- 1Department of Urology, Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan, China
- 2Department of Dermatology, Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan, China
Introduction: Our meta-analysis aimed to evaluate the diagnostic value of 18F-DCFPyL prostate-specific membrane antigen (PSMA) PET in patients with suspected prostate cancer.
Methods: We searched for articles that evaluate the diagnostic value of 18F-DCFPyL PSMA PET in patients with suspected prostate cancer in PubMed, Embase, Cochrane Library, and Web of Science until 1 August 2022. Using the QUADAS-2 instrument, two researchers independently assessed the effectiveness of the studies that were included. The four-grid table data were analyzed by Meta-disc1.4 and Stata 16.0 software. The heterogeneity of each study was tested.
Results: A total of five studies with 258 patients were included, and the pooled sensitivity and specificity of 18F-DCFPyL PSMA PET for primary prostate cancer were 0.92 (95% confidence interval (CI): 0.85–0.96) and 0.59 (95% CI: 0.08–0.96), respectively. 18F-DCFPyL PSMA PET was successful in detecting primary prostate cancer, with an area under the curve (AUC) of 0.92 (95% CI: 0.89–0.94).
Conclusions: 18F-DCFPyL PSMA PET has a strong predictive value for primary prostate cancer and is an effective method for the non-invasive diagnosis of prostate cancer. More prospective articles were needed.
1 Introduction
Prostate cancer (PCa) is one of the major health problems plaguing elderly men. According to statistical analysis, there were 1.4 million new cases of prostate cancer patients worldwide in 2020 (1). Accurate early diagnosis is essential and necessary in individuals with PCa to provide the best care and enhance prognosis (2).
At present, the diagnostic methods of PCa include digital rectal examination (DRE), prostate-specific antigen (PSA), and transrectal ultrasound (TRUS). The gold standard is still the pathological result of puncture biopsy, but there is a risk of bleeding, severe infection, urinary retention, and so on. At present, it is a question that we need to consider whether there are other ways to reduce unnecessary prostate biopsy or even replace puncture biopsy to diagnose prostate cancer to avoid the above risks (3, 4).
Prostate-specific membrane antigen (PSMA) is a type II glycoprotein with a wide range of extracellular domains (44-750A). The protein is rarely expressed in normal prostate tissues but is highly upregulated and overexpressed in PCa cells and tumor vascular cells (5–7). With rapid development, PSMA is playing a transformative role in diagnosis and treatment (8). PET/CT imaging technology targeting 68Ga-PSMA has developed rapidly in recent years. The generator, however, produced the nuclide 68Ga, which had a short half-life and significant electron energy, which reduced its clinical application. In clinical practice, the most commonly used positron nuclide was 18F-DCFPYL. It is based on a glutamate-urea-lysine structure. When compared with 68Ga-PSMA-11, some of its traits include strong affinity, favorable in vivo pharmacokinetics, and good solubility and may have a higher detection rate for small lesions. Therefore, the performance is better, and it is easier to be widely used in clinical practice (9–11).
At present, the research that evaluates 18F-DCFPyL PSMA PET in the diagnosis of suspect PCa is small in scale. Therefore, we collected relevant pieces of literature and summarized their data for meta-analysis to more accurately evaluate the diagnostic value of 18F-DCFPyL PSMA PET in patients with suspected prostate cancer.
2 Methods
2.1 Search strategy
We searched relevant published literature by computer, and the search databases included PubMed, Embase, Cochrane Library, and Web of Science. The search time was from the establishment of the database to 1 August 2022. The search terms are the following phrases: DCFPyL, Prostatic Cancer, Prostatic Cancer, Prostate Cancer, Prostate Cancer, Prostatic Neoplasm, Prostate Neoplasm, Prostate Neoplasms, Prostate tumor, and prostatic tumor. Two researchers independently combined computer search with manual search to avoid missing relevant literature.
2.2 Inclusion criteria
Articles that met the following requirements were only considered for inclusion: 1) untreated patients with suspected prostate cancer, who are patients with prostate abnormalities found in DRE or TRUS, MRI examination, or PSA screening abnormalities. 2) 18F-DCFPyL PSMA PET scan was performed. 3) The number of subjects ≥10. 4) The reference standard for prostate cancer was a histopathological biopsy.
2.3 Exclusion criteria
The following cases are excluded: 1) patients with biochemical recurrence; 2) patients with metastases; 3) case reports, abstracts, comments, letters, reviews, or meta-analyses; 4) data to construct a four-grid table cannot be extracted.
2.4 Quality assessment
Using the QUADAS-2 instrument, two researchers independently assessed the effectiveness of the studies that were included. The patient selection, index test, reference standard, and flow and timing were the domains that were utilized to assess each study. The bias risk determined whether these domains’ applicability was rated as “high”, “poor”, or “unclear”. The disputes among the researchers were ultimately settled by agreement.
2.5 Data extraction
A total of two researchers independently extracted data for each study. General information, characteristics of the literature, patient characteristics, technical information, and results for the total number of patients, True positive (TP), False positive (FP), True negative (TN) and False negative (FN)—all of which are counted—were all extracted from the data. If these values were not available, calculations were made using the findings of sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) tests.
