
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 14 April 2023
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1134933
This article is part of the Research Topic The Role of Tumor Microenvironment in the Development, Treatment and Prognosis of Hepatocellular Carcinoma View all 30 articles
Background: This study aimed to investigate the role of the alpha fetoprotein (AFP) ratio before and after curative resection in the prognosis of patients with hepatocellular carcinoma (HCC) and to develop a novel pre- to postoperative AFP ratio nomogram to predict recurrence free survival (RFS) for HCC patients after curative resection.
Methods: A total of 485 pathologically confirmed HCC patients who underwent radical hepatectomy from January 2010 to December 2018 were retrospectively analyzed. The independent prognostic factors of hepatocellular carcinoma were identified by multivariate COX proportional model analysis, and the nomogram model was constructed. The receiver operating characteristic and the C-index were used to evaluate the accuracy and efficacy of the model prediction, the correction curve was used to assess the calibration of the prediction model, and decision curve analysis was used to evaluate the clinical application value of the nomogram model.
Results: A total of 485 HCC patients were divided into the training cohort (n = 340) and the validation cohort (n = 145) by random sampling at a ratio of 7:3. Using X-tile software, it was found that the optimal cut-off value of the AFP ratio in the training cohort was 0.8. In both cohorts, the relapse-free survival of patients with an AFP ratio <0.8 (high-risk group) was significantly shorter than in those with an AFP ratio ≥0.8 (low-risk group) (P < 0.05). An AFP ratio <0.8 was an independent risk factor for recurrence of HCC after curative resection. Based on the AFP ratio, BCLC stage and cirrhosis diagnosis, a satisfactory nomogram was developed. The AUC of our nomogram for predicting 1-, 3-, and 5-year RFS was 0.719, 0.690, and 0.708 in the training cohort and 0.721, 0.682, and 0.681 in the validation cohort, respectively. Furthermore, our model demonstrated excellent stratification as well as clinical applicability.
Conclusion: The AFP ratio was a reliable biomarker for tumor recurrence. This easy-to-use AFP ratio-based nomogram precisely predicted tumor recurrence in HCC patients after curative resection.
Primary liver cancer is a common type of malignancy with rapid disease development, high aggressiveness, and poor prognosis, which seriously threatens human health and quality of life, of which hepatocellular carcinoma (HCC) accounts for more than 80% of cases (1). According to 2020 global cancer statistics, HCC is the sixth most common cancer and the third leading cause of cancer-related death in the world (2). For patients with early HCC, the radical treatment options include surgical resection, liver transplantation, and radiofrequency ablation. However, for most patients, radical resection, which is advantageous because of its high surgical resection rate and low mortality rate, is the preferred treatment. Unfortunately, the 5-year recurrence rate after radical resection of liver cancer is still high at 60%–70%, and the overall survival rate is still low (3–5).
To date, there have been many reports of the prognostic factors and prognostic models after curative resection of HCC patients, including the Barcelona Clinic Liver Cancer (BCLC) Staging System, American Joint Committee on Cancer (AJCC) Staging System, Cancer of the Liver Italian Program (CLIP) Staging System, Tokyo Scoring System, and Hong Kong Liver Cancer Staging System (6–10). In general, these predictive models have unique predictive performance; however, none are accepted (11–13).
Nobuoka et al. showed that changes in alpha-fetoprotein (AFP) levels before and after hepatectomy for HCC can effectively predict the postoperative prognosis (14). Another study also reported that pre- to postoperative changes in AFP levels are more reliable than changes in routine AFP levels as a prognostic indicator for monitoring recurrence after HCC hepatectomy (15). These studies indicate that the range of pre- to postoperative changes in AFP levels can be used as a predictive indicator of HCC.
Therefore, in this retrospective study, we confirmed the clinical significance of the pre- to postoperative AFP ratio in the postoperative prognosis of patients with HCC and developed a practical and novel nomogram for recurrence free survival (RFS) based on pre- to postoperative AFP ratios.
A total of 485 HCC patients who underwent hepatic resection at the 900th Hospital of Chinese People’s Liberation Army Joint Support Force from January 2010 to December 2018 were included. The inclusion criteria were as follows (1): confirmed diagnosis of primary HCC by two or more imaging modalities (ultrasound, computed tomography, or magnetic resonance imaging) or postoperative histopathologic examination (2); treated by intended cure resection, which was defined as negative margins with no residual tumor based on the histopathologic examination; and (3) well-documented clinical history and detailed follow-up information. The exclusion criteria were as follows (1): confirmed diagnosis of other malignant tumors (2); preoperative transarterial chemoembolization, radiofrequency ablation, or other anti-tumor therapy; and (3) perioperative death. A total of 1,655 patients were identified with primary hepatocellular carcinoma by the computerized medical record system. According to the inclusion and exclusion criteria, we identified 450 patients. They were stochastically dichotomized into the training cohort (n = 340) and the internal validation cohort (n = 145) at a ratio of 7:3. The study was approved by the ethics committee of the 900th Hospital of Chinese People’s Liberation Army Joint Support Force. All research procedures followed the relevant guidelines and regulations. Signed informed consent was obtained from all patients. This study was conducted in accordance with the Declaration of Helsinki.
All data for this study were collected from each participant’s electronic medical record and included gender; age; white blood cell count (WBC); platelet count (PLT); levels of hemoglobin (HB), sodium (Na), alpha-fetoprotein (AFP), albumin (ALB), total bilirubin (Tbil), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and alanine aminotransferase (ALT); tumor-node-metastasis (TNM) stage; Barcelona Clinic Liver Cancer (BCLC) stage; surgical information; and tumor characteristics.
