
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 13 February 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1133828
This article is part of the Research TopicDiagnosis and Treatment of Bone MetastasesView all 17 articles
Denosumab, a fully humanized monoclonal neutralizing antibody, inhibits activation of the RANK/RANKL/OPG signaling pathway through competitive binding with RANKL, thereby inhibiting osteoclast-mediated bone resorption. Denosumab inhibits bone loss; therefore, it is used to treat metabolic bone diseases (including postmenopausal osteoporosis, male osteoporosis, and glucocorticoid-induced osteoporosis), in clinical practice. Since then, multiple effects of denosumab have been discovered. A growing body of evidence suggests that denosumab has a variety of pharmacological activities and broad potential in clinical diseases such as osteoarthritis, bone tumors, and other autoimmune diseases. Currently, Denosumab is emerging as a treatment for patients with malignancy bone metastases, and it also shows direct or indirect anti-tumor effects in preclinical models and clinical applications. However, as an innovative drug, its clinical use for bone metastasis of malignant tumors is still insufficient, and its mechanism of action needs to be further investigated. This review systematically summarizes the pharmacological mechanism of action of denosumab and the current understanding and clinical practice of the use of denosumab for bone metastasis of malignant tumors to help clinicians and researchers deepen their understanding of denosumab.
The receptor activator of NF-kB ligand (RANKL was originally defined as a new member of the tumor necrosis factor receptor (TNFR) family which is expressed on non-dendritic cells and participates in dendritic cell-mediated T cell proliferation and RANK+T cell activation (1). The discovery of RANKL built a bridge between the bone and the immune system and became an important landmark in the rise of bone immunology (2–4). As the RANKL/RANK (Receptor activator of NF-kB) signaling pathway plays an important role in mediating osteoclast differentiation and function (5), the relationship between RANKL and bone metabolism has been extensively studied.
The differentiation and maturation of osteoblasts is regulated by two systems: the RANK/RANKL system and the macrophage colony-stimulating factor/colony-stimulating factor-1 receptor (M-CSF/c-FMS) system (6). The M-CSF/c-FMS system is responsible for regulating the differentiation of early hematopoietic stem cells (HSCs) into osteoclast precursor cells and the survival of osteoclast precursor cells (7), whereas the RANK/RANKL system is an important trigger for the differentiation of osteoclast precursors into functional osteoclasts. RANKL is a homologous trimeric transmembrane protein which has two receptors: the membrane-binding receptor RANK and the soluble bait receptor OPG (8). In bone, RANKL is expressed in the bone matrix, osteoblast precursor cells, and osteoblasts, and RANK is expressed on the membrane surface of osteoclasts and osteoclast precursors as a membrane-binding receptor (9).
The binding of RANKL to RANK leads to the recruitment of TNF receptor associated factor 6 (TRAF6) as an articulatory molecule, which activates the NF-kB, c-Fos/AP1, MAPK, and other signaling pathways (10), leading to increased activation, amplification, and transcription of the downstream signal nuclear factor of activated T cells (NFATc1) (11), which directly mediates the differentiation of osteoclast precursor cells into osteoclasts (12). NFATc1 is a major regulator of osteogenesis (13). NFATc1 is both a major regulator of osteoclast formation and is involved in the regulation of osteoclast-specific genes (TRAP, Cathepsin K, calcitonin receptor) involved in osteoclast differentiation proliferation and survival (14, 15). The osteeoprotegerin (OPG) inhibits the activation of RANK signaling by competitively binding to RANKL and preventing RANKL from binding to its receptor RANK (16) (Figure 1).
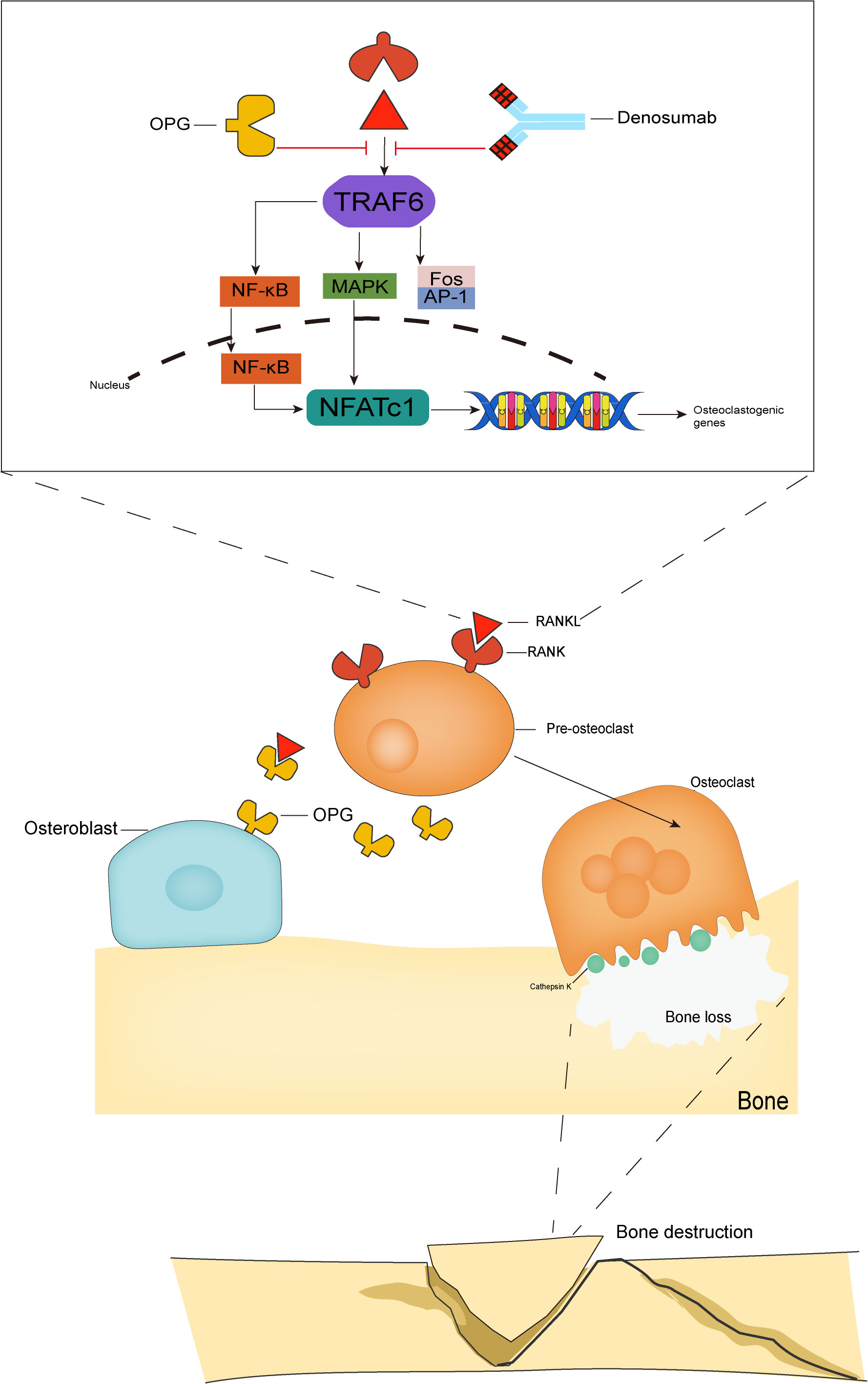
Figure 1 The RANK/RANKL/OPG system. The binding of RANKL to RANK leads to TRAF6 recruitment, which activates NF-kB, MAPK and Fos/AP-1 pathways. These activating signals together lead to the activation of NFATc1, a key transcription factor for downstream osteoclast-associated gene activation, which is the hallmark event of osteoclast formation. The OPG competitively binds RANKL and thus inhibits osteoclast activation, while denosumab, which has the same molecular weight as OPG, also binds to RANKL and inhibits osteoclast activation and maturation.
Denosumab is the first and only clinically available RANKL inhibitor that inhibits osteoclast activity by targeting and blocking the binding between RANK and RANKL. While inhibiting the function of mature osteoclasts (17), it also inhibits the maturation of osteoclast precursor cells, reduces bone resorption, and promotes bone reconstruction, thereby delaying bone-related events.
