
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 17 March 2023
Sec. Breast Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1144021
This article is part of the Research TopicThe Tumor and Microenvironment Crosstalk in Breast Cancer: From Biology to Therapeutic OpportunityView all 12 articles
 May Soe Thu1,2,3
May Soe Thu1,2,3 Korn Chotirosniramit4
Korn Chotirosniramit4 Tanawin Nopsopon4,5,6
Tanawin Nopsopon4,5,6 Nattiya Hirankarn2
Nattiya Hirankarn2 Krit Pongpirul3,4,5,7,8*
Krit Pongpirul3,4,5,7,8*Introduction: Dysbiosis characterises breast cancer through direct or indirect interference in a variety of biological pathways; therefore, specific microbial patterns and diversity may be a biomarker for the diagnosis and prognosis of breast cancer. However, there is still much to determine about the complex interplay of the gut microbiome and breast cancer.
Objective: This study aims to evaluate microbial alteration in breast cancer patients compared with control subjects, to explore intestine microbial modification from a range of different breast cancer treatments, and to identify the impact of microbiome patterns on the same treatment-receiving breast cancer patients.
Methods: A literature search was conducted using electronic databases such as PubMed, Embase, and the CENTRAL databases up to April 2021. The search was limited to adult women with breast cancer and the English language. The results were synthesised qualitatively and quantitatively using random-effects meta-analysis.
Results: A total of 33 articles from 32 studies were included in the review, representing 19 case-control, eight cohorts, and five nonrandomised intervention researches. The gut and breast bacterial species were elevated in the cases of breast tumours, a significant increase in Methylobacterium radiotolerans (p = 0.015), in compared with healthy breast tissue. Meta-analysis of different α-diversity indexes such as Shannon index (p = 0.0005), observed species (p = 0.006), and faint’s phylogenetic diversity (p < 0.00001) revealed the low intestinal microbial diversity in patients with breast cancer. The microbiota abundance pattern was identified in different sample types, detection methods, menopausal status, nationality, obesity, sleep quality, and several interventions using qualitative analysis.
Conclusions: This systematic review elucidates the complex network of the microbiome, breast cancer, and therapeutic options, with the objective of providing a link for stronger research studies and towards personalised medicine to improve their quality of life.
Breast cancer has been a major concern for women for several decades, with an estimated 2.3 million cases in 2020 and a global frequency of 11.7% (1). To date, four subtypes of invasive breast cancer have been identified: luminal subtype A, which exhibits high levels of estrogen receptor (ER) and progesterone receptor (PR), but low expression of human epidermal growth factor receptor 2 (HER2) and cell proliferation index; luminal subtype B, which exhibits ER/PR+, HER2, and high proliferation index; HER2+ breast cancer subtype; and triple-negative breast cancer (TNBC) subtype (2). The likelihood of breast cancer is typically increased by several risk factors, namely, age, sex, obesity, family history, genetic mutation, estrogen level, and sedentary lifestyles (3–5). The human microbiota, on the other hand, has drawn considerable interest as a key risk modulator due to its unique function in controlling steroid hormone metabolism by activating various enzymes, including hydroxysteroid dehydrogenase (6, 7).
The gut microbiota is an abundant ecosystem of highly diversified microorganisms, with the Firmicutes and Bacteroidetes phyla accounting for approximately 90% of the gut microbiota, including Lactobacillus, Clostridium, Enterococcus, Dialister and Ruminicoccus of Firmicutes and Bacteroides, Alistipes, and Prevotella of Bacteroidetes (8). An imbalance of the human gut microbiota known as dysbiosis causes a number of health problems (9–11). A comparison of many breast samples reveals differences in the quantity and microbial diversity of several specific genera between healthy people and patients, despite the absence of conclusive evidence that dysbiosis causes breast cancer (12, 13). It also acknowledges the relationship between different gut microbial profiles and different subtypes of breast cancer (14). Furthermore, although there was no significant variation in abundance between premenopausal breast cancer patients and controls, the structure and functions of the gut microbial community differed between postmenopausal breast cancer patients and healthy controls (15). As a result, the impact of microbial instability in the breast, as well as the function of microbial communities in the development of breast cancers, has been thoroughly established.
Tumour tissue and high-risk tissue had a much lower breast microbial diversity than tumour-neighbouring normal or healthy control tissue adjacent to the tumour. For example, the breast tumour microbiome contained a higher proportion of the Pseudomonadaceae and Enterobacteriaceae families, the genera Pseudomonas, Proteus, Porphyromonas, and Azomonas, compared with other tissues (16). On the other hand, propionibacterium and Staphylococcus were rare in tumour tissue but were important components of healthy control, high-risk, and neighbouring normal tissues (16).
Breast cancer and the oral microbiota, in particular, appear to be linked. The risk of breast cancer has been found to be higher in women who have periodontal disease, caused by specific bacteria such as the red complex (Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola) and the orange complex (Fusobacterium nucleatum, Prevotella intermedia, Prevotella nigrescens, Peptostreptococcus micros, Streptococcus constellatus, Eubacterium nodatum, Campylobacter showae, Campylobacter gracilis, and Campylobacter rectus) (17–19).
The gut microbiota secretes bioactive bacterial metabolites, such as reactivated estrogens, amino acid metabolites, short-chain fatty acids (SCFAs), or secondary bile acids (BAs), which can affect disease progression (20–22). For its estrogen reactivation activity, the role of gut microbial β-glucuronidase (GUS) in the pathogenesis of breast cancer has been proposed, and GUS is encoded by Bacteroidetes and Firmicutes in the human gastrointestinal tract (23). Glutamine-proline-glycine metabolism became active in different subtypes of breast cancer, and amino acid transporter-2 metabolites were up-regulated to serve energy homeostasis and protein and nucleotide biosynthesis (22). SCFAs are essential in cell homeostasis, affecting the colon and other organs through blood flow, and are produced by two major bacterial groups: Bacteroidetes produce propionate and acetate, while Firmicutes produce butyrate (24). BAs, which are soluble derivatives of cholesterol produced in the liver, were previously thought to be carcinogenic agents but can have antineoplastic properties in cases of breast cancer (25).
As a result, the impact of the gut microbiome is multifaceted and important in controlling the host immune system in the pathophysiology of the development of breast cancer and the response and resistance to various cancer therapies (26–28). Through studies in animals, chemotherapy was found to alter the intestinal flora, which can result in adverse effects from early breast cancer treatment including weight gain or neurological disorders (29). Experimental research also showed a link between the gut microbiota and clinical outcomes and therapeutic responsiveness in different subtypes of breast cancer (30, 31). In particular, a study finds that gut bacteria are significantly more prevalent in breast cancer patients than in healthy people, which is detrimental to the prognosis of the disease (29). Thus, for therapeutic purposes and for the prognosis of the disease, detailed insights into the breast cancer oncobiome are important.