The AFP ratio was defined as the ratio of AFP values one week before hepatectomy to the lowest AFP values within 4 months after surgery. The normal range of AFP was defined as 0–20 ng/μL.
Patients received regular follow-up every 3–6 months. At each visit, detailed history and physical examination were performed, and AFP level, liver function, and images were re-examined, Abdominal ultrasound, computed tomography, or magnetic resonance imaging was performed. In some cases, the relevant examinations were performed earlier than scheduled to identify patients with suspected intrahepatic and extrahepatic recurrence and metastasis. Postoperative patients received standardized treatment and regular follow-up, and treatment plans were adjusted if necessary (16). The end point of the follow-up was tumor recurrence, death, or last follow up.
Recurrence free survival was measured from the date of surgery to the date of recurrence, the date of death, or the study closure date of December 31, 2021. Basic descriptive statistics, including mean, median, standard deviation (SD), and frequency (percentage), were used to characterize the dataset in both training and validation cohorts. Measurement data were presented as median or mean ± SD. Mann-Whitney U test was used to compare continuous variables and chi-square or Fisher’s exact test was used to compare categorical variables. X-tile statistical software (17) (version 3.6.1, Yale University, New Haven, CT, USA) was applied to determine the optimal threshold of the AFP ratio for RFS. According to the defined cutoff value, patients were divided into the low AFP ratio group or the high AFP ratio group, and the Kaplan-Meier method was used to draw survival curves. The training cohort was used to generate the nomogram based on multivariate regression to predict the 1-, 3-, and 5-year RFS using the “rms” package. The Web calculator was built by the “shiny” package. The area under the receiver operating characteristic curve and the C-index were used to evaluate the accuracy of the model in predicting survival, and the larger the C-index, the higher the accuracy of the model. Each patient had a total risk score (NomoScore: nomogram risk score) for risk stratification of RFS according to the nomogram. Patients were divided into different risk groups (low-, moderate-, high-) with the cut-off points automatically calculated using X-tile software. Decision curve analysis (DCA) was conducted to determine the clinical benefit of the nomogram by quantifying the net benefits along with the increase in threshold probabilities. Kaplan-Meier curves were plotted using GraphPad Prism 8.0 software. P < 0.05 was considered statistically significant.
A total of 1,655 patient records in the hospital medical record system were reviewed. According to the exclusion criteria, 485 patients were included in our study cohort and randomized at a ratio of 7:3 into the training group (n = 340) and the validation group (n = 145). The process of patient selection is illustrated in Figure 1.
A total of 485 patients diagnosed with HCC were included, with a median follow-up time of 137 months (range, 1–275 months). There was no significant difference in any clinical parameters between the training and validation groups (Table 1).
Using X-tile software, the optimal cutoff value of the AFP ratio for the training group was 0.8, which was divided into high-risk (AFP ratio <0.8) and low-risk (AFP ratio ≥0.8) groups using 0.8 as the cut-off threshold. The correlation between the AFP ratio and other characteristics is shown in Table 2. In the training group, there were significant differences in HBsAg positivity, MVI positivity, and BCLC stage between the high AFP ratio and low AFP ratio groups (P = 0.016, 0.007, 0.017, respectively). In the validation group, there was a significant difference in BCLC stage between the high AFP ratio and low AFP ratio groups (P = 0.031). As predicted, the AFP levels before hepatectomy of the high AFP ratio group were higher than those of the low AFP ratio group. In both cohorts, there were no apparent differences in other clinical and laboratory parameters between the two groups with high and low AFP ratios.
We further investigated the prognostic value of the AFP ratio in HCC patients after curative resection. In the training cohort, the RFS was 42.57 months with high AFP ratio and 15.60 months with low AFP ratio (p = 0.0018; HR, 0. 47, 95% CI, 0.25–0.88). In the validation cohort, the RFS was 38.57 months with high AFP ratio and 8.38 months with low AFP ratio (p = 0.0080; HR, 0.42, 95% CI, 0.18–0.99) (Figure 2).
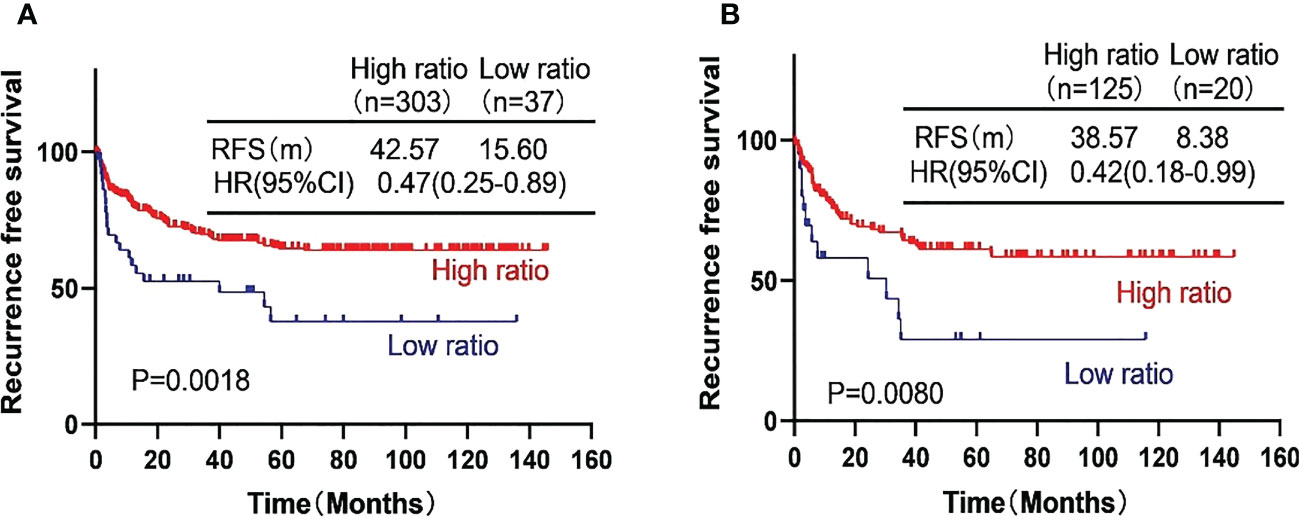
Figure 2 Kaplan-Meier curves showing the recurrence free survival (RFS) of the high-risk subgroup and the low-risk subgroup of hepatocellular carcinoma patients in the training cohort (A) and the validation cohort (B).
