
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 16 June 2023
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1142133
 Feng-Yu Tian1
Feng-Yu Tian1 Jue-Xin Wang1
Jue-Xin Wang1 Gang Huang1
Gang Huang1 Wen An1
Wen An1 Li-Si Ai1
Li-Si Ai1 Sui Wang1
Sui Wang1 Pei-Zhu Wang1
Pei-Zhu Wang1 Yan-Bo Yu1,2,3*
Yan-Bo Yu1,2,3* Xiu-Li Zuo1,2,3
Xiu-Li Zuo1,2,3 Yan-Qing Li1,2,3
Yan-Qing Li1,2,3Objective: The worldwide incidence of primary small intestinal lymphoma (PSIL) is increasing. However, little is known about the clinical and endoscopic characteristics of this disease. The aim of this study was to investigate the clinical and endoscopic data of patients with PSIL, with the goal of enhancing our understanding of the disease, improving diagnostic accuracy, and facilitating more accurate prognosis estimation.
Methods: Ninety-four patients diagnosed with PSIL were retrospectively studied at Qilu Hospital of Shandong University between 2012 and 2021. The clinical data, enteroscopy findings, treatment modalities, and survival times were collected and analyzed.
Results: Ninety-four patients (52 males) with PSIL were included in this study. The median age of onset was 58.5 years (range: 19-80 years). Diffuse large B-cell lymphoma (n=37) was the most common pathological type. Abdominal pain (n=59) was the most frequent clinical presentation. The ileocecal region (n=32) was the most commonly affected site, and 11.7% of patients had multiple lesions. At the time of diagnosis, the majority of patients (n=68) were in stages I-II. A new endoscopic classification of PSIL was developed, including hypertrophic type, exophytic type, follicular/polypoid type, ulcerative type, and diffusion type. Surgery did not show a significant increase in overall survival; chemotherapy was the most commonly administered treatment. T-cell lymphoma, stages III-IV, “B” symptoms, and ulcerative type were associated with poor prognosis.
Conclusion: This study provides a comprehensive analysis of the clinical and endoscopic features of PSIL in 94 patients. This highlights the importance of considering clinical and endoscopic characteristics for accurate diagnosis and prognosis estimation during small bowel enteroscopy. Early detection and treatment of PSIL is associated with a favorable prognosis. Our findings also suggest that certain risk factors, such as pathological type, “B” symptoms, and endoscopic type, may affect the survival of PSIL patients. These results underscore the need for careful consideration of these factors in the diagnosis and treatment of PSIL.
The gastrointestinal (GI) tract is the predominant site of extranodal non-Hodgkin’s lymphoma, accounting for 30-45% of all cases (1). Primary small intestinal lymphoma (PSIL) is a malignant tumor that originates in the lamina propria and submucosal lymphoid tissue of the small intestine. PSIL occurs infrequently, accounting for 20% to 30% of primary gastrointestinal (GI) lymphomas. Although PSIL is rare, its prevalence has been rising globally (1–4). The majority of primary gastrointestinal lymphomas are classified as non-Hodgkin’s lymphoma (NHL) based on histopathology (5, 6).
Primary small intestinal lymphoma (PSIL) is a rare disease that is often misdiagnosed until serious complications such as obstruction and bleeding develop. Although progress has been made in the diagnosis and treatment of gastric lymphomas, PSIL remains poorly characterized, and little is known about its clinical, enteroscopic, and pathological features. Furthermore, the optimal treatment for PSIL remains controversial, and the prognosis is unsatisfactory. The small bowel was previously inaccessible to endoscopists because of its depth, length, and complicated loops, limiting the possibility of large-scale studies on PSIL. Therefore, more research is needed to improve the diagnosis and management of PSIL (7, 8).
The introduction of balloon-assisted enteroscopy, including double-balloon enteroscopy (DBE) and single-balloon enteroscopy (SBE), has led to an increased number of patients being diagnosed with primary small intestinal lymphoma (PSIL) through endoscopic examination. However, the lack of information on the typical endoscopic features of PSIL makes it difficult to accurately diagnose the disease. Therefore, the purpose of this study was to evaluate the clinical and endoscopic features, management, and prognosis of PSIL patients diagnosed at Qilu Hospital of Shandong University over a period of approximately ten years to enhance our understanding of this disease.
From January 2012 to August 2021, a retrospective review of medical records and pathological data was conducted on 94 individuals with small intestine lymphoma who were identified through a database search at the Department of Pathology and the Digestive Endoscopic Center at Qilu Hospital of Shandong University. The patients’ information was anonymized prior to analysis. Histopathological diagnosis was based on the WHO classification (9, 10) and performed through morphologic and immunophenotypic analyses of endoscopically biopsied or surgically resected specimens. Patients were included in the study based on the definition of primary gastrointestinal non-Hodgkin’s lymphoma according to Lewin et al. (11). Those patients who presented with second malignancies or without follow-up information were excluded.
The diagnostic workup included a detailed medical history and physical examination, complete blood cell count, serum chemistry, abdominal ultrasonography, chest/abdomen/pelvis computed tomography (CT) scan, bone marrow aspiration or biopsy, and multiple endoscopic biopsies. If feasible, positron emission tomography (PET) imaging was performed in some patients.
Musshoff’s variation of the Ann Arbor method for gastrointestinal lymphoma was utilized for staging (12), and the imaging modality for clinical staging was CT or PET.
We updated the endoscopic classification system for small intestine lymphoma based on previous studies (5, 13–16), and two competent endoscopists assessed the endoscopic pictures and classified the endoscopic findings into five categories (1): hypertrophic type (2), exophytic tumor type (3), follicular/polypoid type (4), ulcerative type, and (5) diffusion type.
The survival of patients with small intestinal lymphoma was evaluated by the number of days. Overall survival (OS) was calculated from the date of diagnosis until the date of death or the final follow-up visit.
All analyses were performed using SPSS Statistics 23.0 software (SPSS Inc., Chicago, IL, USA). Normal continuous variables were reported as the means ± standard deviations, and significant differences were evaluated using Student’s t test. Nonnormally distributed continuous variables were reported as medians (25th percentile - 75th percentile). Significant differences were evaluated using the Mann−Whitney U test. During the nonparametric tests, proportions were compared using the chi-square test. In cases where the expected values were insufficient to meet the requirements of the chi-square test, Fisher’s exact test was employed. When conducting pairwise comparisons between multiple groups of categorical variables, the Bonferroni correction method was employed, or they were performed by using post hoc testing and observing standardized residuals to determine the significance of the differences. Overall survival was estimated using the life table and Kaplan−Meier product-limit method, and the values were compared using the log-rank test. Differences were considered significant when the P values were less than 0.05.
From January 2012 to August 2021, a total of 94 patients diagnosed with primary small intestinal lymphoma were included in this analysis. Table 1 summarizes the clinical characteristics of these patients. The median age of the cohort was 58.50 (48.00-66.25) years, with a gender distribution of 52 males and 42 females, and an age range of 19 to 80 years. The most common symptom was abdominal pain (n = 59), followed by abdominal distension (n = 26), nausea and vomiting (n = 18), hematemesis or hematochezia (n = 18), diarrhea (n = 10), and mass (n = 3). Twenty-two individuals exhibited “B” symptoms (fever, nocturnal sweats, and weight loss) (Figure 1). Of all patients, 19 (20.2%) acquired illness in the duodenum, 17 (18.1%) in the jejunum, 15 (15.9%) in the ileum, 32 (34.0%) in the ileocecal region, and 11 (11.7%) in more than one location.
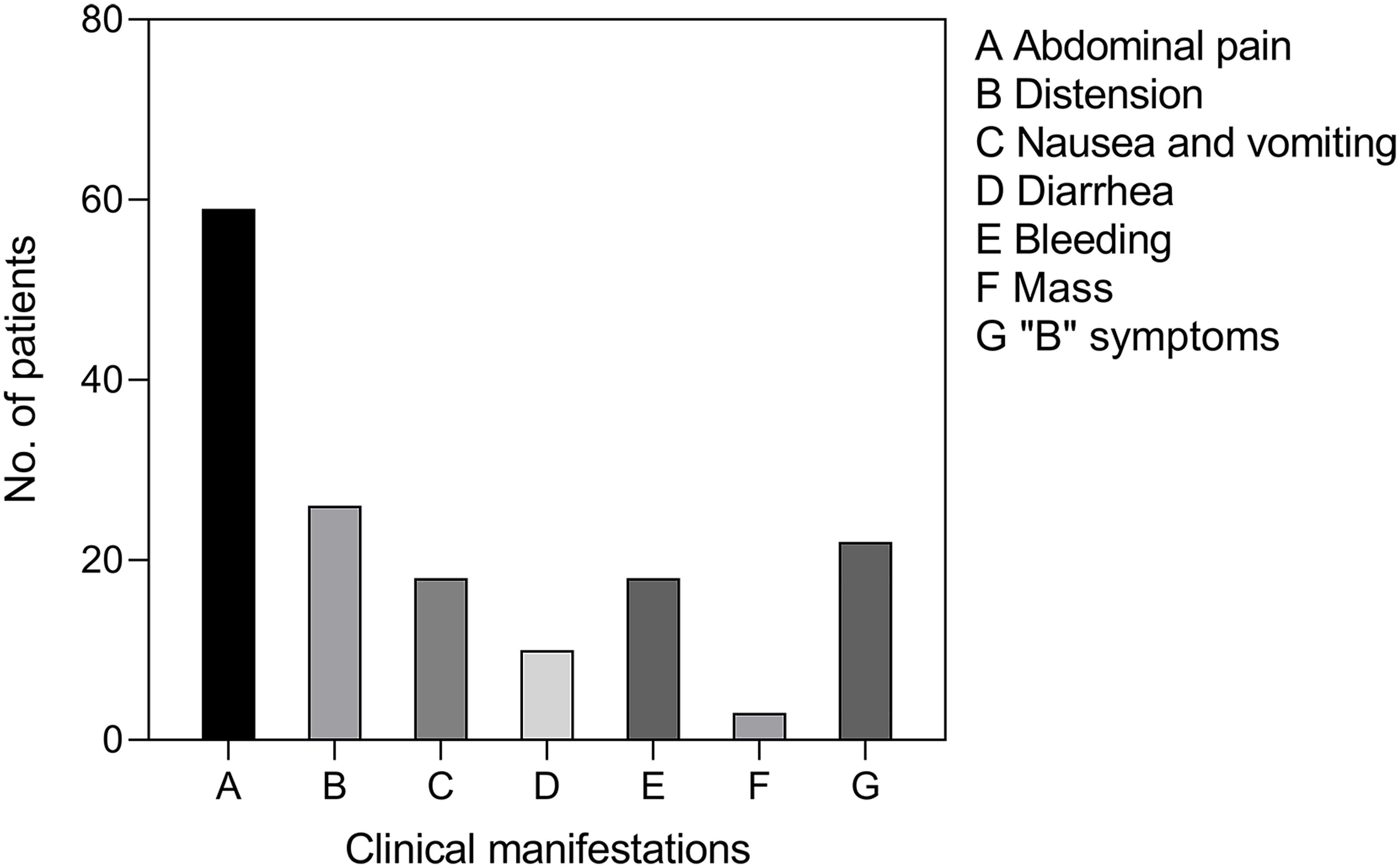
Figure 1 Clinical manifestations of PSIL. Abdominal pain was the most common symptom, and some patients had two or more symptoms. “B” symptoms: symptoms of fever, night sweats, and weight loss.
