
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 06 April 2023
Sec. Hematologic Malignancies
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1135812
This article is part of the Research TopicAdvances in Therapy for Chronic Lymphocytic LeukemiaView all 8 articles
Chronic lymphocytic leukaemia (CLL) is a malignancy of clonally expanded antigen-switched, neoplastic, mature B cells. CLL is characterised by a variable degree of immunosuppression and secondary hypogammaglobulinemia. B-cell depleting therapies have historically been deployed with a proportion of patients becoming resistant to multiple lines of treatment with an associated worsening of immunosuppression and heightened infection risk. Advances in molecular diagnostics and the development of new therapies targeting Bruton’s tyrosine kinase and B-cell lymphoma-2 have resulted in novel insights into the cellular mechanisms associated with an increased infection risk and T-cell escape from the complex tumour environment found in CLL. Generally, immunoglobulin replacement therapy with polyvalent human immunoglobulin G (IgG) is indicated in patients with recurrent severe bacterial infections and low IgG levels, but there is no consensus on the threshold IgG level for initiation of such therapy. A proportion of CLL patients have residual IgG production, with preserved quality of the immunoglobulin molecules, and therefore a definition of ‘IgG quality’ may allow for lower dosing or less frequent treatment with immunoglobulin therapy in such patients. Immunoglobulin therapy can restore innate immunity and in conjunction with CLL targeted therapies may allow T-cell antigen priming, restore T-cell function thereby providing an escape from tumour-associated autoimmunity and the development of an immune-mediated anti-tumour effect. This review aims to discuss the mechanisms by which CLL-targeted therapy may exert a synergistic therapeutic effect with immunoglobulin replacement therapy both in terms of reducing tumour bulk and restoration of immune function.
Chronic lymphocytic leukaemia (CLL) is the most common form of leukaemia in the Western World, with around 4700 new cases diagnosed each year in the United Kingdom (1). Current guidelines suggest that treatment for CLL is indicated only for symptomatic or rapidly progressive disease (2). Therefore, the current standard of care for patients with early CLL is a ‘watch-and-wait’ approach that includes regular physical examination and laboratory testing to assess disease status over time. This approach is based upon research which has demonstrated that pre-emptive therapy for asymptomatic CLL has no effect on overall survival, even when novel targeted agents are utilised, as shown in the recently concluded CLL12 trial (3). The treatment landscape for symptomatic CLL has rapidly evolved with the development of multiple targeted agents that have improved survival outcomes. However, despite these improvements in treatment, CLL remains incurable with infection being the leading direct cause of death (4).
The inherent disease-related immune dysfunction along with secondary hypogammaglobulinemia, decreased cell-mediated immunity (T-cell dysfunction), and immunosuppression related to B-cell directed therapies are the major causes of the increased susceptibility to infection in CLL. Finally, although the mechanism of CLL-associated hypogammaglobulinemia is largely unknown, factors such as T-cell dysfunction and the abnormal cytokine environment (such as increased tumour necrosis factor–alpha [TNF-α] level) may affect immunoglobulin production (5).
Current guidelines recommend immunoglobulin replacement therapy (IgRT) to reduce the risk of bacterial infections and hospitalization in patients with recurrent severe infections and low immunoglobulin G (IgG) levels (6). In addition, there is evidence that IgRT with polyvalent human IgG has immunomodulatory effects on the innate immune system, whilst the effect of such therapy on B-cell function appears to be more complex and poorly defined (7).
IgRT also exerts effects upon other important immune cells, with a resultant increase in CD4+ T-cell numbers, a reduction in exhaustion markers on both CD4+ and CD8+ T-cells, a transient rise in regulatory T-cells (Tregs) along with effects on invariant-natural killer T-cells (iNKT) and alterations in serum IL-2 and TNF-α (7, 8). Whether such changes are IgRT-induced immunomodulatory alterations that may translate into a change in the cytokine-mediated immunosuppressive tumour microenvironment (TME) of CLL is debated. In fact, subcutaneously administered immunoglobulin therapy (scIgRT) at high doses decreased CD83 expression with consequential inhibition of B-cell receptor (BCR) signalling and reduced TNF-α production with in-vitro studies showing an associated decrease in the survival of CLL cells (9). Although such in-vitro findings may not precisely reflect responses to high-dose immunoglobulin therapy in vivo, these results set the stage for a more comprehensive assessment of IgRT activity in CLL.
It is a general characteristic of malignancies that the cancer cell manipulates the immune system to the advantage of the tumour, and CLL is no different in this respect.
CLL almost uniformly evolves from the precursor condition monoclonal B-lymphocytosis (MBL) characterized by the co-expression of CLL-like surface markers (CD19, CD5, and CD23, weak expression of CD20 and CD79b) with less than 5 x 109/L B-cells in the circulation. Progression of MBL to CLL is characterized by continuous clonal evolution with the acquisition of high-risk genetic driver mutations that may lead to symptomatic disease (10–12).
The skewed BCR repertoire found in CLL is illustrated by the utilisation of specific immunoglobulin heavy-chain gene rearrangements found in IGHV 4-34, 3-23, 1-69 as well the expression of highly stereotypic BCRs (13). Similarly, the expression of zeta-associated protein 70 (ZAP70) or CD38, deletions of chromosome 11q or 17p, unmutated immunoglobulin heavy chain (IGHV) genes indicative of pre-germinal centre CD5+ B-cells, mutations in TP53, Ataxia-Telangestasia Mutated (ATM), DDX3X, NOTCH1, spicing factor 3b subunit 1 (SF3B1) genes are all associated with high-risk disease. In contrast, deletions of chromosome 13q and highly mutated IGHV genes (indicative of post-germinal centre CD5+ B-cells) are associated with a favourable prognosis (14). Although to a lesser extent when compared to CLL, high-count MBL can carry CLL-specific genomic aberrations with associated altered T-cell function (10). We have tried to provide an overview of the disruption and chaos of the immune system in patients with CLL (Figure 1) evident at multiple levels of the cellular immune response.
