
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 26 April 2023
Sec. Hematologic Malignancies
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1128636
Objective: To retrospectively analyze the reasons for misdiagnosis of haematolymphoid neoplasms and provide experience for improving the diagnostic level in China.
Methods: A retrospective analysis was performed on 2291 cases of haematolymphoid diseases evaluated by the Department of Pathology of our hospital from 1 July 2019 to 30 June 2021. All 2291 cases were reviewed by two hematopathologist experts and classified according to the 2017 revised WHO classification criteria, supplemented immunohistochemistry (IHC), molecular biology and genetic information as needed. The diagnostic discordance between primary and expert review was evaluated. The possible causes of the diagnostic discrepancies were analyzed for each step involved in the procedure of diagnosis.
Results: In total, 912 cases did not conform to the expert diagnoses among all the 2291 cases, with a total misdiagnosis rate of 39.8%. Among them, misdiagnosis between benign and malignant lesions accounted for 24.3% (222/912), misdiagnosis between haematolymphoid neoplasms and non-haematolymphoid neoplasms accounted for 3.3% (30/912), misdiagnosis among lineages accounted for 9.3% (85/912), misclassification in lymphoma subtypes accounted for 60.8% (554/912), and other misdiagnoses among benign lesions accounted for 2.3% (21/912) of cases, among which misclassification of lymphoma subtypes was the most common.
Conclusion: The accurate diagnosis of haematolymphoid neoplasms is challenging, involving various types of misdiagnosis and complicated causes, however, it is important for precise treatment. Through this analysis, we aimed to highlight the importance of accurate diagnosis, avoid diagnostic pitfalls and to improve the diagnostic level in our country.
The pathological diagnosis of haematolymphoid neoplasms has always been challenging in clinical pathology. In addition, clinical treatment regimens are different for distinct subtypes, leading to a significantly different prognosis (1). To achieve standardized, precise and individualized therapeutic approaches, it is critical to make a precise pathological diagnosis. The overall misdiagnosis rate of haematolymphoid neoplasms is relatively higher (2–6), and the documented rates are as high as 27.3% (3) after the adoption of the World Health Organization (WHO) Classification in the year 2001. Moreover, the expert review was emphasized in the literatures. Recently, a large-sample study in France was carried out with a misdiagnosis rate of up to 19.7% (6). However, there is a lack of related studies in China. Hence, a retrospective analysis of the misdiagnosis of haematolymphoid neoplasms in China is of great significance.
As a specialized hospital for refractory/recurrent haematolymphoid tumors, Beijing GoBroad Boren Hospital established its Department of Pathology on 1 July 2019. By 30 June 2021, the Department of Pathology received consultations of 2291 cases related to haematolymphoid diseases. According to the 2017 revised WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues, we investigated all procedures, including acquisition of clinical information, tissue sampling, fixation, hematoxylin-eosin (HE) and immunohistochemical staining (IHC), interpretation of HE and IHC results, supplemental data such as flow cytometry (FCM), molecular biology and cytogenetics, and pathologists’ comprehension of the diagnostic criteria, and analyzed possible causes of diagnostic discrepancies to provide references for the diagnosis of haematolymphoid neoplasms and improve the accuracy of pathological diagnosis.
A total of 2291 consult cases related to haematolymphoid diseases in the Department of Pathology of our hospital from 1 July 2019 to 30 June 2021 were analyzed, whose samples were reviewed by outside pathologists first from nearly 600 hospitals across the country. There were 1365 males and 926 females aged from 11 days to 91 years. All samples were primarily diagnosed by outside pathologists first, then reviewed by two hematopathologist experts, and finally diagnozed by experts according to the 2017 revised WHO classification criteria.
