
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 07 March 2023
Sec. Cancer Imaging and Image-directed Interventions
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1139720
Low-grade myofibroblastic sarcoma is a rare malignant tumor that arises from mesenchymal tissue and affects the head (particularly the tongue and oral cavity) and neck. It is characterized by local recurrence, with metastases being uncommon. We present a 72-year-old man who initially complained of a painless and progressive mass in the right inguinal region and came for consultation, and a malignant tumor was suspected based on the clinical and pelvic MRI manifestations. The 18F-FDG PET/CT revealed that the multiple lesions were located in the mediastinum, retroperitoneum, pelvis, and inguinal lymph nodes; hence, lymphoma was considered to be a combination of the symptoms. However, the histology of the ultrasound-guided puncture indicated low-grade myofibroblastic sarcoma. The patient was next administered chemotherapy, but the lesions did not undergo remission.
It is common knowledge that positron emission tomography with computed tomography (PET/CT) utilizing 18F-fluoro-deoxyglucose (18F-FDG) is a fairly effective examination for identifying and evaluating malignant tumors. Low-grade myofibroblastic sarcoma (LGMS) has a very low incidence. Such little literature exists on LGMS and 18F-FDG PET/CT imaging of LGMS. In our case, a LGMS patient with 18F-FDG PET/CT abnormalities including lymph nodes in multiple areas of the body had distant metastases. The 18F-FDG PET/CT imaging presentation was mistaken for lymphoma, and the imaging distinction between lymphoma and LGMS is addressed further in the discussion. In addition, the tumor did not diminish appreciably despite receiving two chemotherapy treatments, confirming the LGMS is low susceptibility to chemotherapy.
A 72-year-old man came to our hospital for evaluation with a painless mass in the right inguinal region which had been growing for three months. The mass lacked softness and immobility and was rigid and smooth. The patient has no previous history of tumor or surgery, and no family history of related diseases. The tumor makers revealed a slightly elevated neuron-specific enolase level of 73.09 ng/ml (reference range: 0-29.13 ng/ml) and ferritin level of 582.4ng/ml (reference range: 30-400 ng/ml), in addition to the serum levels of lactic dehydrogenase and creatine kinase, which were 551 U/L (reference range: 114-240 U/L). Other than the indicators listed above, all other laboratory test indicators were within normal range. The pelvic MRI (pictures not shown) revealed multiple enlarged lymph nodes surrounding the right pelvis, right inguinal area, and right common iliac arteries; a malignant tumor was suspected in combination with clinical and imaging characteristics.
Therefore, an 18F-FDG PET/CT was conducted to evaluate the general status of the patient. Except for multiple significantly enhanced 18F-FDG activity foci in the pelvic cavity and inguinal area, the maximum intensity projection (MIP) image (Figure 1A) revealed abnormal activity in the mediastinum and retroperitoneum (arrows). On the axial images (Figure 1B: CT, C: PET, D: fusion), several hypermetabolic foci were observed in the right inguinal region, which corresponded to heterogeneous low-density enlarged lymph nodes (solid arrows), and the maximum standardized uptake value (SUVmax) of the lesion was 12.1. Moreover, the maximum cross-sectional area of the huge bulk was 9.4 cm by 6.0 cm. The abdomen axial imaging (Figure 1E: CT, F: PET, G: fusion) revealed multiple lymph nodes of activity (dotted arrows) along the right common iliac vessels and parts of lesions accompanied by fusion. In the axial pictures, a lymph node with elevated activity (thin arrows) surrounding the descending aorta (SUVmax, 10.2) (Figure 1H: CT, I: PET, J: fusion). A delay 18F-FDG PET/CT imaging was conducted since the moving affected the picture quality, and one of the mediastinum foci disappeared due to the moving’s influence. Nevertheless, the axial imaging demonstrated an elevated 18F-FDG lymph node activity around the aortic arch (arrows), with a SUVmax of 7.1. (Figure 2A: PET, B: CT, C: fusion, D: MIP). All of the above 18F-FDG PET/CT imaging indicated lymphoma.
