- Department of Hepatobiliary Surgery, Suining Central Hospital, Suining, China
The incidence of ampulla of Vater carcinoma, a type of periampullary cancer, has been increasing at an annual percentage rate of 0.9%. However, patients with ampulla of Vater carcinoma have quite different prognoses due to the heterogeneities of the tissue origin of this carcinoma. In addition to TNM staging, histological subtypes and molecular features of ampulla of Vater carcinoma are the key factors for predicting the clinical outcomes of patients. Fortunately, with the development of testing technology, information on the histological subtypes and molecular features of ampulla of Vater carcinoma is increasingly being analyzed in-depth. Patients with the pancreaticobiliary subtype have shorter survival times. In immunohistochemical examination, high cutoff values of positive MUC1 staining can be used to accurately predict the outcome of patients. Mutant KRAS, TP53, negative SMAD4 expression, and microsatellite stability are related to poor prognosis, while the clinical value of BRCA1/BRCA2 mutations is limited for prognosis. Testing the histological subtypes and molecular characteristics of ampulla of Vater carcinoma not only is the key to prognosis analysis but also provides extra information for targeted treatment to improve the clinical outcomes of patients.
1 Introduction
Ampulla of Vater carcinoma (AVC) arises from the complex periampullary region, including the common bile duct, duodenum, and Wirsung duct, and thus, has a mixed tissue origin (1). The incidence rate of AVC is 0.49 per 105 individuals (2, 3). Importantly, the incidence rate of AVC has been increasing (3).
Currently, the standard treatment for AVC is controversial. Pancreaticoduodenectomy (PD) and subsequent chemotherapy, similar to the therapeutic plan for pancreatic adenocarcinoma, have been utilized in many AVC cases (4). However, in contrast to the single tissue origin of pancreatic adenocarcinoma, treatments for AVC may need to depend on the tissue origin of AVC because of the complex anatomical structures of the ampulla of Vater. The substantial heterogeneity of AVC, including complex histological subtypes and molecular features, may be an important reason for different prognoses in different patients.
With the development of testing technology such as immunohistochemical examinations, exome sequencing, and ultradeep sequencing, the analysis of data regarding histological subtypes and molecular features of AVC has been performed in recent years. This promoted the clarification of the pathological mechanism of AVC and the classification of groups of patients with a high risk of death. Importantly, this information could guide further therapy to some degree and influence the prognosis of AVC patients.
In this article, a comprehensive review of recent studies regarding AVC was conducted, especially focusing on the relationship between histological subtypes, molecular features, and clinical outcomes.
2 Ampulla of Vater and AVC
2.1 Ampulla of Vater
The ampulla of Vater, called the ampulla complex, constitutes the dilated junction of the biliary, pancreatic, and digestive tracts, and it lies in the wall of the descending duodenum (5). In the ampulla of Vater, the terminal common bile duct and Wirsung duct are surrounded by the sphincteric system of Oddi, terminating at the major duodenal papilla, which are covered by the duodenal mucosa. The junction of the terminal common bile duct and Wirsung duct in the major duodenal papilla has three variations: 1) separate duodenal openings for the two canals, 2) a double-barreled opening at the apex of the papilla, and 3) an opening in the common duct (5).
The tissues from the ampulla complex, namely, intestinal, pancreatic ductal, and biliary epithelial tissues, have morphological heterogeneities (6). Therefore, when AVC is suspected, the tissue origin of AVC should be assessed.
2.2 AVC
AVC is a type of rare malignancy, and the current official data fail to show its epidemiological characteristics (7, 8). A large retrospective study from the United States reported that the age-adjusted incidence rate of AVC was 7.4 per 1,000,000 person-years between 1999 and 2013 (9). From the Biliary Tract Cancers Pooling Project of 19 studies (10), the incidence rates of AVC in women reached 12 per 1,000,000 person-years between 1980 and 2017.
However, the incidence rate of AVC for all racial groups has been significantly increasing at an annual percentage rate of 0.9% between 1973 and 2005 (P < 0.05) (3). Sometimes, the diagnosis of AVC and cancer of the head of the pancreas may be confusing. From another angle, AVC is a frequent indication for PD, accounting for approximately 21%–30% of diagnoses in patients receiving this procedure (11, 12). Between January 1995 and December 2014, nearly 21.6% (110/510) of patients who received PD therapy were finally diagnosed with AVC, second only to cancer of the head of the pancreas (11). In patients with periampullary cancers receiving PD therapy (12), the proportion of patients diagnosed with AVC was as high as 29.5% (61/207) during 1998–2009. In addition, the National Comprehensive Cancer Network (NCCN) published its first version of clinical practice guidelines for AVC on 9 March 2022.
Therefore, paying more attention to this special group of patients in surgical practice is cost-effective, as the proportion of AVC cases in patients undergoing PD therapy increases.
2.3 Different clinical outcomes of AVC and pancreatic cancer
Although the standard curative treatment for both pancreatic cancer and AVC is PD, patients with AVC have a much better overall survival (OS) than those with pancreatic cancer. The median survival of patients with resected pancreatic cancer was only 14.3 months, and the 5-year OS rate was 20.5% in a multicenter, phase III trial (13). However, the median OS of patients with resected AVC was 64 months and the 5-year OS rates were as high as 53% in a retrospective multicenter cohort study of 887 patients (14), and these results were consistent with those of other studies (51%–58%) (15–17).
The high OS may be related to certain features (18) of AVC, namely, significantly smaller tumor sizes and lower prevalence of lymph node metastases (all P < 0.001) as well as the PD therapy itself. Nevertheless, the strong influence of different histological subtypes (19) and some special genetic mutations (20) on the clinical outcomes of AVC patients should be noted, and the NCCN guidelines mention the relationship of histological subtypes, molecular features, and clinical outcomes.