The received materials contained original slides (HE and IHC) and/or unstained slides or formalin-fixed paraffin-embedded block(s) as well as the clinical information (including tissue site, gender, age, symptoms, and imaging material). The following IHC staining was performed using an automatic IHC staining machine (Leica BOND-MAX). Primary antibodies were purchased from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., and Gene Tech (Shanghai) Company Limited. EBV-encoded small RNAs (EBER) were detected by in situ hybridization (ISH), and the probes and corresponding kits were purchased from Leica Biosystems Nussloch GmbH. All operations were carried out according to the manufacturer’s instructions. The gene rearrangement of MYC, BCL2, BCL6, and CCND1 was detected using fluorescence in situ hybridization (FISH). The probe and kit were purchased from Guangzhou LBP Medicine Science & Technology Co., Ltd. The immunoglobulin (IG) and T-cell receptor (TCR) rearrangement were detected by polymerase chain reaction (PCR) based on the BIOMED-2 standardized clonality analysis system.
Among 2291 cases, 912 were misdiagnosed, and the overall misdiagnosis rate was 39.8%. Among these cases, the misdiagnosis between malignancy and benign lesions accounted for 9.7% (222/2291), the misdiagnosis between haematolymphoid neoplasms and non-haematolymphoid neoplasms accounted for 1.3% (30/2291), the misdiagnosis between tumor lineages accounted for 3.7% (85/2291), the misdiagnosis between lymphoma subtypes accounted for 24.2% (554/2291), and the misdiagnosis of other benign lesions accounted for 0.9% (21/2291). Misdiagnosis of lymphoma subtypes was the most frequent (Figure 1).
Among 2291 cases, there were 1134 cases of non-Hodgkin B-cell neoplasms (B-NHL), including diffuse large B-cell lymphoma (DLBCL), Burkitt lymphoma (BL), high-grade B-cell lymphoma (HGBL), follicular lymphoma (FL), small B-cell lymphoma/chronic lymphocytic leukemia (SLL/CLL), marginal zone B-cell lymphoma (MZL), lymphoplasmacytic lymphoma (LPL), mantle cell lymphoma (MCL), plasmablastic lymphoma (PBL), B-lymphoblastic lymphoma/leukemia (B-LBL/ALL), and plasmacytomas (PCN). Of all the B-NHL, there were 418 cases of DLBCL, accounting for the highest proportion (18.3%, 418/2291), including diffuse large B-cell lymphoma-not otherwise specified (DLBCL-NOS), primary mediastinal large B-cell lymphoma (PMBL), EBV-positive DLBCL and ALK-positive large B-cell lymphoma (LBCL). Among the 418 cases of DLBCL, 254 cases (60.8%, 254/418) had a concordance diagnosis; 24 cases had a misdiagnosis between DLBCL and low-grade B-cell lymphoma (total misdiagnosis rate: 1.1%, 24/2291), and 22 cases had a misdiagnosis between DLBCL and grade 3A FL (1.0%, 22/2291). Among 128 cases of BL, the diagnostic concordance rate was 79.7% (102/128). Among 88 cases of HGBL, the diagnostic concordance rate was only 15.9% (14/88). There were 29 cases of HGBL who were mis-diagnosed as BL, including 4 cases without FISH tests, 12 cases with strong positive BCL2, 13 cases with no MYC rearrangement. Among the 6 cases of HGBLs with MYC and BCL2 and/or BCL6 rearrangements (double-hit HGBLs), 5 cases were undiagnosed for lack of FISH tests, and 1 case was diagnosed as DLBCL with both MYC and BCL6 rearrangements. Among 90 cases of high-grade FL (grade 3A and 3B), the diagnostic concordance rate was 32.2% (29/90), 19 cases were primarily diagnosed as DLBCL, 8 cases were primarily diagnosed as low-grade FL (grade 1-2) and 20 cases had misdiagnoses between high-grade and low-grade FL (grade 1-2) among the 2291 cases (0.9%, 20/2291). Among 249 cases of small B-cell lymphoma (excluding MCL), 141 cases (56.6%) had consistent diagnoses. Among 24 cases of MCL, 19 cases (79.2%) had consistent diagnoses. Among 70 cases of B-LBL/ALL, 9 cases were misdiagnosed as mature B-NHL (12.9%, 9/70), and misdiagnosis between B-LBL/ALL and mature B-NHL occurred in 14 cases of all the 2291 cases (0.6%, 14/2291).
