
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 29 November 2023
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1132417
This article is part of the Research Topic Cancer Control in Primary Care View all 5 articles
 Nor Asiah Muhamad1*
Nor Asiah Muhamad1* Nur Hasnah Ma’amor1
Nur Hasnah Ma’amor1 Izzah Athirah Rosli1
Izzah Athirah Rosli1 Fatin Norhasny Leman1
Fatin Norhasny Leman1 Mohd Hatta Abdul Mutalip2
Mohd Hatta Abdul Mutalip2 Huan-Keat Chan3
Huan-Keat Chan3 Siti Norbayah Yusof4
Siti Norbayah Yusof4 Nor Saleha Ibrahim Tamin5
Nor Saleha Ibrahim Tamin5 Tahir Aris6
Tahir Aris6 Nai Ming Lai7
Nai Ming Lai7 Muhammad Radzi Abu Hassan3,8
Muhammad Radzi Abu Hassan3,8Background: Colorectal cancer (CRC) is a major cause of cancer-related mortality worldwide. It is the second leading cause of cancer death in men and women in Malaysia and poses a major burden on society.
Aims: To determine the overall survival rate of patients diagnosed with CRC and factors contributing to survival.
Methods: Data were obtained from the Malaysia National Cancer Registry. All patients with CRC were identified, and a total of 15,515 patients were screened. A total of 5,675 CRC patients were included from January 1, 2012, to December 31, 2016. Sex, age groups, ethnic groups, stage at diagnosis, cancer sites, and status of treatment received were analysed. The Kaplan–Meier analysis was performed to estimate the 1-, 3-, and 5-year survival of CRC. The log-rank test was conducted to compare the survival between sex, age groups, ethnic groups, stage at diagnosis, cancer sites, and status of treatment received. Multiple Cox regression was conducted to determine the risk of CRC death.
Results: Of 5,675, a total of 2,055 had died, 3,534 were censored, and another 86 were still alive within 5 years of CRC diagnosis. The 1-, 3-, and 5-year survival rates were 68.5%, 34.7%, and 18.4%, respectively with a median survival time of 24 months. Significant differences in survival rates of CRC were observed between age groups (p < 0.001), ethnic groups (p < 0.001), stages at diagnosis (p < 0.001), treatment status (p = 0.003), and treatment modalities (p < 0.001). No significant difference was observed in survival rates of CRC between sex (p = 0.235) and cancer sites (p = 0.410). Those who were 80 years old and above were found to be at higher risk of CRC death compared to those below 80 years old (adjusted hazard ratio (HR): 1.24, 95% CI 1.14–1.36). The risk of CRC death was also found four times higher among those with stage IV compared to those with stage 0 (adjusted HR: 4.28, 95% CI 3.26–5.62).
Conclusion: In general, Malaysian patients with CRC had low survival rates. National health policies should focus on enhancing awareness of CRC, encouraging early screening, and developing strategies for early detection and management to reduce CRC-associated mortality.
Cancer is one of the leading causes of death worldwide, and colorectal cancer (CRC) ranked as the second most common cancer death with 916,000 deaths reported in 2020 (1). In addition, CRC is the third most commonly diagnosed cancer in 2020, globally (2). The age-standardised incidence rate (ASR) and the age-standardised mortality rate (ASMR) for CRC were higher in men compared to women (3). In Malaysia, CRC ranks as the second most common cancer, which contributed to 13.5% of all newly diagnosed cancer cases in 2012–2016 (4). It is the most prevalent cancer in men and the second most prevalent in women (4). The ASR for men was 14.8 per 100,000, which was slightly higher than for women with 11.1 per 100,000 (4). Furthermore, the incidence of CRC was the highest among the Chinese, compared to Malays and Indians in Malaysia (4).
Colorectal cancer is characterised by uncontrolled cell growth that starts in the colon, including the ascending, transverse, descending, and sigmoid colon or rectum (5). Additionally, cancers that are located at the rectosigmoid junction also may be known as CRC, although it only contributes 10% of colorectal cancer diagnosis (6). CRC is identified as a “lifestyle or behavioural” disease and mostly attributed to non-modifiable factors such as age and gender, ethnicity, genetic predisposition, and other modifiable factors such as poor diet habits high in calories and animal fat, alcohol consumption, smoking, infection from Helicobacter pylori, obesity, and inactivity (7–12).
Colorectal cancer is one of the malignancies that are highly preventable and treatable with early detection (4). Early screening and detection lead to a low stage at diagnosis, in which the survival of CRC may be improved. Therefore, many public awareness and health promotion programme on CRC was conducted, and many accomplishments were seen in managing CRC, including the availability of screening tool, advancement in surgical procedure, and other treatment modalities (13). Despite that, more than 70% of CRC patients in Malaysia were diagnosed at late stages, with Malaysia having a low 5-year relative survival rate compared to other developed Asian countries (14).
Cancer survival is a crucial indicator for a better improvement in cancer management (13). By determining survival rates, one country may identify why other countries have better survival and may further implement a better strategy for the prevention, treatment, and care of a particular disease (15). Therefore, in this study, we determined the overall survival rate of CRC and factors associated with increased risks of CRC death in Malaysia based on the data from the Malaysia National Cancer Registry (MNCR) 2012–2016.
A retrospective study was conducted to determine the survival rate of patients with CRC in Malaysia. This study utilised secondary data from the MNCR. This study was conducted from March to September 2022. Data were retrieved from all patients with colorectal cancer registered with MNCR from 2012 to 2016.
MNCR was established in 2007, and the first 5-year report for the incidence period of 2007–2011 was published in 2016 (16). The MNCR 2012–2016 is the second and latest 5-year report by the registry, which was published in 2019 (17). The registry was coordinated by the Clinical Research Centre, Sultanah Bahiyah Hospital, Ministry of Health, Alor Setar, Kedah, Malaysia. The MNCR is a national cancer database that systematically collects data on various types of cancers including colorectal, haematological, lung, breast, and cervical cancers (17). The registry includes data on cancer patients diagnosed in Malaysia between the period of January 1, 2012, and December 31, 2016. The registry captures only Malaysian citizens and residents diagnosed and treated in all the public hospitals within the Ministry of Health and other participating hospitals in Malaysia. Data were provided by appointed healthcare workers from the respective government or private health facilities across 14 states in Malaysia by a standardised cancer notification form. A total of 115,238 newly diagnosed cancer patients were registered from January 1, 2012, to December 31, 2016, of which 15,515 (13.5%) were diagnosed with colorectal cancer.
In this study, the inclusion criteria were as follows: 1) patients diagnosed with CRC between January 1, 2012, and December 31, 2016, and 2) Malaysian citizenship. Patients were excluded when the information on the vital status of dead or alive was not available, if the patients died within a month of CRC diagnosis, or if the information on the date of CRC diagnosis was missing from the study. Therefore, a total of 5,675 patients were included in this study.
