- 1Division of Oncology, Department of Internal Medicine I, University Hospital St. Pölten, St. Pölten, Austria
- 2Karl Landsteiner University of Health Sciences, Krems, Austria
- 3Karl Landsteiner Institute for Oncology and Nephrology, St. Pölten, Austria
- 4Center for Cancer Research, Medical University of Vienna, Vienna, Austria
- 5Comprehensive Cancer Center Vienna, Medical University of Vienna, Vienna, Austria
- 6Medical University Vienna, Department of Medicine I, Division of Oncology, Vienna, Austria
- 7Department of Pulmonology, Klinik Penzing, Vienna, Austria
- 8Division of Oncology, Department of Internal Medicine II, University Hospital Krems, Krems, Austria
Introduction: Metastatic biliary tract cancer (BTC) is a rare and aggressive entity associated with poor prognosis. It represents a major challenge for adequate treatment strategies. In recent years, BTC has become a model for precision medicine in gastrointestinal oncology. Therefore, the analysis of the individual molecular profile in BTC patients may lead to targeted therapies for the benefit of patients.
Methods: In this Austrian, tricentric, real-world, retrospective analysis, we investigated patients diagnosed with metastatic BTC who underwent molecular profiling between 2013 and 2022.
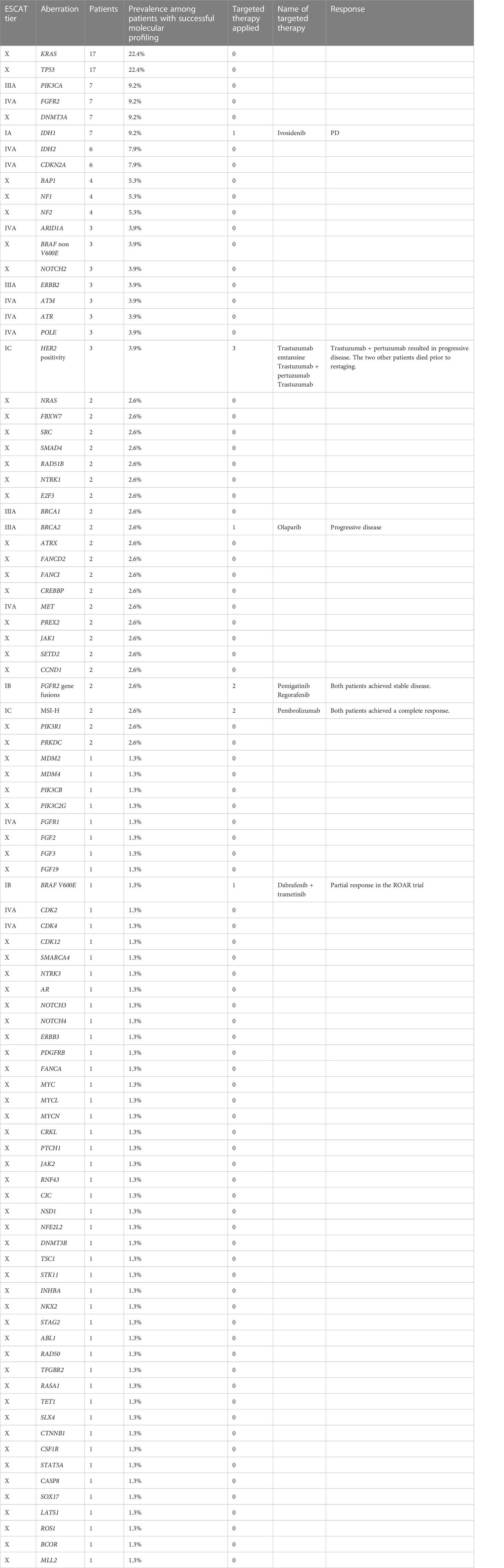
Results: In total, 92 patients were identified in this tricentric analysis and 205 molecular aberrations, including 198 mutations affecting 89 different genes in 61 patients were found. The predominant mutations were in KRAS (n=17; 22.4%), TP53 (n=17; 22.4%), PIK3CA (n=7; 9.2%), FGFR2 (n=7; 9.2%), DNMT3A (n=7; 9.2%), IDH1 (n=7; 9.2%), IDH2 (n=6; 7.9%), CDKN2A (n=6; 7.9%), BAP1 (n=4; 5.3%), NF1 (n=4; 5.3%), and NF2 (n=4; 5.3%). Three patients had HER2 amplification. MSI-H status and FGFR2 fusion genes were each observed in two different patients. One patient had a BRAF V600E mutation. Eventually, 10 patients received targeted therapy, of whom one-half derived clinical benefit.
Conclusions: Molecular profiling of BTC patients is implementable in routine clinical practice and should be regularly employed to detect and exploit molecular vulnerabilities.