3 Histological subtypes of AVC associated with clinical outcomes
3.1 Four histological subtypes of AVC
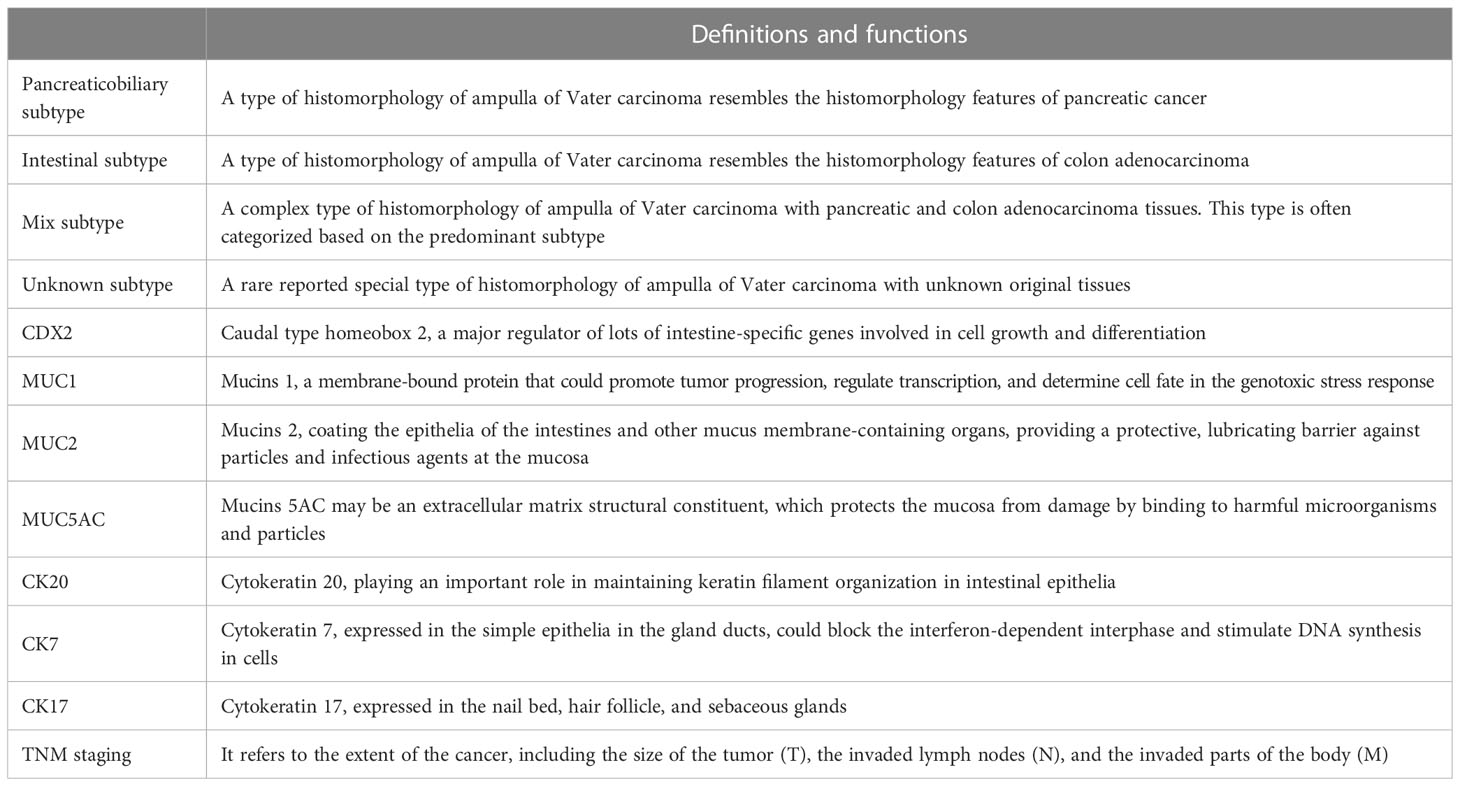
Importantly, the histological classification of solid tumors can be used to predict the clinical outcome of patients (12). According to the anatomical features of the ampulla of Vater, the tissue origin of AVC can be divided into three histological subtypes: the pancreaticobiliary, intestinal, and mixed subtypes (Table 1). Additionally, an unknown histological subtype of AVC has been reported. Interestingly, a study reported that the mixed subtype of AVC could be classified according to the predominant type of tissue, but there were 108 (33.9%) patients with unknown histological subtypes (21). In total, related reports about unknown histological subtypes of AVC are rare. Therefore, the pancreaticobiliary and intestinal subtypes are mainly discussed in this article, and the mixed subtype of AVC is mentioned.
3.2 Proportion of the two histological subtypes
Previous reports have shown that the proportions of the pancreaticobiliary and intestinal subtypes in AVC are variable. An international multicenter retrospective cohort study showed that there was a great proportion of pancreaticobiliary subtype AVC patients (293/547, 53.6%), while the proportion of intestinal subtype AVC patients was 38.6% (211/547), and only 43 AVC patients had the mixed subtype (14). Another retrospective study of 99 patients (18) also reported that there were slightly more patients with the pancreaticobiliary subtype than the intestinal subtype (49.5% vs. 44.4%). Another retrospective study (21) revealed that the number of patients with the pancreaticobiliary subtype was approximately equal to that with the intestinal subtype (n = 105 vs. n = 106). This study classified the mixed subtype of AVC into the intestinal subtype or pancreaticobiliary subtype. Another study (22) with a total of 45 patients between March 2015 and December 2019 revealed that the number of AVC patients with the pancreaticobiliary subtype was lower than that with the intestinal subtype (46.8% vs. 53.2%). The limited samples in the study may have contributed to the above results.
The number of AVC patients with the pancreaticobiliary subtype accounts for a large number of patients, and the 5-year OS of AVC patients with the pancreaticobiliary subtype is only 47% (14). Thus, its negative influence on the outcome of the whole group of AVC patients should be noted.
3.3 Pancreaticobiliary subtype related to poor prognosis
The pancreaticobiliary subtype is significantly associated with poor prognosis (Table 2), which has been validated in several different studies (12, 17, 18, 21–30), including meta-analyses. A large-sample research (21) showed that the median disease-free survival (DFS) time of patients with the intestinal subtype was 58.9 months. However, for those with the pancreaticobiliary subtype, DFS was only 25.3 months (P = 0.0123). The reasons may be that patients with the pancreaticobiliary subtype tend to present with a more advanced T stage and more frequently with regional lymph node involvement and perineural invasion than those with the intestinal subtype (15, 21, 23, 26, 28). Furthermore, a retrospective study of 106 patients (25) analyzed the influence of postoperative complications of PD therapy and the positive node ratio on prognosis from 1996 to 2015, and compared with the intestinal subtype, the pancreaticobiliary subtype was associated with poor prognosis (HR = 2.11, 95% CI = 1.04–4.27, P = 0.04). Furthermore, a previous study of 232 patients (31) indicated that AVC patients with the pancreaticobiliary subtype had a much better prognosis than those with pancreatic cancer (41 vs. 15.6 months, P < 0.001).
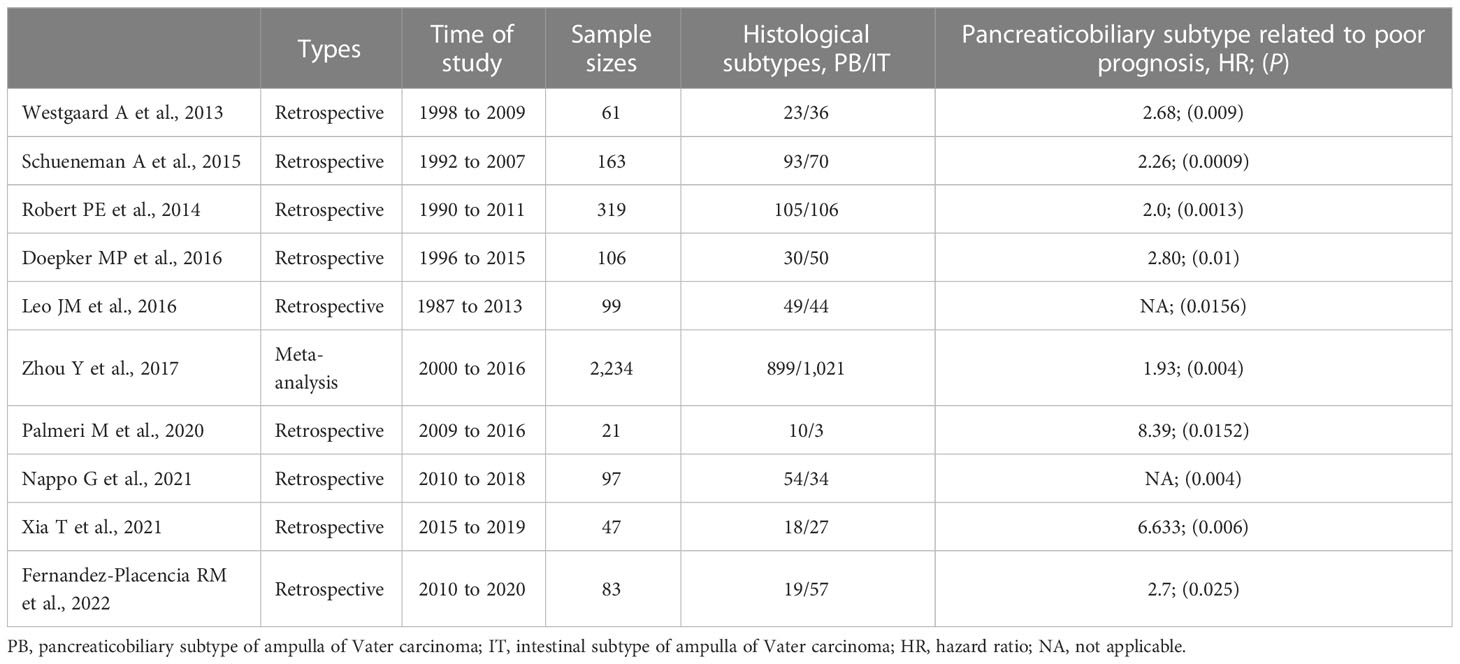
Table 2 Information of those articles concerning the histological subtypes of ampulla of Vater carcinoma and clinical outcomes.