The flow diagram of the retrieval process of the patients’ data is presented in Figure 1. Information on sociodemographics such as sex, age, ethnicity, and clinical characteristics including cancer sites and stage at diagnosis was extracted from the records. Treatment status and treatment modalities received by the patients were also collected. The date of CRC diagnosis, the date of death, or date of last follow-up, and the vital status of the patients (alive or dead) were also retrieved. Missing information on the patient’s date of death or date of last follow-up was cross-checked and verified with the data custodian of the National Registration Department (NRD) using the patients’ National Registration Identity Card (NRIC) numbers.
Sociodemographic variables included in this study were sex (male and female), age, age groups (<80 and ≥80), and ethnic groups (Malays, Chinese, Indians, and other Malaysians). Clinical characteristics included cancer sites based on the International Classification of Diseases for Oncology (ICD-O) (colon, rectosigmoid, and rectum), stage at diagnosis based on the Union for International Cancer Control/American Joint Committee on Cancer (UICC/AJCC) TNM classification system (0 to IV), treatment status (received or not), and treatment modalities received by the patients (surgery, chemotherapy, radiotherapy, other types of treatment). The categorisation of the variables was based on the category specified by the registry, except for age groups. In this study, patients’ age was divided based on the level of risk for CRC. Older adults above 80 years old with CRC had a higher risk of CRC (18).
The date of CRC diagnosis was defined by the MNCR as follows: 1) the date of first histological or cytological confirmation of the malignancy (with the exception of histology or cytology at autopsy), in which the date should be either the date when the specimen was taken (biopsy) or the date of the pathology report, 2) the date of admission to the hospital due to the malignancy, or 3) the date of first consultation at the outpatient clinic due to the malignancy. The date of death refers to the date when the patient died as stated in the death certificate or captured in the cancer registry or when cross-checked with the National Registration Department. The date of the last follow-up referred to the date on which the patient was last known to be alive by the appointed healthcare workers. If the date of death was reported, the patient’s vital status was recorded as dead; meanwhile, if the date of last follow-up was reported, the patient’s vital status was recorded as alive.
The outcome of this study was the overall survival of CRC. The event was defined as the patient’s death. Time-to-event was defined as the difference of months between the date of CRC diagnosis and the date of death or the date of last follow-up. This study evaluated patients for a period of 5 years from the date of diagnosis to death or censored. A censored patient was defined as any patient who was lost to follow-up, dropped out, or has not yet died within 5 years of CRC diagnosis. The survival was estimated to be 1 year, 3 years, and 5 years. From the data, a total of 15,515 patients with CRC were screened. Of these, a total of 5,589 patients who had incomplete information and 4,251 patients who died within a month of diagnosis were excluded. Therefore, the remaining 5,675 patients were included in the study. Out of 5,675 patients included, 2,055 had died, 3,534 were censored, and only 86 were alive within 5 years of CRC diagnosis.
Data were de-identified prior to the analysis by the data custodian. Data were analysed using Stata/IC statistical software version 16 (StataCorp LLC, College Station, TX, USA) and SPSS version 22.0 (IBM Corp., Armonk, NY, USA). The Kaplan–Meier method was performed to determine the 1-, 3-, and 5-year survival rates of CRC. The comparison in survival distributions between sex, age groups, ethnic groups, stage at diagnosis, cancer sites, and status of treatment received was analysed using the log-rank test. Any variables with p-values of less than 0.05 were further analysed using the multivariate Cox proportional hazards regression test using an automatic backward elimination method to determine the associated risk factors of CRC survival. The levels of significance were set at 0.05, and 95% confidence intervals were reported where applicable.
We obtained ethical clearance from the Malaysia Medical Research and Ethics Committee. This study was registered under the National Medical Research Registry (NMRR-22-00326-6JB).
From the study cohort, the mean age of the patients was 60.7 years (standard deviation 12.6), ranging from 13 to 96 years, of which the majority were aged less than 80 years (60.7%). Approximately 56% were men, with a mean age of 61.1 years (SD 12.3), ranging from 18 to 96 years, and 44% of the patients were women, with a mean age of 60.3 years (SD 13.0), ranging from 13 to 94 years. Among the CRC patients, 41% were Malays and 47% were Chinese, and approximately 40% suffered from stage IV, followed by stages III, II, I, and 0. Approximately half of the total patients had CRC located in the colon. In addition, approximately 82% of the patients received CRC treatment, of which most of them had surgery (35.5%); others had chemotherapy, radiotherapy, and other therapies; and some had received more than one type of cancer treatment. The characteristics of patients with CRC are presented in Table 1.
Out of 5,675 patients included, 2,055 had died, 3,534 were censored, and only 86 were alive within 5 years of CRC diagnosis. The 1-, 3-, and 5-year overall survival rates of CRC were 68.5%, 34.7%, and 18.4%, respectively (Table 2). The median survival time for CRC was 24 months. The Kaplan–Meier overall survival curve of CRC is presented in Figure 2. From the log-rank analysis, there was no difference observed in the survival rates between male and female patients (p = 0.235). Similarly, no differences were seen in survival rates between cancer sites (p = 0.410). Conversely, there were significant differences observed in the survival rates between age groups, ethnic groups, stages at diagnosis, treatment status, and treatment modalities. The comparison of the survival curves between age groups, ethnic groups, stages at diagnosis, and treatment status is presented in Figures 3–6.