Introduction
Biliary tract cancer (BTC) is a highly malignant and fatal cancer that arises from the biliary epithelium in the bile duct, gallbladder, or ampulla of Vater. BTC is a molecularly heterogeneous entity encompassing several subentities: gallbladder carcinoma, distal cholangiocarcinoma (CCA), perihilar CCA, intrahepatic CCA (iCCA), and ampullary carcinoma (1, 2). Each of these subtypes has a distinct molecular signature, highlighting the high spatial heterogeneity of this disease group (3, 4). BTC is a relatively rare cancer, with an incidence of about 2/100,000 in the Western world (5); however, its incidence is clearly increasing. Ouyang et al. investigated the burden of BTC in 195 countries between 1990 and 2017 and reported that the incidence of BTC increased by 76%, mortality increased by 65%, and disability-adjusted life-years increased by 52% from 1990 to 2017 (6). BTC is an aggressive malignancy that causes non-specific symptoms. Therefore, this entity is often diagnosed in the advanced stages. Due to late manifestation of symptomatology, paucity of effective treatments, molecular diversity, and poor understanding of its complex molecular mechanisms and pathways, advanced BTC has a dismal prognosis, with a 5-year survival rate of only 2% (2, 4, 7–9).
For many years, systemic chemotherapy was the mainstay of BTC treatment. Recently, the clinical TOPAZ-1 phase 3 trial demonstrated a mild but statistically significant improvement by the addition of durvalumab to cisplatin plus gemcitabine in first line setting, independent of the primary tumor location. The immunochemotherapy led to significantly increased mPFS (7.2 versus 5.7 months, HR 0.75 [0.63–0.89]) and mOS (12.9 versus 11.3 months, HR 0.76 [0.64–0.91]) and higher OS rate at 24 months, with an improvement of 12.1% (23.6% vs 11.5% estimated OS at 24 months) (10). For second line treatment, Lamarca et al. introduced the FOLFOX regime (consisting of folinic acid, 5-fluorouracil, and oxaliplatin), which was evaluated in the ABC-06 phase 3 trial (11).
In recent years, the emergence of next-generation sequencing (NGS), consecutive identification of molecular aberrations, and the development of molecular-guided targeted therapies has heralded the era of precision oncology or molecular oncology.
BTC, particularly small duct iCCA, has evolved as a model disease for molecular oncology, as this entity harbors numerous druggable molecular aberrations. Currently, the targeted drug ivosidenib against IDH1 mutation targeted drugs is approved by both the Food and Drug Administration (FDA). Pemigatinib directed against FGFR2 fusions and rearrangements and pembrolizumab indicated for tumors with microsatellite instability-high (MSI-H) or deficient mismatch repair are approved by both FDA and European Medicines Agency (EMA) (12, 13). Other targetable molecular lesions include BRAF V600E, HER2 positivity, and NTRK fusions (14). Therefore, molecular profiling is essential for modern-day therapeutic management of BTC, especially after failure of first line treatment, and it is recommended by both ESMO and the and the American Society of Clinical Oncology (ASCO).
In this real-world study, we sought to map the molecular profiles of metastatic BTC cases and to specifically target the detected molecular alterations.
Materials and methods
Patients and design of the precision medicine platform
We conducted retrospective analysis of 92 patients with metastatic BTC who underwent molecular profiling at three main Austrian centers: the Medical University of Vienna, the University Hospital St. Poelten, and the University Hospital Krems. Patients needed to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Furthermore, the Institutional Ethics Committee of both Austrian centers approved this analysis (Number 1099/2021). 1
Cancer gene panel sequencing
DNA was isolated from paraffin-embedded tissue samples using a QIAamp Tissue Kit™ (Qiagen, Hilden, Germany). From each specimen, 10 ng DNA was used for sequencing. In selected cases for which tissue samples were unavailable, liquid biopsy was performed. The genetic profile was generated via Ion AmpliSeq Cancer Hotspot Panel v2 (Thermo Fisher Scientific, Waltham, MA, USA), Ion AmpliSeq™ Cancer Hotspot Panel v3 (Thermo Fisher Scientific, Waltham, MA, USA), Oncomine™ Comprehensive Assay v3 (Thermo Fisher Scientific, Waltham, MA, USA) NGS panel, TruSight Oncology 500 Assay (Illumina, San Diego, CA, USA), Oncomine™ Precision Assay GX (Thermo Fisher Scientific, Waltham, MA, USA), FoundationOne CDx (Foundation Medicine, Cambridge, MA, USA), and FoundationOne Liquid CDx (Foundation Medicine, Cambridge, MA, USA).
The FoundationOne Liquid CDx assay was performed using circulating cell- free DNA (cfDNA) isolated from plasma derived from anti-coagulated peripheral whole blood from patients with solid malignant neoplasms. The assay employed a single DNA extraction method to obtain cfDNA from plasma from whole blood. Extracted cfDNA underwent whole-genome shotgun library construction and hybridization- based capture of 324 cancer-related genes.
In this work, the genetic aberrations were ranked and rated according to the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT) to objectify their value as clinical targets based on the available strength of evidence (15, 16).
Immunohistochemistry
Immunohistochemistry was performed using 2-μm-thin tissue sections read by a Ventana Benchmark Ultra Stainer (Ventana, Tucson, Arizona, USA). The following antibody was applied: programmed death-ligand 1 (PD-L1) (clone E1L3N; Cell Signaling Technology).
The immunohistochemical staining intensity for HER2 was scored from 0 to 3+ (0 = negative, 1+ = negative, 2+ = positive, 3+ = positive) according to the scoring guidelines of the Dako HercepTest™ (Agilent Technologies, Vienna, Austria). In the case of HER2 2+, further testing with HER2 in situ hybridization was performed to verify the HER2 gene amplification.