The Cox proportional hazards regression model was used to analyze the AFP ratio and clinical parameters in patients with HCC, and variables with P < 0.05 were included in the multivariate Cox regression analysis. In the training cohort, the results of univariate analysis showed that AFP ratio, tumor capsule, MVI, BCLC stage, and cirrhosis diagnosis were significantly associated with RFS, whereas the results of multivariate analysis showed that AFP ratio, BCLC stage, and cirrhosis diagnosis were important independent factors affecting prognosis. In the validation cohort, univariate and multivariate analyses confirmed that AFP ratio was an independent risk factor for patients with HCC. These results are presented in Tables 3, 4.
Based on the multivariate Cox proportional hazards model of the training group, three variables, including AFP ratio, BCLC stage, and cirrhosis diagnosis, were used to establish satisfactory nomograms for predicting 1-, 3-, and 5-year RFS in HCC patients (Figure 3A). The AUC values for our nomogram for predicting 1-, 3-, and 5-year RFS were 0.719, 0.690, and 0.708 in the training cohort and 0.721, 0.682, and 0.681 in the validation cohort, respectively (Figures 3B, C).
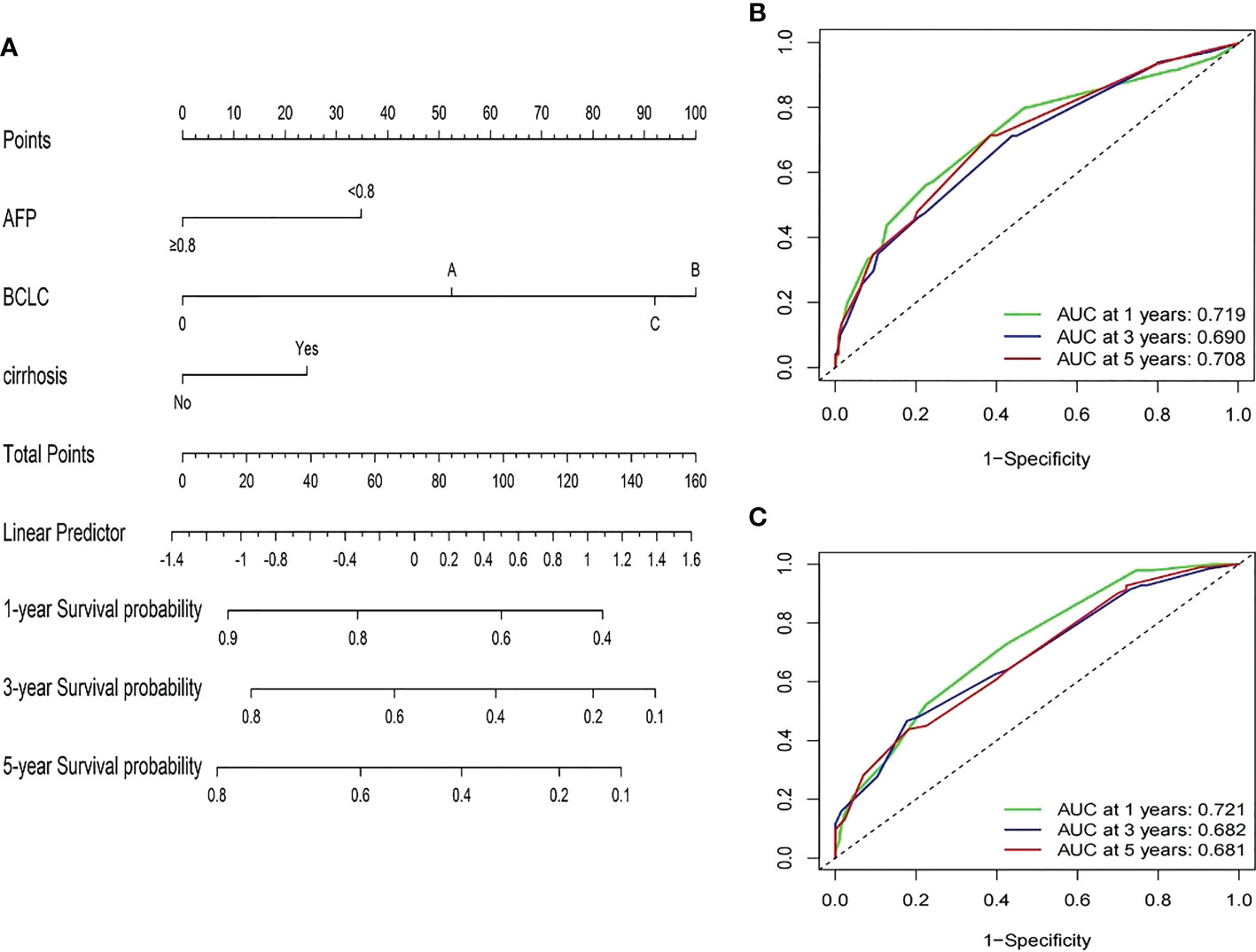
Figure 3 Developed prognosis nomogram model for RFS. (A) nomogram for predicting 1-, 3-, and 5-year RFS of HCC patients after curative resection. (B, C) 1-, 3-, and 5-year ROC values of RFS in the training cohort (B) and the validation cohort (C).
