is also associated with MYC mutations in leukemia (135). Conditional knockout of mouse YTHDF2 using the Cre/LoxP system resulted in an increased number of HSCs, thereby reducing the risk of leukemia due to abnormal HSCs (135, 136). Other findings show that the m6A recognition protein YTHDF2 is widely expressed in human AML and plays an essential role in the pathogenesis and metastasis of AML in mice and humans. YTHDF2 reduces the half-life of different m6A transcripts, including that of the tumor necrosis factor receptor TNFRSF2. These transcripts play a major role in maintaining the functional integrity of LSCs. Interestingly, YTHDF2 is not essential for normal HSC function, and its deletion does not disrupt hematopoiesis. Moreover, a deficiency of YTHDF2 actually enhances HSC activity (137). Therefore, YTHDF2 can be identified as a unique therapeutic target. When it is reduced in expression, LSC proliferation is inhibited, and HSC amplification is promoted. IGF2BP1 is an oncoprotein expressed in various cancers. Elcheva et al. (138) found that IGF2BP1 maintains LSC properties through various stemness pathways, such as self-renewal of the key regulators of HOXB4 and MYB and regulation of ALDH1A1 (aldehyde dehydrogenase 1A1) expression.
Overall, these studies confirm the importance of m6A modification in LSCs, providing insights into the maintenance and self-renewal of LSCs.
4.1.2 m6A in BCSCs
In 2021, the World Health Organization announced that breast cancer had become the most common cancer in the world, overtaking lung cancer (139). As research on breast cancer has advanced, the 5-year survival rate for patients with limited-stage tumors has reached 90%; however, the 5-year survival rate for patients with advanced-stage tumors is still less than 30%. Mortality in breast cancer is mainly due to treatment resistance and malignant metastases. New targeted therapies may help prevent initial or invasive metastases, thus improving survival and clinical outcomes in patients with advanced breast tumors. Only a small percentage of primary breast carcinoma cells have the ability to self-renew, which is an important reason for the formation of metastases or recurrence. These breast cancer cells are referred to as tumor-initiating cells or BCSCs (140).
BCSCs with stem cell properties can generate daughter BCSCs through self-renewal, thus being able to proliferate indefinitely (101, 141). They can also perform a limited number of cell divisions in a short period, producing differentiated breast cancer cells (101, 141). Reports have indicated that breast cancer cells exposed to a hypoxic environment can stimulate significantly increased expression levels of the hypoxia-inducible factors HIF-1α and HIF-2α. Then, the high expression of ALKBH5 was induced in a HIF-dependent manner, which in turn increased NANOG expression levels by removing methylation from the 3’-UTR of NANOG mRNA (142). NANOG, a pluripotent factor that maintains the properties of BCSCs, can promote the renewal of BCSCs. ALKBH5 was shown to enhance the enrichment of BCSCs in the hypoxic tumor microenvironment, and this was also demonstrated in immunodeficient mice (142). In addition to NANOG, ALKBH5 promotes the expression of another pluripotency gene, KLF4. Under hypoxic conditions, m6A in the mRNA of KLF4 is demethylated to maintain the properties of BCSCs (143). Therefore, inhibiting ALKBH5 expression by reducing NANOG and KIF4 expression is an effective strategy for targeting BCSCs in vivo. Moreover, hypoxia-induced lncRNA KB-1980E6.3 could recruit IGF2BP1 and m6A-modified c-Myc mRNA, which in turn increases the stability of c-Myc mRNA and maintains the stemness of BCSCs (144). m6A methylation may also be influenced by anticancer chemicals. Turnip sulfur, a dietary phytochemical, promotes genetic instability by reducing m6A methylation in BCSCs (145).
DROSHA (RNase III) is upregulated in a variety of cancers, although few studies have focused on its mechanism in promoting tumor development. Peng et al. (146) demonstrated that DROSHA cooperates with β-catenin to activate the stemness gene STC1 and maintain BCSC properties. They found that AURKU (Aurora kinase A) kept DROSHA mRNA in a stable state by enhancing m6A modifications to maintain the high expression level of DROSHA in BCSCs, which is primarily based on the ability of AURKU to inhibit the ubiquitination and degradation of METTL14 to stabilize DROSHA mRNA. AURKU also stabilizes m6A-mediated DROSHA mRNA by enhancing IGF2BP2 (146). The above studies suggest that the m6A modification is a potential target for BCSCs.
4.1.3 m6A in GSCs
GBM is the most common and highly destructive primary malignant brain tumor. Recurrence is difficult to avoid, even with surgical resection and intensive radiation therapy. The median survival of patients with GBM is only one year (147). GBMs have significant heterogeneity both inside and outside of tumors. Usually, the cells at the top of the cell hierarchy have stem-like properties. GSCs are self-renewing, resistant to conventional therapy, and maintain long-term tumor growth, which is an important cause of tumor recurrence (148). Hence, studying the mechanisms underlying GSC self-renewal and proliferation could provide a better understanding of GBM tumorigenesis and help identify better therapeutic measures. The m6A modification is essential for GSC self-renewal and tumorigenesis. m6A demethylase ALKBH5 is highly expressed in GSCs, and the proliferation of patient-derived GSCs can be inhibited by silencing the expression of ALKBH5. Integrative transcriptome and m6A-seq analyses showed that ALKBH5 can demethylate the mRNA of the transcription factor FOXM1, thus leading to enhanced expression levels of FOXM1 and the recovery of tumor growth (149, 150).
Recent research progress has also been made on radiation resistance in GBM. ALKBH5, a recognized oncogenic factor, is abnormally elevated in expression in GBM. Previous studies have found that DNA damage repair is upregulated in GSCs (151), which may be one of the reasons for the development of resistance to radiotherapy, and showed that the sensitivity of GSCs to radiotherapy increased when ALKBH5 was downregulated (152). ALKBH5 expression is blocked by ALKBH5 siRNA in GSCs, thus revealing that the expression of genes involved in homologous recombination repair was significantly decreased (152). The results validated by further experiments showed that blocking ALKBH5 could delay DNA damage repair (152) and suggested that inhibitors of ALKBH5 may be a potential therapeutic modality for overcoming resistance and improving sensitivity to radiotherapy.
Reports have also shown that the growth, self-renewal, and tumorigenesis of human GSCs are significantly promoted by knocking down key components of the RNA methyltransferase complex METTL3 or METTL14. In contrast, overexpression of METTL3 or inhibition of FTO activity inhibits the growth and self-renewal of GSCs. Interestingly, inhibition of FTO significantly delays tumor progression and extends the life span of GSC-transplanted mice. An m6A-RIP sequence analysis revealed that the RNA methyltransferases METTL3 and METTL14 significantly promoted the growth and self-renewal of GSCs by regulating the mRNA expression of the GBM-associated gene ADAM19 (153).
Additional studies have confirmed that METTL3 can confer radioresistance to GSCs by enhancing SOX2-dependent DNA repair (154). Enhanced sensitivity of GBM to γ-irradiation is observed when METTL3 is silenced, thus providing evidence for METTL3 as a potential molecular target for GBM treatment (154). Moreover, METTL3 is also required to promote tumorigenesis by maintaining the expression of GSC-specific active transcriptional genes (155).
YTHDF1, a recognition protein of m6A, is also involved in GBM development. Aliaksandr et al. (156) found that tumorsphere formation, stemness marker expression, and migration capacity were decreased after knocking down YTHDF1. They concluded that YTHDF1 is required to maintain CSCs in GBM cell lines. This study found that Musashi-1 (MSI1), an RNA-binding protein, is a marker of poor prognosis that can positively regulate the expression of YTHDF1. Thus, YTHDF1 and MSI1 are potential prognostic markers and therapeutic targets.
4.1.4 m6A in CRSCs
The global cancer statistics report shows that CC is a common cancer that presented high incidence, death, morbidity, and mortality rates in 2021 (157). The mortality and morbidity of CC are rising in China, with 590,000 new cases and 300,000 deaths observed in 2021 (158). Despite improvements in medical care, the 5-year survival rate for patients with CC is only 64.9% (159). In recent years, numerous studies have shown that CSCs play an important role in CC (160–162). Therefore, investigating the molecular regulatory mechanisms of CRSCs in CC renewal and metastasis may be a potential approach to treating CC.
CBX8, also known as human polycomb 3, is significantly expressed in chemotherapy-resistant patients with CC. It is closely associated with stemness markers, such as LGR5, CD133, and CD44, in CC tissues. The RNA Modification Database (RMBase) showed that CBX8 mRNA is distributed with many high-confidence m6A sites. Therefore, researchers have investigated whether there is an association between m6A and CBX8 and queried The Cancer Genome Atlas database, and they showed that METTL3 is significantly upregulated in CC and METTL3-mediated m6A modification induces CBX8 overexpression and maintains the stemness of CC cells (163). This study provides a useful target for reversing the stemness of CC cells and improving the sensitivity of these cells to chemotherapy.
The expression of YTHDF1 is higher in CC than in normal tissues, whereas the expression of other YTH domain family members, YTHDF2, YTHDF3, YTHDC1, and YTHDC2, shows limited differences between colorectal tumors and normal tissues (164). Later studies showed that knocking down YTHDF1 inhibits the activity of the Wnt/β-catenin pathway in CC cells and significantly downregulates the expression of CRSC markers, thereby reducing the tumorigenicity of CRSCs in vitro and the tumor growth of mouse xenografts in vivo (165). Another study investigated the role of YTHDF1 in the Wnt/β-catenin protein signaling pathway in CC (166) and identified the checkpoint TCF7L2, which is activated by β-catenin. YTHDF1 promotes the translation of Wnt signaling effectors, including TCF7L2/TCF4, and activates Wnt signaling while enhancing β-linked protein activity. This process is required to maintain the stemness of intestinal stem cells. The above two studies suggest that the regulation of YTHDF1 plays a key role in the tumorigenicity and maintenance of CRSCs and thus may have revealed a potential therapeutic target for CC.