Tumor metastasis is the process by which cancer cells spread from a primary lesion to other sites. Cancer cells metastasize in three major ways: direct invasion, disseminated metastasis, and vascular and lymphatic metastases (18). Tumor metastasis is a complex biological process (19, 20). Tumor cells metastasizing from the primary site to other tissues and organs generally undergo the steps of reducing intercellular adhesion, destructing the epithelial barriers, escaping from immune surveillance, secondary site colonizing, proliferation and growth and lead to skeletal-related event SREs (21) (Figure 2). The three major primary cancers that are most prone to bone metastasis are breast, lung, and prostate cancers (22). Tumor cells colonize the bone microenvironment from the primary site, resulting in bone disease, which is defined as a SREs. Although all are bone metastases, the different origins of the tumors lead to completely opposite characteristics. When osteoblast-mediated bone formation predominates, the bone shows abnormal proliferation and presents with osteosclerotic malignancy; when osteoclast-mediated bone resorption predominates, the bone shows abnormal resorption and presents with osteolytic malignancy (23), and there are also some mixed lesions in which osteosclerosis and osteolysis abnormalities occur simultaneously (24).
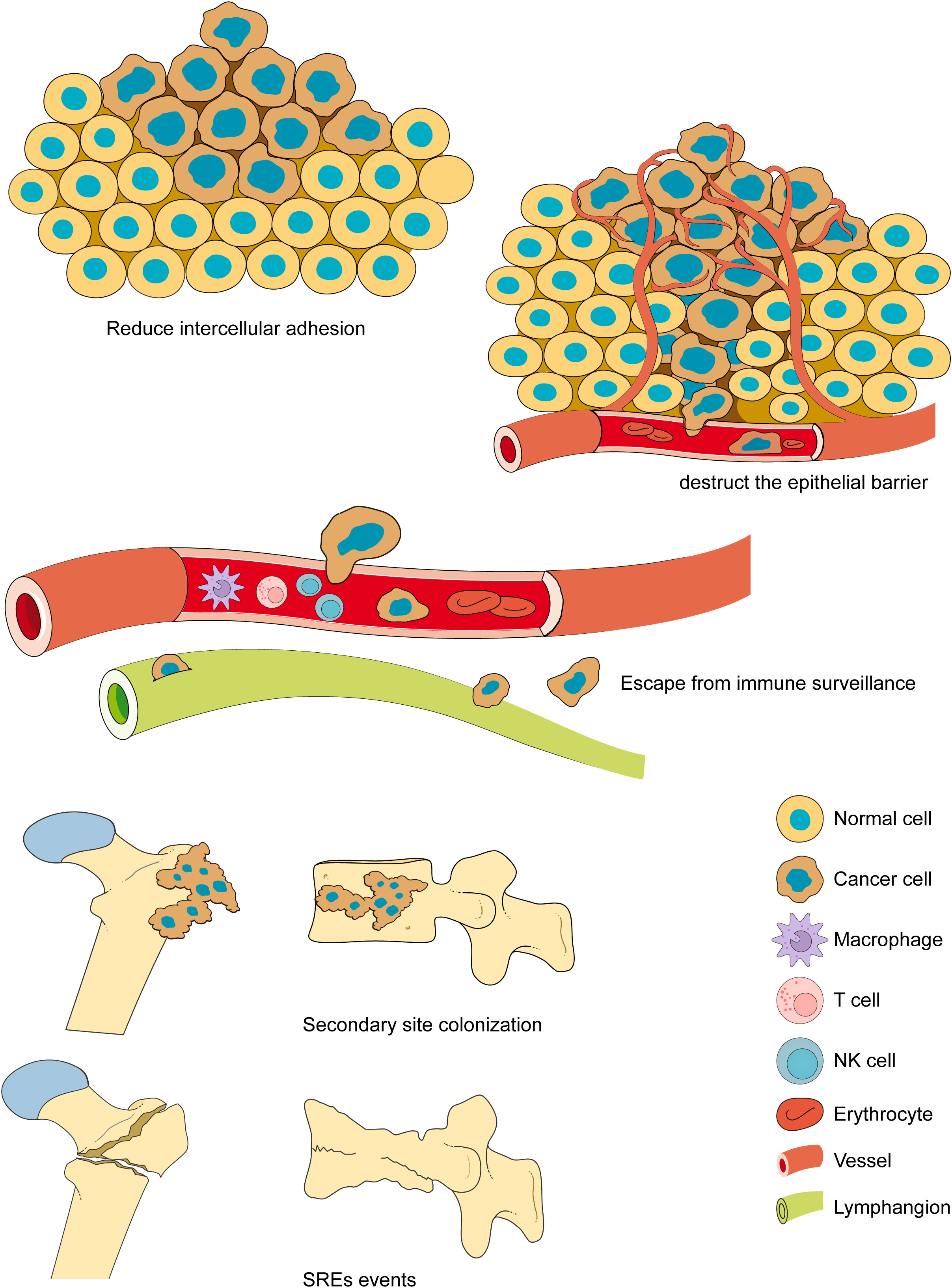
Figure 2 The bone metastasis. The bone metastasis is a complex biological process. Tumor cells metastasizing from the primary site to other tissues and organs generally undergo the steps of reducing intercellular adhesion, overcoming barriers, escaping from immunity, colonizing secondary sites, proliferation and growth and lead to SREs. The bone metastases often lead to serious complications, including fractures, nerve compression, and severe pain, resulting in loss of mobility, a significant increase in medical costs, and a significant reduction in quality of life and survival.
Tumor progression or invasion of other tumors leads to the disruption of bone homeostasis, forming a vicious circle between osteoclasts, osteoblasts, immune cells, and tumor cells (25). Malignant tumors release a variety of cytokines that can directly or indirectly activate osteoblasts or osteoclasts. When tumor cells secrete IL-1 (Interleukin-1), IL-6 (Interleukin-6), TNF-α (Tumor Necrosis Factor alpha), and other inflammatory cytokines, they activate osteoclasts in large quantities, leading to enhanced osteolysis activity, which produces inflammatory cytokines in large quantities, forming a vicious circle in the bone microenvironment promoting pro-tumor transformation and tumor cell progression (26). In addition, the alteration of the bone microenvironment and tumor microenvironment will make the immune cells’ surveillance and clearance effect on the tumor weaken (27). Tumor cells will block the immune response in many ways, resulting in the weakening of immune cell anti-tumor immunity (28). Physicochemical and environmental factors also play an important role in regulating the progression of tumor metastasis. A hypoxic environment and low pH values are conducive to tumor cell proliferation (29), and this hypoxic acidic environment creates a suitable environment for tumor cell growth, leading to increased levels of tumor cell production, migration, invasion, and proliferation (30).
Bone metastases often lead to serious complications, including fractures, nerve compression, and severe pain, resulting in loss of mobility, a significant increase in medical costs, and a significantly lower quality of life and survival rates (31). Metastases occur in approximately 50% of patients with tumors and are the cause of death in 90% of patients with cancer (32). In the past decades, metastases have been treated using systemic approaches, including chemotherapy and immunotherapy, but most patients with new or recurrent metastases still die within 5 years of diagnosis (33). This is especially true for the high incidence and high risk of bone metastases, so there is an urgent need to explore in depth the options to prevent and treat bone metastases (34). Bisphosphonates are well documented (35). Recognizing the development of early metastases in women suffering from breast cancer, which usually occur in bone tissue, attempts have been made to use bisphosphonates for early prevention in women with breast cancer as a nonspecific treatment, decreasing the potential impact of SREs and increasing the overall survival benefit (36).
RANKL has been recognized for its role in mediating dendritic cell survival and T cell proliferation, and subsequently for its crucial roles in mediating osteoclast differentiation and function (37). Thus, it has been intensively studied in the field of bone metabolism, and recently, research has returned to focus on the immune system. Many studies have shown that the RANK/RANKL system plays an essential role in developmental maturation and functional maintenance of the immune system. By genetically engineering RANK- or RANKL-deficient mice, it has been found that RANKL knock out mice show lymph node deficiency and impaired B-cell development (38, 39), and patients with mutations in the TNFRSF11A gene (encoding RANK) show a significant reduction in B-cell numbers (40), which confirms that RANK/RANKL is essential for early T cell and B cell development. In addition, RANK/RANKL intervenes in the interactions between T cells and dendritic cells s, and RANKL enhances dendritic cell formation and function in the absence of co-stimulation and antigen presentation, enhancing the ability of dendritic cells to stimulate the proliferation and differentiation of naive T cells (41). In addition, RANK/RANKL activation triggers intracellular signaling pathways (e.g., MAPK, NF-kB, Fos/AP-1, JNK/ERK/P38), which are involved in tumor proliferation and metabolic activities (42).There are many preclinical studies on the above-mentioned signaling pathways in RANKL activation-induced cancer metastasis. For example, MAPK pathway is involved in RANKL-induced breast cancer cell migration, and inhibition of MAPK pathway activation by specific inhibitors can effectively block RANKL-induced cell migration (43, 44). RANKL induces NF-KB activation leading to enhanced aggressiveness of oral squamous cell carcinoma by suppressing RANKL expression, which inhibits RANKL-induced NF-KB activation thereby suppressing the invasion of oral squamous cell carcinoma into the jawbone (45). However, these intracellular signaling pathways do not exist in isolation, but in crosstalk with each other (46). Until now, evidence on the crosstalk between RANK/RANKL and intracellular signaling pathways to regulate tumor proliferation and metabolism is still incomplete. and needs further study.