Among the various clinical variables studied, a higher number of patients exhibited abnormalities in certain indicators such as hemoglobin (HGB), albumin (ALB), lactate dehydrogenase (LDH), and adenosine deaminase (ADA). However, complete data for these specific indicators were not available for all patients. Among the 86 patients with available data, 38.3% had lower HGB levels, 47.9% had lower ALB levels, and 29.8% had higher LDH levels than the standard range. In the subset of 81 patients with available ADA data, 20.2% had ADA levels above the standard range. Additionally, 35 (40.7%) patients had two or more abnormal laboratory indicators (Table 1).
During computed tomography (CT) examination, intestinal wall thickening of varying degrees, space-occupying lesions in the small intestine, and celiac lymph node enlargement were observed in the patients. Sixty-eight (74.7%) patients had stage I-II disease, and 23 (25.3%) had stage III-IV disease (Table 1).
Diffuse large B-cell lymphoma (DLBCL), which comprises lymphocytes invading the intestinal epithelium (Figures 2 A, B), was the most prevalent subtype (37 patients). Mucosa-associated lymphoid tissue lymphoma (MALT lymphoma) was the second most common pathological type (27 patients), and it is characterized by significant infiltration of lymphocytes of normal size throughout the intestinal wall (Figures 2C, D). Eleven people were diagnosed with follicular lymphoma (FL). The formation of multiple or single submucosal lymphoid follicles was the pathological characteristic of the biopsy tissue (Figure 2E). Monomorphic epitheliotropic T-cell lymphoma (MEITL) and enteropathy-associated T-cell lymphoma (EATL) are primary intestinal lymphomas derived from intraepithelial lymphocytes, which are often characterized by diffuse infiltration of focally medium-sized lymphocytes in the intestinal epithelium (Figure 2F). In our study, we identified 2 patients with EATL and 3 patients with MEITL. In addition, there were 3 patients with mantle cell lymphoma (MCL), 3 patients with peripheral T-cell lymphoma (PTCL), and 2 patients with extranodal NK/T-cell lymphoma (ENKL).
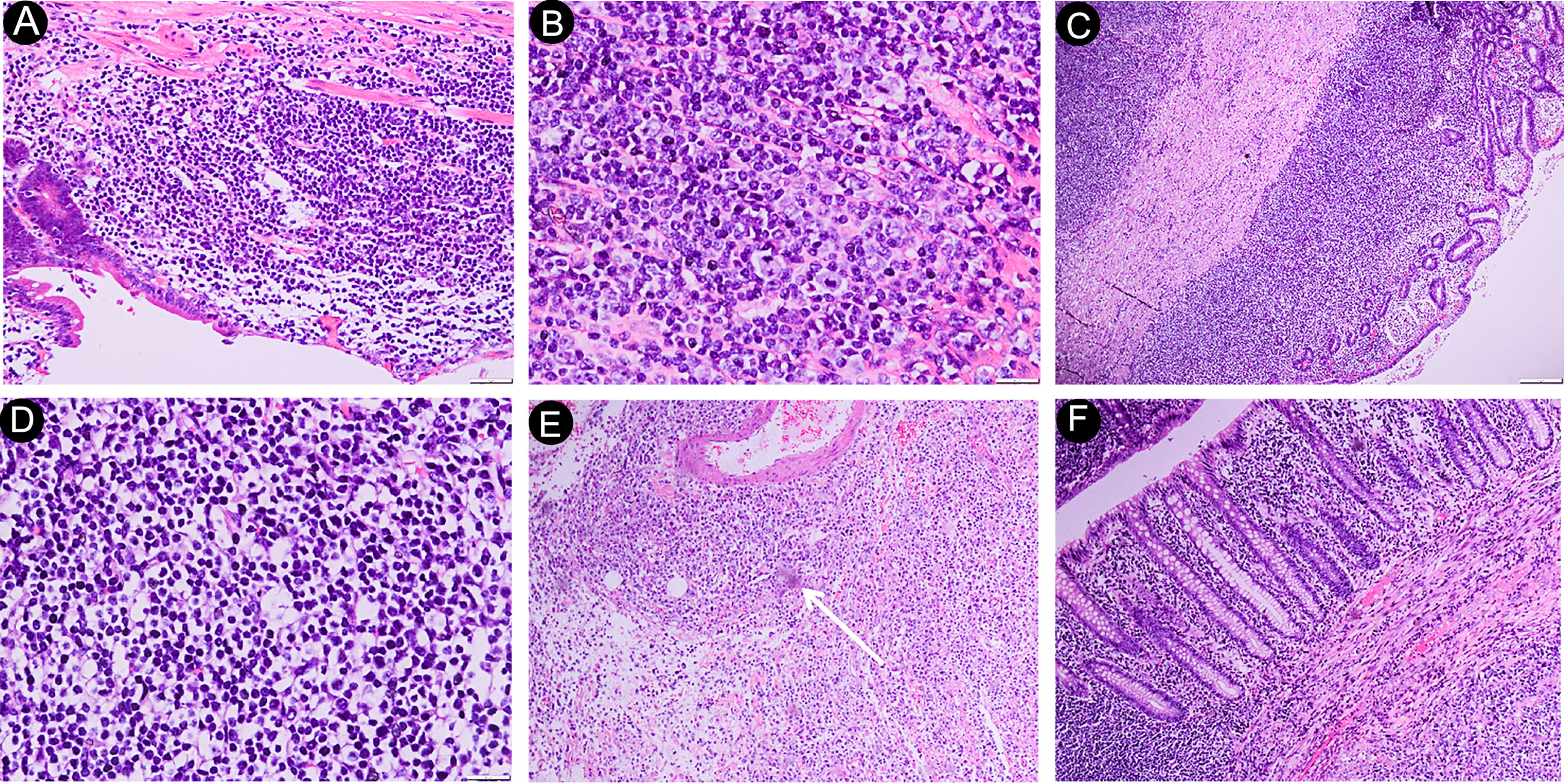
Figure 2 Histological features of PSIL. DLBCL of the ileum, photomicrograph (original magnification ×200, 400; H-E stain) of the specimen shows diffuse large lymphocytes (> 2.5-times larger than normal cells) infiltrating into the intestinal epithelium; no germinal centers were observed; some lymphocytes had nucleoli; lymphoepithelial lesions; part of the muscular layer had been destroyed (A, B). MALT of the jejunum, photomicrograph (original magnification ×40, 400; H-E stain). Histologically, extensive infiltration of normal-sized lymphocytes was observed throughout the corresponding intestinal wall and, occasionally, into the subserosa (C, D). Follicular lymphoma of the duodenum. The photomicrograph (original magnification ×40; H-E stain) shows the presence of individual submucosal lymphoid follicles (arrow), and no apoptotic appearance was observed (E). Monomorphic epitheliotropic intestinal T-cell lymphoma of the jejunum. The photomicrograph (original magnification, ×40; H-E stain) shows small- to focally medium-sized lymphocytes diffusely infiltrating into the intestinal epithelium. The nuclei are oval-shaped or distorted (F). DLBCL, diffuse large B-cell lymphoma; MALT, mucosa-associated lymphoid tissue lymphoma.
We further divided all patients into five pathological subgroups (DLBCL, MALT lymphoma, FL, T-cell lymphoma, and other B-cell lymphoma) to examine the major laboratory indicators and found that the LDH and ADA levels fluctuated significantly in patients with DLBCL. The HGB and ALB levels were lower in T-cell lymphoma and were relatively higher in patients with FL (Figure 3).