2.6 Statistical analysis
The four-grid table data were analyzed by Meta-disc1.4 and Stata 16.0 software. Spearman’s rank correlation coefficient was used to evaluate threshold effect performance, and a p-value <0.05 indicates that the threshold effect is observed (12). If a threshold effect is not observed, the Stata software package midas command was used to analyze the four-grid data. With the use of a bivariate random-effects model, the pooled sensitivity and specificity for 18F-DCFPyL PSMA PET were reported as estimates with 95% confidence intervals (CIs). The summary receiver operating characteristic curve and area under the curve (AUC) were generated by using the summary receiver operating characteristic (SROC) model (13). The heterogeneity of each study was evaluated by heterogeneity index I2 and χ2 test (14). I2 quantifies heterogeneity by calculating the proportion of variation due to heterogeneity rather than due to chance. Homogeneity among the studies was considered to be low, moderate, or high when the I2 value was 25%, 50%, or 75%, respectively. The sources of heterogeneity were identified with a meta-regression analysis when there was obvious heterogeneity. Stata 16.0 was used to make a Deek’s funnel plot to evaluate publication bias (15).
3 Results
3.1 Literature search
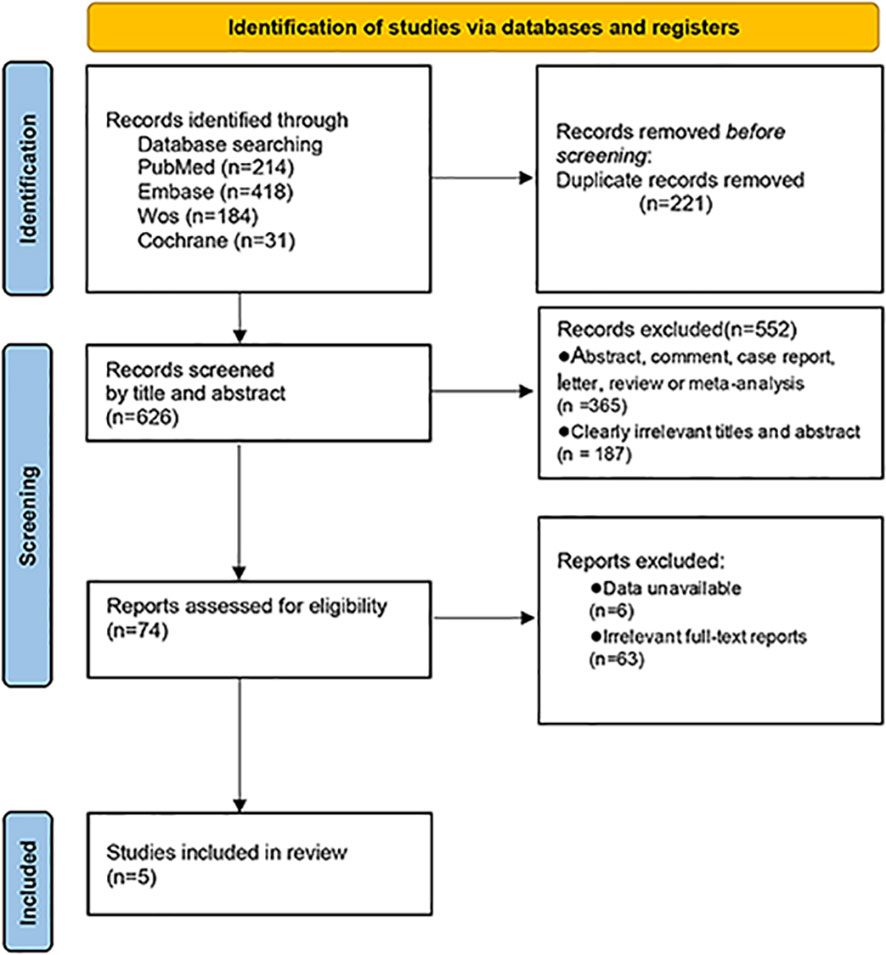
Through the search of PubMed, Cochrane Library, Web of Science databases, and Embase, 847 related articles were initially detected. Endnote X9 software was used for literature management, and 221 duplicate references were deleted. After reading the title and abstract, 552 irrelevant articles were excluded. After reading the full text of the remaining 74 articles that may meet the requirements, 63 articles met the exclusion criteria after reading the full text, and six articles could not directly or indirectly extract the four-grid table data. Finally, five papers were selected (16–20) (Figure 1).
