- 1Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia
- 2Discipline of Genetic Counselling, Graduate School of Health, University of Technology Sydney, Sydney, NSW, Australia
- 3Parkville Familial Cancer Centre, Peter MacCallum Cancer Centre and Royal Melbourne Hospital, Parkville, VIC, Australia
- 4Department of Oncology, Royal Women’s Hospital, Parkville, VIC, Australia
- 5Hunter Genetics, Hunter Family Cancer Service, Newcastle, NSW, Australia
- 6Hereditary Cancer Clinic, Prince of Wales Hospital, Sydney, NSW, Australia
- 7Division of Clinical Cancer Genomics, Department of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center, Duarte, CA, United States
- 8Genomic Medicine Institute, Geisinger, Danville, PA, United States
- 9Cancer Research Division, Cancer Council NSW, Sydney, NSW, Australia
- 10Melbourne School of Population and Global Health, Melbourne University, Melbourne, VIC, Australia
Introduction: “Mainstreaming” is a proposed strategy to integrate genomic testing into oncology. The aim of this paper is to develop a mainstreaming oncogenomics model by identifying health system interventions and implementation strategies for mainstreaming Lynch syndrome genomic testing.
Methods: A rigorous theoretical approach inclusive of conducting a systematic review and qualitative and quantitative studies was undertaken using the Consolidated Framework for Implementation Research. Theory-informed implementation data were mapped to the Genomic Medicine Integrative Research framework to generate potential strategies.
Results: The systematic review identified a lack of theory-guided health system interventions and evaluation for Lynch syndrome and other mainstreaming programs. The qualitative study phase included 22 participants from 12 health organizations. The quantitative Lynch syndrome survey included 198 responses: 26% and 66% from genetic and oncology health professionals, respectively. Studies identified the relative advantage and clinical utility of mainstreaming to improve genetic test access and to streamline care, and adaptation of current processes was recognized for results delivery and follow-up. Barriers identified included funding, infrastructure and resources, and the need for process and role delineation. The interventions to overcome barriers were as follows: embedded mainstream genetic counselors, electronic medical record genetic test ordering, results tracking, and mainstreaming education resources. Implementation evidence was connected through the Genomic Medicine Integrative Research framework resulting in a mainstreaming oncogenomics model.
Discussion: The proposed mainstreaming oncogenomics model acts as a complex intervention. It features an adaptable suite of implementation strategies to inform Lynch syndrome and other hereditary cancer service delivery. Implementation and evaluation of the model are required in future research.
Introduction
Genomic testing (GT) strategies to identify those with hereditary cancer continue to expand. International evidence-based guidelines recommend that those with a significant cancer family history are offered germline GT and counseling (1–3). However, family history-based testing criteria result in suboptimal identification of hereditary cancer (4), and a high proportion of people who harbor pathogenic variants are not referred for GT even if they meet the testing criteria (4–10). Evidence for GT for those with particular tumor subtypes, e.g., epithelial ovarian cancer (11) and triple-negative breast cancer patients under 60 years (12), now allows direct access to GT in routine oncology care (mainstreaming), and guidelines are emerging for endometrial and colorectal cancers (13–15).
Genetic testing for colorectal cancer (CRC) targets deficient mismatch repair (dMMR) genes to identify Lynch syndrome (LS). In the CRC context, the dMMR genes MLH1, MSH2, MSH6, PMS2, or EPCAM are the first to be tested. However, limiting testing to these five genes can miss cases where heritability exists (16). Application of a wider 112-gene panel test with known and candidate genes for CRC in 274 patients found that 25% of cases had a pathogenic variant or variant of unknown significance that could potentially prove pathogenic in the future (17). Additionally, a 25-cancer gene panel testing used in a prospective study of early-onset CRC (<50 years) proved the benefit of this approach identifying 16% of patients (72 of 450) to have a pathogenic variant in at least one cancer predisposition gene (18). Similarly, a prospective cohort of 381 unselected endometrial cancer (EC) cases found that 9.2% had an LS gene and 3.4% had pathogenic variants in other cancer predisposition genes, with the application a 25-cancer gene panel (19). Multigene panel testing is emerging as a more effective approach but is challenged by the clinical utility and lack of evidence of disease causality in some genes included in the panel.
Identifying hereditary cancer and the significant higher risk of cancer development is important to enable access to early detection and risk-reducing measures at an earlier age, reducing cancer-related morbidity and mortality (19–23). Given the suboptimal identification of hereditary cancers, there is a lost opportunity for cancer prevention. Emerging opinion advocates for universal GT for hereditary breast and ovarian cancer (HBOC) and CRC and EC-associated LS at a general population level (24).
In the United Kingdom (UK), general population support exists for access to GT in determining the risk of ovarian cancer (25), CRC (26), and breast cancer at the population-level screening (27, 28). A positive attitude to a population-level GT exists when there are proven benefits, such as prevention and risk-reducing treatments (29).
In contrast, healthcare professionals are more cautious about population-based cancer GT. Fifty percent (166/330 for HBOC and 164/326 for LS responses) of the surveyed United States of America (USA)-based genetic counselors were reluctant to offer population-based GT (30). The main concern was health system readiness to implement large-scale GT. A further decade to prepare was highlighted in terms of workforce shortages, the need to upskill staff in genomics, and infrastructure barriers (31). However, public health initiatives to integrate genomics into health systems are emerging and build on initiatives to improve the identification of HBOC and LS initiated through the United States Preventive Services Task Force in 2005 and 2013 (32, 33).
In 2009, the Evaluation of Genomic Applications in Practice and Prevention recommended all CRC patients to undergo tumor screening for LS with follow-up GT if the screening tests were positive (34). A recent review using the Centers for Disease Control and Prevention Science Impact Framework (35) aimed to track the uptake of these recommendations through the five domains of the framework: disseminating science, creating awareness, catalyzing action, effecting change, and shaping the future (35). Practice change and widespread adoption of recommendations were initially slow to translate. However, after a decade of raising awareness through education, state cancer care planning, policy and national initiatives, and evolving evidence of genomic test utility, integration of screening and testing is beginning to emerge (35).
To accelerate genomics implementation into health services, the concept of a learning healthcare system came to life in 2007 (36) and was defined as an approach to allow for “science, informatics, incentives, and culture align for continuous improvement and innovation, with best practices seamlessly embedded in the delivery process and new knowledge captured as an integral by-product of the delivery experience” (36). Since 2015, the USA has taken the learning health care system approach to integrating genomics (37). The concept of complete learning in the system forges a continuum of clinical research aligned to care and quality improvement, allowing optimal care through continual adaptation to knowledge and evidence (37).
To assist in the translation of genomics into routine care, implementation frameworks to guide services have been utilized. The Implementation of Genomic Medicine Interventions in Clinical Care (IGNITE) framework (38) was developed and identified nine existing constructs from the Consolidated Framework for Implementation Research (CFIR) (39) deemed useful for genomics implementation. Seven additional constructs deemed important in genomics were developed to address patients, families, and communities (39). Further collaboration between IGNITE and the Clinical Sequencing Evidence-Generating Research consortium led to the development of the Genomic Medicine Integrative Research (GMIR) framework (40).
