- Department of Oncology, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
Lung cancer is one of the most devastating diseases worldwide with high incidence and mortality, and the incidence continues to rise. Metastasis is the leading cause of death in lung cancer patients, yet the molecular effectors underlying tumor dissemination remain poorly defined. Research findings in recent years confirmed primed microenvironment of future metastatic sites, called the pre‐metastatic niche, is a prerequisite for overt metastasis. Exosomes have recently emerged as important players in pre‐metastatic niche formation. Natural medicines have traditionally been rich sources of drug discovery. Some of them exhibit favorable anti-lung cancer activity. The review focused on the latest advances in the regulation of the pre‐metastatic niche formation in lung cancer by the contents of exosomes of representative natural medicines. Additionally, the mechanism of natural medicines was summarized in detail, which would provide new insights for anti-cancer new drug development.
1 Introduction
Lung cancer is one of the most prevalent malignancies worldwide, with recent studies showing that more than 2 million people are estimated to be newly diagnosed with lung cancer each year (1), with approximately 236,740 new lung cancer diagnoses and 130,180 deaths in the United States in 2022 (2), and an estimated 820,000 new lung cancer diagnoses and 715,000 lung cancer-related deaths in China in 2020 (3). Lung cancer remains the most common cancer in China today (4). Metastasis is the leading cause of death in lung cancer patients (5), and when present, it usually predicts a poor outcome and is a malignant event that is strongly avoided in clinical practice. Metastasis may be caused by a number of factors, such as the invasive ability of cancer cells, the absence or suppression of immune surveillance function, etc. However, regardless of the mechanism of metastasis, the final manifestation is that the detached cancer cells arrive at a new proliferation area and develop into new tumor tissue. During this process, the area where the tumor colonizes forms a pre-metastatic niche (PMN) (6). The characteristics of the pre-metastatic ecotone include inflammation formation, enhanced angiogenesis and vascular permeability, lymphangiogenesis, immunosuppression, and metabolic reprogramming, and possesses four stages of initiation, licensing, colonization, and progression (7). The reasons for how PMN formation begins before tumor cells arrive are unclear, and tumor-associated exosomes appear to be good mediators of this remote regulation. Exosomes from highly metastatic cancer cells and advanced lung cancer sera have been found to induce migration, invasion and proliferation of non-cancerous recipient cells in the clinic (8), and more basic experiments likewise support the involvement of tumor-derived exosomes in cancer metastasis through multiple mechanisms by remodeling the tumor microenvironment (9). As research continues, new studies have demonstrated the ability of exosomes to seed tumor cells to appropriate ecological niches for the proliferation and formation of “micro-metastases” (10). Having clarified that the involvement of exosomes in inducing pre-metastatic ecological niches is the central mechanism of metastasis occurrence, the search for key interventions is necessary. Natural medicines are a large class of uncommercialized components derived from nature, including some natural compounds of plant origin, herbal medicines and their mixed compositions (i.e., traditional Chinese medicinal preparation) that have been empirically applied (11). They have good effects in the treatment of malignant tumors such as lung cancer (12), but the exosome-related research has not yet become systematic and deserves further exploration. In this paper, we analyze the potential natural medicines intervention targets of each component in PMN formation, and explore the novel antitumor modalities such as natural medicines that can modulate the release of exosomes and natural medicines that artificially encapsulate exosomes and plant-derived exosomes, supporting the clinical challenge that natural medicines can be involved in exosome-mediated formation of pre-metastatic ecological sites in lung cancer.
2 Exosome involvement in lung cancer progression and metastasis
Exosomes are a subtype of extracellular vesicles (EV), a lipid bilayer enclosed vesicle with a diameter of 30-100 nm, and one of the important mediators of intercellular communication (13). Exosomes can be secreted by various cells and can also carry a variety of substances such as lipids, nucleic acids, and proteins, and are widely distributed in body fluids, and this special property leads to their important role in information transfer in tumor metastasis (9). The role of exosomes is mainly determined by their contents, which include proteins, lipids, enzymes, transcription factors, DNA fragments, messenger RNA (mRNA), micro RNA (miRNA), and long non-coding RNA (lncRNA), etc (14). Currently, RNA, DNA and proteins are the most studied ones. Tumor cells can transfer these molecules into stromal cells to ensure communication in the microenvironment and can modify the recipient cell phenotype to be tumorigenic, participating in processes such as induction of angiogenesis, tumor cell migration and proliferation, inflammatory response, immunosuppression, evasion of immune surveillance, and metastasis (14). It has also been demonstrated in clinical settings that exosomes are more abundant in circulating body fluids of lung cancer patients than healthy individuals, and these exosomes can similarly promote the formation of lung cancer microenvironment, increase tumor metastasis, mediate tumor immunosuppression, and participate in radiotherapy resistance in ways that promote lung cancer development (15). miRNA is one of the most prominent components of exosome loading, and several miRNAs have been obtained to promote lung cancer metastasis supported by evidence, such as miR-17-92, several miR-200 family miRNAs (miR-200a, miR-200b, miR-200c, miR-141 and miR-429) including miR-125a-3p/5p, miR-21 and miR-106b-25 family (miR-106b and miR-93), among others (16). Although it is confirmed that exosomes can promote cancer metastasis, the specific mechanisms by which they exert their effects remain complex, and the existing scientific hypotheses are specifically as follows: (1) exosomes from tumor cells induce epithelial mesenchymal transformation and degrade the stroma, such as prostate cancer; (2) tumor-secreted exosomes derived from prostate cancer interfere directly or indirectly with endothelial cells by activating macrophages; (3) circulating tumor cells (CTCs) derived from leukemia, lymphoma and breast cancer and tumors activated platelets derived from lung cancer release exosomes that affect immune cells; (4) exosomes attached to tumors upregulate adhesion molecules on endothelial cells, such as chronic myelogenous leukemia; (5) exosomes can disseminate tumor cells to suitable ecological sites for proliferation and form micro-metastases, such as pancreatic adenocarcinoma and melanoma (10). In this process, the formation of pre-metastatic ecological sites as a direct manifestation of “pre-metastasis”, probably exosomes plays a more specific role.
3 Exosomes are involved in constructing the pre-metastatic microenvironment from multiple perspectives
Exosomes play a crucial role in PMN formation (17). Previous studies have demonstrated that exosomes play different roles in each of the sequential steps necessary to complete the construction of the pre-metastatic ecotone (i.e., angiogenesis and leakage, PMN stromal cell domestication, bone marrow-derived cell domestication and recruitment) (18), and others recognize that PMN formation is preceded by exosomes through the induction of an inflammatory response, which may be the true onset of PMN formation (19) (Figure 1; Table 1).