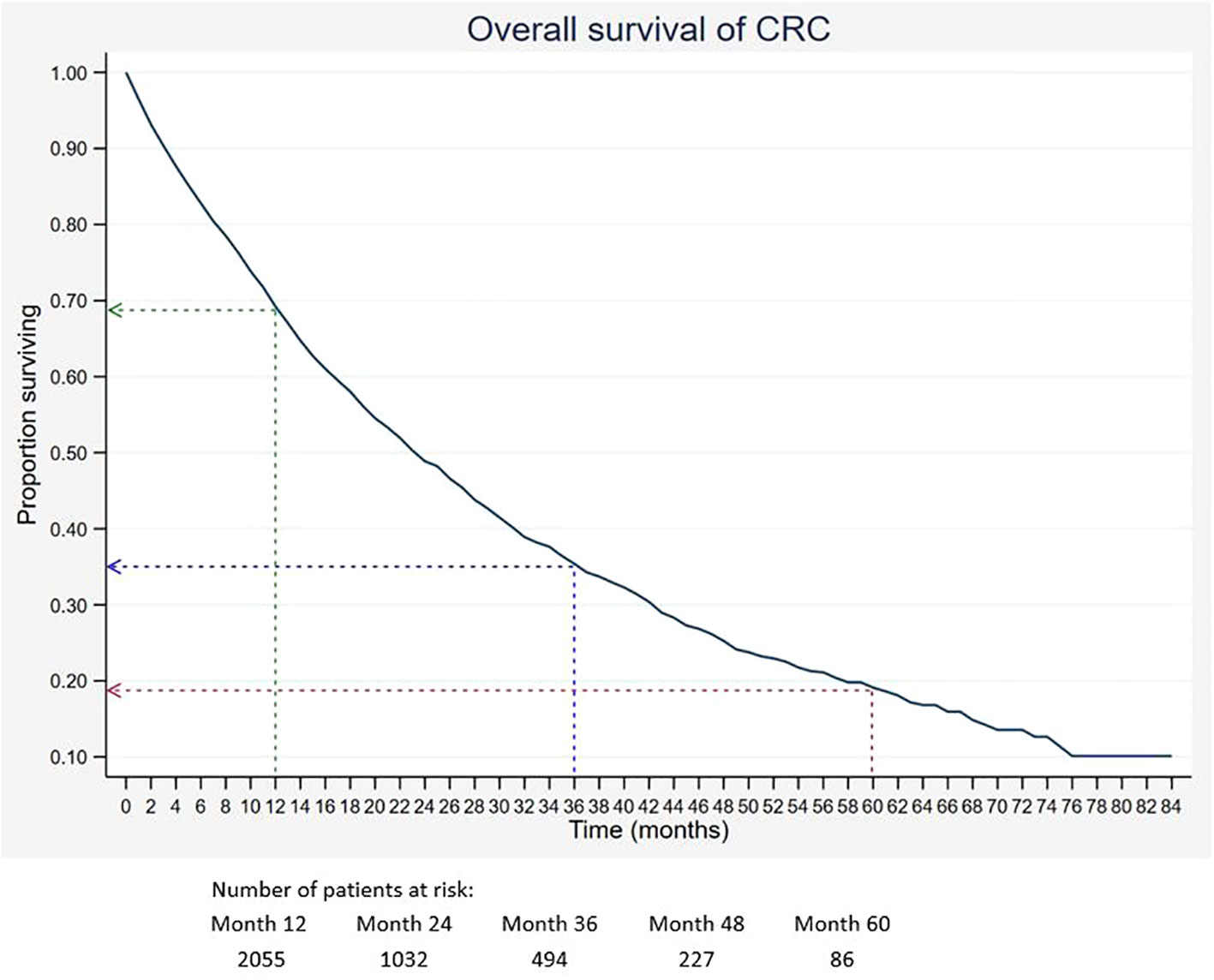
Figure 2 Overall survival of CRC. Green, blue and red dashed-arrows show the 1-, 3- and 5- year survival rates, respectively.
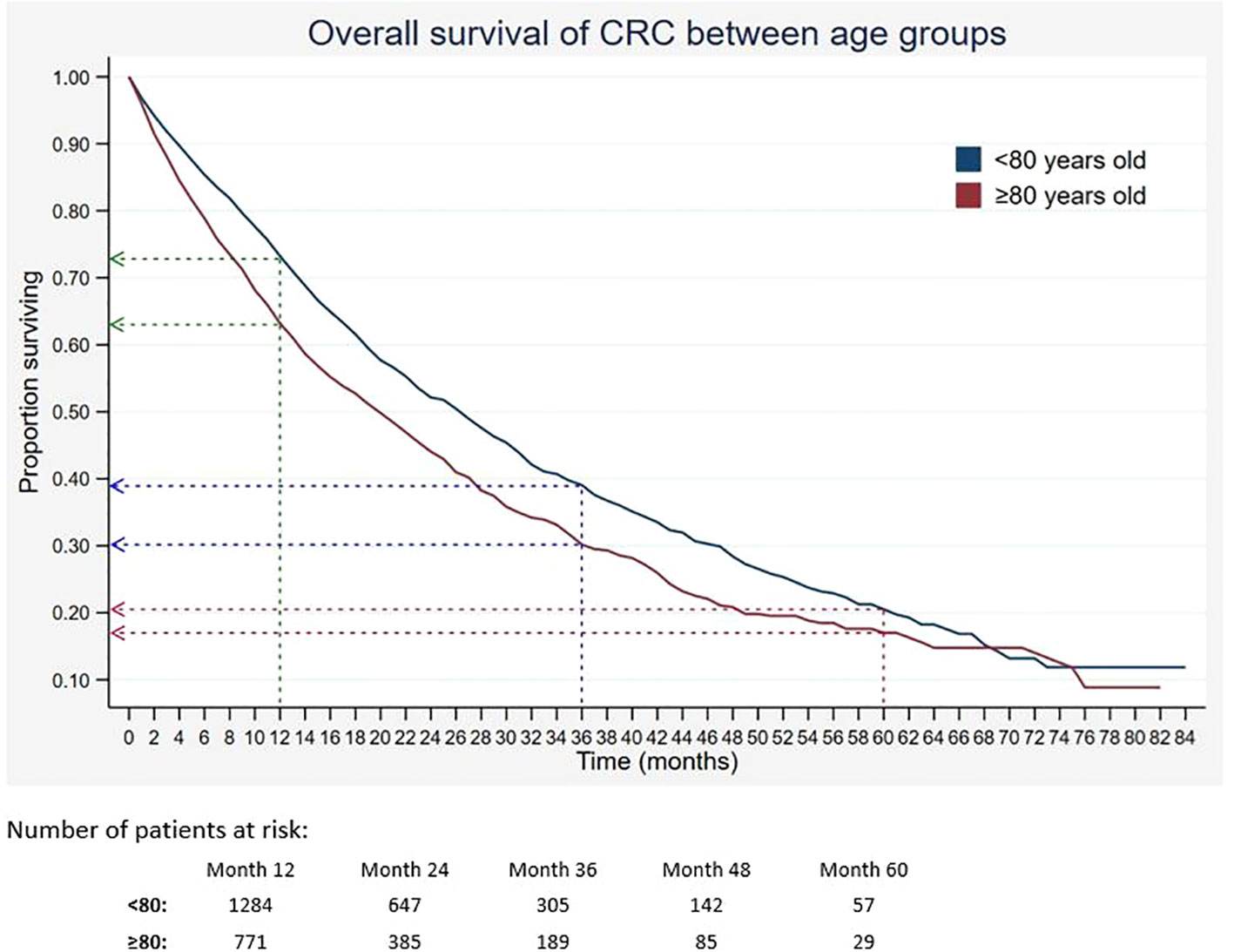
Figure 3 Overall survival of CRC between age groups. Green, blue and red dashed-arrows show the 1-, 3- and 5- year survival rates, respectively.