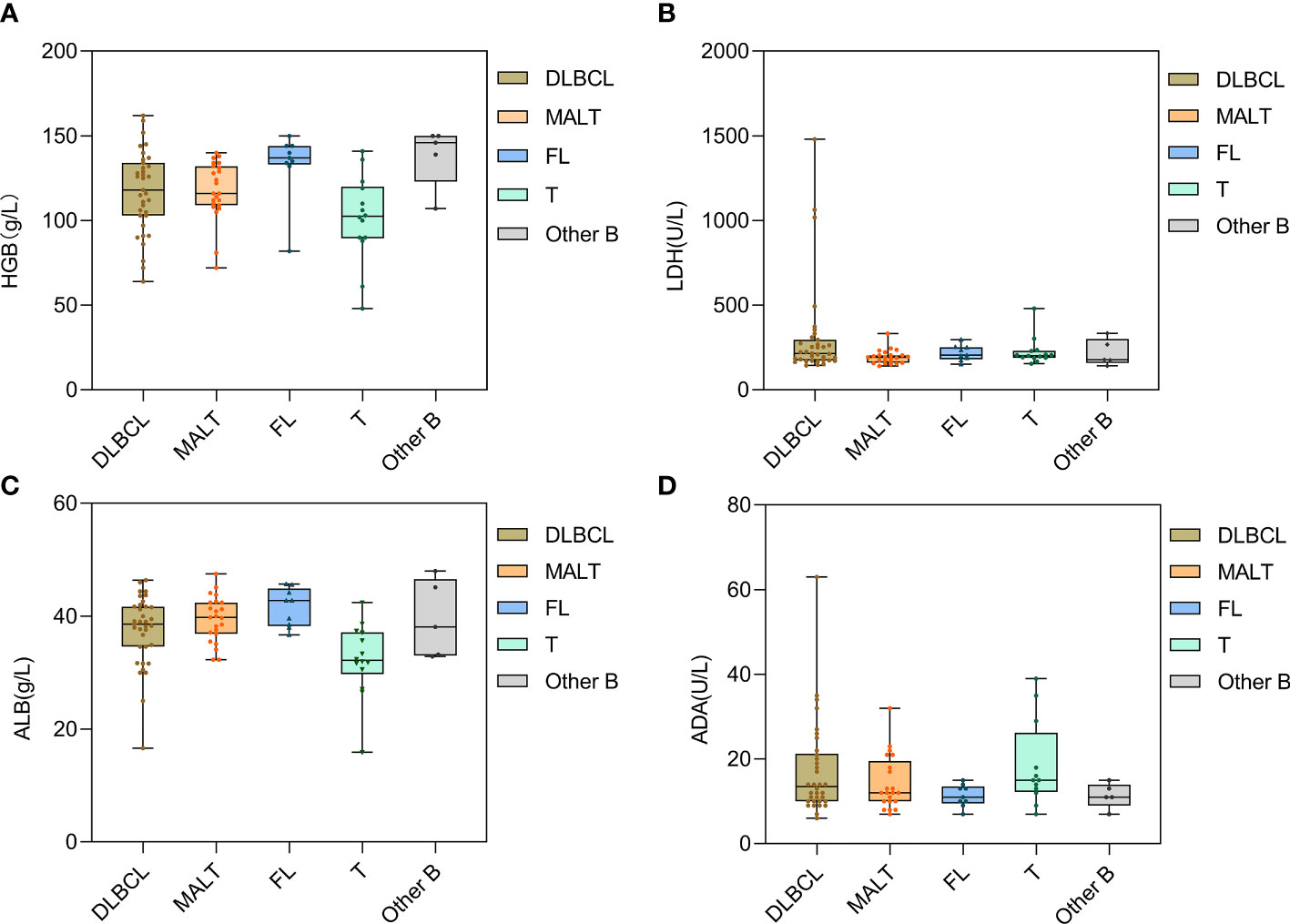
Figure 3 Distribution of laboratory indexes of PSIL in different pathological types. Patients with T-cell lymphoma showed lower HGB and ALB levels (A, C). Patients with DLBCL had large fluctuations in LDH and ADA levels (B, D). DLBCL, diffuse large B-cell lymphoma; MALT, mucosa-associated lymphoid tissue lymphoma; FL, follicular lymphoma; T, T-cell lymphoma; Other B, mantle cell lymphoma and other small B-cell lymphomas. HGB, hemoglobin; LDH, lactate dehydrogenase; ALB, albumin; ADA, adenosine deaminase.
During hospitalization, all patients underwent an enteroscopy examination. The endoscopic features were divided into the following groups: 16 patients had a hypertrophic type, characterized by edematous and irregular folds resulting in intestinal strictures that remained after maximum insufflation; 23 patients had an exophytic type, characterized by tumor-like lesions such as a large, friable, nodular mass with signs of hemorrhage, surface erosion, or moss cover; 17 patients had a follicular/polypoid type, characterized by multiple lymphoid follicular eminences or multiple polypoid lesions; 19 patients had an ulcerative type, characterized by single ulceration or multiple ulcerative lesions; and 19 patients had a diffuse type, characterized by mucosal erosion, punctate or patchy bleeding, mucosal rough hyperplasia, and other endoscopic manifestations. Among these patients, 4 had two or more endoscopic features simultaneously. For statistical analysis purposes, we selected the main endoscopic feature for each patient. All endoscopic characteristics are shown in Figure 4.
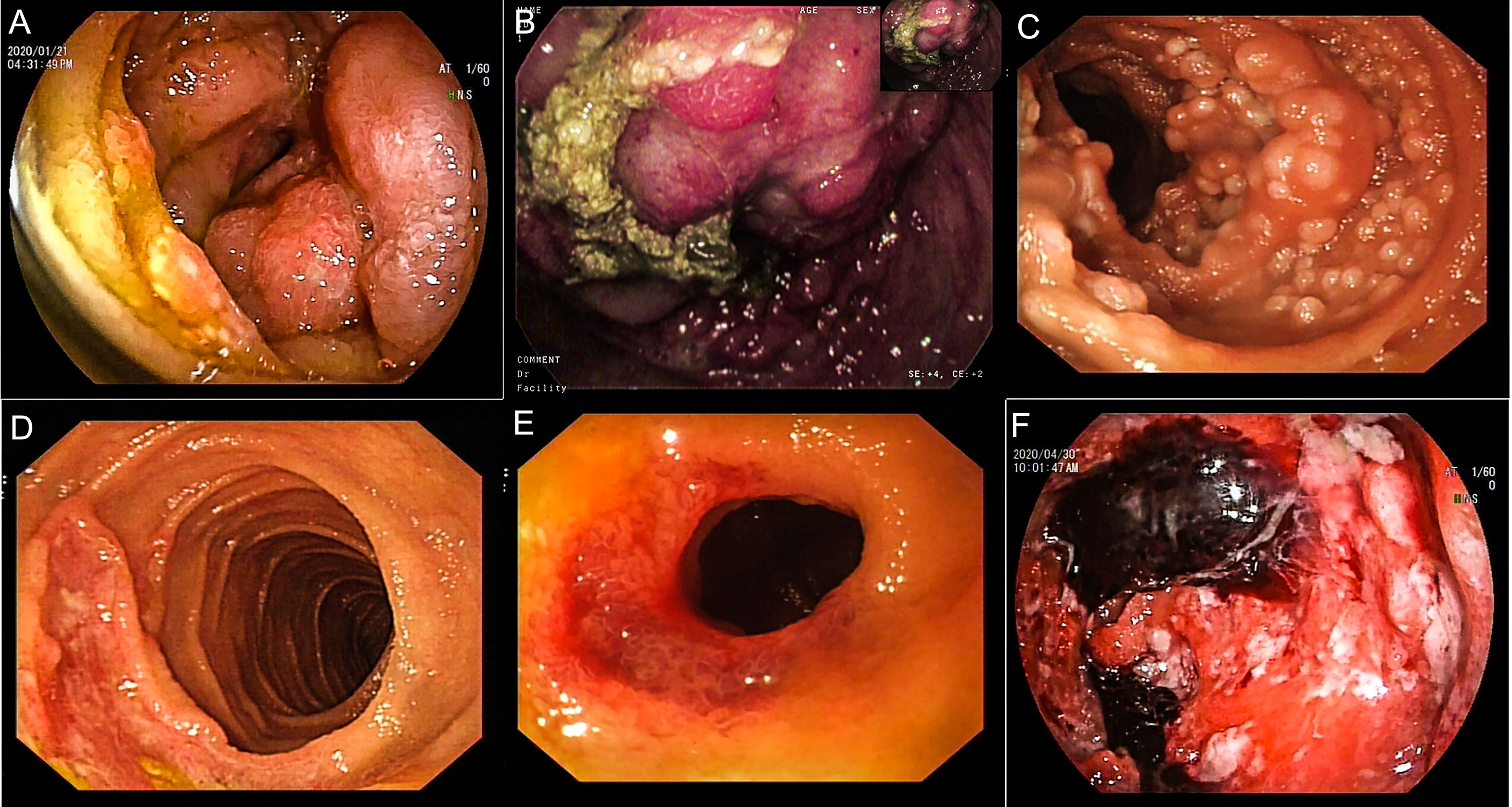
Figure 4 Endoscopic findings of patients with PSIL. Hypertrophic type, irregular mucosal eminence around the duodenum, pathologically confirmed as T-cell lymphoma (A). Exophytic type, ileocecum showed a huge protuberant lesion, surface hyperemia, edema and erosion, pathologically confirmed as MCL (B). Follicular/polypoid type, follicular mucosal eminence diffusely distributed in the proximal small intestine, and pathologically confirmed as FL (C). Ulcerative type, irregular ulcer spots on the ileocecal area, pathologically confirmed as MALT lymphoma (D). Diffusion type, lower jejunum circumferential stenosis, surface ulceration, blood scab attachment, pathology confirmed as MALT (E). Diffusion type, diffuse mucosal hyperemia erosion in the middle part of jejunum, covered with mucus, old blood clots, a rigid wall, a narrow lumen, and DLBCL was confirmed by pathology (F). DLBCL, diffuse large B-cell lymphoma; MALT, mucosa-associated lymphoid tissue lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma.
Abdominal pain was the most prevalent symptom across all endoscopic types. The overall distribution of other clinical symptoms among the different endoscopic types is detailed in Figure 5. The follicular/polypoid form was identified in 75% of asymptomatic patients. Bleeding (including hematemesis and hematochezia) was observed in 33.3% of follicular/polypoid lesions and 41.7% of ulcerative lesions. “B” symptoms were observed in 37.5% of exophytic lesions and 37.5% of ulcerative lesions. Based on Fisher’s exact test and subsequent post hoc testing, it was observed that in FL, the actual value of asymptomatic patients exceeded the expected value, with a standardized residual of 3.7. This suggests that patients with FL are more likely to present without abdominal and systemic symptoms, and the difference is significant (Supplementary Table S1).