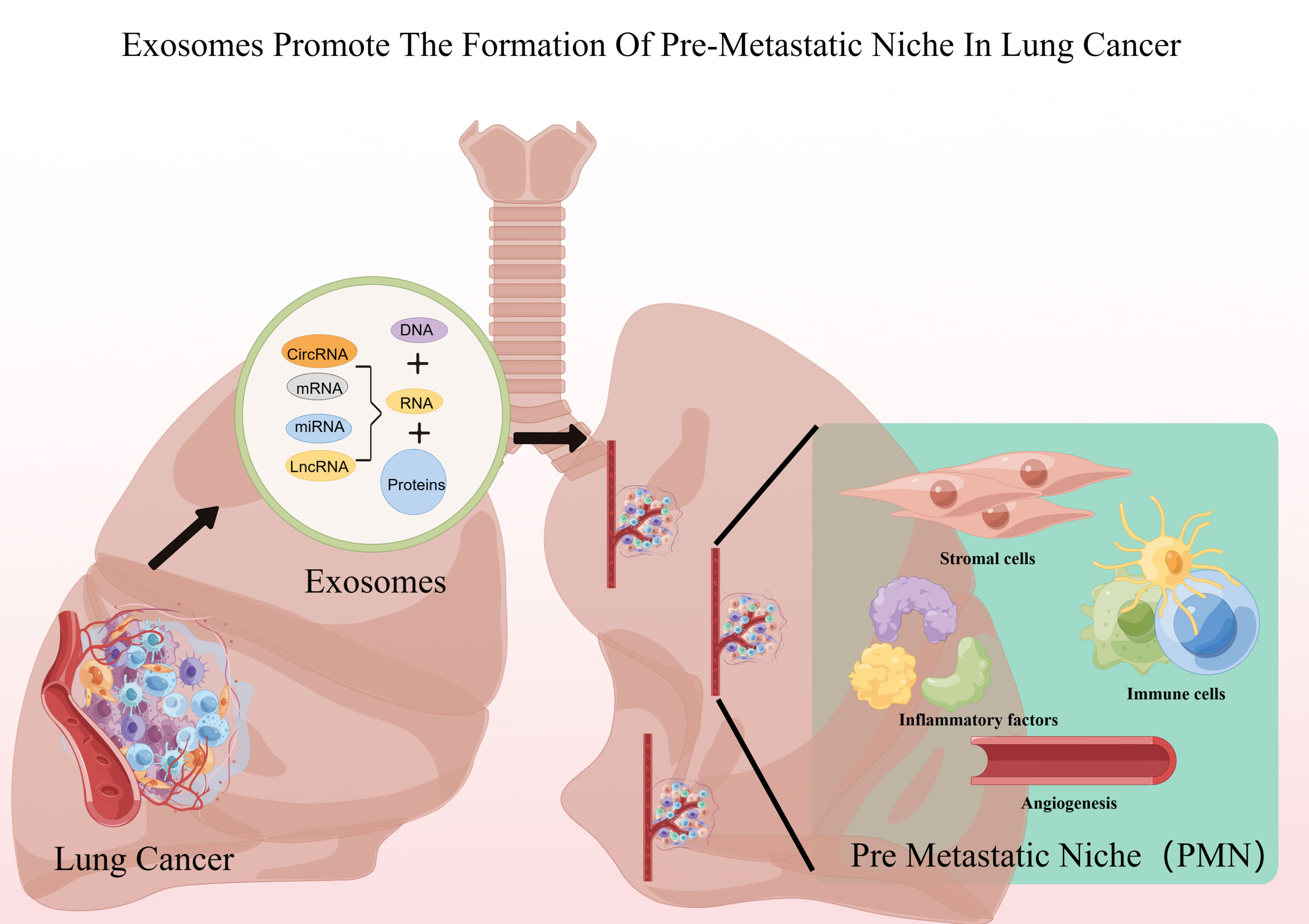
Figure 1 Lung cancer exosomes promote PMN formation. Figure was created by Figdraw (www.figdraw.com).
3.1 Inflammatory reaction
Mild inflammatory response is a favorable condition for lung cancer metastasis (41), but it is worth exploring how to precisely create a suitable PMN environment for pre-metastatic cancer cells. It has been pointed out that the metastasis of tumor cells to target organs is not random, and exosomes expressing integral proteins carrying integrins α6β4 and α6β1 on the surface of exosomes are more inclined to lung fibroblasts and epithelial cells, and tumors are prone to metastasis to the lung (42), therefore, locating after suitable PMN location, the inflammatory response begins to occur. Lung cancer cell-derived exosomes can activate Toll-like receptor 2 (TLR2)/NF-κB signaling via their surface heat shock protein 70, converting naïve MSCs in PMN into a new pro-inflammatory MSC that secretes IL-6, IL-8 and human monocyte chemotactic protein-1(MCP-1) to maintain a low-grade inflammatory microenvironment (20). Liu et al. found that lung epithelial cells with Toll-like receptor 3 (TLR3) deficiency prevented ecotone formation, whereas exosomal RNA reversed this process by activating TLR3, NF-κB and MAPK thereby recruiting neutrophils to maintain the inflammatory environment and facilitating ecotone formation (21). Interestingly, the TLR family seems to be quite importantly present in the inflammatory response prior to PMN formation, not only TLR2 as well as TLR3 above, but also other studies have confirmed that exosomal miR-21 and miR-29a secreted by lung cancer cells can bind to mouse TLR7 (Toll-like receptor 7) and human TLR8 (Toll-like receptor 8) in immune cells, inducing TLR-mediated inflammatory responses, which may eventually provide the right conditions for tumor metastasis (22). Of course, some other inflammation-related factors beyond TLR are also of interest, such as studies confirming that transcripts of TGF-β, lnc-MMP2-2, and IL-10 in lung cancer cell exosomes play a key role in regulating the migratory ability of lung cancer cells by targeting and regulating related genes (9).
3.2 Angiogenesis
Inflammation may be a key factor in the induction of tumor angiogenesis and may likewise be involved in angiogenesis in PMN formation (43). The involvement of tumor-derived exosomes in the induction of inflammatory PMN formation in distant organs to promote metastasis has been supported, as cell migration-inducing and hyaluronan binding protein (CEMIP) of lung tumor origin can induce pro-inflammatory vascular ecotopes to promote metastasis by upregulating cytokines prostaglandin endoperoxide synthase 2(PTGS2), tumor necrosis factor(TNF) and chemokine (C-C Motif) ligand(CCL)/chemokine (C-X-C motif) ligand(CXCL) cytokines (23). Also mediated by inflammatory factors, NSCLC cells use exosomes loaded with LRG1 and promote angiogenesis through TGF-β pathway-mediated expression of pro-angiogenic markers (e.g. VEGFA and Ang1) (24). Hypoxic environment is also a condition for induction of angiogenesis, as exosomal miR-23a of lung cancer cell origin directly inhibits prolyl hydroxylases PHD1 and PHD2, leading to accumulation of hypoxia-inducible factor-1 (HIF-1) in endothelial cells to enhance angiogenesis (25). In lung cancer, not only the exosomes of cancer cells can induce angiogenesis, but also bronchial epithelial cells under smoking induction. For example, bronchial epithelial cells under smoke intervention elevated exosome miR-21 levels, and these miR-21 led to STAT3 activation, which increased VEGF levels in receptor cells and promoted angiogenesis (26).
