
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 24 April 2023
Sec. Cancer Imaging and Image-directed Interventions
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1137510
This article is part of the Research TopicRole of Imaging in Biliary Tract Cancer: Diagnosis, Staging, Response Prediction, and Image-Guided TherapeuticsView all 7 articles
 Giovanni Brandi1,2*†
Giovanni Brandi1,2*† Chiara Deiana1,2†
Chiara Deiana1,2† Linda Galvani1,2
Linda Galvani1,2 Andrea Palloni1
Andrea Palloni1 Angela Dalia Ricci3
Angela Dalia Ricci3 Alessandro Rizzo4
Alessandro Rizzo4 Simona Tavolari1,2
Simona Tavolari1,2Despite representing some of the most common and investigated molecular changes in intrahepatic cholangiocarcinoma (iCCA), the prognostic role of FGFR and IDH1/2 alterations still remains an open question. In this review we provide a critical analysis of available literature data regarding this topic, underlining the strengths and pitfalls of each study reported. Despite the overall poor quality of current available studies, a general trend toward a better overall survival for FGFR2 rearrangements and, possibly, for FGFR2-3 alterations can be inferred. On the other hand, the positive prognostic role of IDH1/2 mutation seems much more uncertain. In this scenario, better designed clinical trials in these subsets of iCCA patients are needed in order to get definitive conclusions on this issue.
Cholangiocarcinoma (CCA) encompasses a group of heterogeneous and rare tumours with poor prognosis, including intrahepatic CCA (iCCA) and extrahepatic (eCCA), with the latter further subdivided into perihilar (pCCA) and distal (dCCA) (1). Overall, these malignancies account for approximately 10-15% of primary liver cancers with an incidence on the rise counting between 0.3 – 6 cases/100’000 inhabitants in Western countries (2, 3). Unfortunately, potentially curative surgical resection is feasible in about 25% of patients at diagnosis and even following radical surgery, relapse rate remains high (4). Most patients present with locally advanced, unresectable, or metastatic disease, and palliative chemotherapy with cytotoxic drugs such as gemcitabine plus cisplatin represents the standard treatment in this setting, with an associated overall survival (OS) of 11.7 months (5). The addition of immunotherapy with the anti PD-L1 drug Durvalumab to the well-known doublet of cisplatin and gemcitabine has recently been proven to be associated with an increased OS, so a change in the first-line setting paradigm could potentially happen soon (6).
FOLFOX regimen, comprised of fluorouracil and oxaliplatin doublet, has been proven to give an advantage compared to active symptom control in second-line setting (7), and therapies directed against different target such as FGFR, IDH, NTRK, Her2 and more, are being investigated.
Large scale sequencing analyses regarding iCCA tumor biology have provided new insights, unravelling the complex and heterogeneous genomic landscape of this disease (8). Currently potential therapeutic targets have been identified in nearly 40% of biliary tract cancers, with the most promising in iCCA patients including isocitrate dehydrogenase 1 (IDH1) and fibroblast growth factor receptor 2 (FGFR2) (9). Anti-FGFR drugs such as Pemigatinib, Infigratinib and Futibatinib and anti-IDH therapies such as Ivosidenib have been approved in pre-treated patients (10, 11).
A whole genome and epigenetic analysis of CCA by Jusakul et al. lead to the definition of four distinct clusters of CCA, with the two clusters pertaining iCCA being defined by the presence of PD-1 or PD-L1 up-regulation, aberration in T- Cells transduction and CD28 co-stimulation (cluster 3), or BAP1, IDH1, IDH2, FGFR alteration, upregulation of FGFR or PI3K pathways and DNA hypermethylation (cluster 4). The study suggested that cluster 4 was associated with the best prognosis of all subtypes (p < 0.001) and, although no specific OS data on FGFR or IDH in iCCA were provided, it was enough to spark interest in the prognostic role of these mutations (12).
Despite the numerous papers that tried to address this topic, the prognostic role of FGFR2 and IDH genetic alterations in iCCA still remains a controversial issue in the medical community, with several studies reporting conflicting results and a poor quality of data.
The aim of this review is to analyze the prognostic relevance of FGFR2 and IDH alteration in iCCA, with a special focus on the data available from each paper (Tables 1, 2) and their subsequent bias or shortcomings (Tables 3, 4).

Table 1 OS in FGFR2/3 altered and OS in FGFR2 rearranged patients compared to OS in iCCA (*) or Biliary Tract Cancer BTC (#) cases.