Although it is not clear whether the RANK/RANKL signaling pathway plays a favorable or unfavorable role in tumor proliferation and metabolism, there is no doubt that RANK/RANKL signaling plays a very important role in tumors. RANK/RANKL is expressed in many tumor tissues (39), and many breast cancer patients show abnormally high levels of RANKL expression in primary lesions, and a positive correlation with the incidence of bone metastases (47). RANK/RANKL is directly involved in tumor proliferation and metabolism and regulates the tumor immune microenvironment (48). The activation of dendritic cells releases a large amount of activated cytokines (including IL-1, IL-6, and IL-12) (41), which increase the number of transcriptional factor Foxp3 regulatory T cells (Foxp3+ Tregs) (49), and induce the differentiation of CD4+ T cells into Th1 cells (50), all of which lead to immunosuppression, and allow tumor cells to escape immune surveillance that promotes tumor progression.
The above evidence seems to indicate a negative aspect of the RANK/RANKL system in the progression of tumors and anti-tumor immunity. Because of the effectiveness of osteoclast inhibition in preventing bone metastases, drugs acting on the RANK/RANKL system have been developed and used to treat bone metastases from malignant neoplasm.
OPG was discovered in the 1990s when genomics was developed and used for target identification and Amgen discovered emerging mRNAs through large-scale sequencing and studied the function of these genes in vivo by overexpressing them in mouse liver. Mice with OPG transfer gene show a phenotype of increased bone density in the lower limb bones. Following the discovery of the OPG phenotype, subsequent studies began to search for a ligand for OPG. The OPG ligand (OPGL) was screened by fluorescence techniques, and a series of subsequent studies revealed that the OPGL sequence was identical to the RANK ligand RANKL, which was then used as a ligand for OPG. Because of its phenotype of increasing bone density, OPG was used to inhibit bone resorption. Hundreds of variants of OPG were developed and used in preclinical animal models, but all showed poor bioactivity and poor pharmacokinetics, and the subsequent OPG immunoglobulin Fc fusion protein (OPG-Fc) had an extended potency enhancer half-life but presented safety risks in phase I trials. Development of OPG-Fc was discontinued and shifted to RANKL. Amgen reconstituted a fully human monoclonal antibody, denosumab, using a modified Ig2 antibody (the modified Ig2 antibody enhances resistance to papain and thus improves efficacy) with little or no cytotoxicity (antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity) and improved pharmacokinetics (51).
Denosumab is a fully synthetic monoclonal neutralizing antibody that acts as an IgG2 subclass immunoglobulin, inhibiting osteoclast differentiation, survival, and activity by competitively binding RANKL, thereby blocking RANK binding to RANKL. Denosumab is considered a highly effective inhibitor of osteoclast bone resorption (52). In vitro studies have shown that denosumab, similar to OPG, has high affinity for soluble and membrane-bound RANKL (53). Denosumab has good pharmacokinetic properties, and although there are individual metabolic differences, its molecular mass and structural properties allow for rapid absorption and a nonlinear metabolic profile that can be sustained in vivo (54). After subcutaneously administration denosumab of 60 mg, maximum serum denosumab concentration was reached on day 10 (range: 2-28 days), with serum levels declining gradually over 3 months (range: 1.5-4.5 months), with a half-life of 26 days (range: 6-52 days). After subcutaneously administration denosumab of 120 mg every 4 weeks, steady-state concentrations were achieved at 6 months. the mean (± standard deviation) serum steady-state trough concentration at 6 months was 20.5 (± 13.5) µg/mL. The mean elimination half-life was 28 days. It can last up to 9 months after a single dose (55). Similar to other monoclonal antibodies, denosumab is likely cleared in vivo by the reticuloendothelial system and is not metabolized by the liver or kidneys (56); therefore, no further impairment of renal function or changes in efficacy or pharmacokinetics have been reported with denosumab in clinical trials, including renal replacement therapy in patients with impaired renal function (57). However, pilot studies on the mechanism of clearance of denosumab, and clinical evaluation of hepatic impairment on the efficacy and pharmacokinetics of denosumab have not yet been conducted (Figure 3).
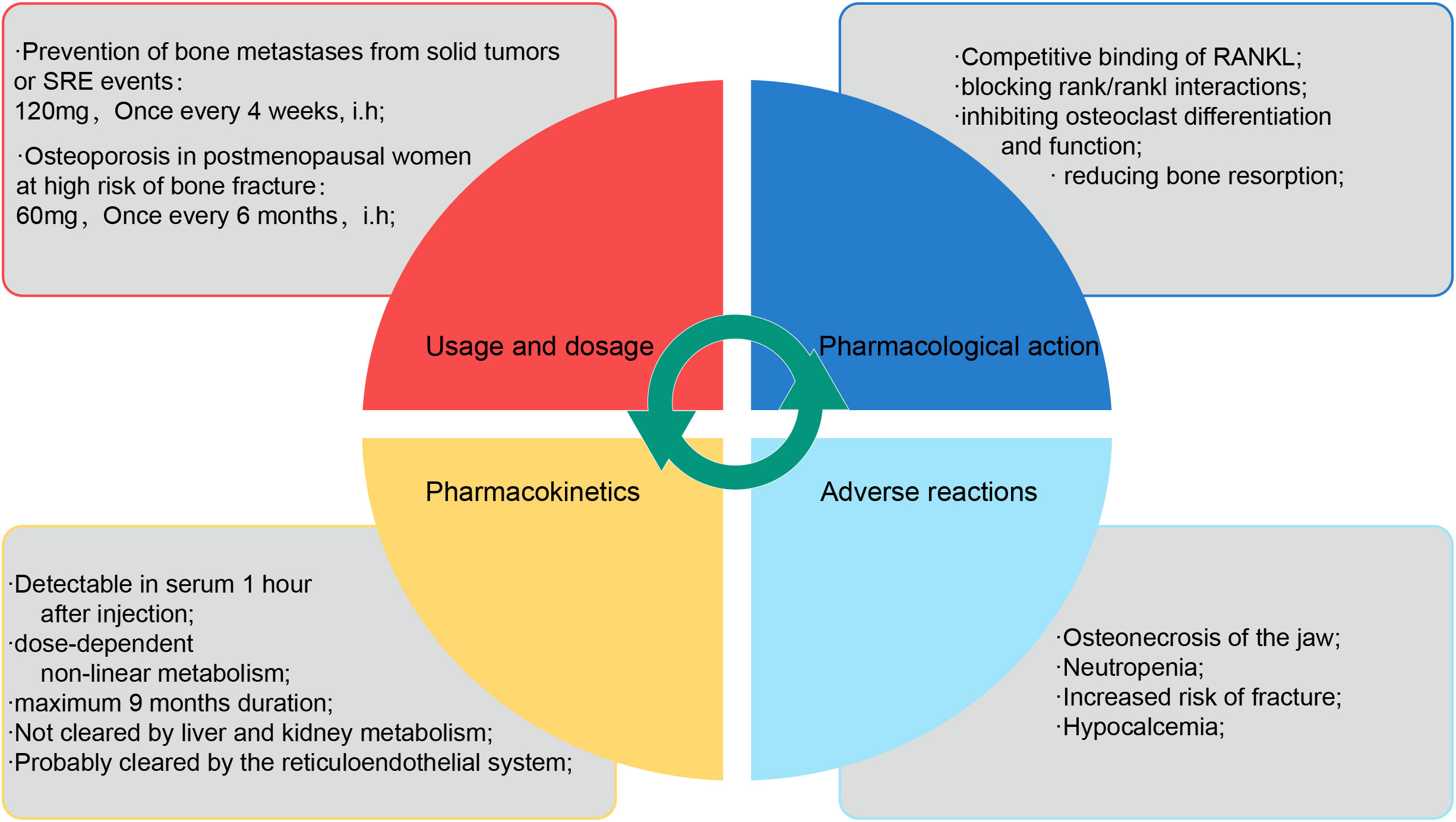
Figure 3 The description of denosumab. The properties of denosumab are briefly described in four dimensions: usage and dosage, pharmacological mechanism, pharmacokinetics and adverse reactions.