Zhao et al. (167) found that berberine has similar effects as FTO inhibitors. Reducing β-catenin can increase FTO in CRSCs and then reduce m6A, resulting in decreased stemness and increased sensitivity to chemotherapy. The study also suggested new strategies for developing new antitumor drugs that target Wnt/beta-catenin.
4.1.5 m6A in HCSCs
Hepatocellular carcinoma (HCC) is a common pathological type of liver cancer and an important cause of death due to cancer. Advanced HCC is poorly treated and generally has a poor prognosis. For advanced HCC and recurrent HCC, effective treatment modalities are lacking. Therefore, understanding the molecular mechanisms of HCSCs may be an avenue to discover effective treatment modalities. YTHDF2 is required for the mRNA of OCT4 (pluripotency factor) to maintain m6A methylation at the 5′-UTR, which in turn promotes the expression of OCT4 protein and the formation of the HCSC phenotype (168). S-adenosylmethionine decarboxylase proenzyme has been identified as an oncoprotein. Recently, researchers have explored the role of S-adenosylmethionine decarboxylase proenzyme in HCC. They announced that increased levels of S-adenosylmethionine decarboxylase proenzyme could promote the expression of stemness genes such as SOX2, KLF4, and NANOG in HCSCs through FTO-mediated m6A demethylation in mRNA (169). All of the above studies provide ideas for targeting HCSCs.
4.1.6 m6A and other solid CSCs
Recently, the role of m6A has also been studied in stem cells of other solid tumors, including lung cancer, pancreatic cancer, bladder cancer, osteosarcoma, ovarian cancer, oral squamous cell carcinoma, and cutaneous squamous cell carcinoma. For example, Liu et al. (170) found that ALKBH5 was highly expressed in CSCs derived from non-small cell lung cancer. p53 could regulate m6A levels by regulating ALKBH5, which in turn affected stemness gene expression in lung CSCs. Hu et al. (171) proclaimed that IGF2BP2 acts as a reader of m6A modification on lncRNA DANCR in pancreatic adenocarcinoma. Its upregulation stabilizes lncRNA DANCR and promotes stemness-like properties of pancreatic adenocarcinoma cells. Gao et al. (172) revealed that METTL3 and m6A modifications were significantly higher in bladder cancer CSCs than in non-CSCs. AFF4 binds to the promoters of SOX2 (stemness gene) and MYC (oncogene) to activate their transcription. METTL3 promotes the self-renewal ability of BCSCs by regulating m6A levels in mRNA and the expression of AFE4. Wang et al. (173) obtained osteosarcoma stem cells with stemness characteristics from osteosarcoma cells treated with progressively increasing concentrations of chemotherapeutic agents. RNA-seq results of these cells showed that the mRNA methylation levels of genes that maintain stemness were significantly increased. They hypothesized that the occurrence of osteosarcoma stem cells is closely related to the maintenance of m6A modification. Huang et al. (174) revealed that FTO was underexpressed in ovarian cancer cells and ovarian CSCs. The self-renewal and tumor formation ability of ovarian CSCs was decreased after overexpression of FTO. Two phosphodiesterase genes (PDE4B and PDE1C) were identified as FTO targets. They play a key role in maintaining ovarian CSC stemness. Omprakash et al. (175) found that DDX3 (a human DEAD-box RNA helicase) reduced the m6A modification of mRNAs of two stemness genes (FOXM1 and NANOG) through ALKBH5 in oral squamous carcinoma, resulting in reduced CSCs. Zhou et al. (176) discovered that the knockdown of METTL3 decreased the self-renewal ability of stem-like cells in cutaneous squamous cell carcinoma. These findings lead us to think that m6A might be related to stem cells in many other tumors. It also further affirms the relationship between m6A and CSCs and provides a direction for finding new therapeutic modalities.
4.2 Other RNA modifications in CSCs
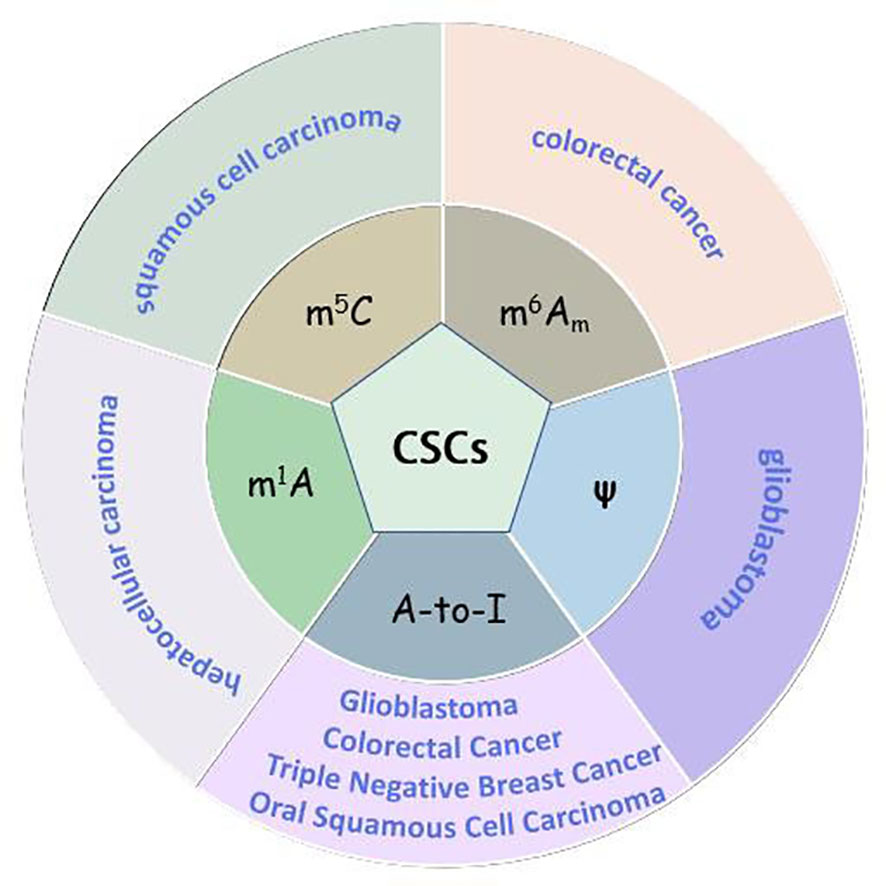
More and more studies have found the relationship between CSCs and other RNA modifications. These findings are exhibited as follows (Figure 3).
4.2.1 m6Am in CSCs
Sun et al. [172] developed an m6Am-seq assay based on demethylation and RNA immunoprecipitation in vitro. This technique can directly differentiate m6Am and 5′-UTR N6-m6A and thus aid in the in-depth study of the functions of both modifications (177). Unlike m6A, the function of m6Am modifications is not well understood in mRNA. Recently, a study found that m6Am levels were elevated in cell lines derived from patients with CC when FTO was expressed at low levels. Moreover, the tumorigenicity and chemical resistance of the cells are enhanced in vivo [173]. After a series of experimental validations, m6Am was found to be a key transcriptomic marker controlling the stem-like cell phenotype in human CC cells. This deepened our understanding of the CSC phenotype and provided insights into developing a drug or therapy that reduces the ability of CSCs to metastasize and resist drugs (178).
4.2.2 m5C in CSCs
m5C modifications play a variety of roles in various life processes, including self-renewal and differentiation of embryonic stem cells (179). Some studies have shown that m5C is closely associated with cancer pathogenesis, including initiation, metastasis, progression, recurrence, and drug resistance (180). However, there are very few studies on the relationship between m5C and CSCs. Blanco et al. (181) investigated the relationship between protein synthesis and cellular stress response and stem cell function and found that the writer of m5C, NSUN2, promotes protein synthesis by protecting tRNA from cleavage. When NSUN2 was inhibited, protein synthesis was decreased. Simultaneously, inhibition of NSUN2 function promoted stem cell function and tumorigenesis when posttranscriptional methylation was decreased in squamous cell carcinoma (181).
4.2.3 ψ in CSCs
Ψ is the most abundant nucleotide modification in rRNA and tRNA and is also present in mammalian and yeast mRNA. It plays a role in RNA folding and secondary structure, stability, and translation. Ψ was the first identified RNA modification associated with cancer (35). PUS7 is a Ψ-depositing enzyme that promotes tumor growth. PUS7 is implicated in the formation and growth of CSCs. Ψ is one of the most abundant RNA modifications in GBM. Cui et al. (182) found that the growth of GSCs was inhibited when PUS7 expression was decreased in hormonal tumors. A specific PUS7-dependent Ψ modification was found in GSCs. In addition, they detected chemical inhibitors of PUS7 that inhibited PUS7-mediated Ψ modifications, suppressed tumorigenesis, and prolonged the lifespan of tumor-bearing mice (182). PUS7-mediated Ψ activates mTOG (mini-5´-terminal oligoguanine), which affects protein synthesis in HSCs. This driver mechanism can damage HSCs and may lead to marrow malignancies in humans (183).
4.2.4 m1A in CSCs
Currently, m1A has been less studied in malignant tumors. Thus, the role of m1A methylation remains largely unknown in tumors. Studies on CSCs and m1A are even rarer. Intriguingly, Wang et al. (184) found that m1A modification of tRNA was significantly elevated in HCCs. The m1A methylation signal also became abnormally upregulated in HCSCs. Therefore, they explored the mechanism and showed that TRMT6/TRMT61A-mediated m1A enhanced PPARδ (peroxisome proliferator-activated receptor δ) protein translation. PPARδ could promote self-renewal and tumor growth in HCSCs.