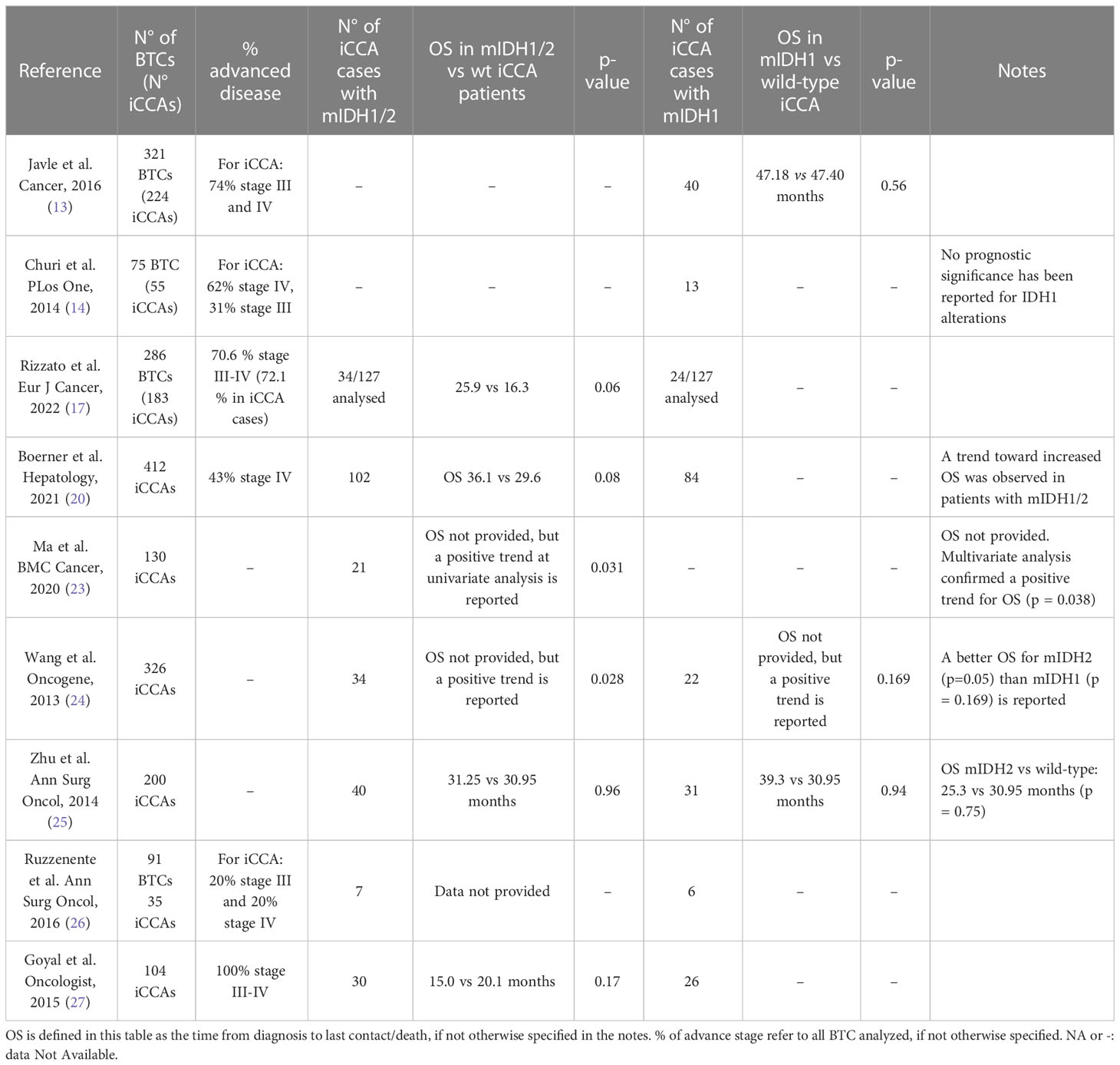
Table 2 OS in IDH1/2 mutated and OS in IDH1 mutated patients compared to OS in iCCA (*) or Biliary Tract Cancer BTC (#) cases.
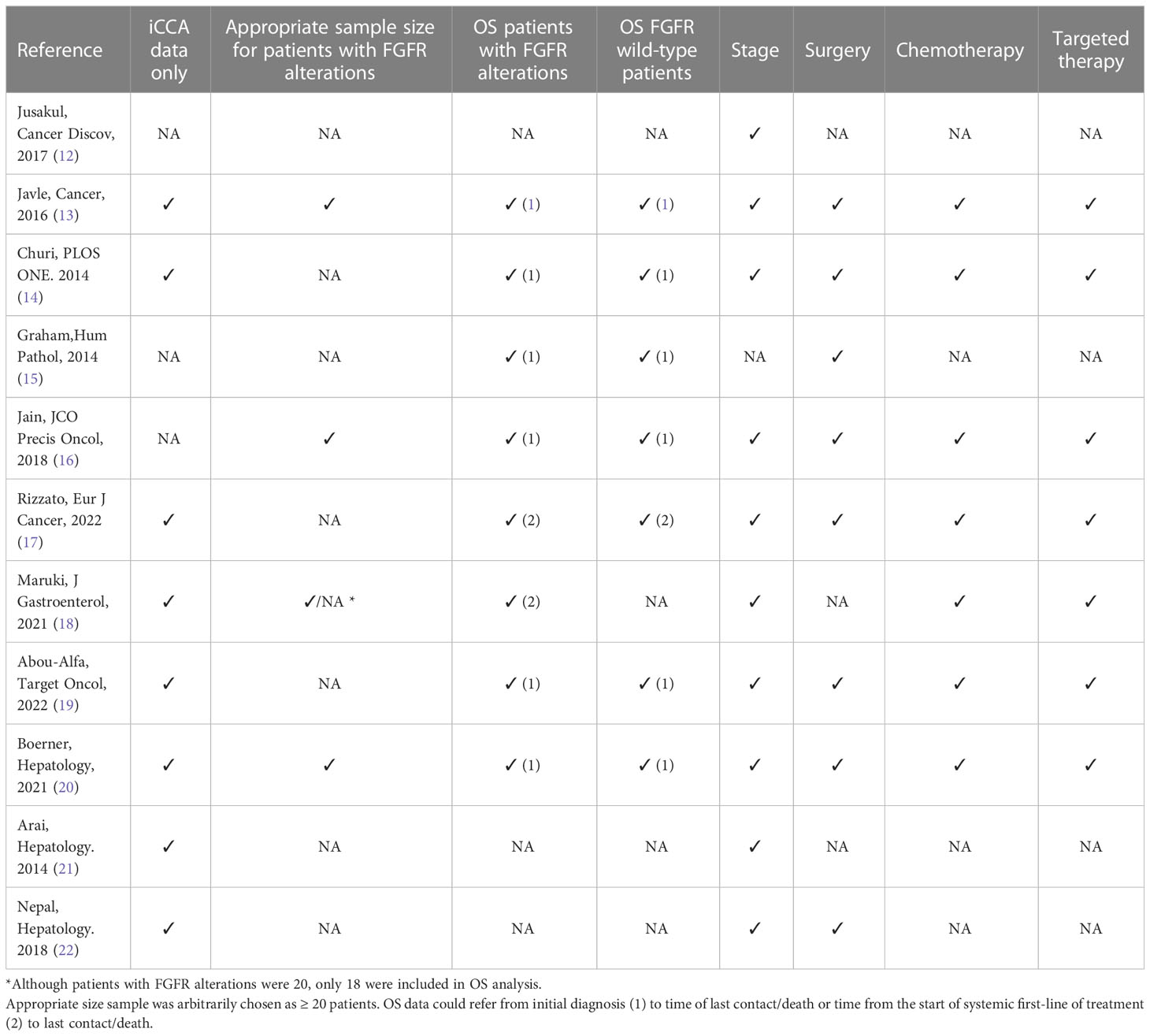
Table 3 Trial’s bias/shortcoming, if relevant data are provided, a checkmark sign is used (✓), otherwise a (NA) standing for Not Available is used.