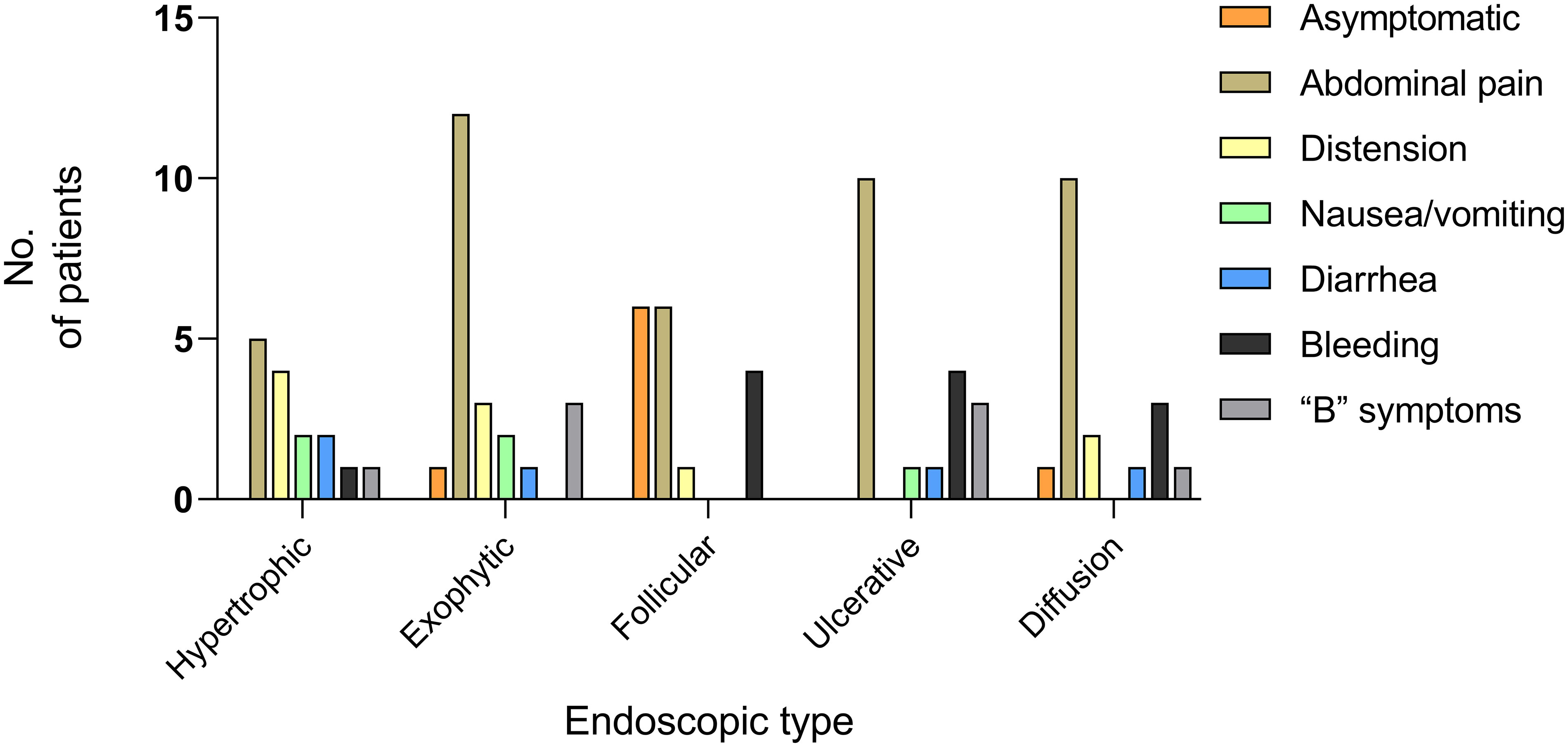
Figure 5 Clinical manifestations of PSIL in five endoscopic types. Abdominal pain emerged as the predominant symptom across all endoscopic types. Among the asymptomatic patients, 75% exhibited the follicular/polypoid type. The clinical symptoms in the data were the main symptoms of the patients. “B” symptoms: symptoms of fever, night sweats, and weight loss.
A total of 52.2% of the exophytic type originated in the ileocecal area, whereas 47.4% of the diffusion type originated in the duodenum (Figure 6). As visualized by imaging analysis, lymph node enlargement was observed in 85.0% of the exophytic type cases, 91.7% of the follicular/polypoid type, and 71.4% of the ulcerative type. However, it was reported in only 50.0% of the hypertrophic type cases and 52.9% of the diffusion type. The differences among the groups were statistically significant (P = 0.043). No significant differences were obtained in thickening and occupying among the subtypes of endoscopic features.
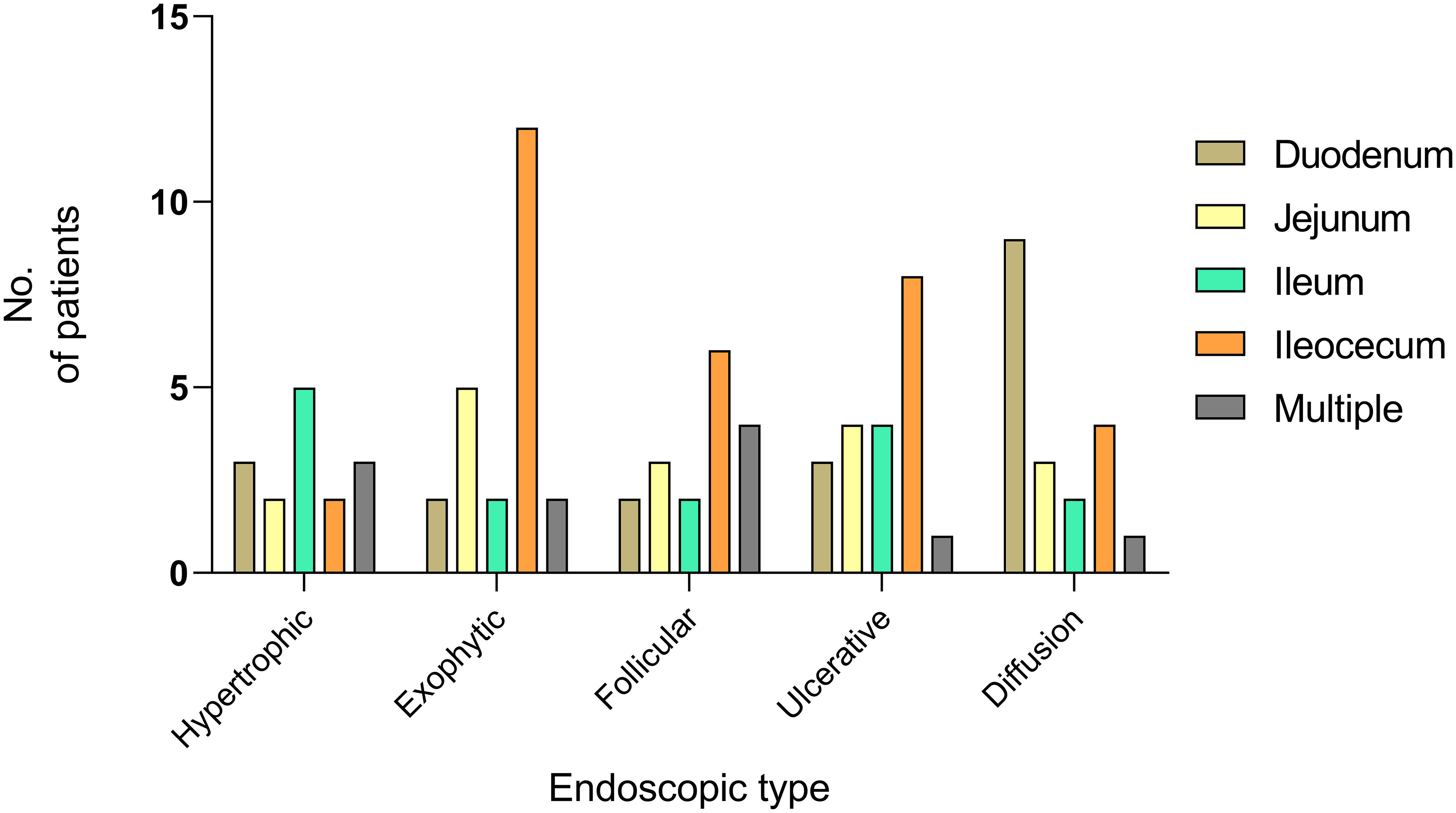
Figure 6 Onset sites of PSIL in different endoscopic types. Fifty-two percent of the exophytic type and 40% of the ulcerative type occurred in the ileocecal region, and 47.4% of patients with the diffusion type developed the disease in duodenum.
A histopathological diagnosis was performed on all patients, and the associations between endoscopic and pathological features were determined using Fisher’s exact test and the Bonferroni correction for multiple group comparisons. The results revealed a statistically significant difference between the groups (P < 0.05). Specifically, within the DLBCL subgroup, the exophytic type demonstrated a higher prevalence than the follicular/polypoid type. In the MALT subgroup, the diffusion type exhibited a greater occurrence than the exophytic type. Furthermore, in the FL subgroup, the follicular/polypoid type was more frequently observed than other types. No significant differences were observed among the different endoscopic types within the T-cell lymphoma subgroup (Table 2).
To investigate the association between clinical and endoscopic characteristics, patients were divided into four categories based on sex (male and female), age (≥ 65, < 65), stage (stages I-II and stages III-IV), and pathological type (B-cell and T-cell lymphoma).
