- 1Shantou University Medical College, Shantou, China
- 2Department of Nursing, First Affiliated Hospital of Shantou University Medical College, Shantou, China
Purpose: Stigma is common in patients with breast cancer after surgery, which has a negative impact on the quality of life (QOL). This study aimed to investigate the QOL of breast cancer patients after surgery and to analyze the multiple chains mediating effects of self-disclosure and social support between stigma and QOL.
Methods: A total 292 patients of breast cancer patients after operation were recruited in this study. A questionnaire survey was conducted using the general information questionnaire, the consumer experiences of stigma questionnaire (CESQ), the distress disclosure index(DDI), the perceived social support scale(PSSS), and the functional assessment of cancer therapy-breast(FACT-B). Path analysis was conducted to test the hypothesized serial multiple mediation model.
Results: The total scores of stigma, self-disclosure, social support and QOL were 15 (10 ~ 22), 39 (31 ~ 46), 58 (50 ~ 67) and 88 (74 ~ 104) respectively. QOL of breast cancer patients after the operation was negatively correlated with stigma (p < 0.01), and positively correlated with self-disclosure and social support (p < 0.01). Self-disclosure and social support played a complete mediating effect between stigma and QOL, and the total mediating effect value was 85. 87%.
Conclusions: Self-disclosure and social support play a complete intermediary role between stigma and QOL. In order to improve the quality of life of patients, medical staff should pay attention to the assessment of stigma, encourage patients to express their emotions, and encourage their families and friends to respond to their expression and needs of patients.
1 Backgrounds
According to the latest Cancer Statistics Report in 2021 (1), breast cancer accounted for the first morbidity of women’s cancer, which posed a serious threat to women’s health and life. Surgery remained the mainstay of treatment for breast cancer patients (2), but increasingly innovative screening techniques allowed early detection of the disease and with the development of better treatment options, the 5-year survival rate of breast cancer has been improved (3). In China, breast-conserving surgery and adjuvant radiotherapy for patients with early-stage breast cancer can make the 5-year overall survival rate of patients > 80% (4). The survival time of breast cancer patients had been prolonged. However, the interpersonal relationship, body image, and psychological status of breast cancer patients after surgery had been affected, which were closely related to the QOL (5, 6). Research had revealed that QOL had become an important outcome measure in breast cancer clinical research and survival research, and could be used as a predictor of mortality rate in breast cancer survivors (7). Research had shown that patients with breast cancer after surgery generally experienced stigma, which had an adverse impact on the QOL of patients (8–10), and patients’ self-disclosure and social support could improve the QOL, which had a positive impact (11–13). The stigma of breast cancer patients is negatively correlated with self-disclosure and social support (14). Self-disclosure could enhance the benefits of social support and promoted their mental health (15). Based on the above analysis of the logical relationship between breast cancer patients’ stigma, social support and self-disclosure variables, the purpose of this study is to investigate the relationship among stigma, self-disclosure, social support, and QOL in breast cancer patients after surgery, and to construct the chain mediation model of self-disclosure and social support. Exploring the influencing factors of QOL of patients, and providing suggestions for intervention programs to improve the QOL for breast cancer patients after surgery.
2 Methods
2.1 Participants and procedures
A total of 292 breast cancer patients from 5 hospitals in Guangdong Province (3 hospitals in Shantou, 1 hospital in Guangzhou, and 1 hospital in Shenzhen) from March 2021 to March 2022 were selected. To increase the sample size and combine with clinical practice, a convenient sampling method is adopted, and the number of participants in each hospital was evenly distributed as far as possible (Figure 1).
Inclusion criteria: (1) pathological diagnosis of breast cancer (stage 0, I, II, III, IV); (2) patients accepted mastectomy, breast-conserving surgery, or breast reconstruction surgery;(3)age ≥ 18 years old; (4) voluntarily participate in this study on the premise of informed consent; (5)clear awareness, reading comprehension and expression ability; (6)awareness of their condition. Exclusion criteria: (1)combined with other malignant tumors or recurrence of breast cancer; (2)mental disorders, unable to cooperate; (3)critical condition, unable to understand or answer questions clearly; (4)combined with myocardial infarction, heart failure and other serious diseases affecting the QOL. Sample size calculation: Combined with popular rules-of-thumb and Monte Carlo analysis method, the minimum sample size is 265 (16–18).
2.2 Measures
2.2.1 Sociodemographic and medical variables
Basic information such as gender, age, marital status, educational level, and religious belief was self-reported. Disease staging, pathological classification, and surgical methods were extracted from medical records.
2.2.2 Consumer experiences of stigma questionnaire
The questionnaire was developed by Wahl et al. in 2013 to provide a tool for assessing stigma in breast cancer patients. This study adopted the Chinese version of the CESQ (19), which mainly included two aspects: the stigma of interpersonal communication and experience of discrimination, with a total of 9 items. Grade 0-5 scoring method was adopted: 0 points (never) -5 points (often), 0-45 points. The higher the score, the higher the level of stigma. In this study, the total Cronbach’s coefficient of CESQ was 0.942.
2.2.3 Distress disclosure index
DDI was used (20), which consisted of 12 items and was scored by the Likert 5-level scoring method, with a total score of 12-60 points. The higher the DDI score, the higher the self-disclosure level. 12-29 points were low self-disclosure, 30-44 points were medium self-disclosure, and 45-60 points were high self-disclosure. In this study, the total Cronbach’s coefficient of DDI was 0.942.
2.2.4 Perceived social support scale
PSSS was a 12-item scale developed by Blumenthal (21) in 1987. This scale was composed of three subscales: family support, friend support, and other support, and each subscale contained four items. Using the Likert 7-level scoring method, 1-7 points represented “extremely disagree” to “extremely agree”. The higher the score, the higher the level of social support, 12-36 points for low support level, 37-60 points for medium support level, and 61-84 points for high support level. In this study, the total Cronbach’s Coefficient of PSSs was 0.903, and the Cronbach’s coefficients of each subscale were 0.926, 0.945, and 0.903 respectively.