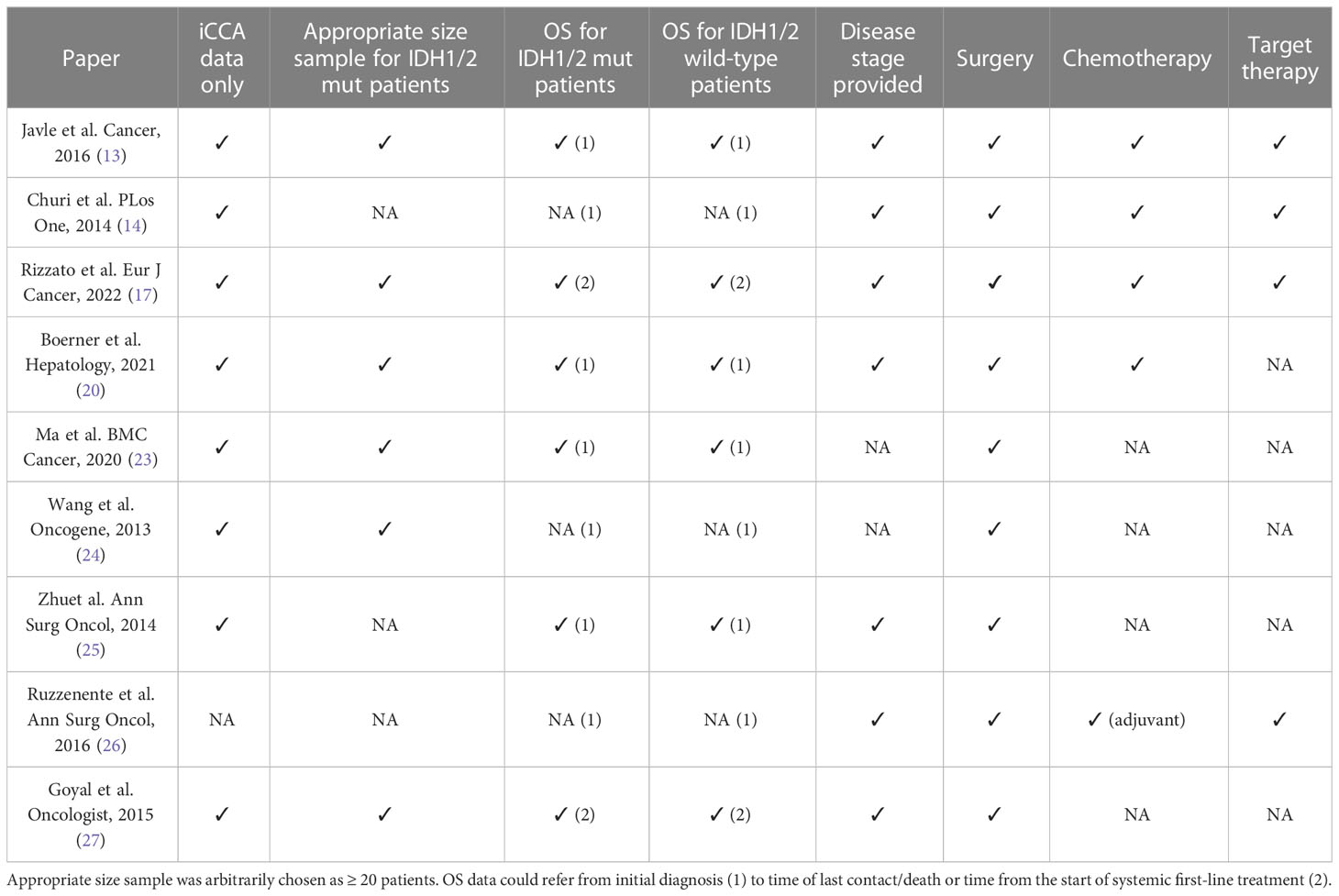
Table 4 Trial’s bias/shortcoming, if relevant data are provided, a checkmark sign is used (✓), otherwise a (NA) standing for Not Available is used.
Fibroblast growth factor receptors (FGFRs) 1-4 are transmembrane tyrosine kinase receptors that, when bound to their ligand FGF, are responsible for the activation of pathways such as RAS/RAF/MAPK and PI3K/AKT, involved in proliferation, migration and anti-apoptotic signals (28).
FGFR2 rearrangements or fusions are the most frequent molecular events detected in iCCA, occurring in a proportion of patients ranging from 3 to 15% of all iCCA cases, whereas mutations and amplifications seem to be rarer (29). The majority of these abnormalities results in a gain-of-function of the receptor, that in turn activates multiple downstream oncogenic signaling pathways (30).
From their first discovery and identification, FGFR2 fusions have been suggested to define a unique clinical iCCA subtype associated with a younger age at onset, female sex, earlier stage and a more indolent disease progression (13, 31). Initial prognostic data on alteration of the FGF pathway and specifically on FGFR2 fusions showed a trend toward better OS data (13, 14).
In a landmark study by Graham and colleagues, the authors reported 13 cases of FGFR2 translocations among 156 surgical cases of biliary tract tumors, with almost all altered cases being iCCAs (12); a significantly longer survival was observed in patients with FGFR2 translocations compared to wild-type ones, with a median OS of 123 vs 37 months, respectively (p = 0.039) (15). Furthermore, of the 12 cases of FGFR2 altered iCCAs, only 3 patients developed recurrence or secondary lesions, all with a statistically longer disease-free interval compared to the FGFR wild-types patients (p = 0.007) (15). It should be noted however that relevant prognostic information, such as stage before resection or systemic treatment, were not provided.
An interesting study by Buckarma analyzed OS for resected iCCA with FGFR2 fusions (12 patients) vs wild-type resected patients (87 patients) and found out that 5 years OS was far longer for altered patients (83 vs 32%, p = 0.01) (32).