3.4 Role of immunohistochemical examination in prognosis
Currently, there is no clear standard to discriminate the different histological subtypes of AVC via only morphological features or combined with immunohistochemical examination. Exploring the auxiliary use of immunohistochemical examination to define the histological subtypes of AVC and predict the clinical outcome is ongoing (19).
A group of patients with a pancreaticobiliary subtype (CDX2 negative and MUC1 positive in immunohistochemical examination) and lymph node metastases had a median survival of 11.9 months, and there were no 5-year survivors in this study of 208 patients from three independent cohorts (19). Considering that MUC1 was the key marker for diagnosing the pancreaticobiliary subtype in previous reports, a study of 163 patients from 1992 until 2007 further showed that a higher cutoff value (≥10%) for defining positive MUC1 staining may have better discrimination power for patients with a significantly worse prognosis (17).
In addition, immunohistochemical examination (Table 1) may play a role in the management and treatment of AVC patients with mixed subtypes. A retrospective study of 99 patients showed that AVC patients with mixed subtypes (negative MUC1, MUC2, CDX2, and CK20) had a similar prognosis to those with the pancreaticobiliary subtype (18). Another study with 97 patients (23) validated the result and described that patients with mixed subtypes had high 1-, 3-, and 5-year OS rates (87.5%, 33.3%, and not reached), similar to those with the pancreaticobiliary subtype (93.7%, 66.9%, and 53.6%, P = 0.4).
Furthermore, some other new key biomarkers for immunohistochemical examination have been assessed. High expression of CK7 and negative expression of CK20 in patients with the pancreaticobiliary subtype were revealed by tissue microarray chip analysis (27, 28). Additionally, the positive rate of CK17 expression in patients with the pancreaticobiliary subtype was very high (20/22, 90.9%) (28). More interestingly, MUC5AC positivity in AVC patients may have a direct and strong correlation with the clinical outcome. Regardless of histological subtype (32), setting the cutoff of MUC5AC positivity at either >25% or 1% (any positivity was regarded as an expression) and testing it on all patients yielded significant survival differences between the MUC5AC-positive versus MUC5AC-negative patients (P = 0.0007 and P = 0.02).
While the sample sizes in those studies were limited, new biomarkers from immunohistochemical examination should be developed and applied for predicting clinical outcomes in the future.
3.5 Other opinions on histological subtypes and outcomes
There seem to be other opinions on the effects of the pancreaticobiliary subtype on clinical outcome. No difference in median OS was found between intestinal subtype patients and pancreaticobiliary subtype patients (33). This result may be attributed to the limited sample sizes (45 cases), and this research was mainly focused on the somatic and germline genetic alterations of AVC patients. A retrospective cohort study showed that N stage, not the pathological subtype, was a stronger predictor of OS (14), which may be mainly due to the higher ratio of patients with the pancreaticobiliary subtype receiving adjuvant chemotherapy. The other reason may be that pancreaticobiliary subtype patients tended to present with a more advanced T stage and more frequently with N stage and perineural invasion than intestinal subtype patients (15).
TNM staging is a critical predictive factor for the clinical outcomes of AVC patients. A study reported the development of a prognostic nomogram for AVC patients based on the Surveillance, Epidemiology, and End Results database, which supported the strong influence of TNM staging on the outcome of AVC patients (26). However, it did not include data on chemotherapy, major comorbidities, and other important factors that could affect prognosis. Additionally, it failed to analyze the prognosis of patients with different histological subtypes. Moreover, a study that built a prognostic score for AVC patients receiving PD therapy (34, 35) based on TNM staging did not show close relationships between the pancreaticobiliary subtype and prognosis (1, 36). Furthermore, in the eighth edition guidelines, the T category has been reconsidered based on the strong prognostic impact of TNM staging, including the depth of duodenal and pancreatic invasion (37).
It is difficult to infer the causal relationship between the histological subtypes and other high-risk factors for death, such as TNM staging. Nevertheless, it is obvious that TNM staging may have a strong independent impact on the prognosis of AVC. However, while it might be weak, the influence of different histological subtypes on clinical outcomes should be noted, as it exists and might be independent.
3.6 Limitations of histological classification for AVC
Based on the different histological subtypes, patients with a high risk of death could be screened. However, it is difficult to select the optimal therapy and ultimately improve the outcome. Additionally, there are some histological subtypes without a clear definition, such as the mixed and unknown subtypes. One retrospective study indicated that the mixed subtype of AVC might have a distinct tumor nature and a significantly poor clinical outcome (38).
4 Molecular classification associated with clinical outcomes
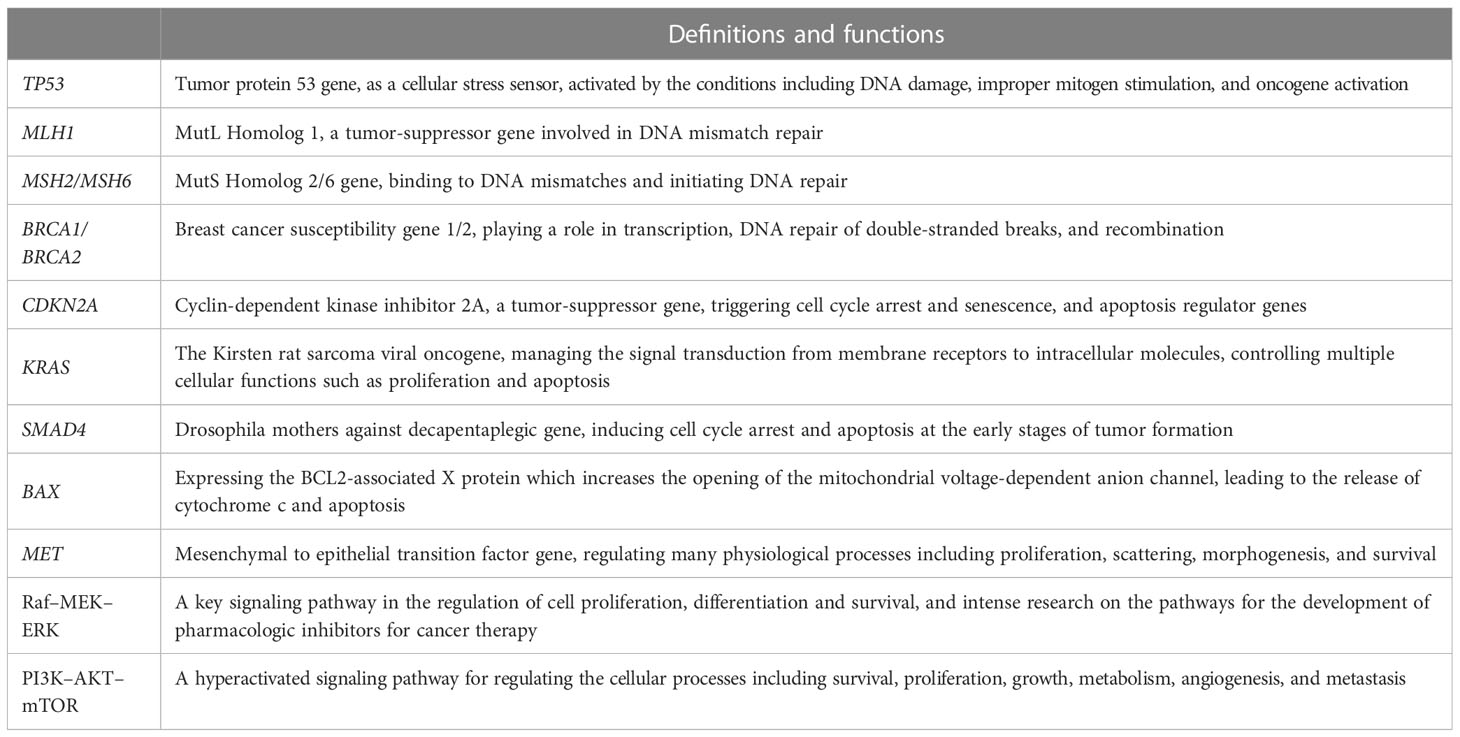
Identifying the molecular characteristics of AVC could be very interesting and impactful. The molecular classification of AVC is another important supplement for histological classification. Molecular classification could overcome the shortcomings of histological classification, as it can be used to predict the prognosis of patients and provide doctors with useful guidance for performing proper chemotherapy, targeted treatment, and immunotherapy (39). Rapid advances in sequencing technologies, including panel sequencing, exome sequencing, and ultradeep sequencing, have permitted in-depth analysis for the characterization of the molecular profile of AVC. Recently, the molecular features of other types of cancer, such as gastric cancer, have been estimated. Therefore, the NCCN guidelines for AVC also suggest testing for inherited mutations in all confirmed AVC patients, including TP53, MLH1, MSH2, MSH6, BRCA1, BRCA2, and CDKN2A (Table 3).