The GMIR framework consists of four domains that cover the organizational contextual factors, interventions, processes, and outcomes to investigate and build strategies for genomic clinical implementation depending on the disease context (40). The GMIR framework has been used in chronic kidney disease and rare undiagnosed pediatric disease contexts (40). However, to date, there is no specific conceptual oncogenomic clinical service model or strategy to guide the health system on the integration of genomic testing into routine oncology care.
With this gap in mind, we aimed to develop an implementation science-informed evidence-based conceptual mainstreaming oncogenomics clinical service model. With the future forecast for health system readiness for population-based cancer GT, our conceptual clinical service model provides generalizable implementation strategies scalable to the population-based level.
Materials and methods
Study design
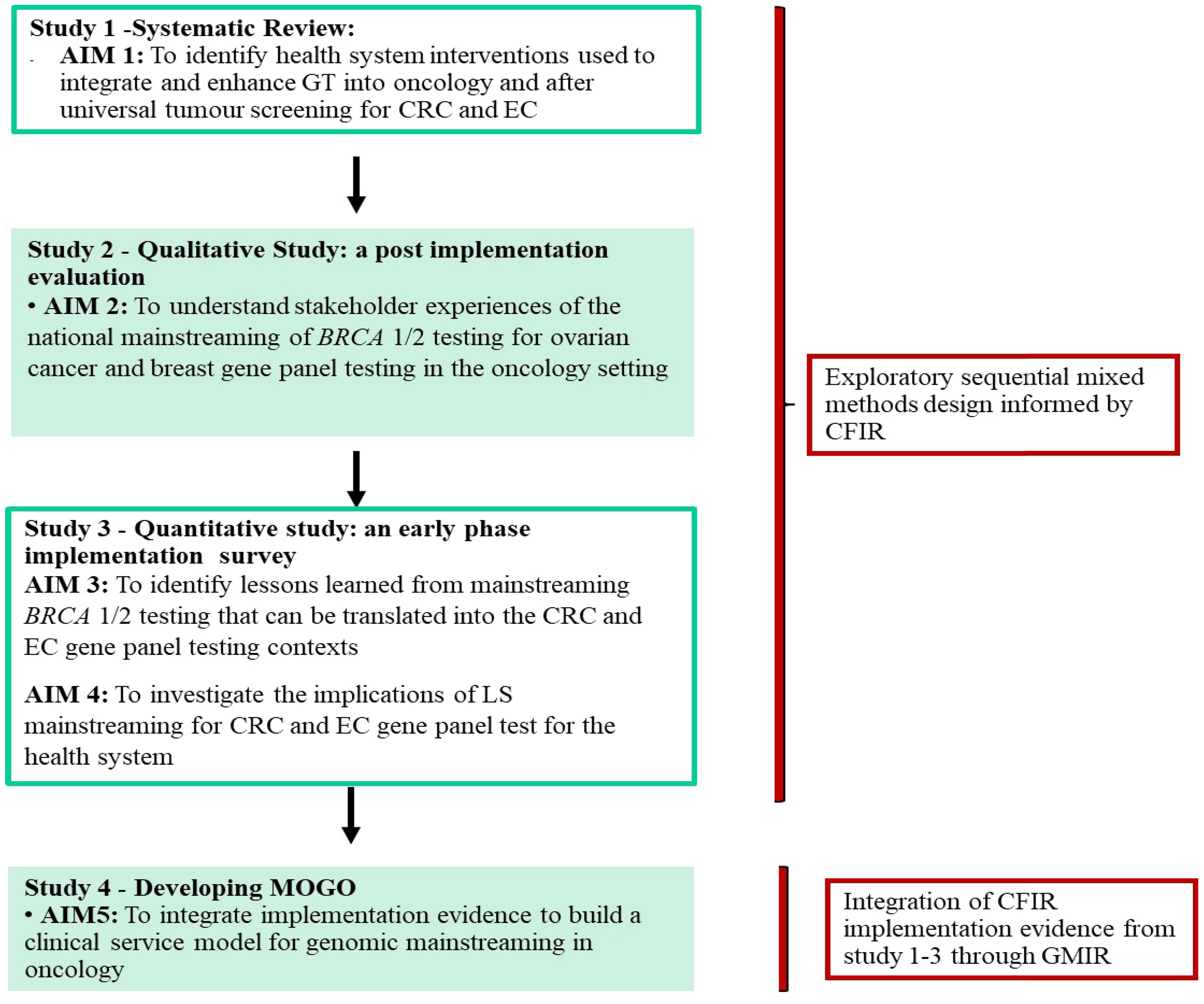
Three studies were completed to inform the development of the conceptual clinical service model (Figure 1). The model combines implementation evidence from a systematic review of GT programs in oncology care (41) (see Supplementary Table 1 for the inclusion/exclusion criteria and the reference list of the included studies), a post-implementation BRCA mainstreaming qualitative study (42), and a quantitative survey with LS stakeholders about the potential implementation of GT mainstreaming in CRC and EC (43). We used an exploratory sequential mixed methods design (44). The systematic review was conducted to identify interventions that have been used to integrate GT into oncology services internationally with outcomes mapped to CFIR (39). The results from the CFIR-guided qualitative BRCA evaluation (42) were used in the design of an LS survey to focus on genomics implementation in the CRC and EC context (38). The full methods of each study are described in the primary publications (41–43).
Data integration and model development
The development of the conceptual clinical service model was through the building and generating of a model concept of mixed methods design and analysis (44). Data integration and analysis were through complementarity, where each set of methods was used to answer a series of questions for the purposes of evaluation and elaboration (45). The qualitative data from the BRCA mainstreaming evaluated key implementation factors that were then used to collect quantitative data to gain a broader understanding in the CRC and EC context. Complementarity of data was achieved by connecting qualitative, quantitative, and systematic review data through sequential dataset building informing the next study stage using the complementary CFIR domains and constructs. The qualitative (qual) and quantitative (quan) data were given the same level of importance and denoted (qual → quan) by the mixed methods notation system (46). Data transformation occurred at the analytical point of integration where the qual results were analyzed and, at a second stage, were then quantified. The connecting of qualitative to quantitative data allowed for narrative weaving by relating the qual and quan findings through a theme-by-CFIR construct basis, with the addition of synthesizing the systematic review findings. Finally, the details of the conceptual clinical service mainstreaming oncogenomics model were generated by matching the synthesized results from the narrative weaving to the four domains of the GMIR (40), either through contextual (health system, clinician, or individual/family factors) or mainstreaming program factors (interventions, process, or outcomes) (Figure 2).
