
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 30 May 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1137785
This article is part of the Research TopicManagement of Peritoneal Surface Malignancies. (Cytoreductive Surgery, HIPEC, PIPAC, and Beyond)View all 11 articles
 Jun Kiat Thaddaeus Tan1,2†
Jun Kiat Thaddaeus Tan1,2† Jolene Si Min Wong1,2,3,4†
Jolene Si Min Wong1,2,3,4† Chin Jin Seo1,2,3,4
Chin Jin Seo1,2,3,4 Cindy Lim5
Cindy Lim5 Hong-Yuan Zhu1,2
Hong-Yuan Zhu1,2 Chin-Ann Johnny Ong1,2,3,4,6,7
Chin-Ann Johnny Ong1,2,3,4,6,7 Claramae Shulyn Chia1,2,3,4*
Claramae Shulyn Chia1,2,3,4*Background: Peritoneal surface malignancies (PSM) present insidiously and often pose diagnostic challenges. There is a paucity of literature quantifying the frequency and extent of therapeutic delays in PSM and its impact on oncological outcomes.
Methods: A review of a prospectively maintained registry of PSM patients undergoing Cytoreductive Surgery and Hyperthermic Intra-peritoneal Chemotherapy (CRS-HIPEC) was conducted. Causes for treatment delays were identified. We evaluate the impact of delayed presentation and treatment delays on oncological outcomes using Cox proportional hazards models.
Results: 319 patients underwent CRS-HIPEC over a 6-years duration. 58 patients were eventually included in this study. Mean duration between symptom onset and CRS-HIPEC was 186.0 ± 37.1 days (range 18-1494 days) and mean duration of between patient-reported symptom onset and initial presentation was 56.7 ± 16.8 days. Delayed presentation (> 60 days between symptom onset and presentation) was seen in 20.7% (n=12) of patients and 50.0% (n=29) experienced a significant treatment delay of > 90 days between 1st presentation and CRS-HIPEC. Common causes for treatment delays were healthcare provider-related i.e. delayed or inappropriate referrals (43.1%) and delayed presentation to care (31.0%). Delayed presentation was a significantly associated with poorer disease free survival (DFS) (HR 4.67, 95% CI 1.11-19.69, p=0.036).
Conclusion: Delayed presentation and treatment delays are common and may have an impact on oncological outcomes. There is an urgent need to improve patient education and streamline healthcare delivery processes in the management of PSM.
Patients with peritoneal surface malignancies (PSM) represent a heterogenous group ranging from primary peritoneal cancer to peritoneal metastases secondary to various primaries. Since its introduction in the 1980s, Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (CRS-HIPEC) has revolutionized the management of peritoneal malignancies (1, 2). When once associated with a dismal prognosis, selected patients receiving optimal treatment now boost a 10 year survival, with rates of up to 63% reported in patients with pseudomyxoma peritonei (1, 3–6). CRS-HIPEC is now widely regarded as central to the management of PSM in selected patients (6–8).
However, PSM are frequently clinically occult with patients presenting insidiously and with nonspecific symptoms thereby posing significant diagnostic challenges (9–16). Establishing a histological diagnosis often proves challenging, and even at the tertiary level, interpretation of potentially indeterminate imaging characteristics and difficulties in determining histological characteristics result in further delay towards timely diagnosis and treatment of such malignancies (9, 17). In addition, general awareness, and knowledge amongst the physician population towards PSM is poor. In a locally conducted survey, up to 50% of survey participants acknowledged that they were unfamiliar with the disease entity and were unaware of the presence of local PSM specialist units for referrals (18), representing a potential source contributing to delayed specialist review.
Several studies suggest a significant correlation between delays incurred in diagnostic evaluation and poorer oncologic outcomes in various tumor histologies (19–25). While the current evidence in literature concurs that delivery of curative surgery in an expedient manner is crucial to the optimal management of PSM (19, 26), the causative factors and overall impact of delays incurred from patient and healthcare-related factors on oncologic outcomes has not been well-studied.
Therefore, we aim to evaluate the incidence and causes of delayed presentation and surgery and examine its impact on oncological outcomes in patients with PSM.
The study was performed in a single tertiary institution. Data was retrieved from a prospectively maintained database of patients treated with CRS-HIPEC for PSM between January 2014 and September 2019. The study was conducted with the approval of the Centralized Institutional Review Board (CIRB) of Singapore Health Services, CIRB reference number 2018/2638.
We included patients undergoing their index CRS-HIPEC surgery after a primary diagnosis of PSM. Patients with (i) recurrent PSM on a background of previously treated peritoneal malignancy, (ii) peritoneal metastases of a previously known and treated primary tumor, or (iii) who underwent neoadjuvant chemotherapy prior to CRS-HIPEC, were excluded.