Another remarkable study was the 2016 paper by Javle et al. (13) as it provided data on patients’ characteristics, treatment received, and it differentiated well between different biliary tract cancer subtypes. At the univariate analysis of the iCCA subset (224 cases, 74% stage III or IV), the 30 FGFR2 rearranged cases had a strong survival advantage (OS NA vs 43 months, p = 0.001) and this positive prognostic correlation with FGFR alteration was confirmed at the multivariate analysis (p = 0.03). However, it was not specified whether with the citation “FGFR alteration”, the authors referred only to FGFR2 fusions or to all FGFR alterations (42 cases in total) found in the iCCA samples.
A favorable prognostic role of FGFR genetic aberrations has been also suggested by another study on 377 CCAs (16). In this cohort of patients, 95 CCAs harbored FGFR genetic aberrations, with most of them being FGFR2 fusions (63 cases), and almost all occurring in iCCAs (60 cases, 95.2%). Patients with FGFR genomic aberrations experienced a longer OS compared to wild-type patients (37 vs 20 months, respectively; p < 0.001) and this advantage was preserved even when considering only stage III or IV patients (24 v 17 months; p < 0.004). However, a pitfall of this study is that no specific OS data on FGFR2 fusions in iCCA were presented, with data from all biliary tract cancer being included.
Of note, in this paper a longer OS was also observed after excluding those patients treated with FGFR inhibitors, showing an OS of 30 in FGFR altered patients vs 20 months in FGFR wild type patients; p = 0.0266) (16).
In fact, it should be noted that the introduction of new lines of therapy with the use of specific anti-FGFR drugs in FGFR altered patients can be a strong confounding factor, as it raises the question of whether the gain in OS seen in some trial could be due to the intrinsic positive prognostic role of FGFR alteration or if it could merely be due to the predictive role to FGFR target therapy.
Several other papers tried to answer this question in their trials. For example, in the BITCOIN trial (17), out of 286 biliary tract cancer cases analyzed, the 24 patients (8.4%) with FGFR2/3 alteration had a better OS compared to FGFR wild-type cases (29.2 vs 14.4 months, p = 0.003). Focusing on the 183 iCCAs, the BITCOIN trial provided OS data from the time of the start of first-line therapy in patients with FGFR2-fusions (13 patients, with 11 undergoing 1st line treatment, OS 29.2 vs 15.0 months, p = 0.03) and in patients with all type pf FGFR2/3 alterations (19 cases, with 17 included in the analysis, OS 24.9 vs 14.8 months, p = 0.02); notably, the survival advantage held true even when censoring the 8 cases who received FGFR targeted therapies (HR = 0.32, 95% CI 0.12–0.85, p = 0.02).
On the other hand, the Japanese PRELUDE study (18) suggests that the OS advantage could be due to targeted therapies. Out of 20 FGFR2-rearranged patients with recurrent or advanced iCCA, 18 underwent first-line chemotherapy (cisplatin/gemcitabine or cisplatin/S-1) with a progression-free survival (PFS) in line with known data (8.9 months compared to the 8.0 months of the ABC-02 trial (5); however, 13 of them had subsequent anti-FGFR2 therapies, obtaining an OS of 38.8 months. It should be noted that the study did not provide PFS or OS data of either FGFR wild-type patients or of those patients with FGFR alterations who did not receive targeted therapy, thus no solid conclusions can be drawn.
What seems clear from the published data is that FGFR alterations are not associated with a different response to first-line chemotherapy compared to FGFR wild-type patients.
A retrospective study (19) on the effect of first-line chemotherapy on FGFR2 altered patient showed that, although OS was longer for altered patients (31.3 vs 21.7 months), this was not due to a better response to first-line treatment (93% of cases platinum based) with a PFS of 6.2 months for altered cases vs 7.2 months for wild-type cases. Interestingly, even after excluding patients who had received an FGFR inhibitor, a slightly longer PFS for second-line treatment was observed (5.6 months for 8 mutated patients vs 3.7 months for 81 wild-type cases). It should be noted however that size sample was small and that only descriptive statistics was employed (p-values were not provided) (19).
Conversely, not all evidences support the hypothesis of a positive prognostic correlation in patients with FGFR2 alterations.
A recent paper in 2021 failed to find a significant correlation between the presence of FGFR2 fusion gene and prolonged OS. Considering a large number of iCCAs (412), both resected and not resected, although numerically the OS was longer for the 47 FGFR2 mutated patients (46.8 vs 29.6 months), it was not statistically relevant (p = 0.20) (20).
Similarly, an Asian cohort study reported no statistically significant survival differences in FGFR2 fusion-positive patients, although the examined cohort was small (9 patients out of 66 iCCAs) and no treatment data was provided (21). Interestingly, another paper on 18 FGFR2 fusion cases out of 122 analyzed resected iCCA cases, reported a trend toward a worse OS (p < 0.0001), however, neither precise data on treatment and stage disease for mutated patients, nor precise OS for wild-type and mutated patients were provided (22).
Isocitrate Dehydrogenase (IDH) 1 and IDH2, residing respectively in the cellular cytoplasm and mitochondria, are NADP+-dependent enzymes which catalyze the oxidative decarboxylation of isocitrate to α-ketoglutarate (α-KG) (33). Somatic mutations in IDH1 gene result in the production of a neomorphic enzyme that leads to the synthesis of the 2-Hydroxyglutarate (D-2HG) oncometabolite, instead of α-KG, thus altering numerous processes involved in epigenetics and cellular metabolism, and favoring tumor maintenance through immune evasion (34–37). Indeed, due to its structural similarity with α-KG, D-2HG is able to inhibit DNA and histone demethylases, inducing a hypermethylated phenotype in target cells and the silencing of genes involved in cell differentiation and immune response (37). Moreover, D-2HG may induce mitochondrial dysfunction, because of succinate dehydrogenase (SDH) inhibition, that results in intracellular succinate increase in levels and mitochondrial respiration inhibition (37).