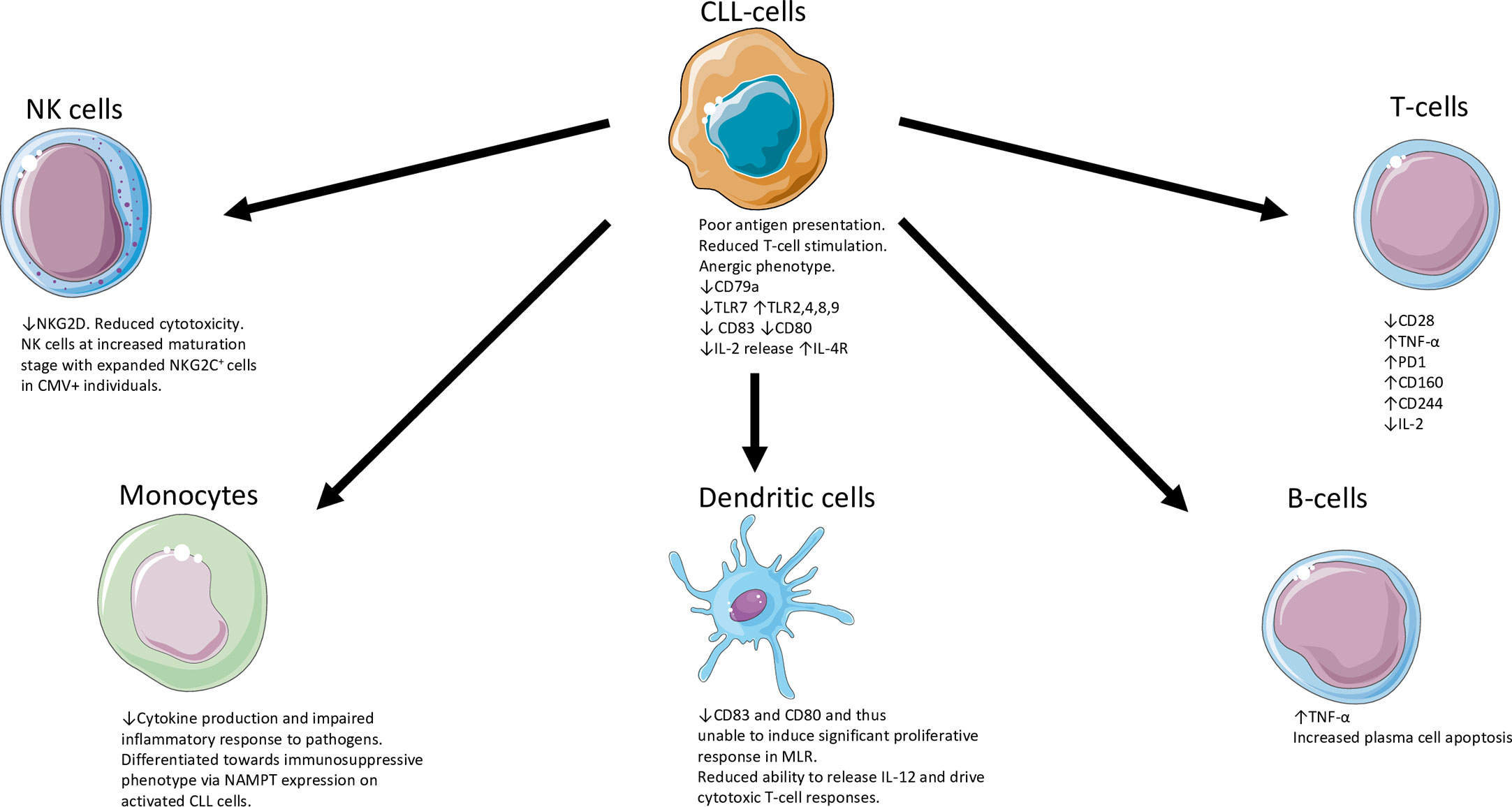
Figure 1 Overview of the disruption and chaos of the immune system in patients with CLL (5, 15–29). CLL, chronic lymphocytic leukaemia; IL-2, interleukin 2; IL-4R, interleukin-4 receptor; NK, natural killer; TLR, Toll-like receptor; MLR, mixed lymphocyte reaction; NAMPT, nicotinamide phosphoribosyltransferase; PD1, Programmed death protein 1; TNF, Tumour necrosis factor; TLR, Toll receptor. The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unsupported license.
The nodal TME provides essential survival signals whereby CLL-cell-secreted soluble factors interact with T-cells and tumour-infiltrating macrophages leading to a tumour supportive M2 phenotype. M2-macrophages upregulate PD-1 expression on T-cells to induce suppression of cytotoxic T-cells and T-cell activation whilst promoting Treg differentiation (15). IL-4 present within the TME upregulates CLL-cell expression of surface IgM (sIgM), CD79b and associated BCR expression that rescues anergic B-CLL cells from signal incompetency as they enter lymph nodes (16). These processes finally shape the immunosuppressive features typical of CLL with the consequential development of hypogammaglobulinemia. Autoimmune haemolytic anaemia and thrombocytopenia are common consequences of such CLL-associated immune dysregulation (5).
The most common and clinically relevant impact of CLL on the affected persons immunological status is hypogammaglobulinaemia (decreased serum immunoglobulin levels of all three isotypes IgG, IgA, and IgM). Such a reduction in immunoglobulins is present in up to a third of persons with CLL at diagnosis, and a further third develop hypogammaglobulinemia as the disease progresses, or because of administered therapy (5). Of note, a proportion of CLL patients retain a degree of residual IgG production, with preserved quality of the immunoglobulin molecules. This residual IgG production may have implications for the ability to mount an immunological response to vaccination in persons with CLL. Generally, the primary immune response to a novel antigen is suppressed in persons with CLL compared to the brisk anamnestic immune response in healthy persons (30). A recent prospective non-interventional trial suggests that multiple doses of a COVID19 vaccine can result in high rates of seroconversion in CLL (94.2%) and MBL (100%) (31). Whether the repeated immune stimulus or a post-treatment recovering immune status is driving the response (or if this is sustained) is unclear and needs further investigation.
The evolution of hypogammaglobulinaemia parallels disease progression with data showing faster rates of decline in immunoglobulin levels in those patients who required treatment (9, 32). Parikh et al. showed that patients with advanced stage CLL were more likely to have hypogammaglobulinemia at diagnosis, and the median time to first treatment (TTFT) was shorter compared to patients with normal IgG (3.8 years vs 7.4 years) (32). Spaner and colleagues showed that patients whose IgG declined at a rate of 0.85 ± 0.14 g/L/year were those who required treatment within 4.5 ± 0.4 years from initial diagnosis (n = 51) versus a slower rate of 0.27 ± 0.04 g/L/year in those remained untreated after 8.5 ± 0.5 years (n = 40) (9). Of note in the same study, patients with indolent disease (n = 25) maintained endogenous IgG levels above 8 g/L. Future studies should clarify whether modification of the dose schedule of IgRT to achieve higher than conventional IgG target levels may impact on the natural history of CLL.