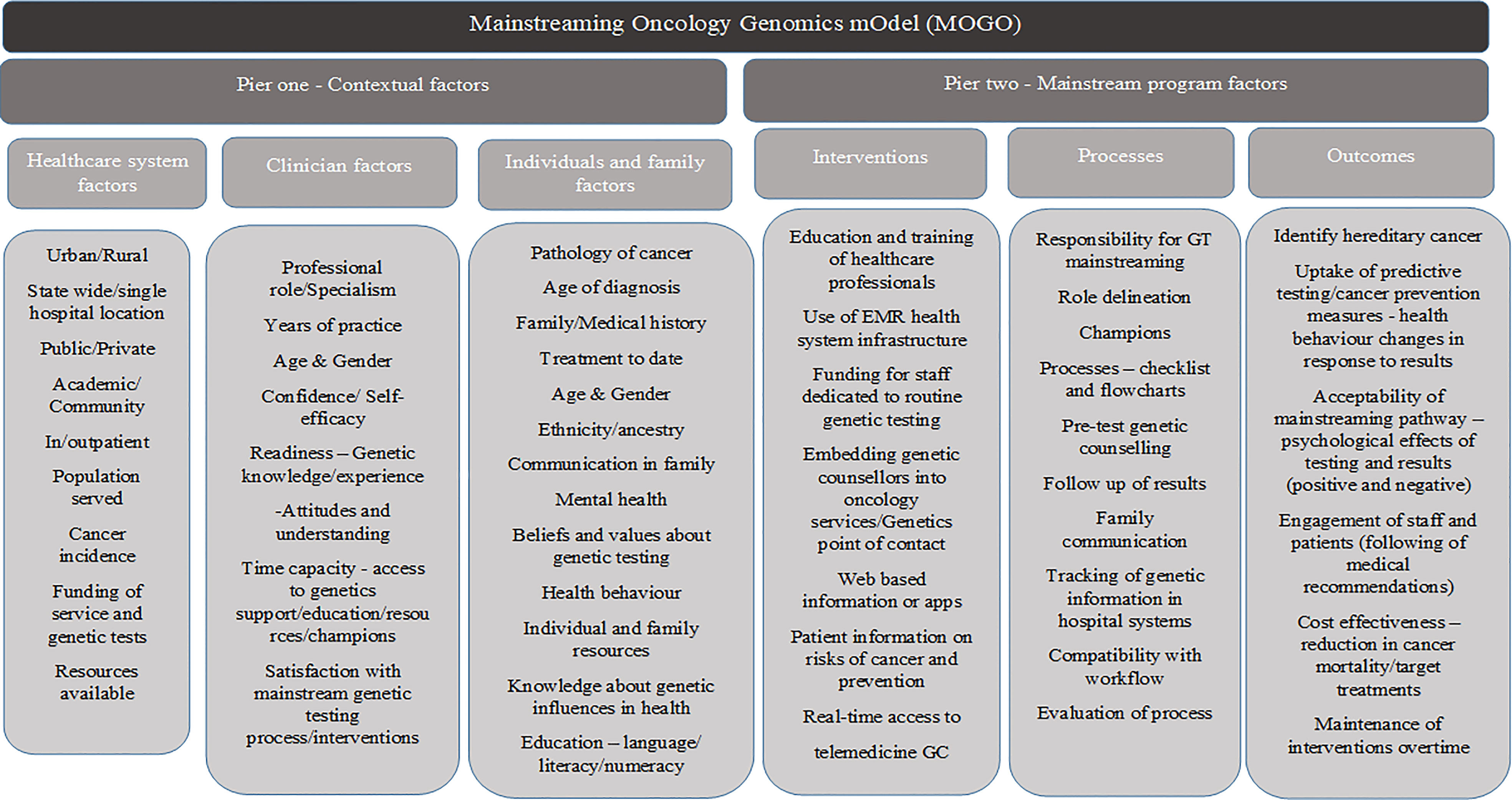
Figure 2 The GMIR-informed mainstreaming oncology genomics model for LS and other GT mainstreaming service delivery in oncology care.
Results
Systematic review
The systematic review (SR) identified 25 intervention studies used globally to integrate GT into oncology care (41) (Supplementary reference list). The CFIR complementary evidence used in the model development pertained to studies that measured patients’ or healthcare professionals’ satisfaction, belief, and feedback of the mainstreaming intervention, the engagement of health professionals through education, or implementing and executing the intervention according to an implementation plan. The intervention characteristics most used in the included studies to facilitate the integration of GT into oncology care were education followed by systems [i.e., electronic medical record (EMR) use and documentation of the process] and interdisciplinary practice (41). A classification of intervention components is described in more detail in the primary study (41). The specific SR results used in the model development are listed in Table 1.
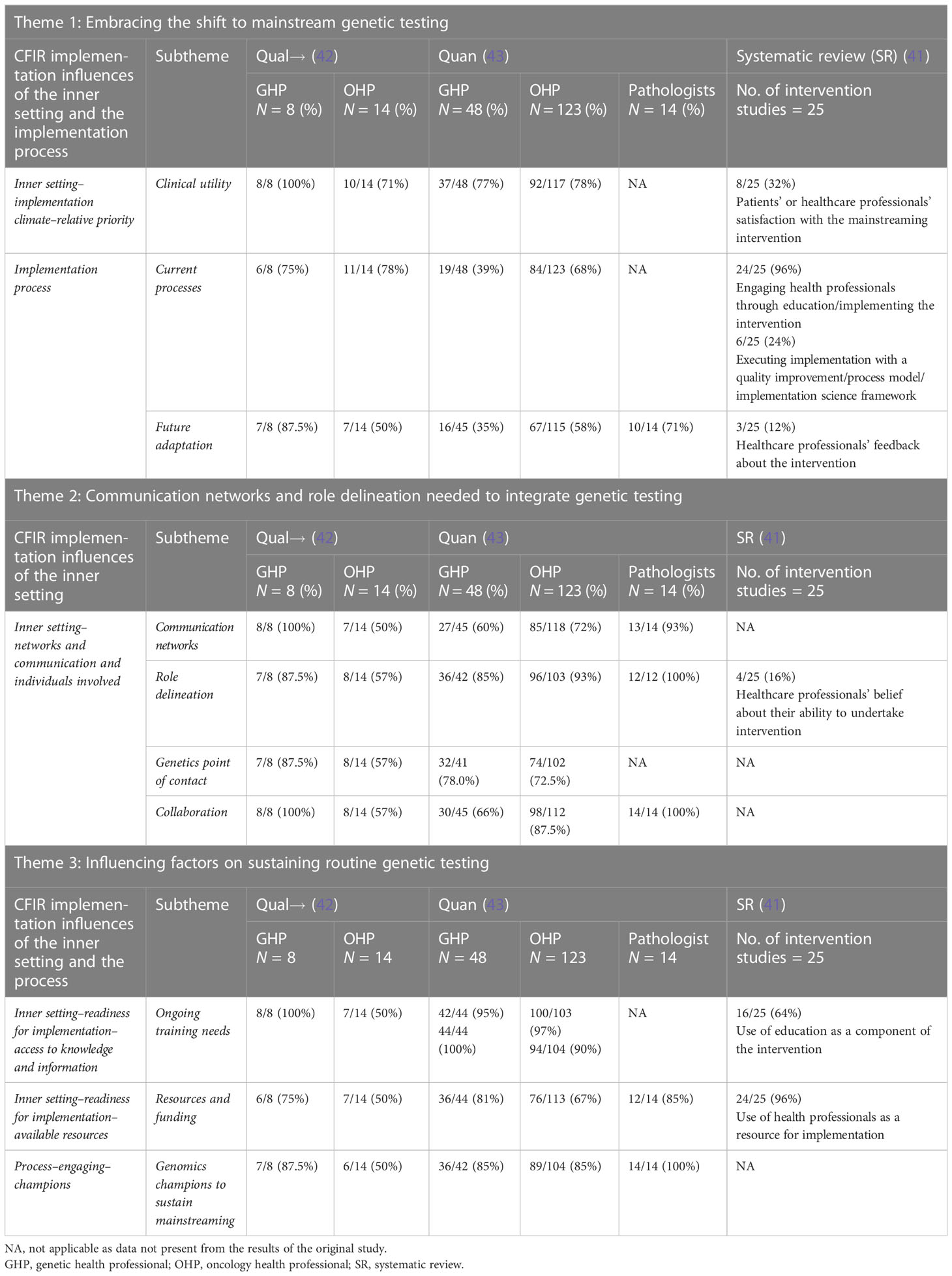
Table 1 Qualitative, quantitative, and systematic review data integration through the CFIR domain and construct and qualitative themes.
