
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Oncol. , 20 February 2023
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1138068
This article is part of the Research Topic Optical Molecular Imaging in Cancer Research-Volume II View all 5 articles
 Zedong Jiang1,2†
Zedong Jiang1,2† Bo Zhou1,2†
Bo Zhou1,2† Xiang Zheng1,2†
Xiang Zheng1,2† Guogang Li1,2
Guogang Li1,2 Zhenzhen Gao1,2
Zhenzhen Gao1,2 Yang Tian1,2
Yang Tian1,2 Chunlong Shao1,2
Chunlong Shao1,2 Shaoyan Xu1,2
Shaoyan Xu1,2 Sheng Yan1,2*
Sheng Yan1,2*Background: Recently, in many Asian centers, laparoscopic anatomical liver resection (LALR) using the indocyanine green (ICG) fluorescence imaging technique has been increasingly applied in resecting hepatocellular carcinoma, even in colorectal liver metastases. However, LALR techniques have not been fully standardized, especially in right superior segments. Due to the anatomical position, prevailing positive staining using a PTCD (percutaneous transhepatic cholangial drainage) needle was superior to negative staining in right superior segments hepatectomy, while it was difficult to manipulate. Herein, we design a novel method of ICG-positive staining for LALR of right superior segments.
Methods: Between April 2021 and October 2022, we retrospectively studied patients in our institute who underwent LALR of right superior segments using a novel method of ICG-positive staining, which comprised a customized puncture needle and an adaptor. Compared to the PTCD needle, the customized needle was not limited by the abdominal wall and could be punctured from the liver dorsal surface, which was more flexible to manipulate. The adapter was attached to the guide hole of the laparoscopic ultrasound (LUS) probe to ensure the precise puncture path of the needle. Guided by preoperative three-dimensional (3D) simulation and intraoperative laparoscopic ultrasound imaging, we punctured the transhepatic needle into the target portal vein through the adaptor and then slowly injected 5-10 ml of 0.025 mg/ml ICG solution into the vessel. LALR can be guided by the demarcation line under fluorescence imaging after injection. Demographic, procedural and postoperative data were collected and analyzed.
Results: In this study, 21 patients underwent LALR of the right superior segments with ICG fluorescence-positive staining, and the procedures had a success rate of 71.4%. The average staining time was 13.0 ± 6.4 min, the operative time was 230.4 ± 71.7 min, R0 resection was 100%, the postoperative hospital stay was 7.1 ± 2.4 days, and no severe puncture complications occurred.
Conclusions: The novel customized puncture needle approach seems to be feasible and safe for ICG-positive staining in LALR of right superior segments, with a high success rate and a short staining time.
It is well established that for HCC (hepatocellular carcinoma), anatomical liver resection is associated with better overall survival and a lower recurrence rate, and recent evidence suggests its role in the treatment of intrahepatic cholangiocarcinoma and potentially for CRLM (colorectal liver metastases) (1).
Combined with the rapid advance of minimally invasive surgery, laparoscopic anatomic liver resection (LALR) has recently become the preferred procedure in liver resection. Segmentectomy or subsegmentectomy, typically involving resection of the tumor-bearing portal pedicles, without disrupting the structural integrity of remnant segments, is considered LALR (2, 3). It is commonly guided by a demarcation line after portal staining or inflow clamping of the target territory (4), injecting the chemical dye into the portal vein of the tumor-bearing segment by ultrasound-guided transhepatic puncture (5), or inducing the ischemic line by clamping the portal pedicle supplying the segment(s) (6).
The right superior segments, including hepatic segments VII (S7) and VIII (S8), are located deep and adjacent to the hepatic veins and inferior vena cava, making it difficult to use hepatic staining and preoperative three-dimensional simulation guidance during laparoscopy (6). Due to the poor anatomical position, right superior segment resection is considered the most difficult in LALR. There is no standardized approach to right superior segments, although many authors have proposed new and different technical approaches, such as the hilar Glissonean-First approach, transparenchymal approach and hepatic vein first approach (7).
Indocyanine green (ICG) was so common among liver surgeons as a reagent for estimating hepatic function that they could hardly think of the other application of ICG for fluorescence imaging (8). With a fusion monitor mounted on the laparoscopic system, the ICG fluorescence imaging technique offers a novel navigation tool to guide liver parenchymal transection by precisely tracing its segmental territorial borderline (9). This technique was first reported by Prof. Aoki (10) in 2008 and then applied to laparoscopic hepatectomy under the name of positive staining (portal staining) and negative staining(systematic staining after target Glissonean pedicle clamping) technique described by Ishizawa et al. (11) in 2012. With the development of the technique, current consensus guidelines for its use (12) were developed in 2021, and an assessment including 51 eligible reports indicated a satisfactory success rate in terms of delineating targeted hepatic segments (13).
