
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 19 June 2023
Sec. Breast Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1136380
This article is part of the Research Topic Overcoming Resistance to Systemic Therapy in Breast Cancer View all 13 articles
 Xiaoyu Liu1†
Xiaoyu Liu1† Peng Zhang2†
Peng Zhang2† Chao Li3
Chao Li3 Xiang Song1,3
Xiang Song1,3 Zhaoyun Liu3
Zhaoyun Liu3 Wenna Shao1
Wenna Shao1 Sumei Li4
Sumei Li4 Xinzhao Wang3,5*
Xinzhao Wang3,5* Zhiyong Yu1,3*
Zhiyong Yu1,3*Background: Inetetamab (cipterbin) is an innovative anti-HER2 humanized monoclonal antibody. The efficacy and safety of a combination of inetetamab and vinorelbine in the first-line treatment of human epidermal receptor positive (HER2+) metastatic breast cancer (MBC) have been confirmed. We aimed to investigate real-world data of inetetamab in complex clinical practice.
Methods: We retrospectively reviewed the medical records of patients who received inetetamab as a salvage treatment at any line setting from July 2020 to June 2022. The main endpoint was progression‐free survival (PFS).
Results: A total of 64 patients were included in this analysis. The median progression‐free survival (mPFS) was 5.6 (4.6–6.6) months. Of the patients, 62.5% received two or more lines of therapy before treatment with inetetamab. The most common chemotherapy and anti-HER2 regimens combined with inetetamab were vinorelbine (60.9%) and pyrotinib (62.5%), respectively. Patients treated with inetetamab plus pyrotinib plus vinorelbine benefited the most (p=0.048), with the mPFS of 9.3 (3.1–15.5) months and an objective response rate of 35.5%. For patients with pyrotinib pretreatment, inetetamab plus vinorelbine plus pyrotinib agents resulted in mPFS of 10.3 (5.2–15.4) months. Regimens (inetetamab plus vinorelbine plus pyrotinib vs. other therapeutic agents) and visceral metastases (yes vs. no) were independent predictors of PFS. Patients with visceral metastases treated with inetetamab plus vinorelbine plus pyrotinib had a mPFS of 6.1(5.1–7.1) months. The toxicity of inetetamab was tolerable, with the most common grade 3/4 adverse event being leukopenia (4.7%).
Conclusions: HER2+ MBC patients pretreated with multiple-line therapies still respond to inetetamab-based treatment. Inetetamab combined with vinorelbine and pyrotinib may be the most effective treatment regimen, with a controllable and tolerable safety profile.
Breast cancer(BC) is the most common cancer and a leading cause of death among women worldwide (1). There are three major breast cancer subtypes: hormone receptor positive(estrogen receptor (ER) positive or progesterone receptor(PR) positive), human epidermal receptor 2 (HER2) positive (HER2+) and triple negative breast cancer (ER negative, PR negative, HER2 negative) (2). HER2 is a transmembrane receptor tyrosine kinase in the epidermal growth factor receptor family that is amplified or overexpressed in approximately 20% of breast cancers, and is associated with poor prognosis in the absence of systemic therapy (3). HER2+ ductal tumors are associated with the presence of calcifications, as well as high tumor grade and increased likelihood of spread to the lymph nodes (4, 5). Without the development and widespread use of anti‐HER2‐targeted drugs, HER2+ BC is an aggressive disease and has poor prognosis (6). Although huge progresses have been achieved in the last few years in understanding and treating HER2+ breast cancer, they remain a disproportionate health burden to patients and huge unmet need (7). Especially, HER2+ metastatic breast cancer(MBC) remains incurable, and novel treatment options are needed. Many anti‐HER2‐targeted drugs have been applied successfully in clinical or currently under review in recent years. Antibody–drug conjugates(ADC) drugs are on rise and provides novel therapeutic advancements in the management of HER2+ MBC (8). But in China, they are expensive and not included in medical insurance which limited their usage.
The innovative drug of recombinant anti-HER2 humanized monoclonal antibody (inetetamab, Cipterbin) for injection independently researched and developed in China is a non-biological analog drug produced by Sansheng Guojian Pharmaceutical (Shanghai) Co., Ltd. (formerly CITIC Guojian Pharmaceutical Co., Ltd.) and approved by the State Food and Drug Administration of China for clinical research on July 2, 2004 (Approval No. 2004L02352). Inetetamab is a monoclonal antibody binding to domain IV of HER2 receptor. The Fab domain of inetetamab is identical with trastuzumab, but whose amino acid sequence at positions 359 (D359, aspartic acid) and 361 (L361, leucine) is different from trastuzumab (E359 (glutamate) and M361 (methionine), respectively) in the constant region of the heavy chain of the Fc domain (9). Previous study confirmed the significant efficacy and good safety of the combination of cipterbin and vinorelbine in the first-line treatment of HER2 positive advanced breast cancer patients who had not received anti-HER2-targeted therapy after previous taxus treatment (10).
Based on the current situation of clinical treatment and needs, we conducted a retrospective study to fill a knowledge gap by investigating the efficacy of inetetamab for HER2+ recurrent and metastatic breast cancer patients pretreated with multi-lines treatment.