In recent decades, numerous studies revealed the impact of the microbiome on different organ-specific cancers and the action of bacterial metabolites in the human host on several signaling pathways, for example, E-cadherin/β-catenin pathway, breaking DNA double strands, promoting apoptosis, and altering cell differentiation (32–34). In particular, there are still several questions between the human microbiome and breast cancer development: “What pattern of the microbiome profile do breast cancer patients have in contrast to nonbreast cancer subjects”; “How different treatments modify the microbiome”; and “What is the microbiome profile in the same treatment”. To address these, a systematic literature review and meta-analysis on breast cancer and microbiome are conducted and the specific objectives are to evaluate microbiota alteration in breast cancer patients compared with nonbreast cancer subjects, to explore microbiota modification from a range of different treatment strategies, and to identify the impact of microbial pattern on the same treatment-receiving breast cancer patients.
The systematic review of the literature was registered on PROSPERO ID 2021 CRD42021288186.
The PRISMA statement guidelines were followed to conduct systematic review and meta-analysis (35). The study search strategy was developed based on the PICO/PECO (Population, Intervention/Exposure, Comparison or Controls, and Outcome) framework (36, 37). Two authors (MT and KC) independently examined each study for inclusion in the systematic review using PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (www.embase.com), and the Cochrane Library (www.cochranelibrary.com). This was conducted using a full search term strategy, as detailed in Supplementary Table S1. The search was limited to adult women with breast cancer and the English language. Studies that included only nonhuman subjects or were not peer reviewed were excluded. Both epidemiological and intervention studies were considered from these databases and mainly focused on the interlink between breast cancer patients and the gut microbiome that was being extracted up to April 2022. Discrepancies were resolved by group discussion at each step.
Article selection was carried out by two independent reviewers (MT and KC) for eligible studies using prespecified inclusion and exclusion criteria, followed by the full text review process. All relevant full-text articles were taken for further data extraction. The inclusion criteria for the meta-analysis were established as follows (1): Epidemiologic studies on how the microbiome profile in breast cancer patients differed from the pattern in nonbreast cancer control and (2) intervention studies on how treatment in breast cancer patients affected the microbiome and vice versa. Exclusion was performed in (1) the studies such as animal studies, in vitro, review articles, non-peer-reviewed articles, protocols, letters, editorial, commentary, recommendations, and guidelines and (2) the studies on breast cancer survivors. Disagreements between review authors were resolved by consensus at every phase of the selection of the systematic review selection.
The two independent authors (MT and KC) performed the data extraction for the following variables: (1) authors, year of publication, study period, study type, and country that implemented the study; (2) demographic characteristics such as menopause, menarche, and hormonal status; (3) related characteristics, including cytokine levels and enzyme activities; and (4) Parameters for the diversity profile. All relevant text, tables, and figures were examined during data extraction, and discrepancies between the two authors were resolved by discussion or consensus.
The two independent authors (MT and KC) evaluated the risk of bias (ROB) in the extracted intervention studies. However, studies are nonrandomised trials, and therefore ROBINS-I (Risk Of Bias in Nonrandomised Studies—of Interventions) tool was applied to assess ROB (38). For included cohort and case-control studies, the two independent authors (MT and KC) performed the ROB evaluation using the Newcastle–Ottawa Quality Assessment Scale (NOS) developed from an ongoing collaboration between the Universities of Newcastle, Australia, and Ottawa, Canada, for quality assessment in a meta-analysis (39). The tool was used to assess the following domains: bias arising from the selection process, bias arising from the comparability process, and bias arising from the outcome/exposure process. Any disagreement was resolved by consensus. If there was not enough information to consider, the corresponding authors were emailed and their response was waited for 2 weeks. In the event of no response, it proceeded with the available data and any disagreement was resolved through discussion.
For intervention studies, mean differences (MDs) and a 95% confidence interval (95% CI) between groups were indicated for microbiome diversity outcomes. The characteristics of the participants, the study period, the type of study, and the location of the study were evaluated for clinical and methodological heterogeneity. The I2 statistics were used for the assessment of statistical heterogeneity (40). The heterogeneity level was as defined in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions. For clinical, methodological, and statistical heterogeneity, the random effects meta-analysis using the DerSimonian and Laird method was utilized by RevMan 5, v.5.4.1 (https://training.cochrane.org/online-learning/core-software/revman/; accessed 31 October 2022).
The literature search found 2,761 articles from the databases, of which 758 duplicates were removed prior to selection. From the initial 2,003 studies, the title and abstract selection were carried out and 1,884 articles were excluded according to the inclusion and exclusion criteria. Next, we retrieved 119 articles for full text screening and checked their eligibility for meta-analysis. Among them, 86 studies were excluded due to the following conditions: 50 studies were articles not peer reviewed, 11 targeted the wrong population, seven raised wrong outcomes, four were protocol papers, three were editorial, three were wrong interventions, three were wrong study design, one were wrong comparator, two were review articles, one was duplicate, and one was not reported in English. Last, 33 articles from 32 studies, with an enrolment of 3,448 participants covering the study period from 2004 to 2019, were included in the systematic review and meta-analysis of the literature, representing 19 case-control, eight cohort, and five non-randomised intervention studies (Figure 1).
The extracted studies were published from 1990 to 2020 in 11 countries, contributed mainly by the United States and China. We included different types of study, such as cohort, case-control, and intervention studies, and the age range participated was 18 to 90 years (Table 1).
Overall, the combined mean of the age of the participant is 54.3, with a standard deviation of 5.4. Regarding the menopausal status of the participants, there are three main groups: premenopausal, perimenopausal, and postmenopausal subjects. Among 66.4% of breast cancer cases, patients with postmenopause are 46% of the cancer patients are ≥ 13 years of age at menarche. In the study, 53.6% of breast cancer patients are from the United States, followed by Ghana with 16% and China with 14.6% (Table 2).
The ROB of the included studies was summarised by the study design group. ROB in case-control studies was evaluated mainly on selection, comparability, and exposure (Figure 2). The recruitment of subjects for 10 researches (14, 15, 17, 19, 41, 42, 50, 52–54) involved independent validation, while the subjects for the remaining research (13, 18, 43–49, 51) were often collected through databases or archival medical records. Four studies had the potential for selection biases: two (41, 42) did not disclose the selection procedure, one (18) used a genomics data repository to find patients but did not make a clear selection statement, and one (50) employed subjects from an Army-related hospital. Although control individuals from six trials (15, 19, 41, 42, 53, 54) were recruited from the community, the majority of control subjects was obtained as hospital controls, such as women who underwent breast reductions or cosmetic surgeries. Amongst them, two studies (48, 50) used mild cases as controls and one study (52) put the other cases of malignancy as controls. All cases and control subjects had comparability according to the study design, and the additional comparability measures were the same Mediterranean diet, age, and hormonal status by menopause. The exposure of the studies (13, 14, 17, 43–48) was identified by surgical records, while the other studies (15, 18, 19, 41, 42, 49–51, 53, 54) used written medical records. The same method of ascertainment and the same rate for both groups followed all the selection of subjects.