None of the included SR studies completed a comprehensive pre- and post-implementation assessment or evaluation, with less focus on implementation outcomes compared with client and service outcomes. These results informed the BRCA qualitative evaluative study design to understand the successes and challenges of BRCA mainstream implementation. The subsequent quantitative study evaluated the potential implementation factors for GT mainstreaming in the LS CRC and EC context.
Qualitative and quantitative data integration
Of the 22 health professionals interviewed for the BRCA mainstreaming evaluation in subsets of breast and/or ovarian cancer (42) context, the majority embraced the shift to mainstream genetic testing due to clinical utility and streamlining the process for patient care (Table 1); for detailed quotes on this theme, refer to O’Shea et al. (42) Similarly, of the 158 completed and 27 partial LS survey responses from genetic health professionals (GHP) and oncology health professionals (OHP) in the CRC and EC context (43), the majority (77% of GHP and 78% of OHP) recognized the relative advantage of aligning GT with dMMR results and agreed that it would streamline care for CRC and EC patients (43) (Table 1).
Optimization of the BRCA mainstreaming process was recognized in the results delivery and follow-up phase. In both BRCA and CRC contexts, communication networks, role delineation, genetics point of contact, and collaboration were key components of the organization’s inner setting that need to function well for mainstreaming to be adopted and maintained. Ongoing training, resources and funding, and champions were highlighted as key measures to sustain mainstreaming into the future (42, 43).
In both contexts, overcoming barriers was recognized, with genomics champions regarded as essential to integrate a genetic testing pathway. Facilitators such as embedding genetic counselors into an oncology clinic, multidisciplinary team documentation, and tracking to follow-up test results along with EMR tracking and flowcharts or checklists for an understandable mainstreaming pathway or process were also identified (Table 2). Many other suggested interventions such as online training, automatic or e-mail reminders, information provision, applications, and telehealth were viewed as suitable to facilitate the adoption of mainstreaming into routine care (Table 2).
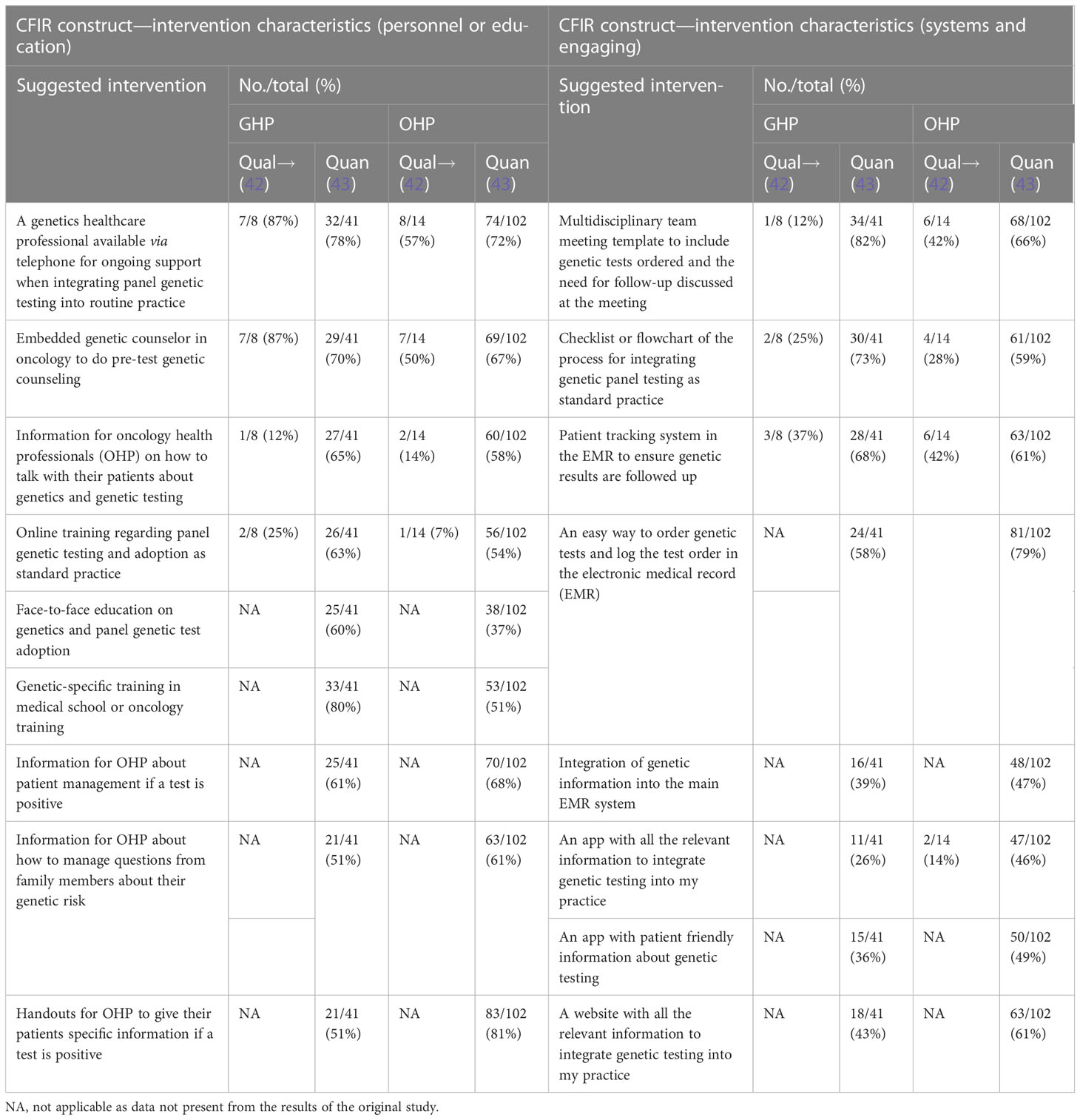
Table 2 Suggested interventions to overcome barriers to mainstream genetic testing in oncology services.