Although CLL represents a malignant proliferation of B-lymphocytes, the proliferating clonal B-cells retain the ability to secrete immunoglobulin (33). Li et al. showed that non-clonal B-cells derived from persons with CLL and CLL-cells have enhanced surface and serum expression of FcμR, and serum FcμR levels correlated significantly with circulating lymphocyte numbers but not with IGHV mutation status or Rai stage (34). This observation may partly explain why serum immunoglobulin levels do not correlate with survival in CLL patients including those treated with Bruton’s tyrosine kinase-inhibitors (BTKi).
In an analysis of 1113 cases with Binet Stage A CLL, low IgA level was associated with a shortened TTFT but neither baseline hypogammaglobulinemia nor the presence of a paraprotein influenced overall survival. However, in this cohort lymphadenopathy, serum β2-microglobulin, CD38, and ZAP70 expression all had prognostic significance (35). A Chinese study of CLL patients with and without a paraprotein found that those with a paraprotein had significantly shorter survival times compared to those without a paraprotein. Factors related to the presence of a paraprotein were advanced Binet stage, autoimmune haemolytic anaemia, elevated serum β2-microglobulin, elevated thymidine kinase, unmutated IGHV, expression of ZAP-70 or CD38-positive and cytogenetic abnormalities such as del(17p13) or del(11q22.3) (36). It is entirely possible that the presence of a paraprotein in CLL may indicate a higher disease burden. Such observations in relation to the presence of a paraprotein and disease burden have also been made in Waldenstrom’s Macroglobulinaemia where autoimmunity associated with a loss of immune tolerance permits the emergence of B-cell clones characterised by a paraprotein which can directly contribute to morbidity (37). In addition, a study by Mozas and colleagues reported 30% prevalence of serum monoclonal components in persons with CLL in a single centre. Such patients with a monoclonal component had shortened survival, irrespective of age, with biclonal paraproteins associated with the shortest survival outcomes (38).
It is generally agreed that persons with CLL with significant hypogammaglobulinemia characterized by an IgG of less than 4g/L, in the presence of recurrent or severe bacterial infection despite antibiotic prophylaxis, are candidates for long-term IgRT (6). IgRT is usually administered at replacement doses of 0.2-0.4g/kg per month (39). The immunoglobulin trough level, monitored whilst on replacement therapy, can be used to assess the effectiveness of IgRT and could help to modify subsequent dosing of IgRT. Measurement of immunoglobulin levels at different times to evaluate the peak and trough levels should help to monitor the response to IgRT. However, the clinical response remains the most important parameter on which to base efficacy assessments of IgRT and close clinical assessment of the nature and frequency of infections should be undertaken whilst on such therapy.
Whilst prophylaxis with IgRT was effective in an early study of patients with recurrent severe infections and a serum IgG<3g/L, a crossover study found that restoration of serum IgG levels did not parallel a decrease in the number of severe infectious episodes (40, 41). This could therefore mean that the ‘quality’ of IgG is more important than the level measured (‘nephelometric quantity’) and that absent functional or specific anti-microbial antibodies could be an important consideration for prophylactic IgRT (42). Of note, a feasibility study showed a small difference in efficacy between prophylactic IgRT and prophylactic oral antibiotics but there was no significant difference in time to first major infection between the two treatment arms (43).
A systematic review and meta-analysis of randomised-controlled trials of immunoglobulin prophylaxis concluded that although IgRT use in lymphoproliferative disorders and plasma cell dyscrasias does not provide a survival benefit such therapy does prevent major infections with decreased numbers of infections (44). However, there is inter-trial heterogeneity with respect to patient selection and such studies are insufficiently powered for mortality outcomes (Table 1). Finally, the evidence with respect to IgRT in CLL mainly relies on studies that pre-date the modern chemoimmunotherapies and targeted agents currently utilised for the treatment of CLL.
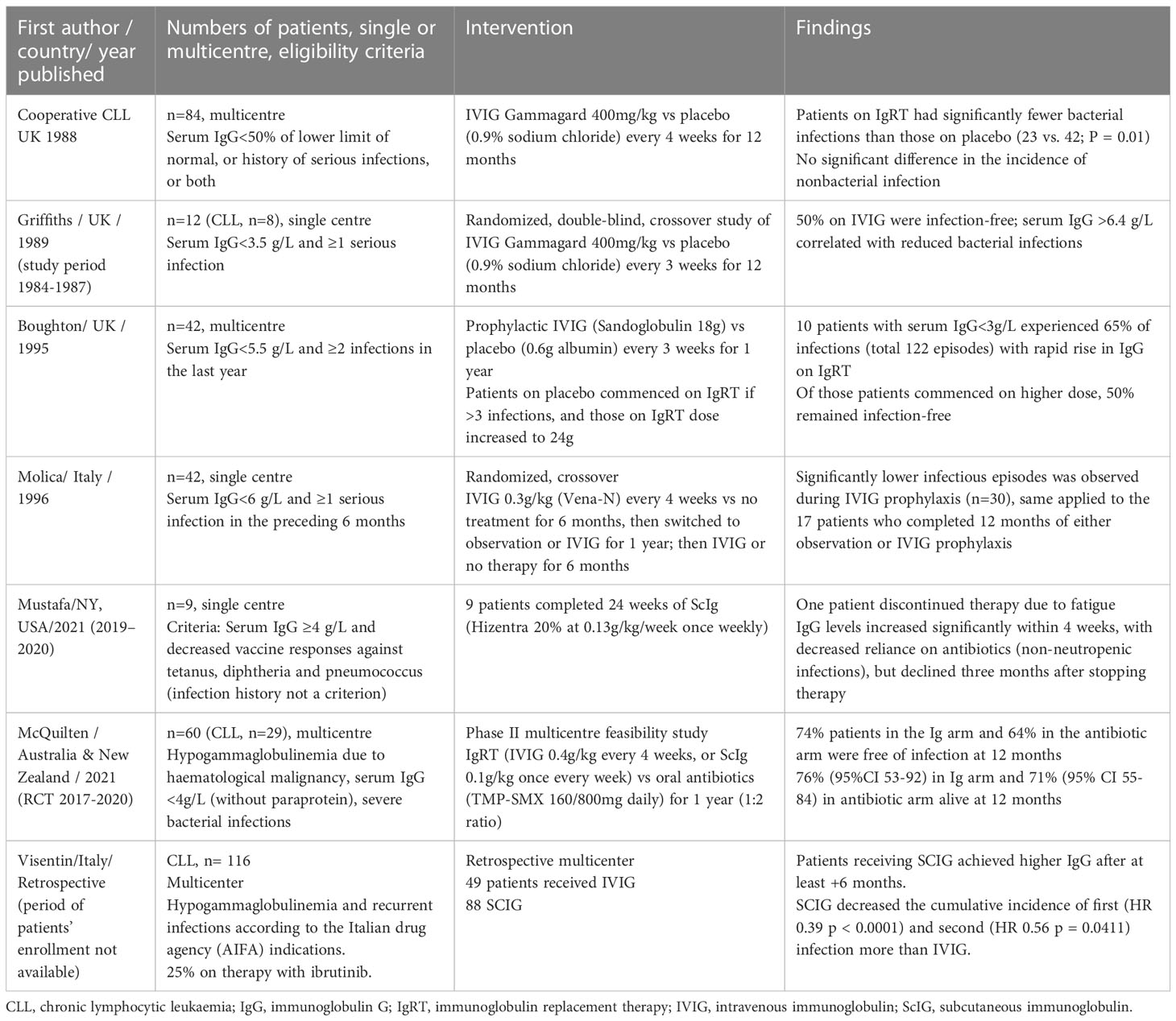
Table 1 Randomised clinical trials on effect of immunoglobulin replacement therapy in CLL patients with hypogammaglobulinemia (40, 43, 45–48).