The selection, comparability, and ROB result in eight cohort studies (55–62) were also evaluated (Figure 3). A study (55) recruited participants from a volunteer group that was not representative of the community, but the other exposed cohorts (56, 57, 59–61) were recruited from nonmastectomy breast surgeries, patients planning breast cancer surgeries, or biopsy−confirmed breast cancer patients. All cohort studies had comparable study controls based on study designs and used medical records prior to outcome analysis. Probably there was sufficient time for follow-up; however, one still needs to outline a claim for the sufficiency. In a cohort study (60), some participants with admixed ancestry were excluded from the final analysis to reduce bias. No statement was found in other studies (55–59) on the suitability of follow-up.
Most research domains were classified as having a low ROB in the ROB assessment of nonrandomized intervention trials (63–67) (Figure 4). Among them, four studies omitted details about the participant selection procedure and a confounding bias existed in one study (64). Another study (66) showed a tendency towards interventions that were not intended.
Due to various sampling locations, different sequencing approaches, and various geographical and biological conditions, there was generally a lot of qualitative data when analysing microbial communities from different research. As a result, case-control, cohort, and non-randomised intervention studies were used to qualitatively analyse all the extracted studies (Tables 3, 4), taking into account the study period, sample information, mean population age, microbial detection methods, microbiome type, and profile of microbial diversity.
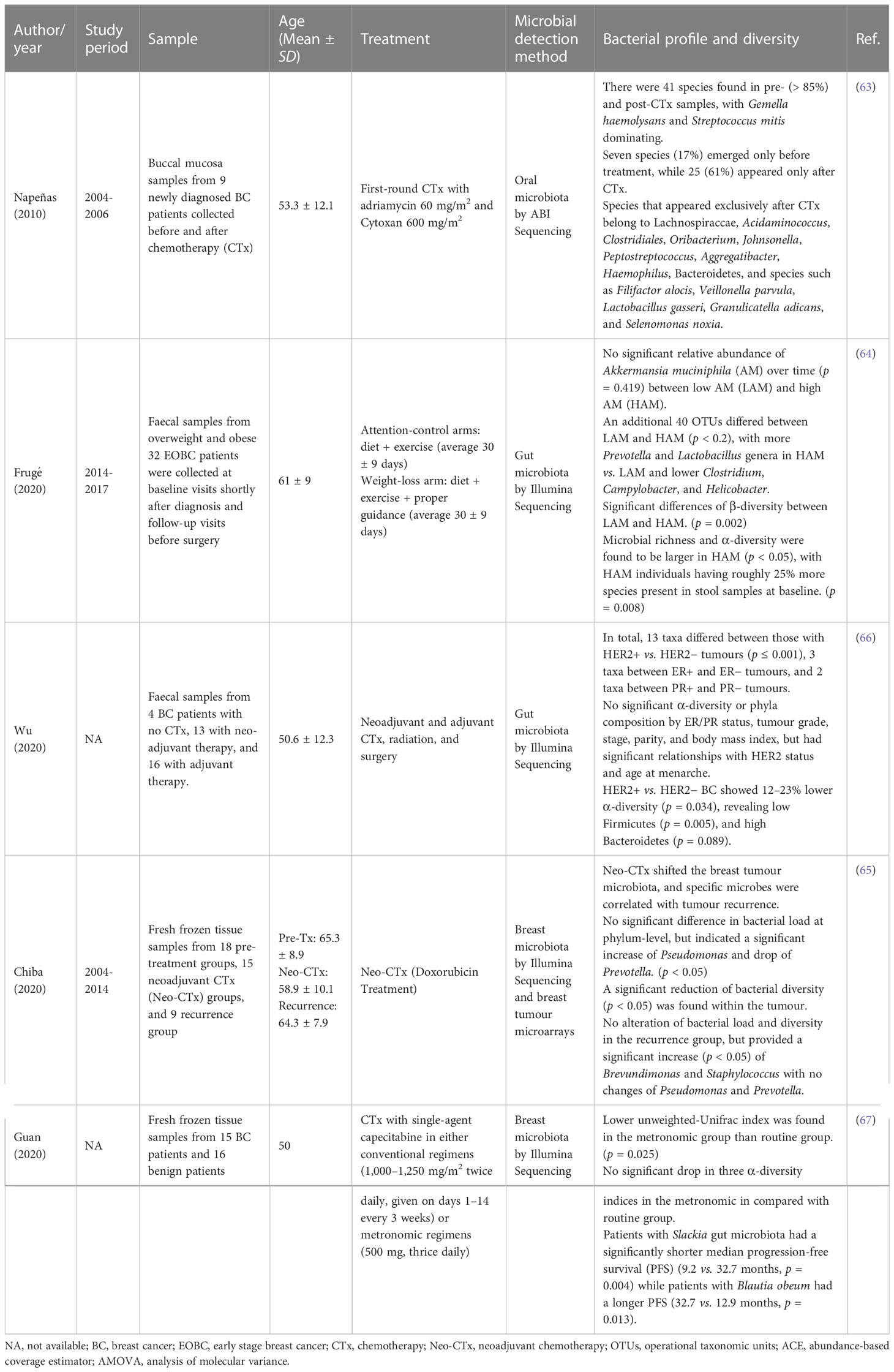
Table 4 Characteristics of non-randomized intervention trials and their microbial profiling and diversity.
A general significant reduction in gut bacterial species observed for the breast cancer group was found in two studies (44, 54) (MD = −20.16; 95% CI = −34.66 to −5.66; p = 0.006); however, the heterogeneity value is high (I2 = 87%; p = 0.006) (Figure 5).