4.2.5 A-to-I in CSCs
Because A-to-I is abundant in the human brain, it has been more extensively studied in the nervous system. Decreased ADAR2 is closely associated with the recurrence and aggressiveness of glioma (98, 99). In addition, ADARs play oncogenic roles in malignant tumors, such as liver, bladder, and lung cancers, through different mechanisms (100–102). However, a small number of studies has shown that ADAR1 plays a role in tumor suppressor genes (103). In conclusion, studies on A-to-I in cancers are gradually increasing, and they revealed that A-to-I is an important mechanism for cancer development and an important target for cancer therapy.
The relationship between A-to-I and cancer stemness has also been reported. For example, Liu et al. (185) demonstrated that ADAR1 oral squamous cell carcinoma is closely associated with stemness. When ADAR1 is knocked down, the expression of SOX2 and POUF51 is reduced, as shown by a protein blotting assay, and the stemness of oral squamous cell carcinoma lines was also found to be attenuated. These studies suggest that ADAR1 can maintain the stemness phenotype of oral squamous cell carcinoma. GSCs are an important factor that contributes to recurrence and drug resistance in GBM. Li et al. (186) found that ADAR1 expression was increased in GSCs. The TYK2/ADAR1/GM2A axis was found to be critical in maintaining the stemness of GSCs; thus, it is likely to be a new clinical strategy for GBM treatment. Lee et al. (187) explored the transcriptome and epitranscriptome landscape of triple-negative breast cancer (TNBC) and identified CSC-like microecological sites in TNBC containing characteristic A-to-I editing. In addition, ADAR1-mediated A-to-I editing leads to an increase in AZIN1 expression, thereby enhancing the stemness of colorectal adenocarcinoma cells (188).
5 Conclusion and future scope
In recent years, technological breakthroughs have rapidly increased our understanding of the mechanisms of RNA modifications. The possible role of posttranscriptional modifications in human diseases has been revealed and recognized. Nevertheless, the epitranscriptome is complex and diverse, and we have probably touched only the tip of the iceberg in terms of its study. Although many RNA modifications contributions to carcinogenesis have been identified, their molecular targets in cancer pathogenesis have not been revealed. Moreover, abnormal expression of RNA modifying enzymes has been found in many cancer cells, but how specific RNA modifications affect different cancer cell subpopulations remains largely unknown. Thus, the field of epitranscriptomics requires comprehensive development, including the creation of new assays, the improvement of assay sensitivity, and the development of detailed research strategies. The detection of RNA modifications by next generation sequencing (NGS) requires selective chemical treatment or antibody immunoprecipitation for specific modification types. At the same time, these methods also face the problems of short reading length, isomer ambiguity, bias and artefacts. Direct RNA sequencing (DRS), commercialized by Oxford Nanopore technologies (ONT), is able to directly detect any given modifications in a single transcript, improving these limitations of NGS-based methods. For example, Zhang et al. (189) presented DirectRMDB, the first ONT-based quantitative mapping database of RNA modifications, which includes 16 types and a total of 904,712 modification sites in 25 species from 39 independent studies. This platform integrates three generation sequencing and eight RNA modification detection technologies, making it possible to easily query specific modifications on RNA isomers.
In addition, thanks to advances in high-throughput sequencing techniques for transcriptome analysis, the emergence of large databases of RNA modifications has become an exciting field that promotes further research into the mechanisms and functions of these RNA modifications. By using machine learning methods, the localization of RNA methylation sites in humans, mice and other species has been predicted more accurately. At the same time, new computational methods are being developed to understand the disease associations of individual RNA methylation sites by utilizing the large amount of apparent transcriptome data accumulated in existing studies (190). Such as DRUM, an online database that incorporates the associations among diseases, genes and m6A RNA methylation sites from gene expression, RNA methylation and disease similarities data with the Random Walk with Restart (RWR) algorithm, was used to query the top m6A sites related to 705 different diseases (191). As a comprehensive database, m6AVar enables systematic association analysis to identify associated variants that may affect RBP binding regions, miRNA targets, and splicing sites. m6AVar can also be used to identify pathogenic variant modifications that cause m6A dysregulation (192). To fully uncover the association between disease-related variants and their epigenetic transcriptome disturbances, Chen et al. established RMDisease, a database of genetic variants that correlate RNA modifications with underlying diseases (193). Recently, the authors have updated RMDisease to RMDiseasev2.0 by collecting all available RM-related variants and annotating their potential participation in diseases and traits (194). Song et al. proposed a comprehensive online platform, m6A-TSHub, to reveal m6A methylation and m6A-relateded mutations in 23 human tissues. This is particularly important for studying the epigenetic transcriptome of diseases associated with specific tissues, especially cancer-related diseases (195). As high-throughput apparent transcriptome data becomes more available, advanced techniques for RM analysis are being developed, and databases linking RNA modification to disease, particularly tumor disease, are being developed to better understand and explore the potential association between modification and tumor disease.
Given that RNA modifications play an important role in a variety of cancers, the use of RNA modifications as diagnostic and prognostic indicators or effective therapeutic strategies has great potential for clinical application. Several current studies related to the link between RNA-modifying enzymes and cancer have also provided a basis for the rational clinical design of efficient and specific RNA inhibitors. For example, through structural and biochemical analysis, the natural product rhein was found to competitively bind to the active site of FTO, thereby preventing FTO from binding to the m6A substrate (196). Therefore, it has an inhibitory effect on m6A demethylation activity (196). Another FTO inhibitor, meclofenamic acid, is an FDA-approved NSAID that also competes with FTO for binding and thus increases m6A levels in mRNAs in cells (197). The successful inhibition of the in vitro and in vivo growth of GSCs by meclofenamic acid 2, an ethylester derivative of meclofenamic acid, was recently reported (146). However, according to current findings, RNA modifications sometimes act on proto-oncogenes or oncogenes. Because of the contradictory role of epitranscriptomics, drug studies should be conducted with great caution to ensure safety. Since RNA-modifying enzymes are involved in a large number of life processes, the effect of small molecule agents on other normal life processes should be taken very seriously when treating tumors. For example, in the absence of RNA methylesterase, the affected organs are usually the brain and testis (198, 199).
With advances in sequencing technology and deciphering of the human genome, our understanding of cancer has changed substantially in the past decade. RNA modifications have been found to play an important role in carcinogenesis and imparts stemness to cancer cell subpopulations. On the one hand, RNA modifications can promote cancer progression by reducing the stability of cancer suppressor genes to eliminate their inhibitory effect. On the other hand, they can enhance the stability and expression of the proto-oncogene transcripts. Cancer genomes have been revealed as complex and heterogeneous, and carcinomas do not arise from a single clone with a single tumor genome. CSCs are a group of cells with self-renewal capabilities that drive tumor growth and reconstitute tumor heterogeneity. CSCs are in a static state during treatment and have strong resistance to treatment; thus, they represent an important cause of tumor recurrence, metastasis, and drug resistance. RNA modifications are closely related to CSC formation and stemness maintenance. In this paper, we discuss recent advances in the relationship between RNA modifications and cancer stemness and the impact of these modifications on cancer formation. The development of new targeted drugs based on the effects of RNA modifications on CSCs represents a very promising treatment direction. Moreover, because of the different abnormal signaling pathways within CSCs, combining drugs from other targets to eradicate CSCs is a promising therapeutic modality. Traditional treatment modalities for cancers mainly target non-CSCs in tumor tissues. Traditional tumor therapy combined with CSC-targeted therapy can kill a large number of tumor cells while eliminating CSCs, thus causing metastasis and recurrence, and it also represents a therapeutic direction that should be further explored.
Author contributions
All authors have contributed to this review. JG has provided the conception. LH has written the first and revised draft. JZ, ZL,XL have revised the draft. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by funding from the Jilin Province Financial and Health Project(A project to improve the ability to diagnose and treat gynecological oncology, 3D517EC03427), Jilin Provincial Department of Finance (CBCT bone registration was used to analyze the effect of volume rotation intensity modulation on target dose, 3D5204889429), Jilin Provincial Department of Finance (Dose-effect analysis of Zeiss intraoperative radiotherapy machine irradiating primary cultured vertebral metastases, 2019SCZT029).