The most frequent mutations of the IDH1 gene are single amino acid changes that occur in the catalytic site, especially at arginine 132 residue, leading to a gain of function of the enzyme (38). The presence of mutated IDH1 among CCA patients is higher in those affected by iCCA compared with those with eCCA, with an overall frequency of 13.1% vs 0.8% respectively (39). The presence of circulating 2-HG has been hypothesized as an alternative biomarker of IDH1/2 mutational status in CCA, and its levels seems to correlate with tumor burden (40). Recently, the ClarIDHy trial, a phase 3 multicenter randomized study, tested the efficacy of the IDH1 inhibitor Ivosidenib in patients with pretreated, non resectable or metastatic CCA with IDH1 mutation, observing an improvement in PFS compared to placebo (mOS 2.7 months vs 1.4 months respectively) (11). This finding represents a new hope in this particularly challenging disease. Despite the efforts made to understand the prognostic significance of IDH1/2 mutations (mIDH1/2) in CCA patients, no clear evidence has been established so far.
Focusing on patients with resected disease, in a work by Ma et al. on 130 resected iCCA patients, the presence of mIDH1/2 showed a significant gain in DFS and OS, showing a trend toward increased OS (p = 0.031 at univariate analysis and 0.038 at multivariate analysis) (23). Another interesting work by Wang et al. (24) on 326 resected iCCA cases, from a Chinese and a US cohort, discovered mIDH1/2 to be associated with longer OS (p = 0.028) and with a longer time to tumor recurrence after resection in the multivariate analysis (p 0.021). Probabilities of tumor recurrence after resection at 1, 4 and 7 years in patients with mIDH1/2 iCCA (10.5%, 45.3% and 45.3%, respectively) were significantly lower than those with wild-type IDH1 or IDH2 (41.7%, 71.5% and 81.3%, respectively). Nonetheless, many important features, such as tumor stage and further treatment strategies, were not provided.
Zhu et al. conducted a very articulate analysis of genomic profiling of 200 iCCA cases, considering data about mIDH1 and mIDH2 patients separately and together, but it did not find a significant association between mIDH1/2 and OS when compared to wild type patients (mOS 31.25 vs 30.95 months respectively, p = 0.96). All the patients included in this study underwent surgical resection, but no information about other subgroups of patients with advanced, non resectable iCCAs were provided (25).
The same result was reached by Ruzzenente et al. (26) in a study on resected iCCA patients. The paper reported no evident influence of mIDH1 on OS in resected iCCA patients; however, despite thorough details regarding differentiation between CCA subtypes and molecular features, no precise data and p-values about survival in iCCA mIDH1/2 patients were provided.
The following studies, on the other hand, aimed to assess the prognostic role of mIDH1/IDH2 in more heterogenous populations, considering patients with both resected and unresectable/advanced disease.
A recent work by Rizzato et al. claimed at the multivariate model a correlation between mIDH1/2 and improved survival in a population of iCCA patients (17), reporting a median OS of 25.9 months compared to 16.3 months in wild-type patients (p = 0.06). In this paper, the benefit appears independent from targeted therapy administration, since no mutated IDH1/2 patients had received IDH1 inhibitors.
In contrast, the previously mentioned work by Javle et al., characterized by a very solid design with relevant features reported, found no prognostic relevance of the presence of mIDH1/2 in iCCA (47.4 vs 47.1 months, p = 0.56) (13).
Consistent with these results, the work by Churi et al. suggested no correlation between mIDH1 and OS (14). Several important characteristics were provided in the paper, such as disease stage and administrated treatments, but it should be noted that the mutated IDH1/2 size simple was very small (13 patients) and p-values and OS are lacking.
Goyal et al. assessed prognostic features of advanced iCCA patients, again reporting no statistically significant association between mIDH1/2 and OS (15.0 vs 20.1 months, p = 0.17), defined both as the time from diagnosis of primary unresectable or metastatic disease and the time from initial diagnosis in the 30 patients diagnosed as early-stage disease who then experienced a recurrence afterwards (27).
Finally, in the previously mentioned work by Boerner et al. focused exclusively on iCCA patients, no statistically significant differences in OS were observed in mIDH1/2 patients, although the presence of the mutation was correlated with a trend toward an improved OS (p = 0.08) (20). Furthermore, this study investigated separately the role of mIDH1 and mIDH2, finding again no association with patients’ outcome.
Several confounding factors should be noted when trying to interpret available data from clinical trials on the prognostic role of FGFR or IDH1/2 molecular alterations.
First of all, most of the trials regarding FGFR included both resected and not resected cases and usually OS data according to stage for mutated patients were not provided. This is of course understandable, given the rarity of the mutations and the subsequent small sample size of the clinical trials (with only 3 studies including more than 20 FGFR altered cases); however, it is worth noting that even the trials with more cases failed to provide this important data. Some findings seem to suggest that FGFR mutated patients are more likely to form a mass-forming cancer, making the surgical approach easier for this subset of CCAs (16). If this was true, it would raise the question of whether the OS advantage observed in FGFR mutated patients could be due to the greater number of patients who underwent resection and not to an intrinsic characteristic of these patients. Thus, separate data set on resected patients vs patients undergoing systemic treatment should be made available.
Regarding the prognostic values of IDH mutations on resected iCCA patients specifically, four studies focused on this subset of patients, but results are heterogeneous, with some paper pointing towards a benefit in OS and DFS, while others denying such benefit. As with FGFR mutated cases however, no OS data according to stage disease were provided across all trials.
Another important question is to rule out whether the presence of FGFR alteration could affect response to systemic therapies. Several trials agree that there is not impact on response to first-line chemotherapy (18, 19). However, this is less clear for further lines of treatment, with one trial suggesting a slightly longer PFS for second-line chemotherapy, although with the strong bias of a retrospective descriptive analysis only (19). Regarding the effect of FGFR target therapies on OS, two trials confirmed the positive prognostic role of FGFR alterations on their subset of patients, even after censoring those who had received targeted drugs (16, 17), thus suggesting that the prognostic role of these molecular changes could be independent to the administered targeted treatments. On the other hand, one trial suggested the opposite (18), ascribing the OS gain to anti-FGFR therapies, although it did not provide OS for FGFR patients who had not received target therapies, making it impossible to draw definitive conclusions. Regarding IDH mutation, data are even scarcer but seeing as no patients received IDH target paper in the mentioned trials, there is no risk of this type of treatment being a confounding factor.