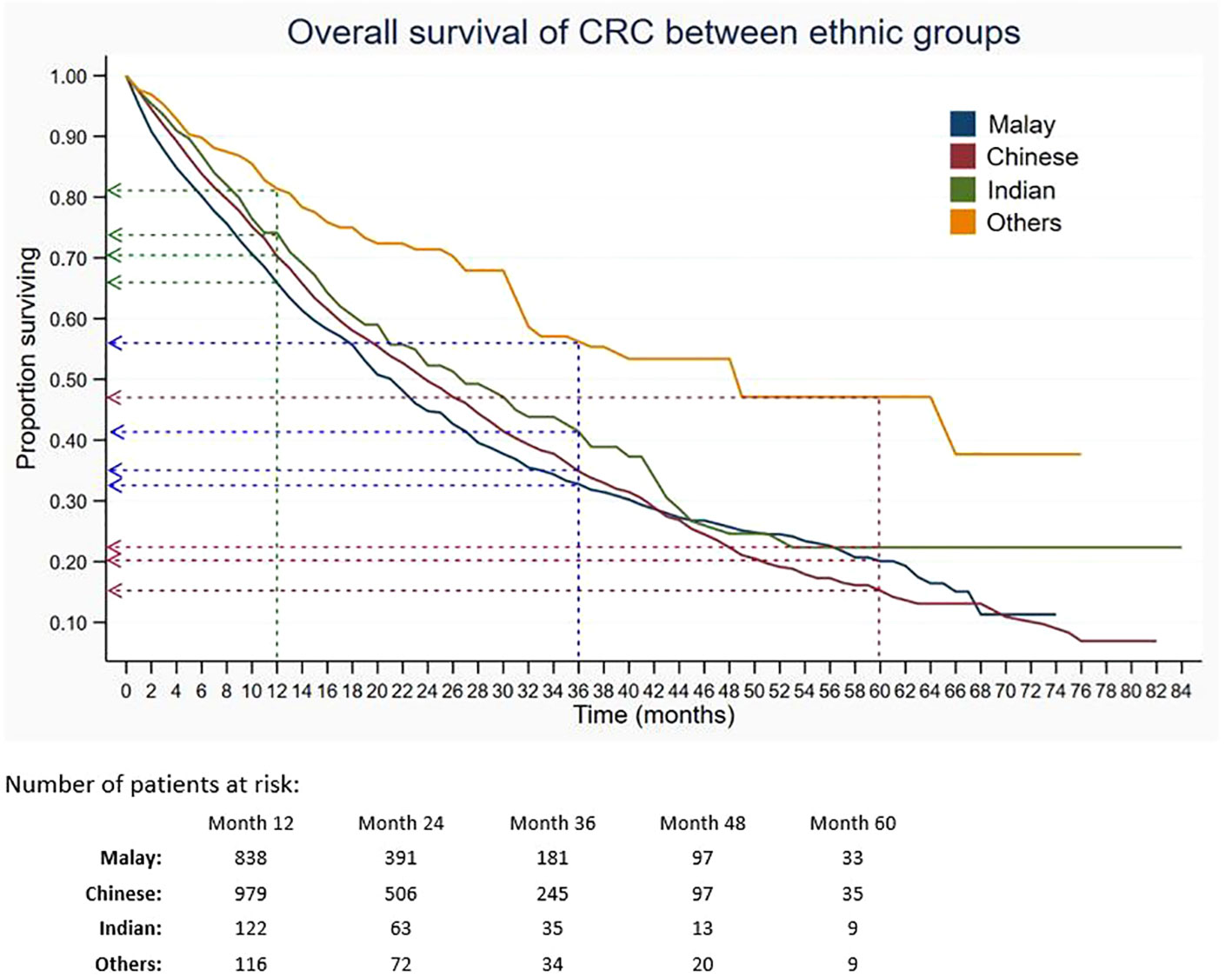
Figure 4 Overall survival of CRC between ethnic groups. Green, blue and red dashed-arrows show the 1-, 3- and 5- year survival rates, respectively.
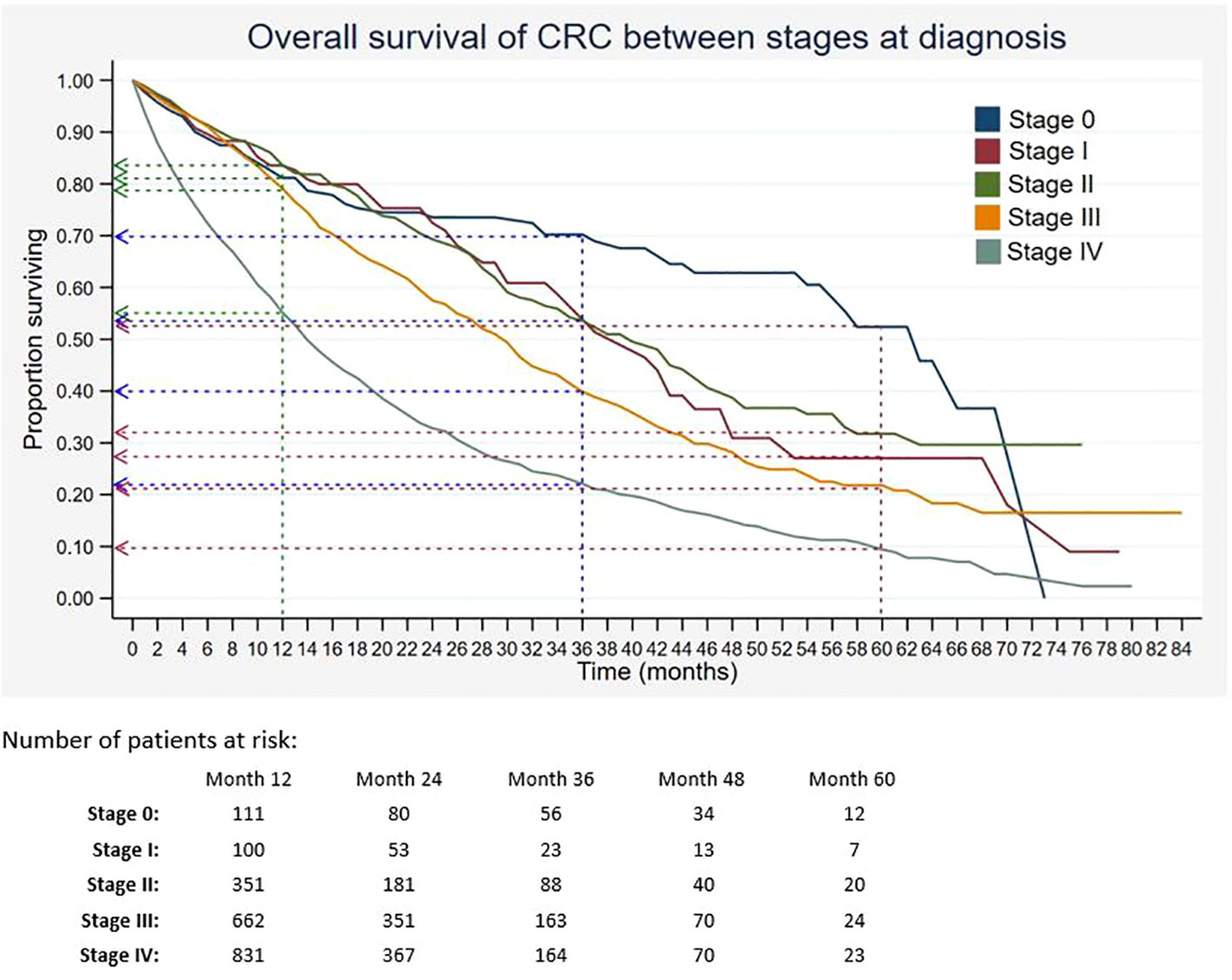
Figure 5 Overall survival of CRC between stages at diagnosis. Green, blue and red dashed-arrows show the 1-, 3- and 5- year survival rates, respectively.