2.2.5 Functional assessment of cancer therapy-breast
The 36-item of Functional Assessment of Cancer Therapy-Breast scale (22) was used to measure participants’ QOL, including physiological status, social/family status, and emotional status, and functional status and additional concerns about breast cancer five dimensions. All items were rated using a 5-point Likert scale, and the full score was 144 points. The higher the score, the higher the QOL of patients. In this study, the total Cronbach’s coefficient was 0.921, and the Cronbach’s coefficient of each dimension ranged from 0.455 to 0.914.
2.3 Data collection and analysis
This study was a cross-sectional design and had been approved by the ethics committee of the hospital. Before the study, researchers were trained and assessed in a unified way, and the researchers were required to use unified guidelines in the survey process. The subjects formally conducted a questionnaire survey after oral or signed informed consent. The completed questionnaire was checked and taken back by the researchers on the spot. Questionnaire exclusion criteria: questionnaire blank ≥ 15%; the answer was single and the content was contradictory. A total of 300 questionnaires were distributed, excluding 8 invalid questionnaires, 292 valid questionnaires were recovered, and the effective recovery rate was 97.3%. Descriptive statistics and correlations were performed in SPSS 26.0. The descriptive data were presented as mean ± SD for variables obeying normal distribution, Md (P25, P75) for variables not obeying normal distribution. The enumeration data were expressed by frequency and constituent ratio. In this study, the scores of stigma, social support, self-disclosure, and quality of life do not obey normal distribution, however, the scores of demographic characteristics are expressed by Md (P25, P75), and the correlation analysis is conducted by Spearman rank correlation analysis. Amos 26. 0 was used to construct the structural equation model, the bootstrap was used to evaluate the direct and indirect effects, and the effects of each path were tested. All tests were performed two-sided, and a p-value of less than 0.05 was considered a significant level.
3 Results
3.1 General information of patients
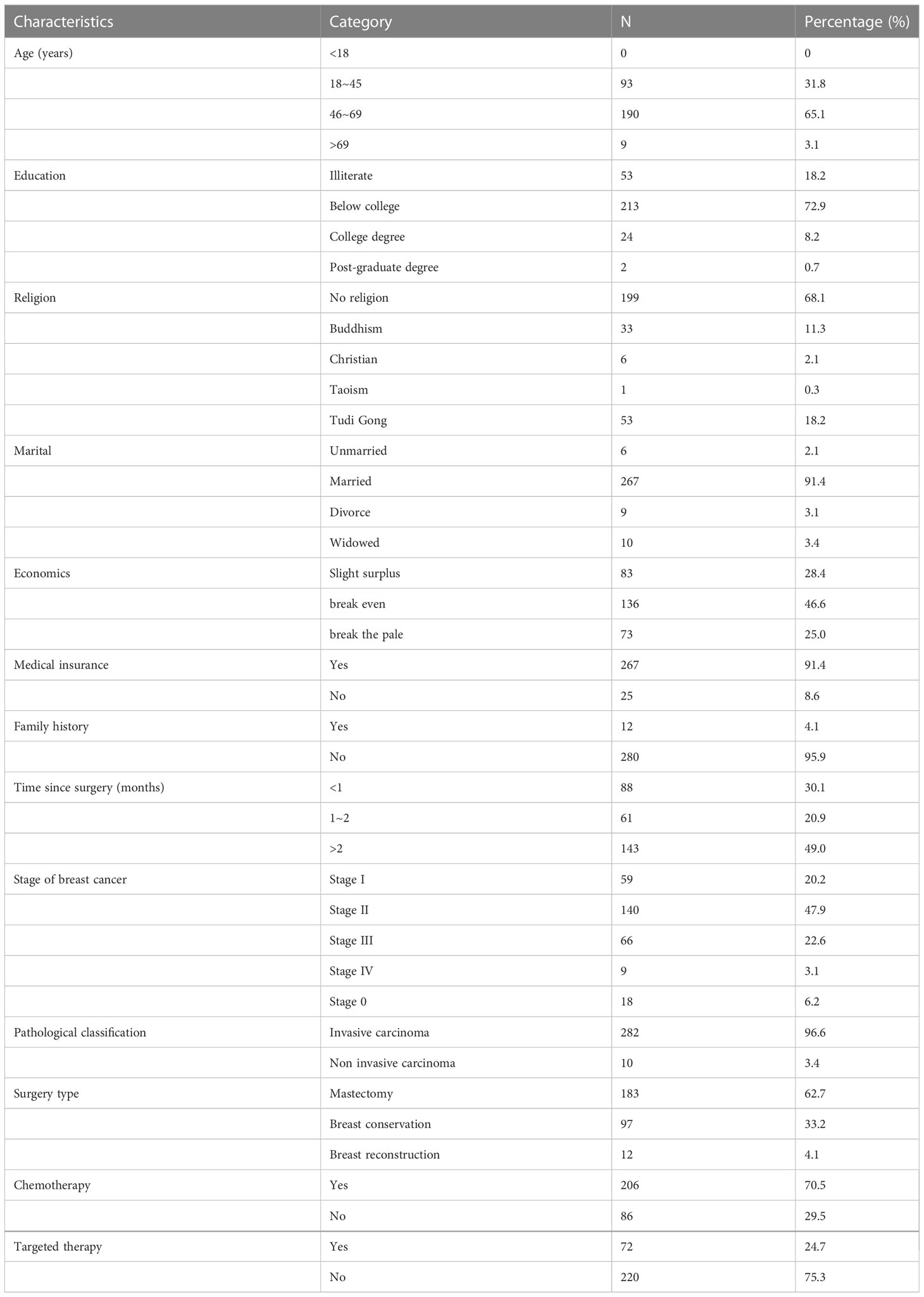
Table 1 showed that the age of 292 patients with breast cancer who participated in the study was mainly 46-69 years old (65.1%), the education of the participants was generally junior college or below (72.9%), and only 26 patients had bachelor’s degree or above (8.9%); Among the religious beliefs, 68.2% of the patients had no religious beliefs, and 11.3% believed in Buddhism; Among the marital status, 91.4% were married; In the economic situation, 46.6% of patients were in the balance of payments. Among 292 cases of breast cancer, 282 cases (96. 6%) were invasive cancer, mastectomy was performed in 183 patients (62. 7%), and breast-conserving surgery was performed in 97 patients (33. 2%).