3.3 Stromal cell domestication
After PMN has undergone the construction of an inflammatory environment and has a vascular supply of nutrients, stromal cells will also be domesticated by exosomes and become the cradle for future tumor cell landings, with the main cell groups including endothelial cells of blood vessels, epithelial cells of trachea or bronchi, and fibroblasts (44). It was demonstrated that the pro-tumorigenicity of miR-210 in A549 cells and their exosomes were increased upon induction of tissue inhibitor of metalloproteinase-1 (TIMP-1) tissue inhibitor, and that miR-210 accumulated in exosomes in vitro and in vivo after overexpression of TIMP-1 in tumor cells. These exosomes promote the formation activity of human umbilical vein endothelial cells (HUVECs), and these transformed stromal cells become paracrine to the malignancy, increasing angiogenesis (27). Exosomes from lung cancer similarly induce lung epithelial cells to exhibit immunomodulatory effects in lung homeostasis and mediate immunosuppressive PMN formation in lung metastases (28). Fibroblasts are a large class of stromal cell populations that can provide ample back-up for lung cancer cells. Circulating Lewis Lung Carcinoma (LLC)-derived exosome miR-3473b can be phagocytosed by lung fibroblasts, causing NF-κB activation in fibroblasts and increasing intrapulmonary colonization of lung tumor cells (29).
3.4 Bone marrow-derived cell domestication and recruitment
After building the basic material basis of PMN, metastatic tumor cells need to find pathway guides and escape from immune cells, and domesticating bone marrow-derived cells to achieve this goal is a “smart” choice for lung cancer cells, and macrophages and dendritic cells (DCs) seem to be more preferred. The macrophages in the tumor microenvironment are called tumor-associated macrophages (TAM) (45). In the NSCLC microenvironment, TAM constitute the major cellular component. They not only act as immunosuppressive cells, enabling NSCLC to immune evade, but also directly promote the proliferation, survival, invasion, and metastasis of cancer cells. TAM include both inflammatory or classically activated (M1) and anti-inflammatory or alternative activated (M2) phenotypes, while the M2 type of TAM has been shown to promote pre-metastatic ecology by secreting TGF-β, SDF-1 and VEGF through STAT3 signaling cascade site formation (46). During excessive primary tumor growth, hypoxic lung cancer cells promote macrophage M2 polarization and induce lung cancer progression by secreting exosomes that affect the PI3K/AKT signaling pathway via miR-21 (30). Another mechanism is that exosomes derived from lung cancer cells secrete TRIM59, which converts macrophages into tumor-promoting macrophages by regulating ABHD5 proteasome degradation, thereby activating the NLRP3 inflammatory vesicle signaling pathway and promoting lung cancer progression by secreting IL-1 (31). DC, as one of the most functional antigen-presenting cells, is necessary for lung cancer cells to achieve immune escape by remotely controlling DC the presence of EGFR in lung cancer exosomes induces tolerogenic DCs, which in turn induce the production of tumor antigen-specific regulatory T cells (Tregs), which suppress tumor antigen-specific CD8 T cells and induce immune tolerance in lung cancer patients (32). Exosomes from LLC lung cancer cells can block the differentiation of bone marrow progenitor cells into CD11c DCs and induce apoptosis at their source (33). In addition, exosomes derived from domesticated bone marrow cells can also suppress immunity and thus promote metastasis, e.g. exosomes from tumor-bearing mouse bone marrow-derived cells (BMDC) carry PD-L1, and PD-L1 on these exosomes is biologically functional and can inhibit CD8 T cell proliferation and activation in vitro and in vivo (34). Of course lung cancer cells can also exert immunosuppressive effects across bone marrow-derived cells, such as exosomes from lung cancer cells that express PD-L1 and promote tumor growth by reducing T cell activity to induce immune escape. The rationale is that exosomal PD-L1 inhibits T-cell secretion of interferon-γ (IFN-γ), reduces cytokine production and induces apoptosis of CD8 T cells to impair immune function and promote lung cancer metastasis (35).
3.5 Other factors affecting the formation of PMN
PMN formation is a sophisticated and complex project in which metabolic alterations, epithelial-mesenchymal transition (EMT) formation and even atmospheric pollutants may be involved, in addition to the standard processes involving inflammation, vasculature, stromal cells, and bone marrow-derived cells. Altered metabolism may be an important pathway in the differentiation of macrophages to an immunosuppressive phenotype by tumor-derived exosomes, such as increased PD-L1 expression through NF-κB-dependent, glycolysis-led metabolic reprogramming (36). Exosomes can be involved in the disruption of lipid metabolism in cancer cells and in the malignant progression of cancer (47). Exosomes derived from lung cancer cells promote lipid degradation in adipocytes, thereby providing energy for uncontrolled cell proliferation (37). In PMN, exosomes may be involved in EMT formation earlier (48), and exosomes have been shown to regulate many cellular functions by activating EMT and inducing pre-metastatic ecotone formation. Specific exosomal miRNAs, including miR-10aand miR-26a are involved in exosomal promotion of EMT in epithelial cells as well as migration and invasion of cancer cells, and may serve as novel biomarkers of the EMT process in lung cancer (38). Cancer-associated fibroblasts (CAF) may also be involved in the induction of EMT, such as SNAI1 delivered to recipient lung cancer cells via exosomes from CAF to promote EMT (39). As an organ in contact with the atmosphere, atmospheric particulate matter (PM) may be involved in the formation of PMN, as shown by a research team that injected LLC cells into C57BL/6 mice via tail vein and PM via nasal drip. The results showed that the lung metastasis foci were increased in the mice dripped with PM, indicating that PM promoted the lung metastasis of LLC cells. In addition, the expression levels of certain genes that promote tumor cell metastasis and promote tumor cell colonization at metastatic sites were significantly increased in the lungs of PM-exposed mice, and some of these genes were upregulated mainly in neutrophils, indicating that PM promotes the formation of pre-metastatic ecological sites in the lungs (40). This interesting finding may be an alternative strategy to reduce lung cancer incidence and improve prognosis in areas with harsh atmospheric conditions and a high prevalence of lung cancer.