During the past decade, the utilisation of BTKi for CLL treatment has grown rapidly, radically changing the treatment landscape of this disease. Both innate and adaptive immunity may be impacted by BTKi treatment. Ibrutinib, the first-in-class BTKi, induces a partial restoration of normal B-cell numbers and humoral immunity during the first two years of therapy. Improvements in serum IgA in BTKi-treated persons were inversely correlated with the infection rate. Acalabrutinib is a newer, more selective BTKi; however, the effect of this agent on humoral and cellular immunity is similar to that observed with ibrutinib and translates into a partial humoral immune reconstitution with a decreased risk of infection, although a degree of immunodeficiency likely persists.
These data pertaining to BTKi-induced immune reconstitution suggests that a reassessment of immunoprophylaxis with IgRT is needed in the era of targeted agents with regards to the timing of discontinuation of IgRT after immune reconstitution is achieved. Studies of infection and immune reconstitution in BTKi-treated persons should also employ uniform definitions of infection severity, and patient-reported outcomes.
The prevailing paradigm underpinning current guidelines of IgRT usage in CLL is that such an approach should be deployed in those patients with reduced serum IgG levels and one major infection. However, the first major infectious episode could be fatal, especially in certain patients. This implies that a pre-emptive treatment could be offered to selected patients. In Figure 2 we weigh the arguments for, and against, an ‘earlier’ or “pre-emptive” IgRT treatment in CLL patients. Apart from symptomatic severe hypogammaglbulinemia, other patient- or CLL-related factors could favour consideration of earlier initiation of prophylactic IgRT. Such patient-specific factors could include the ‘quality’ of low IgG as assessed by a poor vaccine responses (<2.3 x fold response to vaccination), multiple prior lines of therapy, high-risk CLL genomic changes (unmutated IGHV genes, adverse cytogenetic features or a complex karyotype), or the presence of exhausted T-cell phenotype. Some of these are in-vitro findings and require specialised laboratories so therefore may not be routinely available. Prospective studies comparing an earlier versus delayed IgRT usage should clarify the optimal timing of the initiation of IgRT therapy in CLL.
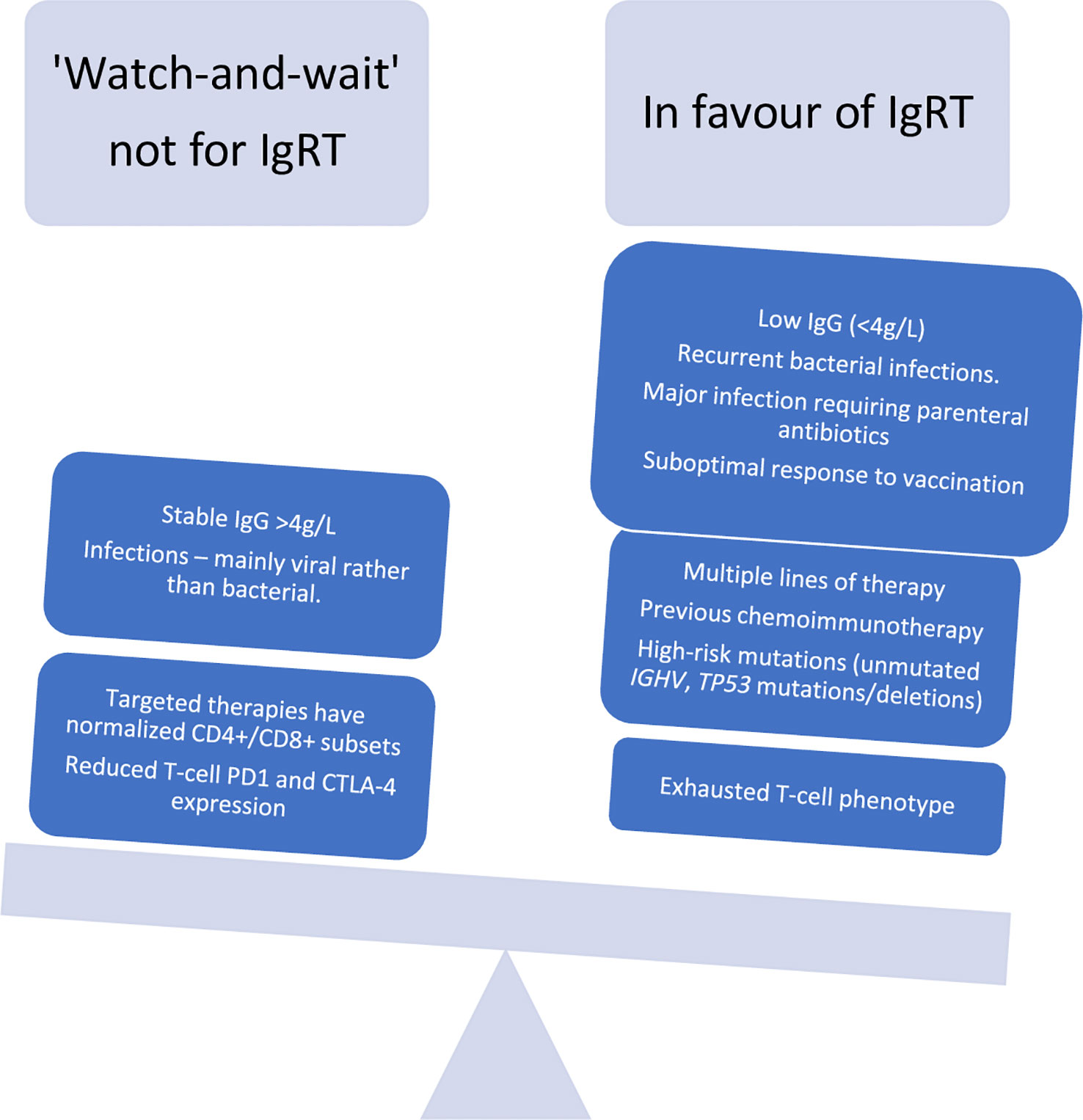
Figure 2 A re-look at indications for immunoglobulin replacement therapy in CLL in the era of targeted therapies.