This retrospective, single-center study enrolled patients with HER2+ MBC treated with inetetamab at Shandong Frist Medical University and Shandong Academy of Medical Sciences between July 2020 and June 2022. The Ethics Committee and Institutional Review Board of Shandong First Medical University and Shandong Academy of Medical Sciences approved this study (SDTHEC2022012020). All investigations were conducted in accordance with the Declaration of Helsinki.
The inclusion criteria for participants were as follows: female sex, age ≥18 years, histologically or cytologically confirmed MBC with documentation of HER2 overexpression, prior trastuzumab therapy with or without other HER2-targeted treatment, at least one cycle of inetetamab, and complete medical records. The exclusion criteria were non-measurable or non-evaluable lesions and those lost to follow-up. There were no limits to the number of prior cytotoxic regimens for metastatic diseases. The last follow-up was conducted in November 2022. Until the last follow-up date, patients who were lost to follow-up were considered as censored data. All data were retrospectively collected from medical records and laboratory results. Patients or their family members (for patients who already died at the study initiation) provided signed informed consent or oral agreement with tape recording.
The characteristics of the patients at the time of initial diagnosis (including age, ECOG performance status, and menstrual status), tumor characteristics (including tumor size, lymph node involvement, grade, histology, and receptor status), treatment regimen in the (neo)adjuvant and metastatic settings (including chemotherapy, anti-HER2, endocrine regimen, surgery, radiotherapy, dose reductions or delays, etc.) were extracted from electronic medical records. Hormone receptor (HR) was defined as estrogen receptor (ER) and/or progesterone receptor (PR) positivity (ER and PR were determined by at least 10% of positively stained nuclei). HER2 positivity was defined as an immunohistochemistry(IHC) score of 3+ or 2+ together with HER2 gene amplification verified by fluorescence in situ hybridization(FISH+). Disease-free interval (DFI) was defined as the time from surgery to diagnosis of metastasis.
Clinical response was evaluated using computed tomography, magnetic resonance imaging, and physical examination according to the Response Evaluation Criteria in Solid Tumors, version 1.1. The main endpoint was progression-free survival (PFS), defined as the time from treatment initiation until disease progression or death. Other endpoints included the objective response rate (ORR), clinical benefit rate (CBR), and safety. ORR was defined as the proportion of patients who achieved a complete response (CR) or partial response (PR). CBR was defined as the proportion of patients who achieved CR, PR, or stable disease (SD). Adverse events (AEs) were graded based on the National Cancer Institute Common Terminology Criteria for AEs, version 4.0.
The median (range) or percentage of patients was used to represent the clinicopathological characteristics. Continuous variables were analyzed by One-way ANOVA. Categorical variables were assessed by the Pearson’s chi-squared test or Fisher’s exact test. The Kaplan–Meier method was used to estimate PFS. Additionally, Cox univariable model was employed to assess the covariate effects on PFS, and then Cox multivariate models were used to assess the factors with relative significant p-values(p ≤ 0.1) in univariate analysis to PFS with hazard ratios(HR.) and corresponding 95% confidence intervals (CIs). Statistical significance for all analyses was set at p<0.05. GraphPad Prism 9.3.1 software was used to perform all statistical analyses.
A total of 69 patients with HER2+ MBC treated with inetetamab were recruited. After considering the exclusion criteria, 64 (92.8%) patients were included in the study (Figure 1).
The baseline characteristics are presented in Table 1. The median age of the patients at diagnosis was 46 years (range: 27–67 years), 54 (84.4%) underwent surgery, 14 patients (21.9%) had stage IV BC as their first diagnosis. Moreover, 53.1% of the patients had more than two metastatic sites, with the four most common metastatic sites being the lymph node (48.4%), local sites (35.9%), bone (32.8%), and liver (32.8%). Half of the patients had visceral metastases, whereas 13 (20.3%) had brain metastases. All patients had been exposed to anti-HER2 therapy, with 64.1% prescribed pyrotinib and 18.8% with lapatinib. More than four-fifths of the patients received trastuzumab during salvage treatment. Furthermore, 62.5% of patients received two or more lines of systemic therapy before inetetamab. These results suggest that, in a real-world setting, patients receiving inetetamab are more likely to be heavily pretreated.
The treatment regimens are shown in Table 2. Most patients were treated with inetetamab in combination with chemotherapy and/or other HER2-targeted therapies. The most common chemotherapy regimens were vinorelbine (n = 39, 60.9%) and abraxane (n = 15, 23.4%). Pyrotinib and inetetamab in combination were administered to 40 (62.5%) patients. Meanwhile, three (3.1%) patients received inetetamab and brain radiotherapy but did not receive any other anti-cancer drugs.
All patients were evaluated for PFS. The median follow-up time was 14.3(12.7–15.9) months. The median progression-free survival (mPFS) was 5.6 (4.6–6.6) months and the ORR was 26.6% (Figure 2A).