4 Natural medicines intervene in the PMN formation link of lung cancer
We have learned above that exosomes are involved in PMN formation in lung cancer, and several reliable and important pathways have been summarized in the cumbersome PMN formation mechanism, and how to intervene in exosomes involved in these pathways may be the key to reduce lung cancer metastasis and improve the prognosis, the current therapeutic options for exosomes are more limited, such as Annette M. Marleau et al. using a AETHLONADPT plasma replacement technique, in which blood containing exosomes is filtered, found that targeted reduction of exosomes may become a treatment for lung cancer. In addition, ceramide may be one of the lipids required for exosome synthesis and its synthesis is mediated by neutral sphingomyelinase 2 (nSMase2). Therefore, the neutral inhibitor of nSMase2, GW4869, could be added to eventually eliminate or reduce exosome production (49). Modification of exosomes for antitumor is a popular direction and there are some promising advances, but there are still relatively few studies targeting exosomes to modulate PMN. Natural medicines have a huge number of potential options that can be explored if they are combined with TCM.
4.1 Regulation of tumor exosome secretion
The formation of PMN is closely related to the release of tumor exosomes, and the regulation of tumor exosome release is a key step, which is currently a hot direction in natural medicines research. Shikonin, a naphthoquinone isolated from Comfrey, has potent antitumor effects, and modulation of exosome miR-628-3p in non-small cell lung cancer cell lines has recently been shown to be a novel mechanism for its inhibition of lung cancer cell proliferation (50). Not only in lung cancer, comfrey can also inhibit MCF-7 cell proliferation by regulating exosomal miR-128 (51). Halofuginone, a small alkaloid extracted from the traditional Chinese herb Changshan, exerts anti-tumor effects by inhibiting the secretion and delivery of exosomal miR-31 through regulation of the HDAC2/cell cycle signaling axis, thereby inhibiting MCF-7 cell proliferation (52). Not only the single components of natural medicines, but also the combination of multiple natural medicines in traditional Chinese medicine can play the role of regulating exosomes. For example, Jinfu Kang preparation, which consists of 12 herbs, has been used in the clinical treatment of lung cancer for a long time, and it has been proved that Jinfu Kang can inhibit CTC-TJH-01 cells by negatively regulating the EGF signaling pathway in CTC-TJH-01 exosomes of lung cancer circulating tumor cells metastasis (53).
4.2 Intervention of inflammatory formation-related exosomes
From the above, we found that Toll-like receptor (TLR) may be an important inflammatory environment-forming molecule during PMN formation, and regulation of TLR-related pathways may be a reliable way to prevent the formation of pre-metastatic inflammatory environment. TLR is an important immune regulator for the regulation of intracellular inflammatory responses, and its downstream may be closely related to the activation of NOD-like receptor thermal protein domain associated protein 3 (NLRP3) (54). Expression of inflammatory vesicles NLRP3 also induces immunosuppressive effects such as M2 macrophages to promote tumor progression (55). Artemisinin (ART) is a classical antimalarial drug derived from the Chinese herb Artemisia annua for the treatment of autoimmune diseases with anti-inflammatory and immunomodulatory properties. New studies have shown that artemisinin can modulate inflammatory factors by mediating exosome release in human renal tubular epithelial cells (HK-2), which may be related to the inhibition of NF-κB/NLRP3 pathway by regulating exosome secretion (56). The same study showed that Zhen Wu Tang can regulate exosomal secretion to inhibit NF-kB/NLRP3 signaling pathway and thus control inflammatory diseases (57). Heat shock proteins are a key pathway for TLR initiation, and kaempferol, a potent flavonoid compound derived from cinnamon leaves, can reduce exosome secretion from cancer cells by regulating the protein expression of heat shock protein HSP 90-α (HSP90AA1), heat shock protein HSP 90-β (HSP90AB1), and ubiquitin-like modifier activator enzyme 1 (UBA1), vacuolar sorting protein 4(VPS4A) are important targets among them (58). TGF-β is also an important molecule in the inflammatory environment, and the combination of the multi-natural medicine Tongxinluo capsules can reduce the expression of TGF-β1-containing exosomes secreted by endothelial cells (59).
4.3 Intervention of angiogenesis-related exosomes
The study of exosomes related to intervention of tumor angiogenesis is not sufficient yet but has good potential for development. Steroidal glycoalkaloids from Solanum lyratum (Solanum lyratum Thunb.), a class of steroidal alkaloid glycosides with important antitumor activity, affect the formation of tumor exosomes, leading to changes in their functions, which in turn inhibit tumor angiogenesis and suppress the development of lung cancer A549 cells (60).
4.4 Intervention of bone marrow-derived cells to domesticate and recruit related exosomes
Modulation of bone marrow-derived cells seems to be another important direction for natural medicines to act, such as ginsenoside Rg1 by attenuating the release of macrophage-derived exosomes miR-21, which has been shown to be an important regulatory mechanism in lung cancer metastasis (61). Dahuang Zhechong Pill (DHZCP) significantly reduced serum exosomal CC chemokine ligand-2 (CCL2) levels and its receptor CCR2, which in exosomes triggers macrophage recruitment and converts M1/M2 macrophages in the liver to the M2 phenotype, and DHZCP modulated the pathway of CCL2 in exosomes reversing the pro-tumor polarization of macrophages (62). In addition to direct action on TAM-derived exosomes, some natural medicines may also act on TAM, the originator of CMPB90-1, a novel natural polysaccharide from Cordyceps sinensis, which translates immunosuppressive TAM by binding to toll-like receptor 2 (TLR2), polarizing TAM to M1 phenotype with antitumor effects and better safety profile. Studies in Chinese medicine compounds are also noteworthy (63).
4.5 Intervention of other pathways related to exosomes
Altered metabolism is a potentially potent player in PMN, and the active ingredient of the traditional Chinese medicine Comfrey is a naphthoquinone compound that inhibits glycolysis in non-small cell lung cancer cells by modulating the exosomal pyruvate kinase M2 pathway (64). EMT has also been published, and Jianshu Huayu Fang (includes ingredients such as Salvia miltiorrhiza Bunge, Poria cocos etc.) is a traditional Chinese medicinal preparation that can treat a wide range of malignancies, including a variety of malignancies, with antitumor effects that are partly EMT-mediated inhibition through exosome-mediated downregulation of intercellular miR-23a-3p transfer and subsequent blockade of Smad signaling, while suggesting that disruption of this exosomal miR-23a-3p/Smad signaling pathway may be an effective anti-tumor approach (65).