4.1 Different types of genetic mutations in AVC
The genetic mutations associated with AVC are very complex (40). KRAS mutations are oncogenic mutations. TP53 and BRCA2 mutations lead to alterations in tumor suppressors, which are closely related to the tumorigenesis of AVC. Gene mutations related to the mismatch repair of DNA (MLH1, MSH2, MSH6) are also tested in AVC patients, and they could cause microsatellite instability (MSI). Mutations in a cell cycle-related gene, CDKN2A, have been identified in AVC patients. SMAD4 mutations could lead to alterations in cell signaling proteins, which also correlated with the clinical outcomes of AVC patients. The alterations in these molecules are summarized and discussed in this review (Table 4). There are many additional changes in the molecular characteristics of AVC patients, including MDM2, ERBB2, ELF3, and PIK3CA (33, 41–43), but the reported clinical values of these gene changes in AVC patients may be limited.
4.2 KRAS mutation in AVC patients
The Kirsten rat sarcoma viral oncogene (KRAS) is a key gene that manages signal transduction from membrane receptors to intracellular molecules, activating the canonical Raf–MEK–ERK, PI3K–AKT–mTOR (Table 2), RalGDS–RalA/B, or TIAM1–RAC1 signaling pathways. The KRAS gene has the ability to control multiple cellular functions, such as proliferation, apoptosis, motility, and survival (44).
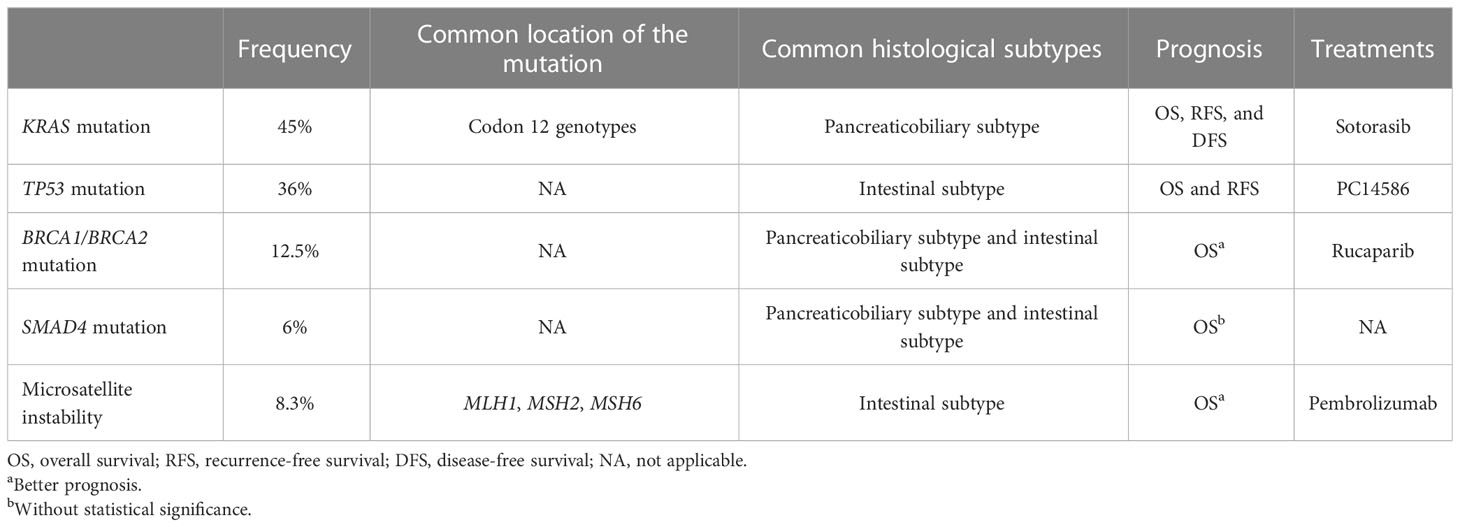
KRAS is one of the most frequently mutated genes in AVC patients (40). The mutation frequency of KRAS was as high as 33% in a study of 146 patients between 1982 and 2008 (45). A meta-analysis study (46) revealed that 45% (175/388) of AVC patients had KRAS mutations, which had a significant correlation with worse recurrence-free survival (HR = 2.74, P = 0.0008). A small sample study reported that KRAS was mutated in 35% of patients (47) and emerged as an independent prognostic factor regardless of the histological subtype (HR = 3.85, P = 0.0018). A large sample study showed that the median OS for AVC patients with mutant KRAS was 22.3 versus 47.7 months in patients carrying the wild-type KRAS gene (20).
Although there were overlapping genomic alterations between the two histological subtypes, a trend has been observed (42) that more frequent KRAS mutations are found in AVC patients with the pancreaticobiliary subtype (61%) than in AVC patients with the intestinal subtype (29%). When histological subtypes were analyzed together (48), concurrent KRAS gene positivity and MET copy number gain were proven to be independent prognostic factors for DFS in AVC patients with the pancreaticobiliary subtype (HR = 4.926, P = 0.047). This may contribute to the findings that KRAS mutation is significantly associated with patients presenting with a large tumor size (38).
Furthermore, there are some specific mutational genotypes of KRAS, especially codon 12 mutation—KRAS genotype G12C. The median survival time of patients with the KRAS genotype G12C mutation was 62 months, which was significantly poorer than that of patients without the KRAS mutation (155 months) in a retrospective study of 146 patients (45). The high mutation rate of KRAS genotype G12C could become a possible therapeutic target. Sotorasib (49), an inhibitor of KRAS genotype G12C, has just been approved by the Food and Drug Administration (FDA) to treat non-small cell lung cancer. Currently, other inhibitors for KRAS genotype G12C mutations, such as MRTX849, are being developed (50). These drugs may be beneficial for AVC patients with KRAS genotype G12C mutations in the future.
4.3 TP53 mutation in AVC patients
Tumor protein 53 gene (TP53) is a cellular stress sensor that is activated by conditions including DNA damage, improper mitogen stimulation, and oncogene activation (51). The downstream target genes transactivated by TP53 are numerous, including cyclin-dependent kinase inhibitor 1A (CDKN1A, encoding p21), which triggers cell cycle arrest and senescence, and apoptosis regulator genes (BAX and PUMA), which trigger apoptosis (52).
TP53 (encoding p53) is the other frequently mutated gene in patients with AVC. In all, 36%–72% of patients have been shown to have high levels of TP53 gene expression (35, 47, 53–57). Another study reported that in 82.4% (56/69) of AVC patients, abnormal p53 immunolabeling was detected (38), including nuclear accumulation of immunolabeled p53 (41.2%) and lack of p53 immunolabeling (41.2%).