However, LALR techniques have not been fully standardized because both selective inflow portal clamping and conventional portal staining, when used, are difficult to perform in the minimally invasive setting (1), especially in right superior segments. Negative staining is technically demanding and has a potential risk for biliary injury during the Glissonean approach in laparoscopic segment VII and VIII segmentectomy (14). Ultrasound-guided positive staining may be superior to negative staining in right superior segments hepatectomy, while the prevailing positive staining using PTCD needles is difficult to manipulate and hard to learn. Herein, we describe a novel method of positive staining in right superior segments hepatectomy to better achieve the staining goal and explore its feasibility, success rate, efficiency and safety.
Between April 2021 and October 2022, patients who underwent LALR at the Second Affiliated Hospital of Zhejiang University were studied retrospectively. The inclusion criteria of this study: diagnosed as HCC or CRLM confined to S7 or S8; resectable by conventional laparoscopic surgery; mono-segmentectomy or sub-segmentectomy of right superior segments, including S7, S8, S8v (ventral segment of S8) or S8d (dorsal segment of S8) resection; and the operations were performed by our novel ICG-positive staining. Patients who received ICG-positive staining for LALR but finally received non-LALR (local hepatectomy or conversion to laparotomy), combined with other organ resections or received segments 2 to 6 resections were excluded from the study. Patients underwent a liver functional reserve test based on the plasma ICG retention rate at 15 min 5-7 days before surgery (0.5 mg/kg) (9). Patients with ICG allergy were prohibited from undergoing this procedure. All operations were performed by the same surgical team. Collection data included demographic data (sex, age, pathology, segments of staining, tumor sizes, hepatitis, cirrhosis), procedural data (staining time, staining success rate, operative time, blood loss, R0 resection) and postoperative data (puncture complication, postoperative hospital days). All patients provided written informed consent for surgery, and this study was approved by the Ethical Review Board of the Second Affiliated Hospital of Zhejiang University.
Preoperative 3D simulation (Vitaworks, Huishi Medical Technology, Shanghai, China) of liver computed tomography (CT) was performed to obtain preliminary knowledge of the location and size of the tumor(s), intrahepatic vascular anatomy and their spatial relationships to the tumor(s). During this preoperative simulation, the number and size of target portal branches, the exact injection point(s), and the resultant positive staining segmental volume were determined (14).
The fluorescence imaging system (HyPixel™ R1, Mindray, Shenzhen, China) can be used with indocyanine green to provide real-time visible light images and near-infrared fluorescence images. After ICG staining, the fluorescent mode helps surgeons determine the superficial and intrahepatic boundaries according to the fluorescence demarcation.
The customized puncture needle is simply connected by a 16-gauge needle (diameter 1.69 mm, length 7 cm) and a soft injection tube (diameter 1.84 mm, length 60 cm) (Figure 1A). The BK laparoscopic ultrasound probe is equipped with a puncture hole that guides the puncture. Accordingly, there is a guide line (switched on if we need it) on ultrasound imaging that matches the path of the puncture guide hole. But the guide hole(13-gauge) is larger than the 16-gauge needle. Therefore, we designed an adaptor (a metal sheath) that can precisely match the 16-gauge needle and the 13-gauge guide hole (Figure 1B) so that it can guide the needle into the target portal vein precisely according to the guide line (Figure 1C). The adaptor was created by an engineer according to our design. It is reusable by high-pressure steam sterilization and can be 3D printed. After our constant practice, we attached the adaptor to the ultrasound hole with a silk thread before the LUS probe entered the abdominal cavity. It was convenient and effective, which could prevent the adaptor from missing.