In addition, a certain inconsistency on data provided furtherly complicates the matter, namely: a) data often refers to all biliary tract cancers and not specifically to iCCA; b) several trials do not distinguish between FGFR2 rearrangements and mutations or between IDH1 and IDH2; c) the definition of OS in not always unanimous, as the starting point can refer to the initial diagnosis in some trials and to the start of first-line chemotherapy in others.
Finally, it should be noted that in the present review we did not consider several other known prognostic factors for iCCA, including the clinical characteristics of the patients (performance status, age, years of diagnosis, presence of hepatic diseases such as hepatolithiasis or cirrhosis) and the histological features of tumor (size, grade, presence of macroscopic vascular invasion, positive resection margins, large duct vs small duct iCCA), as the quality of these data was already poor. Furthermore, another important issue that must be carefully considered when evaluating the possible prognostic role of IDH and FGFR molecular alterations in iCCA is the co-occurrence of other mutations that could potentially influence patients’ survival. In the majority of genomic studies, FGFR2 and IDH alterations tend to be mutually exclusive and to cluster with BAP1 mutations in a subgroup of patients that is distinct from the subgroup of patients carrying TP53, KRAS, SMAD4 and ARID1A genomic alterations (9, 12, 41). Of note, while co-occurrence of IDH/BAP1 or FGFR2-fusion/BAP1 mutations is almost exclusively found in small bile duct iCCA and associates with good prognosis, KRAS, TP53 and SMAD4 mutations are frequently observed in large bile duct iCCA and associate with dismal prognosis (41). The association of FGFR2 alterations with KRAS or TP53 mutations has been also reported in iCCA patients, although with a very low frequency (42). These molecular findings suggest that combined therapeutic strategies targeting these oncogenic drivers could improve the clinical outcome in selected iCCA patients carrying these genetic alterations, deserving further investigation in future clinical studies.
All these above-mentioned prognostic factors should be rendered available and analyzed when assessing the possible prognostic role of FGFR and IDH genetic alterations in iCCA; however, due to poor quality of available data, it is difficult to draw definitive conclusions on this issue from current studies. A general trend toward a better OS for FGFR2 rearrangements and, possibly, for FGFR2-3 alterations could be inferred, likely independent from the treatment provided. However, the presence of few trials reporting no statistical relevance (or even a worse OS) should encourage larger clinical trials to address this issue. Regarding IDH1-2 mutations, on the other hand, only a handful of papers describe a positive prognostic role for these alterations, with most of the paper leaning toward no prognostic correlation.
As the presence of more small retrospective trials is unlikely to resolve the issue, we encourage the creation of accurate and well-designed prospective trials, with a good sample size including only iCCA, with a clear study population in mind (resected vs non resected, advanced vs early stage, FGFR2 translocations vs all FGFR mutations), and in which all confounding prognostic factors are described and taken into account at multivariate analysis, such as clinical characteristics, tumor histopathological features, coexisting mutations and treatment provided (Table 5). Only when all these parameters are available and valuable the prognostic relevance of IDH and FGFR molecular alterations in iCCA can emerge and be clarified.
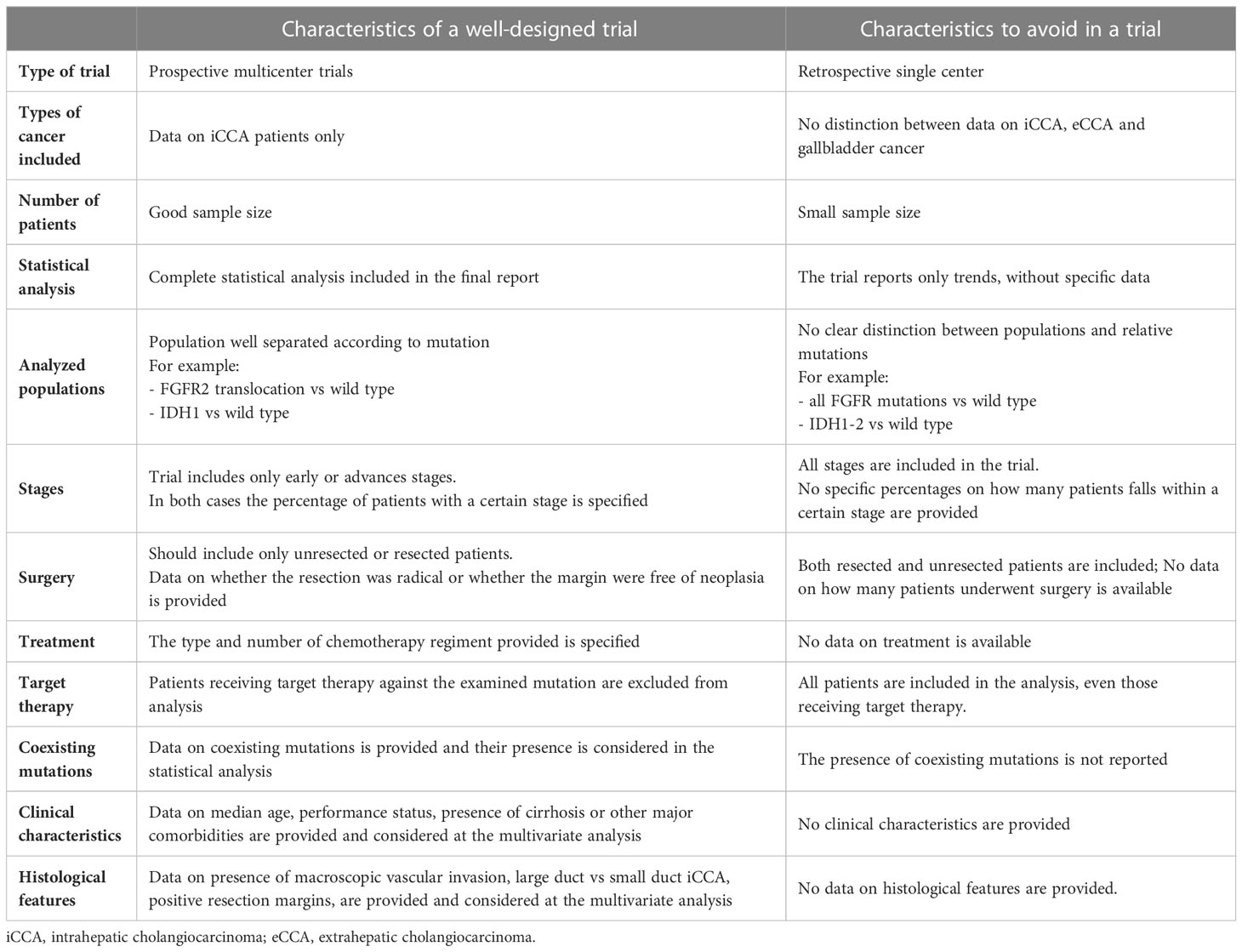
Table 5 “Ideal” parameters to be included in a trial aimed at defining the prognostic role of FGFR or IDH mutations in iCCA.