3.2 The scores and correlation analysis of stigma, self-disclosure, social support, and QOL
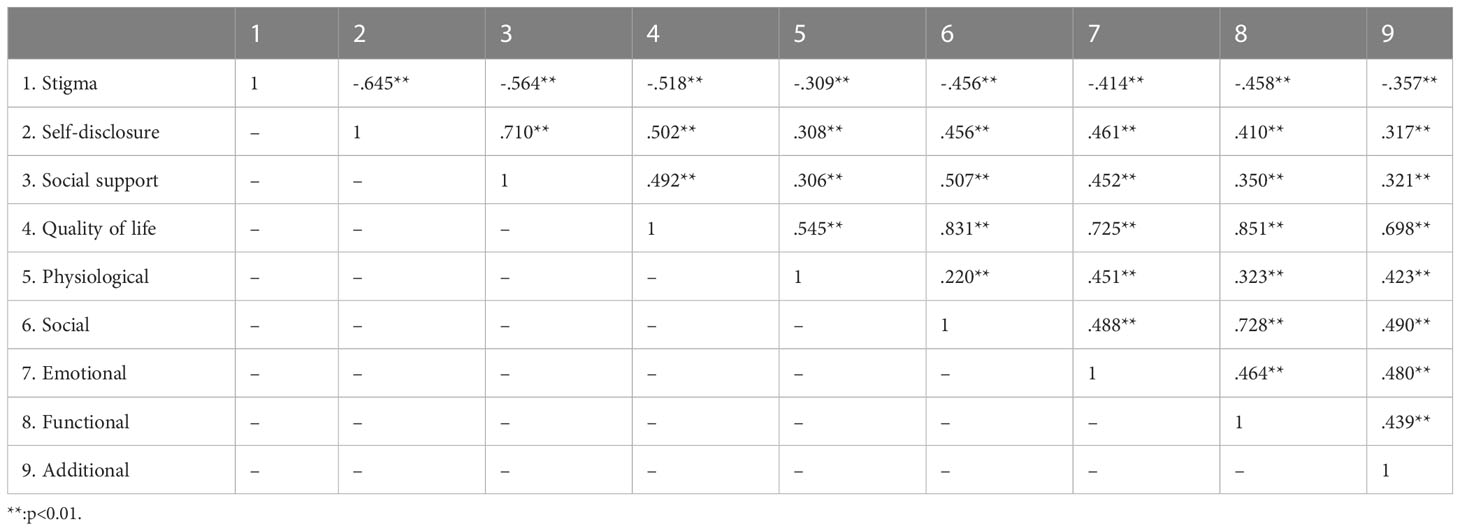
The total score of CESQ was 15 (10~22), the total score of DDI was 39 (31~46), the total score of PSSS was 58 (50~67); The total score of FACT-B was 88 (74~104), and the 5 dimensions were: physiological status, 21 (19~23); social/family status, 17 (11~22); emotional status, 16 (13~19); functional status, 13 (8~17); additional concerns, 24 (21~26), respectively (Table 2). The correlation coefficients among the stigma, self-disclosure, social support, and QOL are shown in Table 3. The total scores of stigma, social support, self-disclosure, and QOL of patients were correlated with each other, and the correlation was statistically significant (P < 0.01). QOL was negatively correlated with stigma(r=-0.518, p<0.01), and positively correlated with self-disclosure(r=0.502, p<0.01) and social support(r=0.492, p<0.01). Stigma was negatively correlated with self-disclosure(r=-0.645, p<0.01) and social support(r=-0.564, p<0.01); Self-disclosure was positively correlated with social support(r=0.710, p<0.01).
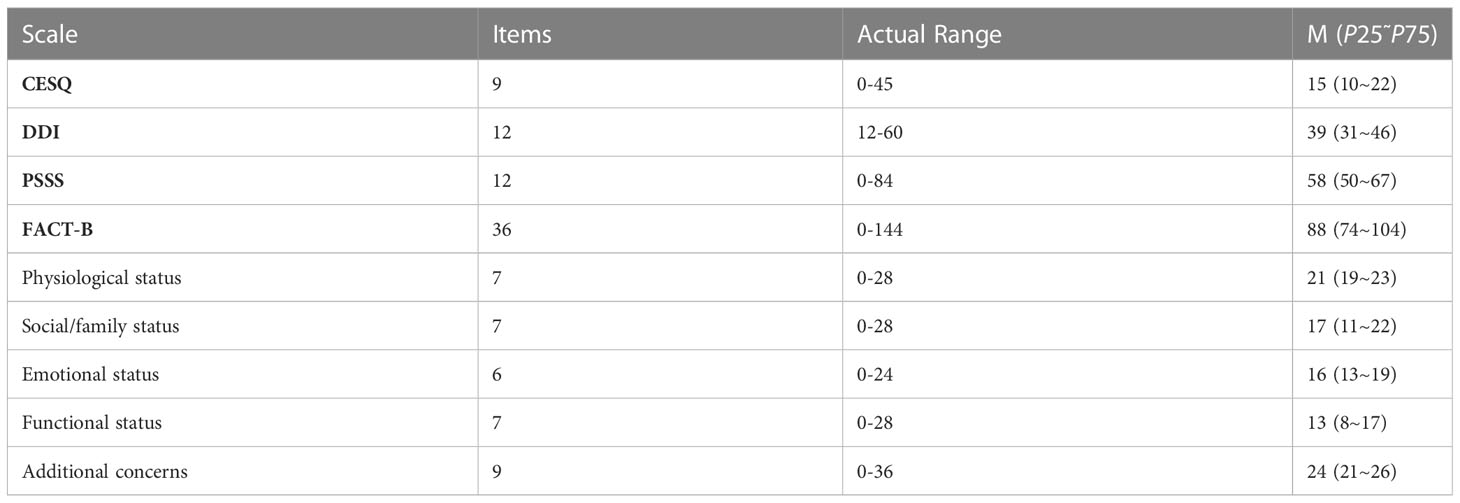
Table 2 Scores for the consumer experiences of stigma questionnaire (CESQ), distress disclosure index (DDI), perceived social support scale (PSSS), functional assessment of cancer therapy-breast (FACT-B).