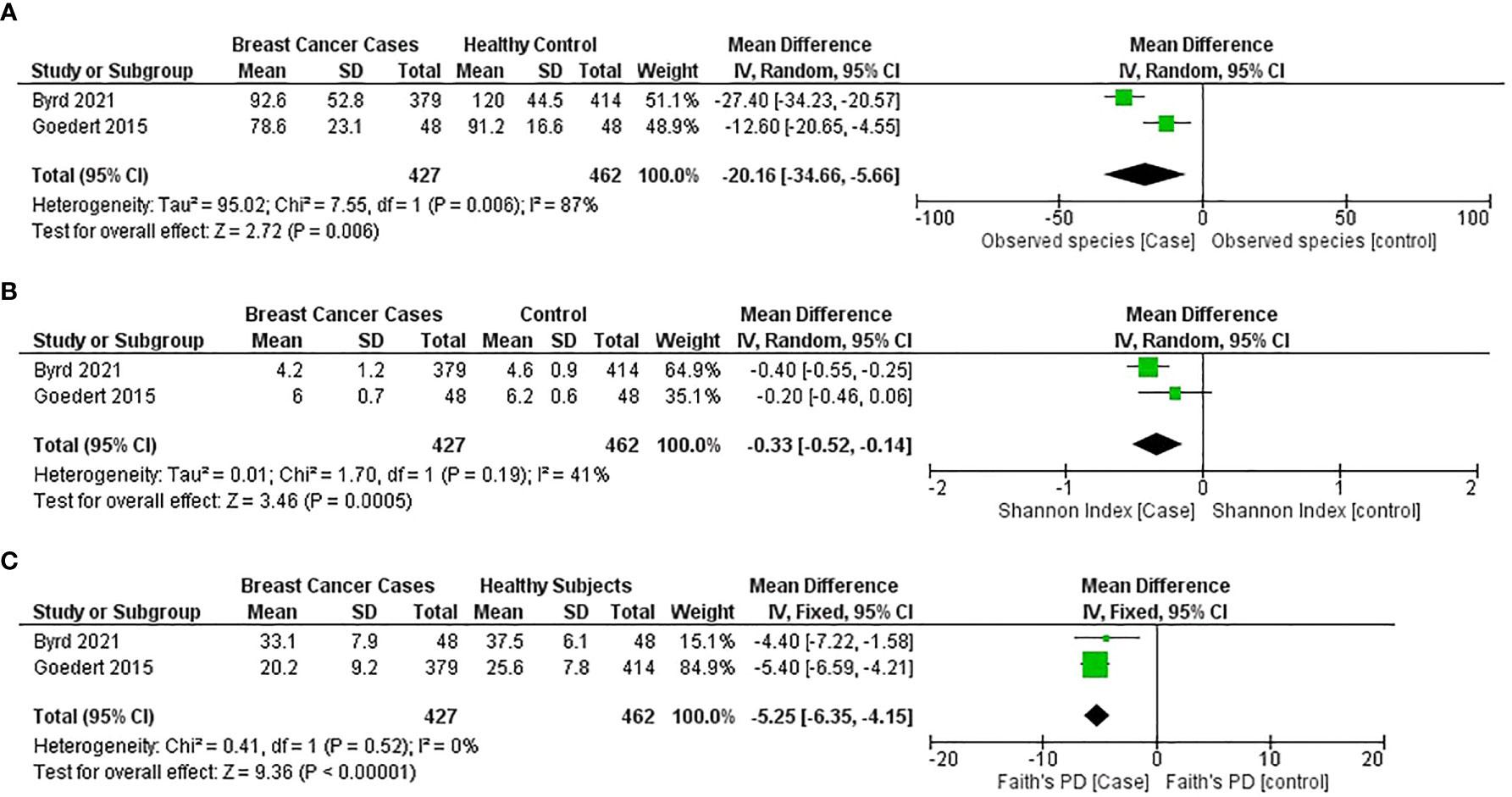
Figure 5 Meta-analysis forest plot representing the risk of breast cancer by different microbial profiling indexes like: (A) observed species; (B) Shannon index; and (C) faith’s PD.
The general estimates of the Shannon index (MD = −0.35; 95% CI = −0.48 to −0.22; p < 0.00001) and the Faith PD index (MD = −5.25; 95% CI = −6.35 to −4.15; p < 0.001) from the studies (44, 54) reported that a significant reduction in gut microbial α-diversity was found in patients with breast cancer compared with healthy subjects (Figures 5B, C).
The systematic review of the literature spanned three decades and included data from nearly a dozen countries on the breast, oral, or gut microbiome and breast cancer. Meta-analysis revealed microbial changes and diversity in breast cancer patients versus controls. The review bridged a gap that allowed us to connect previous microbiome studies in breast cancer patients using qualitative and quantitative meta-analysis tools.
Forest plots from two studies (44, 54) indicated that breast cancer patients have a lower α-diversity as measured by the Shannon index, observed species, and Faith’s phylogenetic diversity (PD), than healthy individuals. For the detection of microbial α-diversity, common parameters include Chao1, Fisher’s alpha, Faith’s PD, Simpson, Abundance-based coverage estimators (ACEs), and Good’s coverage indices (68). Some parameters simply count the number of species or operational taxonomic units (OTUs) present in an area, while others consider the abundance or frequency of the OTUs. As a result, most of the researchers used more than one diversity index, and combining and analysing different indexes in the current study were difficult; therefore, the number of studies for meta-analysis was limited.
The human intestinal microbiota is dominated by two bacterial phyla, Firmicutes and Bacteroidetes, which represent more than 90% of the total community, as well as other subdominant phyla such as Proteobacteria, Actinobacteria, and Verrucomicrobia (69). Bacteroidetes, which make up 45–55% of all bacteria, are all Gramme-negative bacteria (70). When they are particularly high, with values greater than 70–75%, it appears that they put the host at risk of diabetes and possibly other inflammatory diseases, especially when there is a high percentage of Proteobacteria present. Firmicutes, even if present in a lower percentage than Bacteroidetes, are believed to account for 40–45% of the total fecal microbiota under normal conditions (70). The study by Ma et al. found a reduced relative abundance of Firmicutes and Bacteroidetes, increased levels of Proteobacteria, Actinobacteria, and Verrucomicrobia at the phylum level, and decreased abundance of Faecalibacterium prausnitzii in fecal samples from 25 breast cancer patients (50). Furthermore, the Firmicutes/Bacteroidetes (F/B) ratio in breast cancer patients was significantly higher than in controls (53). It was also discovered that the absolute numbers of total bacteria and three bacterial groups (Firmicutes, Faecalibacterium prausnitzii, and Blautia) differed significantly according to the patient’s BMI, as shown in Table 3 (55). All of these demonstrated the gut microbial pattern, as well as a transform in the F/B ratio, which contributes to an increased risk of breast cancer.
A study described that the breast microbiota of tumour tissue has Proteobacteria (48%), Actinobacteria (26.3%), Firmicutes (16.2%), and others (9.5%) of miscellaneous phyla (18). From the included studies (17, 43, 47, 48, 51, 56, 57, 60), an increased relative abundance of Bacillus, Enterobacteriaceae, Staphylococcus, Fusobacterium, Atopobium, Gluconacetobacter, Hydrogenophaga, Lactobacillus, Corynebacterium, Actinomyces, Propionibacteriaceae, Clostridia, Bacteroidia, WPS_2, Ruminococcaceae, Acidaminococcus, Acinetobacter, Akkermansia, Bacteroides, Sutterella, Agrococcus, Ralstonia, Methylobacterium, and Sphingomonas were also discovered. Furthermore, a distinct breast tissue microbiota was found in different breast skin tissues, breast skin swabs, buccal swabs, and deep microbial communities between benign and malignant breast disease (48). Furthermore, there appears to be a geographical difference between the Canadian and Irish breast tissue microbiome (Figure 4), but there is still evidence to prove that (48). Furthermore, the study showed a distinct profile of the breast tissue microbiome with increased species richness compared with the overlying skin tissue, suggesting that the differences may be due to the difference in their environment and ecosystem (48).