In summary, both the qual and quan datasets confirmed the importance of role delineation, organization readiness, genetics support, education, and identification of interventions to overcome barriers for mainstreaming sustainability in oncology services. Adaptation of the BRCA mainstreaming intervention was recognized to ensure a practical fit with the process and to support LS stakeholder consultation in the context of CRC and EC to plan and evaluate the process. Solutions were identified to overcome barriers along with the education, system, documentation, and interdisciplinary practice solutions trialed globally to mainstream GT as identified by the systematic review (41). A specific focus on the implementation and evaluative outcomes of GT mainstreaming programs was lacking and is critical to measure in future programs. The above evidence was combined and matched to the four domains of the GMIR (40) to inform the mainstreaming oncogenomics conceptual clinical service model to guide the translation of GT into oncology services.
Model development
General content
We created a conceptual clinical service model that integrates the GMIR (40) contextual implementation factors, interventions, processes, and outcomes that were found to be important to GT mainstreaming in the oncology setting from our research. The Mainstreaming OncoGenomics Model (MOGO) consists of two piers with interlinking components (Figure 2). The first pier comprises three components describing the organizational factors important to characterize the structure and function of the health system and the clinicians and patients engaged with the system and services. The components of the contextual factors in pier 1 influence GT mainstreaming program design factors in pier 2 through processes, interventions, and outcome measures in context-specific health system organizations. Our intention is that the conceptual clinical service model can guide health organization planning and developing LS and other GT mainstreaming programs in oncology. It does not intend to be prescriptive and highlights the important contextual factors to characterize and suggest interventions, processes, and outcomes to facilitate mainstreaming programs across cancer types. It can act as a single complex intervention unit or an adaptable conceptual model with a suite of implementation strategies to be described and tested in a health system.
Pier 1—contextual factors
The first component of this pier begins with defining the structure and function of the health system or organization where the oncology GT mainstreaming program is being introduced. The health system can be characterized in terms of the type of system or organization, the population served, and cancer incidence. The general description would include reporting on the setting, location, service structure, resources and funding, and the population served. The description of the system is the basis for the study of the related components, i.e., how the clinicians interact in this environment and the resources available to them and how the population being served engage with the system and services offered.
The second component of the health professional factors examines the professional role of those undertaking the GT mainstreaming initiative, along with their years of practice, age, and gender. These factors can impact clinician confidence and self-efficacy to take on mainstreaming, which is linked to their readiness. Clinician readiness extends to their knowledge and experience of genetics, along with their attitudes and understanding of its use. The time capacity to take on a GT mainstreaming role along with access to support and how satisfied they are with the new mainstreaming process in their workflow are important factors to consider.
The third component of this pier is the patient population being served by the organization, characterized by the type of cancer, age, treatment, and medical and family history among others (see Figure 2). GT will be integrated into multiple cancer types, and the context of cancer diagnosis at pathology, communication to the medical team, and workflow to determine GT eligibility require adaptation and description when the model is applied in health systems. Familial factors to consider would be family communication, depression, anxiety, or stress (mental health) with family issues or cancer diagnosis. Patient factors that could impact GT uptake such as belief, value, or knowledge of GT and its influence on health could interact with the health behavior impact. It is important to understand the system, clinician, and patient factors that can impact the mainstreaming program design, potential implementation success, and individual outcomes.
Pier 2—mainstreaming program factors
Three aspects of this pier encompass how the oncology GT mainstream program can be delivered addressing the health system and clinician needs. The first and second components describe interventions and processes to consider for individuals or systems and process planning that can allow routine pre-test genetic counseling and test ordering to be integrated into oncology. Some of the main elements to consider in a mainstreaming program are as follows: the set of interventions to support GT integration and decisions about how to obtain informed consent through pre-test genetic counseling in oncology, for a specific cancer type based on eligible criteria and guidelines; and the process of integrating genetic healthcare in oncology, for example, through an oncology-led patient pathway, i.e., the process from the patients’ initial contact with oncology services to their OHP giving access to GT either confirming or excluding a diagnosis of hereditary cancer and informing treatment management. An iterative learning healthcare systems approach to adapt to the mainstreaming processes by routinely evaluating the MOGO outcomes must be taken into consideration. These can be measured through cancer prevention, reduction in mortality, and cost utility with targeted treatments, along with the acceptability and amount of engagement from staff and patients. Adjusted processes can allow for optimal outcomes and sustained adoption. The specific aspects to consider are described in Figure 2.
Pier 2-related components intersect with pier 1 contextual factors from a healthcare system, clinician, individual, and family perspective. Analysis of contextual factors allows preparation for the mainstreaming program factors such as the expected demand for such a program through the population served, the number and type of cancers diagnosed, and the resources needed to implement such programs. Barriers are important contextual pier 1 factors to be identified and can be overcome in pier 2 by designing suitable interventions as indicated in Figure 2. With a learning healthcare systems approach, pier 2 (component 3) highlights outcome measures to be evaluated to ensure understanding of the need for adaptation of the mainstreaming oncology genomics programs to ensure sustainability.
Discussion
The aim of this paper was to develop a conceptual mainstreaming oncogenomics clinical service model to inform current and future oncology GT mainstreaming programs. Our genomics implementation evidence from a systematic review and qualitative and quantitative data focusing on genomic test integration into oncology services informed the content of the MOGO. Using GMIR as a guiding framework for genomics implementation research, the MOGO can be used as a generalizable foundation for the design and development of LS and other GT mainstreaming programs in oncology and should serve as a scalable learning canvas for future population-based cancer GT. The information contained in the clinical service model highlights the importance of the health system context and readiness and the interventions, process infrastructure, and funding needed to integrate GT mainstreaming programs into the future.
The MOGO consists of two piers to consider in the design and development of LS and other GT mainstream programs. The success and sustainability of a mainstreaming program first lie in understanding the organizational contextual factors (pier 1). The contextual recognition of genomic-specific factors for service delivery was highlighted by the IGNITE model, which extended CFIR to recognize the unique features that genomics information have on the family system (38). The IGNITE patient domain focused on extending the model to recognize the contribution of patient views, emotions, and attitudes toward genetic testing especially in the context of unaffected family members and their community network. The MOGO factors in these facets in the “individual and family factors” domain were informed by our systematic review finding of a lack of patient evaluative outcomes, with only 24% of studies focusing on the patient’s satisfaction of the mainstreaming intervention (41). The MOGO includes evaluative components to address genomics implementation through patient’s experience, satisfaction, health behavior, and overall engagement with the program.