3.2 Study evaluation
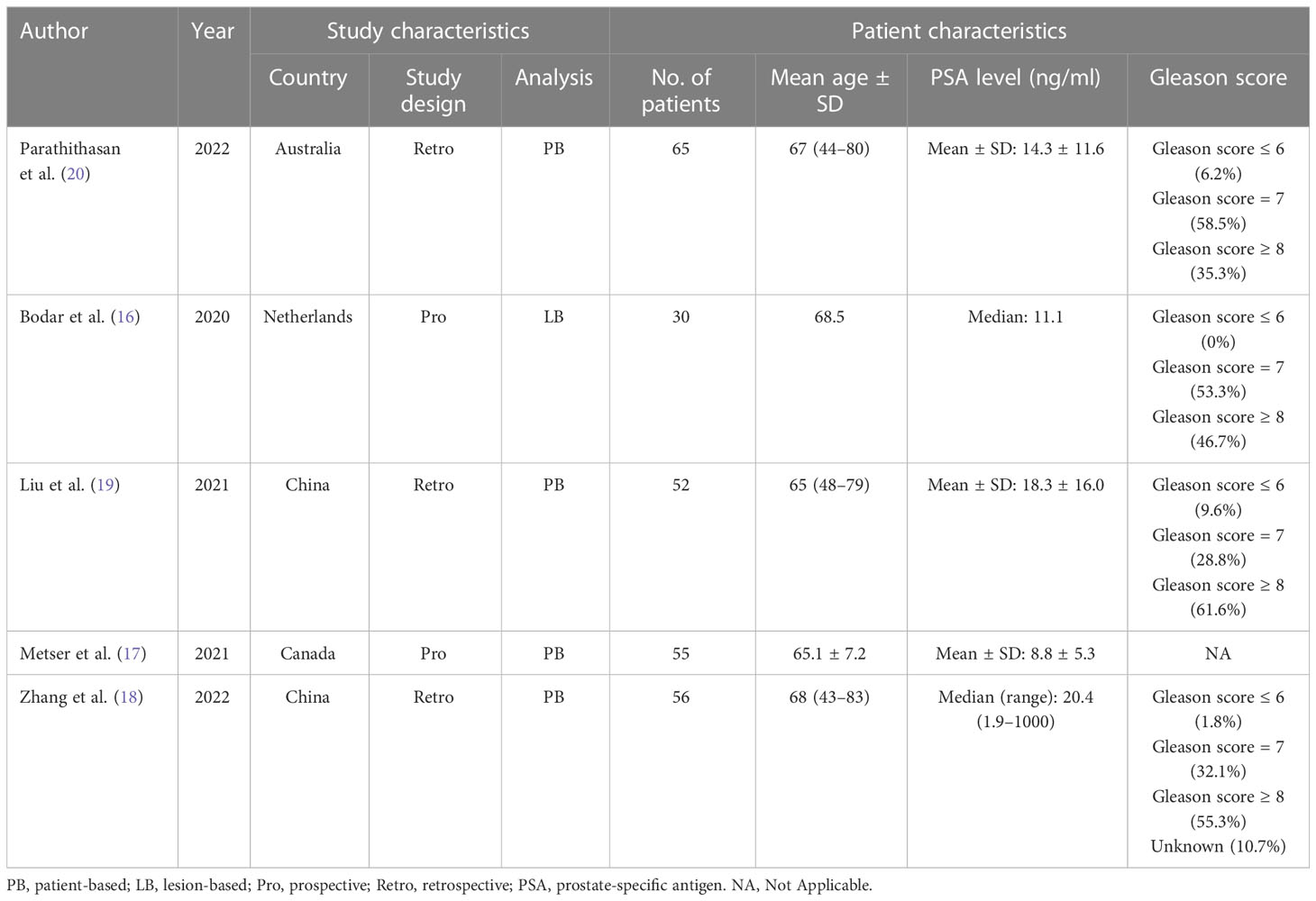
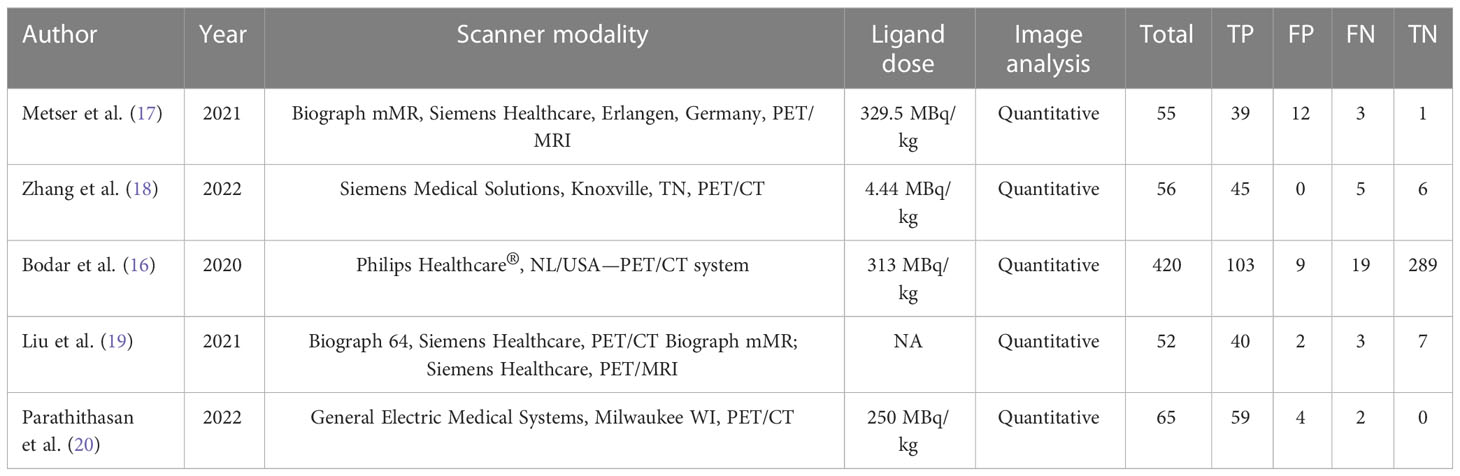
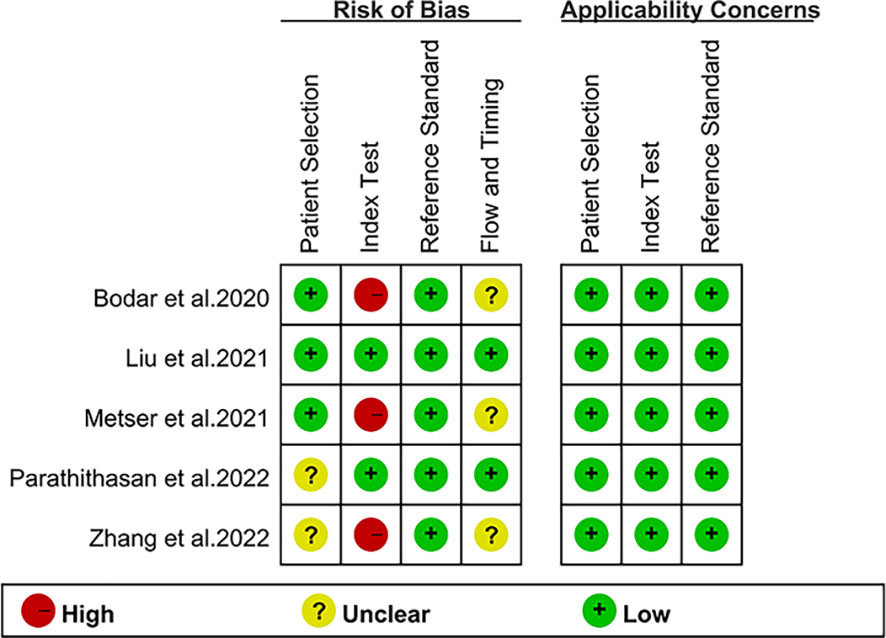
The investigations and patient factors of the five papers, which covered 258 patients, are summarized in Table 1, and the parameters and 18F-DCFPyL PSMA PET reference standards are listed in Table 2. Two of the five studies were designed to prospectively address this study question (16, 17). In four studies, positive PSMA PET results were evaluated per patient, whereas in one study, the data were evaluated per lesion (16). Figure 2 displays the overall findings for each study’s bias risk and applicability issues. The standard of the accepted consensus inclusion research determines the final result.