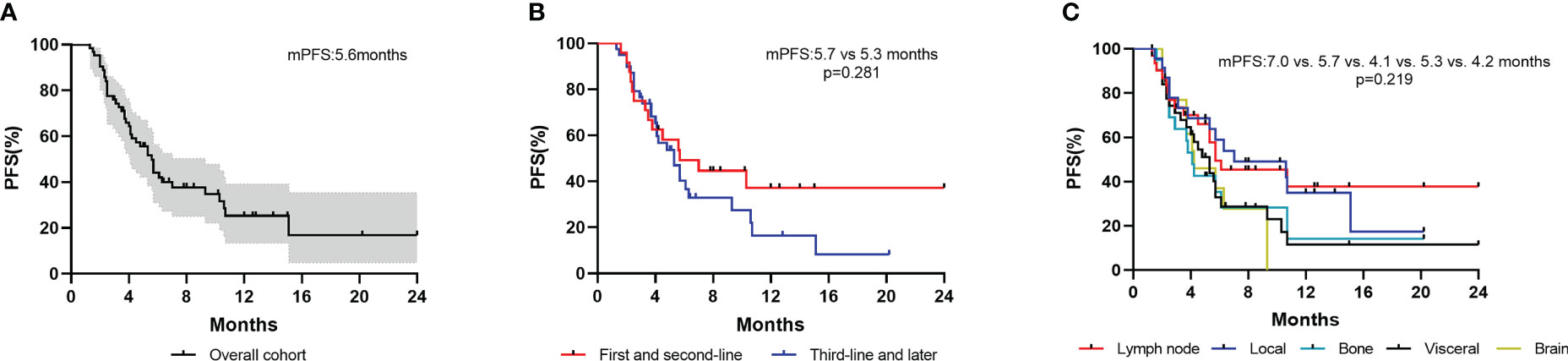
Figure 2 Kaplan–Meier curves of PFS for patients. (A) Overall cohort; (B) Patients stratified by treatment lines; (C) Patients with different metastatic sites.
Patients who received inetetamab‐based therapy as first and later lines of metastatic treatment had a median PFS of 5.7 (1.9–9.5) and 5.3 (3.8–6.8) months, respectively (Figure 2B). Thirty-two patients with visceral metastases showed a median PFS of 5.3 (3.9–6.7) months. A total of 31 patients with lymph node metastases, 23 patients with local metastases, 21 patients with bone metastases and 13 patients with brain metastases had median PFS of 5.7 (0.5–10.9) months, 7.0 (0.8–13.2) months, 4.1(3.4–4.8) months and 4.2 (2.0–6.4) months, respectively (Figure 2C).
To determine the best combination for inetetamab, we firstly investigated the anti-HER2 treatment in the overall cohort. Baseline characteristics were analyzed in Supplementary Tables 1-3. The median PFSs among inetetamab plus pyrotinib, inetetamab plus pertuzumab and inetetamab alone were 6.1 (2.5–9.7) months, 2.5 (1.9–3.1) months, and 5.3 (4.6–6.6) months, respectively. Inetetamab plus pyrotinib was the best combination among the three groups (p=0.005) (Figure 3A). However, the age of patients treated with inetetamab plus pertuzumab is relatively old than other two cohorts(p=0.001) and 6 patients(100%) received at least 3 lines of rescue treatment(p=0.016), which led to the unbalanced baseline characteristics among three cohorts. As vinorelbine and abraxane were the most common combined cytotoxic drugs, we compared the PFS of different chemotherapies. The median PFSs among inetetamab plus vinorelbine, inetetamab plus abraxane and inetetamab plus other therapeutic agents were 5.7 (3.7-7.7) months, 5.7 (4.4–7.0) months, and 4.0 (1.4–6.6) months, respectively (Figure 3B). Furthermore, we compared the efficacy of the combination treatments. The median PFS of inetetamab plus pyrotinib plus vinorelbine was 9.3 (3.1–15.5) months; inetetamab plus pyrotinib plus abraxane, 5.6(0-13.6) months; and inetetamab plus other therapeutic agents, 4.1 (3.0–5.2) months. There were statistically significant differences among the three groups (p = 0.048) (Figure 3C). These findings indicate that inetetamab plus pyrotinib plus vinorelbine may be the most effective inetetamab-based regimen. Thirty-one (48.4%) patients received inetetamab plus pyrotinib plus vinorelbine. The subgroup of patients achieved an ORR of 35.5% and CBR of 48.4%, with CR achieved in three patients, PR achieved in eight patients, and SD achieved in four patients (Figure 3C).