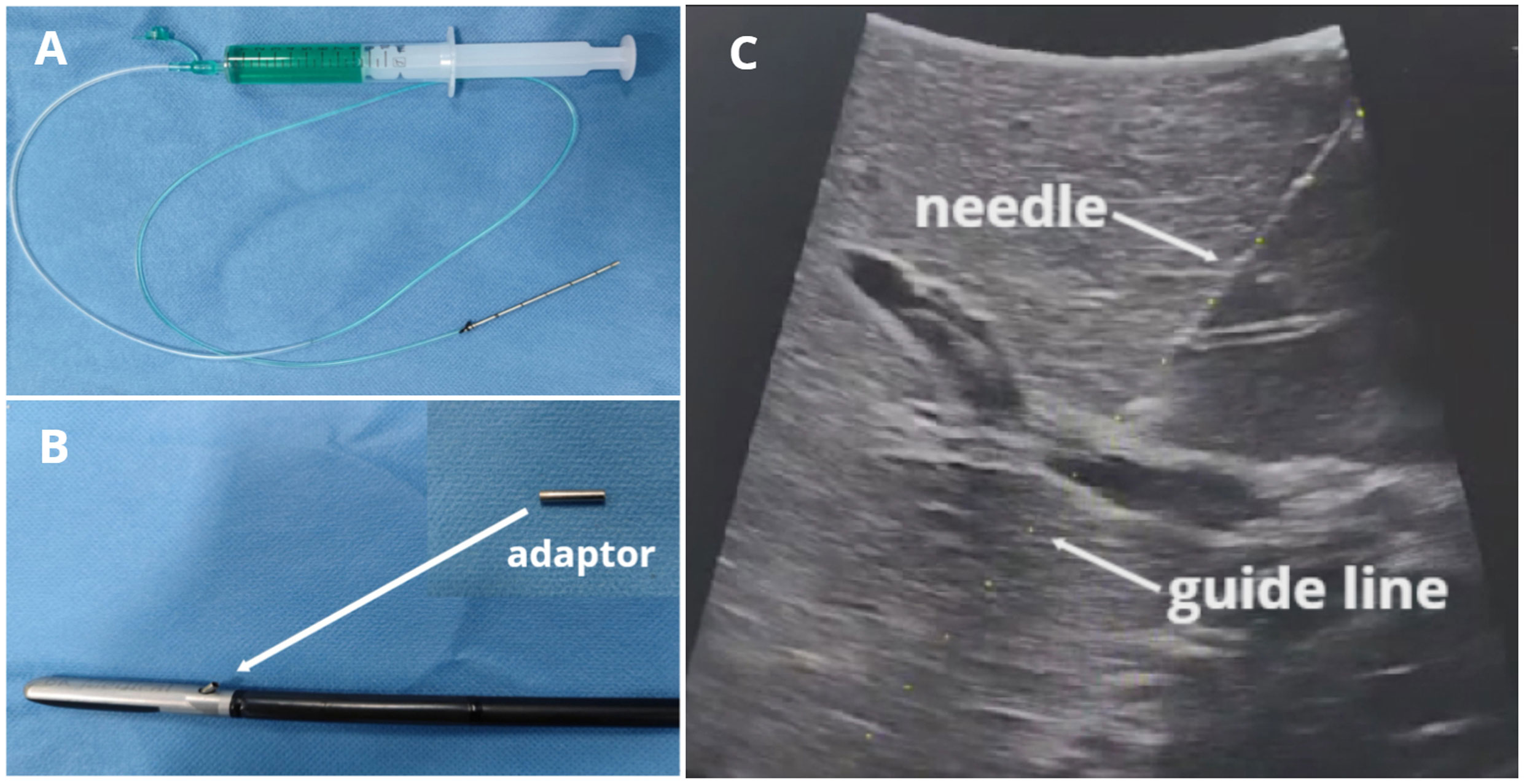
Figure 1 BK LUS-guided precise puncture with our customized needle and adaptor. (A) Puncture needle; (B) Laparoscopic ultrasound probe and adaptor; (C) Puncture needle and the guide line with the same path.
The six-port placements of the operation are illustrated in Figure 2. The first assistant stood at the patient’s left side and located the target portal vein by the LUS (port A). The senior surgeon stood at the patient’s right side, set the customized puncture needle into the abdominal cavity (port B), and punctured the needle through the adaptor into the target portal vein by using a laparoscopic needle holder (port B) and noninvasive forceps (port C). Blood was withdrawn to ensure that the needle tip was in the target portal vein, and 5-10 ml (depending on the segmental volume) of 0.025 mg/ml ICG solution was slowly injected into the vessel. After injection, the fluorescence demarcation line was identified in several seconds and delineated through electrocautery under ICG fluorescence imaging.
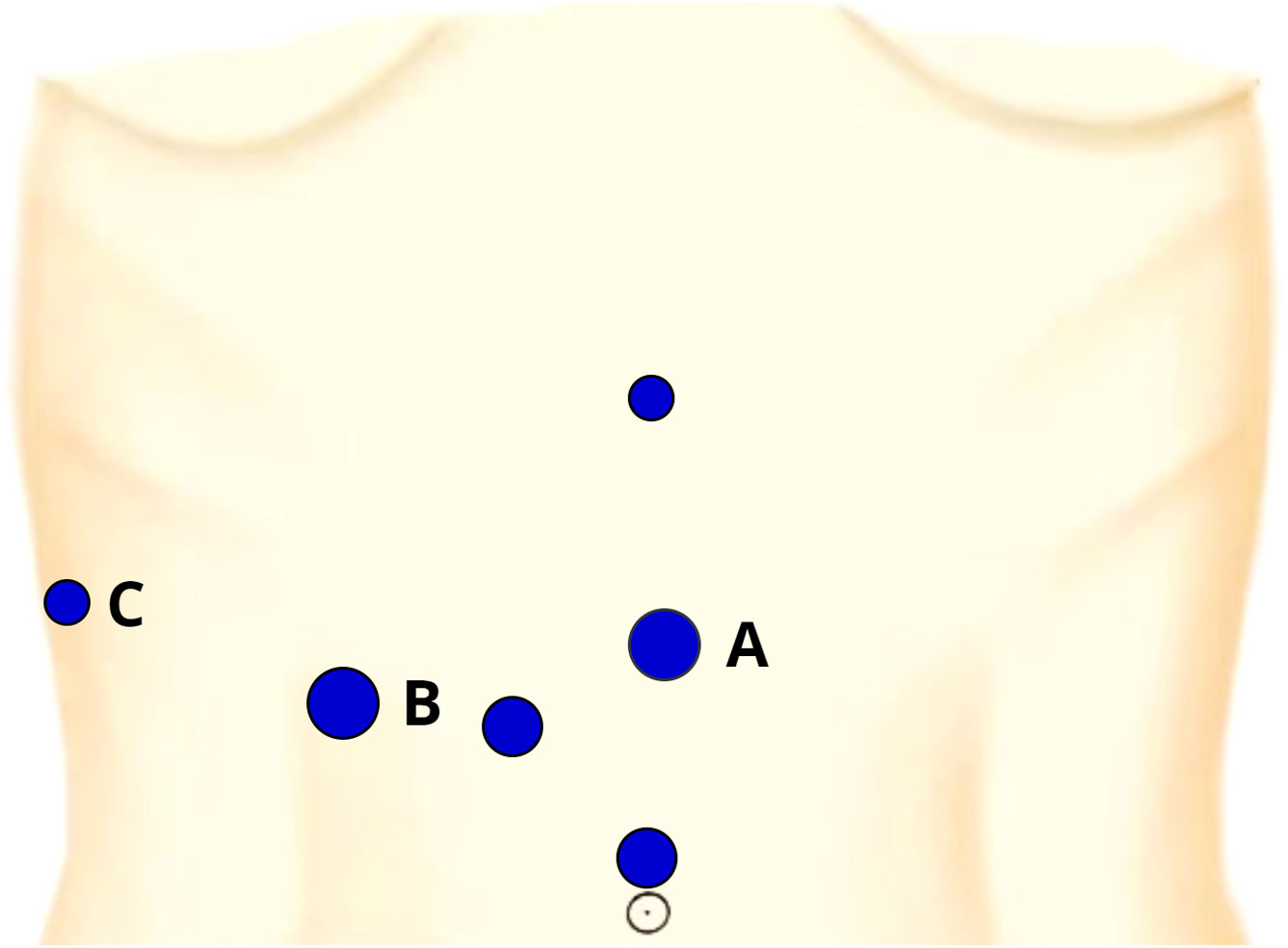
Figure 2 The six-port placements and their entrance for our positive staining. Port (A): a 12-mm trocar located on the left side of the midpoint of xiphoid-navel line, the entrance of the LUS; Port (B): a 12-mm trocar at the junction of 5 cm below the costal margin and the midclavicular line, the entrance of the needle and laparoscopic needle holder; Port (C): a 5-mm trocar located at the junction of the anterior axillary line and the costal margin, the entrance of the laparoscopic noninvasive forceps.