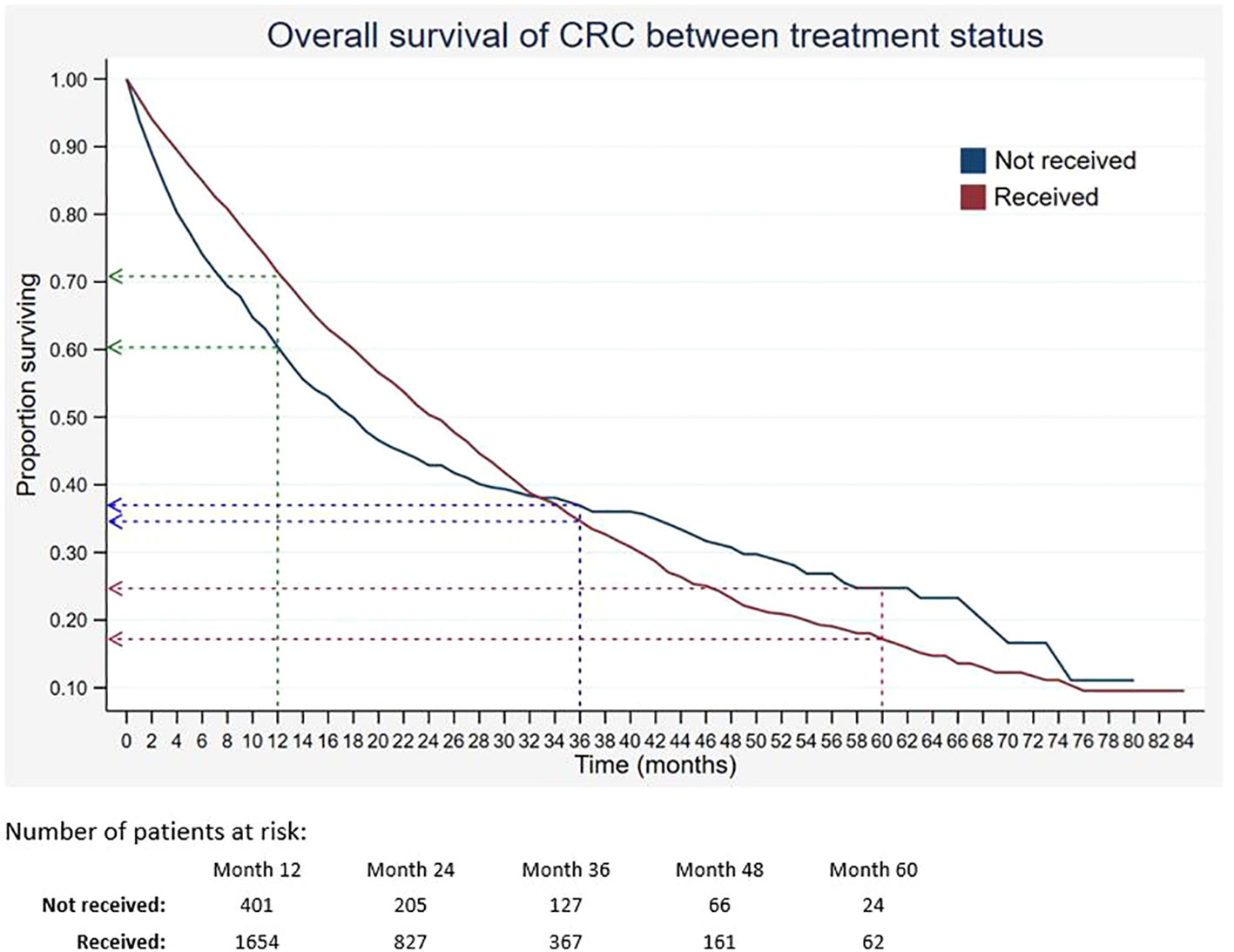
Figure 6 Overall survival of CRC between treatment status. Green, blue and red dashed-arrows show the 1-, 3- and 5- year survival rates, respectively.
From the univariate analysis of stages at diagnosis, stratified by ethnic groups (Table 3), it was found that among patients with stage III and IV, there were significant differences in terms of the survival rates between different ethnic groups (both p < 0.001). In the stratified analysis by treatment modalities (Table 4), there were significant differences in the survival rates between those who suffered from stage I who received different treatment modalities (p = 0.007). Among patients with stage II, a significant difference in the survival rates was also observed between those who received different treatment modalities (p < 0.001). Similar results were found among those with stages III and IV (both p < 0.001).
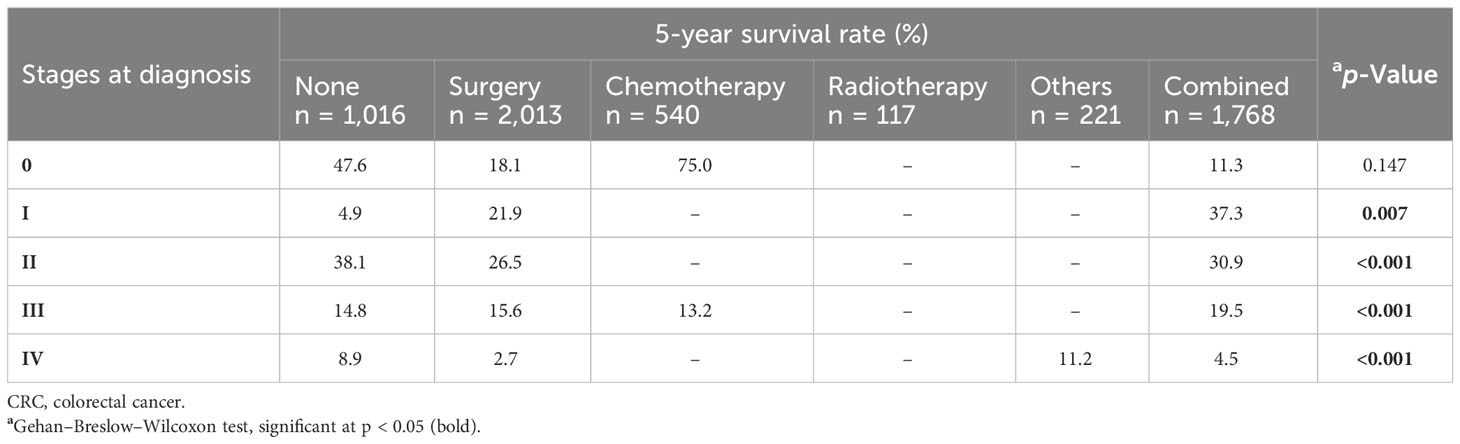
Table 4 Five-year survival rate of CRC patients by stage at diagnosis, stratified by treatment modalities.