There were 424 cases of T/NK-cell tumors, including T-lymphoblastic lymphoma/leukemia (T-LBL/ALL) (98 cases), anaplastic large cell lymphoma (ALCL) (98 cases), NK/T-cell lymphoma (45 cases), EBV-positive T/NK-cell proliferative disease (31 cases), angioimmunoblastic T-cell lymphoma (AITL) (52 cases), peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS) and other mature T-cell non-Hodgkin lymphoma (T-NHL) (99 cases), with diagnostic coincidence rates of 84.7%, 67.4%, 62.2%, 58.1%, 55.8%, and 38.4%, respectively. Excluding T-LBL/ALL, mature T/NK-cell neoplasms had a relatively lower concordance (180/326, 55.2%).
There were 195 cases of classic Hodgkin lymphoma (CHL), including 61 misdiagnosed cases (31.3%). Among 61 misdiagnosed cases, 14 cases were misdiagnosed as benign hyperplasia, 3 cases were misdiagnosed as non-haematolymphoid neoplasms, 13 cases were misdiagnosed as T-cell lymphomas, 2 cases were misdiagnosed as histiocytic neoplasms, 1 case was misdiagnosed as myeloid neoplasms, 10 cases were misdiagnosed as DLBCL (including 2 cases of EBV-positive DLBCL, 2 cases of T-lymphocyte/histiocyte-rich large B-cell lymphoma), 7 cases were misdiagnosed as B-cell lymphoma-unclassified with features between diffuse large B-cell lymphoma and classic Hodgkin lymphoma (DLBC-U, grey zone lymphoma), 3 cases were misdiagnosed as nodular lymphoma primary Hodgkin’s lymphoma (NLPHL), and 8 cases had no definite results in the initial diagnosis. The differential diagnosis between CHL and T-NHL sometimes can be difficult, and PCR-TCR was performed in 4 cases who were primarily diagnosed as AITL.
Mixed phenotype acute leukemia (MPAL) and blast plasmacytoid dendritic cell neoplasm (BPDCN) were rare subtypes of myeloid and histiocytic/dendritic neoplasms, unsurprisingly, with the highest misdiagnosis rates (9/10, 90%; 7/8, 87.5%; respectively). For MPAL, 4 cases were misdiagnosed as T-LBL/ALL, 1 case was misdiagnosed as PTCL, 2 cases were misdiagnosed as B-LBL/ALL, and 2 cases were misdiagnosed as myeloid sarcoma. The diagnosis of MPAL in extramedullary tissue is more challenging, and adequate immunostains and accurate interpretation are necessary. Meanwhile, FCM is of great significance. Two cases were finally diagnosed referring to the subsequent FCM results of bone marrow.
There were 48 cases of non-haematolymphoid neoplasms, of which 13 were misdiagnosed as haematolymphoid neoplasms, with a diagnosis discordance rate of 27.1%. Misdiagnosis between haematolymphoid and non-haematolymphoid neoplasms occurred in 30 cases of all the 2291 cases, and the most commonly involved non-haematolymphoid neoplasms were poorly differentiated carcinomas, sarcoma, neuroendocrine tumors and thymoma.
There were 399 cases of benign hyperplasia (including Castleman disease), including 135 misdiagnosed cases, with a diagnosis discordance rate of 33.8%. Among 135 misdiagnosed cases, 118 cases were misdiagnosed malignant tumor. Notably, 222 out of 2291 cases in this study were misdiagnosed between benignancy and malignancy (that is, submitted diagnosis benign→expert diagnosis malignant, and vice versa) (accounting for 9.7% of the total cases and 24.2% of the total misdiagnosed cases).
Notably, among 554 cases of subtype misdiagnosis, 206 cases (nearly 40%) were only diagnosed as B-NHL or T-NHL at the initial diagnosis, with no clear subtypes. Most of these cases were from hospitals in less developed areas or with lower level, the IHC antibodis were limited and detection means such as FISH, FCM and molecular studies were not available in those laboratories. What’s more, beyond the technology, pathologists’ knowledge and practical experience can also make a big difference in the diagnosis.
The distribution of disease types and the analysis of misdiagnosis types are shown in Tables 1, 2.