Data on patient demographics, onset and duration of symptoms attributable to their primary malignancy, preoperative clinical course and oncologic history was obtained via a thorough retrospective evaluation of prospectively maintained clinical records. Patients with insufficient data pertaining to symptoms prior to initial presentation on clinical records were excluded from this study. Descriptive analyses were performed on these variables and survival outcomes were evaluated. A virtual diagnostic and treatment timeline was generated for each study patient and contributory factors to treatment delays were identified by the authors on a case-by-case basis and analyzed (Figures 1A, B).
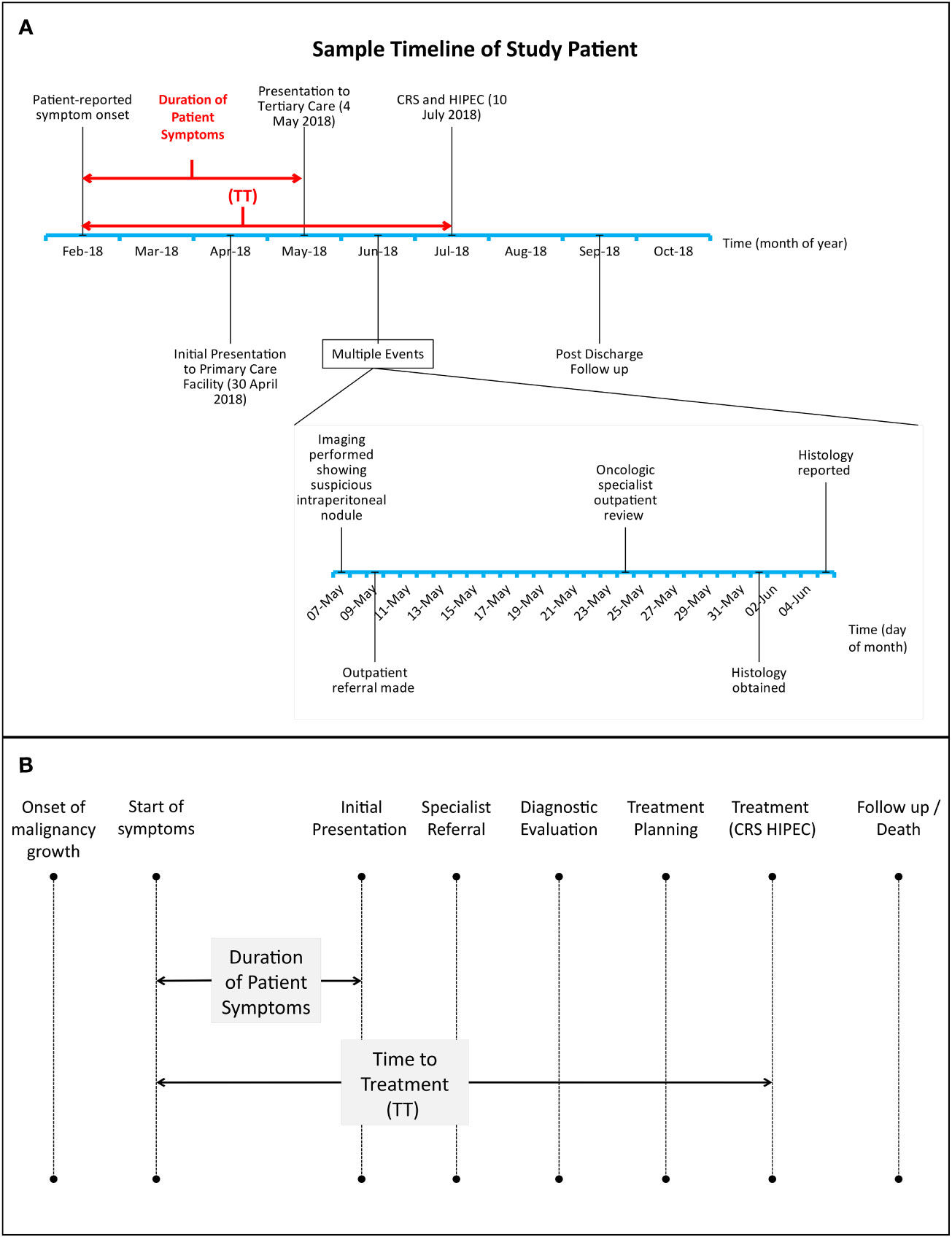
Figure 1 (A, B): (A), sample timeline of study patient treated for high grade serous carcinoma of the fallopian tubes with peritoneal involvement. Dates of initial symptom onset, presentation to tertiary care, further diagnostic evaluation and CRS-HIPEC were recorded for every patient. CRS-HIPEC=Cytoreductive surgery, hyperthermic intraperitoneal chemotherapy. TT=time to treatment. (B), Relationship between duration of patient symptoms and time to treatment (TT) with respect to key time points in cancer-related treatment delays. Adapted from Mou et al. (20).
1. Delayed Presentation: We defined this as a duration of > 60 days between patient-reported symptom onset and 1st presentation at a healthcare institution.