3.3 Structural equation model of stigma, self-disclosure, social support, and QOL
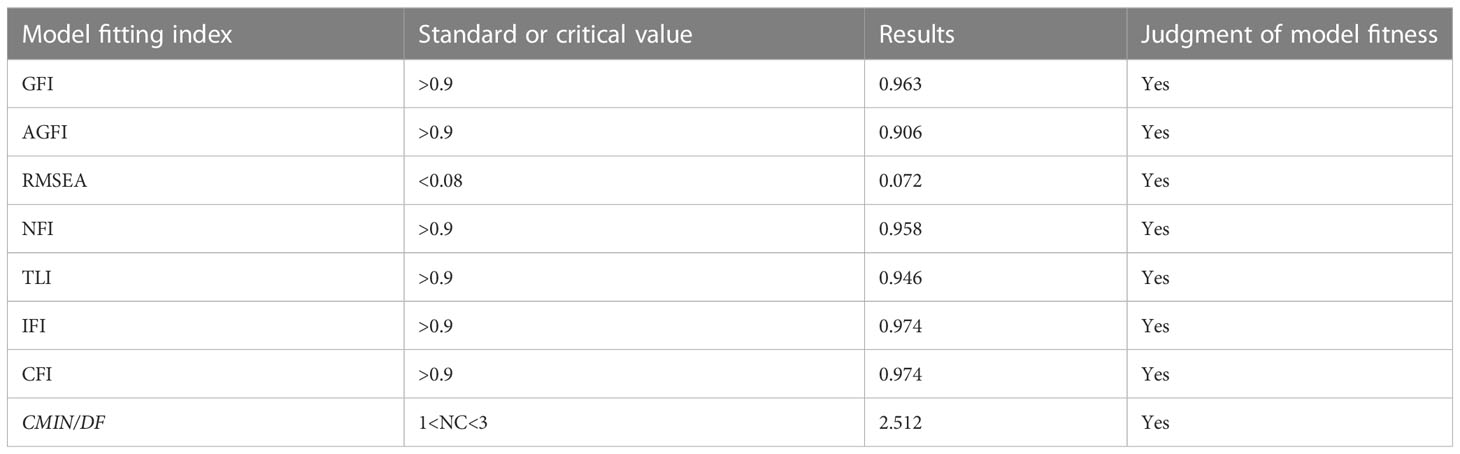
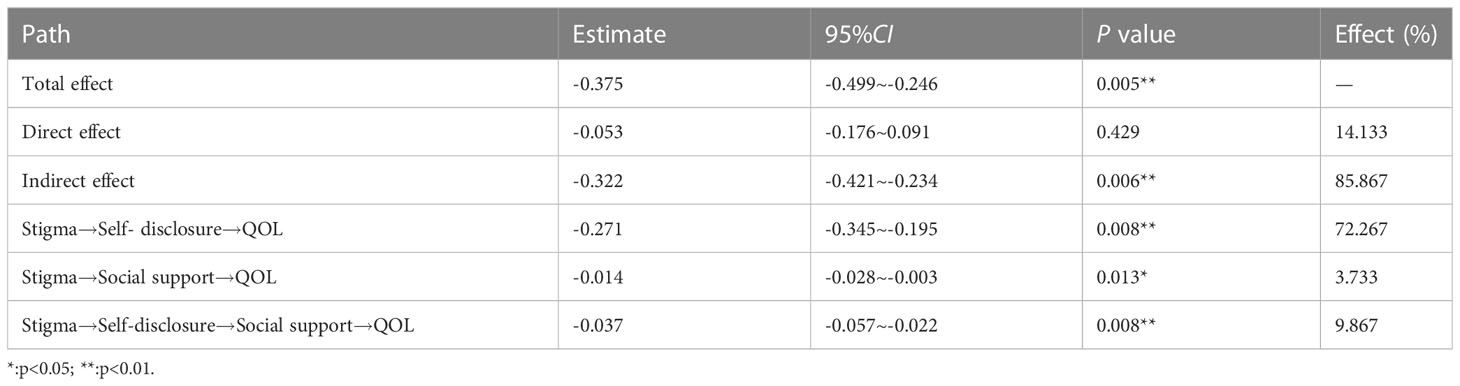
Through a large number of literature research and correlation analysis results, it is hypothesized that QOL can be directly affected by stigma and indirectly affected by self-disclosure and social support. The maximum likelihood method was used to fit the model structure, and the model was corrected according to the correction index. The fitting results of the model: X2/df=2.512, goodness of fit(GFI)=0.963, adjusted goodness of fit (AGFI)=0.906, root mean square error of approximation(RMSEA)=0.072, normed fit index(NFI)=0.958, comparative fit index(CFI)=0.974, which indicated that the fitting degree of the model was good (6) (Table 4). Figure 2 shows that the direct effect of stigma on QOL is not significant ( β=- 0.05, p>0.05), and stigma can indirectly affect the QOL through self-disclosure and social support. Table 5 has shown the path coefficients among the variables detailedly. The results of multiple mediating effect analyses showed that self-disclosure and social support played a complete mediating role in the stigma and QOL of breast cancer patients after surgery. The mediating effect value was -0.322, accounting for 85.87% of the total effect, of which the mediating effect of self-disclosure accounted for 72.27%, the mediating effect of social support accounted for 7.73%, and the chain mediating effect of self-disclosure and social support accounted for 9.87% (Table 6).