For PD-L1, the tumor proportion score was calculated, which is the percentage of viable malignant cells with membrane staining.
The presence of MSI was assessed using the MSI Analysis System Version 1.1 (Promega Corporation, Madison, Wisconsin, USA).
Descriptive statistics
For data description, we used measures of central tendency, including the mean and median. Furthermore, we used the method of frequency distribution to delineate the characteristics of the BTC patients.
Results
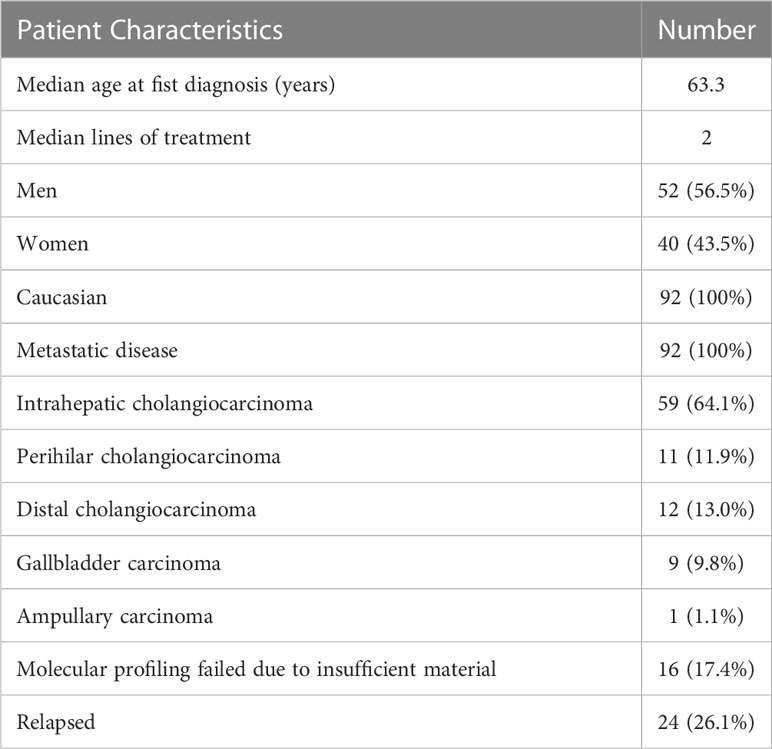
In this tricentric analysis of the main Austrian centers Medical University of Vienna, University Hospital St. Poelten, and University Hospital Krems, the molecular profiles of 92 patients with metastatic BTC between June 2013 and January 2022 were evaluated. All patients were Caucasian, and the cohort comprised 52 men and 40 women.
The most common BTC subentity was iCCA, which was diagnosed in 59 patients (64.1%), followed by extrahepatic (distal and perihilar) CCA (n=23; 24.9%), gallbladder carcinoma (n=9; 9.8%) and ampullary carcinoma (n=1; 1.1%). The histological subtype in all patients was adenocarcinoma. The median age at first diagnosis was 63.3 years, ranging from 34.9 to 86.3 years. Twenty-four patients had a relapsed BTC. All the patients had distant metastases, including 60 patients with liver metastases, 19 patients with osseous metastases, 21 patients with peritoneal carcinomatosis, and four patients with pleural carcinomatosis. Metastases to the spleen, brain, and ovaries were reported in one patient each (Table 1).
In total, we identified 205 molecular aberrations, including 198 mutations affecting 89 different genes in 61 patients. The predominant mutations were in KRAS (n=17; 22.4%), TP53 (n=17; 22.4%), PIK3CA (n=7; 9.2%), FGFR2 (n=7; 9.2%), DNMT3A (n=7; 9.2%), IDH1 (n=7; 9.2%), IDH2 (n=6; 7.9%), CDKN2A (n=6; 7.9%), BAP1 (n=4; 5.3%), NF1 (n=4; 5.3%), and NF2 (n=4; 5.3%). These mutations accounted for 43.7% of all the mutations. BRAF mutations were observed in four patients, including one BRAF V600E mutation in a patient who was subsequently enrolled in the ROAR phase 2 trial (17).
None of the patients harbored the KRAS G12C mutation. MSI-H status and FGFR2 fusion genes (FGFR2::OFD1 and FGFR2::DDX21) were each observed in two different patients. Both patients with MSI-H status also had tumor mutational burden-high (TMB-H). All IDH1 and IDH2 mutations and both FGFR2 fusions genes were found in iCCA patients. HER2 positivity was reported in three patients, of whom two patients had gallbladder carcinoma. The aberrations were categorized according to the ESCAT classification (see Table 2 for the complete list). Four patients underwent molecular analysis via liquid biopsy.
No mutations were detected in 15 patients, including 9 patients with iCCA, 6 patients with pCCA, 1 patient with gallbladder carcinoma and 1 patient with ampullary carcinoma.
2Molecular profiling failed in 16 (17.4%) patients due to lacking or insufficient material. Based on the current ESCAT classification, 19 patients had a targetable molecular aberration with at least ESCAT III tier, representing 25% of all patients (n=76) with a successful molecular analysis (Table 2).