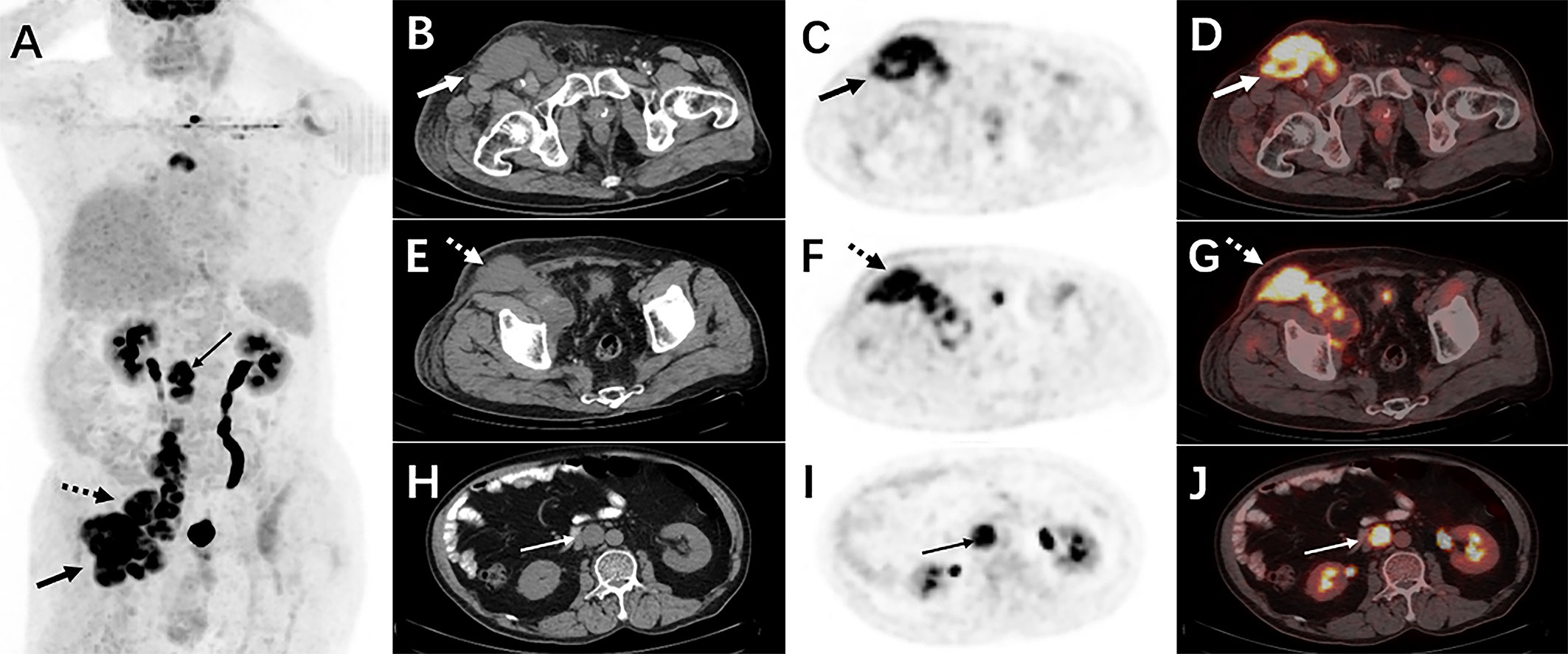
Figure 1 The 18F-FDG PET/CT imaging before treatment. The MIP picture (A) revealed hypermetabolic foci in the retroperitoneum and mediastinum (arrows). On the axial images, several low-density lesions were detected in the right inguinal region (SUVmax 12.1)(solid arrows) (B: CT, C: PET, D: fusion). The image (E: CT, F: PET, G: fusion) revealed numerous lymph nodes with abnormal metabolic confluence and a SUVmax of 9.8. (dotted arrows). Some retroperitoneal hypermetabolic foci (SUVmax 10.2) were found (thin arrows) (H: CT, I: PET, J: fusion).