TP53 mutation has been shown to be related to the clinical outcome of AVC patients. A previous study of 80 patients (47) showed that TP53 mutation is a negative predictor of survival in AVC patients (HR = 3.85, P = 0.0006), regardless of histological subtype. In a retrospective study of 53 patients (58), the clinical prognosis of AVC patients with TP53 overexpression was worse than that of the remaining patients (P = 0.006). Furthermore, a retrospective study, which consisted of 92 patients, reported that TP53-dependent transcription of cyclin-dependent kinase 1 was associated with an increased risk of recurrence-free survival (HR = 3.97, P = 0.02) and worse OS (HR = 11.16, P = 0.005) in patients with AVC after resection therapy (53).
Regarding the features of TP53 mutations in different histological subtypes of AVC, the difference in the proportion of TP53 mutations in the intestinal subtype and pancreaticobiliary subtype patients failed to reach statistical significance (55). A recent retrospective study (57) seemed to reveal that TP53 mutation was more frequent in AVC patients with the intestinal subtype (27/46, 58.6%) than in those with the pancreaticobiliary subtype (13/37, 35.1%, P = 0.042). This may be attributed to the limited sample sizes and the proportion of different histological subtypes in those AVC patients. The frequency of TP53 mutations in patients with different histological subtypes of AVC may need to be investigated in the future.
Currently, there are two chemical compounds, PC14586, a Y220C-selective structural corrector of mutant TP53, and BI 907828, an MDM2-p53 antagonist, in phase I clinical trials (59). Thus, a substantial amount of research must be conducted before these compounds can be applied in clinical practice.
4.4 BRCA1/BRCA2 mutation in AVC patients
Breast cancer susceptibility gene 1/2 (BRCA1/BRCA2) mutations predispose patients to select cancers, especially breast and ovarian cancer (40). However, a few studies have found BRCA2 mutations in AVC patients (33, 60, 61).
The frequency of BRCA2 mutations in AVC patients is low, but it might indicate the risk of cancer in female relatives of AVC patients. A small sample retrospective study showed that the frequency of BRCA2 mutations was 12.5% (2/16) in AVC patients, and the relatives of two patients had a history of cancer (61). For the prognosis, 3 of 44 patients with available germline data had pathogenic alterations in BRCA2, but the OS of the 3 patients were clearly different—84, 18, and 2 months (33). Although it is an indispensable event for some cancers, BRCA2 mutations appear to be biologically neutral in a proportion of other cancers (62), including AVC. Therefore, meaningful pathogenic germline variants of BRCA2 should be identified further. In the different histological subtypes, the frequency of BRCA2 mutations in the pancreaticobiliary subtype could be similar to that in the intestinal subtype (6/13, 46.2% vs. 11/22, 50.0%, P = 0.3) (60).
Additionally, testing the BRCA2 mutation could guide doctors to use rucaparib (a type of poly ADP-ribose polymerase inhibitor, PARPi) for the treatment of patients with advanced ovarian cancer and metastatic castration-resistant prostate cancer (63). Therefore, AVC patients with loss of heterozygosity or mutations in BRCA2 may benefit from the use of PARPi (33), and the clinical outcomes of those patients could be improved to some degree.
4.5 SMAD4 mutation in AVC patients
The SMAD4 gene plays a pivotal role in the switch of TGF-β function by inducing cell cycle arrest and apoptosis at the early stages of tumor formation (64). The frequency of SMAD4 mutation is quite variable, ranging from 6% to 75% (38, 43, 57, 65, 66). Furthermore, a retrospective study reported that two patients (10%) with mutant SMAD4 were identified among 20 patients with KRAS mutation (43).
The clinical outcome of patients with SMAD4 mutations might be similar to that of patients without mutations (40, 66). The mortality rate (66) was higher for AVC patients with negative (absent, trace, or focal) SMAD4 expression (62% vs. 31%) than for patients with positive SMAD4 expression, although the difference may not be statistically significant (P = 0.0865). The reason might contribute to the limited sample size—63 cases. However, an earlier study of 140 patients indicated that the SMAD4 mutation was not related to the 5-year survival rate (67). However, there were no more detailed data in that article. The influence of SMAD4 mutation on prognosis should be validated in a larger sample cohort study.
Regarding the histological subtypes, a previous retrospective study reported that the frequency of SMAD4 mutations in AVC patients with the intestinal subtype (28.2%) was higher than that in patients with the pancreaticobiliary subtype (16.2%), but the P-value was 0.293 (57). The difference in the frequency was not significant (38, 67). In addition, there are currently no available drugs to target SMAD4 mutations.
4.6 Microsatellite instability and AVC
MSI, a molecular phenotype of tumors, is the gain or loss of nucleotides in the short repeating motifs of DNA elements (microsatellite tracts) (68). It was highlighted that if the patient had tumors with MSI phenotypes, analysis of PD-1/PD-L1 expression or tumor mutational burden is unnecessary for receiving immunotherapy—immune checkpoint inhibitor treatments (69). The damaged mismatch repair system (MMR deficiency) for DNA, including the alteration in the MLH1, MSH2, or MSH6 gene, could cause MSI. However, MMR deficiency often has a Lynch-suggestive profile, and routine testing for Lynch syndrome is warranted (29). MSI may be a dependable marker for prognosis.
The overall frequency of MSI phenotypes in AVC patients is unclear (70), but it might be lower than 8.3% (69). Four of 49 (8.2%) tested AVC patients showed MSI phenotypes in next-generation sequencing analysis and showed loss of MLH1 (65). Two of 53 consecutive (6%) patients showed MMR deficiency (70). Despite the low frequency of MSI phenotypes (3%), patients with MSI phenotypes had a better survival than patients with non-MSI periampullary cancers (P < 0.0021). Additionally, the six AVC patients with MSI phenotypes survived from 2 to 8 years after diagnosis (41), which was higher than the AVC patients with microsatellite stability phenotypes (P = 0.04). AVC patients with MSI phenotypes who underwent resection therapy had no evidence of recurrent disease at the cutoff time in a study of 59 patients (65). The AVC patients (29) with MMR deficiency accounted for up to 18% (23/128). Regarding the relationship between histological subtypes and MSI, a study reported that both AVC patients with MSI had the intestinal subtype of AC (65).
Although current articles concerning AVC patients with MSI are limited and the data are discrepant, the value of MSI for immunotherapy is clear. Testing MSI, MMR deficiency, tumor mutational burden, and even the number of tumor-infiltrating lymphocytes could inform the choice of proper therapy for improving the clinical outcome of AVC patients.
4.7 Others
CD340 (HER2) amplification is not a rare event (14/106, 13%) in AVC patients, and it can be reliably identified using routine immunohistochemistry (42). Patients with CD340 amplification may be candidates for targeted therapy (trastuzumab). Other mutant genes have also been reported, although their clinical values are limited. Mutant CDKN2A in AVC patients has been reported (71). Several other molecular alterations in the PI3K, Wnt signaling, and TGF-β pathways and the cell cycle have also been reported in AVC patients (40). Activation of the AKT and MAPK pathways is increased in patients with the pancreaticobiliary subtype with poor clinical outcomes (72).
Interestingly, a new genome algorithm has been developed based on a prospectively sequenced cohort with 3,411 patients for application to panel targeted DNA sequencing data from AVC patients (73). It can be used to predict the outcomes of different AVC patients with mixed subtypes. Additionally, this algorithm was consistent with reports (45, 48), indicating that the genomic scores were higher in patients with the pancreaticobiliary subtype (mutations of the KRAS gene) and associated with lower survival probability.
4.8 Limitations of molecular classification
There are many advantages to the molecular classification of AVC. However, some complex limitations in gene testing and analysis for AVC should be noted. First, the incidence of AVC is low, and the heterogeneity of AVC patients, such as histological origins, is substantial. Therefore, the available samples for gene testing are relatively few, and reliable data in large sample size research are absent. Second, the different methods of gene testing and various definitions of gene mutations could make equivalent analysis very difficult in clinical practice. Third, the role of molecular alterations in the modifications of therapy may be limited. Only 13.3% (397/2,984) of patients harbored the pathogenic germline variant (PGV), and nearly 30% of those patients with high-penetrance variants of PGV had modifications in their therapy (74). In summary, the molecular classification data for AVC patients are hard to come by, and not every finding regarding molecular characteristics in AVC is accurate and has therapeutic applicability. However, the NCCN recommends testing some valuable genes. Therefore, focusing on those key molecular alterations in clinical practice is relatively cost-effective.