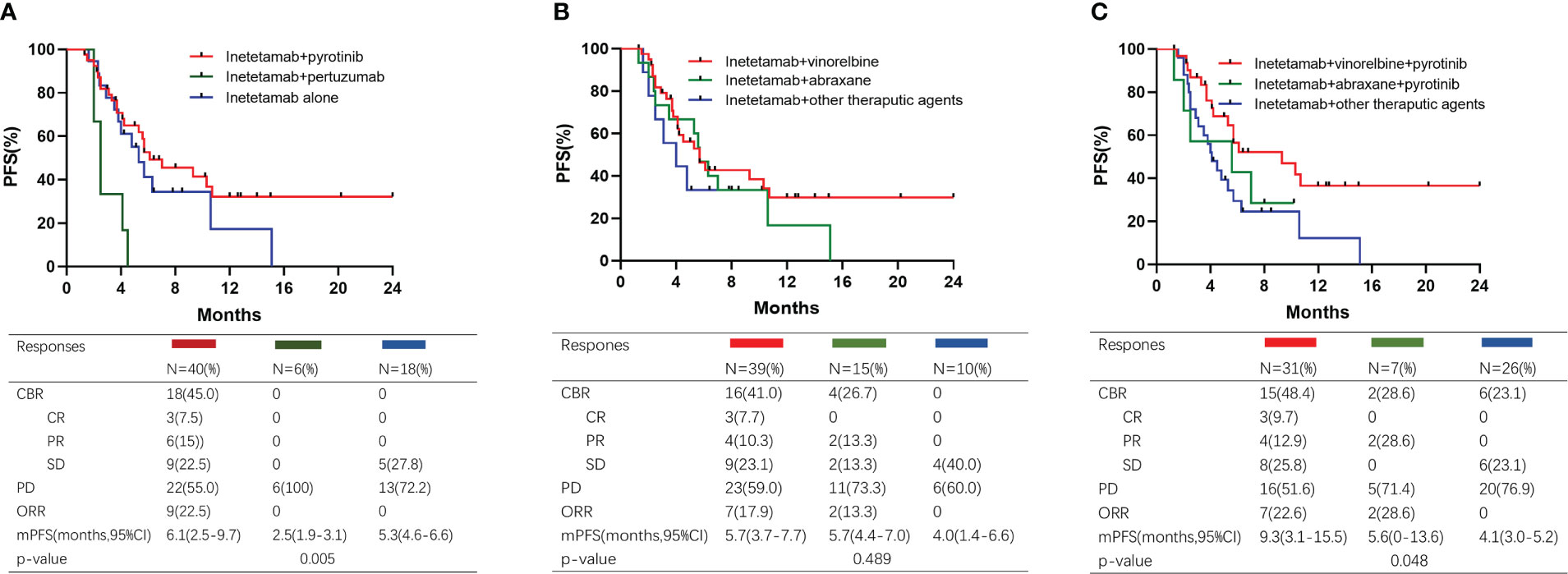
Figure 3 Kaplan–Meier curves of PFS and responses for different treatment. (A) Patients treated with different dual anti-HER2 therapy; (B) Patients treated with different chemotherapy; (C) Patients treated with different combined regimens.
Thirty-five patients received vinorelbine before inetetamab-based therapy. The mPFS of patients with versus without vinorelbine pretreatment was 5.7 (4.0–7.4) months versus 5.6 (3.5–7.7) months, respectively (p=0.750) (Figure 4A). Forty-one patients received pyrotinib before inetetamab‐based therapy. The mPFS of patients with versus without pyrotinib pretreatment was 5.7 (3.5–7.9) months versus 5.3 (3.3–7.3) months, respectively (p=0.988) (Figure 4B). These results indicate that the medication history of vinorelbine and/or pyrotinib had no influence on the efficacy of the drug.
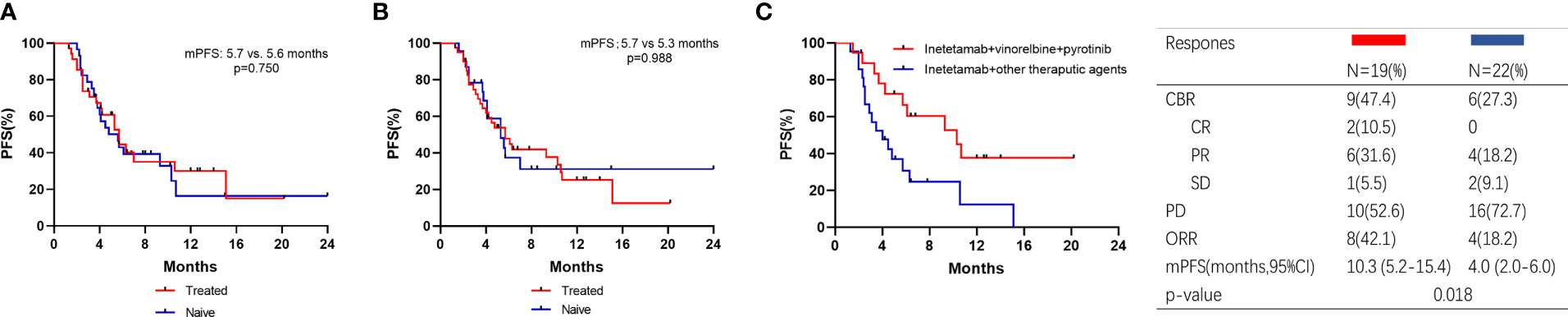
Figure 4 Kaplan–Meier curves of PFS for patients and responses. (A) Patients with vinorelbine‐treated or vinorelbine‐naive; (B) Patients with pyrotinib‐treated or pyrotinib‐naive; (C) Patients who were pyrotinib‐treated received inetetamab + vinorelbine + pyrotinib or other therapeutic agents.
We also analyzed the PFS of the patients pretreated with pyrotinib (Figure 4C). A total of 41 patients were included in this subgroup analysis, with an ORR of 29.3%. Two patients achieved CR and 10 patients achieved PR. Patients exposed to inetetamab plus vinorelbine plus pyrotinib agent had significantly longer PFS (10.3 (5.2–15.4) months) than those exposed to other therapeutic agents (4.0 (2.0–6.0) months) (p=0.018).