In the training cohort, the C-index of the nomogram for the RFS prediction was 0.67, and the calibration curves for the 1-, 3- and 5-year RFS rates overlapped with the standard lines, suggesting excellent agreement between predicted and actual RFS values (Figures 4A–C). In the validation cohort, the C-index of the nomogram for the RFS prediction was 0.64, and the calibration curves for the 1-, 3- and 5-year RFS rates overlapped with the standard lines, also suggesting excellent agreement between predicted and actual RFS values (Figures 4D–F).
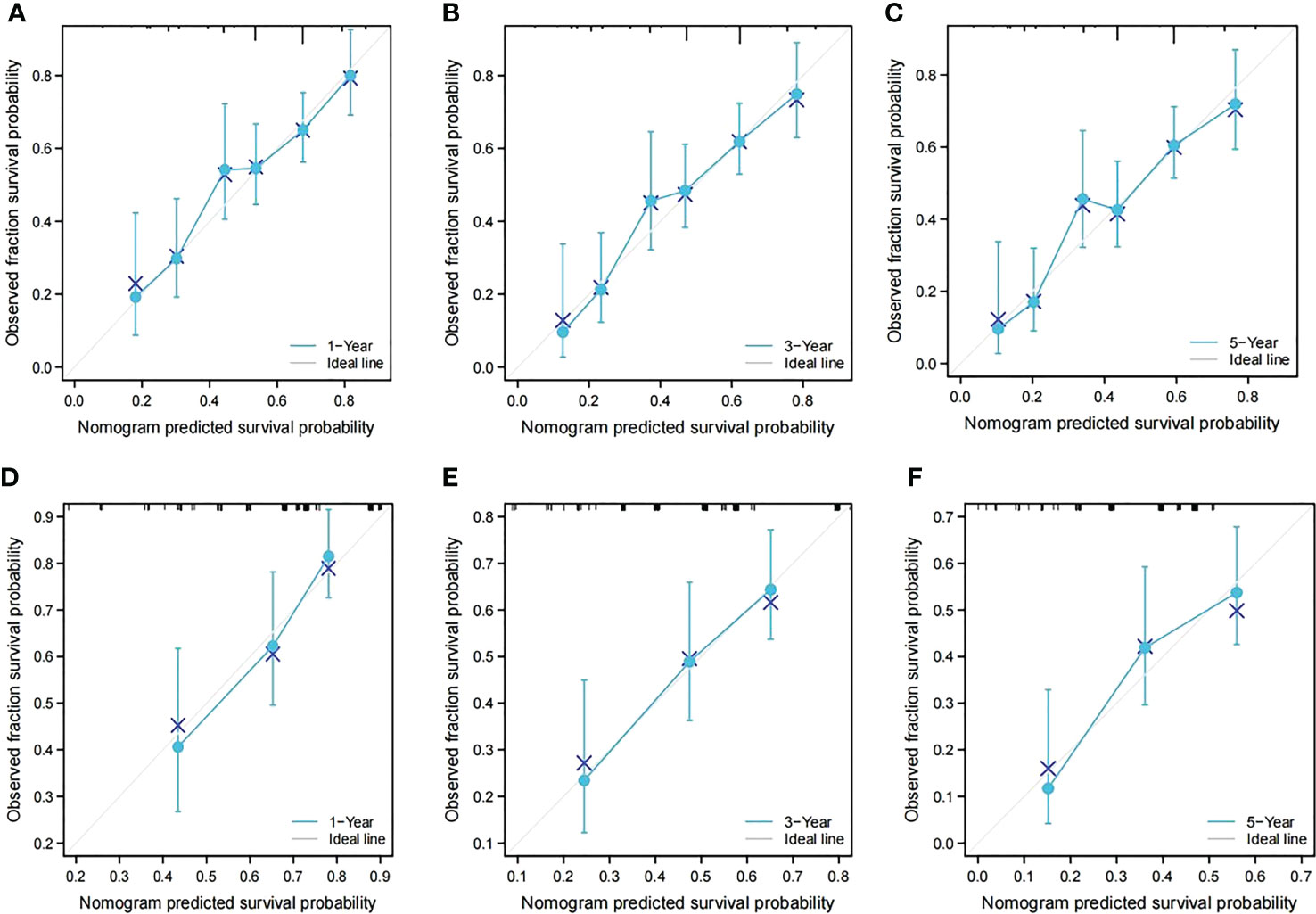
Figure 4 Calibration curves of the prognostic nomogram in both groups. (A) Calibration curve of the nomogram for the training cohort at 1 year. (B) Calibration curve of the nomogram for the training cohort at 3 years. (C) Calibration curve of the nomogram for the training cohort at 5 years. (D) Calibration curve of the nomogram for the validation cohort at 1 year. (E) Calibration curve of the nomogram for the validation cohort at 3 years. (F) Calibration curve of the nomogram for the validation cohort at 5 years.