The overall misdiagnosis rate was 39.8% in our study, indicating that the diagnostic efficacy of haematolymphoid neoplasms in China still lags behind compared to other studies (2–6). This study analyzed the reasons for misdiagnosis from the following aspects.
High-quality tissue specimens and HE-stained sections are essential for pathological diagnosis, especially for haematolymphoid neoplasms. Because of the unique organizational structure of lymphoid tissue, the quality requirements for its slices are relatively higher than that for other types of samples.
To ensure diagnostic accuracy, an excisional biopsy in lymph nodes is preferred (7). For ultrasound-guided core-needle biopsy, it is recommended to obtain at least 3 representative tissues with a core needle no smaller than 18 gauge (G) to ensure enough specimens for subsequent procedures, including HE, IHC, FCM and molecular testing (8).
The tissue should be fixed in 10% neutral formalin solution timely (within 30 minutes). Notably, lymph nodes should be dissected before fixation because the fibrous capsule of the lymph node can resist the penetration of the fixative, resulting in poor fixation. Furthermore, tissue that is poorly fixed can interfere with morphological observation (Figure 2), IHC staining results, and even molecular tests.
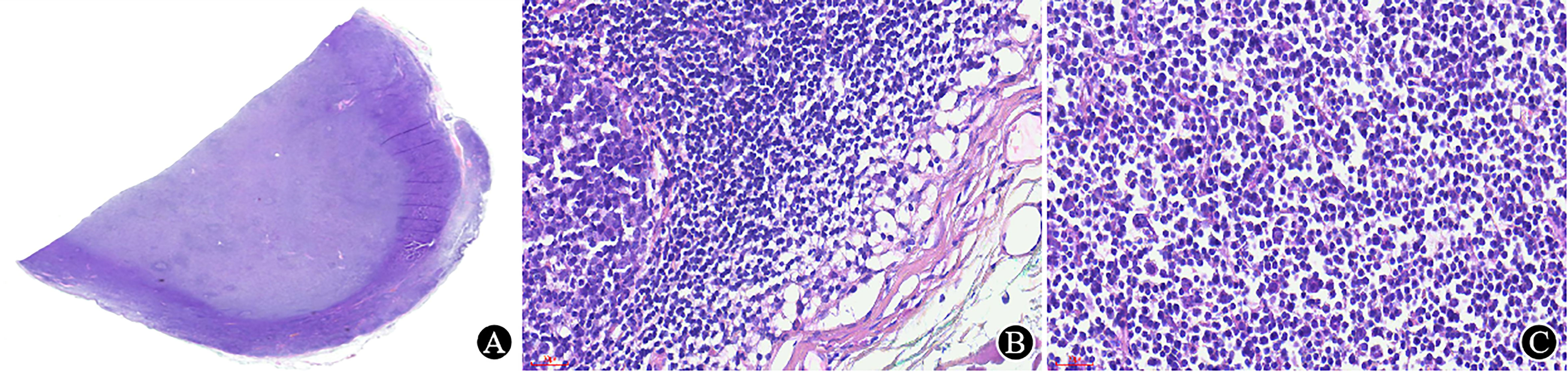
Figure 2 Poorly fixed lymph node. (A) Under low magnification, the lymph nodes is well stained in the periphery and lightly stained in the center (×5.5); (B) The structure near the capsule is well preserved, and the cell morphology is good (×400); (C) The tissue is dissociated, and the cells are shrunken in the area away from the capsule (×400).
High-quality HE sections are the basis of pathological diagnosis. The quality of HE sections depends on each step of tissue processing, and a thickness of 2-4 μm is preferred (7). In our laboratory, a 3 μm slice is required for both HE and IHC, which can clearly display the tissue structure and cellular detail (Figure 3).