The researchers discovered a 10-fold increase in bacterial load in breast tumours, as well as an inverse correlation between bacterial count in tumour tissue and breast cancer stage, with stage 3 patients having the lowest 16S ribosomal DNA copy numbers in a study comparing the breast microbiota between breast tissue and their paired normal tissue (43). A significant increase in Methylobacterium radiotolerans (p = 0.015) was found in breast tumours, while Sphingomonas yanoikuyae (p = 0.009) was found in low abundance in paired normal tissue (43). However, a study found similarities in the breast microbiota between the tumour and adjacent normal tissues using weighted UniFrac distances (57). The number of breast microbiota was found to increase in breast tumours, but there was less diversity compared with normal tissues paired.
Identifying the microbiome of the four BC subtypes may reveal a link between the microbiota and the therapeutic response (71, 72). The oncobiome of each BC cancer subtype is unique and contains a wide range of microbial signatures. ER had the most diverse oncobiome, while TN had the least (31). Furthermore, the presence or absence of specific microbes distinguishes each BC subtype and, therefore, the level of detection of these microbes was predictive of patient outcomes.
Among the multiple drivers of microbial differences, a common element is menopausal status. We found that patients with premenopausal breast cancer had an increased fecal profile of Enterobacteriaceae, aerobic Streptococci, Lactobacilli, and anaerobic bacteria, including Clostridia, Bacteroides, and Lactobacilli (41). A similar behaviour of the anaerobic flora was found in patients with late menopause. The urine microbiome of peri/postmenopausal patients also showed a reduced abundance of Lactobacilli and an elevated profile of many genera, including but not limited to anaerobic bacteria such as Varibaculum, Porphyromonas, Prevotella, Bacteroides, and members of the Clostridia class (17). More details of the microbiota in different groups are described in Tables 3, 4.
Although the role of species in the equilibrium of the GI environment is unclear, an increase in bacterial concentration can modulate estrogen metabolism through deconjugation and contribute to total bacterial enzyme activity (41). For example, the enzymes β-glucuronidase and β-glucosidase are produced by E. coli and S. faecalis, respectively. From an observation study of the enzymatic activity of fecal bacteria, it was recognised that the activity in postmenopausal women was lower than that of premenopausal cases. The expression of high to low enzymes, such as esterase C4, esterase-lipase, leucine and valine acrylamidase, acid phosphatase, α-galactosidase, and β-glucuronidase, was found in healthy subjects, while leucine and valine acrylamidase, β-glucuronidase, and esterase-lipase were higher in women with postmenopausal breast cancer (42). Therefore, it showed intersubject variability for enzymatic activities.
To explore the impact of cancer chemotherapy, Napenas et al. performed a profile of the oral microbiome on nine newly diagnosed breast cancer patients before and after receiving treatment (63). In general, 41 species were detected in total (Supplementary Table S2), and interestingly, > 85% of the detection (33/41) were newly identified species in chemotherapy patients. It revealed that seven species and 25 species appeared only before and after cancer chemotherapy, respectively, and the increase in species per patient had a mean of 2.6 (SD = 4.7, p = 0.052) after chemotherapy (63).
Chiba et al. (65) evaluated modulation of the tumour microbiome by neoadjuvant chemotherapy using breast tumour microarrays (Table 5). It demonstrated no significant changes in total bacterial load in untreated and treated patients; however, bacterial diversity was significantly reduced in the treated tumour. The classification at the phylum level did not show significant changes between the two groups, but the analysis at the genus level showed a significant elevation in Pseudomonas species and a reduction in the abundance of Prevotella in the treated cases (65). In addition, it indicated the modulation of chemotherapy in the tumour microbiome and the correlation of some genera in patients with tumour recurrence (65). Another study by Guan et al. showed significant differences in beta diversity before and after chemotherapy with single agent capecitabine and metronomic regimens (Table 5) and supported the reduction of bacterial diversity in the intervention group but was not statistically significant (67). In general, it was indicated that particular microorganisms are associated with tumour recurrence and that chemotherapy and neoadjuvant chemotherapy change the microbial composition and diversity in breast and oral tumours.
A study investigated the microbiome profile in fecal DNA only in women and control subjects; then, the case and control study discovered a significantly altered microbial community in cases (p = 0.006) compared with controls and less alpha diversity (p = 0.004) (17). Another study (66) revealed that early and late menarche was associated with a low number of OTU (p = 0.036), particularly reduced Firmicute expression (p = 0.048), and a low chao1 index (p = 0.020) (Table 2). Therefore, menopause and menarche status are associated with lower gut microbiome diversity, according to research, but more research is needed in large study populations to identify replicable patterns in taxa impacted by menopause (73, 74).
Goedert et al. (44) showed that a twofold higher level of estrogen expression was found in postmenopausal patients; however, the difference did not change the microbiota and the association with cancer. Banerjee et al. (2018) found that an increased abundance of Brevundimonas was detected in cases of ER+ breast cancer and triple-positive breast cancer (TPBC) compared with cases of ER- breast cancer and TNBC. In addition, a high abundance of Mobiluncus and Mycobacterium was predominantly identified in ER breast cancer samples. Furthermore, Acinetobacter was the most prominent in HR+ breast cancer and HER2+ breast cancer cases, Brevundimonas in TPBC samples, and Caulobacter in TNBC samples (14). Interestingly, a distinct pattern of microbial profile was explored in patients with TNBC using pan-pathogen array technology and was summarised as in (Table 5) (46).
A study also explored the level of expression of antibacterial response genes in tumour tissue, paired normal tissue, and healthy tissue and found that a third of antibacterial genes were significantly down-regulated in breast tumour cases after normalising with a housekeeping gene β-actin, interestingly there are no more up-regulated genes (43). Furthermore, a significant reduction was observed in the transcripts of the microbial sensors, Toll-like receptors (TLR)–2, TLR-5, and TLR-9 (p = 0.0298, p = 0.0201, and p = 0.0021, respectively) was observed in tumour tissue while there was a similar expression level of TLR1, TLR4, and TLR6 in healthy and tumour tissue. Furthermore, tumour tissues showed significantly decreased expression of cytoplasmic microbial sensors (NOD1 and NOD2) and downstream signaling molecules for innate microbial sensors such as CARD6, CARD9, and TRAF6 (p = 0.0207, p = 0.0040, and p = 0.0119, respectively). The levels of bactericidal/permeability increasing protein (BPI), myeloperoxidase (MPO) and proteinase 3 (PRTN3) are significantly reduced (p = 0.0133, 0.002, and 0.0022, respectively) (46). Although further research is needed to confirm the influence of the local microenvironment of breast tissue, these findings demonstrated a significant decrease in antimicrobial responses in breast tumour tissue (43).