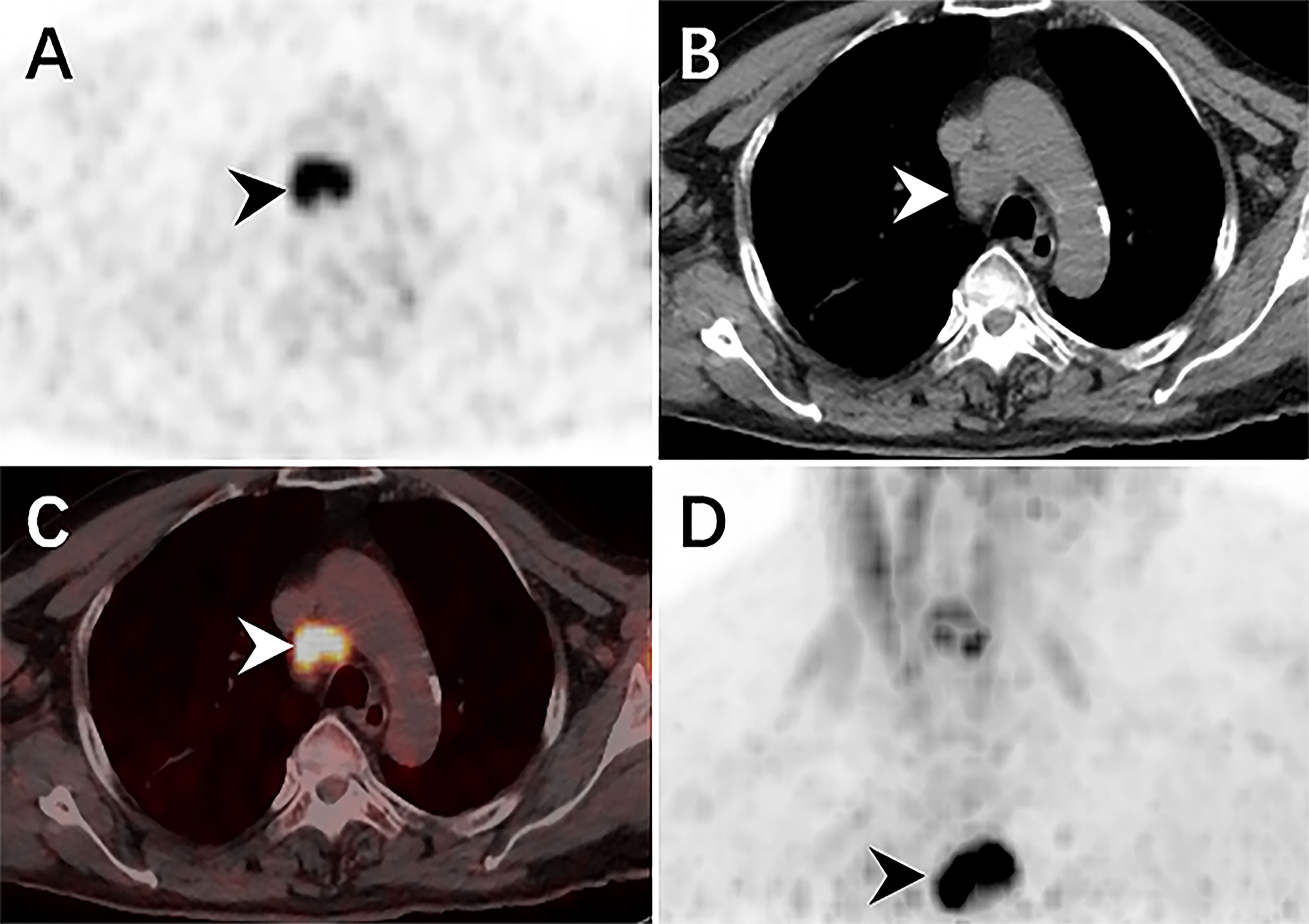
Figure 2 The delay 18F-FDG PET/CT revealed elevated activity foci in the mediastinum, with a SUVmax of 7.0. The CT scan revealed a single, low-density lymph node nearby the aortic arch (arrowheads) (A: PET, B: CT, C: fusion, D: MIP).
Then ultrasound-guided puncture biopsy of the right inguinal mass was performed, and the pathology was low-grade myofibroblastic sarcoma(LGMS). Histopathological examination (hematoxylin-eosin 200) of the specimens obtained from the right inguinal mass revealed spindle-shaped cells arranged in fascicles (Figure 3). Positive immunohistochemistry stains were observed for SMA, TTF-1, and Vimentin, while negative staining was observed for CK, CK20, CK7, MyoD1, Myogenin, P63, and S100. All of the findings were consistent with LGMS.