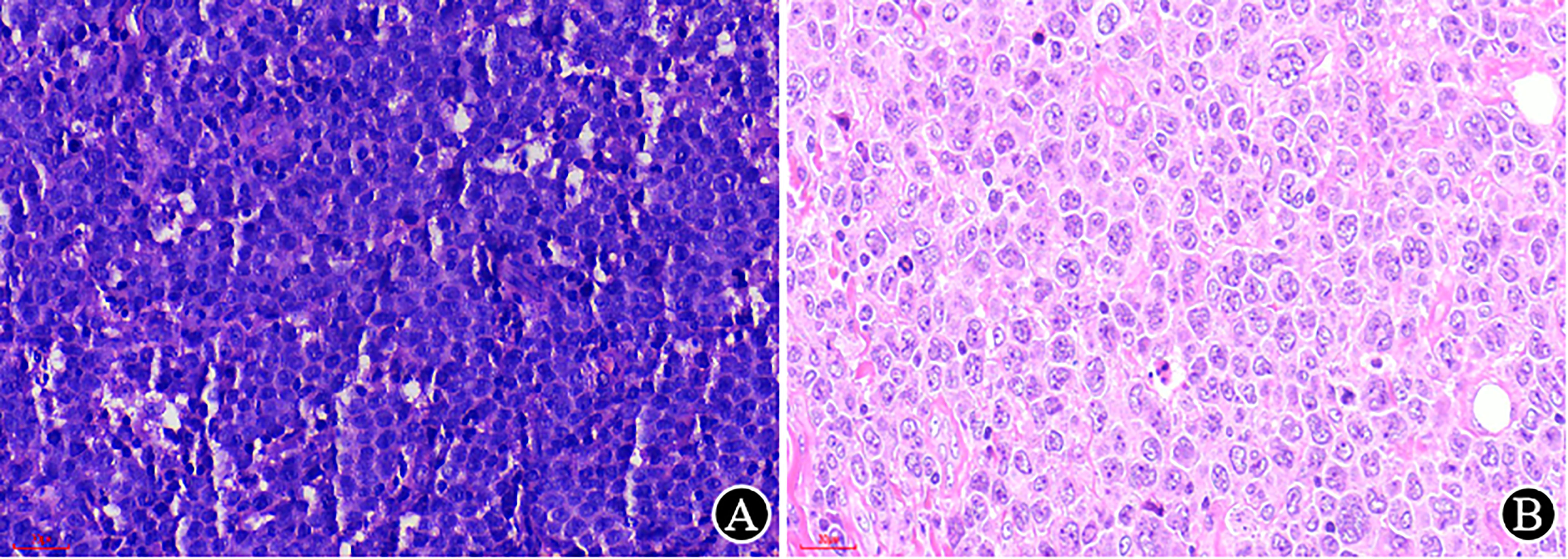
Figure 3 The impact of the thickness of the HE section on morphological observation (×400). (A) The previously prepared slice (at another hospital) is thick, with a medium cell volume and unclear structure; (B) The reprepared slice (at our hospital) is thin, with a clear cellular detail and larger volume.
Immunohistochemistry plays an important role in the accurate diagnosis and definition of disease subtypes in hematopathology. Of all the 912 misdiagnosed cases in this study, IHC is always rechecked or performed significant additional IHC staining. The related errors in this procedure have been analyzed in the literature (9). In our study, the IHC-related errors were discussed from the following two aspects:
In our study, two cases with pleomorphic neoplastic cells were originally diagnosed as DLBCL but were subsequently demonstrated to be CD5- and Cyclin D1-positive in our laboratory, with a CCND1 rearrangement detected by FISH. Classical MCL is one subtype of mature B-cell lymphoma, mainly composed of small to medium lymphoid cells with irregular nuclei; however, the blastoid and pleomorphic variants of MCL can morphologically resemble LBL or DLBCL, which can cause diagnostic confusion. Therefore, it is recommended to routinely examine CD5 and Cyclin D1 for the differential diagnosis of DLBCL, blastoid/pleomorphic variant MCL, DLBCL-type Richter syndrome and primary CD5-positive DLBCL, which is more aggressive (10).