In more than three-fourths (n=69) of the patients, molecular profiling was performed after failure of standard treatments. The median turnaround time in these patients from the decision to perform molecular profiling to the initiation of the targeted therapy was 49 days.
Seventy-six (82.3%) patients received a platinum-based chemotherapy in the first line. The median applied lines of treatment were two (ranging from one to five).
Eventually, 10 patients received targeted therapy based on the individual molecular profile. The patient harboring the BRAF V600E mutation achieved partial response under dabrafenib plus trametinib in the previously published phase 2 ROAR basket trial (17).
Both patients with MSI-H status treated with pembrolizumab achieved complete response. Pembrolizumab treatment is still ongoing in one patient at the time of publication of this report; in the other patient, pembrolizumab treatment was terminated, and he is receiving oncological aftercare.
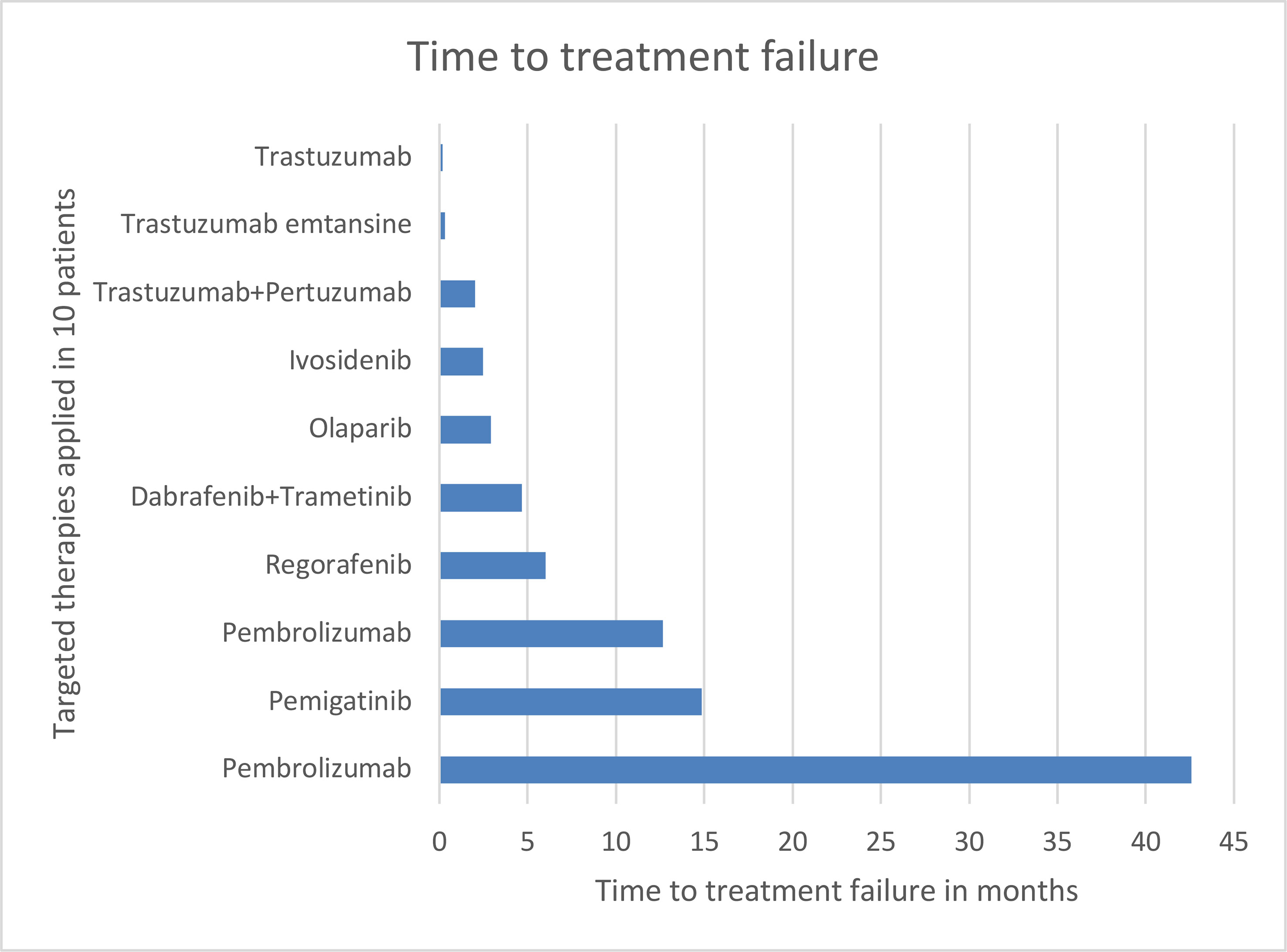
One patient with FGFR2::OFD1 gene fusion was treated with pemigatinib and achieved stable disease. The other patient with FGFR2::DDX21 continues to receive first line treatment. The patient with FGFR2 mutation received regorafenib and achieved stable disease. None of the patients with HER2 amplification with a HER2 score of 3+ who were treated with an anti-HER2 directed therapy achieved a clinical benefit. Olaparib was administered to a patient with BRCA2 mutation; however, he did not respond. Ivosidenib was used to treat an iCCA patient with an IDH1 mutation; however, he experienced progressive disease. See Figure 1 for time to treatment failure.