The MOGO recognizes how clinicians’ factors such as self-efficacy, knowledge, and confidence (pier 2) intertwine with the educational interventions in pier 2 to ready health professionals with the required information to take on the routine pre-test genetic counseling role in oncology. Studies with OHP found that self-efficacy, knowledge, and confidence are the recognized barriers to the integration of GT in mainstream medicine (47). Emerging literature shows cancer specialists’ positive attitudes toward the use of genetic information in precision medicine, with 63.7% favoring its use in treatment and prognostic information but with concerns over cost, knowledge, potential misuse, access, and results delivery (48). To overcome clinician factor barriers, solutions such as a designated point of contact from a genetic service to alleviate concerns, education, or embedding a genetic counselor into routine oncology care are recognized in MOGO. The benefits of these mainstreaming approaches are evident in the USA and Australia (49–51), revealing more patients getting timely access to GT and appropriate follow-up in the system.
The evaluative outcomes highlighted in the MOGO (pier 2) ensure that a learning healthcare system concept of genomics implementation can be taken. Initial intervention design and process planning require real-world system trial and adaptation to achieve optimal outcomes for the patient and the system. A ProvenCare initiative in cancer care by the Geisinger Health System used evidence-based care guidelines to devise protocols and electronic health record (EHR) implementation strategies (52). Success was achieved through a 90% uptake of patient care elements in six hospital sites (52). Dashboard analytics to assess the uptake of the protocol in practice, followed by tracking of outcomes for lung cancer patients, led to the provision of optimal care and translation of the latest evidence into practice (52). Monitoring of future mainstreaming initiatives with EHR infrastructure could allow for similar learning and adaptation to enhance success.
The MOGO recommends the use of the EHR as an important tool in intervention design and reporting of outcomes in GT mainstreaming programs. Several studies in our systematic review used the EHR for streamlining appointments, checking all patients have access to GT, notifying clinicians involved in the genomic healthcare of their patients, and tracking of results to ensure appropriate follow-up (41). An organization’s EHR infrastructure along with clinician, laboratory, and IT collaboration led to the successful EHR integration of genetic information in one US institution (53). This integration allowed for ordering of genetic tests directly in the EHR with results sent promptly into the system and used to notify the clinician.
The above EHR initiatives are examples of how the interventions identified in the MOGO could be operationalized in the oncology context and feed into an evaluative loop assessing patient outcomes such as hereditary cancer identification, cancer prevention through uptake of screening or risk-reducing measures and predictive testing in families, and the utilization of targeted treatments. These outcomes can then be used in a robust cost-effectiveness evaluation of GT mainstreaming programs.
The strength of the MOGO is its underpinning of broad evidence from various health professionals, hospital organizations, disease contexts, and GT mainstreaming intervention evaluations to inform the various components. MOGO was informed by global studies (UK, USA, Australia, and Europe) from the systematic review of evidence encompassing intervention characteristics and evaluation from diverse system structures and contexts. The sequential mixed methodology of building datasets designed with CFIR and model development with data integration and analysis using complimentary CFIR domains and constructs allowed for robust implementation science data to be generated across cancer types and systems. For this reason, the evidence-informed model allows for generalizability across systems and cancer types. The model recognizes the importance of planning, consultation, and role delineation needed for different cancer contexts considering the hospital system infrastructure and resources available. The MOGO intends to be a resource for the system and health professionals designing LS and other GT mainstreaming programs and can be adapted to the inner setting of organizations or individual characteristics to ensure flexibility. The adoption of a MOGO for the evaluation of different GT mainstreaming programs would allow comparative lessons to be learned in diverse programs and in comparison with other countries.
The limitations of the MOGO exist in the lack of direct evidence to inform a social determinant component for contextual factors informing policy, systems, and education needs of an organization and the population it serves. The mixed methodology allows for depth of implementation evidence but the time required to gather and build this evidence to inform future mainstreaming program design may be prohibitive in resource-limited organizations and to keep pace with a rapidly evolving field. The qualitative and quantitative evidence may not represent all stakeholders involved in mainstreaming contexts for breast, ovarian, CRC, and EC, and future implementation research design using hybrid effectiveness methods could facilitate iterative service designs with all stakeholders.
Future research into GT mainstreaming programs needs to focus on the populations’ access and uptake of genetics within oncology services and evaluate the communities’ knowledge about genetic influences in their healthcare. Social policy integrating information about GT into general public education and campaigns could facilitate broader public knowledge, focusing on the laws and regulations regarding the use of genetic test information in individuals and in family healthcare decisions. As a population-based cancer GT emerges, future research in social policy and public needs is important. The qualitative and quantitative evidence may not represent all stakeholders involved in mainstreaming contexts for breast, ovarian, colorectal, and endometrial cancer outside of Australia. Therefore, the MOGO needs to be assessed and trialed by oncology and genetic experts in the field. The outcome domains of the MOGO require future research to identify or create validated measures to evaluate genomic outcomes consistently in oncology.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the University of Sydney. The patients/participants provided their written informed consent to participate in this study.
Author contributions
RO’S, NR, and SL contributed to the conception and design of the study. RO’S performed the analysis. RO’S wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by a Cancer Council New South Wales PhD scholarship and by a Translational Cancer Research Network Clinical PhD Scholarship Top-up award, supported by the Cancer Institute NSW supporting RO’S in the completion of her PhD studies in the Faculty of Medicine and Health at The University of Sydney.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1140135/full#supplementary-material
References
1. eviQ 2020 version 9 BRCA 1 and 2 genetic testing. Available at: https://www.eviq.org.au/cancer-genetics/adult/genetic-testing-for-heritable-pathogenic-variants/620-brca1-and-brca2-genetic-testing#probability-of-a-heritable-pathogenic-variant (Accessed 11.01.2021).
2. National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology. Genetic/Familial high risk assessment: breast, ovarian and pancreatic cancer. version 1.2020. Available at: https://www.nccn.org/professionals/physician_gls/default.aspx#genetics_screening (Accessed 28/06/2020).
3. National Institutes of health and Care Excellence (NICE). Familial breast cancer clinical guideline (2019). Available at: https://www.nice.org.uk/guidance/cg164 (Accessed 28 June 2020).