There were significant variations in HGB between the sexes (P < 0.01). Age (P = 0.233), onset location (P = 0.412), histological type (P = 0.131), endoscopic characteristics (P = 0.812), clinical symptoms (P = 0.372), LDH (P = 0.258), ALB (P = 0.818), and ADA (P = 0.913) were not significantly different between male and female patients. (Supplementary Table S2).
The analysis of patients in distinct age categories revealed statistically significant variations in pathological type distribution (P = 0.048). According to the statistical data, FL was more prevalent among patients under 65 years of age. The onset site (P = 0.088), endoscopic features (P = 0.463), clinical symptoms (P = 0.294), hemoglobin (P = 0.970), LDH (P = 0.493), ALB (P = 0.111), and ADA (P = 0.744) were not significantly different between the age groups (Supplementary Table S3).
Compared with patients in stages I-II, patients in stages III-IV showed lower HGB (P = 0.026) and ALB (P < 0.01) levels, as well as elevated LDH (P < 0.01) and ADA (P < 0.01) levels. Ages, genders, and histopathologic types of patients in stages I-II and stages III-IV did not vary significantly (P > 0.05). (Supplementary Table S4).
The clinical characteristics of B-cell lymphoma and T-cell lymphoma were compared. Based on the pairwise comparisons between the groups, the incidence rate of “B” symptoms was higher in T-cell lymphoma than in B-cell lymphoma, indicating a significant difference (P = 0.038). In addition, T-cell lymphoma was associated with decreased levels of HGB and ALB (P < 0.01). No discernible differences were observed between B-cell and T-cell lymphomas in terms of endoscopic characteristics (P = 0.130), imaging characteristics such as thickening (P = 0.500), occupying (P = 0.549), or lymph node enlargement (P = 0.799), treatment strategy (P = 0.633), or laboratory indicators such as LDH (P = 0.797) and ADA (P = 0.136) (Supplementary Table S5).
Chemotherapy was the primary treatment modality used for 44 patients, while 17 patients chose to undergo surgery before receiving chemotherapy. Among these, four T-cell lymphoma patients died as a result of postoperative chemotherapy complications, such as intestinal obstruction, perforation, gastrointestinal bleeding, and abdominal infection. At the most recent follow-up, an additional 13 patients achieved complete remission (CR) and were still alive. Primary surgery was performed as an emergency measure for four patients experiencing obstruction (one patient), perforation (one patient), and severe abdominal pain (two patients). Of these, three patients (two with DLBCL and one with FL) had complete remission without relapse, while the fourth patient with MCL required emergency surgery due to severe abdominal pain after chemotherapy and died a month later. Five DLBCL patients and two MALT lymphoma patients underwent surgery alone, with two DLBCL patients lost to follow-up after discharge. The remaining three DLBCL patients and two MALT lymphoma patients achieved complete remission.
A larger proportion of patients with MALT lymphoma (15 out of 25) and T-cell lymphoma (8 out of 15) received chemotherapy alone, while a greater percentage of DLBCL patients (12 out of 35) received chemotherapy in combination with surgery. Only six patients with stage I DLBCL received surgery alone. Four patients with FL and seven patients with MALT lymphoma received conservative management, which included but was not limited to appropriate nutritional support, psychological support, physical therapy, and symptom control medication (Table 3).
Eight of 14 patients with the hypertrophic type and 11 of 22 patients with the exophytic type underwent surgery (Table 4). There were no discernible differences in survival rates between the surgical and nonsurgical groups for any endoscopic feature category.
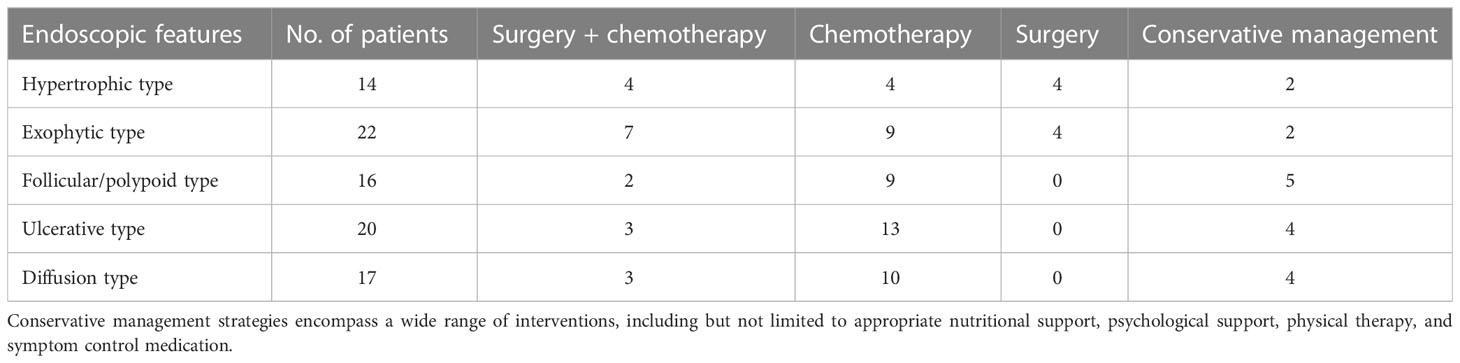
Table 4 Treatment According to the Endoscopic Type in Patients with Primary Small Intestinal Lymphoma.
Survival analyses were performed in 84 patients (of whom 10 were lost to follow-up), and the follow-up period ranging from 30 to 3353 d (mean, 692.6 d). Overall survival (OS) at five years was 62.0% (Table 5). The survival rate of patients in stages I-II was significantly higher than that of patients in stages III-IV (P < 0.01). T-cell lymphoma (P < 0.01) and “B” symptoms (P = 0.014) were related to a worse prognosis. Patients with ulcerative type had a lower OS, whereas patients with follicular/polypoid type had a better survival rate (Figure 7). Other variables, including sex, age, illness location, imaging characteristics (CT), and high LDH, had no influence on the survival rate (Table 5).
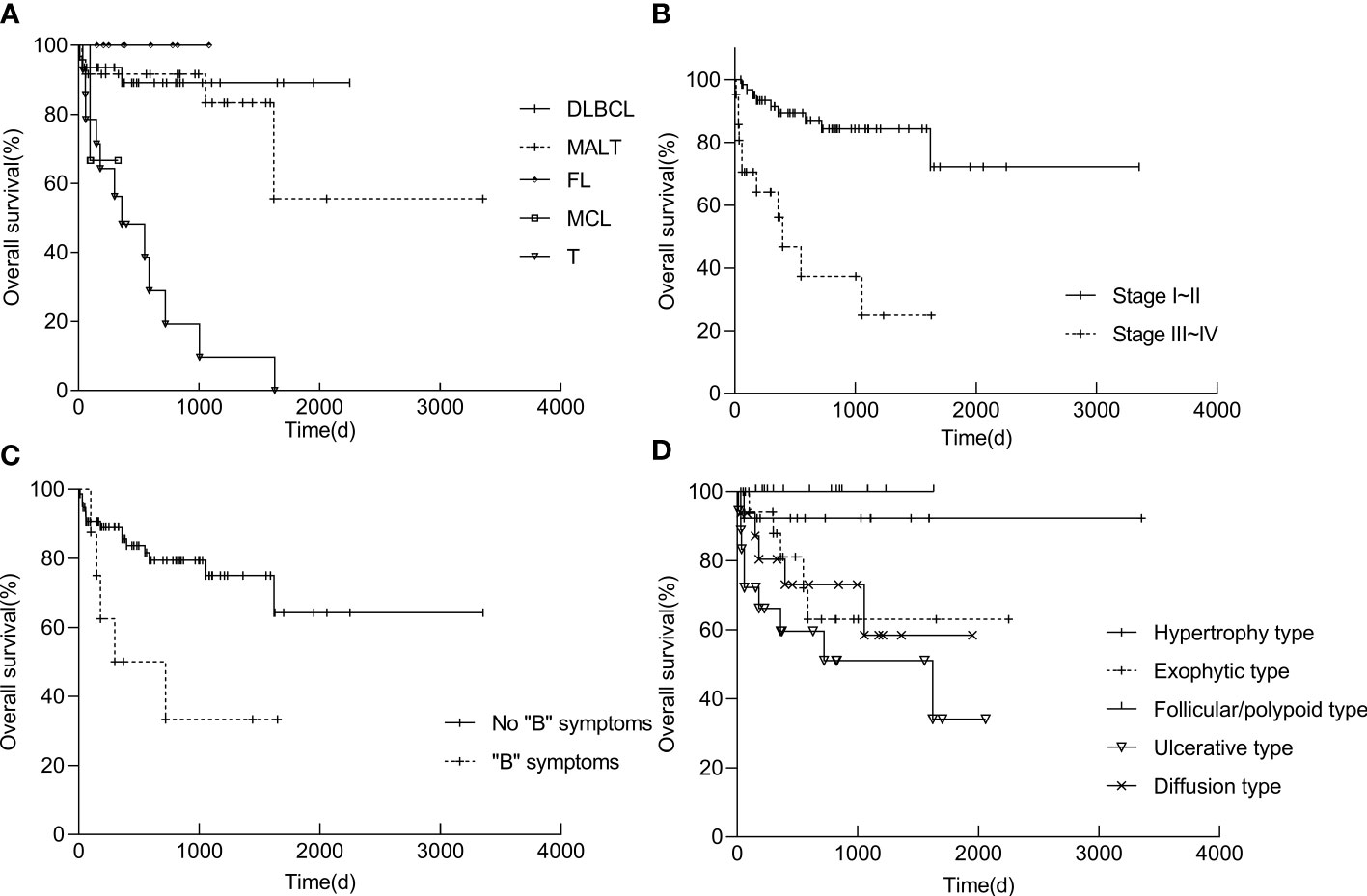
Figure 7 Kaplan−Meier survival curves of PSIL in pathological group (A), stage group (B), symptom group (C) and endoscopic group (D). Statistics of the patient survival rates were determined by 30-3353 days of follow-up. The life table and Kaplan−Meier product−limit method were used, and values were compared using the log rank test. Differences were considered significant when the P values were less than 0.05. DLBCL, diffuse large B-cell lymphoma; MALT, mucosa-associated lymphoid tissue lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; T, T-cell lymphoma; “B” symptoms: symptoms of fever, night sweats, and weight loss.