The univariate analysis indicated that age group (<40 vs. ≥40 years), menstrual status (pre vs. post), hormone receptor status (negative vs. positive), and regimens (inetetamab plus vinorelbine plus pyrotinib vs. other therapeutic agents) were correlated with PFS (p<0.05). Next, we constructed a multivariate model with the above factors, ECOG performance status(0-1 vs ≥2) and visceral metastasis (yes vs. no) as covariates for PFS (Table 3). After adjustment, Cox multivariate regression analysis showed that the regimens (inetetamab plus vinorelbine plus pyrotinib vs. other therapeutic agents) and visceral metastasis (yes vs. no) were independent predictors of PFS (Table 3).
Thirty-two patients (50.0%) exhibited visceral metastasis. Patients with and without visceral metastases had PFS times of 5.3 months and 7.0 months, respectively (Figure 5A). Of the 32 patients, 18 received inetetamab plus vinorelbine plus pyrotinib treatment, with an ORR of 27.8% and a CBR of 33.3%. One patient achieved CR, four achieved PR, and one achieved SD. The median PFS was significantly different for patients who underwent inetetamab plus vinorelbine plus pyrotinib or other therapeutic agents (6.1 (5.1–7.1) vs. 2.9-(0.9–4.9) months, p=0.002; Figure 5B).
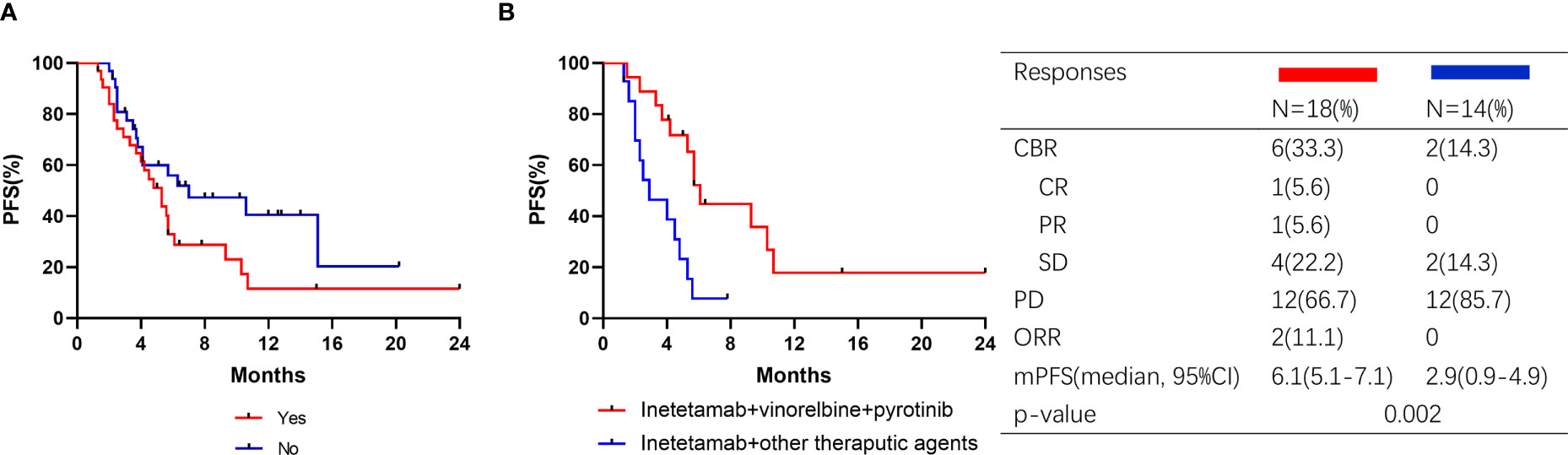
Figure 5 Kaplan–Meier curves of PFS for patients and responses in subgroup. (A) Patients with visceral metastasis or not; (B) Visceral metastasis patients treated with pyrotinib or pyrotinib + trastuzumab.
The safety assessments of etamab-based therapy are listed in Table 4. After the initial etamab-based therapy, 10 (15.6%) patients in the inetetamab group experienced a dose reduction, and two (3.1%) patients interrupted the treatment. The most common grade 3/4 AEs were leukopenia (4.7%) and neutropenia (3.1%). No treatment-related deaths were reported. Overall, the results show that the safety of etamab-based therapy is controllable and tolerable.
This study revealed the real-world clinical practice of inetetamab in HER2+ MBC patients after trastuzumab-based treatment. Previously, the efficacy and safety of inetetamab in combination with chemotherapy as first-line treatment of HER2+ MBC was evaluated (9). But the above study of inetetamab was designed for patients who did not receive any anti-HER2 drugs. Therefore, the role of inetetamab in more heavily treated patients needs further study. To the best of our knowledge, this is the first investigation of the effectiveness of inetetamab in HER2+ MBC patients pretreated with multiline anti-HER2 treatment. Our cohort represented the general population of patients with HER2+ MBC who were usually heavily treated with multiple anti-HER2 agents. Yet, our cohort included a low percentage of patients receiving TDM1(3.1%) and no patient receiving new drugs such as Trastuzumab Deruxtecan (T-DXd) or Tucatinib, which limited our research.