The RANKL inhibitor denosumab has entered clinical trials for malignant bone metastases (Figure 4). A prospective double-blind placebo-controlled phase III trial (58) with 3425 subjects showed that denosumab in women with early-stage hormone receptor-positive postmenopausal breast cancer treated with an aromatase inhibitor was effective in reducing bone mineral density and fractures due to aggressive bone resorption, with no significant variation in the incidence of adverse events (58). Another prospective double-blind placebo-controlled phase I trial with 1432 subjects showed that denosumab was effective in preventing bone metastases in non-metastatic castration-resistant prostate cancer, and that denosumab significantly postponed the onset of first bone metastasis in patients with this type of tumor (59). A randomized phase II clinical study showed that in patients experiencing bone metastases associated with malignant tumors, including prostate, breast, or other tumors, who received bisphosphonates by intravenous injection but still had excessive bone resorption (urinary N-terminal peptide uNTx >100 nmol), increased treatment with denosumab was effective in reducing uNTx levels, inhibiting the bone resorption rate, and reducing the incidence of SREs (60). In addition, a double-blind randomized phase III clinical trial showed that treatment with denosumab (120 mg every 4 weeks) prolonged the time to first bone metastasis radiotherapy compared to conventional bisphosphonate anti-bone metastases (61), implying that the time-lapse to bone metastases was delayed in patients receiving denosumab subcutaneous injections, in addition to prolonging the time to the first SREs occurrence and hypercalcemia, reduced pain levels, and improved the well-being of people suffering from bone metastases. In addition, denosumab effectively reduced serum calcium levels in patients with refractory hypercalcemia whose serum calcium could not be controlled with intravenous bisphosphonate therapy (62), achieving an overall remission rate of 64%, delaying the onset of hypercalcemia in patients with advanced bone metastases, achieving a durable therapeutic response in reducing serum calcium, and being used in patients with renal failure (compared to bisphosphonates). Denosumab is neither metabolized nor excreted by the kidneys compared to bisphosphonates), making denosumab a promising second drug to be approved for the treatment of refractory hypercalcemia after zoledronic acid.
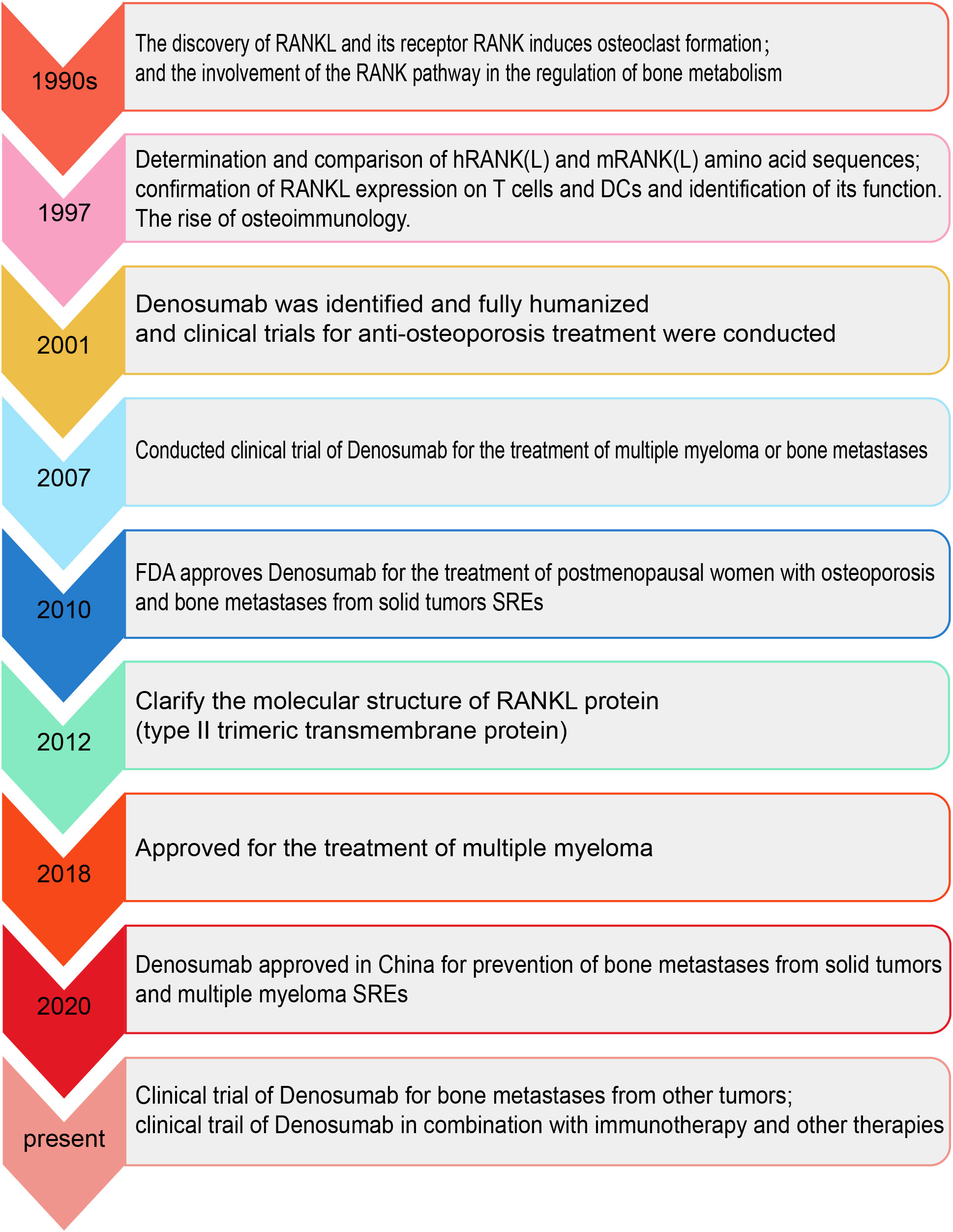
Figure 4 The timeline of RANK/RANKL/OPG system and denosumab. The chronological order shows the important events in the process from the discovery of the RANK/RANKL/OPG system and denosumab to its clinical use.
The use of denosumab has produced alterations in bone metabolism and therefore its application in some specific skeletal metabolic disorders deserves to be noted. Paget’s disease is characterized by local bone metabolism disorder, partial bone overgrowth, disorder of bone reconstruction, abnormal osteoclast metabolism causing bone lysis, compensatory increase of osteoblasts, brittle change of abnormally proliferated bone tissue, bone expansion and loosening, and easy fracture, so some clinical studies abroad use denosumab to intervene in early Paget’s disease. In two reported clinical cases (63, 64), patients with Paget’s disease treated with bisphosphonates for a long time, who progressed to giant cell tumor of bone, received subcutaneous injections of denosumab (120 mg every 4 weeks), and imaging showed a reduction in tumor size and an improvement in clinical symptoms. Treatment of bone metastases is usually systemic, and radiotherapy and chemotherapy are the conventional means of treatment for bone metastases. Patients with bone metastases have usually undergone systemic treatment prior to the development of bone metastases, does the combination of denosumab and chemotherapy produce a synergistic effect? Does the combination of denosumab and chemotherapy have a synergistic effect? Does it have an effect on effectiveness or does it produce drug resistance? Studies in animal models have shown that inhibition of RANKL improves the efficacy of the chemotherapeutic agent cisplatin, but there are no objective data on the effect of denosumab in combination with chemotherapeutic agents at this time (39).
Based on the above clinical study results, denosumab could be considered for use as a more effective anti-bone metastasis drug than bisphosphonates, because it delays the onset of bone metastases, reduces the frequency of SREs, improves patient life treatment, and can also be used in patients with bone metastases who are allergic to bisphosphonates or have renal failure (65). However, caution should be exercised. The clinical application of drugs is different from preclinical studies, because tumorigenesis is a complex process with differences in the nature of the tumor itself, and tumorigenesis leads to systemic metabolic changes. There are already relevant clinical studies that are skeptical of denosumab for bone metastases from malignant tumors.
A large multicenter prospective randomized clinical trial revealed the effect of adjuvant treatment with denosumab on early-stage female breast cancer patients. A total of 4509 female breast cancer patients were enrolled in the study (66), half of whom received denosumab (120 mg once a month) at the start of chemotherapy for five years, primarily to determine whether denosumab could play an anti-metastatic role. Unfortunately, there was no significant difference between the two groups, and no significant improvement or therapeutic effect on bone metastases, in addition to neutropenia in 15% of patients and osteonecrosis of the jaw in 5% of patients. Another randomized open phase III clinical study showed that adding denosumab to standard first-line platinum-based dual therapy did not improve overall survival in patients with advanced non-small cell lung cancer (NSCLC) (67). These clinical trials showed that the combination of denosumab did not benefit patients and imposed a financial burden on these patients. A large randomized trial showed a 9-fold increase in medical costs for monthly denosumab treatment compared with 3-monthly zoledronic acid treatment, but no significant survival extension or other benefits were observed (68).