2. Time to Treatment (TT): Duration between patient-reported symptom onset and CRS-HIPEC surgery.
3. Delayed TT: We defined this as a Time to Treatment of > 90 days.
Given the paucity of evidence for the expected treatment timelines in peritoneal malignancies in the current literature base, the cutoffs for delayed presentation and delayed TT of 60 and 90 days, respectively, were set arbitrarily on agreement with all authors based on our institutional experience with PSM and its expected treatment course.
Factors contributing to delays of any duration were identified on a case-by-case basis and classified into 5 categories: Delayed presentation, healthcare provider, healthcare system, disease or patient-related (Table 1).
CRS and HIPEC performed at our institution included resection of the primary tumor with resection of all macroscopic peritoneal deposits combining peritonectomy procedures as well as resection of any involved intraabdominal visceral organs to achieve complete cytoreduction, with subsequent administration of HIPEC. HIPEC was performed in a closed technique, with administration of mitomycin C at 41-42°C over a duration of 60 minutes.
Differences in characteristics were tested using the Wilcoxon rank sum test for continuous variables or Fisher’s exact test for categorical variables. Kaplan-Meier survival functions were used to analyze the impact of treatment delays with respect to time to treatment and symptom duration. Overall survival (OS) was defined as time from CRS-HIPEC to death from all causes or censored at last follow-up. Disease-free survival (DFS) was defined as time from CRS-HIPEC to disease progression or censored at death or last follow-up. The Cox proportional hazards model was used to model association between survival endpoints and patient characteristics, adjusted for tumor histology. Differences between groups were estimated using the log-rank test. A two-sided p-value of less than 0.05 was considered statistically significant. All analyses were performed in R software (version 4.2.0).
319 patients underwent CRS-HIPEC at the National Cancer Centre Singapore and Singapore General Hospital between January 2014 and September 2019. 60% (n=194) underwent surgery for recurrent peritoneal disease or PM arising from a previously known and treated primary tumor and were excluded from the study. 6.3% (n=20) had insufficient data on preoperative presentation and were excluded. A further 14.7% (n=47) underwent neoadjuvant chemotherapy prior to definitive surgery, and were excluded. Finally, 58 patients were included into this study. Patients were followed up for an average of 12.4 months from time of surgery. A summary of demographic and clinical characteristics of patients studied is listed in Table 2.
Common presenting complaints were varied and include abdominal discomfort, distension and constitutional symptoms including loss of weight and appetite. Patients who were asymptomatic or had extra-abdominal symptoms not attributable to PSM were assigned a symptom duration of zero days. The mean duration between patient-reported symptom onset and CRS-HIPEC (TT) was 186.0 ± 37.1 days (range 18-1494 days) and mean duration of between patient-reported symptom onset and initial presentation to any healthcare institution was 56.7 days (SD ± 16.8, range 0-730). 29(50.0%) experienced prolonged TT of more than 90 days; while 12(20.7%) patients were found to have a delayed presentation of more than 60 days. Among patients included into this study, healthcare-provider related delays (43.1%), delayed presentation (31.0%) were identified as the predominant causes of delayed TT. Among the patients who suffered healthcare provider related delays, 17(68.0%) patients were first evaluated in centers which were not specialized in treatment of peritoneal malignancies, and 6(24.0%) incurred delays after being inappropriately referred from primary care providers to disciplines not equipped to manage peritoneal disease. Patients who encountered treatment delays attributable to delayed presentation predominantly experienced protracted symptoms prior to making a decision to seek medical attention.
Less common causes for treatment delays were disease related issues (20.7%), patient related (3.4%) and healthcare system related delays (1.7%) (Figure 2). A comprehensive list of factors included under each category is shown in Table 3.