The combination of inetetamab, pyrotinib and vinorelbine, as evidence-based, trustworthy and promising drugs, play a synergistic role in efficacy. Pyrotinib, a small-molecule irreversible tyrosine kinase inhibitor(TKI), has attracted much attention due to its unique properties in recent years. According to the National Comprehensive Cancer Network guidelines, pyrotinib is a valid treatment option. A number of reports have verified the therapeutic efficacy of pyrotinib in HER2+ MBC. Several multicenter analyses showed that pyrotinib treatment led to a mPFS time of about 8 months (11, 12) the ORR of 17.1% in two or later line therapy (13). Besides, the clinical benefits and safety of dual HER2 blockade by anti-HER2 monoclonal antibody plus TKI for patients that had progressed during trastuzumab-based treatment regimens were confirmed (14–16). Thus, inetetamab, as an identical monoclonal antibody with trastuzumab, combined with pyrotinib led to a satisfactory efficacy. On the other hand, vinorelbine is a semi-synthetic, antimitotic, microtubule destabilizing drug that has been shown to be effective and well-tolerated for the treatment of MBC (17). It is noteworthy that compared with other chemotherapy drugs, the combined index CI of vinorelbine and trastuzumab was only 0.34 (18–20). It is suggested that the combination of vinorelbine and anti-HER2 monoclonal antibody has the best synergistic effect. In first-line treatment, the combination of vinorelbine with trastuzumab and pertuzumab reached the mPFS of 14.2 months, indicated that vinorelbine plus dual anti-HER2 therapy showed successful anti-tumor activity and few adverse effects (21, 22).. A retrospective study reported that the mPFS of patients treated with metronomic vinorelbine and triweekly trastuzumab was 8.9 months (23). Two multicenter retrospective studies showed pyrotinib plus vinorelbine therapy had promising efficacy and tolerable toxicity in HER2+ MBC, with mPFS of 7.8 and 8.3 months, respectively (24, 25). In addition, pyrotinib combined with vinorelbine in HER2+ MBC was effective regardless of resistant status of trastuzumab (24, 25).
Considering that inetetamab is similar to trastuzumab (9), the combination of inetetamab and trastuzumab should have a good efficacy in metastatic setting. But the small sample data resulted in some analysis biases and the inability to conduct depth analysis. Despite the combination of inetetamab plus pyrotinib plus vinorelbine showed satisfactory outcomes, which was comparable to the mPFS of 9.6 months in the EMILIA study (26), there is a significant gap compared to T-DXd(mPFS=28.8 months) according to the updated results from DESTINY-Breast03 trial (27). Notwithstanding, high prices of TDM1 and T-DXd results in limitations in the ability to use in clinical practice. Whereas, inetetamab plus pyrotinib plus vinorelbine can be considered as an alternative treatment option.
Brain and visceral metastases have poor prognosis and limit treatment for HER2+ MBC (3, 28). For patients with visceral metastasis, the outcomes of the combination regimen are inferior to that reported for pyrotinib-based regimens in the previous multicenter retrospective study (24, 29–32). The reason might be in our study, patients were less sensitive to anti-HER2 treatment after multi-lines treatment, especially after pyrotinib-based treatment. Despite mounting evidence verified that pyrotinib-based combination therapy was efficient to treat HER2+ brain metastasis (11, 33–37), brain metastasis was not a significant factor affecting the efficacy of inetetamab in our study and the recruited patients with brain metastasis was too little for further analysis.
In terms of toxicity, the published results of the large clinical trials indicated that there were no significant change in grades and incidences of AEs, showing that inetetamab and trastuzumab are equivalently safe (9). Inetetamab-based therapy was also tolerated in our study. Yet, the medical records might omit important information about AEs even though we have thoroughly reviewed the patient’s examination results and medical records, which resulted in deviations in our results.
In conclusion, major populations of HER2+ MBC patients previously treated with multiple anti‐HER2 therapies including trastuzumab still responded to inetetamab‐based treatment in clinical practice. Inetetamab combined vinorelbine and pyrotinib might be the most effective inetetamab-based regimen. And the safety of inetetamab was controllable and tolerable. Notwithstanding the efficacy and safety of clinical trials are applicable for two or later‐line inetetamab‐based therapy remains questionable, our study of a series of patients provides real‐world data to further explore inetetamab-based treatment patterns and more experience outside the clinical trials for clinicians in treating general HER2+ MBC patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The Ethics Committee and Institutional Review Board of Shandong First Medical University and Shandong Academy of Medical Sciences (SDTHEC2022012020). The patients/participants provided their written informed consent to participate in this study.
XL and PZ collected the data. XL analyzed data and wrote the manuscript. CL, XS, ZL, WS and SL revised the manuscript. ZY and XW played a role in developing the idea. All authors contributed to manuscript revision, read, and approved the submitted version.
The article was funded by Youth Science Fund Cultivation Program of Shandong First Medical University and Shandong Academy of Medical Sciences(202201-115).
The authors thank the patients, doctors, and nurses for their supports to our study.