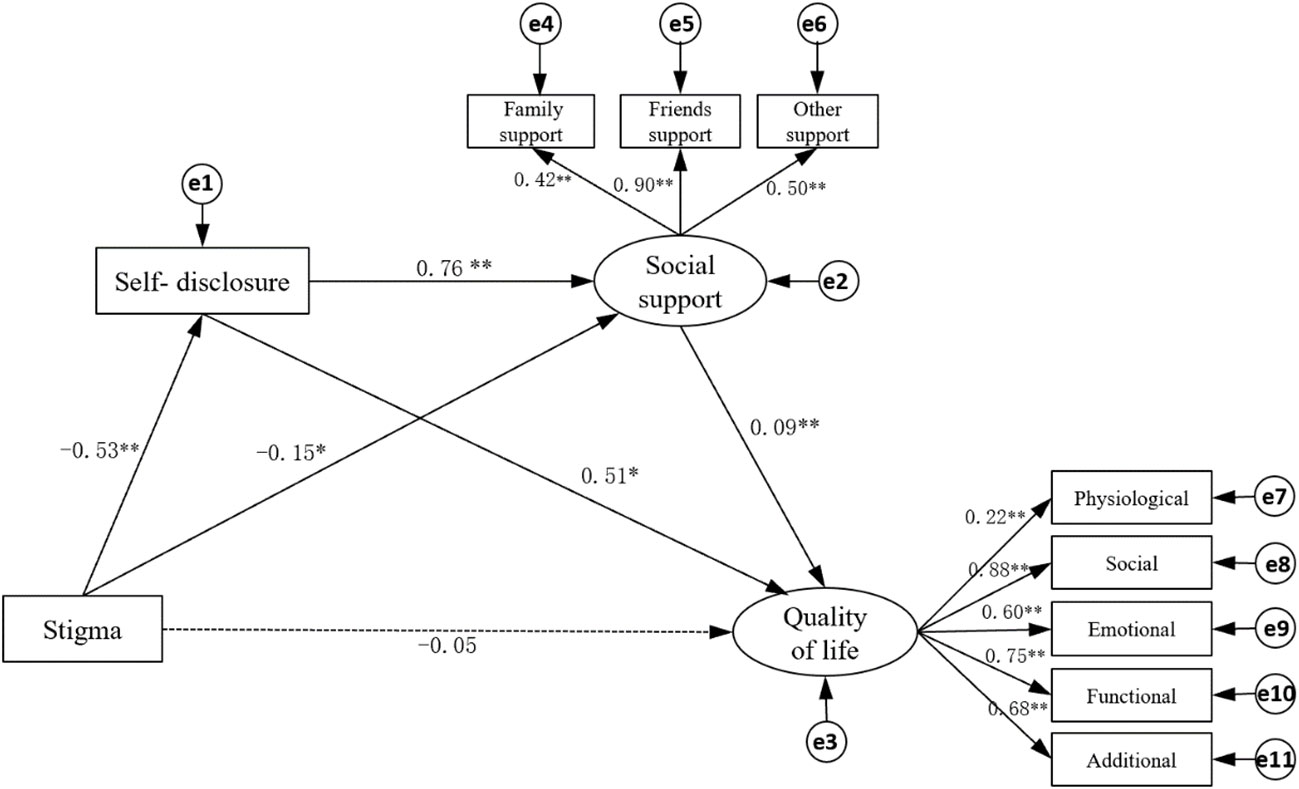
Figure 2 Structural equation model of quality of life of breast cancer patients. The dotted lines mean “not Significant”. The numbers represent correlation. *P < 0.05, ** p < 0.001.
4 Discussion
4.1 QOL in patients with breast cancer after operation
This research has shown that the median total score of QOL was 88 points (range 0-144 points), which is above average. Compared with the research of Criscitiello C et al. (23), the QOL in this study is slightly lower (99.0 ± 21.9). Lu Q et al. (24) showed that compared with their American counterparts, Chinese breast cancer survivors reported a lower QOL. However, in recent years, through effective intervention measures, the QOL of breast cancer patients had been greatly improved (7), and the QOL in this research has exceeded the average level, but it is still slightly lower than that of breast cancer patients in other countries (23). It may be that most of the participants in this study have a low education level, medium economic status, and younger age structure, making them more prone to panic about death, unknown, and financial contraction, which would reduce the quality of life (7, 25). The majority of participants undergo mastectomy (62.7%) and have the lowest score in the functional status dimension of FACT-B (Table 2). Patients with mastectomy are more likely to suffer from physical and psychological discomfort, and their probability of shoulder and motor function limitation is 6 times higher than that of patients with breast-conserving surgery (26). The early postoperative functional score is also lower than that of patients who chose breast-conserving, and the systemic side effects are more severe (24, 27). Therefore, similar to the studies in China (28, 29), the QOL of the participants in this study is above average, in which the score of functional status is the lowest, but the QOL was still slightly lower than that of foreign breast cancer patients.