The use of insufficient immunostains can also cause the misdiagnosis of MPAL as LBL/ALL, the diagnosis of which depends heavily on the immunophenotype. There are two main approaches for MPAL typing, the European Group for the Immunological Characterization of Leukemias (EGIL) and WHO Classification criteria (1, 11). IHC can also provide helpful supplemental information for lineage specificity, especially for antibodies that located in cytoplasm and nucleus. T-cell lineage assignment is specifically relying on cytoplasmic CD3 (cCD3) expression.The confirmation of B-cell component requires multiple markers, which is based on CD19 expression (CD20 is uncommon in MPAL), corroborating with one or two other B cell makers including CD79a, PAX5 or CD22. The most specific maker of myeloid lineage assignment is MPO, together with two or more of myeloid or monocytic makers such as lysozyme, CD33, CD68, CD14. It is necessary to perform a comprehensive panel of antibodies for the diagnosis of MPAL with at least two key antibodies per lineage included.
An optimally immunostained slide relies on every step in tissue processing and immunohistochemical staining. It requires knowledge of the antibodies used, appropriate controls and the expected staining pattern for accurate interpretation of immunostains. Knowledge of the cellular staining location of the targeted antigen is crucial, e.g., CD20 should be located in the membrane, Cyclin D1 in the nucleus, etc., otherwise it should not be regarded as positive in any situation (Figure 4).
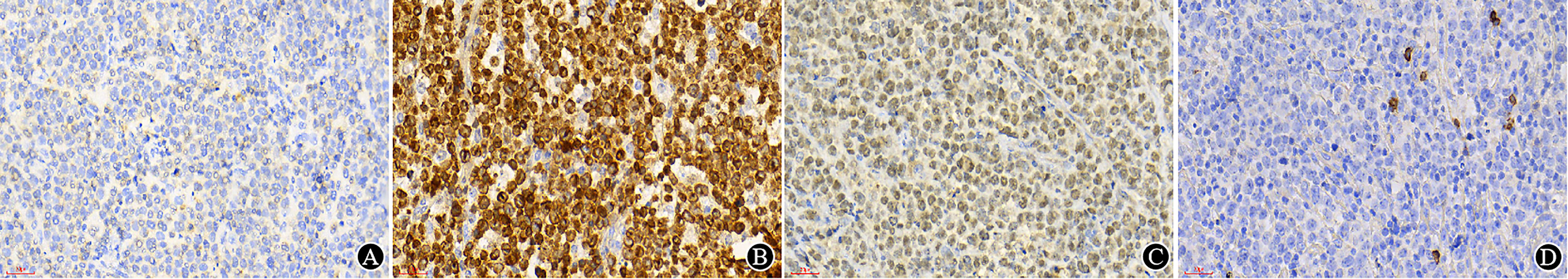
Figure 4 Immunostains and interpretation (×400). (A) In original sections made by other hospitals, CD3 staining is difficult to interpret; (B) After restaining in our hospital, the CD3 results were positive in the cytoplasm; (C) In original sections made by other hospitals, CD19 showed positive signals but was located in the nucleus; (D) After restaining in our hospital, CD19 was found to be negative in tumor cells, and positive signals of the internal controls were localized on the cell membrane.
In some situations, dual-staining can be very helpful to identify the target cells (Figure 5) because it is useful for diagnosis or related to prognosis and treatment in some lymphomas (12).