5 New interventions where natural medicines are viable
Natural medicines can modulate PMN through exosomes not only by modulating tumor-related exosomes, but also by using the properties of exosomes to achieve the role of modulating PMN-related mechanisms. In the current related research, using exosomes to encapsulate natural medicines seems to be a recommended option, and isolating their own exosomes from natural medicines for tumor treatment is also a feasible decision to explore the active ingredients of natural medicines. The isolation of exosomes from natural medicines for tumor treatment is also a feasible decision to explore the active ingredients of natural medicines (Table 2).
5.1 Exosomes loaded with natural medicines
Recently, several studies have shown that exosomes can be used as potential drug delivery system (DDS) in cancer therapy. With its small size, high stability, biocompatibility and safety profile, it can be used as an efficient tumor-targeting vehicle to deliver drugs to proximal or distal cells (66, 67). Exosomes derived from cancer cells are pro-trophic to their parental cells and can therefore be used as trojan horses to target these malignant cells (68). The ability of exosome biogenesis in different human cancer cells is positively correlated with the activation of STAT3/PKM2/SNAP23 pathway, and STAT3 plays a key role in regulating the biogenesis of tumor-derived exosomes (69). Curcumin has been shown to effectively inhibit STAT3 phosphorylation in small cell lung cancer by downregulating downstream regulatory proteins of STAT3, which contributes to the inhibition of cell proliferation, diminished cell colony formation, and reduced cell migration and invasion (70). For example, the anti-inflammatory activity of curcumin was improved when encapsulated in breast cancer cell-derived exosomes due to reduced off-target effects (71). Compared to free curcumin, exosome-encapsulated curcumin showed enhanced anti-lung cancer activity (72). The mechanism may be related to the modulation of exosomal TCF21, thereby inhibiting exosome-induced lung cancer metastasis (73). Exosomes from sources other than tumors also have enhanced effects on natural medicines components, such as norethindrone (NCTD), a derivative of zebularine isolated from the dried body of zebularine, which has multiple pharmacological activities including antitumor, and after using bone mesenchymal stem cell-derived exosomes (BMSC-Exos) were used as drug carriers to encapsulate NCTD, BMSC-Exos-NCTD significantly promoted cellular uptake and reduced cell migration and invasion of cancer (74). The surprise of the exosome encapsulation technology does not stop there, as milk-derived exosomes are equally effective, which provides sufficient carrier support for the prospective application of natural medicines after exosome encapsulation and greatly enhances the clinical feasibility. Celastrol (CEL) is a plant-derived triterpenoid derived from C. rhamnosus, a known inhibitor of Hsp90 and NF-κB activation pathways. milk-derived exosomes loaded with Celastrol (Exo-CEL) exhibited higher efficacy in inhibiting lung cancer cell proliferation in vivo and in vitro compared to free CEL (75).
5.2 Plant-derived exosomes
With advances in assay technology, the exploration of active ingredients of natural medicines has become increasingly sophisticated with a variety of potent monomers emerging, and the recent discovery of plant-derived exosomes for their antitumor effects provides an interesting angle for research. Citrus limon-derived nanovesicles inhibit cancer cell proliferation by inducing TRAIL-mediated cell death in human lung cancer cell line A549 cells and may have a potential role in inhibiting lung cancer metastasis (76). Ginger-derived exosomes were redesigned into nanocarriers designed to deliver adriamycin to colon tumor cells, and these nanocarriers effectively incorporated into and inhibited the growth of tumor model cells without the intrinsic properties of the encapsulated adriamycin exosomes likewise inducing significant antitumor effects by a mechanism that may be related to the regulation of pro-inflammatory cytokine levels (77).
6 Concluding remarks
Exosomes have been studied in tumors at all stages of tumor diagnosis, development, progression, and prognosis. miRNAs encapsulated in exosomes are not easily degraded, making them more suitable biomarker candidates, and thus the diagnostic value of blood-derived exosomal miRNA signatures has been extensively described in lung cancer and other types of cancers (78). Tumor relatively specific non-coding RNAs (miRNA, circRNA, lncRNA), double-stranded DNA and proteins have all been found to be present in exosomes, demonstrating their potential as diagnostic biomarkers (79). Then whether some exosomes can be used as specific tumor markers for lung cancer metastasis may be of interest. In this paper, based on the fact that the formation of PMN in lung cancer is necessary for the occurrence of metastasis, and that blocking this pathway is an important strategy to improve the prognosis of lung cancer patients in the future, we explored the role of exosomes in PMN formation in lung cancer, expecting to provide a reference for the discovery of specific tumor metastasis markers.
Natural medicines are limited by their complex composition, and relevant studies mostly stay on the inhibitory effect of a particular monomer on a certain tumor and the intervention of the corresponding pathway, thus in vitro experiments tend to be more than in vivo experiments, while clinical trials are even scarcer, limiting the application of this treasure trove. However, the complex composition of natural medicines may be more likely to play a role in this complex process of PMN formation, so the potential of natural medicines to intervene in the various pathways of exosome-mediated PMN formation is explored in this paper; unfortunately, relevant studies are still scarce, but there is still evidence available that suggests to us that natural medicines modulating exosomes have potential in PMN formation in lung cancer. Another situation in which natural medicines are limited by their application is their generally low anticancer activity, poor water solubility and poor absorption (80), and it would be beneficial to enhance these capabilities for the promotion of natural medicines. In this paper, we also found that exosome-encapsulated natural products significantly enhanced their antitumor effects and utilization, which is not a promising research direction.
Combining the regulatory role of natural medicines in lung cancer exosomes and the multi-faceted intervention role of PMN, we believe that there are several directions for further expansion of applications in the future clinic. Firstly, by engineering small cell exosomes to encapsulate natural medicines components, standardized production can also enhance their effects, promote new drug development, and make up for the shortage of tumor exosome-related therapeutic tools; secondly, tumor-secreted exosomes have different tissue and organophilic properties, and different kinds of natural medicines components have similar distribution relationships, which can be used precisely to stop metastasis after detecting exosomes of certain biomarkers. Thirdly, some natural medicines have been used in long-term anti-tumor use in a fixed ratio of synergistic effect, and for PMN formation process with complex mechanism, the combination of multiple natural medicines components may achieve better clinical efficacy. The exosomes of natural medicines themselves show us the rich value of natural medicines, which are promising in human efforts to continuously overcome tumor metastasis and improve the prognosis of tumor patients.