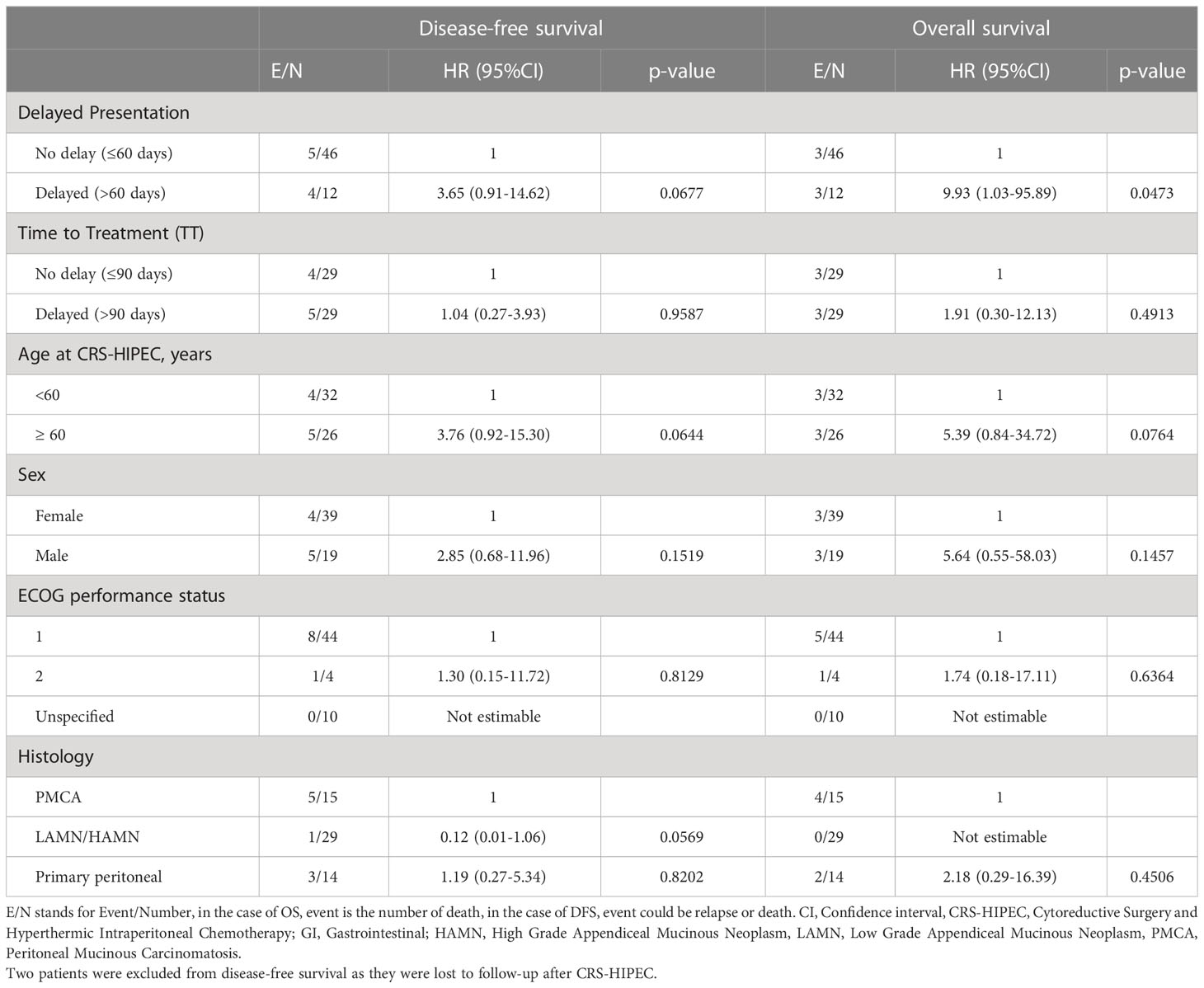
Table 3 Cox regression analysis of disease-free survival and overall survival by demographic and clinical variables.
Patients with delayed presentation demonstrated poorer overall survival compared to those without (p=0.015, Figure 3A), with median overall survival (OS) being 26.0 months for patients with delayed presentation. 1- and 2- year OS was 100% (95% CI 100-100) and 71.4% (95% CI 44.7-100) versus 100% and 100% respectively for patients with and without delayed presentation. Delayed patient presentation was associated with poorer survival (HR 9.93, 95% CI 1.03-95.89, p=0.047), although this was non-significant after adjustment for tumor histology (HR 7.91, 95% CI 0.72-87.15, p=0.091).
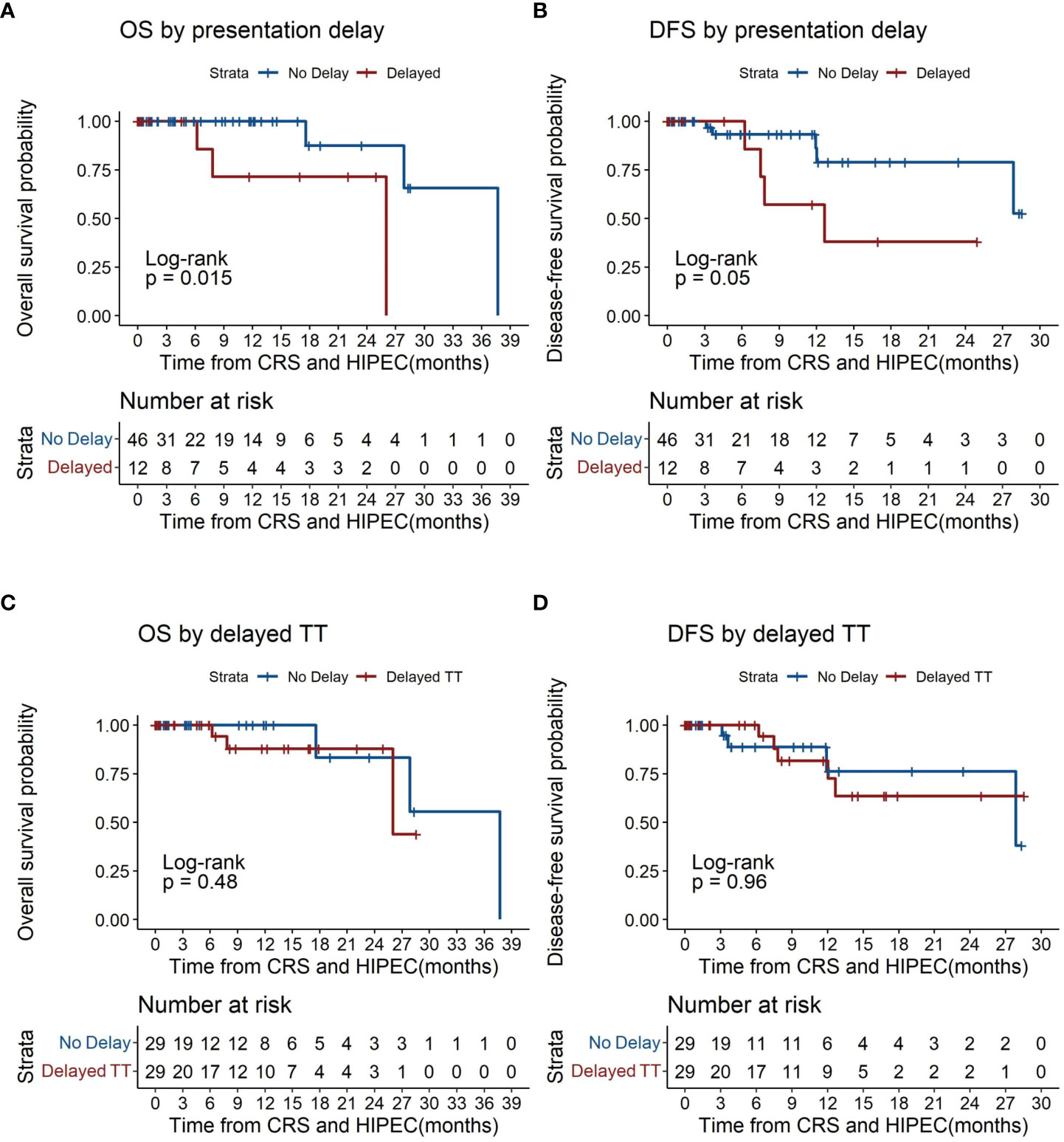
Figure 3 Kaplan-Meier plots of overall survival and disease-free survival by time to treatment (TT). (A) overall survival by presentation delay. (B) disease free survival by presentation delay. (C) overall survival by time to treatment. (D) disease free survival by time to treatment. CRS, Cytoreductive Surgery; HIPEC, Hyperthermic intraperitoneal chemotherapy; TT, time to treatment.
Patients with delayed presentation similarly demonstrated lower rates of DFS (p=0.05, Figure 3B) with median disease free survival (DFS) at 12.7 months for patients with delayed presentation. 1- and 2- year DFS was 100% and 57.1% (95% CI 30.1-100) versus 93.3% (95% CI 84.8-100) and 86.1% (95% CI 71.7-100) respectively for patients with and without delayed presentation. Patients with delayed presentation demonstrated a trend towards poorer DFS (HR 3.65, 95% CI 0.91-14.62, p=0.068) which was significant after correcting for tumor histology (HR 4.67, 95% CI 1.11-19.69, p=0.036) (Table 3).
Overall survival was similar between patients with and without delayed TT (p=0.48, Figure 3C). Among patients with delayed TT, median overall survival (OS) was 26.0 months. 1- and 2- year OS was 100% and 87.8% (95% CI 73.4-100) versus 100% and 100% respectively for those with and without delayed TT. No statistical difference in overall survival was demonstrated between patients with and without delayed TT (HR 1.91, 95% CI 0.30-12.13, p=0.491) (Table 3).