Decision curve analysis can determine the clinical benefit of a nomogram by quantifying the net benefit as well as the increase in threshold probability. The DCA of our nomogram and the independent risk factors, namely, AFP ratio, BCLC stage, and cirrhosis diagnosis, in the training and validation cohorts for 1-, 3-, and 5-year RFS are illustrated in Figure 5. Both the training and validation groups showed that, compared with AFP ratio, BCLC stage, cirrhosis diagnosis, our nomogram provided a better clinical benefit and had significant clinical application in the prediction of HCC recurrence (Figures 5A–F).
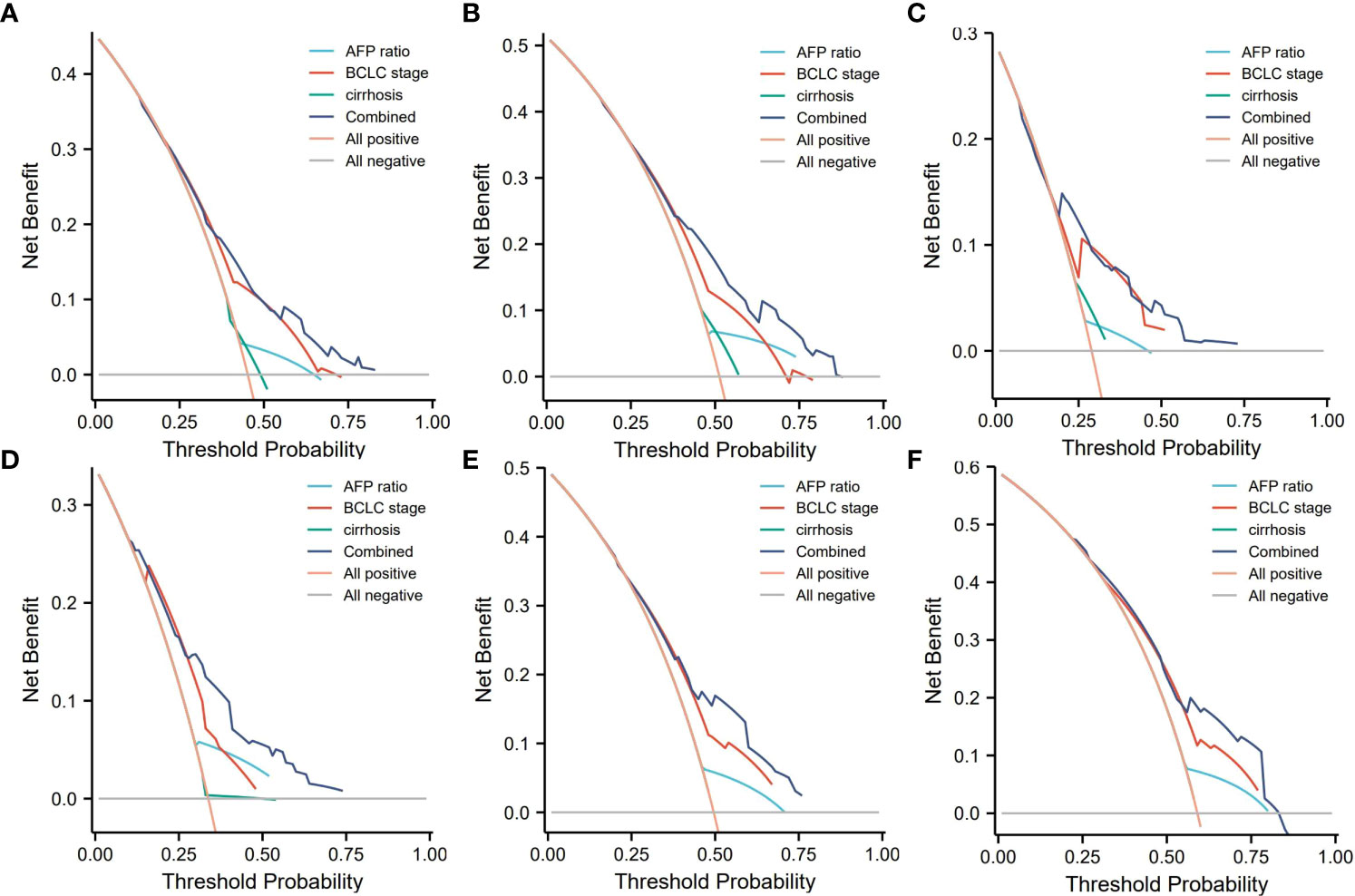
Figure 5 Decision curve analysis (DCA) of the nomogram. (A–C) DCA of 1-, 3-, and 5-year RFS predicted nomograms in the training cohort; (D–F) DCA of the 1-, 3-, and 5-year RFS predicted nomograms in the validation cohort. AFP ratio, alpha-fetoprotein ratio; BCLC, the Barcelona Clinic Liver Cancer staging system.
We further examined nomograms for prognostic evaluation and risk stratification. Each HCC patient received a different score according to the total risk score calculated from the nomogram model, which was divided into different risk groups to determine the discriminative ability of the nomogram for RFS. The optimal cut-off points were automatically calculated by X-tile software. The calculated risk score could classify HCC patients into high-risk (>115), middle-risk (35–114), and low-risk (<34) groups. The Kaplan-Meier method was used to compare the RFS of three different risk groups, and the results showed that the nomogram risk score had a significant discriminatory ability for the risk of patient recurrence (P < 0.05, Figure 6).