Author contributions
JL designed the research study. X-YZ wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NO. 82074402) and the Scientific and technological innovation project of China Academy of Chinese Medical Sciences (CI2021A01802).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72:7–33. doi: 10.3322/caac.21708
3. Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) (2021) 134:783–91. doi: 10.1097/CM9.0000000000001474
4. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) (2022) 135:584–90. doi: 10.1097/CM9.0000000000002108
5. van der Weyden L, Arends MJ, Campbell AD, Bald T, Wardle-Jones H, Griggs N, et al. Genome-wide in vivo screen identifies novel host regulators of metastatic colonization. Nature (2017) 541:233–6. doi: 10.1038/nature20792
6. Ombrato L, Nolan E, Kurelac I, Mavousian A, Bridgeman VL, Heinze I, et al. Metastatic-niche labelling reveals parenchymal cells with stem features. Nature (2019) 572:603–8. doi: 10.1038/s41586-019-1487-6
7. Liu Y, Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell (2016) 30:668–81. doi: 10.1016/j.ccell.2016.09.011
8. Rahman MA, Barger JF, Lovat F, Gao M, Otterson GA, Nana-Sinkam P. Lung cancer exosomes as drivers of epithelial mesenchymal transition. Oncotarget (2016) 7:54852–66. doi: 10.18632/oncotarget.10243
9. Jiang C, Zhang N, Hu X, Wang H. Tumor-associated exosomes promote lung cancer metastasis through multiple mechanisms. Mol Cancer (2021) 20:117. doi: 10.1186/s12943-021-01411-w
10. An T, Qin S, Xu Y, Tang Y, Huang Y, Situ B, et al. Exosomes serve as tumour markers for personalized diagnostics owing to their important role in cancer metastasis. J Extracell Vesicles (2015) 4:27522. doi: 10.3402/jev.v4.27522
11. Jia Q, He Q, Yao L, Li M, Lin J, Tang Z, et al. Utilization of physiologically based pharmacokinetic modeling in pharmacokinetic study of natural medicine: an overview. Molecules (2022) 27:8670. doi: 10.3390/molecules27248670
12. Xiao H, Xue Q, Zhang Q, Li C, Liu X, Liu J, et al. How ginsenosides trigger apoptosis in human lung adenocarcinoma cells. Am J Chin Med (2019) 47:1737–54. doi: 10.1142/S0192415X19500885
13. Zhou L, Lv T, Zhang Q, Zhu Q, Zhan P, Zhu S, et al. The biology, function and clinical implications of exosomes in lung cancer. Cancer Lett (2017) 407:84–92. doi: 10.1016/j.canlet.2017.08.003
14. Thakur A, Parra DC, Motallebnejad P, Brocchi M, Chen HJ. Exosomes: Small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioact Mater (2022) 10:281–94. doi: 10.1016/j.bioactmat.2021.08.029
15. Hu C, Meiners S, Lukas C, Stathopoulos GT, Chen J. Role of exosomal microRNAs in lung cancer biology and clinical applications. Cell Prolif (2020) 53:e12828. doi: 10.1111/cpr.12828
16. Sun T, Kalionis B, Lv G, Xia S, Gao W. Role of exosomal noncoding RNAs in lung carcinogenesis. BioMed Res Int (2015) 2015:125807. doi: 10.1155/2015/125807
17. Meehan K, Vella LJ. The contribution of tumour-derived exosomes to the hallmarks of cancer. Crit Rev Clin Lab Sci (2016) 53:121–31. doi: 10.3109/10408363.2015.1092496
18. Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med (2012) 18:883–91. doi: 10.1038/nm.2753
19. Fang T, Lv H, Lv G, Li T, Wang C, Han Q, et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun (2018) 9:191. doi: 10.1038/s41467-017-02583-0
20. Li X, Wang S, Zhu R, Li H, Han Q, Zhao RC. Lung tumor exosomes induce a pro-inflammatory phenotype in mesenchymal stem cells via NFkappaB-TLR signaling pathway. J Hematol Oncol (2016) 9:42. doi: 10.1186/s13045-016-0269-y
21. Liu Y, Gu Y, Han Y, Zhang Q, Jiang Z, Zhang X, et al. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell (2016) 30:243–56. doi: 10.1016/j.ccell.2016.06.021
22. Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA (2012) 109:E2110–6. doi: 10.1073/pnas.1209414109
23. Rodrigues G, Hoshino A, Kenific CM, Matei IR, Steiner L, Freitas D, et al. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat Cell Biol (2019) 21:1403–12. doi: 10.1038/s41556-019-0404-4
24. Li Z, Zeng C, Nong Q, Long F, Liu J, Mu Z, et al. Exosomal leucine-rich-alpha2-glycoprotein 1 derived from non-small-cell lung cancer cells promotes angiogenesis via TGF-beta signal pathway. Mol Ther Oncolytics (2019) 14:313–22. doi: 10.1016/j.omto.2019.08.001
25. Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC, Tsai PH, et al. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene (2017) 36:4929–42. doi: 10.1038/onc.2017.105
26. Liu Y, Luo F, Wang B, Li H, Xu Y, Liu X, et al. STAT3-regulated exosomal miR-21 promotes angiogenesis and is involved in neoplastic processes of transformed human bronchial epithelial cells. Cancer Lett (2016) 370:125–35. doi: 10.1016/j.canlet.2015.10.011
27. Cui H, Seubert B, Stahl E, Dietz H, Reuning U, Moreno-Leon L, et al. Tissue inhibitor of metalloproteinases-1 induces a pro-tumourigenic increase of miR-210 in lung adenocarcinoma cells and their exosomes. Oncogene (2015) 34:3640–50. doi: 10.1038/onc.2014.300
28. Wang Z, Zhu J, Liu Y, Wang Z, Cao X, Gu Y. Tumor-polarized GPX3(+) AT2 lung epithelial cells promote premetastatic niche formation. Proc Natl Acad Sci USA (2022) 119:e2201899119. doi: 10.1073/pnas.2201899119
29. Du C, Duan X, Yao X, Wan J, Cheng Y, Wang Y, et al. Tumour-derived exosomal miR-3473b promotes lung tumour cell intrapulmonary colonization by activating the nuclear factor-kappaB of local fibroblasts. J Cell Mol Med (2020) 24:7802–13. doi: 10.1111/jcmm.15411
30. Jin J, Yu G. Hypoxic lung cancer cell-derived exosomal miR-21 mediates macrophage M2 polarization and promotes cancer cell proliferation through targeting IRF1. World J Surg Oncol (2022) 20:241. doi: 10.1186/s12957-022-02706-y