Based on the result obtained from the Cox regression analysis, four factors, namely, age groups, ethnic groups, stages at diagnosis, and treatment modalities, were identified as hazards of CRC survival, after adjusting for sex and cancer sites (Table 5). The risk of CRC death was higher among those who were 80 years old and above (adjusted hazard ratio (HR) 1.24, 95% CI 1.14–1.36, p < 0.001). The Chinese had a slightly lower risk of death than the Malays (adjusted HR 0.85, 95% CI 0.78–0.93, p = 0.001). Patients with stage I (adjusted HR 1.51, 95% CI 1.05–2.16, p = 0.027), stage II (adjusted HR 1.46, 95% CI 1.08–1.97, p = 0.013), stage III (adjusted HR 2.17, 95% CI 1.64–2.87, p < 0.001), and stage IV (adjusted HR 4.28, 95% CI 3.26–5.62, p < 0.001) also had higher risk of CRC death compared to those with stage 0. In addition, those who received treatment had a lower risk of death compared to those who did not receive any treatment (adjusted HR 0.66, 95% CI 0.59–0.75, p < 0.001). Furthermore, those who received a single therapy (surgery, chemotherapy, radiotherapy, or others) had a higher risk of CRC death than those who received more than one treatment modality (all p < 0.001).
From this study, the 5-year colorectal cancer survival rate in Malaysia was 18.4%, which was relatively low compared to the overall survival (OS) of CRC in other countries (Thailand (44.0%), Martinique (43.8%), and Singapore (57.0%))),an countries (50.0%), the USA (58.0%), and other high-income countries (like Australia, Canada, Denmark, Norway, Sweden, and the UK), all with survival rates of approximately 67% (19–23). The calculated OS in this study was also lower as compared to the previously reported OS among CRC patients in Hospital Tuanku Ja’afar, one of the tertiary hospitals in Malaysia (46.5%) (24). The delayed presentation of CRC had contributed to the low survival in Malaysia (25). A study examining immunochemical faecal occult blood test (iFOBT) among average-risk individuals in Malaysia reported that the coverage of stool-based screening tests in Malaysia is still low (26). We found no difference in survival based on gender. This is similar to a study conducted at an oncology centre in Brazil (27). However, some studies showed that survival was lower in men (28, 29).
Survival of CRC is significantly determined by certain prognostic factors. Many studies have examined the correlation between demographic and diagnostic characteristics on the survival of patients with CRC. The current finding suggested that survival of CRC decreased with age. Almost 40% of the identified CRC patients were aged 80 years and above. Age was described as one of the predictive factors for death in cancer patients including CRC (30, 31). Furthermore, age has been linked to an increased risk of comorbid conditions among Americans, Japanese, and Danish, which lowers CRC patients’ survival rates (31–33). In light of the findings, it is important to take considerations from multidisciplinary aspects in treatment decisions, as oncological diagnosis may not be the only risk of having a low survival rate.
In this current study, ethnicity was found to be associated with CRC survival. The stage-specific analysis showed that among patients with stage IV, the Chinese had lower 5-year survival than Malays. The finding was in concordance with previous studies. Magaji et al. reported that the Chinese had lower survival of CRC compared to Malays and Indians among University of Malaya Medical Centre patients (34). Muhammad Radzi et al. also reported that the highest incidence and mortality rates were among the Chinese, causing them to have a lower CRC survival, followed by other ethnicities (35). In addition, the Chinese were reported to have less awareness of CRC than Malays, and Malays were better at identifying symptoms compared to the Chinese, contributing to delayed presentation that resulted in poorer survival (36, 37).
Other than age and ethnicity, stage at diagnosis was also found to be significantly associated with survival of CRC. Similarly, other studies showed advanced stages of CRC had lower survival as compared to early stages (19, 23, 38). In the present study, patients with stages III and IV were approximately two times and four times more at risk of dying as compared to stage 0. Previous findings from MNCR in the period between 2008 and 2009 also reported that patients diagnosed with stage IV had the highest risk of death compared to patients with earlier stages (13). In Estonia, patients with advanced stages of colon and rectal cancers have poor 5-year survival rates of 2% and 4%, respectively (38). Similarly, the relative survival at 5 years was 9% among those with late-stage diagnosis in Mumbai, India (39). It was observed that developed countries like Denmark had low 1-year survival among patients who were diagnosed with colon cancer at a late stage, approximately 41% for those with Duke’s stage D compared to 92% for stage A patients (23). This study showed that the majority of CRC cases were diagnosed in late stages. The increase in late diagnoses may be due to the ineffective screening programme in Malaysia. Additionally, there is a scarcity of national data on the incidence of adenomatous polyps or studies that evaluate the effectiveness of different CRC screening tests, which may contribute to the late diagnosis of CRC.
In addition, the 5-year survival of patients who received treatment was lower than that of those who did not receive treatment. The finding was similar to a previous study, in which the estimated 5-year survival rate of those who did not receive chemotherapy was 1.3% higher than that of those who received chemotherapy (40). On the contrary, two studies found that treatments such as systemic therapy, metastasectomy, and surgery significantly increased the relative survival for colorectal cancer (41, 42). The contradicting findings of this current study could be due to the high percentage of patients with advanced stages, in which they had poor prognostic factors that contributed to low survival despite having treatment modalities. Our results showed that patients with late stages who received surgery or chemotherapy had lower survival of CRC than those who did not receive any treatment modalities. It was stated by van den Berg et al. that standard cancer therapy was insufficient to increase survival rates of those with late stages of CRC and had poor prognostic factors (42). Therefore, the need for advancement in treatment as well as implementation of effective screening programs is important.
When compared with a previous study that analysed data of CRC patients from the MNCR record between the years 2008 and 2009, the current study that analysed patients’ records from the year 2012 to 2016 showed lower 3- and 5-year survival rates (13). The finding was due to the increased percentage of patients with advanced stages (III and IV) in the current study (72%), compared to the previous study (59%). An increased percentage of CRC patients with late stages at diagnosis was found to be one of the significant prognostic factors associated with low overall survival of the cancer (19, 20, 24). There were some studies that investigated survival among the Malaysian population (13, 24); however, the risk factors determining survival such as tumour characteristics, lifestyle, treatment, and genetic predisposition among patients with CRC were yet to be demonstrated.
This report may indicate that improvement for a better cancer control programme in Malaysia is needed. Although efforts to implement screening programs and cancer-related promotional activities have been made, the proportion of Malaysians having early screening tests is still low. The reasons why many people do not undergo screening tests are probably due to not having time, as many are busy with work, the long distance between home and hospital, and the cost of the screening test, and most of them do not feel it is necessary, as they believe they are healthy (43). Creativity in attracting people to listen and understand the importance of having an early screening test should be considered. With the undergoing development of a national population-based colorectal cancer screening programme, it is predicted that it will increase early cancer diagnosis and boost survival rates in Malaysia in the future.