Before staining, adequate dissection of the liver is necessary in most cases, especially for the dorsal puncture in S7. After positive staining, the liver parenchyma was transected using a harmonic scalpel (Ethicon, NY, USA) using the clamp-crushing technique. Vessels with a diameter of 2 mm or less were directly dissected using a harmonic scalpel, while vessels with a diameter greater than 2 mm were dissected after clipping with a hemostat clamp or stapler. Hemostasis of the cut surface was achieved using a bipolar sealer or monopolar electrocautery. The hepatic blood inflow was controlled by means of intermittent application of Pringle’s maneuver with 15 min of occlusion and 5 min of release to reduce blood loss during liver parenchymal transection. In the transection, fluorescence imaging can be displayed repeatedly to confirm the demarcations.
In total, 35 patients with HCC or CRLM underwent our novel positive staining for LALR, but 14 patients were excluded, including local hepatectomy(n=2), converted to laparotomy(n=1), combined with other organ resections(n=2), and segments 2 to 6 resections(n=9). Finally, 21 patients underwent LALR of the right superior segments with this novel positive staining method (Figure 3). Among the 21 patients, ten underwent S7 hepatectomy, six underwent S8 hepatectomy, two underwent S8v hepatectomy, and three underwent S8d hepatectomy. Fourteen cases were diagnosed with hepatocellular carcinoma and seven cases were diagnosed with colorectal liver metastasis according to the postoperative pathology. The mean age of the 21 patients (15 males and 6 females) was 61 years, ranging from 28 to 85. The average staining time (including LUS location, needle puncture and ICG injection) was 13.0 ± 6.4 min, the operative time was 230.4 ± 71.7 min, the blood loss was 140.5 ± 106.8 ml, R0 resection was 100%, and the postoperative hospital stay was 7.1 ± 2.4 days (Table 1).