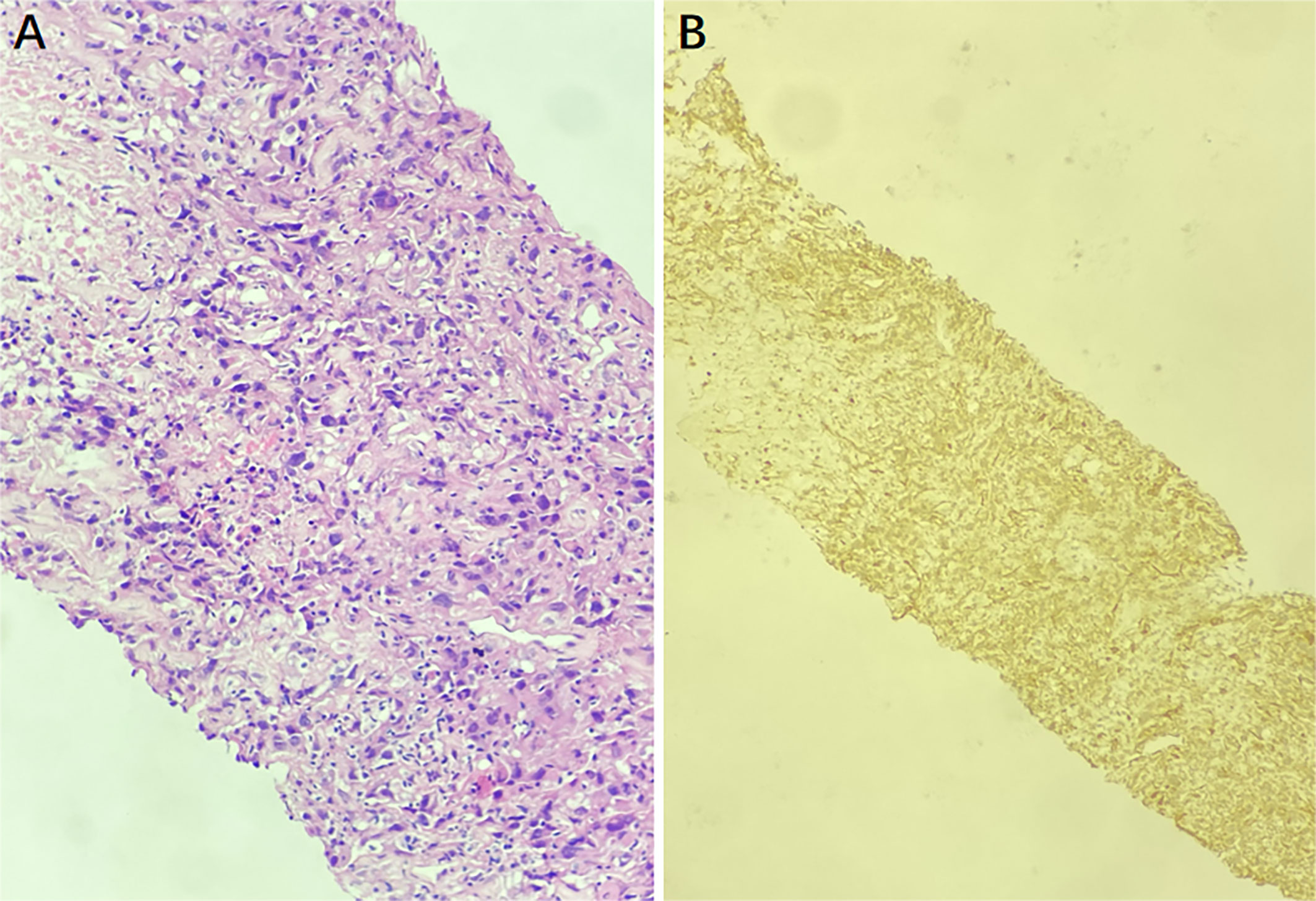
Figure 3 The puncture biopsy specimen pathological findings show infiltration of myofibroblasts, and there is no inflammatory cells (hematoxylin-eosin ×200) (A). The immunohistochemistry staining for SMA was positive, indicating a LGMS (B).