Discussion
In this retrospective, tricentric, real-world analysis, we examined the molecular profiles of 92 patients with BTC. Our work demonstrated that molecular profiling is feasible and implementable in routine clinical practice at university hospitals. We found 198 mutations affecting 89 different genes, which reflects the molecular heterogeneity of BTC. This finding is consistent with the well-described extreme and complex intratumoral heterogeneity of BTC that occurs within the same tumor tissue; vascularization, proliferation, and subclones are all known to be highly variable. The pattern of genetic and epigenetic aberrations varies both spatially and temporally. Tumor biology at metastatic sites is different from the primary site and varies in a relapse (4, 9, 18–23). The mutations identified in this analysis are in line with previous reports. All IDH1 mutations and FGFR2 fusions were found in iCCA. A growing body of evidence reports that the genomic landscape of BTC differs with the location of the carcinoma. Intrahepatic CCA is frequently associated with genomic aberrations in IDH1, IDH2, and BAP1, while perihilar and distal CCA are frequently associated with mutations in KRAS, TP53, and SMAD4 (24–29). Mutations in KRAS, TP53, APC, SMAD4 are frequently seen in ampullary carcinoma (30). HER2 amplifications are mainly found gallbladder carcinoma (31). In our multicentric cohort, three patients displayed HER2 positivity, of whom two patients had gallbladder carcinoma. Based on the current ESCAT classification, 19 patients had a targetable molecular aberration with at least ESCAT III tier, which represents 25% of all patients with a successful molecular analysis. This result underscores that BTC is a target-rich disease (24).
Ten patients received targeted therapy. The drugs were carefully selected for individualized treatment, with consideration of the patient’s molecular information, clinical and treatment history, performance status, comorbidities, and concomitant therapies.
Both patients with MSI-H status were treated with pembrolizumab and achieved complete response, demonstrating a deep and durable clinical benefit. MSI-H is a predictive marker for immunotherapy and is found in less than 5% of BTC patients (32). The multicohort phase 2 trial KEYNOTE-158 included 22 pretreated BTC patients with MSI-H who were treated with pembrolizumab (33). An ORR of 40.9% was achieved. The mPFS and mOS were 4.2 and 24.3 months, respectively. Median DOR was not reached (34). Based on these results, pembrolizumab received the first tumor tissue-agnostic approval from the FDA for the treatment of MSI-H-positive solid tumors in 2017 (35). Recently, the EMA granted approval for this treatment for MSI-H-positive BTC patients who have disease progression during or following at least one prior therapy.
The promising results emphasize the importance of determining the status of microsatellite stability alongside genetic testing.
Although our analysis showed that molecular profiling is implementable in routine clinical practice, only five patients derived a clinical benefit through this approach. There are several reasons for this modest outcome. One reason may be the relatively long median turnaround time of nearly 50 days from the decision to perform molecular profiling after failure of all standard therapies to the initiation of the targeted therapy in patients for whom there was no effective therapy for a highly aggressive cancer. This might explain why two patients who received anti-HER2 therapy died prior to restaging; the targeted therapy was initiated too late to exhibit its full antitumoral potential.
Upfront testing would be reasonable to bridge the turnaround time while the patient is under treatment. In addition, liquid biopsy may be a viable option as it supersedes the need for conventional biopsy, which prolongs the turnaround time due to the need to organize inpatient admission and is associated with intervention-related complications. Therefore, liquid biopsy may help to reduce turnaround time, monitor the disease, and assess the response to therapy (36). Another reason for the modest response may be the complexity of tumor biology, reflected in part by the high degree of heterogeneity of BTC (3, 37, 38).
The molecular profiles of the BTC patients were collected between 2013 and 2022. For a large part of this period, the clinical actionability of the identified molecular lesions were not ranked or standardized. It was not until 2018 that ESCAT tiers to rank and classify the targetable aberrations. Certain molecular alterations were initially not targetable and have only recently become druggable. A prime example is IDH1 mutation. The phase 3 ClarIDHy trial, published in 2020, demonstrated the clinical benefits of ivosidenib in IDH1-mutant, chemotherapy-refractory BTC (27, 39). Seven patients in our analysis were IDH1 mutants; however, only one patient received molecular-guided therapy with ivosidenib since this mutation was identified at a time when ivosidenib was accessible through a compassionate use program. This example demonstrates the advances in precision and molecular oncology in the field of BTC (40).
According to the current ESCAT classification, 19 patients had a targetable molecular aberration with at least ESCAT III tier, representing 25% of all patients in our cohort with a successful molecular analysis. Thus, molecular profiling strongly informs the clinical decision finding, particularly after the failure of the-first line therapy. In future, more and more BTC patients will benefit from molecular profiling and precision oncology due to three main reasons:
Firstly, biomarkers such as IDH1 and FGFR2 fusions are currently investigated in clinical trials in first-line settings which means that - in case that the trials meet the endpoint – the patients will receive front-up a targeted therapy (41–43). The second reason is that new predictive biomarkers are tested in different trials, including biomarkers for predicting the effectiveness of immunotherapies, such as ATM, ATR, BRCA1, BRCA2, FANCA, and POLE (44, 45). Last, but not least, new emergent therapies will likely be a gamechanger in the BTC management, particularly KRAS inhibitors (46, 47).