However, the meta-analysis study has some limitations. First, several matrices for detecting alpha diversity were utilised in different studies, and there are probably no standardised tools for measurement. Therefore, only a few studies were able to analyse quantitatively. Second, some mean values cannot be found in the papers and supplementary files, and the email contacts for 2 weeks were reachable only to some; perhaps the contacts were changed, or the data were not archived for a long period. Therefore, qualitative analysis was applied to the data provided for the articles.
In general, our meta-analysis suggests the fecal, tumour, or oral microbiome profile of breast cancer patients, differences in microbiota abundance by menopausal status, menarche and cancer stages, and the change in the microbial pattern before and after chemotherapy. However, the microbiome investigation is still in its infancy for breast cancer patients, and the sample size is normally limited due to high sequencing costs. Therefore, more studies with a larger cohort of patients would be required to identify the biological and pathological significance of the findings in the meta-analysis. We expected that the review could fill the gap linking to better understand the connection between breast cancer and the microbiome.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
KP and MT contributed for the conceptualization of the study. MT, TN, and KP conducted the methodology. KP and MT applied the software for data visualization. MT, TN, and KC performed the investigation throughout the study. TN, MT, and KP made the formal analysis. MT, KC, TN completed the data curation. MT, and KP prepared the original draft writing. For review and editing, MT, and KP emphasized on it. NH, and KP involved for supervision. KP, and NH worked on the project administration. All authors contributed to the article and approved the submitted version.
MT was supported by the Graduate Scholarship Programme for ASEAN or Non-ASEAN Countries, Chulalongkorn University. KP was funded by the Second Century Fund, Chulalongkorn University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1144021/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 7(3):209–49. doi: 10.3322/caac.21660
2. Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. Lancet (2021) 397(10286):1750–69. doi: 10.1016/S0140-6736(20)32381-3
3. Dashti SG, Simpson JA, Karahalios A, Viallon V, Moreno-Betancur M, Gurrin LC, et al. Adiposity and estrogen receptor-positive, postmenopausal breast cancer risk: Quantification of the mediating effects of fasting insulin and free estradiol. Int J Cancer (2020) 146(6):1541–52. doi: 10.1002/ijc.32504
4. Nyante SJ, Gammon MD, Kaufman JS, Bensen JT, Lin DY, Barnholtz-Sloan JS, et al. Genetic variation in estrogen and progesterone pathway genes and breast cancer risk: an exploration of tumor subtype-specific effects. Cancer Causes Control (2015) 26(1):121–31. doi: 10.1007/s10552-014-0491-2
5. Bogdanova N, Helbig S, Dork T. Hereditary breast cancer: ever more pieces to the polygenic puzzle. Hered Cancer Clin Pract (2013) 11(1):12. doi: 10.1186/1897-4287-11-12
6. Flores R, Shi J, Fuhrman B, Xu X, Veenstra TD, Gail MH, et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med (2012) 10:253. doi: 10.1186/1479-5876-10-253
7. Hussain T, Murtaza G, Kalhoro DH, Kalhoro MS, Metwally E, Chughtai MI, et al. Relationship between gut microbiota and host-metabolism: Emphasis on hormones related to reproductive function. Anim Nutr (2021) 7(1):1–10. doi: 10.1016/j.aninu.2020.11.005
8. Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med (2018) 24(4):392–400. doi: 10.1038/nm.4517
9. Sepsis Lung Microbiome Study G. Could lung bacterial dysbiosis predict ICU mortality in patients with extra-pulmonary sepsis? A proof-of-concept study. Intensive Care Med (2020) 46(11):2118–20. doi: 10.1007/s00134-020-06190-4
10. Zhuang H, Cheng L, Wang Y, Zhang YK, Zhao MF, Liang GD, et al. Dysbiosis of the gut microbiome in lung cancer. Front Cell Infect Microbiol (2019) 9:112. doi: 10.3389/fcimb.2019.00112
11. Sekyere JO, Maningi NE, Matukane SR, Mbelle NM, Fourie PB. An Oxford nanopore-based characterisation of sputum microbiota dysbiosis in patients with tuberculosis: from baseline to 7 days after antibiotic treatment. medRxiv (2021).
12. Laborda-Illanes A, Sanchez-Alcoholado L, Dominguez-Recio ME, Jimenez-Rodriguez B, Lavado R, Comino-Mendez I, et al. Breast and gut microbiota action mechanisms in breast cancer pathogenesis and treatment. Cancers (Basel) (2020) 12(9):2465–92. doi: 10.3390/cancers12092465
13. Urbaniak C, Cummins J, Brackstone M, Macklaim JM, Gloor GB, Baban CK, et al. Microbiota of human breast tissue. Appl Environ Microbiol (2014) 80(10):3007–14. doi: 10.1128/AEM.00242-14
14. Banerjee S, Tian T, Wei Z, Shih N, Feldman MD, Peck KN, et al. Distinct microbial signatures associated with different breast cancer types. Front Microbiol (2018) 9:951. doi: 10.3389/fmicb.2018.00951
15. Zhu J, Liao M, Yao Z, Liang W, Li Q, Liu J, et al. Breast cancer in postmenopausal women is associated with an altered gut metagenome. Microbiome (2018) 6(1):136. doi: 10.1186/s40168-018-0515-3
16. Tzeng A, Sangwan N, Jia M, Liu C-C, Keslar KS, Downs-Kelly E, et al. Human breast microbiome correlates with prognostic features and immunological signatures in breast cancer. Genome Med (2021) 13(1):60. doi: 10.1186/s13073-021-00874-2
17. Wang H, Altemus J, Niazi F, Green H, Calhoun BC, Sturgis C, et al. Breast tissue, oral and urinary microbiomes in breast cancer. Oncotarget (2017) 8(50):88122–38. doi: 10.18632/oncotarget.21490
18. Thompson KJ, Ingle JN, Tang X, Chia N, Jeraldo PR, Walther-Antonio MR, et al. A comprehensive analysis of breast cancer microbiota and host gene expression. PloS One (2017) 12(11):e0188873. doi: 10.1371/journal.pone.0188873
19. Huang YF, Chen YJ, Fan TC, Chang NC, Midha MK, Chen TH, et al. Analysis of microbial sequences in plasma cell-free DNA for early-onset breast cancer patients and healthy females. BMC Med Genomics (2018) 11(Suppl 1):16. doi: 10.1186/s12920-018-0329-y