Hepatocellular carcinoma is a common type of malignancy, and its methods of treatment and effects of treatment are not the same, of which curative resection surgery is the generally accepted treatment of choice (18). In-depth studies have focused on the development, progression, and prognostic factors of HCC, and several improvements have been reported in diagnosis and treatment, surgical techniques, and comprehensive treatment; however, the prognosis of HCC after treatment is still not satisfactory. Studies have demonstrated that the 5-year recurrence and metastasis rate after surgical resection of HCC can be as high as 70% (19), and the 5-year overall survival rate is only 39%–65% (20, 21). The high recurrence rate of HCC after surgical resection is unacceptable, because it affects the prognosis of millions of HCC patients worldwide, so the development of a scoring model that can accurately predict the postoperative recurrence of HCC patients can help clinicians to individualize the preoperative treatment methods, surgical methods, and postoperative treatment options, which is critical for improving the long-term survival of HCC patients.
Alpha-fetoprotein is a serum biomarker used in the clinical diagnosis and postoperative monitoring of HCC, and the positive detection rate in HCC patients is approximately 70%–80%. In HCC patients with a negative detection rate, AFP is still an important indicator for predicting the prognosis of HCC (22, 23). Previous studies have demonstrated that high AFP levels in HCC patients are significantly associated with poor prognosis (24, 25). Some liver cancer staging systems, such as the Biomarker Combined Japan Integrated Staging (bm-JIS) and the CLIP score, also utilize serum AFP levels (8, 26), whereas AFP levels ≤400 ng/mL are included in the Hangzhou criteria as a patient selection criterion before liver transplantation (27).
However, the effectiveness of preoperative or postoperative AFP levels alone in predicting recurrence after HCC resection remains inadequate. Serum AFP levels may reflect tumor burden, and a decrease in the serum AFP level after surgery is considered to indicate a good response to treatment (28, 29). Thus, dynamic changes between preoperative and postoperative AFP levels may predict HCC prognosis more accurately than preoperative or postoperative AFP levels alone. Toro et al. indicated that AFP levels before and after treatment were associated with survival in HCC patients (30), whereas Nobuoka et al. showed that AFP levels changed from positive preoperatively to negative postoperatively and could be used to predict postoperative recurrence of HCC (14). Luo et al. also demonstrated that changes in preoperative to postoperative AFP levels could be used to assess recurrence and survival after radiofrequency ablation in HCC patients, and that the preoperative to postoperative AFP ratio could be used as a potential assessment index (31). Taken collectively, these findings suggested a strong association between changes in AFP levels before and after treatment and HCC prognosis.
In this study, we confirmed the prognostic value of the ratio of preoperative AFP to postoperative AFP in HCC patients after surgery. The optimal cut-off value of the AFP ratio in this study was 0.83, and HCC patients with AFP ratios less than 0.8 had significantly lower recurrence-free survival than those with AFP ratios greater than 0.8 in training and validation groups. Univariate and multivariate analyses showed that AFP ratio was an independent risk predictor for postoperative RFS in HCC patients. In conclusion, our results indicated that the AFP ratio was an important prognostic indicator for HCC patients undergoing curative resection surgery.
Nomograms transform complex regression equations into visual graphs, and they are reliable tools for integrating and quantifying significant risk factors for the prognosis of a variety of diseases (32, 33). Accurate prognostic evaluations can help physicians follow patients and select individualized treatment measures based on risk-benefit assessment scores. Our study developed a novel nomogram based on independent risk predictors of postoperative RFS in HCC patients. The nomogram included three variables, namely, AFP ratio, BCLC stage, and cirrhosis. Currently, BCLC staging is the most widely used staging system worldwide, and its unique advantage lies in the comprehensive consideration of the general condition, tumor condition, and liver function of HCC patients, and the preferred treatment is proposed according to the stage (34). Several studies have reported that the BCLC stage was an independent factor for the prognosis of HCC patients, which has better survival stratification and prognostic ability than other liver cancer staging modalities such as TNM stage, Japan Integrated Staging (JIS), and CLIP score (35, 36). Cirrhosis, a chronic persistent liver injury, is the result of multiple etiologies that lead to hepatocyte necrosis, which in turn causes severe liver lesions, thus becoming a risk factor for early HCC and postoperative recurrence. Many prognostic studies have demonstrated that cirrhosis was an independent predictor of HCC prognosis (37, 38). Our study confirmed BCLC stage and cirrhosis as independent and significant risk factors for poor disease-free survival after curative liver resection.
In this study, we combined the prediction model constructed by the pre- to postoperative AFP ratio, BCLC stage, and cirrhosis diagnosis, and comprehensively considered the comprehensive effects of three major aspects, namely, laboratory examination indicators, clinical characteristics, and imaging characteristics. The C-index of the model in the training group was 0.67, and the AUC values for predicting 1-, 3-, and 5-year RFS were 0.719, 0.690, and 0.708, respectively. In the validation group, the C-index was 0.64, and the AUC values for predicting 1-, 3-, and 5-year RFS were 0.721, 0.682, and 0.681, respectively. In addition, DCA showed that our nomogram achieved a higher net benefit than any single prognostic indicator such as BCLC stage and cirrhosis diagnosis in predicting 1-, 3-, and 5-year RFS. In summary, the nomogram prediction model we created based on the AFP ratio had high reliability and high clinical application value, and it can help clinicians individualize the treatment of HCC patients.
Although our nomogram showed satisfactory clinical performance, many limitations remain. First, this investigation was a single-center study, and the sample size was insufficient, which may bias the study results. Second, retrospective study analysis associated with selection bias in data collection and postoperative follow-up, and finally, in this study, the main cause of HCC was hepatitis B virus infection, which is different from the common cause in Western countries, which may affect AFP secretion. Thus, in the future, this model requires larger cohorts and prospective studies to validate its stratification strategy and prognostic ability.