Another reason for the modest response in our study is the remarkable percentage (17.4%) of failure of molecular profiling due to insufficient material, which is comparable with the percentage reported by Lamarca et al. (48).
These explanations highlight the importance of performing upfront testing in all BTC patients, as in case of failure, early testing would allow enough time to re-biopsy the patient to collect sufficient material for repeat testing. Further, upfront testing now has a therapeutic consequence as it impacts therapy sequencing after failure of the first line therapy (48).
This study has an important limitation: it was a non-randomized, retrospective analysis of patients without an adequate control group. However, this study demonstrated the potential and challenges of precision oncology in a real-world setting for BTC management.
Molecular profiling and molecular oncology are integral elements of the modern therapeutic management of BTC patients and should be implemented as upfront testing in routine clinical practice.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving human participants were reviewed and approved by Kommission für scientific integrity und Ethik der Karl Landsteiner Privatuniversität. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Contributions: (I) Conception and Design: HT, GP (II) Administrative support: All authors (III) Provision of study materials or patients: All authors (IV) Collection and assembly of data: HT (V) Data analysis and interpretation: All authors (VI) Manuscript writing: All authors; All authors contributed to the article and approved the submitted version.
Funding
We acknowledge support by Open Access Publishing Fund of Karl Landsteiner University of Health Sciences, Krems, Austria.
Conflict of interest
HT received honoraria from Servier, Pierre Fabre, Amgen, BMS, Lilly, Ipsen, Eisai, and Astra Zeneca. GP received honoraria for speaker or advisory role from Roche, Merck Serono, Amgen, Servier, Bayer, BMS, Pierre Fabre, Lilly, Daiichy Sanyo, Astra Zeneca, PeerVoice, CECOG and MSD. JS declares honorarium payments from Abbvie, Amgen, Gilead, Janssen, Merck, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Servier as an invited speaker or expert consulting.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bridgewater J, Imber C. New advances in the management of biliary tract cancer. HPB (Oxford) (2007) 9(2):104–11. doi: 10.1080/13651820701216216
2. Ciombor KK, Goff LW. Advances in the management of biliary tract cancers. Clin Adv Hematol Oncol (2013) 11(1):28–34.
3. Personeni N, Lleo A, Pressiani T, Colapietro F, Openshaw MR, Stavraka C, et al. Biliary tract cancers: Molecular heterogeneity and new treatment options. Cancers (Basel) (2020) 12(11). doi: 10.3390/cancers12113370
4. Brandi G, Farioli A, Astolfi A, Biasco G, Tavolari S. Genetic heterogeneity in cholangiocarcinoma: a major challenge for targeted therapies. Oncotarget (2015) 6(17):14744–53. doi: 10.18632/oncotarget.4539
5. Bridgewater JA, Goodman KA, Kalyan A, Mulcahy MF. Biliary tract cancer: Epidemiology, radiotherapy, and molecular profiling. Am Soc Clin Oncol Educ Book (2016) 35:e194–203. doi: 10.1200/EDBK_160831
6. Ouyang G, Liu Q, Wu Y, Liu Z, Lu W, Li S, et al. The global, regional, and national burden of gallbladder and biliary tract cancer and its attributable risk factors in 195 countries and territories, 1990 to 2017: A systematic analysis for the global burden of disease study 2017. Cancer (2021) 127(13):2238–50. doi: 10.1002/cncr.33476
7. Patel T. Cholangiocarcinoma–controversies and challenges. Nat Rev Gastroenterol Hepatol (2011) 8(4):189–200. doi: 10.1038/nrgastro.2011.20
8. Sahu S, Sun W. Targeted therapy in biliary tract cancers-current limitations and potentials in the future. J Gastrointest Oncol (2017) 8(2):324–36. doi: 10.21037/jgo.2016.09.16
9. Bragazzi MC, Ridola L, Safarikia S, Di Matteo S, Costantini D, Nevi L, et al. New insights into cholangiocarcinoma: multiple stems and related cell lineages of origin. Ann Gastroenterol (2018) 31(1):42–55.