It has been shown that scIgRT is safe, efficacious, and well tolerated when compared to intravenous IgRT (ivIgRT), providing patients with primary or secondary immunodeficiency disorders with an alternative route of Ig administration (49, 50). Some of the key benefits of scIgRT include: no requirement for venous access, the option to deliver therapy at a time and location of the persons choosing as little medical expertise is needed for administration (infusion pump required but generally very simple to use, after adequate training). scIgRT is typically administered more frequently (such as biweekly, weekly, and even daily based on patient need), resulting in steady state concentrations with fewer fluctuations in IgG plasma levels. More recent scIgRT products utilise a vial of human normal immunoglobulin (Immune Globulin 10%) and one vial of recombinant human hyaluronidase (rHuPH20) that increases the permeability of the subcutaneous tissue by temporarily depolymerizing hyaluronan, allowing larger volume of infusions and treatment intervals to be gradually prolonged from once weekly to once every 3 or 4 weeks. The route of administration also plays a major role in the types of adverse events (AEs) observed in persons receiving IgRT therapy, with systemic AEs associated with intravenous administration and local reactions more commonly seen with subcutaneous administration. Our experience with scIgRT in elderly persons with significant co-morbidities has been positive, but ivIgRT is also effective if there is also clinical supervision to monitor for side-effects associated with increased fluid volumes and rates of infusion. Of note, one meta-analysis determined that the systemic AE rate for scIgRT was 0.43%. A recent retrospective study compared the efficacy of subcutaneous immunoglobulin with an intravenous formulation in patients with CLL and secondary antibody deficiency and found that scIgRT results in higher IgG levels and a decreased rate of infections particularly when IgG levels of 6 g/L is reached (51).
Although limited to few retrospective analyses, the results of efficacy and safety analyses suggest that scIgRT should be preferred in clinical practice for persons with CLL and symptomatic hypogammaglobulinaemia.
The financial cost of IgRT is significant due to high procurement costs and indefinite usage. Unnecessary and indiscriminate use of IgRT could result in product shortages, adverse outcomes and higher healthcare costs. Keegan and colleagues reported on the demand, supply, and patterns of utilisation of IgRT in CLL in Australia and revealed a sustained increased usage of 5.5% per annum but with significant regional variation across geographical areas (52).
Numerous other studies have demonstrated that scIgRT compared to ivIgRT results in reduced resource use and is therefore more cost effective. A study comparing the cost-effectiveness of scIgRT and ivIgRT suggests that significant cost savings could be made with a subcutaneous formulation as opposed to an intravenous preparation (53). A Canadian analysis indicated that every 37 patients treated with scIgRT in preference to ivIgRT resulted in sufficient financial savings to fund one nursing full-time equivalent (54).
Another approach to maximise cost-effectiveness is to de-escalate or withdraw IgRT when this approach is no longer necessary. Current guidelines do not provide clear advice with respect to the timing and indications for safe IgRT discontinuation. Therefore, in the absence of evidence-based recommendations for IgRT discontinuation clinical expertise and medical judgment should guide decision making. A reasonable approach could be a gradual IgRT discontinuation at one-year in patients with history of underlying disease in remission, a normal IgG trough level and evidence of immune reconstitution if CLL-directed therapy has been deployed. Following discontinuation, the criteria for the recommencement of IgRT should be the same as those for the primary indication.
There is a need therefore to develop a clinical trial wherein the efficacy, safety of IgRT replacement is compared with less costly prophylactic oral antibiotics in persons with CLL with secondary hypogammaglobulinemia.
IgRT is administered at distinct dosage in two different clinical contexts:- immunodeficient patients are treated with replacement doses of immunoglobulin, typically 0.2-0.4g/kilogram every 3 to 4 weeks whilst patients with autoimmune and inflammatory diseases are administered with very high doses of IgRT such as 0.5-2g/kilogram often over a single, or several days (39). In CLL, IgRT is generally believed to replace the missing antibodies and thereby prevent recurrent infections. However, the most important question remains: does IgRT mediate more complex mechanisms of immune reconstitution possibly with an associated with a direct anti-tumour effect rather than just preventing infections in CLL?
Spaner et al. showed that scIgRT preparations have the ability to impair BCR signalling, activation, and cytokine secretion by CLL-cells when stimulated in vitro by BCR ligation (9). This IgRT regimen decreased TNF-α and β2-microglobulin level (a marker of lymphocyte turnover), whilst a more intensive IgRT regimen decreased BCR activation. These findings could indicate that rather than acting solely as a replacement therapy, administered immunoglobulin may have activity against CLL-cells in-vivo. As immunoglobulin isotypes IgA and IgM may also be suppressed in CLL, the isotype content of immunoglobulin preparations could have different effects. Colado and colleagues compared effects of the pentaglobin (IVIgGMA) preparation which includes all immunoglobulin isotypes, versus standard preparations which contained only IgG, on leukemic and T-cells obtained from CLL patients (55). This study found that CLL-cell BCR signalling was reduced by both preparations. Whilst the addition of IVIgGMA to in-vitro cultures decreased venetoclax-induced T-cell apoptosis, this did not affect CLL-cell apoptosis in response to this drug. This may indicate an intrinsic CLL-cell resistance to apoptosis in the presence of IgRT and is perhaps supportive role of BTKi therapy as opposed to BCL-2 inhibition in CLL patients treated with IgRT.