3.3 Diagnostic performance
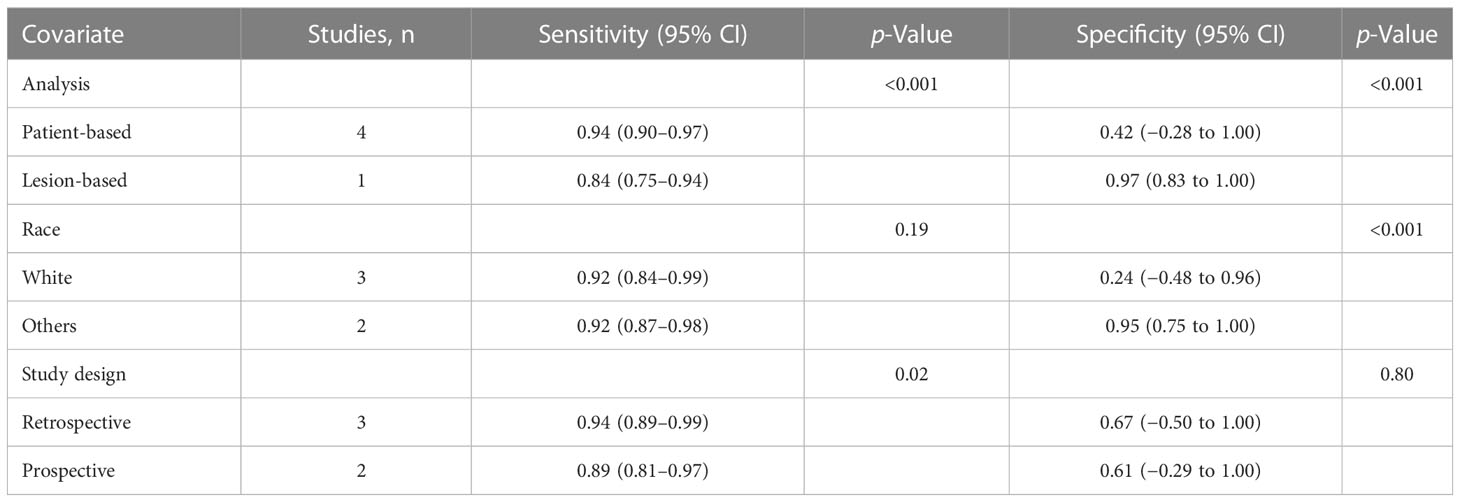
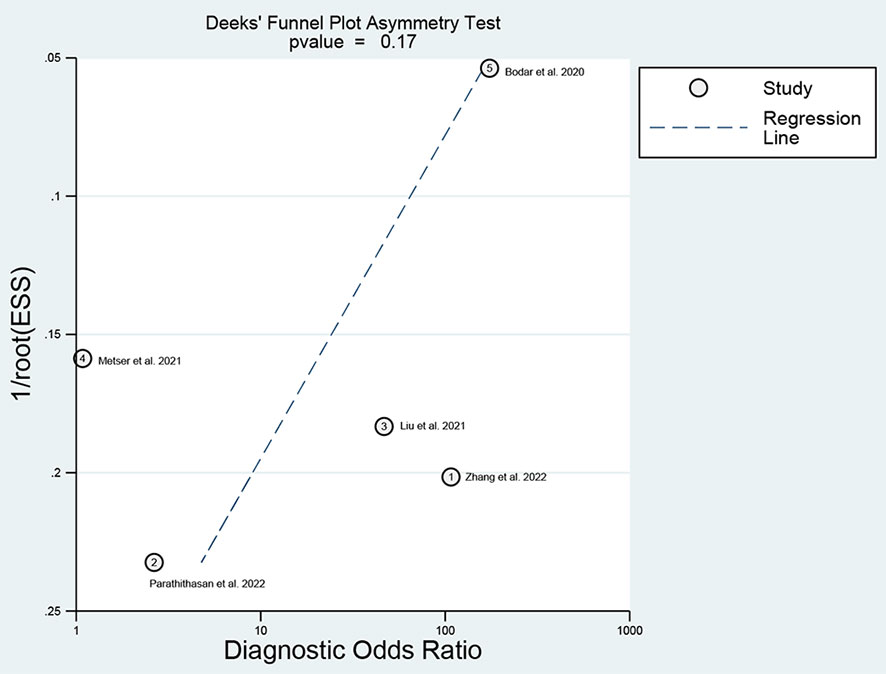
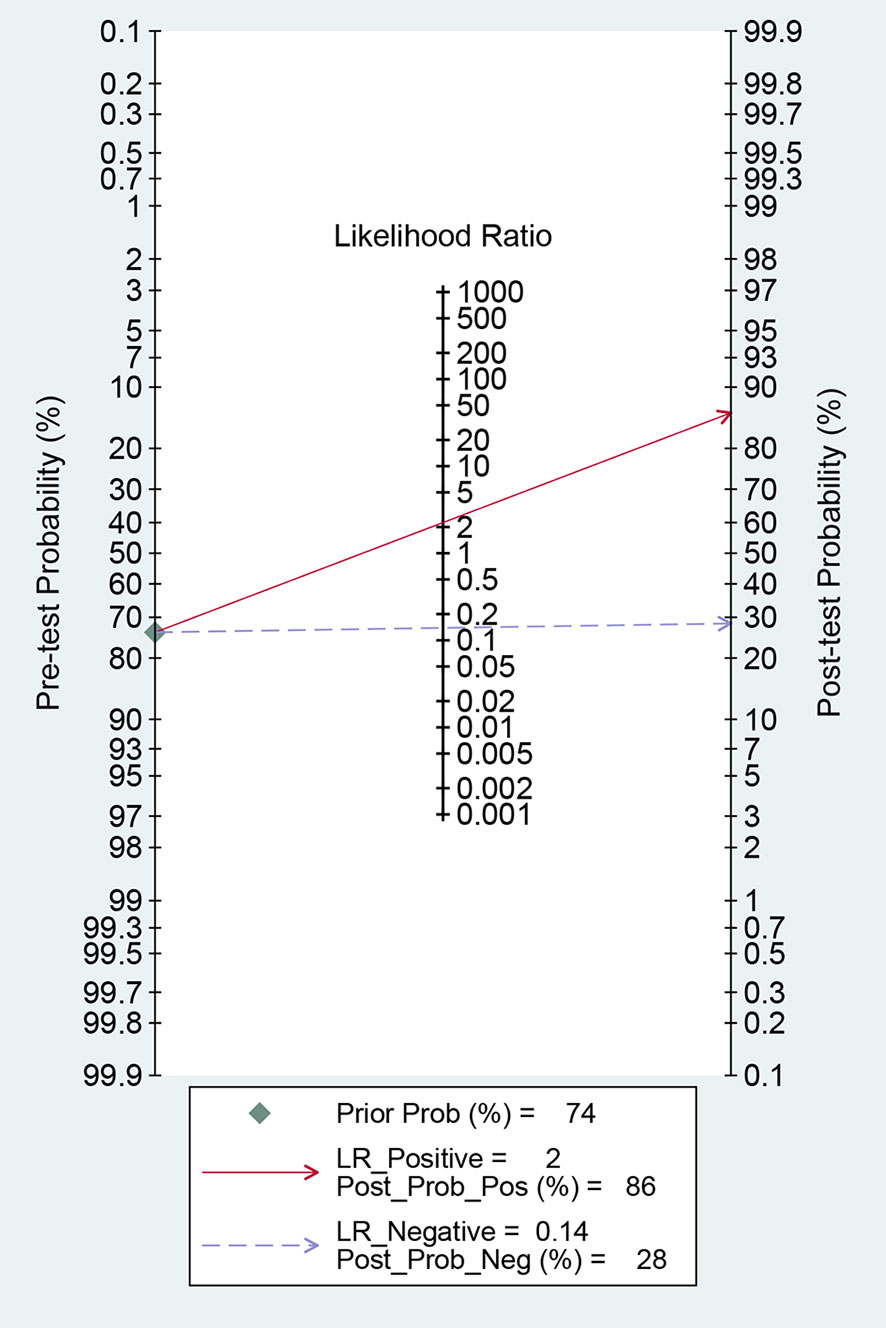
For 18F-DCFPyL PSMA PET, the results of Spearman’s rank correlation coefficient demonstrated that a threshold effect was not observed (Spearman’s correlation coefficient = 0.700, p = 0.188). The pooled sensitivity and specificity of 18F-DCFPyL PSMA PET for primary prostate cancer were 0.92 (95% CI: 0.85–0.96) and 0.59 (95% CI: 0.08–0.96), respectively (Figure 3). Figure 4 shows the SROC curve for the AUC of 0.92 of 18F-DCFPyL PSMA PET (95% CI: 0.89–0.94). We tried to find out the cause of heterogeneity by using meta-regression analysis. It demonstrated that diverse races (specificity p < 0.001) and analysis (specificity p < 0.001) were two potential causes of heterogeneity (Table 3). No publication bias was discovered (p = 0.17), as shown in Figure 5. To facilitate clinical analysis, the Fagan diagram was created. It might be seen in Figure 6. The 18F-DCFPyL PSMA PET posttest probability was 86%, which was greater than the pretest likelihood of 74%.