No significant differences were found in disease free survival between patients with and without delayed TT (p=0.96, Figure 3D). Among patients with delayed TT, median DFS was 4 months. 1- and 2- year DFS was 100% and 81.6% (95% CI 64.7-100) versus 100% and 100% respectively for those with and without delayed TT (Table 3).
In view of the limited number of events and small sample size, 95% confidence intervals for median OS and DFS for patients with and without delayed TT, and similarly for those with and without presentation delay, could not be estimated.
Our study demonstrates insight into the factors contributing to treatment delays in PSM, which consist of several potentially actionable causes predominantly attributable to provider-related delays incurred in work processes involved in transfer of care between healthcare institutions (43.1%). These findings represent, to our knowledge, the first attempt in current literature at describing actionable factors contributing to treatment delays in peritoneal malignancies. Our findings also suggest that delayed presentation may result in poorer overall survival and disease-free survival among patients with PSM. Interestingly, however, a delayed time to treatment of >90 days did not result in any significant difference in overall survival or disease-free survival in our study cohort. While the literature consistently supports the early diagnosis and treatment of peritoneal malignancies (27–29) the quantitative effect of diagnostic and treatment delays on oncologic outcomes in such patients has not been sufficiently studied. Furthermore, given the highly litigated nature of oncologic practice in general, delayed evaluation and treatment of such conditions may prove also to be a significant source of financial and legal burden to healthcare systems worldwide (30). Therefore, quantifying the causative factors of treatment delays and identifying the impact of such treatment delays on oncologic outcomes is crucial to addressing public health concerns pertaining to the evaluation and treatment of peritoneal malignancies.