In addition, the increased incidence of adverse events associated with the use of denosumab cannot be ignored, with frequently reported adverse events being osteonecrosis of the jaw, neutropenia, and increased risk of fracture (61, 69).
Based on the above studies, we must carefully evaluate the use of denosumab for the prophylaxis and treatment of bone metastases in malignant tumors. The following questions require careful consideration;
● Whether denosumab is only indicated for certain specific tumor types?
● Whether the use of denosumab brings more clinical benefits and medical cost savings to patients with malignant tumors?
● Whether the combination of denosumab with first-line chemotherapeutic agents may superimpose adverse effects and how to prevent rebound effects after denosumab discontinuation are worthy of prudent evaluation.
Therefore, joint efforts by researchers and clinicians are required.
In 2020, there will be approximately 19.3 million new cancer cases and 10 million cancer deaths worldwide, and with the growing population base and aging population, this number is expected to increase by 47% by 2040, with the global cancer burden reaching 28.4 million cases (70, 71). Approximately 50% of patients with tumors develop metastases, and the majority of patients with tumors die from a variety of complications caused by metastases, rather than from other causes. Bone metastatic disease is most common in some specific cancers, among which the incidence of bone metastasis in breast cancer is approximately 70%, prostate cancer is about 85%, cancer bone metastasis is about 40%, and the incidence of bone metastasis in multiple myeloma is as high as 95% (22). Given the high incidence of these tumors, many bone metastases occur each year, causing great pain and devastation to patients. Most bone metastases occur in the spine, pelvis, ribs, and other important areas (72), causing pain, compression, bone destruction, pathological fractures, and other serious SREs (73). Approximately 40% of patients with bone metastases experience SREs during antitumor treatment (73).
The evolution of malignant tumor metastasis to the bone is a complex process (74). The metastatic spread of tumor cells includes reducing intercellular adhesion, destructing the epithelial barrier, escaping from immune surveillance, secondary site colonization and SREs events happening (75). However, when tumor cells colonize the bone, it also provides favorable support for the rapid proliferation of tumor cells. The relationship between tumor cells and the bone microenvironment has been compared to the relationship between seeds and soil (76), and the various cells and blood supply in the skeletal system provide a natural breeding ground for tumor cells to colonize and proliferate. However, not all types of tumors develop bone metastasis, and it seems that the characteristics of the seed (i.e., tumor cells) interacting with the soil (i.e., bone microenvironment), play a more important role in the spread of malignant tumor bone metastasis (77), which may explain why some specific primary tumor types (e.g., breast cancer and prostate cancer), are more prone to bone metastasis (78). In addition, differences in the primary foci classify bone metastases into different types, and bone metastases are classified into osteosclerotic malignancies, osteolytic malignancies, and mixed malignancies (79). Osteolytic malignancies are usually primary tumors of breast or lung cancer, whereas osteosclerotic malignancies are usually highly associated with prostate cancer. However, this division is simple and rough, and when bone metastases from malignant tumors occur, skeletal lesions within the bone microenvironment are often complex (80). When bone metastases occur in most solid tumors, there is both an accelerated process of osteolytic destruction and bone formation and reconstruction, and bone metastases in different parts of a single patient’s body may be different, i.e. osteolytic bone destruction may occur in one part of the bone and another part of In other words, osteolytic bone destruction may occur in one part of the skeleton, while another part of the skeleton, on the contrary, may develop sclerotic osteogenic lesions or mixed bone metastases. The complexity of bone metastases along with the resistance of neoplastic cells to metastases poses a great challenge for their treatment (81).
The current standard of care for bone metastases from malignant tumors includes bisphosphonates and the RANKL inhibitor, denosumab (82), both drug types which target osteoclast inhibition (83). Bisphosphonates have been used clinically for many years, and a large amount of preclinical evidence fully demonstrates their anti-tumor cell metastatic ability (84, 85). Their action on osteoclasts leads to reduced bone resorption, which may establish a bone microenvironment unfavorable to tumor cell attachment (86). In addition, nitrogen-containing bisphosphonates can inhibit tumor angiogenesis and modulate immunity to exert indirect antitumor activity (87). Due to its good pharmacological activity and cost-effectiveness, zoledronic acid has been the standard of care for the prevention of bone metastases from malignant tumors and other SREs for nearly a decade (88), significantly improving the quality of life and survival of patients with bone metastases (89). However, the pharmacological properties of zoledronic acid have led to adverse effects (mainly acute reactions and renal impairment), making it unavailable to some patients with bone metastases (90), and until the advent of denosumab, these patients had no choice.
Denosumab was approved by the FDA in 2010 for the treatment of bone metastases from solid tumors, and has since become a breakthrough treatment for bone metastases from malignant tumors. Several clinical trials have confirmed that it is as effective as zoledronic acid (91, 92). Several large multicenter prospective double-blind randomized controlled clinical trials have shown that denosumab is effective in delaying the time to first bone metastasis, reducing the incidence of SREs, reducing pain levels in patients with bone metastases, and improving the quality of life in patients with malignancies (93, 94). However, with further clinical studies and the gradual expansion of the included population, the effectiveness of denosumab was gradually questioned (95), and several clinical studies showed that denosumab did not differ from zoledronic acid in terms of overall survival and disease progression (96). In addition, the treatment of bone metastases from malignant tumors is a long-term process, and the cost of the drug is an issue that must be considered. With conflicting clinical data from different institutional centers, more clinical studies and longer follow-up periods are needed to obtain credible evidence about the role of denosumab in malignant bone metastases, its advantages over zoledronic acid, and to analyze the various reported adverse effects from denosumab. The answers are thus both necessary and urgent.
Although clinical studies and conclusions about denosumab are still to be optimized, there is no doubt about its advantages over zoledronic acid. The emergence of denosumab brings hope to patients with bone metastases from malignant tumors combined with renal abnormalities as it is not metabolized and excreted by the kidneys (97). This makes it available for patients with bone metastases from malignant tumors treated with renal replacement therapy. This is particularly important for elderly prostate cancer patients, who are at a high risk of bone metastases. They often suffer from renal insufficiency due to malignant tumor proliferation-induced urinary tract obstruction, for which bisphosphonates are absolutely contraindicated, and who desperately need a drug that can control bone metastases (93). In addition, denosumab reduces the rate of bone resorption by competitively binding to RANKL, resulting in a durable reduction in serum calcium levels. This provides a new approach for the treatment of previously intractable hypercalcemia with bone metastases (98). Denosumab has also been reported to delay pain progression and reduce overall pain levels and analgesic drug use (99), but these reports suffer from inadequate sample sizes and are highly susceptible to subjective evaluations, which are currently unreliable.
Another question that needs to be answered is how does denosumab function in different types of tumors? Although it was approved by the FDA in 2010 for the treatment of bone metastases from solid tumors, it is not yet known whether denosumab is effective for all types of bone metastases from solid tumors (100). Clinical trials in NSCLC have shown that bone metastasis is very common in non-small cell lung cancer; one clinical trial confirmed that 50%-60% of NSCLC tumor tissues express RANKL and RANK, and the trial showed that denosumab can directly block RANKL to inhibit bone metastasis in NSCLC. However, subsequent clinical trials have indicated the opposite conclusions, since adding denosumab to standard first-line platinum-based dual therapy did not improve overall survival in advanced NSCLC. Data from different studies are conflicting, so there are questions about whether denosumab is effective in NSCLC. A more fundamental issue is that the mechanism of denosumab, which acts on osteoclasts to exert anti-metastatic effects by reducing bone resorption, may be effective in osteolytic bone metastases where osteoclast-mediated bone resorption predominates. However, there is a lack of evidence to demonstrate whether it is effective in sclerotic bone metastases where osteoblast-mediated bone formation predominates.
To minimize the impact of malignant tumor bone metastases on patients and reduce the occurrence of SREs events, the control of bone metastases often requires comprehensive treatment rather than a single therapeutic measure, and the treatment usually includes multiple treatment measures such as radiotherapy, chemotherapy, surgical treatment, immunotherapy, and palliative care. Combination therapy with denosumab is therefore a major clinical issue, most notably denosumab in combination with immunotherapy (101). RANKL, as a bridge between the skeletal and immune systems, will inevitably crosstalk with the immune system; therefore, the outcomes arising from the combination of immunotherapy and denosumab must be seriously considered. Several studies are currently underway to examine the effects of denosumab monotherapy and combination immunotherapy to assess whether the combination has a beneficial effect on progression-free survival and overall survival of patients (102).