4. Febbraro T, Robison K, Scalia Wilbura J, Laprisea J, Bregara A, Lopesa V, et al. Adherence patterns to national comprehensive cancer network (NCCN) guidelines for referral to cancer genetic professionals. Gyn Onc (2015) 138:109–14. doi: 10.1016/j.ygyno.2015.04.029
5. Powell CB, Littell R, Hoodfar E, Sinclair F, Pressman A. Does the diagnosis of breast or ovarian cancer trigger referral to genetic counseling? Int J Gynecol Cancer (2013) 23:431–6. doi: 10.1097/IGC.0b013e318280f2b4
6. Demsky R, McCuaig J, Maganti M, Murphy KJ, Rosen B, Armel SR. Keeping it simple: genetics referrals for all invasive serous ovarian cancers. Gyn Onc (2013) 130:329–33. doi: 10.1016/j.ygyno.2013.05.003
7. Wright JD, Chen L, Tergas AI, Accordino M, Ananth CV, Neugut AI, et al. Underuse of BRCA testing in patients with breast and ovarian cancer. Am J Obstet Gynecol (2016) 214:761–3. doi: 10.1016/j.ajog.2016.02.011
8. Pi S, Nap-Hill E, Telford J, Enns R. Recognition of lynch syndrome amongst newly diagnosed colorectal cancers at st. paul’s hospital. Can J Gastroenterol Hepatol (2017) 2017(6):9625638. doi: 10.1155/2017/9625638
9. Singh H, Schiesser R, Anand G, Richardson P, El- Serag HB. Underdiagnoses of lynch syndrome involves more than family history criteria. Clin Gastroenterol Hepatol (2010) 8:523–9. doi: 10.1016/j.cgh.2010.03.010
10. Armel SR, Volenik A, Demsky R, Malcolmson J, Maganti M, McCuaig J. Setting a baseline: a 7-year review of referral rates and outcomes for serous ovarian cancer prior to implementation of oncologist mediated genetic testing. Gynae Oncol (2020) 158:440–5. doi: 10.1016/j.ygyno.2020.05.014
11. Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian ovarian cancer study group. J Clin Onc (2012) 30:2654–63. doi: 10.1200/JCO.2011.39.8545
12. Hartman AR, Kaldate RR, Sailer LM, Painter L, Grier CE, Endsley RR, et al. Prevalence of BRCA mutations in an unselected population of triple-negative breast cancer. Cancer (2012) 118:2787–95. doi: 10.1002/cncr.26576
13. National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology. Genetic/Familial high-risk assesment: colorectal, V 1.2020. Available at: www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf (Accessed 21 July 2020).
14. Monahan KJ, Bradshaw N, Dolwani S, Desouza B, Dunlop MG, East JE, et al. Hereditary CRC guidelines eDelphi consensus group guidelines for the management of hereditary colorectal cancer from the British society of gastroenterology (BSG)/Association of coloproctology of great Britain and Ireland (ACPGBI)/ united kingdom cancer genetics group (UKCGG). Gut (2020) 69:411–44. doi: 10.1136/gutjnl-2019-319915
15. EviQ. Mismatch repair (MMR) genetic testing (2019). Australia: Cancer Institute NSW. Available at: https://www.eviq.org.au/cancer-genetics/adult/genetic-testing-for-heritable-pathogenic-variants/619-mismatch-repair-mmr-genetic-testing (Accessed 28 June 2020).
16. Hansen MF, Johansen J, Sylvander AE, Bjørnevoll I, Talseth-Palmer BA, Lavik LAS, et al. Use of multigene-panel identifies pathogenic variants in several CRC-predisposing genes in patients previously tested for lynch syndrome. Clin Genet (2017) 92:405–14. doi: 10.1111/cge.12994
17. Pearlman R, Frankel WL, Swanson B, Zhao W, Yilmaz A, Miller K, et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol (2017) 3:464–71. doi: 10.1001/jamaoncol.2016.5194
18. Ring KL, Bruegl AS, Allen BA, Elkin EP, Singh N, Hartman AR, et al. Germline multi-gene hereditary cancer panel testing in an unselected endometrial cancer cohort. Mod Pathol (2016) 29:1381–9. doi: 10.1038/modpathol.2016.135
19. Evans DG, Harkness EF, Howell A, Wilson M, Hurley E, Holmen MM, et al. Intensive breast screening in BRCA2 mutation carriers is associated with reduced breast cancer specific and all cause mortality. Hered Cancer Clin Pract (2016) 14:8. doi: 10.1186/s13053-016-0048-3
20. Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA (2010) 304:967–75. doi: 10.1001/jama.2010.1237
21. Marchetti C, De Felice F, Palaia I, Perniola G, Musella A, Musio D, et al. Risk-reducing salpingooophorectomy: a meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Womens Health (2014) 14:150. doi: 10.1186/s12905-014-0150-5
22. Vasen HF, Abdirahman M, Brohet R, Langers AM, Kleibeuker JH, van Kouwen M, et al. One to 2-year surveillance intervals reduce risk of colorectal cancer in families with lynch syndrome. Gastroenterology (2010) 138:2300–6. doi: 10.1053/j.gastro.2010.02.053
23. Dove-Edwin I, Sasieni P, Adams J, Thomas HJ. Prevention of colorectal cancer by colonoscopic surveillance in individuals with a family history of colorectal cancer: 16 year, prospective, follow up study. BMJ (2005) 331:1047. doi: 10.1136/bmj.38606.794560
24. Evans O, Manchanda R. Population-based genetic testing for precision prevention (2020). Cancer Prev Res (2020) 13:643–8. doi: 10.1158/1940-6207.CAPR-20-0002
25. Meisel SF, Side L, Fraser L, Gessler S, Wardle J, Lanceley A. Population-based, risk-stratified genetic testing for ovarian cancer risk: a focus group study. Public Health Genomics (2013) 16:184–91. doi: 10.1159/000352028
26. Veldwijk J, Lambooij M, Kallenberg F, van Kranen HJ, Bredenoord AL, Dekker EH, et al. Preferences for genetic testing for colorectal cancer within a population-based screening program: a discrete choice experiment. Eur J Hum Genet (2016) 24:361–6. doi: 10.1038/ejhg.2015.117
27. Meisel SF, Rahman B, Side L, Fraser L, Gessler S, Lanceley A, et al. Genetic testing and personalized ovarian cancer screening: a survey of public attitudes. BMC Womens Health (2016) 16:46. doi: 10.1186/s12905-016-0325-3
28. Meisel SF, Fraser LSM, Side L, Gessler S, Hann KEJ, Wardle J, et al. Anticipated health behaviour changes and perceived control in response to disclosure of genetic risk of breast and ovarian cancer: a quantitative survey study among women in the UK. BMJ Open (2017) 7:e017675. doi: 10.1136/bmjopen-2017-017675
29. Severin F, Schmidtke J, Muhlbacher A, Rogowski WH. Eliciting preferences for priority setting in genetic testing: a pilot study comparing best-worst scaling and discrete choice experiments. Eur J Hum Genet (2013) 21:1202–8. doi: 10.1038/ejhg.2013.36
30. De Simone LM, Arjunan A, Vogel Postula KJ, Maga T, Bucheit LA. Genetic counselors’ perspectives on population-based screening for BRCA-related hereditary breast and ovarian cancer and lynch syndrome. J Genet Couns (2020) 00:1–12. doi: 10.1002/jgc4.1305
31. Hann KEJ, Fraser L, Side L, Gessler S, Waller J, Sanderson SC, et al. Health care professionals’ attitudes towards population-based genetic testing and riskstratification for ovarian cancer: a crosssectional survey. BMC Women's Health (2017) 17:132. doi: 10.1186/s12905-017-0488-6
32. United States Preventive Services Task Force (USPSTF). Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: recommendation statement. Ann Intern Med (2005) 143:355–61. doi: 10.7326/0003-4819-143-5-200509060-00011
33. Moyer VA, United States Preventive Services Task Force. Risk assessment,genetic counseling, and genetic testing for BRCA-related cancer in women: U.S. preventive services task force recommendation statement. Ann Intern Med (2014) 160:271–81. doi: 10.7326/M13-2747
34. Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP working group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from lynch syndrome in relatives. Genet Med (2009) 11:35–41. doi: 10.1097/GIM.0b013e31818fa2ff
35. Centers for Disease Control and Prevention. CDC Science making a difference–five domains of influence. Available at: https://www.cdc.gov/od/science/impact/index.htm (Accessed 5 February 2021).