The overall success rate was 71.4%. Six failure cases were classified into three types in our practice as follows: FI (Failure I)- unable to puncture into the target portal vein (n=2), poor location or thin vessel (<3 mm); FII (Failure II)- nontumor-bearing segment staining (n=2), backflow or diffusion. Of note, slight contamination could also be considered successful, as we could distinguish the surgical boundaries according to the intensity of fluorescence in fluorescent mode or grayscale in grayscale-mode; FIII (Failure III)- tumor-bearing segments with incomplete staining (n=2), puncture of the tributary of the target vessel or incomplete puncture of multiple target branches. In FI, one patient underwent S7 hepatectomy, deep puncture and limited needle length led to puncture failure, and in the other patient who underwent S8d hepatectomy, two relatively thin target vessels led to failure. In FII, two patients underwent S8 hepatectomy, ICG back flowed into S5 (segment V) in one patient, and ICG diffused into S7 because of vascular anatomic variation in another patient. In FIII, one patient who underwent S8 hepatectomy underwent puncture of two of multiple (>3) target branches, and the other patient who underwent S7 hepatectomy underwent puncture into the tributary of target P7 (portal vein of segment VII), leading to incomplete staining (Table 2). In failure cases, LALR can be completed with negative staining or the method inducing ischemic demarcation (an incomplete LALR with the help of LUS and landmark veins) after inflow clamping of the target territory.
There were 4 (19.0%) patients with postoperative complications. Two patients with slight bile leakage recovered with conservative treatment, and two patients with pleural fluids recovered after thoracic tube drainage. No Clavien−Dindo IV or V complications occurred in this study.
A 48-year-old male patient with a single HCC (33 mm x 24 mm) confined to Segment 7 (Patient 5). We performed this novel method with a customized needle to puncture P7 from the liver dorsal surface and injected 5 ml ICG solution. Figure 4 (See Video 1, Supplementary digital materials)
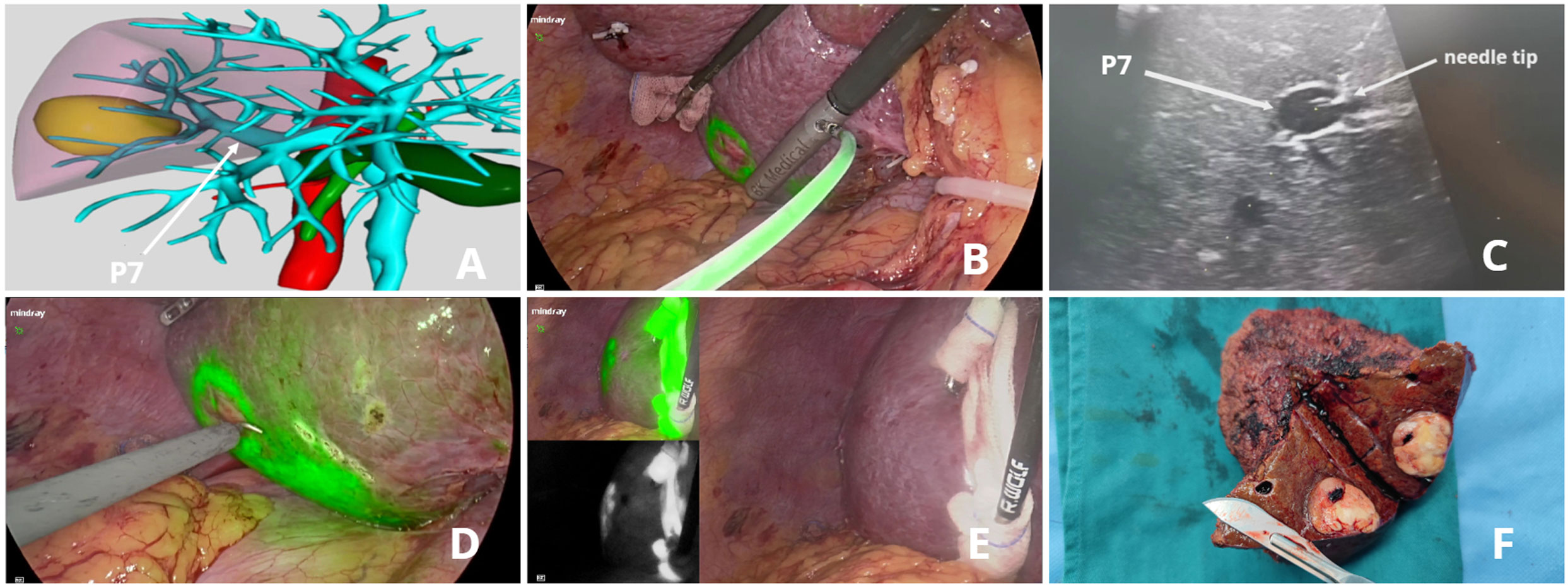
Figure 4 ICG fluorescence-guided S7 segmentectomy. (A) preoperative 3D simulation of CT identified the tumor confined to segment 7, located at the tumor-bearing portal branch (P7); (B) ICG solution injection from the liver dorsal surface; (C) LUS guided puncture to P7: ICG solution was floating in P7 through the needle tip; (D) fluorescent delineation of tumor-bearing S7 on the liver dorsal surface; (E) liver ventral surface demarcation; (F) specimen.