In summary, the bone metastasis of malignant tumors has become a major challenge in tumor treatment. The various mechanisms mediating the growth of metastasis and the special skeletal microenvironment bring great challenges to the treatment of bone metastasis, and the emergence of denosumab provides a new path for the treatment of bone metastasis. However, since the understanding of denosumab is not yet perfect, more research is needed in the future to explore the great therapeutic potential of denosumab and provide a more solid and rational basis for clinical use.
BS, JL, CM, and YZ conceived the idea for the study and provided critical revision of the manuscript. LS, DH, and CM collected the information. BS, JL, and DH participated in study design, supervising, writing and drafting of the manuscript. All authors contributed to the article and approved the submitted version.
This study was partially funded by the National Natural Science Foundation of China (Project Number: 82174182, 81974546, 81974249, and 81901144).
The authors would like to thank Editage (www.editage.cn) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yasuda H. Discovery of the RANKL/RANK/OPG system. J Bone mineral Metab (2021) 39(1):2–11. doi: 10.1007/s00774-020-01175-1
2. Nagy V, Penninger JM. The RANKL-RANK story. Gerontology. (2015) 61(6):534–42. doi: 10.1159/000371845
3. Ono T, Hayashi M, Sasaki F, Nakashima T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflammation regeneration. (2020) 40:2. doi: 10.1186/s41232-019-0111-3
4. Okamoto K, Takayanagi H. Osteoimmunology. Cold Spring Harbor Perspect Med (2019) 9(1). doi: 10.1101/cshperspect.a031245
5. Cao X. RANKL-RANK signaling regulates osteoblast differentiation and bone formation. Bone Res (2018) 6:35. doi: 10.1038/s41413-018-0040-9
6. Boyce BF. Advances in the regulation of osteoclasts and osteoclast functions. J Dental Res (2013) 92(10):860–7. doi: 10.1177/0022034513500306
7. Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. (2007) 40(2):251–64. doi: 10.1016/j.bone.2006.09.023
8. Luan X, Lu Q, Jiang Y, Zhang S, Wang Q, Yuan H, et al. Crystal structure of human RANKL complexed with its decoy receptor osteoprotegerin. J Immunol (Baltimore Md 1950). (2012) 189(1):245–52. doi: 10.4049/jimmunol.1103387
9. Eriksen EF. Cellular mechanisms of bone remodeling. Rev endocrine Metab Disord (2010) 11(4):219–27. doi: 10.1007/s11154-010-9153-1
10. Chen Y, Chen J, Chen J, Yu H, Zheng Y, Zhao J, et al. Recent advances in seafood bioactive peptides and their potential for managing osteoporosis. Crit Rev Food Sci Nutr (2022) 62(5):1187–203. doi: 10.1080/10408398.2020.1836606
11. Lorenzo J. The many ways of osteoclast activation. J Clin Invest (2017) 127(7):2530–2. doi: 10.1172/JCI94606
12. Gohda J, Akiyama T, Koga T, Takayanagi H, Tanaka S, Inoue J. RANK-mediated amplification of TRAF6 signaling leads to NFATc1 induction during osteoclastogenesis. EMBO J (2005) 24(4):790–9. doi: 10.1038/sj.emboj.7600564
13. Lu J, Hu D, Ma C, Shuai B. Advances in our understanding of the mechanism of action of drugs (including traditional Chinese medicines) for the intervention and treatment of osteoporosis. Front Pharmacol (2022) 13:938447. doi: 10.3389/fphar.2022.938447
14. Zeng XZ, Zhang YY, Yang Q, Wang S, Zou BH, Tan YH, et al. Artesunate attenuates LPS-induced osteoclastogenesis by suppressing TLR4/TRAF6 and PLCγ1-Ca(2+)-NFATc1 signaling pathway. Acta pharmacologica Sinica. (2020) 41(2):229–36. doi: 10.1038/s41401-019-0289-6
15. Pang M, Rodríguez-Gonzalez M, Hernandez M, Recinos CC, Seldeen KL, Troen BR. AP-1 and mitf interact with NFATc1 to stimulate cathepsin K promoter activity in osteoclast precursors. J Cell Biochem (2019) 120(8):12382–92. doi: 10.1002/jcb.28504
16. Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol (2014) 5:511. doi: 10.3389/fimmu.2014.00511
17. Polyzos SA, Makras P, Tournis S, Anastasilakis AD. Off-label uses of denosumab in metabolic bone diseases. Bone. (2019) 129:115048. doi: 10.1016/j.bone.2019.115048
18. Bergers G, Fendt SM. The metabolism of cancer cells during metastasis. Nat Rev Cancer. (2021) 21(3):162–80. doi: 10.1038/s41568-020-00320-2
19. Suhail Y, Cain MP, Vanaja K, Kurywchak PA, Levchenko A, Kalluri R, et al. Systems biology of cancer metastasis. Cell systems. (2019) 9(2):109–27. doi: 10.1016/j.cels.2019.07.003
20. Valastyan S, Weinberg RA. Tumor metastasis: Molecular insights and evolving paradigms. Cell. (2011) 147(2):275–92. doi: 10.1016/j.cell.2011.09.024
21. Chiang AC, Massagué J. Molecular basis of metastasis. New Engl J Med (2008) 359(26):2814–23. doi: 10.1056/NEJMra0805239
22. Coleman RE, Croucher PI, Padhani AR, Clézardin P, Chow E, Fallon M, et al. Bone metastases. Nat Rev Dis primers. (2020) 6(1):83. doi: 10.1016/B978-0-323-47674-4.00056-6
23. Verron E, Schmid-Antomarchi H, Pascal-Mousselard H, Schmid-Alliana A, Scimeca JC, Bouler JM. Therapeutic strategies for treating osteolytic bone metastases. Drug Discovery Today (2014) 19(9):1419–26. doi: 10.1016/j.drudis.2014.04.004
24. Clézardin P. Pathophysiology of bone metastases from solid malignancies. Joint Bone spine. (2017) 84(6):677–84. doi: 10.1016/j.jbspin.2017.05.006
25. Sethakorn N, Heninger E, Sánchez-de-Diego C, Ding AB, Yada RC, Kerr SC, et al. Advancing treatment of bone metastases through novel translational approaches targeting the bone microenvironment. Cancers. (2022) 14(3). doi: 10.3390/cancers14030757
26. Yin JJ, Pollock CB, Kelly K. Mechanisms of cancer metastasis to the bone. Cell Res (2005) 15(1):57–62. doi: 10.1038/sj.cr.7290266
27. Mohme M, Riethdorf S, Pantel K. Circulating and disseminated tumour cells - mechanisms of immune surveillance and escape. Nat Rev Clin Oncol (2017) 14(3):155–67. doi: 10.1038/nrclinonc.2016.144
28. Santini D, Galluzzo S, Zoccoli A, Pantano F, Fratto ME, Vincenzi B, et al. New molecular targets in bone metastases. Cancer Treat Rev (2010) 36 Suppl 3:S6–s10. doi: 10.1016/S0305-7372(10)70013-X
29. Yang C, Tian Y, Zhao F, Chen Z, Su P, Li Y, et al. Bone microenvironment and osteosarcoma metastasis. Int J Mol Sci (2020) 21(19). doi: 10.3390/ijms21196985
30. Hiraga T. Hypoxic microenvironment and metastatic bone disease. Int J Mol Sci (2018) 19(11). doi: 10.3390/ijms19113523
31. Clézardin P, Coleman R, Puppo M, Ottewell P, Bonnelye E, Paycha F, et al. Bone metastasis: Mechanisms, therapies, and biomarkers. Physiol Rev (2021) 101(3):797–855. doi: 10.1152/physrev.00012.2019
32. Ganesh K, Massagué J. Targeting metastatic cancer. Nat Med (2021) 27(1):34–44. doi: 10.1038/s41591-020-01195-4
33. Coleman RE. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat Rev (2001) 27(3):165–76. doi: 10.1053/ctrv.2000.0210
34. Ban J, Fock V, Aryee DNT, Kovar H. Mechanisms, diagnosis and treatment of bone metastases. Cells. (2021) 10(11). doi: 10.3390/cells10112944
35. Stresing V, Daubiné F, Benzaid I, Mönkkönen H, Clézardin P. Bisphosphonates in cancer therapy. Cancer letters. (2007) 257(1):16–35. doi: 10.1016/j.canlet.2007.07.007
36. Barni S, Mandalà M, Cazzaniga M, Cabiddu M, Cremonesi M. Bisphosphonates and metastatic bone disease. Ann Oncol Off J Eur Soc Med Oncol (2006) 17 Suppl 2:ii91–95. doi: 10.1093/annonc/mdj935
37. Walsh MC, Choi Y. Regulation of T cell-associated tissues and T cell activation by RANKL-RANK-OPG. J Bone mineral Metab (2021) 39(1):54–63. doi: 10.1007/s00774-020-01178-y
38. Cheng ML, Fong L. Effects of RANKL-targeted therapy in immunity and cancer. Front Oncol (2014) 3:329. doi: 10.3389/fonc.2013.00329
39. van Dam PA, Verhoeven Y, Trinh XB. The non-Bone-Related role of RANK/RANKL signaling in cancer. Adv Exp Med Biol (2020) 1277:53–62. doi: 10.1007/978-3-030-50224-9_3
40. Rao S, Cronin SJF, Sigl V, Penninger JM. RANKL and RANK: From mammalian physiology to cancer treatment. Trends Cell Biol (2018) 28(3):213–23. doi: 10.1016/j.tcb.2017.11.001
41. Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. (1997) 390(6656):175–9. doi: 10.1038/36593
42. Hu J, Hu WX. Targeting signaling pathways in multiple myeloma: Pathogenesis and implication for treatments. Cancer letters. (2018) 414:214–21. doi: 10.1016/j.canlet.2017.11.020