4.2 Relationship between stigma and QOL in patients with breast cancer after operation
This research explores the relationship between stigma and QOL and its influence path in patients with breast cancer after surgery. Among them, the stigma of patients is above average, which is similar to most studies, indicating that patients with breast cancer after surgery generally experience stigma (8, 9). The results show that stigma is negatively correlated with QOL and its dimensions (Table 3). By constructing a structural equation model (Figure 1), we further explain the path of stigma affecting QOL: the direct effect of stigma on QOL is not significant, and the way of influence is mainly through indirect effects. Similar to previous studies, there was a negative correlation between stigma and QOL (4, 10), and stigma prevented patients from seeking medical help and adhering to treatment (10, 30). For cancer patients, it is an obstacle to maintaining health-related QOL (31, 32). Hatzenbuehler ML et al. (33) proposed that there were mediating effects regulated by different mechanisms between stigma and QOL. According to different mediating effects, there is a direct effect between stigma and QOL (34), and it can also be completely affected by indirect effects (8). In this study, the effect of stigma on QOL is completely mediated by self-disclosure and social support.
4.3 Chain mediating effect of self-disclosure and social support between stigma and QOL in breast cancer patients after operation
In this survey, self-disclosure and social support of patients after breast cancer surgery are negatively correlated with stigma and positively correlated with QOL (Table 3). The results of Figure 1 and Table 6 show that there are three indirect effects of stigma on QOL: stigma→self-disclosure→QOL; stigma→social support→QOL; stigma→social support→QOL; stigma→self-disclosure→social support→QOL.
4.3.1 Mediating effect of social support between stigma and QOL
The results of this study show that the indirect effect value of stigma → social support →QOL is -0.014, accounting for 3.73% of the total indirect effect. The stigma experience can improve the QOL through the increase of social support. The breast cancer patients who have finished surgery often have social support needs (35), when patients receive more social support, their stigma would be lower (36). Social support can improve the QOL of patients (12, 13), and buffer the pressure by promoting their mental health and physical health (36, 37). At the same time, based on the theory of “stress buffer hypothesis”, social support can be used as a “ direct driver” to improve personal well-being and health, and as a “pre-factor” to improve individuals’ positive coping styles and psychological status, so as to have a positive impact on life and health (4). Therefore, while paying attention to the physical condition of breast cancer patients after surgery, medical staff should give more care to patients, mobilize their families and friends to support them, understand their inner feelings and needs, and give feedback timely.
4.3.2 The chain mediating effect of self-disclosure and social support between stigma and QOL
The results of this study show that the indirect effect value of stigma → self-disclosure →QOL was -0.271, accounting for 84.16% of the total indirect effect, ranking first among the three mediating effect paths. Chinese breast cancer patients believe that coping with disease and misfortune is a private matter, and they are reluctant to disclose their diagnosis, treatment, and disease-related thoughts and feelings to others (38). Women who do not disclose their diagnosis and related concerns are more likely to blame themselves, which may increase the risk of depression that affected emotional well-being and reduce the QOL (39). According to the “social cognitive processing theory”, individual adaptation to cancer can be facilitated by emotional disclosure, which helps to improve psychological adaptation to cancer in the social environment (40, 41). The results show that the indirect effect value of stigma→self-disclosure→social support→QOL was -0.037, accounting for 9.87% of the total indirect effect. Similar to the path of this research, the results of Taniguchi E et al. (15) showed that the characteristics of self-disclosure implied stigma and indirectly promoted psychological well-being through social support, which was a prerequisite for social support. At the same time, self-disclosure enhanced the benefits of social support and was a “booster” of social support. The research of R Rüsch N et al. (42) showed that the better family and friends’ attitude towards patients’ self-disclosure, the better the QOL of patients. Therefore, when medical staff is concerned about the stigma experienced by patients after breast cancer surgery, we should encourage patients to express their emotions and also encourage their families and friends to respond effectively to the expression, express their concern and support for patients, then improve the QOL of patients as much as possible.
To sum up, the QOL of breast cancer patients after surgery still needs to be improved, which can be affected by stigma, self-disclosure, and social support. Stigma can affect QOL through multiple mediating effects of self-disclosure and social support. To improve the QOL of patients, we should encourage patients and their families to express themselves and carry out relevant psychological counseling activities.
4.4 Study limitations
There are some limitations to this study. First, this study uses a cross-sectional design, only the independent time point data are collected, and it can not assess patients at different times. A longitudinal study design can be introduced in the later research to explore the trajectory of patients’ QOL at different times. Secondly, this study adopted a convenient sampling method to collect data in five hospitals, and the representativeness of the sample needs to be improved. Although we used convenient sampling, we tried our best to achieve “stratified sampling” by hospital and operation. In the future, we will continue to expand the sample size, increase the hospitals included in the study, adopt a random sampling method, and include more influencing factors for analysis to obtain more accurate conclusions.
5 Conclusions
The QOL of patients with breast cancer after surgery is at the upper middle level, which is higher than before, but it can still be improved compared with other countries. Stigma, self-disclosure, social support, and QOL are correlated with each other, and self-disclosure and social support play a multiple chain mediation effects between stigma and QOL. To improve the QOL of patients with breast cancer after surgery, medical staff should not only pay attention to the physical condition of the patients, but also pay attention to the evaluation of their stigma experience, encourage patients to express their emotions, and also encourage their families and friends to respond to the expression and needs of the patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
All methods were performed in accordance with the relevant guidelines and regulations or declaration of Helsinki. The study was approved by the ethics committee of Shantou University Medical College (Approval No: SUMC-2021-54). Informed consent was obtained from all individual participants and legal guardian. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by LB, SZ and GF. The first draft of the manuscript was written by LB and all authors commented on previous versions of the manuscript. GF ultimately modified the manuscript. All authors read and approved the final manuscript.