Acknowledgments
We acknowledge and appreciate our colleagues for their valuable efforts and comments on this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cheon H, Paik JH, Choi M, Yang H-J, Son J-H. Detection and manipulation of methylation in blood cancer DNA using terahertz radiation. Sci Rep (2019) 9(1):6413. doi: 10.1038/s41598-019-42855-x
2. Antoun E, Kitaba NT, Titcombe P, Dalrymple KV, Garratt ES, Barton SJ, et al. Maternal dysglycaemia, changes in the infant's epigenome modified with a diet and physical activity intervention in pregnancy: Secondary analysis of a randomised control trial. PloS Med (2020) 17(11):e1003229. doi: 10.1371/journal.pmed.1003229
3. Parira T, Figueroa G, Laverde A, Casteleiro G, Gomez Hernandez ME, Fernandez-Lima F, et al. Novel detection of post-translational modifications in human monocyte-derived dendritic cells after chronic alcohol exposure: Role of inflammation regulator H4k12ac. Sci Rep (2017) 7(1):11236. doi: 10.1038/s41598-017-11172-6
4. Dong X, Zhang J, Yang F, Wu J, Cai R, Wang T, et al. Effect of luteolin on the methylation status of the opcml gene and cell growth in breast cancer cells. Exp Ther Med (2018) 16(4):3186–94. doi: 10.3892/etm.2018.6526
5. Zhou M, Yin X, Zheng L, Fu Y, Wang Y, Cui Z, et al. Mir-181d/Rbp2/Nf-κb P65 feedback regulation promotes chronic myeloid leukemia blast crisis. Front Oncol (2021) 11:654411. doi: 10.3389/fonc.2021.654411
6. Liu X, Wang X, Liu N, Zhu K, Zhang S, Duan X, et al. Tet2 is involved in DNA hydroxymethylation, cell proliferation and inflammatory response in keratinocytes. Mol Med Rep (2020) 21(4):1941–9. doi: 10.3892/mmr.2020.10989
7. Yoon KJ, Vissers C, Ming GL, Song H. Epigenetics and Epitranscriptomics in temporal patterning of cortical neural progenitor competence. J Cell Biol (2018) 217(6):1901–14. doi: 10.1083/jcb.201802117
8. Cohn WE. Some results of the applications of ion-exchange chromatography to nucleic acid chemistry. J Cell Physiol Suppl (1951) 38(Suppl. 1):21–40. doi: 10.1002/jcp.1030380405
9. Li X, Zhu P, Ma S, Song J, Bai J, Sun F, et al. Chemical pulldown reveals dynamic pseudouridylation of the mammalian transcriptome. Nat Chem Biol (2015) 11(8):592–7. doi: 10.1038/nchembio.1836
10. Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, et al. Topology of the human and mouse M6a rna methylomes revealed by M6a-seq. Nature (2012) 485(7397):201–6. doi: 10.1038/nature11112
11. Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mrna methylation reveals enrichment in 3' utrs and near stop codons. Cell (2012) 149(7):1635–46. doi: 10.1016/j.cell.2012.05.003
12. Huang T, Chen W, Liu J, Gu N, Zhang R. Genome-wide identification of mrna 5-methylcytosine in mammals. Nat Struct Mol Biol (2019) 26(5):380–8. doi: 10.1038/s41594-019-0218-x
13. Pandolfini L, Barbieri I, Bannister AJ, Hendrick A, Andrews B, Webster N, et al. Mettl1 promotes let-7 microrna processing Via M7g methylation. Mol Cell (2019) 74(6):1278–90.e9. doi: 10.1016/j.molcel.2019.03.040
14. Zhang LS, Liu C, Ma H, Dai Q, Sun HL, Luo G, et al. Transcriptome-wide mapping of internal N(7)-methylguanosine methylome in mammalian mrna. Mol Cell (2019) 74(6):1304–16.e8. doi: 10.1016/j.molcel.2019.03.036
15. Xu L, Liu X, Sheng N, Oo KS, Liang J, Chionh YH, et al. Three distinct 3-methylcytidine (M(3)C) methyltransferases modify trna and mrna in mice and humans. J Biol Chem (2017) 292(35):14695–703. doi: 10.1074/jbc.M117.798298
16. Ramanathan A, Robb GB, Chan SH. Mrna capping: Biological functions and applications. Nucleic Acids Res (2016) 44(16):7511–26. doi: 10.1093/nar/gkw551
17. Sun WJ, Li JH, Liu S, Wu J, Zhou H, Qu LH, et al. Rmbase: A resource for decoding the landscape of rna modifications from high-throughput sequencing data. Nucleic Acids Res (2016) 44(D1):D259–65. doi: 10.1093/nar/gkv1036
18. Boccaletto P, Stefaniak F, Ray A, Cappannini A, Mukherjee S, Purta E, et al. Modomics: A database of rna modification pathways. 2021 update. Nucleic Acids Res (2022) 50(D1):D231–d5. doi: 10.1093/nar/gkab1083
19. Deng S, Zhang H, Zhu K, Li X, Ye Y, Li R, et al. M6a2target: A comprehensive database for targets of M6a writers, erasers and readers. Brief Bioinform (2021) 22(3). doi: 10.1093/bib/bbaa055
20. Tang Y, Chen K, Song B, Ma J, Wu X, Xu Q, et al. M6a-atlas: A comprehensive knowledgebase for unraveling the N6-methyladenosine (M6a) epitranscriptome. Nucleic Acids Res (2021) 49(D1):D134–d43. doi: 10.1093/nar/gkaa692
21. Ma J, Song B, Wei Z, Huang D, Zhang Y, Su J, et al. M5c-atlas: A comprehensive database for decoding and annotating the 5-methylcytosine (M5c) epitranscriptome. Nucleic Acids Res (2022) 50(D1):D196–d203. doi: 10.1093/nar/gkab1075
22. Jiang S, Xie Y, He Z, Zhang Y, Zhao Y, Chen L, et al. M6asnp: A tool for annotating genetic variants by M6a function. Gigascience (2018) 7(5). doi: 10.1093/gigascience/giy035
23. Song B, Chen K, Tang Y, Wei Z, Su J, de Magalhães JP, et al. Consrm: Collection and Large-scale prediction of the evolutionarily conserved rna methylation sites, with implications for the functional epitranscriptome. Brief Bioinform (2021) 22(6). doi: 10.1093/bib/bbab088
24. Hussain S. Shaping and reshaping transcriptome plasticity during evolution. Trends Biochem Sci (2017) 42(9):682–4. doi: 10.1016/j.tibs.2017.06.009
25. Nombela P, Miguel-López B, Blanco S. The role of M(6)a, M(5)C and Ψ rna modifications in cancer: Novel therapeutic opportunities. Mol Cancer (2021) 20(1):18. doi: 10.1186/s12943-020-01263-w
26. Uddin MB, Wang Z, Yang C. Dysregulations of functional rna modifications in cancer, cancer stemness and cancer therapeutics. Theranostics (2020) 10(7):3164–89. doi: 10.7150/thno.41687
27. Lence T, Akhtar J, Bayer M, Schmid K, Spindler L, Ho CH, et al. M(6)a modulates neuronal functions and sex determination in drosophila. Nature (2016) 540(7632):242–7. doi: 10.1038/nature20568
28. Tong J, Cao G, Zhang T, Sefik E, Amezcua Vesely MC, Broughton JP, et al. M(6)a mrna methylation sustains treg suppressive functions. Cell Res (2018) 28(2):253–6. doi: 10.1038/cr.2018.7
29. Najafi M, Farhood B, Mortezaee K. Cancer stem cells (Cscs) in cancer progression and therapy. J Cell Physiol (2019) 234(6):8381–95. doi: 10.1002/jcp.27740
30. Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell (2014) 14(3):275–91. doi: 10.1016/j.stem.2014.02.006
31. Barbieri I, Kouzarides T. Role of rna modifications in cancer. Nat Rev Cancer (2020) 20(6):303–22. doi: 10.1038/s41568-020-0253-2
32. Huang H, Weng H, Chen J. M(6)a modification in coding and non-coding rnas: Roles and therapeutic implications in cancer. Cancer Cell (2020) 37(3):270–88. doi: 10.1016/j.ccell.2020.02.004
33. Liu L, Li H, Hu D, Wang Y, Shao W, Zhong J, et al. Insights into N6-methyladenosine and programmed cell death in cancer. Mol Cancer (2022) 21(1):32. doi: 10.1186/s12943-022-01508-w
34. Xue C, Chu Q, Zheng Q, Jiang S, Bao Z, Su Y, et al. Role of main rna modifications in cancer: N(6)-methyladenosine, 5-methylcytosine, and pseudouridine. Signal Transduct Target Ther (2022) 7(1):142. doi: 10.1038/s41392-022-01003-0
35. Delaunay S, Frye M. Rna modifications regulating cell fate in cancer. Nat Cell Biol (2019) 21(5):552–9. doi: 10.1038/s41556-019-0319-0
36. Frye M, Harada BT, Behm M, He C. Rna modifications modulate gene expression during development. Science (2018) 361(6409):1346–9. doi: 10.1126/science.aau1646
37. Meyer KD, Jaffrey SR. Rethinking M(6)a readers, writers, and erasers. Annu Rev Cell Dev Biol (2017) 33:319–42. doi: 10.1146/annurev-cellbio-100616-060758
38. Canaani D, Kahana C, Lavi S, Groner Y. Identification and mapping of N6-methyladenosine containing sequences in simian virus 40 rna. Nucleic Acids Res (1979) 6(8):2879–99. doi: 10.1093/nar/6.8.2879
39. Narayan P, Ayers DF, Rottman FM, Maroney PA, Nilsen TW. Unequal distribution of N6-methyladenosine in influenza virus mrnas. Mol Cell Biol (1987) 7(4):1572–5. doi: 10.1128/mcb.7.4.1572-1575.1987