5 Future prospects
The close relationships between histological subtypes, molecular features, and clinical outcomes of AVC patients would be proven. It was mentioned that some other factors, such as safer surgical therapy (75) and efficient management in the perioperative period (76), could be included in the prognostic analysis for reaching reliable conclusions. With the rapid accumulation of data, the development of data sharing platforms, and the decrease of the cost of gene testing and analysis, the conclusions will be verified in the near future.
6 Conclusions
The histological subtypes and molecular alterations of patients with AVC play an important role in predicting and improving clinical outcomes. In the analysis of the histological subtypes of AVC, the pancreaticobiliary subtype is obviously related to poor clinical outcomes. Immunohistochemical examinations are the key to prognosis analysis. Testing the molecular characteristics of tumors not only helps to predict the prognosis of patients with mixed or unknown histological subtypes but also provides information for selecting targeted treatments for AVC patients.
Author contributions
HL: conceptualization, methodology and investigation, formal analysis, and writing of the original draft. YZ: acquisition of data and article revision. Y-kW: conceptualization, methodology, supervision, and article revision. All authors approved the final manuscript and were accountable for the contents of the article. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Sichuan Provincial Science and Technology Department (Grant No. 2019YJ0704).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Neoptolemos JP, Moore MJ, Cox TF, Valle JW, Palmer DH, McDonald AC, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. Jama (2012) 308(2):147–56. doi: 10.1001/jama.2012.7352
2. Henson DE, Schwartz AM, Nsouli H, Albores-Saavedra J. Carcinomas of the pancreas, gallbladder, extrahepatic bile ducts, and ampulla of vater share a field for carcinogenesis: a population-based study. Arch Pathol Lab Med (2009) 133(1):67–71. doi: 10.5858/133.1.67
3. Albores-Saavedra J, Schwartz AM, Batich K, Henson DE. Cancers of the ampulla of vater: demographics, morphology, and survival based on 5,625 cases from the SEER program. J Surg Oncol (2009) 100(7):598–605. doi: 10.1002/jso.21374
4. Rizzo A, Dadduzio V, Lombardi L, Ricci AD, Gadaleta-Caldarola G. Ampullary carcinoma: an overview of a rare entity and discussion of current and future therapeutic challenges. Curr Oncol (Toronto Ont). (2021) 28(5):3393–402. doi: 10.3390/curroncol28050293
5. Avisse C, Flament JB, Delattre JF. Ampulla of vater. anatomic, embryologic, and surgical aspects. Surg Clin North Am (2000) 80(1):201–12. doi: 10.1016/s0039-6109(05)70402-3
6. Pea A, Riva G, Bernasconi R, Sereni E, Lawlor RT, Scarpa A, et al. Ampulla of vater carcinoma: molecular landscape and clinical implications. World J gastrointestinal Oncol (2018) 10(11):370–80. doi: 10.4251/wjgo.v10.i11.370
7. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
8. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA: Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
9. Van Dyke AL, Shiels MS, Jones GS, Pfeiffer RM, Petrick JL, Beebe-Dimmer JL, et al. Biliary tract cancer incidence and trends in the united states by demographic group, 1999-2013. Cancer (2019) 125(9):1489–98. doi: 10.1002/cncr.31942
10. Jackson SS, Adami HO, Andreotti G, Beane-Freeman LE, de González AB, Buring JE, et al. Associations between reproductive factors and biliary tract cancers in women from the biliary tract cancers pooling project. J Hepatol (2020) 73(4):863–72. doi: 10.1016/j.jhep.2020.04.046
11. Williams JL, Chan CK, Toste PA, Elliott IA, Vasquez CR, Sunjaya DB, et al. Association of histopathologic phenotype of periampullary adenocarcinomas with survival. JAMA Surg (2017) 152(1):82–8. doi: 10.1001/jamasurg.2016.3466
12. Westgaard A, Pomianowska E, Clausen OP, Gladhaug IP. Intestinal-type and pancreatobiliary-type adenocarcinomas: how does ampullary carcinoma differ from other periampullary malignancies? Ann Surg Oncol (2013) 20(2):430–9. doi: 10.1245/s10434-012-2603-0
13. Versteijne E, van Dam JL, Suker M, Janssen QP, Groothuis K, Akkermans-Vogelaar JM, et al. Neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: long-term results of the Dutch randomized PREOPANC trial. J Clin Oncol (2022) 40(11):1220–30. doi: 10.1200/jco.21.02233
14. Moekotte AL, Lof S, Van Roessel S, Fontana M, Dreyer S, Shablak A, et al. Histopathologic predictors of survival and recurrence in resected ampullary adenocarcinoma: international multicenter cohort study. Ann Surg (2020) 272(6):1086–93. doi: 10.1097/sla.0000000000003177
15. Kim WS, Choi DW, Choi SH, Heo JS, You DD, Lee HG. Clinical significance of pathologic subtype in curatively resected ampulla of vater cancer. J Surg Oncol (2012) 105(3):266–72. doi: 10.1002/jso.22090
16. Zhou J, Zhang Q, Li P, Shan Y, Zhao D, Cai J. Prognostic factors of carcinoma of the ampulla of vater after surgery. Tumour Biol (2014) 35(2):1143–8. doi: 10.1007/s13277-013-1153-9
17. Schueneman A, Goggins M, Ensor J, Saka B, Neishaboori N, Lee S, et al. Validation of histomolecular classification utilizing histological subtype, MUC1, and CDX2 for prognostication of resected ampullary adenocarcinoma. Br J Cancer. (2015) 113(1):64–8. doi: 10.1038/bjc.2015.172
18. Leo JM, Kalloger SE, Peixoto RD, Gale NS, Webber DL, Owen DA, et al. Immunophenotyping of ampullary carcinomata allows for stratification of treatment specific subgroups. J Clin pathology. (2016) 69(5):431–9. doi: 10.1136/jclinpath-2015-203337
19. Chang DK, Jamieson NB, Johns AL, Scarlett CJ, Pajic M, Chou A, et al. Histomolecular phenotypes and outcome in adenocarcinoma of the ampulla of vater. J Clin Oncol (2013) 31(10):1348–56. doi: 10.1200/jco.2012.46.8868
20. Schultz NA, Roslind A, Christensen IJ, Horn T, Høgdall E, Pedersen LN, et al. Frequencies and prognostic role of KRAS and BRAF mutations in patients with localized pancreatic and ampullary adenocarcinomas. Pancreas (2012) 41(5):759–66. doi: 10.1097/MPA.0b013e31823cd9df
21. Robert PE, Leux C, Ouaissi M, Miguet M, Paye F, Merdrignac A, et al. Predictors of long-term survival following resection for ampullary carcinoma: a large retrospective French multicentric study. Pancreas (2014) 43(5):692–7. doi: 10.1097/mpa.0000000000000112
22. Xia T, Wu X, Mou Y, Xu Y, Zhou Y, Lu C, et al. Clinicopathological prognostic factors and chemotherapeutic outcome for two histopathological types of ampulla of vater adenocarcinoma. Front Oncol (2021) 11:616108. doi: 10.3389/fonc.2021.616108