Furthermore, the patient successfully completed two cycles of ifosfamide and bevacizumab chemotherapy. The CT of the chest, abdomen and pelvic cavity indicated no change in the quantity or volume of lesions, and the largest mass measured approximately 8.9cm × 7.1cm (Figure 4). Due to the lack of a significant reduction in lesions following treatment, the patient abandoned treatment and died the next year. Informed consent has been obtained for this retrospective study.
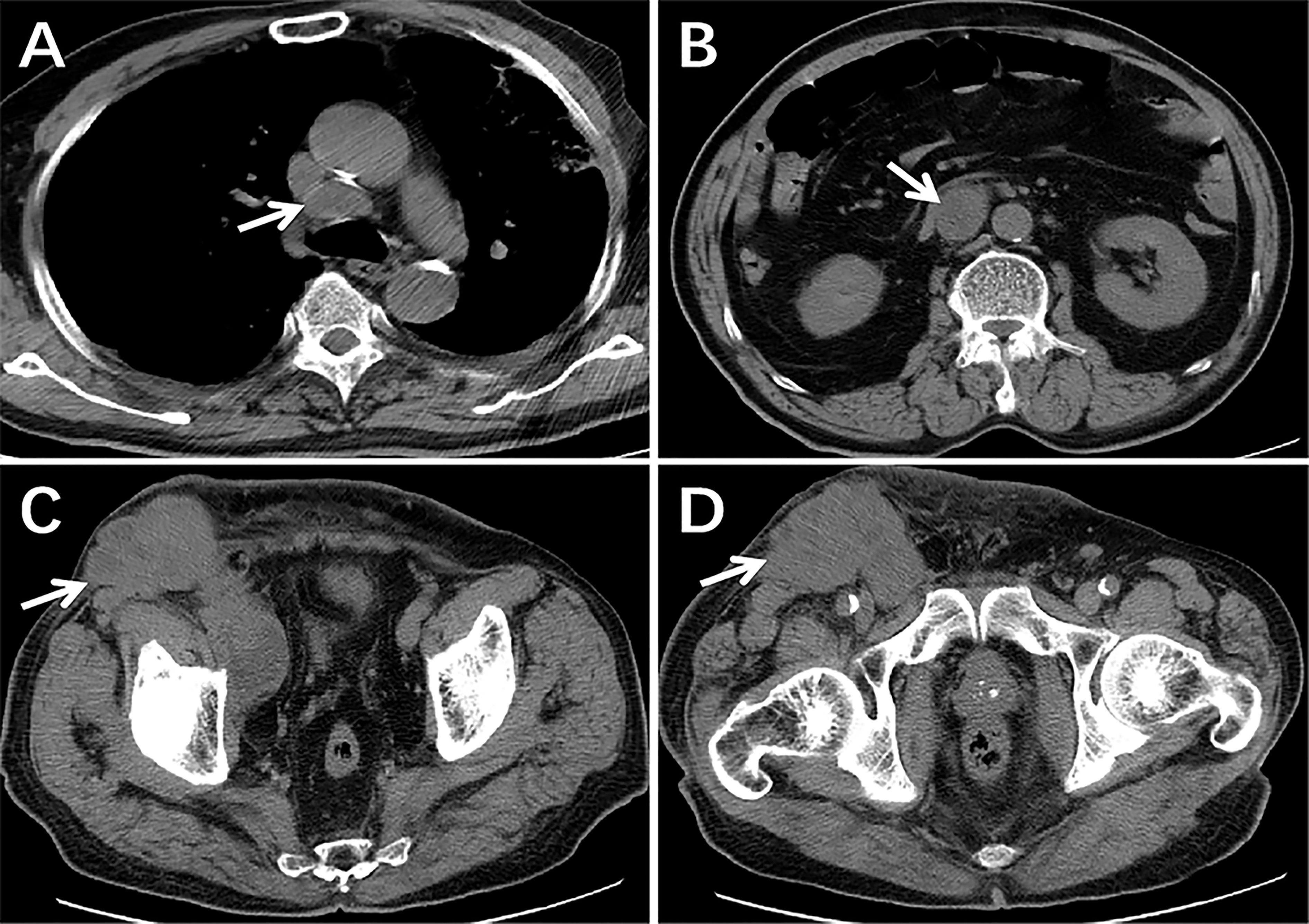
Figure 4 The CT scans following two cycles of treatment. There were still lesions in the mediastinum, retroperitoneum, pelvic, and inguinal regions and the size and quantity of lymph nodes had not decreased significantly from pre-treatment imaging (arrows) (A–D).