Colado et al. also showed that the proliferation of T-cells from persons with CLL in response to TCR-stimulation or IL-15 was differentially affected by two different immunoglobulin preparations (55). There was enhanced inhibition of T-cell proliferation in response to anti-CD3 and IL-15 with intravenous IgG compared with IVIgGMA preparations. Moreover, IgM-enriched immunoglobulin preparations showed no benefits when used for the treatment of haematological patients with sepsis. This would suggest that polyclonal IgG based immunoglobulin preparations have more protective and immunomodulatory actions than enriched preparations.
In conclusion, in the era of targeted therapy, IgRT should be offered to CLL patients as suggested by current guidelines (2). However such guidance can be criticised as the evidence is mainly derived from historic studies where CLL-directed treatment was predominantly with chemoimmunotherapy as opposed to the targeted agents currently in widespread use. There is little evidence in relation to the role of IgRT in persons with CLL treated with BTKi or inhibitors of BCL-2. The results of a recent, large, retrospective study suggests that adherence to IgRT guidelines positively correlates with fewer and less severe infections (6). This finding corroborates the results of a recent European expert conference on recommendations for the diagnosis and treatment of antibody deficiency with IgRT in patients with malignant hematologic diseases (56) This consensus document supports the initiation of IgRT in persons with IgG of less than 4g/L and at least one major infection treated with parenteral antibiotics or three infections over a twelve month period.
Prospective studies to assess the cost-effectiveness of IgRT and the impact on quality of life for persons with CLL are required. Furthermore, long-term IgRT for persons with CLL may not be necessary therefore a systematic approach to IgRT discontinuation is needed. Expert opinion suggests that IgRT could be discontinued after six months without an infection in the presence of evidence of immune reconstitution.
Finally, the successful navigation of the complex TME in CLL and the development of an understanding of the disease- and treatment-related immunosuppression is a significant challenge, but the development of BTKi has permitted fascinating insights into immune escape mechanisms present in CLL patients. We suggest that a mechanism of immunomodulation with a combination of B-cell-directed therapies and polyclonal immunoglobulin in high-risk CLL is possible and worthy of future investigation. Such combined modality immunomodulation could represent a novel therapeutic approach for CLL treatment.
SK conceived the idea and wrote the main body of the paper. DA and SM directed, reviewed, and revised the manuscript. All authors contributed to the article and approved the submitted version.
We thank Professor WAC Sewell for his useful suggestions and comments on the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. HMRN. Incidence . Available at: https://hmrn.org/statistics/incidence (Accessed February 16, 2023).
2. Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood (2018) 131:2745–60. doi: 10.1182/BLOOD-2017-09-806398
3. Langerbeins P, Zhang C, Robrecht S, Cramer P, Fürstenau M, Al-Sawaf O, et al. The CLL12 trial: ibrutinib vs placebo in treatment-naïve, early-stage chronic lymphocytic leukemia. Blood (2022) 139:177–87. doi: 10.1182/BLOOD.2021010845
4. Kay NE, Hampel PJ, van Dyke DL, Parikh SA. CLL update 2022: A continuing evolution in care. Blood Rev (2022) 54:100930. doi: 10.1016/J.BLRE.2022.100930
5. Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood (2015) 126:573–81. doi: 10.1182/blood-2015-03-567388
6. Link H, Kerkmann M, Holtmann L. Immunoglobulin substitution in patients with secondary antibody deficiency in chronic lymphocytic leukemia and multiple myeloma: a representative analysis of guideline adherence and infections. Support Care Cancer (2022) 30:5187–200. doi: 10.1007/S00520-022-06920-Y
7. Quinti I, Mitrevski M. Modulatory effects of antibody replacement therapy to innate and adaptive immune cells. Front Immunol (2017) 8:697. doi: 10.3389/FIMMU.2017.00697
8. Sewell WAC, North ME, Cambronero R, Webster ADB, Farrant J. In vivo modulation of cytokine synthesis by intravenous immunoglobulin. Clin Exp Immunol (1999) 116:509–15. doi: 10.1046/J.1365-2249.1999.00924.X
9. Spaner DE, Venema R, Huang J, Norris P, Lazarus A, Wang G, et al. Association of blood IgG with tumor necrosis factor-alpha and clinical course of chronic lymphocytic leukemia. EBioMedicine (2018) 35:222–32. doi: 10.1016/J.EBIOM.2018.08.045
10. Strati P, Shanafelt TD. Monoclonal b-cell lymphocytosis and early-stage chronic lymphocytic leukemia: diagnosis, natural history, and risk stratification. Blood (2015) 126:454–62. doi: 10.1182/BLOOD-2015-02-585059
11. Landgren O, Albitar M, Ma W, Abbasi F, Hayes RB, Ghia P, et al. B-cell clones as early markers for chronic lymphocytic leukemia. N Engl J Med (2009) 360:659–67. doi: 10.1056/NEJMOA0806122
12. Barrio S, Shanafelt TD, Ojha J, Chaffee KG, Secreto C, Kortüm KM, et al. Genomic characterization of high-count MBL cases indicates that early detection of driver mutations and subclonal expansion are predictors of adverse clinical outcome. Leukemia 2017 31:1 (2016) 31:170–6. doi: 10.1038/leu.2016.172
13. Gemenetzi K, Agathangelidis A, Zaragoza-Infante L, Sofou E, Papaioannou M, Chatzidimitriou A, et al. B cell receptor immunogenetics in b cell lymphomas: Immunoglobulin genes as key to ontogeny and clinical decision making. Front Oncol (2020) 10:67/BIBTEX. doi: 10.3389/FONC.2020.00067/BIBTEX
14. Pérez-Carretero C, González-Gascón-y-marín I, Rodríguez-Vicente AE, Quijada-álamo M, Hernández-Rivas JÁ, Hernández-Sánchez M, et al. The evolving landscape of chronic lymphocytic leukemia on diagnosis, prognosis and treatment. Diagnostics (2021) 11(5):853. doi: 10.3390/diagnostics11050853
15. Arruga F, Gyau BB, Iannello A, Deaglio S, Vitale N, Vaisitti T. Immune response dysfunction in chronic lymphocytic leukemia: Dissecting molecular mechanisms and microenvironmental conditions. Int J Mol Sci (2020) 21. doi: 10.3390/IJMS21051825
16. Guo B, Zhang L, Chiorazzi N, Rothstein TL. IL-4 rescues surface IgM expression in chronic lymphocytic leukemia. Blood (2016) 128:553–62. doi: 10.1182/BLOOD-2015-11-682997