This study utilised data from the national registry, which has the most representative data on the incidence and mortality of CRC in different sex and ethnicities in Malaysia, in which there was at least one representative hospital across Malaysia. The hospitals were the major referral public and private hospitals treating CRC in Malaysia, providing the best data on CRC patients.
A few limitations were observed in this study. Firstly, the study had a retrospective design, which relied largely on the data extracted from the registry. Therefore, some data such as co-morbidities, family history of malignancy, and behaviours or lifestyles such as smoking and diet were unavailable. Secondly, some data on clinical characteristics including stage at diagnosis were missing and, hence, were excluded from this study. It is suggested that further studies collect more comprehensive information for a better understanding of the survival of CRC. Thirdly, considering that this study utilised a hospital-based dataset, the total of cases collected might be less than the number of cases in the population-based registration system.
The study reported poor CRC survival among the Malaysian population. Late presentation with advanced stage of CRC had a significant impact on the low survival of CRC. The survival rates of colorectal cancer among patients were comparable with those of some Asian countries. However, the survival rates were lower as compared to those of developed countries. Patients of Chinese ethnicity had lower survival rates compared to Malays or Indians. More advanced staging and late presentation were important predictors of colorectal cancer survival. The majority of the patients presented in an advanced stage, which caused the treatments to become ineffective. Therefore, early screening and treatment are important to improve the survival of patients with CRC. The efficiency of different screening approaches should be identified.
The datasets was obtained after receiving the ethical approval from the Malaysia Medical Research and Ethics Committee (MREC). Requests to access these datasets should be directed to the Malaysia National Cancer Registry (MNCR).
The studies involving humans were approved by Malaysia Medical Research and Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
NAM, NHM, and IAR carried out the study design, study selection, data extraction, and statistical analysis and drafted the manuscript. FL, MHAM, and CK participated in data extraction and drafted the manuscript. SY and NI participated in interoperating the data and drafted the manuscript. TA, LM, and MRAH participated in the discussion for any discrepancies and supervised the study. All authors contributed to the article and approved the submitted version.
The authors would like to thank the Director General of Health Malaysia for his permission to publish this report. The authors would also like to thank all participating centres that contributed to the registry data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. World Health Organization (WHO). Cancer (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/cancer (Accessed 2023 Aug 16).
2. Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol (2021) 14(10):101174. doi: 10.1016/j.tranon.2021.101174
3. IARC. Globocan 2020. Number of new cases in 2020, both sexes, all ages (2020). Available at: https://gco.iarc.fr/today/ (Accessed 2023 May 16).
4. Ministry of Health Malaysia. National strategic plan for colorectal cancer (NSPCRC) 2021 – 2025. (2021). pp. 1–68. Putrajaya, Malaysia: Ministry of Health Malaysia.
5. American Cancer Society. What is colorectal cancer (2020). Available at: https://www.cancer.org/cancer/colon-rectal-cancer/about/what-is-colorectal-cancer.html (Accessed 2023 Mar 17).
6. Hui C, Baclay R, Liu K, Sandhu N, Loo P, Von Eyben R, et al. Rectosigmoid cancer - rectal cancer or sigmoid cancer? Am J Clin Oncol Cancer Clin Trials (2022) 45(8):333–7. doi: 10.1097/COC.0000000000000931
7. Beckmann KR, Bennett A, Young GP, Cole SR, Joshi R, Adams J, et al. Sociodemographic disparities in survival from colorectal cancer in South Australia: A population-wide data linkage study. BMC Health Serv Res [Internet]. (2016) 16(1):1–14. doi: 10.1186/s12913-016-1263-3
8. Chen PC, Lee JC, Der WJ. Estimation of life-year loss and lifetime costs for different stages of colon adenocarcinoma in Taiwan. PloS One (2015) 10(7):1–11. doi: 10.1371/journal.pone.0133755
9. Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, et al. Colorectal cancer. In: ESMO handb cancer sr patient (2015) 1(15065):1–51. doi: 10.1038/nrdp.2015.65.
10. Perron L, Daigle JM, Vandal N, Guertin MH, Brisson J. Characteristics affecting survival after locally advanced colorectal cancer in Quebec. Curr Oncol (2015) 22(6):e485–92. doi: 10.3747/co.22.2692
11. Wang R, Wang MJ, Ping J. Clinicopathological features and survival outcomes of colorectal cancer in young versus elderly. Med (United States). (2015) 94(35):e1402. doi: 10.1097/MD.0000000000001402
12. World Health Organization. IARC Monographs evaluate consumption of red meat and processed meat and cancer risk. Int Agency Res Cancer (2015), 1–2.
13. Muhammad Radzi AH, Mohd Azri MS, Shahrul Aiman S, Noor Syahireen M, Ibtisam I, Faizah A. Survival analysis and prognostic factors for colorectal cancer patients in Malaysia. Asian Pacific J Cancer Prev (2016) 17(7):3575–81. doi: 10.14456/apjcp.2016.136/APJCP.2016.17.7.3575
14. Veettil SK, Lim KG, Chaiyakunapruk N, Ching SM, Abu Hassan MR. Colorectal cancer in Malaysia: Its burden and implications for a multiethnic country. Asian J Surg [Internet]. (2017) 40(6):481–9. doi: 10.1016/j.asjsur.2016.07.005
15. OECD. Cancer Care. Assuring quality to improve survival (2013). Available at: https://www.oecd-ilibrary.org/content/publication/9789264181052-en.
16. Azizah AM, Nor Saleha IT, Noor Hashimah A, Asmah ZA, Mastulu W. Malaysian national cancer registry report (MNCR) 2007-2011. Putrajaya, Malaysia: National Cancer Institute, Ministry of Health (2016).
17. Azizah AM, Hashimah B, Nirmal K, Siti Zubaidah AR, Puteri NA, Nabihah A, et al. Malaysia national cancer registry report (MNCR) 2012-2016. Putrajaya: National Cancer Institute, Ministry of Health (2019) p. 1–116.
18. Mongan J, Kalady MF, Peppone L, Mohile SG. Management of colorectal cancer in the elderly (2010). Available at: https://www.consultant360.com/articles/management-colorectal-cancer-elderly (Accessed 2023 May 17).