LGMS is a rare and low-grade malignant bone and soft tissue tumor that originates from mesenchymal tissue. The most common patients are older males, and the clinical presentation is a painless, progressive enlargement resembling lymphoma symptoms. The definitive diagnosis of LGMS is determined by histopathology. LGMS has a varied immunophenotype, but typically displays myogenic antibodies similar to SMA. In addition, Vimentin is the specific marker for fibrosarcoma, and it is positive in the majority of LGMS patients. The immunohistochemistry staining of SMA and Vimentin was positive in our case.
LGMS mainly involves the head and neck, particularly the oral cavity and tongue (1–4). LGMS can be observed in the bone, muscle, breast, and pleura in addition to a few individual case reports (5–10). However, very few studies have reported lymph node involvement in LGMS. And it is generally accepted that LGMS has a higher risk of recurrence and a lower metastatic incidence. But our research revealed that a patient with LGMS had lymph nodes in the abdominal cavity, pelvic cavity, and mediastinum region. According to a study (11), 18F-FDG PET/CT is of considerable value in the diagnosis of LGMS and effectively detects occult soft tissue malignancy (12). On the 18F-FDG PET/CT images, our case demonstrates intense 18F-FDG activity foci corresponding to multiple enlarged lymph nodes with mutual fusion throughout the body, similar to lymphoma. Through further review of the literature (13), we found the most notable distinctions between this case of LGMS and typical lymphoma: lymphoma involving lymph nodes is generally homogeneous in density and mostly symmetrical in distribution, whereas LGMS had apparent liquefied necrosis and was located on one side in this case. Additionally, the lymph nodes involved in lymphoma are frequently distributed bilaterally in the inguinal region, whereas in this case, the lymph nodes involved in the LGMS were located on one side only. Lymphoma involving large lymph nodes is typically created by the fusion of numerous lymph nodes, but in this case, there is no sign of fusion in the right inguinal mass, supporting the notion that this is the primary focus. Therefore, LGMS should be suspected if numerous lymph nodes with necrosis especially were involved on 18F-FDG PET/CT.
Other diseases, in addition to lymphoma, should be considered in the differential diagnosis. Firstly, lymph node hypertrophy is a result of inflammation. In this case, the patient had no fever symptoms and no abnormal laboratory testing for inflammatory indices, therefore inflammation was ruled out. The second differential diagnosis is myxofibrosarcoma. The main difference between the two from a pathological perspective is that myxofibrosarcoma had more mucus, whereas the pathology specimen from our patient had no mucus. Additionally, SMA, a distinctive myogenic antibody that aids in differentiating between the two, is typically present in LGMS.
The research findings demonstrate that complete surgical resection of the lesion is the most effective treatment for LGMS and generally has a promising prognosis (14), but LGMS is not sensitive to radiotherapy and chemotherapy (9). In our study, however, the patient was elder and had extensive lesions, so his body could not tolerate surgery and he chose chemotherapy. Furthermore, there was no substantial reduction of the lesion after two cycles of ifosfamide and bevacizumab chemotherapy, confirming that chemotherapy is insensitive to LGMS and has poor therapeutic efficacy.