17. Scrivener S, Kaminski ER, Demaine A, Prentice AG. Analysis of the expression of critical activation/interaction markers on peripheral blood T cells in b-cell chronic lymphocytic leukaemia: evidence of immune dysregulation. Br J Haematol (2001) 112:959–64. doi: 10.1046/J.1365-2141.2001.02672.X
18. Frydecka I, Kosmaczewska A, Bocko D, Ciszak L, Wolowiec D, Kuliczkowski K, et al. Alterations of the expression of T-cell-related costimulatory CD28 and downregulatory CD152 (CTLA-4) molecules in patients with b-cell chronic lymphocytic leukaemia. Br J Cancer (2004) 90:2042–8. doi: 10.1038/SJ.BJC.6601833
19. Jain P, Javdan M, Feger FK, Chiu PY, Sison C, Damle RN, et al. Th17 and non-Th17 interleukin-17-expressing cells in chronic lymphocytic leukemia: delineation, distribution, and clinical relevance. Haematologica (2011) 97:599–607. doi: 10.3324/HAEMATOL.2011.047316
20. Podhorecka M, Dmoszynska A, Rolinski J. Intracellular IFN-γ expression by CD3+/CD8+ cell subset in b-CLL patients correlates with stage of the disease. Eur J Haematol (2004) 73:29–35. doi: 10.1111/j.1600-0609.2004.00258.x
21. Gallego A, Vargas JA, Castejón R, Citores MJ, Romero Y, Millán I, et al. Production of intracellular IL-2, TNF-alpha, and IFN-gamma by T cells in b-CLL. Cytometry B Clin Cytom (2003) 56:23–9. doi: 10.1002/CYTO.B.10052
22. Dazzi F, D’Andrea E, Biasi G, de Silvestro G, Gaidano G, Schena M, et al. Failure of b cells of chronic lymphocytic leukemia in presenting soluble and alloantigens. Clin Immunol Immunopathol (1995) 75:26–32. doi: 10.1006/CLIN.1995.1048
23. Pang N, Zhang R, Li J, Zhang Z, Yuan H, Chen G, et al. Increased IL-10/IL-17 ratio is aggravated along with the prognosis of patients with chronic lymphocytic leukemia. Int Immunopharmacol (2016) 40:57–64. doi: 10.1016/J.INTIMP.2016.07.008
24. Lad DP, Varma S, Varma N, Sachdeva MUS, Bose P, Malhotra P. Regulatory T-cell and T-helper 17 balance in chronic lymphocytic leukemia progression and autoimmune cytopenias. Leuk Lymphoma (2015) 56:2424–8. doi: 10.3109/10428194.2014.986479
25. Hofland T, Endstra S, Gomes CKP, de Boer R, de Weerdt I, Bobkov V, et al. Natural killer cell hypo-responsiveness in chronic lymphocytic leukemia can be circumvented in vitro by adequate activating signaling. Hemasphere (2019) 3. doi: 10.1097/HS9.0000000000000308
26. de Weerdt I, van Hoeven V, Marius Munneke J, Endstra S, Hofland T, Hazenberg MD, et al. Innate lymphoid cells are expanded and functionally altered in chronic lymphocytic leukemia. Haematologica (2016) 101:e461–4. doi: 10.3324/HAEMATOL.2016.144725
27. Rip J, de Bruijn MJW, Appelman MK, Singh SP, Hendriks RW, Corneth OBJ. Toll-like receptor signaling drives btk-mediated autoimmune disease. Front Immunol (2019) 10:95/BIBTEX. doi: 10.3389/FIMMU.2019.00095/BIBTEX
28. Muzio M, Fonte E, Caligaris-Cappio F. Toll-like receptors in chronic lymphocytic leukemia. Mediterr J Hematol Infect Dis (2012) 4. doi: 10.4084/MJHID.2012.055
29. van Attekum MHA, van Bruggen JAC, Slinger E, Lebre MC, Reinen E, Kersting S, et al. CD40 signaling instructs chronic lymphocytic leukemia cells to attract monocytes via the CCR2 axis. Haematologica (2017) 102:2069–76. doi: 10.3324/HAEMATOL.2016.157206
30. Mellinghoff SC, Cornely OA. SARS-CoV-2 vaccination in CLL: how often is enough? Blood (2022) 140:2655–7. doi: 10.1182/BLOOD.2022018586
31. Shen Y, Freeman JA, Holland J, Naidu K, Solterbeck A, van Bilsen N, et al. Multiple COVID-19 vaccine doses in CLL and MBL improve immune responses with progressive and high seroconversion. Blood (2022) 140:2709–21. doi: 10.1182/BLOOD.2022017814
32. Parikh SA, Leis JF, Chaffee KG, Call TG, Hanson CA, Ding W, et al. Hypogammaglobulinemia in newly diagnosed chronic lymphocytic leukemia: Natural history, clinical correlates, and outcomes. Cancer (2015) 121:2883–91. doi: 10.1002/CNCR.29438
33. Fu SM, Chiorazzi N, Kunkel HG. Differentiation capacity and other properties of the leukemic cells of chronic lymphocytic leukemia*. Immunol Rev (1979) 48:23–44. doi: 10.1111/J.1600-065X.1979.TB00297.X
34. Li FJ, Kubagawa Y, McCollum MK, Wilson L, Motohashi T, Bertoli LF, et al. Enhanced levels of both the membrane-bound and soluble forms of IgM fc receptor (FcμR) in patients with chronic lymphocytic leukemia. Blood (2011) 118:4902–9. doi: 10.1182/BLOOD-2011-04-350793
35. Shvidel L, Tadmor T, Braester A, Bairey O, Rahimi-Levene N, Herishanu Y, et al. Serum immunoglobulin levels at diagnosis have no prognostic significance in stage a chronic lymphocytic leukemia: a study of 1113 cases from the Israeli CLL study group. Eur J Haematol (2014) 93:29–33. doi: 10.1111/EJH.12290
36. Xu W, Wang YH, Fan L, Fang C, Zhu DX, Wang DM, et al. Prognostic significance of serum immunoglobulin paraprotein in patients with chronic lymphocytic leukemia. Leuk Res (2011) 35:1060–5. doi: 10.1016/J.LEUKRES.2010.12.005
37. Stone MJ, Merlini G, Pascual V. Autoantibody activity in waldenström’s macroglobulinemia. Clin Lymphoma (2005) 5:225–9. doi: 10.3816/CLM.2005.N.004
38. Mozas P, Piñeyroa JA, Nadeu F, Magnano L, Rivero A, Rivas-Delgado A, et al. Serum monoclonal component in chronic lymphocytic leukemia: baseline correlations and prognostic impact. Haematologica (2021) 106:1754–7. doi: 10.3324/HAEMATOL.2020.263228
39. Kerr J, Quinti I, Eibl M, Chapel H, Späth PJ, Sewell WAC, et al. Is dosing of therapeutic immunoglobulins optimal? a review of a three-decade long debate in Europe. Front Immunol (2014) 5:629/BIBTEX. doi: 10.3389/FIMMU.2014.00629/BIBTEX
40. Boughton BJ, Jackson N, Lim S, Smith N. Randomized trial of intravenous immunoglobulin prophylaxis for patients with chronic lymphocytic leukaemia and secondary hypogammaglobulinaemia. Clin Lab Haematol (1995) 17:75–80. doi: 10.1111/J.1365-2257.1995.TB00322.X
41. Favre O, Leimgruber A, Nicole A, Spertini F. Intravenous immunoglobulin replacement prevents severe and lower respiratory tract infections, but not upper respiratory tract and non-respiratory infections in common variable immune deficiency. Allergy: Eur J Allergy Clin Immunol (2005) 60:385–90. doi: 10.1111/j.1398-9995.2005.00756.x
42. Dhalla F, Lucas MM, Schuh AA, Bhole MM, Jain RR, Patel SY, et al. Antibody deficiency secondary to chronic lymphocytic leukemia: Should patients be treated with prophylactic replacement immunoglobulin? J Clin Immunol (2014) 34:277–82. doi: 10.1007/S10875-014-9995-5
43. McQuilten Z, Weinkove R, Crispin P, Degelia A, Dendle CG, Gilbertson M, et al. RATIONAL: A randomised controlled feasibility trial comparing prophylactic immunoglobulin with antibiotics in patients with acquired hypogammaglobulinaemia secondary to haematological malignancies. Hemasphere (2021) 5:99–100.