40. Zhong S, Li H, Bodi Z, Button J, Vespa L, Herzog M, et al. Mta is an arabidopsis messenger rna adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell (2008) 20(5):1278–88. doi: 10.1105/tpc.108.058883
41. Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, et al. A Mettl3-Mettl14 complex mediates mammalian nuclear rna N6-adenosine methylation. Nat Chem Biol (2014) 10(2):93–5. doi: 10.1038/nchembio.1432
42. Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, Guttman M, et al. M(6)a rna methylation promotes xist-mediated transcriptional repression. Nature (2016) 537(7620):369–73. doi: 10.1038/nature19342
43. Knuckles P, Lence T, Haussmann IU, Jacob D, Kreim N, Carl SH, et al. Zc3h13/Flacc is required for adenosine methylation by bridging the mrna-binding factor Rbm15/Spenito to the M(6)a machinery component Wtap/Fl(2)D. Genes Dev (2018) 32(5-6):415–29. doi: 10.1101/gad.309146.117
44. Yue Y, Liu J, Cui X, Cao J, Luo G, Zhang Z, et al. Virma mediates preferential M(6)a mrna methylation in 3'utr and near stop codon and associates with alternative polyadenylation. Cell Discovery (2018) 4:10. doi: 10.1038/s41421-018-0019-0
45. Wen J, Lv R, Ma H, Shen H, He C, Wang J, et al. Zc3h13 regulates nuclear rna M(6)a methylation and mouse embryonic stem cell self-renewal. Mol Cell (2018) 69(6):1028–38.e6. doi: 10.1016/j.molcel.2018.02.015
46. Wang Y, Li Y, Toth JI, Petroski MD, Zhang Z, Zhao JC. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol (2014) 16(2):191–8. doi: 10.1038/ncb2902
47. Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, et al. Mammalian wtap is a regulatory subunit of the rna N6-methyladenosine methyltransferase. Cell Res (2014) 24(2):177–89. doi: 10.1038/cr.2014.3
48. Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR, Qian SB. Dynamic M(6)a mrna methylation directs translational control of heat shock response. Nature (2015) 526(7574):591–4. doi: 10.1038/nature15377
49. Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, et al. Alkbh5 is a mammalian rna demethylase that impacts rna metabolism and mouse fertility. Mol Cell (2013) 49(1):18–29. doi: 10.1016/j.molcel.2012.10.015
50. Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, et al. N6-methyladenosine in nuclear rna is a major substrate of the obesity-associated fto. Nat Chem Biol (2011) 7(12):885–7. doi: 10.1038/nchembio.687
51. Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, Hewitson KS, et al. The obesity-associated fto gene encodes a 2-Oxoglutarate-Dependent nucleic acid demethylase. Science (2007) 318(5855):1469–72. doi: 10.1126/science.1151710
52. Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, Brüning JC, et al. Inactivation of the fto gene protects from obesity. Nature (2009) 458(7240):894–8. doi: 10.1038/nature07848
53. Shi H, Wei J, He C. Where, when, and how: Context-dependent functions of rna methylation writers, readers, and erasers. Mol Cell (2019) 74(4):640–50. doi: 10.1016/j.molcel.2019.04.025
54. Roundtree IA, He C. Nuclear M(6)a reader Ythdc1 regulates mrna splicing. Trends Genet (2016) 32(6):320–1. doi: 10.1016/j.tig.2016.03.006
55. Roundtree IA, Luo GZ, Zhang Z, Wang X, Zhou T, Cui Y, et al. Ythdc1 mediates nuclear export of N(6)-methyladenosine methylated mrnas. Elife (2017) 6. doi: 10.7554/eLife.31311
56. Kasowitz SD, Ma J, Anderson SJ, Leu NA, Xu Y, Gregory BD, et al. Nuclear M6a reader Ythdc1 regulates alternative polyadenylation and splicing during mouse oocyte development. PloS Genet (2018) 14(5):e1007412. doi: 10.1371/journal.pgen.1007412
57. Hsu PJ, Zhu Y, Ma H, Guo Y, Shi X, Liu Y, et al. Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res (2017) 27(9):1115–27. doi: 10.1038/cr.2017.99
58. Wojtas MN, Pandey RR, Mendel M, Homolka D, Sachidanandam R, Pillai RS. Regulation of M(6)a transcripts by the 3'→5' rna helicase Ythdc2 is essential for a successful meiotic program in the mammalian germline. Mol Cell (2017) 68(2):374–87.e12. doi: 10.1016/j.molcel.2017.09.021
59. Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, et al. N6-Methyladenosine-Dependent regulation of messenger rna stability. Nature (2014) 505(7481):117–20. doi: 10.1038/nature12730
60. Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, et al. Ythdf3 facilitates translation and decay of N(6)-Methyladenosine-Modified rna. Cell Res (2017) 27(3):315–28. doi: 10.1038/cr.2017.15
61. Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, et al. N(6)-methyladenosine modulates messenger rna translation efficiency. Cell (2015) 161(6):1388–99. doi: 10.1016/j.cell.2015.05.014
62. Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, et al. 5' utr M(6)a promotes cap-independent translation. Cell (2015) 163(4):999–1010. doi: 10.1016/j.cell.2015.10.012
63. Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, et al. Recognition of rna N(6)-methyladenosine by Igf2bp proteins enhances mrna stability and translation. Nat Cell Biol (2018) 20(3):285–95. doi: 10.1038/s41556-018-0045-z
64. Feng M, Xie X, Han G, Zhang T, Li Y, Li Y, et al. Ybx1 is required for maintaining myeloid leukemia cell survival by regulating Bcl2 stability in an M6a-dependent manner. Blood (2021) 138(1):71–85. doi: 10.1182/blood.2020009676
65. Chen H, Gu L, Orellana EA, Wang Y, Guo J, Liu Q, et al. Mettl4 is an snrna M(6)Am methyltransferase that regulates rna splicing. Cell Res (2020) 30(6):544–7. doi: 10.1038/s41422-019-0270-4
66. Mauer J, Sindelar M, Despic V, Guez T, Hawley BR, Vasseur JJ, et al. Fto controls reversible M(6)Am rna methylation during snrna biogenesis. Nat Chem Biol (2019) 15(4):340–7. doi: 10.1038/s41589-019-0231-8
67. Mauer J, Luo X, Blanjoie A, Jiao X, Grozhik AV, Patil DP, et al. Reversible methylation of M(6)a(M) in the 5' cap controls mrna stability. Nature (2017) 541(7637):371–5. doi: 10.1038/nature21022
68. Sendinc E, Valle-Garcia D, Dhall A, Chen H, Henriques T, Navarrete-Perea J, et al. Pcif1 catalyzes M6am mrna methylation to regulate gene expression. Mol Cell (2019) 75(3):620–30.e9. doi: 10.1016/j.molcel.2019.05.030
69. Sun H, Zhang M, Li K, Bai D, Yi C. Cap-specific, terminal N(6)-methylation by a mammalian M(6)Am methyltransferase. Cell Res (2019) 29(1):80–2. doi: 10.1038/s41422-018-0117-4
70. Akichika S, Hirano S, Shichino Y, Suzuki T, Nishimasu H, Ishitani R, et al. Cap-Specific terminal N (6)-methylation of rna by an rna polymerase Ii-associated methyltransferase. Science (2019) 363(6423). doi: 10.1126/science.aav0080
71. Boulias K, Toczydłowska-Socha D, Hawley BR, Liberman N, Takashima K, Zaccara S, et al. Identification of the M(6)Am methyltransferase Pcif1 reveals the location and functions of M(6)Am in the transcriptome. Mol Cell (2019) 75(3):631–43.e8. doi: 10.1016/j.molcel.2019.06.006
72. Mauer J, Jaffrey SR. Fto, M6am, and the hypothesis of reversible epitranscriptomic mrna modifications. FEBS Lett (2018) 592(12):2012–22. doi: 10.1002/1873-3468.13092
73. Khoddami V, Cairns BR. Identification of direct targets and modified bases of rna cytosine methyltransferases. Nat Biotechnol (2013) 31(5):458–64. doi: 10.1038/nbt.2566
74. Yang X, Yang Y, Sun BF, Chen YS, Xu JW, Lai WY, et al. 5-methylcytosine promotes mrna export - Nsun2 as the methyltransferase and alyref as an M(5)C reader. Cell Res (2017) 27(5):606–25. doi: 10.1038/cr.2017.55
75. Xing J, Yi J, Cai X, Tang H, Liu Z, Zhang X, et al. Nsun2 promotes cell growth Via elevating cyclin-dependent kinase 1 translation. Mol Cell Biol (2015) 35(23):4043–52. doi: 10.1128/mcb.00742-15
76. Motorin Y, Lyko F, Helm M. 5-methylcytosine in rna: Detection, enzymatic formation and biological functions. Nucleic Acids Res (2010) 38(5):1415–30. doi: 10.1093/nar/gkp1117
77. Fang L, Wang W, Li G, Zhang L, Li J, Gan D, et al. Cigar-seq, a Crispr/Cas-based method for unbiased screening of novel mrna modification regulators. Mol Syst Biol (2020) 16(11):e10025. doi: 10.15252/msb.202010025
78. Xue M, Shi Q, Zheng L, Li Q, Yang L, Zhang Y. Gene signatures of M5c regulators may predict prognoses of patients with head and neck squamous cell carcinoma. Am J Transl Res (2020) 12(10):6841–52.