In our case, lymphoma was suspected based on either the clinical symptoms or the 18F-FDG PET/CT imaging. Nevertheless, the patient was identified with LGMS after undergoing a biopsy. Even though LGMS has a very low incidence, the disease is potentially severe. Therefore, early diagnosis and therapy are essential for the prognosis of LGMS patients, and we should consider the possibility of LGMS when a patient presents with multiple lymph node involvement.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Institutional Tangshan Gongren Hospital. The patients/participants provided their written informed consent to participate in this study.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HZ wrote the manuscript. LH, BH and XZ preformed image acquisition and prepared clinical data. LZ reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Science &Technology Program of Hebei (182777145).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Jayasooriya PR, Athukorala C, Attygalla M, Mendis BRRN, Lombardi T. Low-grade myofibroblastic sarcoma of the oral cavity: A report of three cases illustrating an emerging disease in children. Dermatopatho (Basel) (2021) 8(1):1–9. doi: 10.3390/dermatopathology8010001
2. Padmawar NS, Bhadange S, Mustilwar RG, Mopagar VP, Vadvadgi VH, Joshi SR. Aberrant location of low-grade myofibroblastic sarcoma of the gingiva in posterior maxilla. Int J Clin Pediatr Dent (2021) 14(6):816–9. doi: 10.5005/jp-journals-10005-2077
3. Mikami Y, Fujii S, Kohashi KI, Yamada Y, Moriyama M, Kawano S, et al. Low-grade myofibroblastic sarcoma arising in the tip of the tongue with intravascular invasion: A case report. Oncol Lett (2018) 16(3):3889–94. doi: 10.3892/ol.2018.9115
4. Bai Y, Li X, Yin Z. Management of low-grade myofibroblastic sarcoma of the larynx. Ear Nose Throat J (2020) 99(7):NP82–3. doi: 10.1177/0145561319840140
5. Hou W, Su M, Li Q, Tian R. Low-grade myofibroblastic sarcoma demonstrated on 99mTc-MDP bone scan and 18F-FDG PET/CT. Clin Nucl Med (2020) 45(7):549–51. doi: 10.1097/RLU.0000000000003073
6. Yonezawa H, Yamamoto N, Hayashi K, Takeuchi A, Miwa S, Igarashi K, et al. Low-grade myofibroblastic sarcoma of the levator scapulae muscle: a case report and literature review. BMC Musculoskelet Disord (2020) 21(1):836. doi: 10.1186/s12891-020-03857-3
7. Guillen Astete CA, Larena Grijalva C. Myofibroblastic sarcoma of the trapezius muscle. Reumatol Clin (Engl Ed) (2019) 15(5):e47–8. doi: 10.1016/j.reuma.2017.06.011
8. Myong NH, Min JW. Low-grade myofibroblastic sarcoma arising in fibroadenoma of the breast-a case report. Diagn Pathol (2016) 11:33. doi: 10.1186/s13000-016-0480-8
9. Wu X, Guo L, Li S, Zheng Y, Fan B, Zhou C. Low-grade myofibroblastic sarcoma with abdominal pain, a stuffy nose, hearing loss, and multiple cavity effusion: a case report and literature review. J Int Med Res (2020) 48(1):300060519895661. doi: 10.1177/0300060519895661
10. Wang L, Li LX, Chen DQ, Yang L, Li SK, Cheng C. Low-grade myofibroblastic sarcoma: clinical and imaging findings. BMC Med Imaging (2019) 19(1):36. doi: 10.1186/s12880-018-0287-z
11. Niu R, Wang JF, Zhang DC, Shao XL, Qiu C, Wang YT. Low-grade myofibroblastic sarcoma of gastric cardia on 18F-FDG positron emission tomography/computed tomography: An extremely rare case report. Med (Baltimore) (2018) 97(4):e9720. doi: 10.1097/MD.0000000000009720
12. Morii T, Mochizuki K, Sano H, Fujino T, Harasawa A, Satomi K. Occult myofibroblastic sarcoma detected on FDG-PET performed for cancer screening. Ann Nucl Med (2008) 22(9):811–5. doi: 10.1007/s12149-008-0194-4
13. Deng Qr, Han Lj, Hu Ss, Zhai H, Chen Lg. The value of 18F-FDG PET/CT imaging in the differential diagnosis between extensive lymph node metastasis and non-hodgkin’s lymphom. J Med Theor & Prac (2022) 35(17):2884–7. doi: 10.19381/j.issn.1001-7585.2022.17.002
Keywords: myofibroblastic sarcoma, cancer, FDG, PET/CT, metastasis
Citation: Zhang H, He L, Hu B, Zhang X and Zheng L (2023) Case report: Low-grade myofibroblastic sarcoma resembling lymphoma on 18F-FDG PET/CT. Front. Oncol. 13:1139720. doi: 10.3389/fonc.2023.1139720
Received: 07 January 2023; Accepted: 22 February 2023;
Published: 07 March 2023.
Edited by:
Venkatesan Renugopalakrishnan, Harvard University, United StatesReviewed by:
Bilgin Kadri Aribas, Bülent Ecevit University, TürkiyeCopyright © 2023 Zhang, He, Hu, Zhang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lichun Zheng, bm16aGVuZ2xjaEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.