10. Oh D-Y, He AR, Qin S, Chen L-T, Okusaka T, Vogel A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evidence (2022) 1(8):EVIDoa2200015. doi: 10.1056/EVIDoa2200015
11. Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. A randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin / 5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced / metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. J Clin Oncol (2019) 37(15_suppl):4003–3. doi: 10.1200/JCO.2019.37.15_suppl.4003
12. Casak SJ, Pradhan S, Fashoyin-Aje LA, Ren Y, Shen Y-L, Xu Y, et al. FDA approval summary: Ivosidenib for the treatment of patients with advanced unresectable or metastatic, chemotherapy refractory cholangiocarcinoma with an IDH1 mutation. Clin Cancer Res (2022) 28(13):2733–7. doi: 10.1158/1078-0432.CCR-21-4462
13. Patel TH, Marcus L, Horiba MN, Donoghue M, Chatterjee S, Mishra-Kalyani PS, et al. FDA approval summary: Pemigatinib for previously treated, unresectable locally advanced or metastatic cholangiocarcinoma with FGFR2 fusion or other rearrangement. Clin Cancer Res (2022). doi: 10.1158/1078-0432.CCR-22-2036
14. Vogel A, Bridgewater J, Edeline J, Kelley RK, Klümen HJ, Malka D, et al. Biliary tract cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol (2022). doi: 10.1016/j.annonc.2022.10.506
15. Mateo J, Chakravarty D, Dienstmann R, Jezdic S, Gonzalez-Perez A, Lopez-Bigas N, et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO scale for clinical actionability of molecular targets (ESCAT). Ann Oncol (2018) 29(9):1895–902. doi: 10.1093/annonc/mdy263
16. Verdaguer H, Sauri, Acosta DA, Guardiola M, Sierra A, Hernando J, et al. ESMO scale for clinical actionability of molecular targets driving targeted treatment in patients with cholangiocarcinoma. Clin Cancer Res (2022) 28(8):1662–71. doi: 10.1158/1078-0432.CCR-21-2384
17. Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, et al. Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: updated analysis from the phase II ROAR basket study. Ann Oncol (2022) 33(4):406–15. doi: 10.1016/j.annonc.2021.12.014
18. Walter D, Döring C, Feldhahn M, Battke F, Hartmann S, Winkelmann R, et al. Intratumoral heterogeneity of intrahepatic cholangiocarcinoma. Oncotarget (2017) 8(9):14957–68. doi: 10.18632/oncotarget.14844
19. Bogenberger JM, DeLeon TT, Arora M, Ahn DH, Borad MJ. Emerging role of precision medicine in biliary tract cancers. NPJ Precis Oncol (2018) 2:21. doi: 10.1038/s41698-018-0064-z
20. Ahn DH, Bekaii-Saab T. Biliary cancer: intrahepatic cholangiocarcinoma vs. extrahepatic cholangiocarcinoma vs. gallbladder cancers: classification and therapeutic implications. J Gastrointest Oncol (2017) 8(2):293–301.
21. Shiao MS, Chiablaem K, Charoensawan V, Ngamphaiboon N, Jinawath N. Emergence of intrahepatic cholangiocarcinoma: How high-throughput technologies expedite the solutions for a rare cancer type. Front Genet (2018) 9:309. doi: 10.3389/fgene.2018.00309
22. Wardell CP, Fujita M, Yamada T, Simbolo M, Fassan M, Karlic R, et al. Genomic characterization of biliary tract cancers identifies driver genes and predisposing mutations. J Hepatol (2018) 68(5):959–69. doi: 10.1016/j.jhep.2018.01.009
23. Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the european network for the study of cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol (2016) 13(5):261–80. doi: 10.1038/nrgastro.2016.51
24. Jain A, Javle M. Molecular profiling of biliary tract cancer: a target rich disease. J Gastrointest Oncol (2016) 7(5):797–803. doi: 10.21037/jgo.2016.09.01
25. Churi CR, Shroff R, Wang Y, Rashid A, Kang HC, Weatherly J, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PloS One (2014) 9(12):e115383. doi: 10.1371/journal.pone.0115383
26. Doherty M, Chiu J, McNamara M, Horgan AM, Serra S, Kamel-Reid S, et al. Molecular profiling of advanced biliary cancer: Lost in translation from bench to bedside. J Clin Oncol (2016) 34(4_suppl):283–3. doi: 10.1200/jco.2016.34.4_suppl.283
27. Wu MJ, Shi L, Merritt J, Zhu AX, Bardeesy N. Biology of IDH mutant cholangiocarcinoma. Hepatology (2022) 75(5):1322–37. doi: 10.1002/hep.32424
28. Weinberg BA, Xiu J, Lindberg MR, Shields AF, Hwang JJ, Poorman K, et al. Molecular profiling of biliary cancers reveals distinct molecular alterations and potential therapeutic targets. J Gastrointest Oncol (2019) 10(4):652–62. doi: 10.21037/jgo.2018.08.18
29. Valle JW, Lamarca A, Goyal L, Barriuso J, Zhu AX. New horizons for precision medicine in biliary tract cancers. Cancer Discovery (2017) 7(9):943–62. doi: 10.1158/2159-8290.CD-17-0245
30. Perkins G, Bouchet-Doumenq C, Svrcek M, Colussi O, Voron T, Sauvanet A, et al. Genomic profiling of ampullary adenocarcinoma (AA): Insights from a comparative analysis of pancreatic and intestinal adenocarcinoma and opportunities for targeted therapies use. J Clin Oncol (2017) 35(4_suppl):300–0. doi: 10.1200/JCO.2017.35.4_suppl.300