44. Raanani P, Gafter-Gvili A, Paul M, Ben-Bassat I, Leibovici L, Shpilberg O. Immunoglobulin prophylaxis in hematological malignancies and hematopoietic stem cell transplantation. Cochrane Database Syst Rev (2008) 8(4):CD006501. doi: 10.1002/14651858.CD006501.PUB2
45. Cooperative Group for the Study of Immunoglobulin in Chronic Lymphocytic Leukemia. Intravenous immunoglobulin for the prevention of infection in chronic lymphocytic leukemia. New Engl J Med (1988) 319:902–7. doi: 10.1056/nejm198810063191403
46. Griffiths H, Brennan V, Lea J, Bunch C, Lee M, Chapel H. Crossover study of immunoglobulin replacement therapy in patients with low-grade b-cell tumors. Blood (1989) 73:366–8. doi: 10.1182/BLOOD.V73.2.366.366
47. Molica S, Musto P, Chiurazzi F, Specchia G, Brugiatelli M, Cicoira L, et al. Prophylaxis against infections with low-dose intravenous immunoglobulins (IVIG) in chronic lymphocytic leukemia. results of a crossover study. Haematologica (1996) 81:121–6. doi: 10.3324/%x
48. Mustafa SS, Jamshed S, Vadamalai K, Ramsey A. Subcutaneous immunoglobulin replacement for treatment of humoral immune dysfunction in patients with chronic lymphocytic leukemia. PloS One (2021) 16:e0258529. doi: 10.1371/JOURNAL.PONE.0258529
49. Vacca A, Melaccio A, Sportelli A, Solimando AG, Dammacco F, Ria R. Subcutaneous immunoglobulins in patients with multiple myeloma and secondary hypogammaglobulinemia: a randomized trial. Clin Immunol (2018) 191:110–5. doi: 10.1016/J.CLIM.2017.11.014
50. Sleasman JW, Lumry WR, Hussain I, Wedner HJ, Harris JB, Courtney KL, et al. Immune globulin subcutaneous, human - klhw 20% for primary humoral immunodeficiency: an open-label, phase III study. Immunotherapy (2019) 11:1371–86. doi: 10.2217/IMT-2019-0159
51. Visentin A, Molinari MC, Pravato S, Cellini A, Angotzi F, Cavaretta CA, et al. A retrospective study on the efficacy of subcutaneous immunoglobulin as compared to intravenous formulation in patients with chronic lymphocytic leukemia and secondary antibody deficiency. Curr Oncol (2023) 30:274–83. doi: 10.3390/CURRONCOL30010022
52. Keegan A, Dennington PM, Dhondy N, Mulligan SP. Immunoglobulin replacement therapy in chronic lymphocytic leukaemia patients with hypogammaglobulinaemia and infection. Eur J Haematol (2022) 108:460–8. doi: 10.1111/EJH.13754
53. Windegger TM, Nghiem S, Nguyen KH, Fung YL, Scuffham PA. Cost-utility analysis comparing hospital-based intravenous immunoglobulin with home-based subcutaneous immunoglobulin in patients with secondary immunodeficiency. Vox Sang (2019) 114:237–46. doi: 10.1111/VOX.12760
54. Membe SK, Ho C, Cimon K, Morrison A, Kanani A, Roifman CM. Economic assessment of different modalities of immunoglobulin replacement therapy. Immunol Allergy Clin North Am (2008) 28:861–74. doi: 10.1016/J.IAC.2008.06.008
55. Colado A, Elías EE, Sarapura Martínez VJ, Cordini G, Morande P, Bezares F, et al. Immunomodulatory effects of different intravenous immunoglobulin preparations in chronic lymphocytic leukemia. Sci Rep (2021) 11:12926–6. doi: 10.1038/S41598-021-92412-8
Keywords: CLL, hypogammaglobulinemia, Ig replacement, immunomodulation, restoration immunity
Citation: Khan S, Allsup D and Molica S (2023) An updated perspective on immunoglobulin replacement in chronic lymphocytic leukaemia in the era of targeted therapies. Front. Oncol. 13:1135812. doi: 10.3389/fonc.2023.1135812
Received: 01 January 2023; Accepted: 27 March 2023;
Published: 06 April 2023.
Edited by:
Lydia Scarfò, Vita-Salute San Raffaele University, ItalyReviewed by:
Yair Herishanu, Tel Aviv Sourasky Medical Center, IsraelCopyright © 2023 Khan, Allsup and Molica. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefano Molica, c3RlZmFuby5tb2xpY2FAbmhzLm5ldA==, c21vbGljYUBsaWJlcm8uaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.