Treatment paradigm of iCCA is progressively shifting towards precision oncology; in this scenario, a better understanding of the prognostic role of genetic aberrations driving iCCA carcinogenesis appears mandatory to improve the clinical outcome of these patients.
GB and ST: conception of the study, data interpretation and drafting of the article; CD, LG, AP: drafting of the article; AR and ADR: critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
The work reported in this publication was funded by the Italian Ministry of Health, RC-2022-2773354, and it was supported by “Fondazione Donato-Venturi” to GB.
GB received a research grant from INCYTE and IPSEN and he is a member of the advisory board for INCYTE, LILLY and TAIHO.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma — evolving concepts and therapeutic strategies. Nat Rev Clin Oncol (2018) 15(2):95–111. doi: 10.1038/nrclinonc.2017.157
2. Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol (2020) 17(9):557–88. doi: 10.1038/s41575-020-0310-z
3. Izquierdo-Sanchez L, Lamarca A, Casta AL, Buettner S, Utpatel K, Klümpen HJ, et al. Cholangiocarcinoma landscape in Europe: diagnostic, prognostic and therapeutic insights from the ENSCCA registry. J Hepatol (2022) 76(5):1109–21. doi: 10.1016/j.jhep.2021.12.010
4. Kelley RK, Bridgewater J, Gores GJ, Zhu AX. Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol (2020) 72(2):353–63. doi: 10.1016/j.jhep.2019.10.009
5. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med (2010) 362(14):1273–81. doi: 10.1056/NEJMoa0908721
6. Oh DY, Ruth HA, Qin S, Chen LT, Okusaka T, Vogel A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid (2022) 1(8):EVIDoa2200015. doi: 10.1056/EVIDoa2200015
7. Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol (2021) 22(5):690–701. doi: 10.1016/S1470-2045(21)00027-9
8. Mahipal A, Kommalapati A, Tella SH, Lim A, Kim R. Novel targeted treatment options for advanced cholangiocarcinoma. Expert Opin Investig Drugs (2018) 27(9):709–20. doi: 10.1080/13543784.2018.1512581
9. Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, et al. Genomic spectra of biliary tract cancer. Nat Genet (2015) 47(9):1003–10. doi: 10.1038/ng.3375
10. Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol (2020) 21(5):671–84. doi: 10.1016/S1470-2045(20)30109-1
11. Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol (2020) 21(6):796–807. doi: 10.1016/S1470-2045(20)30157-1
12. Jusakul A, Cutcutache I, Yong CH, Lim JQ, Huang MN, Padmanabhan N, et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov (2017) 7(10):1116–35. doi: 10.1158/2159-8290.CD-17-0368
13. Javle M, Bekaii-Saab T, Jain A, Wang Y, Kelley RK, Wang K, et al. Biliary cancer: utility of next-generation sequencing for clinical management. Cancer (2016) 122(24):3838–47. doi: 10.1002/cncr.30254
14. Churi CR, Shroff R, Wang Y, Rashid A, Kang HC, Weatherly J, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PloS One (2014) 9(12):e115383. doi: 10.1371/journal.pone.0115383
15. Graham RP, Barr Fritcher EG, Pestova E, Schulz J, Sitailo LA, Vasmatzis G, et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum Pathol (2014) 45(8):1630–8. doi: 10.1016/j.humpath.2014.03.014
16. Jain A, Borad MJ, Kelley RK, Wang Y, Abdel-Wahab R, Meric-Bernstam F, et al. Cholangiocarcinoma with FGFR genetic aberrations: a unique clinical phenotype. JCO Precis Oncol (2018) 2):1–12. doi: 10.1200/PO.17.00080
17. Rizzato M, Brignola S, Munari G, Gatti M, Dadduzio V, Borga C, et al. Prognostic impact of FGFR2/3 alterations in patients with biliary tract cancers receiving systemic chemotherapy: the BITCOIN study. Eur J Cancer (2022) 166:165–75. doi: 10.1016/j.ejca.2022.02.013
18. Maruki Y, Morizane C, Arai Y, Ikeda M, Ueno M, Ioka T, et al. Molecular detection and clinicopathological characteristics of advanced/recurrent biliary tract carcinomas harboring the FGFR2 rearrangements: a prospective observational study (PRELUDE study). J Gastroenterol (2021) 56(3):250–60. doi: 10.1007/s00535-020-01735-2
19. Abou-Alfa GK, Bibeau K, Schultz N, Yaqubie A, Millang B, Ren H, et al. Effect of FGFR2 alterations on overall and progression-free survival in patients receiving systemic therapy for intrahepatic cholangiocarcinoma. Target Oncol (2022) 17(5):517–27. doi: 10.1007/s11523-022-00906-w
20. Boerner T, Drill E, Pak LM, Nguyen B, Sigel CS, Doussot A, et al. Genetic determinants of outcome in intrahepatic cholangiocarcinoma. Hepatology (2021) 74(3):1429–44. doi: 10.1002/hep.31829
21. Arai Y, Totoki Y, Hosoda F, Shirota T, Hama N, Nakamura H, et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology (2014) 59(4):1427–34. doi: 10.1002/hep.26890
22. Nepal C, O’Rourke CJ, Oliveira DVNP, Taranta A, Shema S, Gautam P, et al. Genomic perturbations reveal distinct regulatory networks in intrahepatic cholangiocarcinoma. Hepatology (2018) 68(3):949–63. doi: 10.1002/hep.29764