The diversity of PSIL in terms of patient features, stage, histologic subtypes, clinical symptoms, therapy, and prognosis has been widely acknowledged. While primary gastrointestinal lymphoma, particularly primary gastric lymphoma, has been the subject of numerous studies (1, 17–19), PSIL has received relatively little attention, with few studies conducted in mainland China (1, 20–22). In 2007, L. Yin et al. performed a retrospective study on 34 PSIL patients, which represents one of the few studies on this topic in China (7). In this study, we present the largest retrospective cohort of PSIL patients reported to date, providing a comprehensive analysis of clinical and histological characteristics, establishing an endoscopic classification, and conducting a survival analysis to investigate the long-term survival outcomes of PSIL patients.
Primary gastrointestinal lymphoma has been reported to have a male predominance and an average age of onset of ≥50 years, especially in the ileocecal region, with a male-to-female ratio up to 2.7:1 (11, 23–32). In our research, the average age was 56.4 years, and there was only a slight male preponderance for the entire small intestine (male to female ratio of 1.2:1) and the ileocecal region (male to female ratio of 1.3:1), which is lower than that reported in previous studies.
PSIL may manifest clinically in a variety of ways, with pain being the most prevalent symptom in all instances, followed by obstructive symptoms such as distension, nausea, and vomiting, as well as bleeding and diarrhea. The occurrence of “B” symptoms varies from 11.9% to 25% (7, 23, 33, 34).
Regarding illness location, we noticed that 34.1% of PSIL occurred in the ileocecal area, confirming prior findings that the ileocecal region is the most prevalent site for PSIL (1, 6, 23, 35). Koch P et al. noted that ileocecal lymphoma may indicate a good prognosis (23). In our series, no significant differences were observed in the outcomes among different disease locations. This overall improvement in prognosis may be attributed to the advancements made in the detection and treatment of PSIL.
Most authors have utilized the Ann Arbor classification with revisions by Musshoff or the Lugano staging method to determine the extent of GI lymphoma (12, 36). With the exception of the research by Wang W et al. (37), which revealed more patients with stage III-IV (72%) disease, most patients presented with early-stage (I-II) disease (17, 22, 29, 38). In our study, the majority of patients (74.4%) were classified as stages I-II, while 25.3% were classified as stages III-IV. Our findings confirm that early-stage disease (stages I-II) is associated with higher 5-year overall survival rates (73.0% for stages I-II vs. 27.0% for stages III-IV), consistent with previous reports (20, 21, 23, 39).
DLBCL and MALT lymphoma were the predominant pathological subtypes in our cohort, consistent with prior research (11, 17, 26, 40–42). MALT lymphoma is commonly found in the stomach, possibly due to the association with Helicobacter pylori infection as a significant risk factor. The B-cell phenotype represents more than 90% of gastrointestinal lymphomas (43, 44). In contrast to European countries, China appears to have a higher incidence of T-cell lymphoma in primary gastrointestinal lymphoma (1, 18, 23, 37). The small intestine is more susceptible to T-cell lymphoma, accounting for 16.6%-25.0% of cases (23, 29). In the current research, T and B lymphocyte phenotypes accounted for 14.9% and 85.1% of small intestine cases, respectively.
Based upon the presenting symptoms, investigations are usually requested in the diagnosis of PSIL, including endoscopy, CT, or PET (45). In our study, the CT findings of PSIL included wall thickening, lymph node hypertrophy, and lymph node occupation. These signs are indicative of the proliferation and lymphatic metastasis of intestinal lymphoma, which have important implications for the CT diagnosis of small intestinal lymphoma. Both SBE and DBE offer the benefit of tissue diagnosis during examination (46–48). A study of 13 patients with FL found that DBE indicated multifocal FL lesions in the jejunum and/or ileum in all patients, while abdominal CT and PET only detected GI tract involvement in one and two patients, respectively (49). In our study of 94 patients with PSIL, 88.3% obtained histological diagnoses through enteroscopy (including DBE and SBE).
Asyia Ahmad et al. initially classified gastrointestinal MALT lymphoma into the exogenous type, hypertrophic type, and ulcerative type based on endoscopy findings (5). In 2010, Angelo Zullo updated this classification and proposed a modified version, including the ulcerative type, exophytic type, hypertrophic type, petechial hemorrhage type, and normal/hyperemic type (15, 16). Another study on NK/T-cell lymphoma in the gastrointestinal tract identified four endoscopic types: superficial/erosive, ulcerative, ulceroinfiltrative, and infiltrative (13). In our study, we developed a novel categorization of five endoscopic subtypes of PSIL based on previous research. In this study, we comprehensively investigated various types of lymphomas. To ensure the inclusiveness of the new endoscopic classification system for these lymphoma types, we made selective updates to the existing endoscopic classifications reported in prior studies. Notably, in the case of follicular lymphoma (FL), a predominant presentation of multiple lymphoid follicles in the intestinal wall was observed during endoscopic examinations. To emphasize its distinctive features, we introduced a novel endoscopic phenotype termed the follicular/polypoid type. Furthermore, certain patients exhibited benign alterations such as superficial erosion or mild bleeding, which had been previously categorized as the normal/hyperemic type or superficial/erosive type in earlier investigations. By combining the endoscopic characteristics of both types, we defined this entity as the diffusion type. In DLBCL, exophytic endoscopic manifestations were significantly more common than the follicular/polypoid type, while in MALT lymphoma, the diffusion type was notably more prevalent than the exophytic type. This may be because exophytic masses tend to develop from particular pathogenic types, such as DLBCL. However, the majority of low-grade malignant GI lymphoma manifests with diffuse submucosal tumor infiltration (5, 50). Given that the follicular/polypoid type of FL is the most common and that the diagnosis under endoscopy was made in the majority of patients before nonspecific abdominal symptoms, we posit that precise recognition of endoscopic features holds considerable value in facilitating early detection and intervention of FL.
The most prevalent symptom across all the endoscopic types was abdominal discomfort. The fact that the majority of asymptomatic patients had follicular/polypoid endoscopic characteristics suggests that patients with the follicular/polypoid type may receive their pathology diagnosis by chance prior to the onset of nonspecific symptoms.
The treatment of gastrointestinal lymphoma remains controversial. Chemotherapy has been the mainstay of treatment for extranodal lymphomas, while the role of surgery has been less clear (51). In nearly half of previous studies, the combination of surgery and chemotherapy was found to improve overall survival (8, 20, 39, 52–59). However, some studies suggested that the combination of surgery and chemotherapy had no effect on OS or that its impact was uncertain (1, 39, 60–63). Surgical resection is preferred for patients with significant gastrointestinal bleeding, blockage, or perforation (37, 64). In our series, patients with prevalent B-cell pathological types, such as DLBCL, MALT lymphoma, and FL, achieved a high long-term survival rate after surgery, despite the presence of severe complications. However, for T-cell lymphoma and MCL, the postoperative prognosis was poor, regardless of whether patients received selective surgery before chemotherapy or emergency surgery due to severe complications. Therefore, we propose that surgery may improve the survival of some pathological types with relatively good prognoses, but this remains to be conclusively indicated. For certain pathological types, including T-cell lymphoma and MCL, surgery appears to have no discernible influence on prognosis. Further studies with larger sample sizes are needed to evaluate the efficacy of various therapeutic approaches in improving prognosis.
T-cell lymphoma and stages III-IV have previously been shown to indicate a lower survival rate in numerous studies (2, 65–67). In this investigation, we found that “B” symptoms and ulcerative endoscopy type were also associated with a lower survival rate. Contrary to previous studies (2, 7, 17, 18), we did not observe any association between normal LDH levels and prolonged OS, or between the ileocecal region and improved survival. Interestingly, patients with shown factors such as T-cell lymphoma, stages III-IV, “B” symptoms and ulcerative type were often accompanied by abnormal levels of LDH, ADA, HGB and ALB. We believe that these laboratory indicators have the potential to evaluate prognosis; however, additional large-sample randomized controlled trials are necessary to confirm this association.
However, this study has several limitations. First, the sample size of a single center is relatively small, and some results may require further validation through large-scale, multicenter studies. Second, the incidence of PSIL was relatively low, and the study covered a wide range of pathological types, with some types having a small number of cases. Therefore, prognostic judgment based on endoscopic features and laboratory examinations may have certain limitations. Finally, we updated the endoscopic classification system based on previous studies. However, as previous studies mainly focused on a single pathological type of lymphoma, including different types of lymphoma in our study may cause some bias in the number of specific endoscopic features.
In conclusion, this study provides a comprehensive analysis of the clinical and histological characteristics of PSIL, establishing an endoscopic classification and performing a survival analysis. The ileocecal region is the most common site for PSIL. DLBCL and MALT lymphoma were the predominant pathological subtypes. The novel categorization of the five endoscopic subtypes of PSIL developed in this study has potential for use in diagnosis and prognosis. Poor prognosis was associated with stages III-IV, “B” symptoms, T-cell lymphoma, and ulcerative type. The requirement for surgical resection should be determined based on individual patient characteristics. Overall, these findings provide valuable insights into the clinical features and outcomes of PSIL and can help improve the diagnosis, treatment, and prognosis of this disease.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Research Ethics Committee of Shandong University Qilu Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
F-YT performed the clinical reviews and data collection, analyzed the data and drafted the manuscript. F-YT and GH reviewed the endoscopic images. SW, WA, L-SA collected and interpreted the data. P-ZW analyzed the data. Y-BY obtained the funding, designed the study, reviewed the endoscopic images and critically revised the manuscript. X-LZ and Y-QL designed the study and critically revised the manuscript. All authors discussed the results and commented on the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China [NSFC 82070540]; and the Taishan Scholars Program of Shandong Province(tsqn202211309).