A 68-year-old female patient with a single HCC (24 mm × 18 mm) confined to segment 8 (Patient 15). P8 (portal vein of segment VIII) was punctured from the liver ventral surface, and 5 ml ICG diluted solution was injected. Figure 5 (See Video 2, Supplementary digital materials)

Figure 5 ICG fluorescence-guided S8 segmentectomy. (A) Preoperative 3D simulation of CT identified the tumor confined to segment 8, located in the tumor-bearing portal branch and the landmark hepatic veins; (B) LUS-guided puncture to the portal branch feeding S8; (C) ICG solution injection process under fluorescence imaging; (D) fluorescent delineation of tumor-bearing S8; (E) intrahepatic parenchymal borderline in the transection; (F) S8 pedicle dissection and division.
Laparoscopic anatomical liver resections guided by a demarcation line after portal staining or inflow clamping of the target area have been established as essential methods for curative treatment of hepatocellular carcinoma (HCC) and have subsequently been applied to other malignancies (15). However, located deep-seated and surrounded by the ribs and the diaphragm (16), there is still no standardized approach to right superior segments.
With the emergence of fluorescence imaging techniques, ICG staining is increasingly applied in LALR. However, both conventional positive staining and negative staining of ICG injection are technically demanding and have a poor success rate. Xu et al.’s (9) 36 cases of LALR guided using ICG concluded that segmental staining was successful in 19 procedures (53%, 19/36), including negative staining and positive staining in 52% (14/27) and 56% (5/9), respectively. Diffusion of ICG might be the biggest problem with negative staining, which may lead to a smaller than expected resection area and a narrower surgical margin (17). Lan et al. (17) reported 24 LALR procedures using a positive staining “hepatic pedicle first” approach, representing a 79.17% success rate. However, both negative staining and the “hepatic pedicle first” approach require dissection of the target pedicle, which is technically demanding and time-consuming. Moreover, the pedicles of S7/8 are rather difficult to dissect through the first porta. Similarly, the other approach, in which the Glissonean pedicle supplying the tumor-bearing segment(s) is clamped to induce an ischemic demarcation line on the liver surface, is mainly feasible for Segments 1 to 6 and difficult for the superoposterior segments (6). Indeed, the method inducing ischemic demarcation has other problems, including identification in intrahepatic parenchymal borderlines and undetectable demarcation in some difficult settings. Percutaneous transhepatic puncture with a PTCD (percutaneous transhepatic cholangial drainage) needle is a common method of positive staining in Asian centers. In Takeshi Aoki’s report, two cases of preoperative percutaneous ICG injection failed, representing an 86% (12/14) success rate (14). We have practiced this method and experienced several complications using the procedure. First, to perform this method, surgeons must be skillful at the interpretation of the preoperative 3D simulation, proficient in LUS and comfortable with ultrasound-guided puncture. Second, due to the limitations of the abdominal wall and PTCD needle inflexibility, a precise transhepatic puncture is quite difficult in laparoscopic settings. Surgeons used educated guesses to determine puncture points and experienced manipulation to puncture or adjust the needle. In other words, percutaneous transhepatic puncture cannot be replicated easily. Compared to the method using a PTCD needle, our method guided by the guide line and guide hole is more precise and easier to learn. In addition, our customized needle with a soft injection tube is more flexible to manipulate and adjust in the abdominal cavity.
A new positive staining method using a laser fixed on the LUS probe to irradiate the abdominal wall to determine the puncture point seems more innovative, while the abdominal wall might be a big hurdle. The abdominal wall is quite thick in some patients which might influence the puncture path and slight deviation of the puncture path probably cause puncture difficulties. Compared to this method, our method is not limited by the abdominal wall with simpler workmanship. Another approach was reported by Ueno et al., who used preoperative interventional radiology techniques to stain the targeted arterial branch by performing angiography in a hybrid operation room. This procedure is helpful but may not be popular in centers because of its additional time and cost (18).
Our method of positive staining is new, with a relatively high success rate (71.4%) and short staining time (13.0 ± 6.4 min). To our knowledge, this is the first report describing this method for positive staining in LALR of right superior segments. It seems to be effective and efficient. In addition, this set of puncture needles is user-friendly and cost-effective. Our new device was mainly used for ICG-positive staining in laparoscopic anatomical liver resection of S7 and S8. Besides, it also could be used in LALR of segments 2 to 6, but the total 9 cases of five segments were relatively few. In fact, in our clinical practice, S5, S6(segment VI) and left liver resections were generally performed by negative staining, as it seemed easier and more effective than positive staining. Laparoscopic anatomical liver resection of right superior segments (S7 and S8) is the best indication of our method, and it is also our main aim in this study.