31. Liang M, Chen X, Wang L, Qin L, Wang H, Sun Z, et al. Cancer-derived exosomal TRIM59 regulates macrophage NLRP3 inflammasome activation to promote lung cancer progression. J Exp Clin Cancer Res (2020) 39:176. doi: 10.1186/s13046-020-01688-7
32. Huang SH, Li Y, Zhang J, Rong J, Ye S. Epidermal growth factor receptor-containing exosomes induce tumor-specific regulatory T cells. Cancer Invest (2013) 31:330–5. doi: 10.3109/07357907.2013.789905
33. Ning Y, Shen K, Wu Q, Sun X, Bai Y, Xie Y, et al. Tumor exosomes block dendritic cells maturation to decrease the T cell immune response. Immunol Lett (2018) 199:36–43. doi: 10.1016/j.imlet.2018.05.002
34. Sun Y, Guo J, Yu L, Guo T, Wang J, Wang X, et al. PD-L1(+) exosomes from bone marrow-derived cells of tumor-bearing mice inhibit antitumor immunity. Cell Mol Immunol (2021) 18:2402–9. doi: 10.1038/s41423-020-0487-7
35. Kim DH, Kim H, Choi YJ, Kim SY, Lee JE, Sung KJ, et al. Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp Mol Med (2019) 51:1–13. doi: 10.1038/s12276-019-0295-2
36. Morrissey SM, Zhang F, Ding C, Montoya-Durango DE, Hu X, Yang C, et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab (2021) 33:2040–2058 e10. doi: 10.1016/j.cmet.2021.09.002
37. Hu W, Ru Z, Xiao W, Xiong Z, Wang C, Yuan C, et al. Adipose tissue browning in cancer-associated cachexia can be attenuated by inhibition of exosome generation. Biochem Biophys Res Commun (2018) 506:122–9. doi: 10.1016/j.bbrc.2018.09.139
38. Tang YT, Huang YY, Li JH, Qin SH, Xu Y, An TX, et al. Alterations in exosomal miRNA profile upon epithelial-mesenchymal transition in human lung cancer cell lines. BMC Genomics (2018) 19:802. doi: 10.1186/s12864-018-5143-6
39. You J, Li M, Cao LM, Gu QH, Deng PB, Tan Y, et al. Snail1-dependent cancer-associated fibroblasts induce epithelial-mesenchymal transition in lung cancer cells via exosomes. QJM (2019) 112:581–90. doi: 10.1093/qjmed/hcz093
40. Liu J, Li S, Fei X, Nan X, Shen Y, Xiu H, et al. Increased alveolar epithelial TRAF6 via autophagy-dependent TRIM37 degradation mediates particulate matter-induced lung metastasis. Autophagy (2022) 18:971–89. doi: 10.1080/15548627.2021.1965421
41. Zhang R, Dong Y, Sun M, Wang Y, Cai C, Zeng Y, et al. Tumor-associated inflammatory microenvironment in non-small cell lung cancer: correlation with FGFR1 and TLR4 expression via PI3K/Akt pathway. J Cancer (2019) 10:1004–12. doi: 10.7150/jca.26277
42. Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature (2015) 527:329–35. doi: 10.1038/nature15756
43. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov (2022) 12:31–46. doi: 10.1158/2159-8290.CD-21-1059
44. Lambrechts D, Wauters E, Boeckx B, Aibar S, Nittner D, Burton O, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med (2018) 24:1277–89. doi: 10.1038/s41591-018-0096-5
45. Conway EM, Pikor LA, Kung SH, Hamilton MJ, Lam S, Lam WL, et al. Macrophages, inflammation, and lung cancer. Am J Respir Crit Care Med (2016) 193:116–30. doi: 10.1164/rccm.201508-1545CI
46. Chen XW, Yu TJ, Zhang J, Li Y, Chen HL, Yang GF, et al. CYP4A in tumor-associated macrophages promotes pre-metastatic niche formation and metastasis. Oncogene (2017) 36:5045–57. doi: 10.1038/onc.2017.118
47. Luo X, Zhao X, Cheng C, Li N, Liu Y, Cao Y. The implications of signaling lipids in cancer metastasis. Exp Mol Med (2018) 50:1–10. doi: 10.1038/s12276-018-0150-x
48. Tucci M, Mannavola F, Passarelli A, Stucci LS, Cives M, Silvestris F. Exosomes in melanoma: a role in tumor progression, metastasis and impaired immune system activity. Oncotarget (2018) 9:20826–37. doi: 10.18632/oncotarget.24846
49. Menck K, Sonmezer C, Worst TS, Schulz M, Dihazi GH, Streit F, et al. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J Extracell Vesicles (2017) 6:1378056. doi: 10.1080/20013078.2017.1378056
50. Pan J, Li M, Yu F, Zhu F, Wang L, Ning D, et al. Up-regulation of p53/miR-628-3p pathway, a novel mechanism of shikonin on inhibiting proliferation and inducing apoptosis of A549 and PC-9 non-small cell lung cancer cell lines. Front Pharmacol (2021) 12:766165. doi: 10.3389/fphar.2021.766165
51. Wei Y, Li M, Cui S, Wang D, Zhang CY, Zen K, et al. Shikonin inhibits the proliferation of human breast cancer cells by reducing tumor-derived exosomes. Molecules (2016) 21:777. doi: 10.3390/molecules21060777
52. Xia X, Wang X, Zhang S, Zheng Y, Wang L, Xu Y, et al. miR-31 shuttled by halofuginone-induced exosomes suppresses MFC-7 cell proliferation by modulating the HDAC2/cell cycle signaling axis. J Cell Physiol (2019) 234:18970–84. doi: 10.1002/jcp.28537
53. Que ZJ, Luo B, Wang CT, Qian FF, Jiang Y, Li Y, et al. Proteomics analysis of tumor exosomes reveals vital pathways of Jinfukang inhibiting circulating tumor cells metastasis in lung cancer. J Ethnopharmacol (2020) 256:112802. doi: 10.1016/j.jep.2020.112802
54. Tang X, Pan L, Zhao S, Dai F, Chao M, Jiang H, et al. SNO-MLP (S-nitrosylation of muscle LIM protein) facilitates myocardial hypertrophy through TLR3 (Toll-like receptor 3)-mediated RIP3 (Receptor-interacting protein kinase 3) and NLRP3 (NOD-like receptor pyrin domain containing 3) inflammasome activation. Circulation (2020) 141:984–1000. doi: 10.1161/CIRCULATIONAHA.119.042336
55. Sharma BR, Kanneganti TD. NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol (2021) 22:550–9. doi: 10.1038/s41590-021-00886-5
56. Bai L, Li J, Li H, Song J, Zhou Y, Lu R, et al. Renoprotective effects of artemisinin and hydroxychloroquine combination therapy on IgA nephropathy via suppressing NF-kappaB signaling and NLRP3 inflammasome activation by exosomes in rats. Biochem Pharmacol (2019) 169:113619. doi: 10.1016/j.bcp.2019.08.021