In conclusion, the AFP ratio-based RFS nomogram prognostic model showed great potential predictive accuracy in HCC patients after curative resection and could be used in clinical practice to accurately assess RFS and identify high-risk patients, so as to develop more precise individualized treatment plans, and then increase the survival time of patients.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
CY and HW collected and statistically analyzed the medical records, as well as wrote the manuscript. JL and FY collected medical records and performed data analysis. LL, YJ, and QC provided practical advice, grant support, and administrative support, and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by Fujian Natural Science Foundation (No.: 2020Y0078) and Joint Support Forces Joint Medical Key Specialty.
We are grateful to the contributors of the GEO and TCGA for sharing the HCC expression profile data set on open access. In addition, we would like to acknowledge to all the people who have given us help on our article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1134933/full#supplementary-material
1. Villanueva A. Hepatocellular carcinoma. N Engl J Med (2019) 380(15):1450–62. doi: 10.1056/NEJMra1713263
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Chan AC, Chan SC, Chok KS, Cheung TT, Chiu DW, Poon RT, et al. Treatment strategy for recurrent hepatocellular carcinoma: Salvage transplantation, repeated resection, or radiofrequency ablation? Liver Transpl (2013) 19(4):411–9. doi: 10.1002/lt.23605
4. Du ZG, Wei YG, Chen KF, Li B. Risk factors associated with early and late recurrence after curative resection of hepatocellular carcinoma: A single institution's experience with 398 consecutive patients. Hepatobiliary Pancreat Dis Int HBPD Int (2014) 13(2):153–61. doi: 10.1016/s1499-3872(14)60025-4
5. Feng LH, Dong H, Lau WY, Yu H, Zhu YY, Zhao Y, et al. Novel microvascular invasion-based prognostic nomograms to predict survival outcomes in patients after R0 resection for hepatocellular carcinoma. J Cancer Res Clin Oncol (2017) 143(2):293–303. doi: 10.1007/s00432-016-2286-1
6. Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Network JNCCN (2021) 19(5):541–65. doi: 10.6004/jnccn.2021.0022
7. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology (2018) 67(1):358–80. doi: 10.1002/hep.29086
8. Capuano G, Daniele B, Gaeta GB, Gallo C, Perrone F, Pignata S, et al. A new prognostic system for hepatocellular carcinoma: A retrospective study of 435 patients: the cancer of the liver Italian program (CLIP) investigators. Hepatology (1998) 28(3):751–5. doi: 10.1002/hep.510280322
9. Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, et al. Construction of the Chinese university prognostic index for hepatocellular carcinoma and comparison with the TNM staging system, the okuda staging system, and the cancer of the liver Italian program staging system: A study based on 926 patients. Cancer (2002) 94(6):1760–9. doi: 10.1002/cncr.10384
10. Tateishi R, Yoshida H, Shiina S, Imamura H, Hasegawa K, Teratani T, et al. Proposal of a new prognostic model for hepatocellular carcinoma: An analysis of 403 patients. Gut (2005) 54(3):419–25. doi: 10.1136/gut.2003.035055
11. Kamarajah SK, Frankel TL, Sonnenday C, Cho CS, Nathan H. Critical evaluation of the American joint commission on cancer (AJCC) 8th edition staging system for patients with hepatocellular carcinoma (HCC): A surveillance, epidemiology, end results (SEER) analysis. J Surg Oncol (2018) 117(4):644–50. doi: 10.1002/jso.24908
12. Liao R, Wei XF, Che P, Yin KL, Liu L. Nomograms incorporating the CNLC staging system predict the outcome of hepatocellular carcinoma after curative resection. Front Oncol (2021) 11:755920. doi: 10.3389/fonc.2021.755920
13. Cammà C, Cabibbo G. Prognostic scores for hepatocellular carcinoma: None is the winner. Liver Int Off J Int Assoc Study Liver (2009) 29(4):478–80. doi: 10.1111/j.1478-3231.2009.01994.x
14. Nobuoka D, Kato Y, Gotohda N, Takahashi S, Nakagohri T, Konishi M, et al. Postoperative serum alpha-fetoprotein level is a useful predictor of recurrence after hepatectomy for hepatocellular carcinoma. Oncol Rep (2010) 24(2):521–8. doi: 10.3892/or_00000888
15. Sun LY, He Y, Liu Q, Wang F. Effect of delta α-fetoprotein on the detection of liver cancer recurrence. Trans Cancer Res (2020) 9(10):6263–74. doi: 10.21037/tcr-20-1874
16. Guidelines for diagnosis and treatment of primary liver cancer in China. Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi = Chin J Hepatol (2020) 28(2):112–28. doi: 10.3760/cma.j.issn.1007-3418.2020.02.004
17. Camp RL, Dolled-Filhart M, Rimm DL. X-Tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res an Off J Am Assoc Cancer Res (2004) 10(21):7252–9. doi: 10.1158/1078-0432.Ccr-04-0713
18. Dhir M, Melin AA, Douaiher J, Lin C, Zhen WK, Hussain SM, et al. A review and update of treatment options and controversies in the management of hepatocellular carcinoma. Ann Surg (2016) 263(6):1112–25. doi: 10.1097/sla.0000000000001556
19. Maennich D, Marshall L. Hepatocellular carcinoma. Nat Rev Dis Primers (2021) 7(1):7. doi: 10.1038/s41572-021-00245-6