31. Chen L, Xu L, Shen L, Luo R, Jiang D, Wang Y, et al. HER2 positivity is affected by the papillary structure and has a bidirectional prognostic value for gallbladder carcinoma. Front Genet (2021) 12:831318. doi: 10.3389/fgene.2021.831318
32. Silva VW, Askan G, Daniel TD, Lowery M, Klimsra DS, Abou-Alfa GK, et al. Biliary carcinomas: pathology and the role of DNA mismatch repair deficiency. Chin Clin Oncol (2016) 5(5):62. doi: 10.21037/cco.2016.10.04
33. Piha-Paul SA, Oh D-Y, Ueno M, Malka D, Chung HC, Nagrial A, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer (2020) 147(8):2190–8. doi: 10.1002/ijc.33013
34. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, Jesus-Acosta AD, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite Instability/Mismatch repair-deficient cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol (2020) 38(1):1–10. doi: 10.1200/JCO.19.02105
35. Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: Pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res (2019) 25(13):3753–8. doi: 10.1158/1078-0432.CCR-18-4070
36. Arrichiello G, Nacca V, Paragliola F, Giunta EF. Liquid biopsy in biliary tract cancer from blood and bile samples: current knowledge and future perspectives. Explor Target Antitumor Ther (2022) 3(3):362–74. doi: 10.37349/etat.2022.00087
37. Ahn KS, Kang KJ. Molecular heterogeneity in intrahepatic cholangiocarcinoma. World J Hepatol (2020) 12(12):1148–57. doi: 10.4254/wjh.v12.i12.1148
38. Kendre G, Murugesan K, Brummer T, Segatto O, Saborowski A, Vogel A. Charting co-mutation patterns associated with actionable drivers in intrahepatic cholangiocarcinoma. J Hepatol (2022). doi: 10.1016/j.jhep.2022.11.030
39. Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol (2020) 21(6):796–807. doi: 10.1016/S1470-2045(20)30157-1
40. Kam AE, Masood A, Shroff RT. Current and emerging therapies for advanced biliary tract cancers. Lancet Gastroenterol Hepatol (2021) 6(11):956–69. doi: 10.1016/S2468-1253(21)00171-0
41. Bekaii-Saab TS, Valle JW, Van Cutsem E, Rimassa L, Furuse J, Ioka T, et al. FIGHT-302: Phase III study of first-line (1L) pemigatinib (PEM) versus gemcitabine (GEM) plus cisplatin (CIS) for cholangiocarcinoma (CCA) with FGFR2 fusions or rearrangements. J Clin Oncol (2020) 38(4_suppl):TPS592–2. doi: 10.1200/JCO.2020.38.4_suppl.TPS592
42. Borad MJ, Bridgewater JA, Morizane C, Shroff RT, Oh D-Y, Moehler M, et al. A phase III study of futibatinib (TAS-120) versus gemcitabine-cisplatin (gem-cis) chemotherapy as first-line (1L) treatment for patients (pts) with advanced (adv) cholangiocarcinoma (CCA) harboring fibroblast growth factor receptor 2 (FGFR2) gene rearrangements (FOENIX-CCA3). J Clin Oncol (2020) 38(4_suppl):TPS600–0. doi: 10.1200/JCO.2020.38.4_suppl.TPS600
43. Abou-Alfa GK, Borbath I, Goyal L, Lamarca A, Macarulla T, Oh D-Y, et al. PROOF 301: A multicenter, open-label, randomized, phase 3 trial of infigratinib versus gemcitabine plus cisplatin in patients with advanced cholangiocarcinoma with an FGFR2 gene fusion/rearrangement. J Clin Oncol (2022) 40(16_suppl):TPS4171–TPS4171. doi: 10.1200/JCO.2022.40.16_suppl.TPS4171
44. Hachem S, Kassis Y, Hachem MC, Zouein J, Gharios J, Kourie HR. BRCAness in biliary tract cancer: a new prognostic and predictive biomarker? biomark Med (2023). doi: 10.2217/bmm-2022-0664
45. Giorgione R, Risalti M, Bartolini I, Rossi G, Pillozzi S, Muiesan P, et al. The emerging role of immunotherapy in biliary tract cancer: a review of new evidence and predictive biomarkers. Immunotherapy (2022) 14(7):567–76. doi: 10.2217/imt-2021-0257
46. Wang H, Chi L, Yu F, Dai H, Gao C, Si X, et al. Annual review of KRAS inhibitors in 2022. Eur J Med Chem (2023) 249:115124. doi: 10.1016/j.ejmech.2023.115124
47. Haider K, Sharma A, Yar MS, Yakkala PA, Shafi S, Kamal A. Novel approaches for the development of direct KRAS inhibitors: structural insights and drug design. Expert Opin Drug Discovery (2022) 17(3):247–57. doi: 10.1080/17460441.2022.2029842
Keywords: biliary tract cancer, molecular aberrations, molecular profiling, precision medicine, precision oncology
Citation: Taghizadeh H, Schmalfuss T, Maj-Hes A, Singer J and Prager GW (2023) Austrian tricentric real-life analysis of molecular profiles of metastatic biliary tract cancer patients. Front. Oncol. 13:1143825. doi: 10.3389/fonc.2023.1143825
Received: 13 January 2023; Accepted: 25 April 2023;
Published: 10 May 2023.
Edited by:
Manoj Pandey, Banaras Hindu University, IndiaReviewed by:
Takashi Kokudo, University of Tokyo, JapanGianluca Russo, University of Naples Federico II, Italy
Copyright © 2023 Taghizadeh, Schmalfuss, Maj-Hes, Singer and Prager. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gerald W. Prager, Z2VyYWxkLnByYWdlckBtZWR1bml3aWVuLmFjLmF0
 Hossein Taghizadeh
Hossein Taghizadeh Theresa Schmalfuss1,2,4,5
Theresa Schmalfuss1,2,4,5 Josef Singer
Josef Singer