XW was employed by REMEGEN, LTD.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1136380/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Barzaman K, Karami J, Zarei Z, Hosseinzadeh A, Kazemi MH, Moradi-Kalbolandi S, et al. Breast cancer: biology, biomarkers, and treatments. Int Immunopharmacol (2020) 84:106535. doi: 10.1016/j.intimp.2020.106535
3. Waks AG, Winer EP. Breast cancer treatment: a review. JAMA (2019) 321:288–300. doi: 10.1001/jama.2018.19323
4. Radenkovic S, Konjevic G, Isakovic A, Stevanovic P, Gopcevic K, Jurisic V. HER2-positive breast cancer patients: correlation between mammographic and pathological findings. Radiat Prot Dosimetry (2014) 162:125–8. doi: 10.1093/rpd/ncu243
5. O'Grady S, Morgan MP. Microcalcifications in breast cancer: from pathophysiology to diagnosis and prognosis. Biochim Biophys Acta Rev Cancer (2018) 1869:310–20. doi: 10.1016/j.bbcan.2018.04.006
6. Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science (1989) 244:707–12. doi: 10.1126/science.2470152
7. Society CSoCOCAACoBC. Expert consensus on diagnosis and management of human epidermal growth factor receptor 2 positive breast cancer (version 2021). Natl Med J China (2021) 101:6. doi: 10.21037/tbcr-21-42
8. Najjar MK, Manore SG, Regua AT, Lo HW. Antibody-drug conjugates for the treatment of HER2-positive breast cancer. Genes (Basel) (2022) 13(11):2065. doi: 10.3390/genes13112065
9. Wang T, Zhang P, Di L, Wang X, Yang J, Tong Z, et al. Efficacy and safety of inetetamab in combination with chemotherapy as first-line treatment of HER2-positive metastatic breast cancer: a subgroup analysis in the HOPES study. Trans Breast Cancer Res (2022) 3:15–5. doi: 10.21037/tbcr-21-42
10. Bian L, Xu BH, Di LJ, Wang T, Wang XJ, Jiao SC, et al. Phase III randomized controlled, multicenter, prospective study of recombinant anti-HER2 humanized monoclonal antibody (Cipterbin) combined with vinorelbine in patients with HER2 positive metastatic breast cancer: the HOPES study. Zhonghua Yi Xue Za Zhi (2020) 100:2351–57. doi: 10.3760/cma.j.cn112137‑20200116‑00105
11. Anwar M, Chen Q, Ouyang D, Wang S, Xie N, Ouyang Q, et al. Pyrotinib treatment in patients with HER2-positive metastatic breast cancer and brain metastasis: exploratory final analysis of real-world, multicenter data. Clin Cancer Res (2021) 27:4634–41. doi: 10.1158/1078-0432.CCR-21-0474
12. Zhang L, Wu X, Zhou J, Zhu M, Yu H, Zhang Y, et al. Pyrotinib in the treatment of women with HER2-positive advanced breast cancer: a multicenter, prospective, real-world study. Front Oncol (2021) 11:699323. doi: 10.3389/fonc.2021.699323
13. Hua Y, Li W, Jin N, Cai D, Sun J, Sun C, et al. Treatment with pyrotinib-based therapy in lapatinib-resistant HER2-positive metastatic breast cancer: a multicenter real-world study. Ther Adv Med Oncol (2022) 14:17588359221085232. doi: 10.1177/17588359221085232
14. Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol (2010) 28:1124–30. doi: 10.1200/JCO.2008.21.4437
15. Han Y, Wang J, Liu W, Yuan P, Li Q, Zhang P, et al. Trastuzumab treatment after progression in HER2-positive metastatic breast cancer following relapse of trastuzumab-based regimens: a meta-analysis. Cancer Manag Res (2019) 11:4699–706. doi: 10.2147/CMAR.S198962
16. Xie XF, Zhang QY, Huang JY, Chen LP, Lan XF, Bai X, et al. Pyrotinib combined with trastuzumab and chemotherapy for the treatment of human epidermal growth factor receptor 2-positive metastatic breast cancer: a single-arm exploratory phase II trial. Breast Cancer Res Treat (2022) 197(1):93–101. doi: 10.21203/rs.3.rs-1752120/v1
17. Romero A, Rabinovich MG, Vallejo CT, Perez JE, Rodriguez R, Cuevas MA, et al. Vinorelbine as first-line chemotherapy for metastatic breast carcinoma. J Clin Oncol (1994) 12:336–41. doi: 10.1200/JCO.1994.12.2.336
18. Foucquier J, Guedj M. Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect (2015) 3:e00149. doi: 10.1002/prp2.149
19. Pegram MD, Konecny GE, O'Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst (2004) 96:739–49. doi: 10.1093/jnci/djh131
20. Pegram M, Hsu S, Lewis G, Pietras R, Beryt M, Sliwkowski M, et al. Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene (1999) 18:2241–51. doi: 10.1038/sj.onc.1202526
21. Andersson M, Lidbrink E, Bjerre K, Wist E, Enevoldsen K, Jensen AB, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. J Clin Oncol (2011) 29:264–71. doi: 10.1200/JCO.2010.30.8213
22. Reinhorn D, Kuchuk I, Shochat T, Nisenbaum B, Sulkes A, Hendler D, et al. Taxane versus vinorelbine in combination with trastuzumab and pertuzumab for first-line treatment of metastatic HER2-positive breast cancer: a retrospective two-center study. Breast Cancer Res Treat (2021) 188:379–87. doi: 10.1007/s10549-021-06198-4