23. Nappo G, Galvanin J, Gentile D, Capretti G, Pulvirenti A, Bozzarelli S, et al. Long-term outcomes after pancreatoduodenectomy for ampullary cancer: the influence of the histological subtypes and comparison with the other periampullary neoplasms. Pancreatology (2021) 21(5):950–6. doi: 10.1016/j.pan.2021.03.005
24. Zhou Y, Li D, Wu L, Si X. The histopathologic type predicts survival of patients with ampullary carcinoma after resection: a meta-analysis. Pancreatology (2017) 17(2):273–8. doi: 10.1016/j.pan.2017.01.007
25. Doepker MP, Thompson ZJ, Centeno BA, Kim RD, Wong J, Hodul PJ. Clinicopathologic and survival analysis of resected ampullary adenocarcinoma. J Surg Oncol (2016) 114(2):170–5. doi: 10.1002/jso.24281
26. Li HB, Zhao FQ, Zhou J. Prognostic nomogram for disease-specific survival in patients with non-metastatic ampullary carcinoma after surgery. Ann Surg Oncol (2019) 26(4):1079–85. doi: 10.1245/s10434-018-07115-8
27. Palmeri M, Funel N, Franco GD, Furbetta N, Gianardi D, Guadagni S, et al. Tissue microarray-chip featuring computerized immunophenotypical characterization more accurately subtypes ampullary adenocarcinoma than routine histology. World J Gastroenterol (2020) 26(43):6822–36. doi: 10.3748/wjg.v26.i43.6822
28. Liu FF, Shen DH, Wang HL, Ma YT, Yuan F, Liu J, et al. [Combined application of immunohistochemical markers to identify pathologic subtypes of ampullary carcinoma and its clinical significance]. Zhonghua bing li xue za zhi = Chin J Pathol (2019) 48(2):92–7. doi: 10.3760/cma.j.issn.0529-5807.2019.02.003
29. Xue Y, Balci S, Aydin Mericoz C, Taskin OC, Jiang H, Pehlivanoglu B, et al. Frequency and clinicopathologic associations of DNA mismatch repair protein deficiency in ampullary carcinoma: routine testing is indicated. Cancer (2020) 126(21):4788–99. doi: 10.1002/cncr.33135
30. Fernandez-Placencia RM, Montenegro P, Guerrero M, Serrano M, Ortega E, Bravo M, et al. Survival after curative pancreaticoduodenectomy for ampullary adenocarcinoma in a south American population: a retrospective cohort study. World J Gastrointest Surg (2022) 14(1):24–35. doi: 10.4240/wjgs.v14.i1.24
31. Reid MD, Balci S, Ohike N, Xue Y, Kim GE, Tajiri T, et al. Ampullary carcinoma is often of mixed or hybrid histologic type: an analysis of reproducibility and clinical relevance of classification as pancreatobiliary versus intestinal in 232 cases. Modern Pathol (2016) 29(12):1575–85. doi: 10.1038/modpathol.2016.124
32. Xue Y, Reid MD, Balci S, Quigley B, Muraki T, Memis B, et al. Immunohistochemical classification of ampullary carcinomas: critical reappraisal fails to confirm prognostic relevance for recently proposed panels, and highlights MUC5AC as a strong prognosticator. Am J Surg Pathol (2017) 41(7):865–76. doi: 10.1097/pas.0000000000000863
33. Wong W, Lowery MA, Berger MF, Kemel Y, Taylor B, Zehir A, et al. Ampullary cancer: evaluation of somatic and germline genetic alterations and association with clinical outcomes. Cancer (2019) 125(9):1441–8. doi: 10.1002/cncr.31951
34. Colussi O, Voron T, Pozet A, Hammel P, Sauvanet A, Bachet JB, et al. Prognostic score for recurrence after whipple's pancreaticoduodenectomy for ampullary carcinomas; results of an AGEO retrospective multicenter cohort. Eur J Surg Oncol (2015) 41(4):520–6. doi: 10.1016/j.ejso.2015.01.010
35. Perkins G, Svrcek M, Bouchet-Doumenq C, Voron T, Colussi O, Debove C, et al. Can we classify ampullary tumours better? clinical, pathological and molecular features. results of an AGEO study. Br J Cancer. (2019) 120(7):697–702. doi: 10.1038/s41416-019-0415-8
36. Shaib WL, Sharma R, Brutcher E, Kim S, Maithel SK, Chen Z, et al. Treatment utilization and surgical outcome of ampullary and duodenal adenocarcinoma. J Surg Oncol (2014) 109(6):556–60. doi: 10.1002/jso.23529
37. Imamura T, Yamamoto Y, Sugiura T, Okamura Y, Ito T, Ashida R, et al. The prognostic relevance of the new 8th edition of the union for international cancer control classification of TNM staging for ampulla of vater carcinoma. Ann Surg Oncol (2019) 26(6):1639–48. doi: 10.1245/s10434-019-07238-6
38. Asano E, Okano K, Oshima M, Kagawa S, Kushida Y, Munekage M, et al. Phenotypic characterization and clinical outcome in ampullary adenocarcinoma. J Surg Oncol (2016) 114(1):119–27. doi: 10.1002/jso.24274
39. Lee J, Kim ST, Kim K, Lee H, Kozarewa I, Mortimer PGS, et al. Tumor genomic profiling guides patients with metastatic gastric cancer to targeted treatment: the VIKTORY umbrella trial. Cancer Discov (2019) 9(10):1388–405. doi: 10.1158/2159-8290.Cd-19-0442
40. Jayaramayya K, Balachandar V, Santhy KS. Ampullary carcinoma-a genetic perspective. Mutat Res Rev Mutat Res (2018) 776:10–22. doi: 10.1016/j.mrrev.2018.03.002
41. Gingras MC, Covington KR, Chang DK, Donehower LA, Gill AJ, Ittmann MM, et al. Ampullary cancers harbor ELF3 tumor suppressor gene mutations and exhibit frequent WNT dysregulation. Cell Rep (2016) 14(4):907–19. doi: 10.1016/j.celrep.2015.12.005
42. Hechtman JF, Liu W, Sadowska J, Zhen L, Borsu L, Arcila ME, et al. Sequencing of 279 cancer genes in ampullary carcinoma reveals trends relating to histologic subtypes and frequent amplification and overexpression of ERBB2 (HER2). Modern Pathol (2015) 28(8):1123–9. doi: 10.1038/modpathol.2015.57
43. Mikhitarian K, Pollen M, Zhao Z, Shyr Y, Merchant NB, Parikh A, et al. Epidermal growth factor receptor signaling pathway is frequently altered in ampullary carcinoma at protein and genetic levels. Modern Pathol (2014) 27(5):665–74. doi: 10.1038/modpathol.2013.185
44. Román M, Baraibar I, López I, Nadal E, Rolfo C, Vicent S, et al. KRAS oncogene in non-small cell lung cancer: clinical perspectives on the treatment of an old target. Mol cancer. (2018) 17(1):33. doi: 10.1186/s12943-018-0789-x
45. Valsangkar NP, Ingkakul T, Correa-Gallego C, Mino-Kenudson M, Masia R, Lillemoe KD, et al. Survival in ampullary cancer: potential role of different KRAS mutations. Surgery (2015) 157(2):260–8. doi: 10.1016/j.surg.2014.08.092
46. Kim BJ, Jang HJ, Kim JH, Kim HS, Lee J. KRAS mutation as a prognostic factor in ampullary adenocarcinoma: a meta-analysis and review. Oncotarget (2016) 7(36):58001–6. doi: 10.18632/oncotarget.11156
47. Mafficini A, Amato E, Cataldo I, Rusev BC, Bertoncello L, Corbo V, et al. Ampulla of vater carcinoma: sequencing analysis identifies TP53 status as a novel independent prognostic factor and potentially actionable ERBB, PI3K, and WNT pathways gene mutations. Ann surgery. (2018) 267(1):149–56. doi: 10.1097/sla.0000000000001999
48. Kwon MJ, Kim JW, Jeon JY, Nam ES, Cho SJ, Park HR, et al. Concurrent MET copy number gain and KRAS mutation is a poor prognostic factor in pancreatobiliary subtype ampullary cancers. Pathol Res Pract (2017) 213(4):381–8. doi: 10.1016/j.prp.2017.01.004