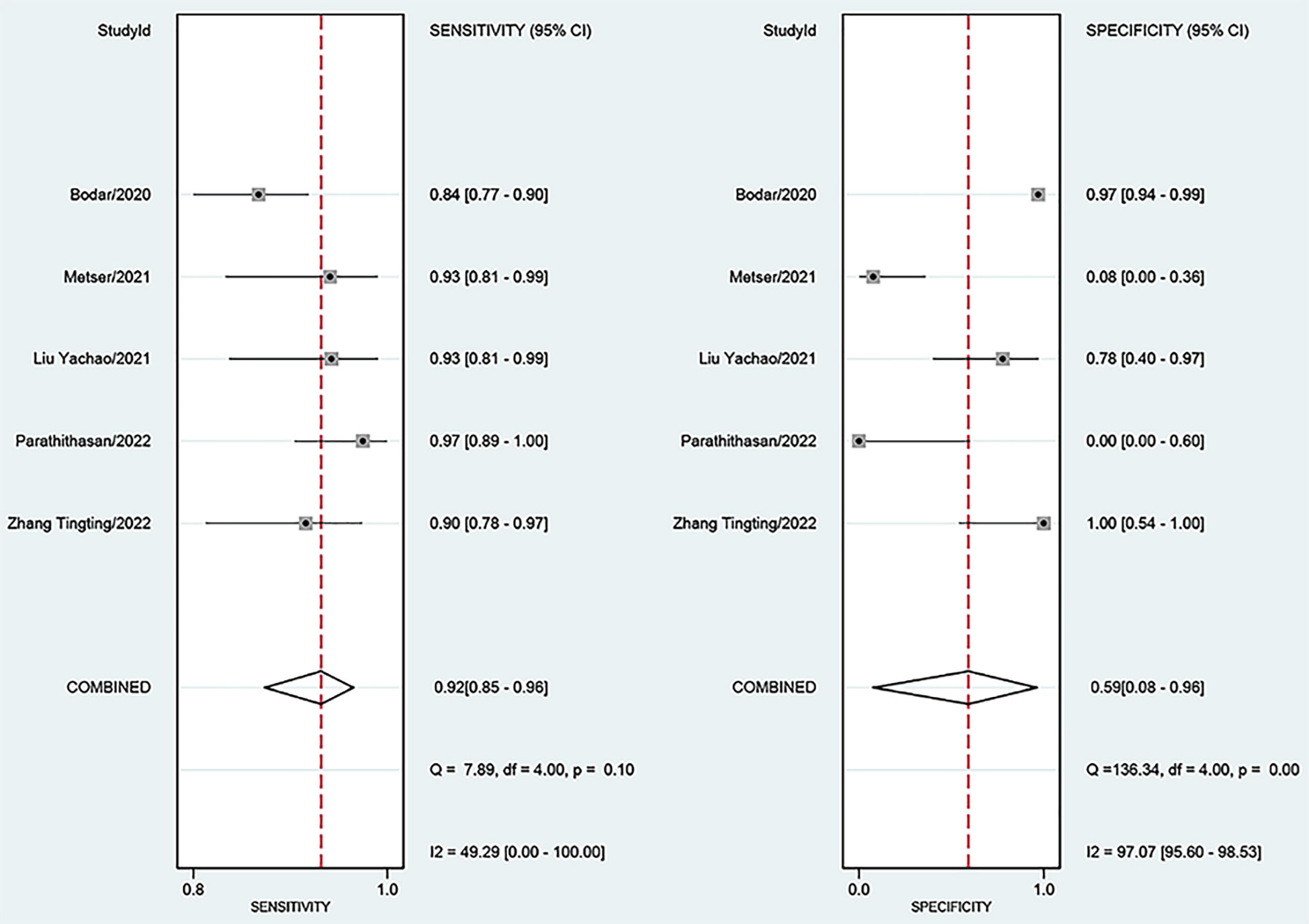
Figure 3 Forest plot showing the sensitivity and specificity of 18F-DCFPyL PSMA PET for primary prostate cancer. PSMA, prostate-specific membrane antigen.
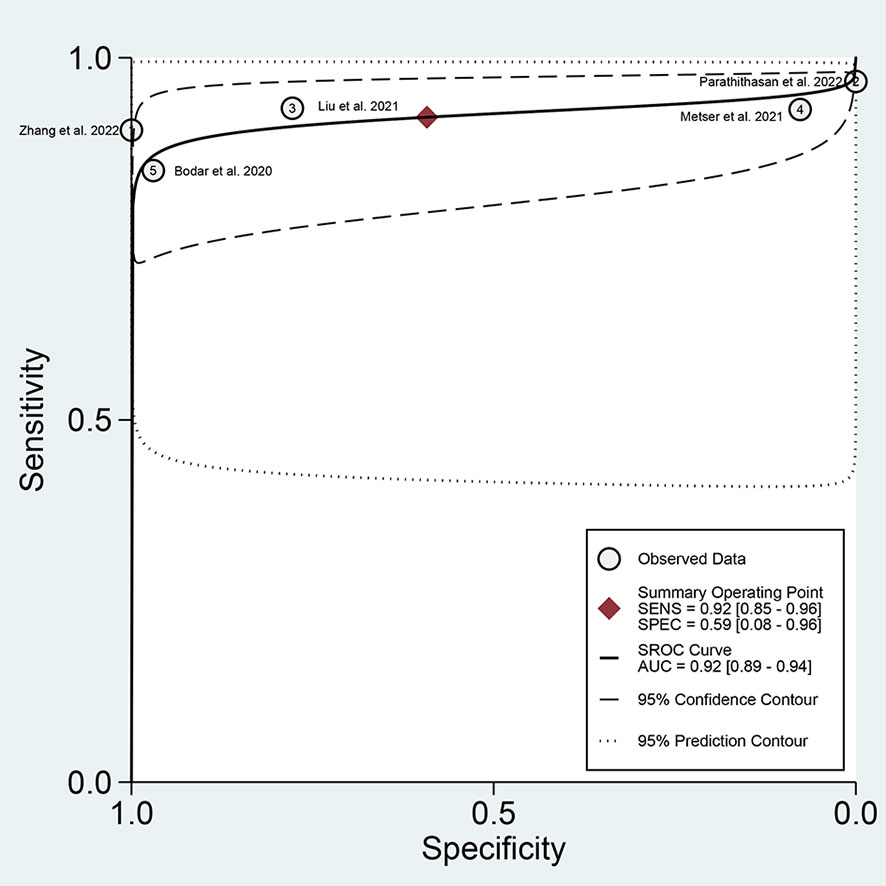
Figure 4 SROC curve for 18F-DCFPyL PSMA PET with an AUC value. SROC, summary receiver operating characteristic; PSMA, prostate-specific membrane antigen; AUC, area under the curve.