Funding
Science and Technology Bureau of Guangdong Province in China (210728156901595), General program of Guangdong Natural Science Foundation (2022A1515012192), Medical research fund project of Guangdong Province (A2021500, A2022535), Shantou science and technology plan’s medical and health category project (190716185262435, SFK [2019] No. 106-10),Philosophy and Social Sciences Planning Foundation Discipline Co-construction Project of Guangdong Province of 2022 (GD22XXW10).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
2. Zehra S, Doyle F, Barry M, Walsh S, Kell MR. Health-related quality of life following breast reconstruction compared to total mastectomy and breast-conserving surgery among breast cancer survivors: a systematic review and meta-analysis. Breast Cancer (2020) 27(4):534–66. doi: 10.1007/s12282-020-01076-1
3. International Agency For Research on Cancer. Available at: https://www.iarc.fr/faq/latest-global-cancer-data-2020-qa/.html.
4. Cui C, Wang L, Wang X. Health-related quality of life and social constraints among Chinese breast cancer patients: a cross-sectional study. Health Qual Life Outcomes (2021) 19(1):238. doi: 10.1186/s12955-021-01871-0
5. Yfantis A, Intas G, Tolia M, Nikolaou M, Tsoukalas N, Lymperi M, et al. Health-related quality of life of young women with breast cancer. review of the literature. J BUON (2018) 23(1):1–6.
6. Cho YU, Lee BG, Kim SH. Coping style at diagnosis and its association with subsequent health-related quality of life in women with breast cancer: a 3-year follow-up study. Eur J Oncol Nurs (2020) 45:101726. doi: 10.1016/j.ejon.2020.101726
7. Mokhatri-Hesari P, Montazeri A. Health-related quality of life in breast cancer patients: review of reviews from 2008 to 2018. Health Qual Life Outcomes (2020) 18(1):338. doi: 10.1186/s12955-020-01591-x
8. Chu Q, Wong CCY, Chen L, Shin LJ, Chen L, Lu Q. Self-stigma and quality of life among Chinese American breast cancer survivors: a serial multiple mediation model. Psychooncology (2021) 30(3):392–9. doi: 10.1002/pon.5590
9. Wong CCY, Pan-Weisz BM, Pan-Weisz TM, Yeung NCY, Mak WWS, Lu Q. Self-stigma predicts lower quality of life in Chinese American breast cancer survivors: exploring the mediating role of intrusive thoughts and posttraumatic growth. Qual Life Res (2019) 28(10):2753–60. doi: 10.1007/s11136-019-02213-w
10. Scott N, Crane M, Lafontaine M, Seale H, Currow D. Stigma as a barrier to diagnosis of lung cancer: patient and general practitioner perspectives. Prim Health Care Res Dev (2015) 16(6):618–22. doi: 10.1017/S1463423615000043
11. Truong C, Gallo J, Roter D, Joo J. The role of self-disclosure by peer mentors: using personal narratives in depression care. Patient Educ Couns (2019) 102(7):1273–9. doi: 10.1016/j.pec.2019.02.006
12. Ho PJ, Gernaat SAM, Hartman M, Verkooijen HM. Health-related quality of life in Asian patients with breast cancer: a systematic review. BMJ Open (2018) 8(4):e020512. doi: 10.1136/bmjopen-2017-020512
13. Kugbey N, Oppong Asante K, Meyer-Weitz A. Depression, anxiety and quality of life among women living with breast cancer in Ghana: mediating roles of social support and religiosity. Support Care Cancer (2020) 28(6):2581–8. doi: 10.1007/s00520-019-05027-1
14. Tang WZ, Yusuf A, Jia K, Iskandar YHP, Mangantig E, Mo XS, et al. Correlates of stigma for patients with breast cancer: a systematic review and meta-analysis. Support Care Cancer (2022) 31(1):55. doi: 10.1007/s00520-022-07506-4
15. Taniguchi E, Thompson CM. Mental illness self-disclosure among college students: a pre-requisite of social support or a booster of social support benefits? J Ment Health (2021) 30(3):323–32. doi: 10.1080/09638237.2021.1922626
16. Bentler PM, Chou C-P. Practical issues in structural modeling. Sociological Methods Res (1987) 16(1):78–117. doi: 10.1177/0049124187016001004
17. Muthe’n LK, Muthe’n BO. How to use a Monte Carlo study to decide on sample size and determine power. Struct Equation Model (2002) 9(4):599–620. doi: 10.1207/S15328007SEM0904_8
18. Hu L-t, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equation Model (1999) 6(1):1–55. doi: 10.1080/10705519909540118
19. Li R, Jiang Z, Gong S, Liu D. Reliability and validity of the Chinese version of the sickness experience questionnaire in breast cancer patients. Chin J Pract Nursing (2019) 35(28):2195–9. doi: 10.3760/cma.j.issn.1672-7088.2019.28.008
20. Kahn JH, Hessling RM. Measuring the tendency to conceal versus disclose psychological distress. J Soc Clin Psychol (2001) 20(1):41–65. doi: 10.1521/jscp.20.1.41.22254
21. Blumenthal JA, Burg MM, Barefoot J, Williams RB, Haney T, Zimet G. Social support, type a behavior, and coronary artery disease. Psychosom Med (1987) 49(4):331–40. doi: 10.1097/00006842-198707000-00002
22. FACT-B (Functional Assessment of Cancer Therapy). Questionnaires for patients with breast cancer . Available at: https://www.facit.org/FACITOrg/Questionnaires (Accessed September 25, 2019).