Sufficient preoperative and intraoperative preparations would reduce the failure rates. Considering the complicated intrahepatic anatomical structure and vascular variations, it is indispensable to perform preoperative 3D simulation and study it carefully to determine the target vessel(s) or even the exact point(s) on the vessel(s) to be punctured. Over the last decade, it has been proven safe and effective to use 3D simulation software for pre-hepatectomy assessment, virtual hepatectomy, and measurement of liver volumes in blood flow areas of the portal vein (19). Moreover, it has been reported that 3D reconstruction achieves better outcomes than 2D imaging. Jihua Jiang’s meta-analysis of 16 studies showed that the 3D liver reconstruction group was significantly superior to the conventional surgery group in terms of operation time and intraoperative blood loss (20).
In our practice of this study, S7 staining had a better outcome since P7 is generally a single large branch or has fewer small branches. In the P7 puncture, the needle puncture depth might be > 8 cm from the ventral liver surface. However, deep vessels were difficult to puncture, which meant more parenchymal puncture with a higher risk of injury. Therefore, we could puncture from the dorsal liver surface instead, as Patient 5 presented. S8 often has two main portal branches (P8v and P8d, ventral branch of P8 and dorsal branch of P8) or more, so we can dye its subsegments according to our needs. If we want to dye the complete S8, we must identify which branches to be dyed through preoperative 3D reconstruction to avoid omission. When S8 branches were >3 times and the branch diameters were relatively thin, negative staining was chosen instead. Of note, we had undergone a failure of S8 staining because of backflow. We targeted the puncture site of P8 through LUS. However, the puncture point on the vessel was too close to the proximal portal venous bifurcation, making ICG backflow to S5 apparent and even visible in LUS imaging. Therefore, similar to the conclusion of Pro. Makuuchi, the puncture point of the portal venous branch must be 1 to 2 centimeters distal from the ligating point because the dye regurgitates in the portal vein and flows into the other branches, which should be preserved (5). In addition, a slow injection can avoid backflow to some extent.
There are several limitations to our staining procedure. First, backflow, diffusion and multiple thin target vessel(s) are still tricky challenges when using this method. In addition, the needle and adaptor we devised may be a transitional product. An advanced product could be an integrated needle with LUS. Third, this study was retrospective with a relatively small sample size. A prospective study with a large sample size should be developed in our future study.
In conclusion, this novel method of positive staining in LALR of right superior segments seems to be effective, efficient and convenient. Further study may involve the integrated design of a puncture needle with LUS including workmanship, materials and specifications. With the refinements of laparoscopic instruments and the improvement of surgeons’ techniques, this method can be applied further.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethical Review Board of the Second Affiliated Hospital of Zhejiang University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
SY and BZ performed the operation on all patients and designed the research. ZJ and XZ wrote the manuscript. GL, ZG, YT, CS, and SX participated in the operation and collected the data. ZJ, BZ, and XZ analyzed and interpreted the data and revised this article. All authors contributed to the article and approved the submitted version.
This research is supported by grants from the National Natural Science Foundation of China (SY, Grant No. 82270684 and YT, Grant No. 82000619).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1138068/full#supplementary-material
Supplementary Video 1 | Positive staining in laparoscopic anatomical S7 segmentectomy.
Supplementary Video 2 | Positive staining in laparoscopic anatomical S8 segmentectomy.
1. Felli E, Ishizawa T, Cherkaoui Z, Diana M, Tripon S, Baumert TF, et al. Laparoscopic anatomical liver resection for malignancies using positive or negative staining technique with intraoperative indocyanine green-fluorescence imaging. HPB (Oxford) (2021) 23(11):1647–55. doi: 10.1016/j.hpb.2021.05.006
2. Kang W-H, Kim K-H, Jung D-H, Park G-C, Kim S-H, Cho H-D, et al. Long-term results of laparoscopic liver resection for the primary treatment of hepatocellular carcinoma: Role of the surgeon in anatomical resection. Surg Endosc (2018) 32(11):4481–90. doi: 10.1007/s00464-018-6194-0
3. Makuuchi M. Surgical treatment for HCC – special reference to anatomical resection. Int J Surg (2013) 11:S47–9. doi: 10.1016/S1743-9191(13)60015-1
4. Inoue Y, Arita J, Sakamoto T, Ono Y, Takahashi M, Takahashi Y, et al. Anatomical liver resections guided by 3-dimensional parenchymal staining using fusion indocyanine green fluorescence imaging. Ann Surg (2015) 262(1):105–11. doi: 10.1097/SLA.0000000000000775
5. Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet (1985) 161:346–50.