43. Zhang L, Teng Y, Zhang Y, Liu J, Xu L, Qu J, et al. Proteasome inhibitor bortezomib (PS-341) enhances RANKL-induced MDA-MB-231 breast cancer cell migration. Mol Med Rep (2012) 5(2):580–4. doi: 10.3892/mmr.2011.678
44. Tang ZN, Zhang F, Tang P, Qi XW, Jiang J. RANKL-induced migration of MDA-MB-231 human breast cancer cells via src and MAPK activation. Oncol Rep (2011) 26(5):1243–50. doi: 10.3892/or.2011.1368
45. Shin M, Matsuo K, Tada T, Fukushima H, Furuta H, Ozeki S, et al. The inhibition of RANKL/RANK signaling by osteoprotegerin suppresses bone invasion by oral squamous cell carcinoma cells. Carcinogenesis. (2011) 32(11):1634–40. doi: 10.1093/carcin/bgr198
46. Renema N, Navet B, Heymann MF, Lezot F, Heymann D. RANK-RANKL signalling in cancer. Bioscience Rep (2016) 36(4). doi: 10.1042/BSR20160150
47. Infante M, Fabi A, Cognetti F, Gorini S, Caprio M, Fabbri A. RANKL/RANK/OPG system beyond bone remodeling: Involvement in breast cancer and clinical perspectives. J Exp Clin Cancer Res CR. (2019) 38(1):12. doi: 10.1186/s13046-018-1001-2
48. Li B, Wang P, Jiao J, Wei H, Xu W, Zhou P. Roles of the RANKL-RANK axis in immunity-implications for pathogenesis and treatment of bone metastasis. Front Immunol (2022) 13:824117. doi: 10.3389/fimmu.2022.824117
49. Francisconi CF, Vieira AE, Azevedo MCS, Tabanez AP, Fonseca AC, Trombone APF, et al. RANKL triggers treg-mediated immunoregulation in inflammatory osteolysis. J Dental Res (2018) 97(8):917–27. doi: 10.1177/0022034518759302
50. Vernal R, Díaz-Zúñiga J, Melgar-Rodríguez S, Pujol M, Diaz-Guerra E, Silva A, et al. Activation of RANKL-induced osteoclasts and memory T lymphocytes by porphyromonas gingivalis is serotype dependant. J Clin periodontology. (2014) 41(5):451–9. doi: 10.1111/jcpe.12236
51. Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, et al. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug discovery. (2012) 11(5):401–19. doi: 10.1038/nrd3705
52. Dougall WC, Holen I, González Suárez E. Targeting RANKL in metastasis. BoneKEy Rep (2014) 3:519. doi: 10.1038/bonekey.2014.14
53. Kostenuik PJ, Nguyen HQ, McCabe J, Warmington KS, Kurahara C, Sun N, et al. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J Bone mineral Res Off J Am Soc Bone Mineral Res (2009) 24(2):182–95. doi: 10.1359/jbmr.081112
54. Keizer RJ, Huitema AD, Damen CW, Schellens JH, Beijnen JH. [The pharmacokinetics of monoclonal antibodies]. Nederlands tijdschrift voor geneeskunde. (2007) 151(12):683–8.
55. Gül G, Sendur MA, Aksoy S, Sever AR, Altundag K. A comprehensive review of denosumab for bone metastasis in patients with solid tumors. Curr Med Res opinion. (2016) 32(1):133–45. doi: 10.1185/03007995.2015.1105795
56. Wohlrab J. Pharmacokinetic characteristics of therapeutic antibodies. J der Deutschen Dermatologischen Gesellschaft = J German Soc Dermatol JDDG. (2015) 13(6):530–4. doi: 10.1111/ddg.12648
57. Iseri K, Watanabe M, Yoshikawa H, Mitsui H, Endo T, Yamamoto Y, et al. Effects of denosumab and alendronate on bone health and vascular function in hemodialysis patients: A randomized, controlled trial. J Bone mineral Res Off J Am Soc Bone Mineral Res (2019) 34(6):1014–24. doi: 10.1002/jbmr.3676
58. Gnant M, Pfeiler G, Dubsky PC, Hubalek M, Greil R, Jakesz R, et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet (London England) (2015) 386(9992):433–43. doi: 10.1016/S0140-6736(15)60995-3
59. Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet (London England) (2012) 379(9810):39–46. doi: 10.1016/S0140-6736(11)61226-9
60. Doshi S, Sutjandra L, Zheng J, Sohn W, Peterson M, Jang G, et al. Denosumab dose selection for patients with bone metastases from solid tumors. Clin Cancer Res an Off J Am Assoc Cancer Res (2012) 18(9):2648–57. doi: 10.1158/1078-0432.CCR-11-2944
61. Raje N, Terpos E, Willenbacher W, Shimizu K, García-Sanz R, Durie B, et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: An international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol (2018) 19(3):370–81. doi: 10.1016/S1470-2045(18)30072-X
62. Hu MI, Glezerman IG, Leboulleux S, Insogna K, Gucalp R, Misiorowski W, et al. Denosumab for treatment of hypercalcemia of malignancy. J Clin Endocrinol Metab (2014) 99(9):3144–52. doi: 10.1210/jc.2014-1001
63. Verma V, Puri A, Shah S, Rekhi B, Gulia A. Giant cell tumor developing in paget's disease of bone: A case report with review of literature. J orthopaedic Case Rep (2016) 6(4):103–7. doi: 10.13107/jocr.2250-0685.594
64. Tanaka T, Slavin J, McLachlan SA, Choong P. Anti-osteoclastic agent, denosumab, for a giant cell tumor of the bone with concurrent paget's disease: A case report. Oncol letters. (2017) 13(4):2105–8. doi: 10.3892/ol.2017.5693
65. Zhang Z, Pu F, Shao Z. The skeletal-related events of denosumab versus zoledronic acid in patients with bone metastases: A meta-analysis of randomized controlled trials. J Bone Oncol (2017) 9:21–4. doi: 10.1016/j.jbo.2017.09.003
66. Coleman R, Finkelstein DM, Barrios C, Martin M, Iwata H, Hegg R, et al. Adjuvant denosumab in early breast cancer (D-CARE): An international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol (2020) 21(1):60–72. doi: 10.1016/S1470-2045(19)30687-4
67. Peters S, Danson S, Hasan B, Dafni U, Reinmuth N, Majem M, et al. A randomized open-label phase III trial evaluating the addition of denosumab to standard first-line treatment in advanced NSCLC: The European thoracic oncology platform (ETOP) and European organisation for research and treatment of cancer (EORTC) SPLENDOUR trial. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2020) 15(10):1647–56. doi: 10.1016/j.jtho.2020.06.011
68. Shapiro CL, Moriarty JP, Dusetzina S, Himelstein AL, Foster JC, Grubbs SS, et al. Cost-effectiveness analysis of monthly zoledronic acid, zoledronic acid every 3 months, and monthly denosumab in women with breast cancer and skeletal metastases: CALGB 70604 (Alliance). J Clin Oncol Off J Am Soc Clin Oncol (2017) 35(35):3949–55. doi: 10.1200/JCO.2017.73.7437
69. Chen J, Zhou L, Liu X, Wen X, Li H, Li W. Meta-analysis of clinical trials to assess denosumab over zoledronic acid in bone metastasis. Int J Clin pharmacy. (2021) 43(1):2–10. doi: 10.1007/s11096-020-01105-1
70. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
71. Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: New estimates from GLOBOCAN 2020. Gastroenterology. (2022) 163(3):649–658.e642. doi: 10.1053/j.gastro.2022.05.054
72. Demers LM, Costa L, Lipton A. Biochemical markers and skeletal metastases. Cancer. (2000) 88(12 Suppl):2919–26. doi: 10.1002/1097-0142(20000615)88:12+<2919::AID-CNCR7>3.0.CO;2-Z
73. Hong S, Youk T, Lee SJ, Kim KM, Vajdic CM. Bone metastasis and skeletal-related events in patients with solid cancer: A Korean nationwide health insurance database study. PloS One (2020) 15(7):e0234927. doi: 10.1371/journal.pone.0234927
74. Roodman GD. Mechanisms of bone metastasis. New Engl J Med (2004) 350(16):1655–64. doi: 10.1056/NEJMra030831
75. Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal transduction targeted Ther (2020) 5(1):28. doi: 10.1038/s41392-020-0134-x
76. Liu Q, Zhang H, Jiang X, Qian C, Liu Z, Luo D. Factors involved in cancer metastasis: a better understanding to "seed and soil" hypothesis. Mol cancer. (2017) 16(1):176. doi: 10.1186/s12943-017-0742-4
77. Fidler IJ. The pathogenesis of cancer metastasis: The 'seed and soil' hypothesis revisited. Nat Rev Cancer. (2003) 3(6):453–8. doi: 10.1038/nrc1098
78. Fornetti J, Welm AL, Stewart SA. Understanding the bone in cancer metastasis. J Bone mineral Res Off J Am Soc Bone Mineral Res (2018) 33(12):2099–113. doi: 10.1002/jbmr.3618
79. Chappard D, Bouvard B, Baslé MF, Legrand E, Audran M. Bone metastasis: Histological changes and pathophysiological mechanisms in osteolytic or osteosclerotic localizations. A review. Morphologie Bull l'Association Des anatomistes. (2011) 95(309):65–75. doi: 10.1016/j.morpho.2011.02.004
80. Ponzetti M, Rucci N. Switching homes: How cancer moves to bone. Int J Mol Sci (2020) 21(11). doi: 10.3390/ijms21114124
81. Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res an Off J Am Assoc Cancer Res (2008) 14(9):2519–26. doi: 10.1158/1078-0432.CCR-07-2223
82. Landherr L, Nagykálnai T. [Treatment of bone metastases: Bisphosphonates and denosumab]. Magyar onkologia. (2017) 61(2):175–80.