36. Institute of Medicine. The learning healthcare system: workshop summary. Olsen LA, Aisner D, McGinnis JM, editors. Washington, DC: Natl. Acad. Press (2007). Available at: https://www.ncbi.nlm.nih.gov/books/NBK53494.
37. Roundtable on Translating Genomic-Based Research for Health, Board on Health Sciences Policy, Institute of Medicine. Genomics-enabled learning health care systems: gathering and using genomic information to improve patient care and research: workshop summary. Washington (DC: Natl. Acad. Press (US (2015).
38. Orlando LA, Sperber NR, Voils C, Nichols M, Myers RA, Wu R, et al. Developing a common framework for evaluating the implementation of genomic medicine interventions in clinical care: the IGNITE network’s common measures working group. Genet Med (2018) 20:655–63. doi: 10.1038/gim.2017.144
39. Damschroder L, Aron D, Keith R, Kirsh S, Alexander J, Lowery J. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci (2009) 4:50. doi: 10.1186/1748-5908-4-50
40. Horowitz CR, Orlando LA, Slavotinek AM, Peterson J, Angelo F, Biesecker B, et al. The genomic medicine integrative research framework: a conceptual framework for conducting genomic medicine research. Am J Hum Genet (2019) 104:1088–96. doi: 10.1016/j.ajhg.2019.04.006
41. O’Shea R, Taylor N, Crook A, Jacobs C, Jung Kang Y, Lewis S, et al. Health system interventions to integrate genetic testing in routine oncology services: a systematic review. PloS One (2021) 16(5):e0250379. doi: 10.1371/journal.pone.0250379
42. O’Shea R, Rankin NM, Kentwell M, Gleeson M, Salmon L, Tucker KM, et al. How can Australia integrate routine genetic sequencing in oncology: a qualitative study through an implementation science lens. Genet Med (2020) 22:1507–16. doi: 10.1038/s41436-020-0838-x
43. O’Shea R, Rankin NM, Kentwell M, Gleeson M, Tucker KM, Hampel H, et al. Stakeholders’ views of integrating universal tumour screening and genetic testing for colorectal and endometrial cancer into routine oncology. Eu J Hum Genet (2021) 29:1634–44. doi: 10.1038/s41431-021-00871-4
44. Creswell JW, Plano Clark VL. Designing and conducting mixed methods research. 2nd ed. Thousand Oaks, CA: Sage (2011).
45. Fetters MD, Curry LA, Creswell JW. Achieving integration in mixed methods designs: principles and practices. Health Serv Res (2013) 48:2134–56. doi: 10.1111/1475-6773.12117
46. Morse JM. Approaches to qualitative-quantitative methodological triangulation. Nurs Res (1991) 40:120–3. doi: 10.1097/00006199-199103000-00014
47. White S, Jacobs C, Phillips J. Mainstreaming genetics and genomics: a systematic review of the barriers and facilitators for nurses and physicians in secondary and tertiary care. Genet Med (2020) 22:1149–55. doi: 10.1038/s41436-020-0785-6
48. Vetsch J, Wakefield CE, Techakesari P, Warby M, Ziegler DS, O'Brien TA, et al. Healthcare professionals’ attitudes toward cancer precision medicine: a systematic review. Semin Oncol (2019) 46:291–303. doi: 10.1053/j.seminoncol.2019.05.001
49. Kentwell M, Dow E, Antill Y, Wrede CD, McNally O, Higgs E, et al. Mainstreaming cancer genetics: a model integrating germline BRCA testing into routine ovarian cancer clinics. Gynecol Oncol (2017) 145:130–6. doi: 10.1016/j.ygyno.2017.01.030
50. Senter L, O'Malley DM, Backes FJ, Copeland LJ, Fowler JM, Salani R, et al. Genetic consultation embedded in a gynecologic oncology clinic improves compliance with guideline-based care. Gynecol Oncol (2017) 147:110–4. doi: 10.1016/j.ygyno.2017.07.141
51. Rana HQ, Kipnis L, Hehir K, Cronin A, Jaung T, Stokes SM, et al. Embedding a genetic counselor into oncology clinics improves testing rates and timeliness for women with ovarian cancer. Gynecol Oncol (2020) 21:S0090–8258(20)34111-1. doi: 10.1016/j.ygyno.2020.11.003
52. Katlic MR, Facktor MA, Berry SA, McKinley KE, Bothe A Jr., Steele GD Jr. ProvenCare lung cancer: a multi-institutional improvement collaborative. CA Cancer J Clin (2011) 61:382–96. doi: 10.3322/caac.20119
Keywords: Lynch syndrome, routine genetic testing, oncology service delivery, mainstreaming, oncogenomics model
Citation: O’Shea R, Crook A, Jacobs C, Kentwell M, Gleeson M, Tucker KM, Hampel H, Rahm AK, Taylor N, Lewis S and Rankin NM (2023) A mainstreaming oncogenomics model: improving the identification of Lynch syndrome. Front. Oncol. 13:1140135. doi: 10.3389/fonc.2023.1140135
Received: 08 January 2023; Accepted: 24 April 2023;
Published: 26 May 2023.
Edited by:
Gad Rennert, Technion Israel Institute of Technology, IsraelReviewed by:
Farnoosh Abbas Aghababazadeh, University Health Network (UHN), CanadaMujeeb Zafar Banday, Government Medical College (GMC), India
Alfonso De Stefano, G. Pascale National Cancer Institute Foundation (IRCCS), Italy
Copyright © 2023 O’Shea, Crook, Jacobs, Kentwell, Gleeson, Tucker, Hampel, Rahm, Taylor, Lewis and Rankin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rosie O’Shea, cm9zaWUub3NoZWFAc3lkbmV5LmVkdS5hdQ==
†These authors have contributed equally to this work
 Rosie O’Shea
Rosie O’Shea Ashley Crook2†
Ashley Crook2† Chris Jacobs
Chris Jacobs Katherine M. Tucker
Katherine M. Tucker Alanna Kulchak Rahm
Alanna Kulchak Rahm Sarah Lewis
Sarah Lewis