We thank all the patients and the authors involved in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1142133/full#supplementary-material
1. Wang GB, Xu GL, Luo GY, Shan HB, Li Y, Gao XY, et al. Primary intestinal non-hodgkin's lymphoma: a clinicopathologic analysis of 81 patients. World J Gastroenterol (2011) 17(41):4625–31. doi: 10.3748/wjg.v17.i41.4625
2. Peng JC, Zhong L, Ran ZH. Primary lymphomas in the gastrointestinal tract. J Dig Dis (2015) 16(4):169–76. doi: 10.1111/1751-2980.12234
3. Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer (1972) 29(1):252–60. doi: 10.1002/1097-0142(197201)29:1<252::aid-cncr2820290138>3.0.co;2-#
4. Muller AM, Ihorst G, Mertelsmann R, Engelhardt M. Epidemiology of non-hodgkin's lymphoma (Nhl): trends, geographic distribution, and etiology. Ann Hematol (2005) 84(1):1–12. doi: 10.1007/s00277-004-0939-7
5. Ahmad A, Govil Y, Frank BB. Gastric mucosa-associated lymphoid tissue lymphoma. Am J Gastroenterol (2003) 98(5):975–86. doi: 10.1111/j.1572-0241.2003.07424.x
6. Ghimire P, Wu GY, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol (2011) 17(6):697–707. doi: 10.3748/wjg.v17.i6.697
7. Yin L, Chen CQ, Peng CH, Chen GM, Zhou HJ, Han BS, et al. Primary small-bowel non-hodgkin's lymphoma: a study of clinical features, pathology, management and prognosis. J Int Med Res (2007) 35(3):406–15. doi: 10.1177/147323000703500316
8. Iida T, Nozawa H, Sonoda H, Toyama K, Kawai K, Hata K, et al. Upfront surgery for small intestinal non-hodgkin's lymphoma. Anticancer Res (2020) 40(4):2373–7. doi: 10.21873/anticanres.14206
9. Jaffe ES. The 2008 who classification of lymphomas: implications for clinical practice and translational research. Hematol Am Soc Hematol Educ Program (2009) 2009:523–31. doi: 10.1182/asheducation-2009.1.523
10. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood (2016) 127(20):2375–90. doi: 10.1182/blood-2016-01-643569
11. Lewin KJ, Ranchod M, Dorfman RF. Lymphomas of the gastrointestinal tract. a study of 117 cases presenting with gastrointestinal disease. Cancer (1978) 42(2):693–707. doi: 10.1002/1097-0142(197808)42:2<693::aid-cncr2820420241>3.0.co;2-j
12. Mushoff K. Klinische stadieneiteilung der nicht-Hodgkin lymphoma. Stranlentherapie (1977) 153:218–21.
13. Kim JH, Lee JH, Lee J, Oh SO, Chang DK, Rhee PL, et al. Primary nk-/T-Cell lymphoma of the gastrointestinal tract: clinical characteristics and endoscopic findings. Endoscopy (2007) 39(2):156–60. doi: 10.1055/s-2006-945114
14. Seifert E, Schulte F, Weismüller J, De Mas C, Stolte M. Endoscopic and bioptic diagnosis of malignant non-hodgkin's lymphoma of the stomach. Endoscopy (1993) 25(08):497–501. doi: 10.1055/s-2007-1010384
15. Zullo A, Hassan C, Andriani A, Cristofari F, Cardinale V, Spinelli GP, et al. Primary low-grade and high-grade gastric malt-lymphoma presentation. J Clin Gastroenterol (2010) 44(5):340–4. doi: 10.1097/MCG.0b013e3181b4b1ab
16. Zullo A, Hassan C, Cristofari F, Perri F, Morini S. Gastric low-grade mucosal-associated lymphoid tissue-lymphoma: helicobacter pylori and beyond. World J Gastrointest Oncol (2010) 2(4):181–6. doi: 10.4251/wjgo.v2.i4.181
17. d'Amore F, Brincker H, Grønbaek K, Thorling K, Pedersen M, Jensen M, et al. Non-hodgkin's lymphoma of the gastrointestinal tract: a population-based analysis of incidence, geographic distribution, clinicopathologic presentation features, and prognosis. Danish lymphoma study group. J Clin Oncol (1994) 12(8):1673–84. doi: 10.1200/JCO.1994.12.8.1673
18. Gurney K, Cartwright R, Gilman E. Descriptive epidemiology of gastrointestinal non-hodgkin’s lymphoma in a population-based registry. Br J Cancer (1999) 79(11):1929–34. doi: 10.1038/sj.bjc.6690307
19. Nakamura S, Matsumoto T, Iida M, Yao T, Tsuneyoshi M. Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer (2003) 97(10):2462–73. doi: 10.1002/cncr.11415
20. Kim SJ, Choi CW, Mun YC, Oh SY, Kang HJ, Lee SI, et al. Multicenter retrospective analysis of 581 patients with primary intestinal non-Hodgkin lymphoma from the consortium for improving survival of lymphoma (Cisl). BMC Cancer (2011) 11:321. doi: 10.1186/1471-2407-11-321
21. Gou HF, Zang J, Jiang M, Yang Y, Cao D, Chen XC. Clinical prognostic analysis of 116 patients with primary intestinal non-Hodgkin lymphoma. Med Oncol (2012) 29(1):227–34. doi: 10.1007/s12032-010-9783-x
22. Lightner AL, Shannon E, Gibbons MM, Russell MM. Primary gastrointestinal non-hodgkin’s lymphoma of the small and Large intestines: a systematic review. J Gastrointest Surg (2016) 20(4):827–39. doi: 10.1007/s11605-015-3052-4
23. Koch P, del Valle F, Berdel WE, Willich NA, Reers B, Hiddemann W, et al. Primary gastrointestinal non-hodgkin’s lymphoma: i. anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German multicenter study git nhl 01/92. J Clin Oncol (2001) 19(18):3861–73. doi: 10.1200/JCO.2001.19.18.3861
24. Radaszkiewicz T, Dragosics B, Bauer P. Gastrointestinal malignant lymphomas of the mucosa-associated lymphoid tissue: factors relevant to prognosis. Gastroenterology (1992) 102(5):1628–38. doi: 10.1016/0016-5085(92)91723-H
25. Ruskone-Fourmestraux A, Aegerter P, Delmer A, Brousse N, Galian A, Rambaud JC. Primary digestive tract lymphoma: a prospective multicentric study of 91 patients. groupe d'etude des lymphomes digestifs. Gastroenterology (1993) 105(6):1662–71. doi: 10.1016/0016-5085(93)91061-l
26. Morton J, Leyland M, Vaughan Hudson G, Vaughan Hudson B, Anderson L, Bennett M, et al. Primary gastrointestinal non-hodgkin's lymphoma: a review of 175 British national lymphoma investigation cases. Br J Cancer (1993) 67(4):776–82. doi: 10.1038/bjc.1993.141
27. Liang R, Todd D, Chan TK, Chiu E, Lie A, Kwong YL, et al. Prognostic factors for primary gastrointestinal lymphoma. Hematol Oncol (1995) 13(3):153–63. doi: 10.1002/hon.2900130305
28. Azab MB, Henry-Amar M, Rougier P, Bognel C, Theodore C, Carde P, et al. Prognostic factors in primary gastrointestinal non-hodgkin's lymphoma. a multivariate analysis, report of 106 cases, and review of the literature. Cancer (1989) 64(6):1208–17. doi: 10.1002/1097-0142(19890915)64:6<1208::aid-cncr2820640608>3.0.co;2-z
29. Hansen P, Vogt K, Skov R, Pedersen-Bjergaard U, Jacobsen M, Ralfkiaer E. Primary gastrointestinal non-hodgkin's lymphoma in adults: a population-based clinical and histopathologic study. J Intern Med (1998) 243(7):71–8. doi: 10.1046/j.1365-2796.1998.00317.x
30. Hong YW, Kuo IM, Liu YY, Yeh TS. The role of surgical management in primary small bowel lymphoma: a single-center experience. Eur J Surg Oncol (2017) 43(10):1886–93. doi: 10.1016/j.ejso.2017.06.016
31. Juarez-Salcedo LM, Sokol L, Chavez JC, Dalia S. Primary gastric lymphoma, epidemiology, clinical diagnosis, and treatment. Cancer Control (2018) 25(1):1073274818778256. doi: 10.1177/1073274818778256
32. Thieblemont C, Cascione L, Conconi A, Kiesewetter B, Raderer M, Gaidano G, et al. A malt lymphoma prognostic index. Blood (2017) 130(12):1409–17. doi: 10.1182/blood-2017-03-771915
33. Howdle PD, Jalal PK, Holmes GK, Houlston RS. Primary small-bowel malignancy in the uk and its association with coeliac disease. QJM (2003) 96(5):345–53. doi: 10.1093/qjmed/hcg058
34. Feng L, Zhang G, Hu Z, Zou Y, Chen F, Zhang G, et al. Diagnosis and treatment of 81 patients with primary gastrointestinal lymphoma. Zhong Nan Da Xue Xue Bao Yi Xue Ban (2009) 34(7):582–8.