20. Kovacs T, Miko E, Vida A, Sebo E, Toth J, Csonka T, et al. Cadaverine, a metabolite of the microbiome, reduces breast cancer aggressiveness through trace amino acid receptors. Sci Rep (2019) 9(1):1300. doi: 10.1038/s41598-018-37664-7
21. Al-Ansari MM, AlMalki RH, Dahabiyeh LA, Abdel Rahman AM. Metabolomics-microbiome crosstalk in the breast cancer microenvironment. Metabolites. (2021) 11(11):758–70. doi: 10.3390/metabo11110758
22. Miko E, Kovacs T, Sebo E, Toth J, Csonka T, Ujlaki G, et al. Microbiome-microbial metabolome-cancer cell interactions in breast cancer-familiar, but unexplored. Cells (2019) 8(4):293–326. doi: 10.3390/cells8040293
23. Sui Y, Wu J, Chen J. The role of gut microbial beta-glucuronidase in estrogen reactivation and breast cancer. Front Cell Dev Biol (2021) 9:631552. doi: 10.3389/fcell.2021.631552
24. Mirzaei R, Afaghi A, Babakhani S, Sohrabi MR, Hosseini-Fard SR, Babolhavaeji K, et al. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. BioMed Pharmacother (2021) 139:111619. doi: 10.1016/j.biopha.2021.111619
25. Režen T, Rozman D, Kovács T, Kovács P, Sipos A, Bai P, et al. The role of bile acids in carcinogenesis. Cell Mol Life Sci (2022) 79(5):243. doi: 10.1007/s00018-022-04278-2
26. Durack J, Lynch SV. The gut microbiome: Relationships with disease and opportunities for therapy. J Exp Med (2019) 216(1):20–40. doi: 10.1084/jem.20180448
27. Rashidi A, Ebadi M, Rehman TU, Elhusseini H, Nalluri H, Kaiser T, et al. Gut microbiota response to antibiotics is personalized and depends on baseline microbiota. Microbiome. (2021) 9(1):211. doi: 10.1186/s40168-021-01170-2
28. Bose M, Mukherjee P. Role of microbiome in modulating immune responses in cancer. Mediators Inflamm (2019) 2019:4107917. doi: 10.1155/2019/4107917
29. Terrisse S, Derosa L, Iebba V, Ghiringhelli F, Vaz-Luis I, Kroemer G, et al. Intestinal microbiota influences clinical outcome and side effects of early breast cancer treatment. Cell Death Differ (2021) 28(9):2778–96. doi: 10.1038/s41418-021-00784-1
30. Dieleman S, Aarnoutse R, Ziemons J, Kooreman L, Boleij A, Smidt M. Exploring the potential of breast microbiota as biomarker for breast cancer and therapeutic response. Am J Pathol (2021) 191(6):968–82. doi: 10.1016/j.ajpath.2021.02.020
31. Banerjee S, Wei Z, Tian T, Bose D, Shih NNC, Feldman MD, et al. Prognostic correlations with the microbiome of breast cancer subtypes. Cell Death Dis (2021) 12(9):831. doi: 10.1038/s41419-021-04092-x
32. Nougayrede JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science (2006) 313(5788):848–51. doi: 10.1126/science.1127059
33. Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating e-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe (2013) 14(2):195–206. doi: 10.1016/j.chom.2013.07.012
34. Wang TC, Goldenring JR, Dangler C, Ito S, Mueller A, Jeon WK, et al. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with helicobacter felis infection. Gastroenterology (1998) 114(4):675–89. doi: 10.1016/S0016-5085(98)70581-5
35. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ (2021) 372:n160. doi: 10.1136/bmj.n160
36. Huang X, Lin J, Demner-Fushman D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu Symp Proc (2006) 2016:359–63.
37. Morgan RL, Whaley P, Thayer KA, Schunemann HJ. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int (2018) 121(Pt 1):1027–31. doi: 10.1016/j.envint.2018.07.015
38. Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ (2016) 355:i4919. doi: 10.1136/bmj.i4919
39. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. (2021). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
40. Higgins JPT, Altman DG JACS. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, MS. C, editors. Cochrane handbook for systematic reviews of interventions. version 5.2.0. Cochrane (2017). Available at: https://training.cochrane.org/handbook.
41. Bertazzoni Minelli E, Beghini AM, Vesentini S, Marchiori L, Nardo G, Cerutti R, et al. Intestinal microflora as an alternative metabolic source of estrogens in women with uterine leiomyoma and breast cancer. NY Acad Sci (1990). doi: 10.1111/j.1749-6632.1990.tb34337.x
42. Benini A, Bertazzoni EM, Vesentini S, Marchiori L, Nardo G. Intestinal ecosystem and metabolic capacity in women with breast cancer. Pharmacol Res (1992) 25:184–5. doi: 10.1016/1043-6618(92)90352-C
43. Xuan C, Shamonki JM, Chung A, Dinome ML, Chung M, Sieling PA, et al. Microbial dysbiosis is associated with human breast cancer. PloS One (2014) 9(1):e83744. doi: 10.1371/journal.pone.0083744
44. Goedert JJ, Jones G, Hua X, Xu X, Yu G, Flores R, et al. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: a population-based case-control pilot study. J Natl Cancer Inst (2015) 107(8):184–5. doi: 10.1093/jnci/djv147
45. Goedert JJ, Hua X, Bielecka A, Okayasu I, Milne GL, Jones GS, et al. Postmenopausal breast cancer and oestrogen associations with the IgA-coated and IgA-noncoated faecal microbiota. Br J Cancer (2018) 118(4):471–9. doi: 10.1038/bjc.2017.435
46. Banerjee S, Wei Z, Tan F, Peck KN, Shih N, Feldman M, et al. Distinct microbiological signatures associated with triple negative breast cancer. Sci Rep (2015) 5:15162. doi: 10.1038/srep15162
47. Urbaniak C, Gloor GB, Brackstone M, Scott L, Tangney M, Reid G. The microbiota of breast tissue and its association with breast cancer. Appl Environ Microbiol (2016) 82(16):5039–48. doi: 10.1128/AEM.01235-16
48. Hieken TJ, Chen J, Hoskin TL, Walther-Antonio M, Johnson S, Ramaker S, et al. The microbiome of aseptically collected human breast tissue in benign and malignant disease. Sci Rep (2016) 6:30751. doi: 10.1038/srep30751
49. Smith A, Pierre JF, Makowski L, Tolley E, Lyn-Cook B, Lu L, et al. Distinct microbial communities that differ by race, stage, or breast-tumor subtype in breast tissues of non-Hispanic black and non-Hispanic white women. Sci Rep (2019) 9(1):11940. doi: 10.1038/s41598-019-48348-1
50. Ma J, Sun L, Liu Y, Ren H, Shen Y, Bi F, et al. Alter between gut bacteria and blood metabolites and the anti-tumor effects of faecalibacterium prausnitzii in breast cancer. BMC Microbiol (2020) 20(1):82. doi: 10.1186/s12916-020-01751-2