A large proportion of study patients suffered delays attributable to provider-related delays incurred by processes involved in transfer of care between institutions (43.1%) and delayed presentation (31.0%) predominantly contributed by a lack of patient awareness regarding signs and symptoms of peritoneal disease. These findings suggest that treatment delays are predominantly influenced by factors within the healthcare system that can be further mitigated with improved professional education and streamlining of administrative processes. Considering the well-documented challenges involved in evaluation of peritoneal malignancies given their significant heterogeneity, varying nature of initial presentation and overall rare occurrence (14, 15, 17, 31–34), healthcare professionals at large would benefit from better awareness on the initial presentations, appropriate evaluation strategies and treatment options for peritoneal malignancies. Additionally, treatment delays incurred here are also contributed at least in part by patients’ failure to recognize symptoms that suggest underlying peritoneal malignancies which resulted in delayed presentation to primary care. Findings from our study similarly highlight the importance of early patient recognition of symptoms and early appropriate clinical evaluation in optimizing treatment outcomes for peritoneal surface malignancies.
This study’s findings on the association between delayed presentation and poorer overall survival and disease-free survival must be interpreted with due consideration given to the limited sample size and relatively short follow up duration of 12.4 months post CRS-HIPEC, also taking into consideration the effects of disease biology on symptom progression and presentation. The detrimental effect of delayed presentation on survival outcomes may potentially be attributable to poorer disease biology, with more indolent biology presenting with more clinically occult symptoms (28, 29) which translates to a delayed presentation to medical attention, and ultimately poorer survival outcomes. However, our study notably demonstrated that tumor histology and patient comorbidities appear relatively consistent in both non-delayed and delayed time to treatment groups (Table 2), suggesting that such factors were not likely to have influenced the duration of time to treatment. This study further demonstrated an independent association between delayed presentation and reduced disease free survival which persisted after correction for tumor histology (HR 4.67, 95% CI 1.11-19.69, p=0.036), which suggests that delayed presentation of more than 60 days may adversely impact disease outcomes in PSM regardless of the contributory primary histology. The persistent correlation between delayed presentation and poorer survival outcomes after correction for histology would, however, suggest that delayed presentation results in poorer survival due to other independent factors, which has been similarly demonstrated for some other biologically distinct malignancies, delayed presentation in soft tissue sarcoma for instance having demonstrated to be associated with higher likelihood of distal metastases on diagnosis, portending poorer prognoses and poorer survival outcomes (23).
While age does not appear to significantly influence survival outcomes in patients with PM based on our data, older patients 60 years of age and above with peritoneal malignancies demonstrate a tendency towards poorer survival (HR 5.39, 95% CI 0.84-34.72, p=0.076) and lower DFS (HR 3.76, 95% CI 0.92-15.30) compared to younger patients (Table 3). This emphasizes the importance of early detection of PM in particular for older patients, although more studies with a longer follow up duration would be required to prove a significant correlation between age and oncological outcomes in PM.
This study bears several other limitations. Firstly, data collected on pre-hospital symptoms relies on patient-reported duration and severity of symptoms which may bear an inherent recall bias at the time of consult. Secondly, median follow up time was relatively short (12.4 months) with a small sample size of 58, which also did not include any patients previously treated with neoadjuvant chemotherapy or patients with PSM secondary to previously treated primary tumors, thus limiting the analytic power of this study as well as generalizability of results to a small subset of patients with primary PSM who received upfront CRS-HIPEC. Thirdly, this study does not account for the impact of adjuvant treatment regimens on the overall outcomes of patients undergoing CRS-HIPEC. With greater amounts of data obtained from further follow up, further studies can be conducted on the individual treatment outcomes tailored to peritoneal malignancies of each subtype with further subgroup analyses being performed these cases.
Treatment delays are predominantly contributed by healthcare-provider related factors which can be further optimized by streamlined referral processes and wider awareness towards evaluation and management of peritoneal malignancies among healthcare workers. Delayed presentation of >60 days appears to be associated with poorer disease free survival in index-presentation peritoneal surface malignancies receiving upfront CRS-HIPEC. Further studies evaluating the effects of treatment delays on survival outcomes in peritoneal malignancies would be useful in improving treatment protocols and optimizing outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Centralized Institutional Review Board (CIRB) of Singapore Health Services, CIRB reference number 2018/2638. The patients/participants provided their written informed consent to participate in this study.