23. Liu CT, Hsieh MC, Su YL, Hung CM, Pei SN, Liao CK, et al. Metronomic vinorelbine is an excellent and safe treatment for advanced breast cancer: a retrospective, observational study. J Cancer (2021) 12:5355–64. doi: 10.7150/jca.60682
24. Li Y, Qiu Y, Li H, Luo T, Li W, Wang H, et al. Pyrotinib combined with vinorelbine in HER2-positive metastatic breast cancer: a multicenter retrospective study. Front Oncol (2021) 11:664429. doi: 10.3389/fonc.2021.664429
25. Xie Y, Li Y, Ting L, Sang D, Yuan P, Li W, et al. Pyrotinib plus vinorelbine versus lapatinib plus capecitabine in patients with previously treated HER2-positive metastatic breast cancer: a multicenter, retrospective study. Front Oncol (2021) 11:699333. doi: 10.3389/fonc.2021.699333
26. Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med (2012) 367:1783–91. doi: 10.1056/NEJMoa1209124
27. Hurvitz SA, Hegg R, Chung WP, Im SA, Jacot W, Ganju V, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet (2023) 401:105–17. doi: 10.1016/S0140-6736(22)02420-5
28. Deluche E, Antoine A, Bachelot T, Lardy-Cleaud A, Dieras V, Brain E, et al. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008-2016. Eur J Cancer (2020) 129:60–70. doi: 10.1016/j.ejca.2020.01.016
29. Sun Y, Chen B, Li J, Peng L, Li S, Yu X, et al. Real-world analysis of the efficacy and safety of a novel irreversible HER2 tyrosine kinase inhibitor pyrotinib in patients with HER2-positive metastatic breast cancer. Cancer Manag Res (2021) 13:7165–74. doi: 10.2147/CMAR.S321428
30. Yin S, Chi Y, Du Y, Wang J, Shan C, Yi W, et al. Efficacy and safety of pyrotinib-containing regimen in the patients with HER2-positive metastatic breast cancer: a multicenter real-world study. Cancer Med (2022) 12(3):2333–44. doi: 10.1002/cam4.5056
31. Li C, Bian X, Liu Z, Wang X, Song X, Zhao W, et al. Effectiveness and safety of pyrotinib-based therapy in patients with HER2-positive metastatic breast cancer: a real-world retrospective study. Cancer Med (2021) 10:8352–64. doi: 10.1002/cam4.4335
32. Lin Y, Lin M, Zhang J, Wang B, Tao Z, Du Y, et al. Real-world data of pyrotinib-based therapy in metastatic HER2-positive breast cancer: promising efficacy in lapatinib-treated patients and in brain metastasis. Cancer Res Treat (2020) 52:1059–66. doi: 10.4143/crt.2019.633
33. Ma X, Li Y, Li L, Gao C, Liu D, Li H, et al. Pyrotinib-based treatments in HER2-positive breast cancer patients with brain metastases. Ann Med (2022) 54:3085–95. doi: 10.1080/07853890.2022.2139411
34. Nader-Marta G, Martins-Branco D, Agostinetto E, Bruzzone M, Ceppi M, Danielli L, et al. Efficacy of tyrosine kinase inhibitors for the treatment of patients with HER2-positive breast cancer with brain metastases: a systematic review and meta-analysis. ESMO Open (2022) 7:100501. doi: 10.1016/j.esmoop.2022.100501
35. Gao M, Fu C, Li S, Chen F, Yang Y, Wang C, et al. The efficacy and safety of pyrotinib in treating HER2-positive breast cancer patients with brain metastasis: a multicenter study. Cancer Med (2022) 11:735–42. doi: 10.1002/cam4.4481
36. Yao JH, Xie ZY, Li M, Zhang ML, Ci HF, Yang Y. Metastatic brain tumors respond favorably to pyrotinib in a HER2-positive breast cancer following failure using trastuzumab. Am J Transl Res (2020) 12:5874–81.
37. Chen Q, Ouyang D, Anwar M, Xie N, Wang S, Fan P, et al. Effectiveness and safety of pyrotinib, and association of biomarker with progression-free survival in patients with HER2-positive metastatic breast cancer: a real-world, multicentre analysis. Front Oncol (2020) 10:811. doi: 10.3389/fonc.2020.00811
Keywords: breast cancer, inetetamab, human epidermal receptor 2 positive, monoclonal antibody, real-word data
Citation: Liu X, Zhang P, Li C, Song X, Liu Z, Shao W, Li S, Wang X and Yu Z (2023) Efficacy and safety of inetetamab-containing regimens in patients with HER2-positive metastatic breast cancer: a real-world retrospective study in China. Front. Oncol. 13:1136380. doi: 10.3389/fonc.2023.1136380
Received: 03 January 2023; Accepted: 05 June 2023;
Published: 19 June 2023.
Edited by:
Chunyan Dong, Tongji University, ChinaReviewed by:
Marzia Locatelli, European Institute of Oncology (IEO), ItalyCopyright © 2023 Liu, Zhang, Li, Song, Liu, Shao, Li, Wang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiyong Yu, enl5dUBzZGZtdS5lZHUuY24=; Xinzhao Wang, MDh3YW5neGluemhhb0AxNjMuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.