51. Klann E, Williamson JM, Tagliamonte MS, Ukhanova M, Asirvatham JR, Chim H, et al. Microbiota composition in bilateral healthy breast tissue and breast tumors. Cancer Causes Control (2020) 31(11):1027–38. doi: 10.1007/s10552-020-01338-5
52. Uzan-Yulzari A, Morr M, Tareef-Nabwani H, Ziv O, Magid-Neriya D, Armoni R, et al. The intestinal microbiome, weight, and metabolic changes in women treated by adjuvant chemotherapy for breast and gynecological malignancies. BMC Med (2020) 18(1):281. doi: 10.1186/s12916-020-01751-2
53. He C, Liu Y, Ye S, Yin S, Gu J. Changes of intestinal microflora of breast cancer in premenopausal women. Eur J Clin Microbiol Infect Dis (2021) 40(3):503–13. doi: 10.1007/s10096-020-04036-x
54. Byrd DA, Vogtmann E, Wu Z, Han Y, Wan Y, Clegg-Lamptey JN, et al. Associations of fecal microbial profiles with breast cancer and nonmalignant breast disease in the Ghana breast health study. Int J Cancer (2021) 148(11):2712–23. doi: 10.1002/ijc.33473
55. Luu TH, Michel C, Bard JM, Dravet F, Nazih H, Bobin-Dubigeon C. Intestinal proportion of blautia sp. is associated with clinical stage and histoprognostic grade in patients with early-stage breast cancer. Nutr Cancer (2017) 69(2):267–75. doi: 10.1080/01635581.2017.1263750
56. Meng S, Chen B, Yang J, Wang J, Zhu D, Meng Q, et al. Study of microbiomes in aseptically collected samples of human breast tissue using needle biopsy and the potential role of in situ tissue microbiomes for promoting malignancy. Front Oncol (2018) 8:318. doi: 10.3389/fonc.2018.00318
57. Costantini L, Magno S, Albanese D, Donati C, Molinari R, Filippone A, et al. Characterization of human breast tissue microbiota from core needle biopsies through the analysis of multi hypervariable 16S-rRNA gene regions. Sci Rep (2018) 8(1):16893. doi: 10.1038/s41598-018-35329-z
58. Shi J, Geng C, Sang M, Gao W, Li S, Yang S, et al. Effect of gastrointestinal microbiome and its diversity on the expression of tumor-infiltrating lymphocytes in breast cancer. Oncol Lett (2019) 17(6):5050–6. doi: 10.3389/fonc.2018.00318
59. Yoon HJ, Kim HN, Bang JI, Lim W, Moon BI, Paik NS, et al. Physiologic intestinal (18)F-FDG uptake is associated with alteration of gut microbiota and proinflammatory cytokine levels in breast cancer. Sci Rep (2019) 9(1):18273. doi: 10.1038/s41598-019-54680-3
60. Thyagarajan S, Zhang Y, Thapa S, Allen MS, Phillips N, Chaudhary P, et al. Comparative analysis of racial differences in breast tumor microbiome. Sci Rep (2020) 10(1):14116. doi: 10.1038/s41598-020-71102-x
61. Di Modica M, Gargari G, Regondi V, Bonizzi A, Arioli S, Belmonte B, et al. Gut microbiota condition the therapeutic efficacy of trastuzumab in HER2-positive breast cancer. Cancer Res (2021) 81(8):2195–206. doi: 10.1158/0008-5472.CAN-20-1659
62. Yao ZW, Zhao BC, Yang X, Lei SH, Jiang YM, Liu KX. Relationships of sleep disturbance, intestinal microbiota, and postoperative pain in breast cancer patients: a prospective observational study. Sleep Breath (2021) 25(3):1655–64. doi: 10.1007/s11325-020-02246-3
63. Napenas JJ, Brennan MT, Coleman S, Kent ML, Noll J, Frenette G, et al. Molecular methodology to assess the impact of cancer chemotherapy on the oral bacterial flora: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod (2010) 109(4):554–60. doi: 10.1016/j.tripleo.2009.11.015
64. Fruge AD, van der Pol W, Rogers LQ, Morrow CD, Tsuruta Y, Demark-Wahnefried W. Fecal akkermansia muciniphila is associated with body composition and microbiota diversity in overweight and obese women with breast cancer participating in a presurgical weight loss trial. J Acad Nutr Diet (2020) 120(4):650–9. doi: 10.1016/j.jand.2018.08.164
65. Chiba A, Bawaneh A, Velazquez C, Clear KYJ, Wilson AS, Howard-McNatt M, et al. Neoadjuvant chemotherapy shifts breast tumor microbiota populations to regulate drug responsiveness and the development of metastasis. Mol Cancer Res (2020) 18(1):130–9. doi: 10.1158/1541-7786.MCR-19-0451
66. Wu AH, Tseng C, Vigen C, Yu Y, Cozen W, Garcia AA, et al. Gut microbiome associations with breast cancer risk factors and tumor characteristics: A pilot study. Breast Cancer Res Treat (2020) 182(2):451–63. doi: 10.1007/s10549-020-05702-6
67. Guan X, Ma F, Sun X, Li C, Li L, Liang F, et al. Gut microbiota profiling in patients with HER2-negative metastatic breast cancer receiving metronomic chemotherapy of capecitabine compared to those under conventional dosage. Front Oncol (2020) 10:902. doi: 10.3389/fonc.2020.00902
68. Thukral AK. A review on measurement of alpha diversity in biology. Agric Res J (2017) 54(1):10. doi: 10.5958/2395-146X.2017.00001.1
69. Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature (2010) 464(7285):59–65. doi: 10.1038/nature08821
70. Cazzaniga M, Zonzini GB, Di Pierro F, Moricoli S, Bertuccioli A. Gut microbiota, metabolic disorders and breast cancer: Could berberine turn out to be a transversal nutraceutical tool? A narrative analysis. Int J Mol Sci (2022) 23(20):12538. doi: 10.3390/ijms232012538
71. Rea D, Coppola G, Palma G, Barbieri A, Luciano A, Del Prete P, et al. Microbiota effects on cancer: from risks to therapies. Oncotarget (2018) 9(25):17915–27. doi: 10.18632/oncotarget.24681
72. Zitvogel L, Ma Y, Raoult D, Kroemer G, Gajewski TF. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science (2018) 359(6382):1366–70. doi: 10.1126/science.aar6918
73. Peters BA, Santoro N, Kaplan RC, Qi Q. Spotlight on the gut microbiome in menopause: Current insights. Int J Womens Health (2022) 14:1059–72. doi: 10.2147/IJWH.S340491
Keywords: breast cancer, dysbiosis, gut microbiome, microbial diversity, estrogen
Citation: Thu MS, Chotirosniramit K, Nopsopon T, Hirankarn N and Pongpirul K (2023) Human gut, breast, and oral microbiome in breast cancer: A systematic review and meta-analysis. Front. Oncol. 13:1144021. doi: 10.3389/fonc.2023.1144021
Received: 13 January 2023; Accepted: 02 March 2023;
Published: 17 March 2023.
Edited by:
Ira Ida Skvortsova, Innsbruck Medical University, AustriaReviewed by:
Sona Ciernikova, Slovak Academy of Sciences, SlovakiaCopyright © 2023 Thu, Chotirosniramit, Nopsopon, Hirankarn and Pongpirul. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Krit Pongpirul, ZG9jdG9ya3JpdEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.