79. Chen X, Li A, Sun BF, Yang Y, Han YN, Yuan X, et al. 5-methylcytosine promotes pathogenesis of bladder cancer through stabilizing mrnas. Nat Cell Biol (2019) 21(8):978–90. doi: 10.1038/s41556-019-0361-y
80. Shi M, Zhang H, Wu X, He Z, Wang L, Yin S, et al. Alyref mainly binds to the 5' and the 3' regions of the mrna in vivo. Nucleic Acids Res (2017) 45(16):9640–53. doi: 10.1093/nar/gkx597
81. Sun J, Yan L, Shen W, Meng A. Maternal Ybx1 safeguards zebrafish oocyte maturation and maternal-to-Zygotic transition by repressing global translation. Development (2018) 145(19). doi: 10.1242/dev.166587
82. Barker GR, Hollinshead JA. The degradation of ribonucleic acid in the cotyledons of pisum arvense. Biochem J (1967) 103(1):230–7. doi: 10.1042/bj1030230
83. Schwartz S, Bernstein D, Mumbach A, Jovanovic M, Herbst RH, León-Ricardo BX, et al. Transcriptome-wide mapping reveals widespread dynamic-regulated Pseudouridylation of Ncrna and Mrna. Cell (2014) 159(1):148–62. doi: 10.1016/j.cell.2014.08.028
84. Arnez JG, Steitz TA. Crystal structure of unmodified Trna(Gln) complexed with glutaminyl-trna synthetase and atp suggests a possible role for pseudo-uridines in stabilization of rna structure. Biochemistry (1994) 33(24):7560–7. doi: 10.1021/bi00190a008
85. Duan J, Li L, Lu J, Wang W, Ye K. Structural mechanism of substrate rna recruitment in H/Aca rna-guided pseudouridine synthase. Mol Cell (2009) 34(4):427–39. doi: 10.1016/j.molcel.2009.05.005
86. Rintala-Dempsey AC, Kothe U. Eukaryotic stand-alone pseudouridine synthases - rna modifying enzymes and emerging regulators of gene expression? RNA Biol (2017) 14(9):1185–96. doi: 10.1080/15476286.2016.1276150
87. Zhang C, Jia G. Reversible rna modification N(1)-methyladenosine (M(1)a) in mrna and trna. Genomics Proteomics Bioinf (2018) 16(3):155–61. doi: 10.1016/j.gpb.2018.03.003
88. Li X, Xiong X, Wang K, Wang L, Shu X, Ma S, et al. Transcriptome-wide mapping reveals reversible and dynamic N(1)-methyladenosine methylome. Nat Chem Biol (2016) 12(5):311–6. doi: 10.1038/nchembio.2040
89. Chujo T, Suzuki T. Trmt61b is a methyltransferase responsible for 1-methyladenosine at position 58 of human mitochondrial trnas. Rna (2012) 18(12):2269–76. doi: 10.1261/rna.035600.112
90. Waku T, Nakajima Y, Yokoyama W, Nomura N, Kako K, Kobayashi A, et al. Nml-mediated rrna base methylation links ribosomal subunit formation to cell proliferation in a P53-dependent manner. J Cell Sci (2016) 129(12):2382–93. doi: 10.1242/jcs.183723
91. Saikia M, Fu Y, Pavon-Eternod M, He C, Pan T. Genome-wide analysis of N1-Methyl-Adenosine modification in human trnas. Rna (2010) 16(7):1317–27. doi: 10.1261/rna.2057810
92. Liu F, Clark W, Luo G, Wang X, Fu Y, Wei J, et al. Alkbh1-mediated trna demethylation regulates translation. Cell (2016) 167(3):816–28.e16. doi: 10.1016/j.cell.2016.09.038
93. Woo HH, Chambers SK. Human Alkbh3-induced M(1)a demethylation increases the csf-1 mrna stability in breast and ovarian cancer cells. Biochim Biophys Acta Gene Regul Mech (2019) 1862(1):35–46. doi: 10.1016/j.bbagrm.2018.10.008
94. Dai X, Wang T, Gonzalez G, Wang Y. Identification of yth domain-containing proteins as the readers for N1-methyladenosine in rna. Anal Chem (2018) 90(11):6380–4. doi: 10.1021/acs.analchem.8b01703
95. Levanon EY, Eisenberg E, Yelin R, Nemzer S, Hallegger M, Shemesh R, et al. Systematic identification of abundant a-to-I editing sites in the human transcriptome. Nat Biotechnol (2004) 22(8):1001–5. doi: 10.1038/nbt996
96. Nishikura K. A-to-I editing of coding and non-coding rnas by adars. Nat Rev Mol Cell Biol (2016) 17(2):83–96. doi: 10.1038/nrm.2015.4
97. Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat Rev Cancer (2008) 8(10):755–68. doi: 10.1038/nrc2499
98. Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into scid mice. Nature (1994) 367(6464):645–8. doi: 10.1038/367645a0
99. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature (2001) 414(6859):105–11. doi: 10.1038/35102167
100. Qi W, Liang W, Jiang H, Miuyee Waye M. The function of mirna in hepatic cancer stem cell. BioMed Res Int (2013) 2013:358902. doi: 10.1155/2013/358902
101. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U.S.A. (2003) 100(7):3983–8. doi: 10.1073/pnas.0530291100
102. Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U.S.A. (2003) 100(25):15178–83. doi: 10.1073/pnas.2036535100
103. Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell (2005) 121(6):823–35. doi: 10.1016/j.cell.2005.03.032
104. O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. . Nat (2007) 445(7123):106–10. doi: 10.1038/nature05372
105. Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res (2005) 65(23):10946–51. doi: 10.1158/0008-5472.Can-05-2018
106. Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and mullerian inhibiting substance responsiveness. Proc Natl Acad Sci U.S.A. (2006) 103(30):11154–9. doi: 10.1073/pnas.0603672103
107. Dianat-Moghadam H, Heidarifard M, Jahanban-Esfahlan R, Panahi Y, Hamishehkar H, Pouremamali F, et al. Cancer stem cells-emanated therapy resistance: Implications for liposomal drug delivery systems. J Control Release (2018) 288:62–83. doi: 10.1016/j.jconrel.2018.08.043
108. Batlle E, Clevers H. Cancer stem cells revisited. Nat Med (2017) 23(10):1124–34. doi: 10.1038/nm.4409
109. Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, et al. Targeting notch, hedgehog, and wnt pathways in cancer stem cells: Clinical update. Nat Rev Clin Oncol (2015) 12(8):445–64. doi: 10.1038/nrclinonc.2015.61
110. Dreesen O, Brivanlou AH. Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev (2007) 3(1):7–17. doi: 10.1007/s12015-007-0004-8
111. El Hout M, Dos Santos L, Hamaï A, Mehrpour M. A promising new approach to cancer therapy: Targeting iron metabolism in cancer stem cells. Semin Cancer Biol (2018) 53:125–38. doi: 10.1016/j.semcancer.2018.07.009
112. Arend RC, Londoño-Joshi AI, Samant RS, Li Y, Conner M, Hidalgo B, et al. Inhibition of Wnt/β-catenin pathway by niclosamide: A therapeutic target for ovarian cancer. Gynecol Oncol (2014) 134(1):112–20. doi: 10.1016/j.ygyno.2014.04.005
113. Londoño-Joshi AI, Arend RC, Aristizabal L, Lu W, Samant RS, Metge BJ, et al. Effect of niclosamide on basal-like breast cancers. Mol Cancer Ther (2014) 13(4):800–11. doi: 10.1158/1535-7163.Mct-13-0555
114. Prabhu VV, Lulla AR, Madhukar NS, Ralff MD, Zhao D, Kline CLB, et al. Cancer stem cell-related gene expression as a potential biomarker of response for first-in-Class imipridone Onc201 in solid tumors. PloS One (2017) 12(8):e0180541. doi: 10.1371/journal.pone.0180541
115. Greaves M, Maley CC. Clonal evolution in cancer. Nature (2012) 481(7381):306–13. doi: 10.1038/nature10762
116. Lin S, Liu J, Jiang W, Wang P, Sun C, Wang X, et al. Mettl3 promotes the proliferation and mobility of gastric cancer cells. Open Med (Wars) (2019) 14:25–31. doi: 10.1515/med-2019-0005
117. Ma JZ, Yang F, Zhou CC, Liu F, Yuan JH, Wang F, et al. Mettl14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N(6) -Methyladenosine-Dependent primary microrna processing. Hepatology (2017) 65(2):529–43. doi: 10.1002/hep.28885
118. Toh TB, Lim JJ, Chow EK-H. Epigenetics in cancer stem cells. Mol Cancer (2017) 16(1):29. doi: 10.1186/s12943-017-0596-9
119. Liu S, Dontu G, Wicha MS. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res (2005) 7(3):86–95. doi: 10.1186/bcr1021
120. Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: Challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discovery (2009) 8(10):806–23. doi: 10.1038/nrd2137
121. Gomez-Cabrero A, Wrasidlo W, Reisfeld RA. Imd-0354 targets breast cancer stem cells: A novel approach for an adjuvant to chemotherapy to prevent multidrug resistance in a murine model. PloS One (2013) 8(8):e73607. doi: 10.1371/journal.pone.0073607
122. Frosina G. DNA Repair and resistance of gliomas to chemotherapy and radiotherapy. Mol Cancer Res (2009) 7(7):989–99. doi: 10.1158/1541-7786.Mcr-09-0030
123. Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature (2006) 444(7120):756–60. doi: 10.1038/nature05236
125. Vu LP, Pickering BF, Cheng Y, Zaccara S, Nguyen D, Minuesa G, et al. The N(6)-methyladenosine (M(6)a)-forming enzyme Mettl3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med (2017) 23(11):1369–76. doi: 10.1038/nm.4416