JKT, JSM and CSC contributed to conception and design of the study. JKT, JCO and JSM organized the database. JKT and CL performed the statistical analysis. JKT and JSM wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This study is supported by the NCCS Cancer Fund (Research) and SingHealth Duke-NUS Academic Medicine Centre, facilitated by Joint Office of Academic Medicine (JOAM). JCO is supported by the National Research Council Clinician Scientist-Individual Research Grant (CIRG21jun-0038). All the funding sources had no role in the study design, data interpretation or writing of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol (2012) 30(20):2449–56. doi: 10.1200/jco.2011.39.7166
2. Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol (2006) 7(1):69–76. doi: 10.1016/s1470-2045(05)70539-8
3. Sugarbaker PH. Epithelial appendiceal neoplasms. Cancer J (2009) 15(3):225–35. doi: 10.1097/PPO.0b013e3181a9c781
4. Jimenez W, Sardi A, Nieroda C, Sittig M, Milovanov V, Nunez M, et al. Predictive and prognostic survival factors in peritoneal carcinomatosis from appendiceal cancer after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol (2014) 21(13):4218–25. doi: 10.1245/s10434-014-3869-1
5. Mercier F, Bakrin N, Bartlett DL, Goere D, Quenet F, Dumont F, et al. Peritoneal carcinomatosis of rare ovarian origin treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a multi-institutional cohort from psogi and big-renape. Ann Surg Oncol (2018) 25(6):1668–75. doi: 10.1245/s10434-018-6464-z
6. Bonnot PE, Piessen G, Kepenekian V, Decullier E, Pocard M, Meunier B, et al. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (Cyto-chip study): a propensity score analysis. J Clin Oncol (2019) 37(23):2028–40. doi: 10.1200/jco.18.01688
7. van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med (2018) 378(3):230–40. doi: 10.1056/NEJMoa1708618
8. Mirnezami R, Mehta AM, Chandrakumaran K, Cecil T, Moran BJ, Carr N, et al. Cytoreductive surgery in combination with hyperthermic intraperitoneal chemotherapy improves survival in patients with colorectal peritoneal metastases compared with systemic chemotherapy alone. Br J Cancer (2014) 111(8):1500–8. doi: 10.1038/bjc.2014.419
9. Pai RK, Longacre TA. Appendiceal mucinous tumors and pseudomyxoma peritonei: histologic features, diagnostic problems, and proposed classification. Adv Anat Pathol (2005) 12(6):291–311. doi: 10.1097/01.pap.0000194625.05137.51
10. Mahdavi A, Malviya VK, Herschman BR. Peritoneal tuberculosis disguised as ovarian cancer: an emerging clinical challenge. Gynecol Oncol (2002) 84(1):167–70. doi: 10.1006/gyno.2001.6479
11. Tajima T, Tajiri T, Mukai M, Sugiyama T, Hasegawa S, Yamamoto S, et al. Single-center analysis of appendiceal neoplasms. Oncol Lett (2018) 15(5):6393–9. doi: 10.3892/ol.2018.8134
12. Tsuda B, Kajiwara H, Sakota N, Amatsu S, Tanaka A, Ikeda M. Difficult diagnosis of peritoneal serous papillary carcinoma in a 63-Year-Old woman: a case report. Tokai J Exp Clin Med (2019) 44(3):49–53.
13. Wong AK, Seidman JD, Barbuto DA, McPhaul LW, Silva EG. Mucinous metaplasia of the fallopian tube: a diagnostic pitfall mimicking metastasis. Int J Gynecol Pathol (2011) 30(1):36–40. doi: 10.1097/PGP.0b013e3181f45f28
14. Hart WR. Diagnostic challenge of secondary (Metastatic) ovarian tumors simulating primary endometrioid and mucinous neoplasms. Pathol Int (2005) 55(5):231–43. doi: 10.1111/j.1440-1827.2005.01819.x
15. Choudry HA, Pai RK, Parimi A, Jones HL, Pingpank JF, Ahrendt SS, et al. Discordant diagnostic terminology and pathologic grading of primary appendiceal mucinous neoplasms reviewed at a high-volume center. Ann Surg Oncol (2019) 26(8):2607–14. doi: 10.1245/s10434-019-07447-z
16. Lowes H, Rowaiye B, Carr NJ, Shepherd NA. Complicated appendiceal diverticulosis versus low-grade appendiceal mucinous neoplasms: a major diagnostic dilemma. Histopathology (2019) 75(4):478–85. doi: 10.1111/his.13931
17. Amin A, Carr N. Diagnostic concordance in cases of appendiceal mucinous neoplasia referred to a tertiary referral centre. J Clin Pathol (2019) 72(9):639–41. doi: 10.1136/jclinpath-2019-205945
18. Wong JSM, Ng IAT, Paik B, Ong CJ, Seo CJ, Lee PP, et al. Attitudes, knowledge & awareness amongst physicians in the management of peritoneal-surface based malignancies. Asian J Surg (2022) 45(12):2829–31. doi: 10.1016/j.asjsur.2022.06.054