6. Takasaki K. Glissonean pedicle transection method for hepatic resection: A new concept of liver segmentation. J Hepatobiliary Pancreat Surg (1998) 5(3):286–91. doi: 10.1007/s005340050047
7. Anselmo A, Sensi B, Bacchiocchi G, Siragusa L, Tisone G. All the routes for laparoscopic liver segment VIII resection: A comprehensive review of surgical techniques. Front Oncol (2022) 12:864867. doi: 10.3389/fonc.2022.864867
8. Nakaseko Y, Ishizawa T, Saiura A. Fluorescence-guided surgery for liver tumors. J Surg Oncol (2018) 118(2):324–31. doi: 10.1002/jso.25128
9. Xu Y, Chen M, Meng X, Lu P, Wang X, Zhang W, et al. Laparoscopic anatomical liver resection guided by real-time indocyanine green fluorescence imaging: Experience and lessons learned from the initial series in a single center. Surg Endosc (2020) 34(10):4683–91. doi: 10.1007/s00464-020-07691-5
10. Aoki T, Yasuda D, Shimizu Y, Odaira M, Niiya T, Kusano T, et al. Image-guided liver mapping using fluorescence navigation system with indocyanine green for anatomical hepatic resection. World J Surg (2008) 32(8):1763–7. doi: 10.1007/s00268-008-9620-y
11. Ishizawa T, Zuker NB, Kokudo N, Gayet B. Positive and negative staining of hepatic segments by use of fluorescent imaging techniques during laparoscopic hepatectomy. Arch Surg (2012) 147(4):393–4. doi: 10.1001/archsurg.2012.59
12. Wang X, Teh CSC, Ishizawa T, Aoki T, Cavallucci D, Lee SY, et al. Consensus guidelines for the use of fluorescence imaging in hepatobiliary surgery. Ann Surg (2021) 274(1):97–106. doi: 10.1097/SLA.0000000000004718
13. Ishizawa T, McCulloch P, Stassen L, van den Bos J, Regimbeau JM, Dembinski J, et al. Assessing the development status of intraoperative fluorescence imaging for anatomy visualisation, using the IDEAL framework. BMJ Surg Interv Health Technol (2022) 4(1):e000156. doi: 10.1136/bmjsit-2022-000156
14. Aoki T, Koizumi T, Mansour DA, Fujimori A, Kusano T, Matsuda K, et al. Ultrasound-guided preoperative positive percutaneous indocyanine green fluorescence staining for laparoscopic anatomical liver resection. J Am Coll Surg (2020) 230(3):e7–e12. doi: 10.1016/j.jamcollsurg.2019.11.004
15. Urade T, Sawa H, Iwatani Y, Abe T, Fujinaka R, Murata K, et al. Laparoscopic anatomical liver resection using indocyanine green fluorescence imaging. Asian J Surg (2020) 43(1):362–8. doi: 10.1016/j.asjsur.2019.04.008
16. Monden K, Sadamori H, Hioki M, Ohno S, Takakura N. Intrahepatic glissonean approach for laparoscopic bisegmentectomy 7 and 8 with root-side hepatic vein exposure. Ann Surg Oncol (2022) 29(2):970–1. doi: 10.1245/s10434-021-10839-9
17. Lan X, Tang Y, Wei W, Jiang K, Chen K, Du C, et al. Indocyanine green fluorescence staining based on the "hepatic pedicle first" approach during laparoscopic anatomic liver resection. Surg Endosc (2022) 36(11):8121–31. doi: 10.1007/s00464-022-09237-3
18. Ueno M, Hayami S, Sonomura T, Tanaka R, Kawai M, Hirono S, et al. Indocyanine green fluorescence imaging techniques and interventional radiology during laparoscopic anatomical liver resection (with video). Surg Endosc (2018) 32(2):1051–5. doi: 10.1007/s00464-017-5997-8
19. Fang C, An J, Bruno A, Cai X, Fan J, Fujimoto J, et al. Consensus recommendations of three−dimensional visualization for diagnosis and management of liver diseases. Hepatol Int (2020) 14(4):437–53. doi: 10.1007/s12072-020-10052-y
Keywords: laparoscopic anatomical liver resection, right superior segments, ICG-positive staining, novel method, three-dimensional (3D) simulation, laparoscopic ultrasound, fluorescence imaging
Citation: Jiang Z, Zhou B, Zheng X, Li G, Gao Z, Tian Y, Shao C, Xu S and Yan S (2023) A novel method of ultrasound-guided positive staining using indocyanine green fluorescence in laparoscopic anatomical liver resection of segments VII and VIII. Front. Oncol. 13:1138068. doi: 10.3389/fonc.2023.1138068
Received: 05 January 2023; Accepted: 09 February 2023;
Published: 20 February 2023.
Edited by:
Nicolas Golse, Hôpital Paul Brousse, FranceReviewed by:
Takashi Kokudo, The University of Tokyo, JapanCopyright © 2023 Jiang, Zhou, Zheng, Li, Gao, Tian, Shao, Xu and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheng Yan, c2hlbmd5YW5Aemp1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.