126. Barbieri I, Tzelepis K, Pandolfini L, Shi J, Millán-Zambrano G, Robson SC, et al. Promoter-bound Mettl3 maintains myeloid leukaemia by M(6)a-dependent translation control. Nature (2017) 552(7683):126–31. doi: 10.1038/nature24678
127. Weng H, Huang H, Wu H, Qin X, Zhao BS, Dong L, et al. Mettl14 inhibits hematopoietic Stem/Progenitor differentiation and promotes leukemogenesis Via mrna M(6)a modification. Cell Stem Cell (2018) 22(2):191–205.e9. doi: 10.1016/j.stem.2017.11.016
128. Sorci M, Ianniello Z, Cruciani S, Larivera S, Ginistrelli LC, Capuano E, et al. Mettl3 regulates wtap protein homeostasis. Cell Death Dis (2018) 9(8):796. doi: 10.1038/s41419-018-0843-z
129. Lin S, Choe J, Du P, Triboulet R, Gregory RI. The M(6)a methyltransferase Mettl3 promotes translation in human cancer cells. Mol Cell (2016) 62(3):335–45. doi: 10.1016/j.molcel.2016.03.021
130. Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin (2016) 66(4):271–89. doi: 10.3322/caac.21349
131. Li Z, Weng H, Su R, Weng X, Zuo Z, Li C, et al. Fto plays an oncogenic role in acute myeloid leukemia as a N(6)-methyladenosine rna demethylase. Cancer Cell (2017) 31(1):127–41. doi: 10.1016/j.ccell.2016.11.017
132. Su R, Dong L, Li Y, Gao M, Han L, Wunderlich M, et al. Targeting fto suppresses cancer stem cell maintenance and immune evasion. Cancer Cell (2020) 38(1):79–96.e11. doi: 10.1016/j.ccell.2020.04.017
133. Shen C, Sheng Y, Zhu AC, Robinson S, Jiang X, Dong L, et al. Rna demethylase Alkbh5 selectively promotes tumorigenesis and cancer stem cell self-renewal in acute myeloid leukemia. Cell Stem Cell (2020) 27(1):64–80.e9. doi: 10.1016/j.stem.2020.04.009
134. Wang J, Li Y, Wang P, Han G, Zhang T, Chang J, et al. Leukemogenic chromatin alterations promote aml leukemia stem cells Via a Kdm4c-Alkbh5-Axl signaling axis. Cell Stem Cell (2020) 27(1):81–97.e8. doi: 10.1016/j.stem.2020.04.001
135. Wang H, Zuo H, Liu J, Wen F, Gao Y, Zhu X, et al. Loss of Ythdf2-mediated M(6)a-dependent mrna clearance facilitates hematopoietic stem cell regeneration. Cell Res (2018) 28(10):1035–8. doi: 10.1038/s41422-018-0082-y
136. Li Z, Qian P, Shao W, Shi H, He XC, Gogol M, et al. Suppression of M(6)a reader Ythdf2 promotes hematopoietic stem cell expansion. Cell Res (2018) 28(9):904–17. doi: 10.1038/s41422-018-0072-0
137. Paris J, Morgan M, Campos J, Spencer GJ, Shmakova A, Ivanova I, et al. Targeting the rna M(6)a reader Ythdf2 selectively compromises cancer stem cells in acute myeloid leukemia. Cell Stem Cell (2019) 25(1):137–48.e6. doi: 10.1016/j.stem.2019.03.021
138. Elcheva IA, Wood T, Chiarolanzio K, Chim B, Wong M, Singh V, et al. Rna-binding protein Igf2bp1 maintains leukemia stem cell properties by regulating Hoxb4, myb, and Aldh1a1. Leukemia (2020) 34(5):1354–63. doi: 10.1038/s41375-019-0656-9
139. Jacobs AT, Martinez Castaneda-Cruz D, Rose MM, Connelly L. Targeted therapy for breast cancer: An overview of drug classes and outcomes. Biochem Pharmacol (2022) 204:115209. doi: 10.1016/j.bcp.2022.115209
140. Xiang L, Gilkes DM, Hu H, Takano N, Luo W, Lu H, et al. Hypoxia-inducible factor 1 mediates taz expression and nuclear localization to induce the breast cancer stem cell phenotype. Oncotarget (2014) 5(24):12509–27. doi: 10.18632/oncotarget.2997
141. Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res (2009) 69(4):1302–13. doi: 10.1158/0008-5472.Can-08-2741
142. Zhang C, Samanta D, Lu H, Bullen JW, Zhang H, Chen I, et al. Hypoxia induces the breast cancer stem cell phenotype by hif-dependent and Alkbh5-mediated M6a-demethylation of nanog mrna. Proc Natl Acad Sci U.S.A. (2016) 113(14):E2047–56. doi: 10.1073/pnas.1602883113
143. Zhang C, Zhi WI, Lu H, Samanta D, Chen I, Gabrielson E, et al. Hypoxia-inducible factors regulate pluripotency factor expression by Znf217- and Alkbh5-mediated modulation of rna methylation in breast cancer cells. Oncotarget (2016) 7(40):64527–42. doi: 10.18632/oncotarget.11743
144. Zhu P, He F, Hou Y, Tu G, Li Q, Jin T, et al. A novel hypoxic long noncoding rna kb-1980e6.3 maintains breast cancer stem cell stemness Via interacting with Igf2bp1 to facilitate c-myc mrna stability. Oncogene (2021) 40(9):1609–27. doi: 10.1038/s41388-020-01638-9
145. Lewinska A, Adamczyk-Grochala J, Deregowska A, Wnuk M. Sulforaphane-induced cell cycle arrest and senescence are accompanied by DNA hypomethylation and changes in microrna profile in breast cancer cells. Theranostics (2017) 7(14):3461–77. doi: 10.7150/thno.20657
146. Peng F, Xu J, Cui B, Liang Q, Zeng S, He B, et al. Oncogenic aurka-enhanced N6-methyladenosine modification increases drosha mrna stability to transactivate Stc1 in breast cancer stem-like cells. Cell Res (2021) 31(3):345–61. doi: 10.1038/s41422-020-00397-2
147. Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med (2008) 359(5):492–507. doi: 10.1056/NEJMra0708126
148. Virolle T. [Cancer stem cells in glioblastoma]. Bull Cancer (2017) 104(12):1075–9. doi: 10.1016/j.bulcan.2017.10.012
149. Dixit D, Xie Q, Rich JN, Zhao JC. Messenger rna methylation regulates glioblastoma tumorigenesis. Cancer Cell (2017) 31(4):474–5. doi: 10.1016/j.ccell.2017.03.010
150. Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, et al. M(6)a demethylase Alkbh5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining Foxm1 expression and cell proliferation program. Cancer Cell (2017) 31(4):591–606.e6. doi: 10.1016/j.ccell.2017.02.013
151. Ahmed SU, Carruthers R, Gilmour L, Yildirim S, Watts C, Chalmers AJ. Selective inhibition of parallel DNA damage response pathways optimizes radiosensitization of glioblastoma stem-like cells. Cancer Res (2015) 75(20):4416–28. doi: 10.1158/0008-5472.Can-14-3790
152. Kowalski-Chauvel A, Lacore MG, Arnauduc F, Delmas C, Toulas C, Cohen-Jonathan-Moyal E, et al. The M6a rna demethylase Alkbh5 promotes radioresistance and invasion capability of glioma stem cells. Cancers (Basel) (2020) 13(1):40. doi: 10.3390/cancers13010040
153. Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, et al. M(6)a rna methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep (2017) 18(11):2622–34. doi: 10.1016/j.celrep.2017.02.059
154. Visvanathan A, Patil V, Arora A, Hegde AS, Arivazhagan A, Santosh V, et al. Essential role of Mettl3-mediated M(6)a modification in glioma stem-like cells maintenance and radioresistance. Oncogene (2018) 37(4):522–33. doi: 10.1038/onc.2017.351
155. Visvanathan A, Patil V, Abdulla S, Hoheisel JD, Somasundaram K. N6-methyladenosine landscape of glioma stem-like cells: Mettl3 is essential for the expression of actively transcribed genes and sustenance of the oncogenic signaling. Genes (Basel) (2019) 10(2):141. doi: 10.3390/genes10020141
156. Yarmishyn AA, Yang Y-P, Lu K-H, Chen Y-C, Chien Y, Chou S-J, et al. Musashi-1 promotes cancer stem cell properties of glioblastoma cells Via upregulation of Ythdf1. Cancer Cell Int (2020) 20(1):597. doi: 10.1186/s12935-020-01696-9
157. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
158. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and united states, 2022: Profiles, trends, and determinants. Chin Med J (2022) 135(5):584–90. doi: 10.1097/cm9.0000000000002108
159. Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin (2014) 64(2):104–17. doi: 10.3322/caac.21220
160. Zeuner A, Todaro M, Stassi G, De Maria R. Colorectal cancer stem cells: From the crypt to the clinic. Cell Stem Cell (2014) 15(6):692–705. doi: 10.1016/j.stem.2014.11.012
161. Ordóñez-Morán P, Dafflon C, Imajo M, Nishida E, Huelsken J. Hoxa5 counteracts stem cell traits by inhibiting wnt signaling in colorectal cancer. Cancer Cell (2015) 28(6):815–29. doi: 10.1016/j.ccell.2015.11.001
162. Lettini G, Sisinni L, Condelli V, Matassa DS, Simeon V, Maddalena F, et al. Trap1 regulates stemness through Wnt/β-catenin pathway in human colorectal carcinoma. Cell Death Differ (2016) 23(11):1792–803. doi: 10.1038/cdd.2016.67
163. Zhang Y, Kang M, Zhang B, Meng F, Song J, Kaneko H, et al. M6a modification-mediated Cbx8 induction regulates stemness and chemosensitivity of colon cancer Via upregulation of Lgr5. Mol Cancer (2019) 18(1):185. doi: 10.1186/s12943-019-1116-x