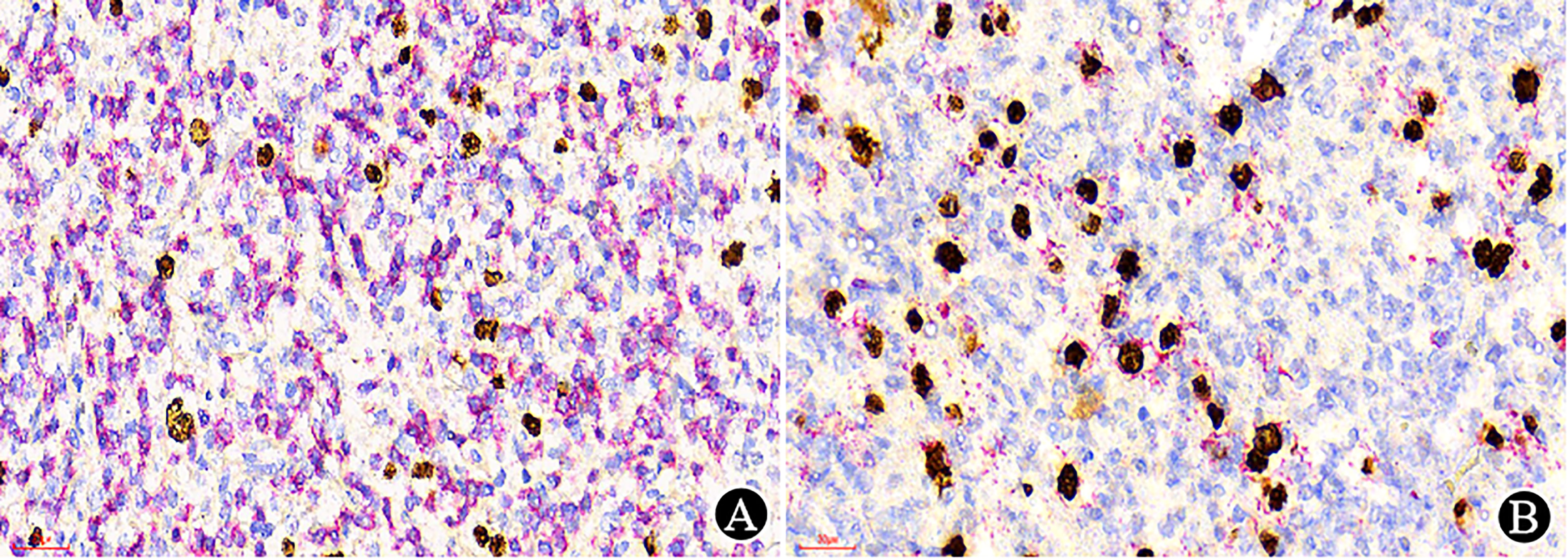
Figure 5 Dual-staining with EBER (nuclear stain in brown) and CD3/CD20 (membranous and cytoplasmic in red) (×400). (A) EBER/CD3 dual-staining showed no dual-staining positive cells; (B) EBER/CD20 dual-staining demonstrated dual-positive cells.
The importance and necessity of a comprehensive diagnosis was emphasized in the 2017 revised WHO classification. In addition to IHC, FCM and molecular and cytogenetic tests play an important role in diagnostic hematopathology.
FCM is an important tool in hematopathology due to its advantages of high sensitivity, strong specificity and short detection period. As previously mentioned, FCM has superiority in the diagnosis of MPAL, and the use of a comprehensive panel is vital to prevent misdiagnosis (13). Meanwhile, multiparameter FCM is also crucial for elucidating MPAL cases with a single population of blasts at diagnosis (biphenotypic leukaemia) or a leukaemia containing separate blast populations (bilineage leukaemia) (14, 15). In view of significant differences in clinical treatment regimens between different lineages and between immature and mature haematolymphoid malignancies, an accurate diagnosis is critical (16, 17). It is recommended that, in addition to routine histopathology and IHC, an FCM test be performed in bone marrow or extramedullary tissue as much as possible to accurately locate and analyze cell differentiation. For some special or small specimen, as for instance, FCM on brain biopsy rinse fluid is a potentially useful strategy for multidisciplinary diagnosis of central nervous system (CNS) lymphoma (18, 19).
Molecular testing is necessary for some cases. PCR-based IG and TCR gene rearrangements are important in the diagnosis of haematolymphoid tumors (20). At the same time, next-generation sequencing (NGS), which has been widely used, shows its superiority in the diagnosis, prognosis and treatment evaluation of lymphoma (21). Significant lymphoid proliferation in the lesion gastric mucosa sometimes makes it difficult to identify MALToma on the basis of morphology and immunohistochemistry only; however, IG gene rearrangement is necessary for the differential diagnosis. Based on the additional PCR-IG gene rearrangement, 11 cases modified the diagnoses from benign/reactive to MALToma, 9 cases from MALToma to benign/reactive in this study. The diagnosis of gastric MALToma should be made with caution because approximately 90% of gastric MALToma cases are related to Helicobacter pylori (H. pylori) infection, and approximately 50% to 90% of cases can achieve complete remission after receiving antibiotic eradication therapy (22). It should be noted that IG and TCR gene rearrangement may have lineage crossover. For example, IG rearrangement may occur in some cases of AITL, and immature haematolymphoid malignancies can often have cross-lineage rearrangements (20, 23). Nevertheless, the molecular results should be interpreted on the basis of histopathology, and attention should be given to the possibility of false negative or positive results.