19. Fernández-de Castro JD, Baiocchi Ureta F, Fernández González R, Pin Vieito N, Cubiella Fernández J. The effect of diagnostic delay attributable to the healthcare system on the prognosis of colorectal cancer. Gastroenterol Hepatol (2019) 42(9):527–33. doi: 10.1016/j.gastrohep.2019.03.012
20. Mou J, Bolieu EL, Pflugeisen BM, Amoroso PJ, Devine B, Baldwin LM, et al. Delay in treatment after cancer diagnosis in adolescents and young adults: does facility transfer matter? J Adolesc Young Adult Oncol (2019) 8(3):243–53. doi: 10.1089/jayao.2018.0128
21. Gómez I, Seoane J, Varela-Centelles P, Diz P, Takkouche B. Is diagnostic delay related to advanced-stage oral cancer? a meta-analysis. Eur J Oral Sci (2009) 117(5):541–6. doi: 10.1111/j.1600-0722.2009.00672.x
22. Felippu AW, Freire EC, Silva Rde A, Guimarães AV, Dedivitis RA. Impact of delay in the diagnosis and treatment of head and neck cancer. Braz J Otorhinolaryngol (2016) 82(2):140–3. doi: 10.1016/j.bjorl.2015.10.009
23. Nakamura T, Matsumine A, Matsubara T, Asanuma K, Uchida A, Sudo A. The symptom-to-Diagnosis delay in soft tissue sarcoma influence the overall survival and the development of distant metastasis. J Surg Oncol (2011) 104(7):771–5. doi: 10.1002/jso.22006
24. Goedhart LM, Gerbers JG, Ploegmakers JJ, Jutte PC. Delay in diagnosis and its effect on clinical outcome in high-grade sarcoma of bone: a referral oncological centre study. Orthop Surg (2016) 8(2):122–8. doi: 10.1111/os.12239
25. Caplan LS, Helzlsouer KJ. Delay in breast cancer: a review of the literature. Public Health Rev (1992) 20(3-4):187–214.
26. Chua TC, Liauw W, Zhao J, Morris DL. Upfront compared to delayed cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei is associated with considerably lower perioperative morbidity and recurrence rate. Ann Surg (2011) 253(4):769–73. doi: 10.1097/SLA.0b013e31820b4dba
27. Foster JM, Sleightholm RL, Wahlmeier S, Loggie B, Sharma P, Patel A. Early identification of dpam in at-risk low-grade appendiceal mucinous neoplasm patients: a new approach to surveillance for peritoneal metastasis. World J Surg Oncol (2016) 14(1):243. doi: 10.1186/s12957-016-0996-0
28. Nutu OA, Marcacuzco Quinto AA, Manrique Municio A, Justo Alonso I, Calvo Pulido J, García-Conde M, et al. Mucinous appendiceal neoplasms: incidence, diagnosis and surgical treatment. Cir Esp (2017) 95(6):321–7. doi: 10.1016/j.ciresp.2017.05.008
29. Shaib WL, Goodman M, Chen Z, Kim S, Brutcher E, Bekaii-Saab T, et al. Incidence and survival of appendiceal mucinous neoplasms: a seer analysis. Am J Clin Oncol (2017) 40(6):569–73. doi: 10.1097/coc.0000000000000210
30. Hwang R, Park HY, Sheppard W, Bernthal NM. Delayed diagnosis is the primary cause of sarcoma litigation: analysis of malpractice claims in the united states. Clin Orthop Relat Res (2020) 478(10):2239–53. doi: 10.1097/corr.0000000000001340
31. Arnold CA, Graham RP, Jain D, Kakar S, Lam-Himlin DM, Naini BV, et al. Knowledge gaps in the appendix: a multi-institutional study from seven academic centers. Mod Pathol (2019) 32(7):988–96. doi: 10.1038/s41379-019-0216-x
32. Zhang W, Tan C, Xu M, Wu X. Appendiceal mucinous neoplasm mimics ovarian tumors: challenges for preoperative and intraoperative diagnosis and clinical implication. Eur J Surg Oncol (2019) 45(11):2120–5. doi: 10.1016/j.ejso.2019.08.004
33. Al-Sukhni E, LeVea C, Skitzki J, Kane J 3rd, Francescutti V. Key gaps in pathologic reporting for appendiceal mucinous neoplasms: time for universal synoptic reporting? Ann Diagn Pathol (2016) 24:52–4. doi: 10.1016/j.anndiagpath.2016.06.004
Keywords: peritoneal malignancy, cytoreductive surgery, hyperthermic intraperitoneal chemotherapy, delay, survival
Citation: Tan JKT, Wong JSM, Seo CJ, Lim C, Zhu H-Y, Ong C-AJ and Chia CS (2023) Incidence and outcomes of delayed presentation and surgery in peritoneal surface malignancies. Front. Oncol. 13:1137785. doi: 10.3389/fonc.2023.1137785
Received: 04 January 2023; Accepted: 18 May 2023;
Published: 30 May 2023.
Edited by:
Naoual Bakrin, Hospices Civils de Lyon, FranceReviewed by:
Dario Baratti, Fondazione IRCCS Istituto Nazionale Tumori, ItalyCopyright © 2023 Tan, Wong, Seo, Lim, Zhu, Ong and Chia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claramae Shulyn Chia, Y2xhcmFtYWUuY2hpYS5zLmxAc2luZ2hlYWx0aC5jb20uc2c=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.