164. Nishizawa Y, Konno M, Asai A, Koseki J, Kawamoto K, Miyoshi N, et al. Oncogene c-myc promotes epitranscriptome M(6)a reader Ythdf1 expression in colorectal cancer. Oncotarget (2018) 9(7):7476–86. doi: 10.18632/oncotarget.23554
165. Bai Y, Yang C, Wu R, Huang L, Song S, Li W, et al. Ythdf1 regulates tumorigenicity and cancer stem cell-like activity in human colorectal carcinoma. Front Oncol (2019) 9:332. doi: 10.3389/fonc.2019.00332
166. Han B, Yan S, Wei S, Xiang J, Liu K, Chen Z, et al. Ythdf1-mediated translation amplifies wnt-driven intestinal stemness. EMBO Rep (2020) 21(4):e49229. doi: 10.15252/embr.201949229
167. Zhao Z, Zeng J, Guo Q, Pu K, Yang Y, Chen N, et al. Berberine suppresses stemness and tumorigenicity of colorectal cancer stem-like cells by inhibiting M6a methylation. Front Oncol (2021) 11:775418. doi: 10.3389/fonc.2021.775418
168. Zhang C, Huang S, Zhuang H, Ruan S, Zhou Z, Huang K, et al. Ythdf2 promotes the liver cancer stem cell phenotype and cancer metastasis by regulating Oct4 expression Via M6a rna methylation. Oncogene (2020) 39(23):4507–18. doi: 10.1038/s41388-020-1303-7
169. Bian X, Shi D, Xing K, Zhou H, Lu L, Yu D, et al. Amd1 upregulates hepatocellular carcinoma cells stemness by fto mediated mrna demethylation. Clin Transl Med (2021) 11(3):e352. doi: 10.1002/ctm2.352
170. Liu X, Wang Z, Yang Q, Hu X, Fu Q, Zhang X, et al. Rna demethylase Alkbh5 prevents lung cancer progression by regulating emt and stemness Via regulating P53. Front Oncol (2022) 12:858694. doi: 10.3389/fonc.2022.858694
171. Hu X, Peng W-X, Zhou H, Jiang J, Zhou X, Huang D, et al. Igf2bp2 regulates dancr by serving as an N6-methyladenosine reader. Cell Death Differentiation (2020) 27(6):1782–94. doi: 10.1038/s41418-019-0461-z
172. Gao Q, Zheng J, Ni Z, Sun P, Yang C, Cheng M, et al. The m6A methylation-regulated Aff4 promotes self-renewal of bladder cancer stem cells. Stem Cells Int (2020) 2020:8849218. doi: 10.1155/2020/8849218
173. Wang Y, Zeng L, Liang C, Zan R, Ji W, Zhang Z, et al. Integrated analysis of transcriptome-wide M6a methylome of osteosarcoma stem cells enriched by chemotherapy. Epigenomics (2019) 11(15):1693–715. doi: 10.2217/epi-2019-0262
174. Huang H, Wang Y, Kandpal M, Zhao G, Cardenas H, Ji Y, et al. Fto-dependent N6-methyladenosine modifications inhibit ovarian cancer stem cell self-renewal by blocking camp signaling. Cancer Res (2020) 80(16):3200–14. doi: 10.1158/0008-5472.Can-19-4044
175. Shriwas O, Priyadarshini M, Samal SK, Rath R, Panda S, Das Majumdar SK, et al. Ddx3 modulates cisplatin resistance in oscc through Alkbh5-mediated M6a-demethylation of Foxm1 and nanog. Apoptosis (2020) 25(3):233–46. doi: 10.1007/s10495-020-01591-8
176. Zhou R, Gao Y, Lv D, Wang C, Wang D, Li Q. Mettl3 mediated M6a modification plays an oncogenic role in cutaneous squamous cell carcinoma by regulating Δnp63. Biochem Biophys Res Commun (2019) 515(2):310–7. doi: 10.1016/j.bbrc.2019.05.155
177. Sun H, Li K, Zhang X, Liu J, Zhang M, Meng H, et al. M(6)Am-seq reveals the dynamic M(6)Am methylation in the human transcriptome. Nat Commun (2021) 12(1):4778. doi: 10.1038/s41467-021-25105-5
178. Relier S, Ripoll J, Guillorit H, Amalric A, Achour C, Boissière F, et al. Fto-mediated cytoplasmic M(6)a(M) demethylation adjusts stem-like properties in colorectal cancer cell. Nat Commun (2021) 12(1):1716. doi: 10.1038/s41467-021-21758-4
179. Frye M, Blanco S. Post-transcriptional modifications in development and stem cells. Development (2016) 143(21):3871–81. doi: 10.1242/dev.136556
180. Gao Y, Fang J. Rna 5-methylcytosine modification and its emerging role as an epitranscriptomic mark. RNA Biol (2021) 18(sup1):117–27. doi: 10.1080/15476286.2021.1950993
181. Blanco S, Bandiera R, Popis M, Hussain S, Lombard P, Aleksic J, et al. Stem cell function and stress response are controlled by protein synthesis. Nature (2016) 534(7607):335–40. doi: 10.1038/nature18282
182. Cui Q, Yin K, Zhang X, Ye P, Chen X, Chao J, et al. Targeting Pus7 suppresses trna pseudouridylation and glioblastoma tumorigenesis. Nat Cancer (2021) 2(9):932–49. doi: 10.1038/s43018-021-00238-0
183. Guzzi N, Cieśla M, Ngoc PCT, Lang S, Arora S, Dimitriou M, et al. Pseudouridylation of trna-derived fragments steers translational control in stem cells. Cell (2018) 173(5):1204–16.e26. doi: 10.1016/j.cell.2018.03.008
184. Wang Y, Wang J, Li X, Xiong X, Wang J, Zhou Z, et al. N1-methyladenosine methylation in trna drives liver tumourigenesis by regulating cholesterol metabolism. Nat Commun (2021) 12(1):6314. doi: 10.1038/s41467-021-26718-6
185. Liu X, Fu Y, Huang J, Wu M, Zhang Z, Xu R, et al. Adar1 promotes the epithelial-to-Mesenchymal transition and stem-like cell phenotype of oral cancer by facilitating oncogenic microrna maturation. J Exp Clin Cancer Res (2019) 38(1):315. doi: 10.1186/s13046-019-1300-2
186. Jiang L, Hao Y, Shao C, Wu Q, Prager BC, Gimple RC, et al. Adar1-mediated rna editing links ganglioside catabolism to glioblastoma stem cell maintenance. J Clin Invest (2022) 132(6):e143397. doi: 10.1172/jci143397
187. Lee AC, Lee Y, Choi A, Lee H-B, Shin K, Lee H, et al. Spatial epitranscriptomics reveals a-to-I editome specific to cancer stem cell microniches. Nat Commun (2022) 13(1):2540. doi: 10.1038/s41467-022-30299-3
188. Shigeyasu K, Okugawa Y, Toden S, Miyoshi J, Toiyama Y, Nagasaka T, et al. Azin1 rna editing confers cancer stemness and enhances oncogenic potential in colorectal cancer. JCI Insight (2018) 3(12):e99926. doi: 10.1172/jci.insight.99976
189. Zhang Y, Jiang J, Ma J, Wei Z, Wang Y, Song B, et al. Directrmdb: A database of post-transcriptional rna modifications unveiled from direct rna sequencing technology. Nucleic Acids Res (2023) 51(D1):D106–d16. doi: 10.1093/nar/gkac1061
190. Ma J, Zhang L, Chen S, Liu H. A brief review of rna modification related database resources. Methods (2022) 203:342–53. doi: 10.1016/j.ymeth.2021.03.003
191. Tang Y, Chen K, Wu X, Wei Z, Zhang SY, Song B, et al. Drum: Inference of disease-associated M(6)a rna methylation sites from a multi-layer heterogeneous network. Front Genet (2019) 10:266. doi: 10.3389/fgene.2019.00266
192. Zheng Y, Nie P, Peng D, He Z, Liu M, Xie Y, et al. M6avar: A database of functional variants involved in M6a modification. Nucleic Acids Res (2018) 46(D1):D139–d45. doi: 10.1093/nar/gkx895
193. Chen K, Song B, Tang Y, Wei Z, Xu Q, Su J, et al. Rmdisease: A database of genetic variants that affect rna modifications, with implications for epitranscriptome pathogenesis. Nucleic Acids Res (2021) 49(D1):D1396–d404. doi: 10.1093/nar/gkaa790
194. Song B, Wang X, Liang Z, Ma J, Huang D, Wang Y, et al. Rmdisease V2.0: An updated database of genetic variants that affect rna modifications with disease and trait implication. Nucleic Acids Res (2023) 51(D1):D1388–d96. doi: 10.1093/nar/gkac750
195. Song B, Huang D, Zhang Y, Wei Z, Su J, Pedro de Magalhães J, et al. M6a-tshub: Unveiling the context-specific M(6)a methylation and M6a-affecting mutations in 23 human tissues. Genomics Proteomics Bioinf (2022). doi: 10.1016/j.gpb.2022.09.001
196. Chen B, Ye F, Yu L, Jia G, Huang X, Zhang X, et al. Development of cell-active N6-methyladenosine rna demethylase fto inhibitor. J Am Chem Soc (2012) 134(43):17963–71. doi: 10.1021/ja3064149
197. Huang Y, Yan J, Li Q, Li J, Gong S, Zhou H, et al. Meclofenamic acid selectively inhibits fto demethylation of M6a over Alkbh5. Nucleic Acids Res (2015) 43(1):373–84. doi: 10.1093/nar/gku1276
198. Scheper GC, van der Knaap MS, Proud CG. Translation matters: Protein synthesis defects in inherited disease. Nat Rev Genet (2007) 8(9):711–23. doi: 10.1038/nrg2142
Keywords: epitranscriptomics, RNA modification, cancer stem cell, stemness, RNA-modifying enzyme
Citation: Hao L, Zhang J, Liu Z, Lin X and Guo J (2023) Epitranscriptomics in the development, functions, and disorders of cancer stem cells. Front. Oncol. 13:1145766. doi: 10.3389/fonc.2023.1145766
Received: 16 January 2023; Accepted: 10 February 2023;
Published: 17 March 2023.
Edited by:
Rachel Ellsworth, Walter Reed National Military Medical Center, United StatesCopyright © 2023 Hao, Zhang, Liu, Lin and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Guo, Z3VvamllQGpsdS5lZHUuY24=
 Linlin Hao
Linlin Hao Jian Zhang2
Jian Zhang2 Jie Guo
Jie Guo