83. Vallet S, Smith MR, Raje N. Novel bone-targeted strategies in oncology. Clin Cancer Res an Off J Am Assoc Cancer Res (2010) 16(16):4084–93. doi: 10.1158/1078-0432.CCR-10-0600
84. Wellington K, Goa KL. Zoledronic acid: A review of its use in the management of bone metastases and hypercalcaemia of malignancy. Drugs. (2003) 63(4):417–37. doi: 10.2165/00003495-200363040-00009
85. Santini D, Fratto ME, Vincenzi B, Galluzzo S, Tonini G. Zoledronic acid in the management of metastatic bone disease. Expert Opin Biol Ther (2006) 6(12):1333–48. doi: 10.1517/14712598.6.12.1333
86. Mundy GR, Yoneda T, Hiraga T. Preclinical studies with zoledronic acid and other bisphosphonates: Impact on the bone microenvironment. Semin Oncol (2001) 28(2 Suppl 6):35–44. doi: 10.1016/S0093-7754(01)90263-5
87. Van Acker HH, Anguille S, Willemen Y, Smits EL, Van Tendeloo VF. Bisphosphonates for cancer treatment: Mechanisms of action and lessons from clinical trials. Pharmacol Ther (2016) 158:24–40. doi: 10.1016/j.pharmthera.2015.11.008
88. O'Carrigan B, Wong MH, Willson ML, Stockler MR, Pavlakis N, Goodwin A. Bisphosphonates and other bone agents for breast cancer. Cochrane Database systematic Rev (2017) 10(10):Cd003474. doi: 10.1002/14651858.CD003474.pub4
89. Saad F. Zoledronic acid: Past, present and future roles in cancer treatment. Future Oncol (London England). (2005) 1(2):149–59. doi: 10.1517/14796694.1.2.149
90. Singireesu S, Mondal SK, Yerramsetty S, Misra S. Zoledronic acid induces micronuclei formation, mitochondrial-mediated apoptosis and cytostasis in kidney cells. Life Sci (2018) 203:305–14. doi: 10.1016/j.lfs.2018.04.059
91. Peddi P, Lopez-Olivo MA, Pratt GF, Suarez-Almazor ME. Denosumab in patients with cancer and skeletal metastases: A systematic review and meta-analysis. Cancer Treat Rev (2013) 39(1):97–104. doi: 10.1016/j.ctrv.2012.07.002
92. Ford JA, Jones R, Elders A, Mulatero C, Royle P, Sharma P, et al. Denosumab for treatment of bone metastases secondary to solid tumours: Systematic review and network meta-analysis. Eur J Cancer (Oxford Engl 1990). (2013) 49(2):416–30. doi: 10.1016/j.ejca.2012.07.016
93. Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol Off J Am Soc Clin Oncol (2011) 29(9):1125–32. doi: 10.1200/JCO.2010.31.3304
94. Chawla S, Blay JY, Rutkowski P, Le Cesne A, Reichardt P, Gelderblom H, et al. Denosumab in patients with giant-cell tumour of bone: A multicentre, open-label, phase 2 study. Lancet Oncol (2019) 20(12):1719–29. doi: 10.1016/S1470-2045(19)30663-1
95. Snedecor SJ, Carter JA, Kaura S, Botteman MF. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A cost-effectiveness analysis. J Med economics. (2013) 16(1):19–29. doi: 10.3111/13696998.2012.719054
96. Menshawy A, Mattar O, Abdulkarim A, Kasem S, Nasreldin N, Menshawy E, et al. Denosumab versus bisphosphonates in patients with advanced cancers-related bone metastasis: Systematic review and meta-analysis of randomized controlled trials. Supportive Care Cancer Off J Multinational Assoc Supportive Care Cancer. (2018) 26(4):1029–38. doi: 10.1007/s00520-018-4060-1
97. Chen F, Pu F. Safety of denosumab versus zoledronic acid in patients with bone metastases: A meta-analysis of randomized controlled trials. Oncol Res Treat (2016) 39(7-8):453–9. doi: 10.1159/000447372
98. Roukain A, Alwan H, Bongiovanni M, Sykiotis GP, Kopp PA. Denosumab for the treatment of hypercalcemia in a patient with parathyroid carcinoma: A case report. Front endocrinology. (2021) 12:794988. doi: 10.3389/fendo.2021.794988
99. Martin M, Bell R, Bourgeois H, Brufsky A, Diel I, Eniu A, et al. Bone-related complications and quality of life in advanced breast cancer: Results from a randomized phase III trial of denosumab versus zoledronic acid. Clin Cancer Res an Off J Am Assoc Cancer Res (2012) 18(17):4841–9. doi: 10.1158/1078-0432.CCR-11-3310
100. Kurata T, Nakagawa K. Efficacy and safety of denosumab for the treatment of bone metastases in patients with advanced cancer. Japanese J Clin Oncol (2012) 42(8):663–9. doi: 10.1093/jjco/hys088
101. Li HS, Lei SY, Li JL, Xing PY, Hao XZ, Xu F, et al. Efficacy and safety of concomitant immunotherapy and denosumab in patients with advanced non-small cell lung cancer carrying bone metastases: A retrospective chart review. Front Immunol (2022) 13:908436. doi: 10.3389/fimmu.2022.908436
102. Ferrara R, Imbimbo M, Malouf R, Paget-Bailly S, Calais F, Marchal C, et al. Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer. Cochrane Database systematic Rev (2021) 4(4):Cd013257. doi: 10.1002/14651858.CD013257.pub3
Keywords: denosumab, RANK/RANKL/OPG system, bone metastasis, skeletal-related events, osteoclast
Citation: Lu J, Hu D, Zhang Y, Ma C, Shen L and Shuai B (2023) Current comprehensive understanding of denosumab (the RANKL neutralizing antibody) in the treatment of bone metastasis of malignant tumors, including pharmacological mechanism and clinical trials. Front. Oncol. 13:1133828. doi: 10.3389/fonc.2023.1133828
Received: 29 December 2022; Accepted: 01 February 2023;
Published: 13 February 2023.
Edited by:
Wenwen Zhang, Nanjing Medical University, ChinaReviewed by:
Jingfeng Li, Zhongnan Hospital, Wuhan University, ChinaCopyright © 2023 Lu, Hu, Zhang, Ma, Shen and Shuai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Shuai, Ym9fc2h1YWlAaHVzdC5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.