20. Nathan H, Schulick RD, Choti MA, Pawlik TM. Predictors of survival after resection of early hepatocellular carcinoma. Ann Surg (2009) 249(5):799–805. doi: 10.1097/SLA.0b013e3181a38eb5
21. Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol (2018) 68(3):526–49. doi: 10.1016/j.jhep.2017.09.016
22. Li J, Li J, Bao Y, Pan K, Lin X, Liu X, et al. Low frequency of peripheral lymphocyte in chronic hepatitis b patients predicts poor progression to hepatocellular carcinoma. J Clin Lab Anal (2016) 30(3):208–15. doi: 10.1002/jcla.21838
23. Debes JD, Romagnoli PA, Prieto J, Arrese M, Mattos AZ, Boonstra A, et al. Serum biomarkers for the prediction of hepatocellular carcinoma. Cancers (2021) 13(7):1–13. doi: 10.3390/cancers13071681
24. Chan MY, She WH, Dai WC, Tsang SHY, Chok KSH, Chan ACY, et al. Prognostic value of preoperative alpha-fetoprotein (AFP) level in patients receiving curative hepatectomy- an analysis of 1,182 patients in Hong Kong. Trans Gastroenterol Hepatol (2019) 4:52. doi: 10.21037/tgh.2019.06.07
25. Sharma Y, Weaver MJ, Ludwig DR, Fowler K, Vachharajani N, Chapman WC, et al. Serum alpha-fetoprotein level per total tumor volume as a predictor of recurrence of hepatocellular carcinoma after resection. Surgery (2018) 163(5):1002–7. doi: 10.1016/j.surg.2017.10.063
26. Kitai S, Kudo M, Minami Y, Ueshima K, Chung H, Hagiwara S, et al. A new prognostic staging system for hepatocellular carcinoma: Value of the biomarker combined Japan integrated staging score. Intervirology (2008) 51 Suppl 1:86–94. doi: 10.1159/000122599
27. Chen J, Xu X, Wu J, Ling Q, Wang K, Wang W, et al. The stratifying value of hangzhou criteria in liver transplantation for hepatocellular carcinoma. PloS One (2014) 9(3):e93128. doi: 10.1371/journal.pone.0093128
28. Peng SY, Chen WJ, Lai PL, Jeng YM, Sheu JC, Hsu HC. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: Significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int J Cancer (2004) 112(1):44–50. doi: 10.1002/ijc.20279
29. Sherman M. The resurrection of alphafetoprotein. J Hepatol (2010) 52(6):939–40. doi: 10.1016/j.jhep.2010.02.006
30. Toro A, Ardiri A, Mannino M, Arcerito MC, Mannino G, Palermo F, et al. Effect of pre- and post-treatment α-fetoprotein levels and tumor size on survival of patients with hepatocellular carcinoma treated by resection, transarterial chemoembolization or radiofrequency ablation: A retrospective study. BMC Surg (2014) 14:40. doi: 10.1186/1471-2482-14-40
31. Luo B, Liu L, Bi J, Bao S, Zhang Y. Role of the pre- to postoperative alpha-fetoprotein ratio in the prognostic evaluation of liver cancer after radiofrequency ablation. Int J Biol Markers (2022) 37(3):306–13. doi: 10.1177/03936155221101075
32. Cho CS, Gonen M, Shia J, Kattan MW, Klimstra DS, Jarnagin WR, et al. A novel prognostic nomogram is more accurate than conventional staging systems for predicting survival after resection of hepatocellular carcinoma. J Am Coll Surgeons (2008) 206(2):281–91. doi: 10.1016/j.jamcollsurg.2007.07.031
33. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol Off J Am Soc Clin Oncol (2008) 26(8):1364–70. doi: 10.1200/jco.2007.12.9791
34. Kinoshita A, Onoda H, Fushiya N, Koike K, Nishino H, Tajiri H. Staging systems for hepatocellular carcinoma: Current status and future perspectives. World J Hepatol (2015) 7(3):406–24. doi: 10.4254/wjh.v7.i3.406
35. Zhang J, Zeng Q, Zhang P, Chen Y, Cao J. Efficacy evaluation and prognostic risk factors analysis of precise hepatectomy in the treatment of intermediate and advanced hepatocellular carcinoma. Eur J Gastroenterol Hepatol (2023) 35(1):120–6. doi: 10.1097/meg.0000000000002460
36. Guglielmi A, Ruzzenente A, Pachera S, Valdegamberi A, Sandri M, D'Onofrio M, et al. Comparison of seven staging systems in cirrhotic patients with hepatocellular carcinoma in a cohort of patients who underwent radiofrequency ablation with complete response. Am J Gastroenterol (2008) 103(3):597–604. doi: 10.1111/j.1572-0241.2007.01604.x
37. Nguyen-Khac V, Brustia R, Rhaiem R, Regnault H, Sessa A, Mule S, et al. Liver resection for single large hepatocellular carcinoma: a prognostic factors study. Ann Hepatol (2022) 27(6):100739. doi: 10.1016/j.aohep.2022.100739
Keywords: alpha fetoprotein ratio, hepatocellular carcinoma, nomogram, recurrence free survival, curative resection
Citation: Yang C, Wang H, Liu J, Yang F, Lv L, Jiang Y and Cai Q (2023) Pre- to postoperative alpha-fetoprotein ratio-based nomogram to predict tumor recurrence in patients with hepatocellular carcinoma. Front. Oncol. 13:1134933. doi: 10.3389/fonc.2023.1134933
Received: 31 December 2022; Accepted: 20 March 2023;
Published: 14 April 2023.
Edited by:
Zhigang Ren, First Affiliated Hospital of Zhengzhou University, ChinaReviewed by:
Christian Cotsoglou, Ospedale di Vimercate - ASST Brianza, ItalyCopyright © 2023 Yang, Wang, Liu, Yang, Lv, Jiang and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiucheng Cai, Y2FpcWl1Y2hlbmcyMjExQDE2My5jb20=; Yi Jiang, amlhbmd5aTgxODJAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.