49. Blair HA. Sotorasib: first approval. Drugs (2021) 81(13):1573–9. doi: 10.1007/s40265-021-01574-2
50. Huang L, Guo Z, Wang F, Fu L. KRAS mutation: from undruggable to druggable in cancer. Signal Transduct Target Ther (2021) 6(1):386. doi: 10.1038/s41392-021-00780-4
51. Bykov VJN, Eriksson SE, Bianchi J, Wiman KG. Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer. (2018) 18(2):89–102. doi: 10.1038/nrc.2017.109
52. Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell (2009) 137(3):413–31. doi: 10.1016/j.cell.2009.04.037
53. Guo R, Overman M, Chatterjee D, Rashid A, Shroff S, Wang H, et al. Aberrant expression of p53, p21, cyclin D1, and Bcl2 and their clinicopathological correlation in ampullary adenocarcinoma. Hum Pathol (2014) 45(5):1015–23. doi: 10.1016/j.humpath.2013.12.016
54. Saraggi D, Galuppini F, Fanelli GN, Remo A, Urso EDL, Bao RQ, et al. MiR-21 up-regulation in ampullary adenocarcinoma and its pre-invasive lesions. Pathol Res Pract (2018) 214(6):835–9. doi: 10.1016/j.prp.2018.04.018
55. Perysinakis I, Minaidou E, Mantas D, Sotiropoulos GC, Leontara V, Tsipras H, et al. Differentiation and prognostic markers in ampullary cancer: role of p53, MDM2, CDX2, mucins and cytokeratins. Pathol Res Pract (2016) 212(11):1039–47. doi: 10.1016/j.prp.2016.09.004
56. Gleeson FC, Kerr SE, Kipp BR, Voss JS, Minot DM, Tu ZJ, et al. Targeted next generation sequencing of endoscopic ultrasound acquired cytology from ampullary and pancreatic adenocarcinoma has the potential to aid patient stratification for optimal therapy selection. Oncotarget (2016) 7(34):54526–36. doi: 10.18632/oncotarget.9440
57. Mishra SK, Kumari N, Krishnani N, Singh RK, Mohindra S. Identification and prevalence of potentially therapeutic targetable variants of major cancer driver genes in ampullary cancer patients in India through deep sequencing. Cancer Genet (2021) 41-8:258–9. doi: 10.1016/j.cancergen.2021.08.001
58. Park SH, Kim YI, Park YH, Kim SW, Kim KW, Kim YT, et al. Clinicopathologic correlation of p53 protein overexpression in adenoma and carcinoma of the ampulla of vater. World J surgery. (2000) 24(1):54–9. doi: 10.1007/s002689910011
59. Glimmers of hope for targeting p53. Cancer Discov (2022) 12(8):Of5. doi: 10.1158/2159-8290.Cd-nd2022-0009
60. Kumari N, Singh RK, Mishra SK, Raghvendra L, Mohindra S, Krishnani N. Prevalence and spectrum of pathogenic germline variants in intestinal and pancreatobiliary type of ampullary cancer. Pathol Res Pract (2021) 217:153309. doi: 10.1016/j.prp.2020.153309
61. Pinto P, Peixoto A, Santos C, Rocha P, Pinto C, Pinheiro M, et al. Analysis of founder mutations in rare tumors associated with hereditary Breast/Ovarian cancer reveals a novel association of BRCA2 mutations with ampulla of vater carcinomas. PloS One (2016) 11(8):e0161438. doi: 10.1371/journal.pone.0161438
62. Jonsson P, Bandlamudi C, Cheng ML, Srinivasan P, Chavan SS, Friedman ND, et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature (2019) 571(7766):576–9. doi: 10.1038/s41586-019-1382-1
63. Abida W, Patnaik A, Campbell D, Shapiro J, Bryce AH, McDermott R, et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol (2020) 38(32):3763–72. doi: 10.1200/jco.20.01035
64. Zhao M, Mishra L, Deng CX. The role of TGF-β/SMAD4 signaling in cancer. Int J Biol Sci (2018) 14(2):111–23. doi: 10.7150/ijbs.23230
65. Harthimmer MR, Stolborg U, Pfeiffer P, Mortensen MB, Fristrup C, Detlefsen S. Mutational profiling and immunohistochemical analysis of a surgical series of ampullary carcinomas. J Clin Pathol (2019) 72(11):762–70. doi: 10.1136/jclinpath-2019-205912
66. Alkhasawneh A, Duckworth LV, George TJ, Desai NV, Sommerfeld AJ, Lu X, et al. Clinical, morphologic, and immunophenotypic characteristics of ampullary carcinomas with an emphasis on SMAD4 expression. J Gastrointest Oncol (2016) 7(6):974–81. doi: 10.21037/jgo.2016.06.14
67. McCarthy DM, Hruban RH, Argani P, Howe JR, Conlon KC, Brennan MF, et al. Role of the DPC4 tumor suppressor gene in adenocarcinoma of the ampulla of vater: analysis of 140 cases. Modern Pathol (2003) 16(3):272–8. doi: 10.1097/01.Mp.0000057246.03448.26
68. Hause RJ, Pritchard CC, Shendure J, Salipante SJ. Classification and characterization of microsatellite instability across 18 cancer types. Nat Med (2016) 22(11):1342–50. doi: 10.1038/nm.4191
69. Luchini C, Bibeau F, Ligtenberg MJL, Singh N, Nottegar A, Bosse T, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol (2019) 30(8):1232–43. doi: 10.1093/annonc/mdz116
70. Agaram NP, Shia J, Tang LH, Klimstra DS. DNA Mismatch repair deficiency in ampullary carcinoma: a morphologic and immunohistochemical study of 54 cases. Am J Clin Pathol (2010) 133(5):772–80. doi: 10.1309/ajcpgdde8plldrcc
71. Imai Y, Tsurutani N, Oda H, Nakatsuru Y, Inoue T, Ishikawa T. p16INK4 gene mutations are relatively frequent in ampullary carcinomas. Japanese J Cancer Res Gann. (1997) 88(10):941–6. doi: 10.1111/j.1349-7006.1997.tb00312.x
72. Overman MJ, Zhang J, Kopetz S, Davies M, Jiang ZQ, Stemke-Hale K, et al. Gene expression profiling of ampullary carcinomas classifies ampullary carcinomas into biliary-like and intestinal-like subtypes that are prognostic of outcome. PloS One (2013) 8(6):e65144. doi: 10.1371/journal.pone.0065144
73. Chakraborty S, Ecker BL, Seier K, Aveson VG, Balachandran VP, Drebin JA, et al. Genome-derived classification signature for ampullary adenocarcinoma to improve clinical cancer care. Clin Cancer Res (2021) 27(21):5891–9. doi: 10.1158/1078-0432.Ccr-21-1906
74. Samadder NJ, Riegert-Johnson D, Boardman L, Rhodes D, Wick M, Okuno S, et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncol (2021) 7(2):230–7. doi: 10.1001/jamaoncol.2020.6252
75. Huscher CGS, Lazzarin G. Coronary artery stent for securing pancreatico-jejunal anastomosis after PD: the "Huscher technique". Pancreatology (2022) 22(7):1057–8. doi: 10.1016/j.pan.2022.08.005
Keywords: ampulla of Vater carcinoma, molecular features, clinical outcomes, relationships, histological subtypes
Citation: Liang H, Zhu Y and Wu Y-k (2023) Ampulla of Vater carcinoma: advancement in the relationships between histological subtypes, molecular features, and clinical outcomes. Front. Oncol. 13:1135324. doi: 10.3389/fonc.2023.1135324
Received: 31 December 2022; Accepted: 03 May 2023;
Published: 18 May 2023.
Edited by:
John Gibbs, Hackensack Meridian Health, United StatesReviewed by:
Gianni Lazzarin, Abano Terme Hospital, ItalyGregory Tiesi, Jersey Shore University Medical Center (JSUMC), United States
Copyright © 2023 Liang, Zhu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ya-kun Wu, MzUzODI3NTE0QHFxLmNvbQ==
 Hao Liang
Hao Liang Yu Zhu
Yu Zhu Ya-kun Wu
Ya-kun Wu