4 Discussion
PCa has become one of the major malignant tumors in the male reproductive system that causes more trouble to older men, which occurs in the prostate epithelium. The diagnosis of tumors has received more attention in recent years in cancer research investigations, which include but are not limited to blood biomarkers, tissue pathology analysis, and imaging methods (21). It has become clear that the best cancer screening techniques should be precise, non-invasive, and practical, especially at the early stage (22). However, the biological behavior of PCa and its clinical manifestations have a great deal of variation, which makes the diagnosis extremely challenging. Therefore, high sensitivity and precision imaging equipment are essential because most patients miss the ideal therapy window.
PSMA is a transmembrane protein located in prostate epithelial cells. Compared with normal prostate tissue, almost all prostate cancer cells can highly express PSMA in the cell membrane. Studies related to 68Ga-PSMA PET have shown good diagnostic and staging values in primary prostate cancer (23–25). However, its clinical application is limited because of the short half-life and low PET images. However, the new imaging agent 18F-DCFPYL is popular because of its high affinity and good in vivo pharmacokinetics. The clinical comparison showed that its various properties were better than those of 68GA-PSMA-11 (10, 11, 26).
Therefore, in this study, we evaluated the diagnostic value of 18F-DCFPyL PSMA PET in patients with suspected prostate cancer. In a previous meta-analysis, the combined sensitivity estimates for 18F-DCFPyL PSMA PET in identifying prostate cancer was 0.91, and the specificity was 0.90. However, it was restricted to research comparing PET/CT to CT, but our literature was not. In contrast, for analysis, patients with biochemical recurrence or distant metastasis were mainly included, whereas our study involved untreated patients with suspected prostate cancer. The Bodar et al. (16) prospective studies showed that the sensitivity of 18F-DCFPyL PSMA PET for prostate cancer diagnosis was 61.4%, and the specificity was 88.3%. Metser et al. (17) also conducted a prospective study and concluded a sensitivity of 86% and a specificity of 32%. In our study, the sensitivity and specificity of 18F-DCFPyL PSMA PET for the diagnosis of prostate cancer are 0.92 (95% CI: 0.85–0.96) and 0.59 (95% CI: 0.08–0.96), respectively. 18F-DCFPyL PSMA PET showed better accuracy than multiparameter MRI and CT in the diagnosis of prostate cancer. Meissner et al. (27) described for the first time a possible biopsy-free diagnostic pathway for PC in selected men with a high suspicion of significant malignancy in both multiparametric MRI (mpMRI) and PSMA PET. We expect that in the near future, under certain circumstances, patients will be exempted from biopsies.
There were still certain limitations to this study. First, the number of articles included in the study is limited, and the total number of patients is not large enough, which may bring selection bias. Second, there are not enough prospective studies in the included articles, which may affect the sensitivity and specificity. In the end, when we combined the general PET specificity, there was greater heterogeneity. The cause of heterogeneity was examined using meta-regression analysis. It demonstrated that diverse races (specificity p <0.001) and the patient-based and lesion-based analyses (specificity p <0.001) were two potential causes of heterogeneity. Of course, there could be other causes that are sources of heterogeneity.
5 Conclusions
In conclusion, 18F-DCFPyL PSMA PET has a high diagnostic value for primary prostate cancer and is an effective method for the non-invasive diagnosis of prostate cancer. We hope that in the future, more prospective articles will be added to analyze whether 18F-DCFPyL PSMA PET can be used as a diagnostic tool to avoid prostate biopsy in patients with primary prostate cancer.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
WP: project development, data collection, and manuscript writing. SD: project development and data analysis. SC: data collection. ZD: manuscript writing. All authors have read and approved the final version of the manuscript.
Funding
Analysis of feasibility, safety, and efficacy of treatment of renal ureteral stones under local anesthesia: a prospective randomized controlled trial (CBY22-QNA58). The funder was not involved in any study design, data collection, analysis and interpretation, report writing, and article submission for publication.
Acknowledgments
We would like to thank the researchers and study participants for their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49.
2. Liu J, Chen Z, Wang T, Liu L, Zhao L, Guo G, et al. Influence of four radiotracers in PET/CT on diagnostic accuracy for prostate cancer: A bivariate random-effects meta-analysis. Cell Physiol Biochem: Int J Exp Cell Physiol Biochem Pharmacol (2016) 39(2):467–80.
3. Stefanova V, Buckley R, Flax S, Spevack L, Hajek D, Tunis A, et al. Transperineal prostate biopsies using local anesthesia: Experience with 1,287 patients. prostate cancer detection rate, complications and patient tolerability. J Urol (2019) 201(6):1121–6.
4. Liss MA, Ehdaie B, Loeb S, Meng MV, Raman JD, Spears V, et al. An update of the American urological association white paper on the prevention and treatment of the more common complications related to prostate biopsy. J Urol (2017) 198(2):329–34.
5. Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res: an Off J Am Assoc Cancer Res (1997) 3(1):81–5.
6. Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem (2004) 91(3):528–39.
7. Ross JS, Sheehan CE, Fisher HA, Kaufman RP Jr., Kaur P, Gray K, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res (2003) 9(17):6357–62.