35. Beaton C, Davies M, Beynon J. The management of primary small bowel and colon lymphoma–a review. Int J Colorectal Dis (2012) 27(5):555–63. doi: 10.1007/s00384-011-1309-2
36. Rosenberg SA. Report of the committee on the staging of hodgkin's disease. Cancer Res: AACR (1966) 26(6):1310.
37. Wang W, Lin P, Yao H, Jia X, Sun J. Clinical analysis of primary gastrointestinal non-hodgkin's lymphoma. Pak J Med Sci (2017) 33(6):1406–11. doi: 10.12669/pjms.336.13631
38. Otter R, Bieger R, Kluin P, Hermans J, Willemze R. Primary gastrointestinal non-hodgkin's lymphoma in a population-based registry. Br J Cancer (1989) 60(5):745–50. doi: 10.1038/bjc.1989.351
39. Lee J, Kim WS, Kim K, Ko YH, Kim JJ, Kim YH, et al. Intestinal lymphoma: exploration of the prognostic factors and the optimal treatment. Leukemia lymphoma (2004) 45(2):339–44. doi: 10.1080/10428190310001593111
40. Kim SJ, Kang HJ, Kim JS, Oh SY, Choi CW, Lee SI, et al. Comparison of treatment strategies for patients with intestinal diffuse Large b-cell lymphoma: surgical resection followed by chemotherapy versus chemotherapy alone. Blood (2011) 117(6):1958–65. doi: 10.1182/blood-2010-06-288480
41. Salles G, Herbrecht R, Tilly H, Berger F, Brousse N, Gisselbrecht C, et al. Aggressive primary gastrointestinal lymphomas: review of 91 patients treated with the lnh-84 regimen. a study of the groupe d'etude des lymphomes agressifs. Am J Med (1991) 90(1):77–84. doi: 10.1016/0002-9343(91)90509-v
42. Herrmann R, Panahon AM, Barcos MP, Walsh D, Stutzman L. Gastrointestinal involvement in non-hodgkin's lymphoma. Cancer (1980) 46(1):215–22. doi: 10.1002/1097-0142(19800701)46:1<215::AID-CNCR2820460136>3.0.CO;2-6
43. Chan J. Gastrointestinal lymphomas: an overview with emphasis on new findings and diagnostic problems. Semin Diagn Pathol (1996) 13(4):260–96.
44. Song L-N, Cen X-N, Ou J-P, Liang Z-Y, Qiu Z-X, Wang W-S, et al. Clinical and prognostic analysis of 101 cases of primary gastrointestinal non-hodgkin's lymphoma. J Exp Hematol (2013) 21(2):387–91. doi: 10.7534/j.issn.1009-2137.2013.02.026
45. Ding D, Pei W, Chen W, Zuo Y, Ren S. Analysis of clinical characteristics, diagnosis, treatment and prognosis of 46 patients with primary gastrointestinal non-Hodgkin lymphoma. Mol Clin Oncol (2014) 2(2):259–64. doi: 10.3892/mco.2013.224
46. Mitsui K, Tanaka S, Yamamoto H, Kobayashi T, Ehara A, Yano T, et al. Role of double-balloon endoscopy in the diagnosis of small-bowel tumors: the first Japanese multicenter study. Gastrointest Endosc (2009) 70(3):498–504. doi: 10.1016/j.gie.2008.12.242
47. Yamagami H, Oshitani N, Hosomi S, Suekane T, Kamata N, Sogawa M, et al. Usefulness of double-balloon endoscopy in the diagnosis of malignant small-bowel tumors. Clin Gastroenterol Hepatol (2008) 6(11):1202–5. doi: 10.1016/j.cgh.2008.05.014
48. Almeida N, Figueiredo P, Lopes S, Gouveia H, Leitão MC. Double-balloon enteroscopy and small bowel tumors: a south-European single-center experience. Dig Dis Sci (2009) 54(7):1520–4. doi: 10.1007/s10620-008-0512-7
49. Akamatsu T, Kaneko Y, Ota H, Miyabayashi H, Arakura N, Tanaka E. Usefulness of double balloon enteroscopy and video capsule endoscopy for the diagnosis and management of primary follicular lymphoma of the gastrointestinal tract in its early stages. Dig Endosc (2010) 22(1):33–8. doi: 10.1111/j.1443-1661.2009.00915.x
50. Kolve M, Fischbach W, Greiner A, Wilms K. Differences in endoscopic and clinicopathological features of primary and secondary gastric non-hodgkin's lymphoma. Gastrointest Endosc (1999) 49(3):307–15. doi: 10.1016/S0016-5107(99)70006-4
51. Zelenetz AD, Gordon LI, Chang JE, Christian B, Abramson JS, Advani RH, et al. Nccn Guidelines(R) insights: b-cell lymphomas, version 5.2021. J Natl Compr Canc Netw (2021) 19(11):1218–30. doi: 10.6004/jnccn.2021.0054
52. Ibrahim E, Ezzat A, El-Weshi A, Martin J, Khafaga Y, Al Rabih W, et al. Primary intestinal diffuse Large b-cell non-hodgkin's lymphoma: clinical features, management, and prognosis of 66 patients. Ann Oncol (2001) 12(1):53–8. doi: 10.1023/A:1008389001990
53. Tang TC, Kuo MC, Chang H, Dunn P, Wang PN, Wu JH, et al. Primary colonic lymphoma: an analysis of 74 cases with localized Large-cell lymphoma. Eur J Haematol (2011) 87(1):28–36. doi: 10.1111/j.1600-0609.2011.01632.x
54. Lai YL, Lin JK, Liang WY, Huang YC, Chang SC. Surgical resection combined with chemotherapy can help achieve better outcomes in patients with primary colonic lymphoma. J Surg Oncol (2011) 104(3):265–8. doi: 10.1002/jso.21927
55. Lee HS, Park LC, Lee EM, Shin SH, Ye BJ, Oh SY, et al. Comparison of therapeutic outcomes between surgical resection followed by r-chop and r-chop alone for localized primary intestinal diffuse Large b-cell lymphoma. Am J Clin Oncol (2014) 37(2):182–7. doi: 10.1097/COC.0b013e318271b125
56. Khosla D, Kumar R, Kapoor R, Kumar N, Bera A, Sharma SC. A retrospective analysis of clinicopathological characteristics, treatment, and outcome of 27 patients of primary intestinal lymphomas. J Gastrointest Cancer (2013) 44(4):417–21. doi: 10.1007/s12029-013-9519-1
57. Drolet S, Maclean AR, Stewart DA, Dixon E, Paolucci EO, Buie WD. Primary colorectal lymphoma-clinical outcomes in a population-based series. J Gastrointest Surg (2011) 15(10):1851–7. doi: 10.1007/s11605-011-1572-0
58. She WH, Day W, Lau PY, Mak KL, Yip AW. Primary colorectal lymphoma: case series and literature review. Asian J Surg (2011) 34(3):111–4. doi: 10.1016/j.asjsur.2011.08.004
59. Daum S, Ullrich R, Heise W, Dederke B, Foss H-D, Stein H, et al. Intestinal non-hodgkin’s lymphoma: a multicenter prospective clinical study from the German study group on intestinal non-hodgkin’s lymphoma. J Clin Oncol (2003) 21(14):2740–6. doi: 10.1200/JCO.2003.06.026
60. Li B, Shi Y-K, He X-H, Zou S-M, Zhou S-Y, Dong M, et al. Primary non-Hodgkin lymphomas in the small and Large intestine: clinicopathological characteristics and management of 40 patients. Int J Hematol (2008) 87(4):375–81. doi: 10.1007/s12185-008-0068-5
61. Kobayashi H, Nagai T, Omine K, Sato K, Ozaki K, Suzuki T, et al. Clinical outcome of non-surgical treatment for primary small intestinal lymphoma diagnosed with double-balloon endoscopy. Leuk Lymphoma (2013) 54(4):731–6. doi: 10.3109/10428194.2012.725850
62. Tse E, Gill H, Loong F, Kim SJ, Ng SB, Tang T, et al. Type ii enteropathy-associated T-cell lymphoma: a multicenter analysis from the Asia lymphoma study group. Am J Hematol (2012) 87(7):663–8. doi: 10.1002/ajh.23213
63. Wang S-L, Liao Z-X, Liu X-F, Yu Z-H, Gu D-Z, Qian T-N, et al. Primary early-stage intestinal and colonic non-hodgkin’s lymphoma: clinical features, management, and outcome of 37 patients. World J Gastroenterol (2005) 11(37):5905. doi: 10.3748/wjg.v11.i37.5905
64. Maguire LH, Geiger TM, Hardiman KM, Regenbogen SE, Hopkins MB, Muldoon RL, et al. Surgical management of primary colonic lymphoma: big data for a rare problem. J Surg Oncol (2019) 120(3):431–7. doi: 10.1002/jso.25582
65. Bautista-Quach MA, Ake CD, Chen M, Wang J. Gastrointestinal lymphomas: morphology, immunophenotype and molecular features. J Gastrointest Oncol (2012) 3(3):209–25. doi: 10.3978/j.issn.2078-6891.2012.024
66. Cardona DM, Layne A, Lagoo AS. Lymphomas of the gastro-intestinal tract - pathophysiology, pathology, and differential diagnosis. Indian J Pathol Microbiol (2012) 55(1):1–16. doi: 10.4103/0377-4929.94847
Keywords: primary small intestinal lymphoma, enteroscopy, gastrointestinal oncology, treatment and prognosis, clinical presentation
Citation: Tian F-Y, Wang J-X, Huang G, An W, Ai L-S, Wang S, Wang P-Z, Yu Y-B, Zuo X-L and Li Y-Q (2023) Clinical and endoscopic features of primary small bowel lymphoma: a single-center experience from mainland China. Front. Oncol. 13:1142133. doi: 10.3389/fonc.2023.1142133
Received: 18 April 2023; Accepted: 01 June 2023;
Published: 16 June 2023.
Edited by:
Abdelkrim Hmadcha, Valencian International University, SpainReviewed by:
Muhamad Alhaj Moustafa, Mayo Clinic Florida, United StatesCopyright © 2023 Tian, Wang, Huang, An, Ai, Wang, Wang, Yu, Zuo and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan-Bo Yu, eXV5YW5ibzIwMDBAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.