19. Kittrongsiri K, Wanitsuwan W, Prechawittayakul P, Sangroongruangsri S, Cairns J, Chaikledkaew U. Survival analysis of colorectal cancer patients in a Thai hospital-based cancer registry. Expert Rev Gastroenterol Hepatol (2020) 14(4):291–300. doi: 10.1080/17474124.2020.1740087
20. Joachim C, Macni J, Drame M, Pomier A, Escarmant P, Veronique-Baudin J, et al. Overall survival of colorectal cancer by stage at diagnosis: Data from the Martinique Cancer Registry. Med (Baltimore). (2019) 98(35):e16941. doi: 10.1097/MD.0000000000016941
21. Teo MCC, Soo KC. Cancer trends and incidences in Singapore. Jpn J Clin Oncol (2013) 43(3):219–24. doi: 10.1093/jjco/hys230
22. Allemani C, Rachet B, Weir HK, Richardson LC, Lepage C, Faivre J, et al. Colorectal cancer survival in the USA and Europe: A CONCORD high-resolution study. BMJ Open (2013) 3(9). doi: 10.1136/bmjopen-2013-003055
23. Maringe C, Walters S, Rachet B, Butler J, Fields T, Finan P, et al. Stage at diagnosis and colorectal cancer survival in six high-income countries: A population-based study of patients diagnosed during 2000-2007. Acta Oncol (Madr). (2013) 52(5):919–32. doi: 10.3109/0284186X.2013.764008
24. Lim KG, Lee CS, Chin DHJ, Ooi YS, Veettil SK, Ching SM, et al. Clinical characteristics and predictors of 5-year survival among colorectal cancer patients in a tertiary hospital in Malaysia. J Gastrointest Oncol (2020) 11(2):250–9. doi: 10.21037/jgo.2020.02.04
25. Ibrahim NRW, Chan HK, Soelar SA, Azmi AN, Said RM, Hassan MRA. Incidence, clinico-demographic profiles and survival rates of colorectal cancer in Northern Malaysia: Comparing patients above and below 50 years of age. Asian Pacific J Cancer Prev (2020) 21(4):1057–61. doi: 10.31557/APJCP.2020.21.4.1057
26. Tamin NSI, Razalli KA, Sallahuddin SN, Chan HK, Hassan MRA. A 5-year evaluation of using stool-based test for opportunistic colorectal cancer screening in primary health institutions across Malaysia. Cancer Epidemiol (2020) 69:101829. doi: 10.1016/j.canep.2020.101829
27. Aguiar Junior S, de Oliveira MM E, Silva DRM, de Mello CAL, VF C, Curado MP. Survival of patients with colorectal cancer in a cancer center. Arq Gastroenterol (2020) 57(2):172–7. doi: 10.1590/s0004-2803.202000000-32
28. Agüero F, Murta-Nascimento C, Gallén M, Andreu-García M, Pera M, Hernández C, et al. Colorectal cancer survival: Results from a hospital-based cancer registry. Rev Esp Enfermedades Dig. (2012) 104(11):572–7. doi: 10.4321/S1130-01082012001100004
29. Weiss JM, Pfau PR, O’Connor ES, King J, LoConte N, Kennedy G, et al. Mortality by stage for right- versus left-sided colon cancer: Analysis of surveillance, epidemiology, and end results-medicare data. J Clin Oncol (2011) 29(33):4401–9. doi: 10.1200/JCO.2011.36.4414
30. Lee M, Cronin KA, Gail MH, Feuer EJ. Predicting the absolute risk of dying from colorectal cancer and from other causes using population-based cancer registry data. Stat Med (2012) 31(5):489–500. doi: 10.1002/sim.4454
31. Morishima T, Matsumoto Y, Koeda N, Shimada H, Maruhama T, Matsuki D, et al. Impact of comorbidities on survival in gastric, colorectal, and lung cancer patients. J Epidemiol (2019) 29(3):110–5. doi: 10.2188/jea.JE20170241
32. Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, et al. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. (2014) 120(9):1290–314. doi: 10.1002/cncr.28509
33. Jørgensen TL, Hallas J, Friis S, Herrstedt J. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br J Cancer. (2012) 106(7):1353–60. doi: 10.1038/bjc.2012.46
34. Magaji BA, Moy FM, Roslani AC, Law CW. Survival rates and predictors of survival among colorectal cancer patients in a Malaysian tertiary hospital. BMC Cancer. (2017) 17(1):1–8. doi: 10.1186/s12885-017-3336-z
35. Muhammad Radzi AH, Ibtisam I, Mohd Azri MS, Faizah A, Wan Khamizar WK, Zabedah O, et al. Incidence and mortality rates of colorectal cancer in Malaysia. Epidemiol Health (2016) 38:e2016007. doi: 10.4178/epih.e2016007
36. Su TT, Goh JY, Tan J, Muhaimah AR, Pigeneswaren Y, Khairun NS, et al. Level of colorectal cancer awareness: A cross sectional exploratory study among multi-ethnic rural population in Malaysia. BMC Cancer (2013) 13(1):1. doi: 10.1186/1471-2407-13-376
37. Loh KW, Majid HA, Dahlui M, Roslani AC, Su TT. Sociodemographic predictors of recall and recognition of colorectal cancer symptoms and anticipated delay in help-seeking in a multiethnic Asian population. Asian Pacific J Cancer Prev (2013) 14(6):3799–804. doi: 10.7314/APJCP.2013.14.6.3799
38. Innos K, Soplepmann J, Suuroja T, Melnik P, Aareleid T. Survival for colon and rectal cancer in Estonia: Role of staging and treatment. Acta Oncol (Madr). (2012) 51(4):521–7. doi: 10.3109/0284186X.2011.633928
39. Yeole BB, Sunny L, Swaminathan R, Sankaranarayanan R, Parkin DM. Population-based survival from colorectal cancer in Mumbai, (Bombay) India. Eur J Cancer. (2001) 37(11):1402–8. doi: 10.1016/S0959-8049(01)00108-3
40. Lv Z, Liang Y, Liu H, Mo D. Association of chemotherapy with survival in stage II colon cancer patients who received radical surgery: a retrospective cohort study. BMC Cancer. (2021) 21(1):1–11. doi: 10.1186/s12885-021-08057-3
41. Brouwer NPM, Bos ACRK, Lemmens VEPP, Tanis PJ, Hugen N, Nagtegaal ID, et al. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int J Cancer. (2018) 143(11):2758–66. doi: 10.1002/ijc.31785
42. van den Berg I, Coebergh van den Braak RRJ, van Vugt JLA, Ijzermans JNM, Buettner S. Actual survival after resection of primary colorectal cancer: results from a prospective multicenter study. World J Surg Oncol (2021) 19(1):1–10. doi: 10.1186/s12957-021-02207-4
Keywords: colorectal neoplasm, survival time, cumulative survival, hazard, Malaysia
Citation: Muhamad NA, Ma’amor NH, Rosli IA, Leman FN, Abdul Mutalip MH, Chan H-K, Yusof SN, Tamin NSI, Aris T, Lai NM and Abu Hassan MR (2023) Colorectal cancer survival among Malaysia population: data from the Malaysian National Cancer Registry. Front. Oncol. 13:1132417. doi: 10.3389/fonc.2023.1132417
Received: 12 January 2023; Accepted: 13 November 2023;
Published: 29 November 2023.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Per J. Nilsson, Karolinska Institutet (KI), SwedenCopyright © 2023 Muhamad, Ma’amor, Rosli, Leman, Abdul Mutalip, Chan, Yusof, Tamin, Aris, Lai and Abu Hassan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nor Asiah Muhamad, bm9yYXNpYWhkckBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.