8. Morris MJ, Rowe SP, Gorin MA, Saperstein L, Pouliot F, Josephson D, et al. Diagnostic performance of (18)F-DCFPyL-PET/CT in men with biochemically recurrent prostate cancer: Results from the CONDOR phase III, multicenter study. Clin Cancer Res: an Off J Am Assoc Cancer Res (2021) 27(13):3674–82.
9. Koerber SA, Utzinger MT, Kratochwil C, Kesch C, Haefner MF, Katayama S, et al. (68)Ga-PSMA-11 PET/CT in newly diagnosed carcinoma of the prostate: Correlation of intraprostatic PSMA uptake with several clinical parameters. J Nucl Med (2017) 58(12):1943–8.
10. Rowe SP, Macura KJ, Mena E, Blackford AL, Nadal R, Antonarakis ES, et al. PSMA-based [(18)F]DCFPyL PET/CT is superior to conventional imaging for lesion detection in patients with metastatic prostate cancer. Mol Imaging Biol (2016) 18(3):411–9.
11. Rowe SP, Mana-Ay M, Javadi MS, Szabo Z, Leal JP, Pomper MG, et al. PSMA-based detection of prostate cancer bone lesions with ¹⁸F-DCFPyL PET/CT: A sensitive alternative to (⁹m)Tc-MDP bone scan and Na¹⁸F PET/CT? Clin Genitourinary Cancer (2016) 14(1):e115–8.
12. Leeflang MM. Systematic reviews and meta-analyses of diagnostic test accuracy. Clin Microbiol Infect (2014) 20(2):105–13.
13. Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol (2005) 58(10):982–90.
14. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj (2003) 327(7414):557–60.
15. Deeks JJ. Systematic reviews in health care: Systematic reviews of evaluations of diagnostic and screening tests. Bmj (2001) 323(7305):157–62.
16. Bodar YJL, Jansen BHE, van der Voorn JP, Zwezerijnen GJC, Meijer D, Nieuwenhuijzen JA, et al. Detection of prostate cancer with (18)F-DCFPyL PET/CT compared to final histopathology of radical prostatectomy specimens: is PSMA-targeted biopsy feasible? the DeTeCT trial. World J Urol (2021) 39(7):2439–46.
17. Metser U, Ortega C, Perlis N, Lechtman E, Berlin A, Anconina R, et al. Detection of clinically significant prostate cancer with (18)F-DCFPyL PET/multiparametric MR. Eur J Nucl Med Mol Imaging (2021) 48(11):3702–11.
18. Zhang T, Yang S, Lin L, Wang S, Xia D, Chen D, et al. Role of (18) f-DCFPyL PET/CT in patients with suspected prostate cancer. Hell J Nucl Med (2022) 25(1):11–8.
19. Liu Y, Dong Y, Liu J, Zhang X, Lin M, Xu B. Comparison between (18) f-DCFPyL PET and MRI for the detection of transition zone prostate cancer. Prostate (2021) 81(16):1329–36.
20. Parathithasan N, Perry E, Taubman K, Hegarty J, Talwar A, Wong LM, et al. Combination of MRI prostate and 18F-DCFPyl PSMA PET/CT detects all clinically significant prostate cancers in treatment-naive patients: An international multicentre retrospective study. J Med Imaging Radiat Oncol (2022) 66(7):927–35.
21. van der Leest M, Cornel E, Israël B, Hendriks R, Padhani AR, Hoogenboom M, et al. Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naïve men with elevated prostate-specific antigen: A Large prospective multicenter clinical study. Eur Urol (2019) 75(4):570–8.
23. Eder M, Wängler B, Knackmuss S, LeGall F, Little M, Haberkorn U, et al. Tetrafluorophenolate of HBED-CC: a versatile conjugation agent for 68Ga-labeled small recombinant antibodies. Eur J Nucl Med Mol Imaging (2008) 35(10):1878–86.
24. Schwenck J, Rempp H, Reischl G, Kruck S, Stenzl A, Nikolaou K, et al. Comparison of (68)Ga-labelled PSMA-11 and (11)C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging (2017) 44(1):92–101.
25. Wang X, Wen Q, Zhang H, Ji B. Head-to-Head comparison of (68)Ga-PSMA-11 PET/CT and multiparametric MRI for pelvic lymph node staging prior to radical prostatectomy in patients with intermediate to high-risk prostate cancer: A meta-analysis. Front Oncol (2021) 11:737989.
26. Rowe SP, Gorin MA, Pomper MG. Imaging of prostate-specific membrane antigen using [(18)F]DCFPyL. PET Clinics (2017) 12(3):289–96.
Keywords: 18F-DCFPyL PSMA PET, prostate cancer, diagnostic performance, meta-analysis, systemic review and meta-analysis
Citation: Pang W, Cheng S, Du Z and Du S (2023) The diagnostic performance of 18F-DCFPyL PET in patients with suspected prostate cancer: A systemic review and meta-analysis. Front. Oncol. 13:1145759. doi: 10.3389/fonc.2023.1145759
Received: 16 January 2023; Accepted: 15 February 2023;
Published: 07 March 2023.
Edited by:
Orhan K Oz, University of Texas Southwestern Medical Center, United StatesReviewed by:
Yin Xi, University of Texas Southwestern Medical Center, United StatesFrancesco Claps, The Netherlands Cancer Institute (NKI), Netherlands
Copyright © 2023 Pang, Cheng, Du and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuang Du, ZHVzaHVhbmcwOTI4QDE2My5jb20=
 Wenyang Pang1
Wenyang Pang1 Shulin Cheng
Shulin Cheng Zhongbo Du
Zhongbo Du