57. Li H, Lu R, Pang Y, Li J, Cao Y, Fu H, et al. Zhen-Wu-Tang protects igA nephropathy in rats by regulating exosomes to inhibit NF-κB/NLRP3 pathway. Front Pharmacol (2020) 11:1080. doi: 10.3389/fphar.2020.01080
58. Ku WC, Sridharan B, Chen JY, Li JY, Yang SY, Lee MJ. Kaempferitrin-treated hepG2 differentially expressed exosomal markers and affect extracellular vesicle sizes in the secretome. Biomolecules (2021) 11:187. doi: 10.3390/biom11020187
59. Wu XM, Gao YB, Xu LP, Zou DW, Zhu ZY, Wang XL, et al. Tongxinluo Inhibits Renal Fibrosis in Diabetic Nephropathy: Involvement of the Suppression of Intercellular Transfer of TGF-[Formula: see text]1-Containing Exosomes from GECs to GMCs. Am J Chin Med (2017) 45:1075–92. doi: 10.1142/S0192415X17500586
60. Du X, Wang JN, Sun J, Wu T, Cao XQ, Liu LY, et al. Steroidal glycoalkaloids from Solanum lyratum inhibit the pro-angiogenic activity of A549-derived exosomes. Fitoterapia (2020) 141:104481. doi: 10.1016/j.fitote.2020.104481
61. Zhai K, Duan H, Wang W, Zhao S, Khan GJ, Wang M, et al. Ginsenoside Rg1 ameliorates blood-brain barrier disruption and traumatic brain injury via attenuating macrophages derived exosomes miR-21 release. Acta Pharm Sin B (2021) 11:3493–507. doi: 10.1016/j.apsb.2021.03.032
62. Chen C, Yao X, Xu Y, Zhang Q, Wang H, Zhao L, et al. Dahuang Zhechong Pill suppresses colorectal cancer liver metastasis via ameliorating exosomal CCL2 primed pre-metastatic niche. J Ethnopharmacol (2019) 238:111878. doi: 10.1016/j.jep.2019.111878
63. Bi S, Huang W, Chen S, Huang C, Li C, Guo Z, et al. Cordyceps militaris polysaccharide converts immunosuppressive macrophages into M1-like phenotype and activates T lymphocytes by inhibiting the PD-L1/PD-1 axis between TAMs and T lymphocytes. Int J Biol Macromol (2020) 150:261–80. doi: 10.1016/j.ijbiomac.2020.02.050
64. Dai Y, Liu Y, Li J, Jin M, Yang H, Huang G. Shikonin inhibited glycolysis and sensitized cisplatin treatment in non-small cell lung cancer cells via the exosomal pyruvate kinase M2 pathway. Bioengineered (2022) 13:13906–18. doi: 10.1080/21655979.2022.2086378
65. Xie CF, Feng KL, Wang JN, Luo R, Fang CK, Zhang Y, et al. Jianpi Huayu decoction inhibits the epithelial-mesenchymal transition of hepatocellular carcinoma cells by suppressing exosomal miR-23a-3p/Smad signaling. J Ethnopharmacol (2022) 294:115360. doi: 10.1016/j.jep.2022.115360
66. Surman M, Drozdz A, Stepien E, Przybylo M. Extracellular vesicles as drug delivery systems - methods of production and potential therapeutic applications. Curr Pharm Des (2019) 25:132–54. doi: 10.2174/1381612825666190306153318
67. Gaurav I, Thakur A, Iyaswamy A, Wang X, Chen X, Yang Z. Factors affecting extracellular vesicles based drug delivery systems. Molecules (2021) 26:1544. doi: 10.3390/molecules26061544
68. Dai X, Ye Y, He F. Emerging innovations on exosome-based onco-therapeutics. Front Immunol (2022) 13:865245. doi: 10.3389/fimmu.2022.865245
69. Fan M, Sun W, Gu X, Lu S, Shen Q, Liu X, et al. The critical role of STAT3 in biogenesis of tumor-derived exosomes with potency of inducing cancer cachexia in vitro and in vivo. Oncogene (2022) 41:1050–62. doi: 10.1038/s41388-021-02151-3
70. Yang CL, Liu YY, Ma YG, Xue YX, Liu DG, Ren Y, et al. Curcumin blocks small cell lung cancer cells migration, invasion, angiogenesis, cell cycle and neoplasia through Janus kinase-STAT3 signalling pathway. PloS One (2012) 7:e37960. doi: 10.1371/journal.pone.0037960
71. Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther (2010) 18:1606–14. doi: 10.1038/mt.2010.105
72. Song H, Liu B, Dong B, Xu J, Zhou H, Na S, et al. Exosome-based delivery of natural products in cancer therapy. Front Cell Dev Biol (2021) 9:650426. doi: 10.3389/fcell.2021.650426
73. Wu H, Zhou J, Zeng C, Wu D, Mu Z, Chen B, et al. Curcumin increases exosomal TCF21 thus suppressing exosome-induced lung cancer. Oncotarget (2016) 7:87081–90. doi: 10.18632/oncotarget.13499
74. Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics (2021) 11:3183–95. doi: 10.7150/thno.52570
75. Aqil F, Kausar H, Agrawal AK, Jeyabalan J, Kyakulaga AH, Munagala R, et al. Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Exp Mol Pathol (2016) 101:12–21. doi: 10.1016/j.yexmp.2016.05.013
76. Raimondo S, Naselli F, Fontana S, Monteleone F, Lo Dico A, Saieva L, et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget (2015) 6:19514–27. doi: 10.18632/oncotarget.4004
77. Zhang M, Xiao B, Wang H, Han MK, Zhang Z, Viennois E, et al. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol Ther (2016) 24:1783–96. doi: 10.1038/mt.2016.159
78. Drula R, Ott LF, Berindan-Neagoe I, Pantel K, Calin GA. MicroRNAs from liquid biopsy derived extracellular vesicles: recent advances in detection and characterization methods. Cancers (Basel) (2020) 12:2009. doi: 10.3390/cancers12082009
79. Yang X, Zhang Y, Zhang Y, Zhang S, Qiu L, Zhuang Z, et al. The key role of exosomes on the pre-metastatic niche formation in tumors. Front Mol Biosci (2021) 8:703640. doi: 10.3389/fmolb.2021.703640
Keywords: lung cancer, pre-metastatic niche, natural medicines, exosomes, anti-cancer
Citation: Zhu X-y and Li J (2023) Potential targets of natural medicines: preventing lung cancer pre-metastatic niche formation by regulating exosomes. Front. Oncol. 13:1137007. doi: 10.3389/fonc.2023.1137007
Received: 03 January 2023; Accepted: 11 August 2023;
Published: 28 August 2023.
Edited by:
Jianhui Tian, Shanghai Municipal Hospital of Traditional Chinese Medicine, ChinaReviewed by:
Soumya Basu, Dr. D. Y. Patil Biotechnology & Bioinformatics Institute, IndiaErxi Wu, Baylor Scott and White Health, United States
Copyright © 2023 Zhu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Li, cWZtMjAyMGppZWxpQHllYWgubmV0
 Xiao-yu Zhu
Xiao-yu Zhu Jie Li
Jie Li