23. Ma B, Meng H, Tian Y, Wang Y, Song T, Zhang T, et al. Distinct clinical and prognostic implication of IDH1/2 mutation and other most frequent mutations in large duct and small duct subtypes of intrahepatic cholangiocarcinoma. BMC Cancer (2020) 20:318. doi: 10.1186/s12885-020-06804-6
24. Wang P, Dong Q, Zhang C, Kuan PF, Liu Y, Jeck WR, et al. Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas. Oncogene (2013) 32(25):3091–100. doi: 10.1038/onc.2012.315
25. Zhu AX, Borger DR, Kim Y, Cosgrove D, Ejaz A, Alexandrescu S, et al. Genomic profiling of intrahepatic cholangiocarcinoma: refining prognosis and identifying therapeutic targets. Ann Surg Oncol (2014) 21(12):3827–34. doi: 10.1245/s10434-014-3828-x
26. Ruzzenente A, Fassan M, Conci S, Simbolo M, Lawlor RT, Pedrazzani C, et al. Cholangiocarcinoma heterogeneity revealed by multigene mutational profiling: clinical and prognostic relevance in surgically resected patients. Ann Surg Oncol (2016) 23(5):1699–707. doi: 10.1245/s10434-015-5046-6
27. Goyal L, Govindan A, Sheth RA, Nardi V, Blaszkowsky LS, Faris JE, et al. Prognosis and clinicopathologic features of patients with advanced stage isocitrate dehydrogenase (IDH) mutant and IDH wild-type intrahepatic cholangiocarcinoma. Oncologist (2015) 20(9):1019–27. doi: 10.1634/theoncologist.2015-0210
28. Dieci MV, Arnedos M, Andre F, Soria JC. Fibroblast growth factor receptor inhibitors as a cancer treatment: from a biologic rationale to medical perspectives. Cancer Discov (2013) 3(3):264–79. doi: 10.1158/2159-8290.CD-12-0362
29. Mahipal A, Tella SH, Kommalapati A, Anaya D, Kim R. FGFR2 genomic aberrations: achilles heel in the management of advanced cholangiocarcinoma. Cancer Treat Rev (2019) 78:1–7. doi: 10.1016/j.ctrv.2019.06.003
30. Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer (2010) 10(2):116–29. doi: 10.1038/nrc2780
31. Zhu Z, Dong H, Wu J, Dong W, Guo X, Yu H, et al. Targeted genomic profiling revealed a unique clinical phenotype in intrahepatic cholangiocarcinoma with fibroblast growth factor receptor rearrangement. Transl Oncol (2021) 14(10):101168. doi: 10.1016/j.tranon.2021.101168
32. Buckarma E, de la Cruz G, Truty M, Nagorney D, Cleary S, Kendrick M, et al. Impact of FGFR2 gene fusions on survival of patients with intrahepatic cholangiocarcinoma following curative intent resection. HPB (Oxford) (2022) 24(10):1748–56. doi: 10.1016/j.hpb.2022.05.1341
33. Reitman ZJ, Parsons DW, Yan H. IDH1 and IDH2: not your typical oncogenes. Cancer Cell (2010) 17(3):215–6. doi: 10.1016/j.ccr.2010.02.024
34. Pietrak B, Zhao H, Qi H, Quinn C, Gao E, Boyer JG, et al. A tale of two subunits: how the neomorphic R132H IDH1 mutation enhances production of αHG. Biochemistry (2011) 50(21):4804–12. doi: 10.1021/bi200499m
35. Chowdhury R, Yeoh KK, Tian YM, Hillringhaus L, Bagg EA, Rose NR, et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep (2011) 12(5):463–9. doi: 10.1038/embor.2011.43
36. Wu MJ, Shi L, Dubrot J, Merritt J, Vijay V, Wei TY, et al. Mutant IDH inhibits IFNγ-TET2 signaling to promote immunoevasion and tumor maintenance in cholangiocarcinoma. Cancer Discov (2022) 12(3):812–35. doi: 10.1158/2159-8290.CD-21-1077
37. Raineri S, Mellor J. IDH1: linking metabolism and epigenetics. Front Genet (2018) 9:493. doi: 10.3389/fgene.2018.00493
38. Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature (2009) 462(7274):739–44. doi: 10.1038/nature08617
39. Boscoe AN, Rolland C, Kelley RK. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: a systematic literature review. J Gastrointest Oncol (2019) 10(4):751–65. doi: 10.21037/jgo.2019.03.10
40. Borger DR, Goyal L, Yau T, Poon RT, Ancukiewicz M, Deshpande V, et al. Circulating oncometabolite 2-hydroxyglutarate is a potential surrogate biomarker in patients with isocitrate dehydrogenase-mutant intrahepatic cholangiocarcinoma. Clin Cancer Res Off J Am Assoc Cancer Res (2014) 20(7):1884–90. doi: 10.1158/1078-0432.CCR-13-2649
41. Lowery MA, Ptashkin R, Jordan E, Berger MF, Zehir A, Capanu M, et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clin Cancer Res (2018) 24:4154–61. doi: 10.1158/1078-0432.CCR-18-0078
Keywords: FGFR, IDH, cholangiocarcinoma, prognosis, molecular alteration
Citation: Brandi G, Deiana C, Galvani L, Palloni A, Ricci AD, Rizzo A and Tavolari S (2023) Are FGFR and IDH1-2 alterations a positive prognostic factor in intrahepatic cholangiocarcinoma? An unresolved issue. Front. Oncol. 13:1137510. doi: 10.3389/fonc.2023.1137510
Received: 04 January 2023; Accepted: 06 April 2023;
Published: 24 April 2023.
Edited by:
Avinash Kambadakone, Massachusetts General Hospital, Harvard Medical School, United StatesReviewed by:
Tommaso Stecca, ULSS2 Marca Trevigiana, ItalyCopyright © 2023 Brandi, Deiana, Galvani, Palloni, Ricci, Rizzo and Tavolari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giovanni Brandi, Z2lvdmFubmkuYnJhbmRpQHVuaWJvLml0
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.