23. Criscitiello C, Spurden D, Piercy J, Rider A, Williams R, Mitra D, et al. Health-related quality of life among patients with HR+/HER2- early breast cancer. Clin Ther (2021) 43(7):1228–1244.e4. doi: 10.1016/j.clinthera.2021.04.020
24. Lu Q, You J, Kavanagh A, Warmoth K, Meng Z, Chen Z, et al. Differences in quality of life between American and Chinese breast cancer survivors. Support Care Cancer (2016) 24(9):3775–82. doi: 10.1007/s00520-016-3195-1
25. Fernández de Larrea-Baz N, Pérez-Gómez B, Guerrero-Zotano Á, Casas AM, Bermejo B, Baena-Cañada JM, et al. Primary breast cancer and health related quality of life in Spanish women: the EpiGEICAM case-control study. Sci Rep (2020) 10(1):7741. doi: 10.1038/s41598-020-63637-w
26. Vidt ME, Potochny J, Dodge D, Green M, Sturgeon K, Kass R, et al. The influence of mastectomy and reconstruction on residual upper limb function in breast cancer survivors. Breast Cancer Res Treat (2020) 182(3):531–41. doi: 10.1007/s10549-020-05717-z
27. Słowik AJ, Jabłoński MJ, Michałowska-Kaczmarczyk AM, Jach R. Evaluation of quality of life in women with breast cancer, with particular emphasis on sexual satisfaction, future perspectives and body image, depending on the method of surgery. badanie jakości życia kobiet z rakiem piersi, ze szczególnym uwzględnieniem satysfakcji seksualnej i perspektyw na przyszłość oraz obrazu ciała w zależności od zastosowanej metody leczenia operacyjnego. Psychiatr Pol (2017) 51(5):871–88. doi: 10.12740/PP/OnlineFirst/63787
28. Xia Q, Fu HB, Yu Y, Wang QF. Analysis on life quality and influencing factors in breast cancer patients. Maternal Child Health Care China. (2021) 36(5):1132–5. doi: 10.19829/j.zgfybj.issn.1001-4411.2021.05.050
29. Xu Y, Zhang H, Jiang C, Yan Y. Correlation among supportive care needs,psychological resilience and quality of life in breast cancer patients receiving postoperative chemotherapy. Chin J Breast Dis (Electronic Edition) (2021) 15(6):352–8. doi: 10.3877/cma.j.issn.1674-0807.2021.06.004
30. Nyblade L, Stockton M, Travasso S, Krishnan S. A qualitative exploration of cervical and breast cancer stigma in karnataka, India. BMC Womens Health (2017) 17(1):58. doi: 10.1186/s12905-017-0407-x
31. Nyblade L, Stockton MA, Giger K, Bond V, Ekstrand ML, Lean RM, et al. Stigma in health facilities: why it matters and how we can change it. BMC Med (2019) 17(1):25. doi: 10.1186/s12916-019-1256-2
32. Johnson LA, Schreier AM, Swanson M, Moye JP, Ridner S. Stigma and quality of life in patients with advanced lung cancer. Oncol Nurs Forum (2019) 46(3):318–28. doi: 10.1188/19.ONF.318-328
33. Hatzenbuehler ML, Phelan JC, Link BG. Stigma as a fundamental cause of population health inequalities. Am J Public Health (2013) 103(5):813–21. doi: 10.2105/AJPH.2012.301069
34. Yeung NCY, Lu Q, Mak WWS. Self-perceived burden mediates the relationship between self-stigma and quality of life among Chinese American breast cancer survivors. Support Care Cancer (2019) 27(9):3337–45. doi: 10.1007/s00520-018-4630-2
35. Fong AJ, Scarapicchia TMF, McDonough MH, Wrosch C, Sabiston CM. Changes in social support predict emotional well-being in breast cancer survivors. Psychooncology (2017) 26(5):664–71. doi: 10.1002/pon.4064
36. Zamanian H, Amini-Tehrani M, Jalali Z, Daryaafzoon M, Ramezani F, Malek N, et al. Stigma and quality of life in women with breast cancer: mediation and moderation model of social support, sense of coherence, and coping strategies. Front Psychol (2022) 13:657992. doi: 10.3389/fpsyg.2022.657992
37. Mikal JP, Grande SW, Beckstrand MJ. Codifying online social support for breast cancer patients: retrospective qualitative assessment. J Med Internet Res (2019) 21(10):e12880. doi: 10.2196/12880
38. Liu JE, Mok E, Wong T. Perceptions of supportive communication in Chinese patients with cancer: experiences and expectations. J Adv Nurs (2005) 52(3):262–70. doi: 10.1111/j.1365-2648.2005.03583.x
39. Lee M, Song Y, Zhu L, Ma GX. Coping strategies and benefit-finding in the relationship between non-disclosure and depressive symptoms among breast cancer survivors in China. Am J Health Behav (2017) 41(4):368–77. doi: 10.5993/AJHB.41.4.1
40. Lepore SJ. A social-cognitive processing model of emotional adjustment to cancer. In: Baum A, Andersen BL, editors. Psychosocial interventions for cancer, vol. p . Washington, DC: American Psychological Association (2001). p. 99–116.
41. Lepore SJ, Revenson TA. Social constraints on disclosure and adjustment to cancer. Soc Pers Psychol Compass (2007) 1(1):313–33. doi: 10.1111/j.1751-9004.2007.00013.x
Keywords: breast cancer, stigma, self-disclosure, social support, quality of life, structural equation model
Citation: Bu L, Chen X, Zheng S and Fan G (2023) Construction of the structural equation model of stigma, self-disclosure, social support, and quality of life of breast cancer patients after surgery—a multicenter study. Front. Oncol. 13:1142728. doi: 10.3389/fonc.2023.1142728
Received: 12 January 2023; Accepted: 21 April 2023;
Published: 18 May 2023.
Edited by:
Luigi Cavanna, Azienda Ospedaliera di Piacenza, ItalyReviewed by:
Jianfei Xie, Central South University, ChinaMasoud Sadeghi, Islamic Azad University, Iran
Copyright © 2023 Bu, Chen, Zheng and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaoyan Zheng, c3l6aGVuZ0BzdHUuZWR1LmNu; Guanhua Fan, ZmFuZ2hAc3R1LmVkdS5jbg==
 Liuxiu Bu1
Liuxiu Bu1 Guanhua Fan
Guanhua Fan