FISH can provide diagnostic and prognostic prediction information by detecting specific gene sequence amplification, deletion and gene rearrangement on chromosomes in tumor tissue. The 2017 revised WHO classification proposes a new independent subtype, high-grade B-cell lymphoma (HGBL). Regardless of HGBL-NOS or HGBL with MYC, BCL2 and/or BCL6 rearrangements (double/triple-hit lymphoma), the detection of MYC, BCL2 and BCL6 by FISH is necessary for a clear diagnosis (1).
For newly diagnosed patients, it is recommended that an MICM (Morphology, Immunology, Cytogenetics and Molecular) examination be as thorough as possible to provide comprehensive data for monitoring and follow-up.
Accurate clinical information (including gender, age, symptoms, and auxiliary examinations) is indispensable for pathological diagnosis. Some studies suggest that clinical information accounts for approximately 10% of the information required for the diagnosis of lymphomas (24). The diagnosis of DLBCL in children should exclude BL and B-LBL/ALL first. It is necessary to rule out skeletal lesions in the diagnosis of extraosseous (extramedullary) plasmacytomas, and a clear mass is a prerequisite for the diagnosis of primary DLBCL of the uterine cervix. The 2017 revised WHO lists pediatric-type follicular lymphoma (PTFL) as an independent subtype of B-NHL. PTFL primarily involves the lymph nodes of the head and neck with localized lesions, and its pathological grade is usually FL-3A in morphology. Its immunophenotype and genetic characteristics overlap with those of common FL, but its clinical manifestations are different from those of common FL (1, 25). Whether a localized lesion is found by clinical evaluation is crucial for the diagnosis of PTFL. This is a rare type of lymphoma with a better prognosis, and the clinical treatments are significantly different from those for common FL (25, 26). Therefore, attention should be given to clinical information to improve the accuracy of diagnosis.
Haematolymphoid neoplasms have a wide variety and complex classification. With the rapid development of new technologies such as FCM, cytogenetics, and molecular pathology, the understanding of haematolymphoid neoplasms has been further deepened. Despite the application of innovative technologies, diagnosis in hematopathology is sometimes difficult. Meanwhile, due to variations in knowledge, training background and practical experience, pathologists’ subjective judgments can lead to different conclusions from the same specimen.
HGBL was proposed as an independent subtype in the 2017 revised WHO classification, including 1. HGBL with MYC, BCL2 and/or BCL6 rearrangements, i.e., double/triple-hit lymphoma; 2. HGBL-NOS. In this study, 54 cases were corrected to HGBL, and most of the original diagnoses were DLBCL or BL. One more thing is that some pathologists can confuse the definition of HGBL with just simply highly aggressive lymphoma. This suggests that pathologists should strictly grasp the diagnostic criteria of HGBL, including the morphology and immunophenotype; furthermore, FISH is necessary to detect MYC, BCL2 and BCL6 rearrangements. For patients who were diagnosed with DLBCL or BL, MYC, BCL2, and BCL6 rearrangements should be detected by FISH to exclude double/triple-hit lymphoma. What is noteworthy is that MYC rearrangement is found not only in BL and HGBL with MYC and BCL2 and/or BCL6 rearrangements but also in DLBCL, FL, PBL, B-LBL/ALL, and even in myeloid tumors (27, 28).
FL is a common subtype of B-cell lymphoma, which can be divided into low-grade FL (grades 1 and 2) and high-grade FL (grades 3A and 3B) according to the WHO classification. The pathological grading of FL should be accurate, since low-grade and some grade 3A FL patients have indolent clinical features and can be followed up or treated locally, while grade 3B FL patients should be treated according to the treatment strategy of DLBCL (29). Pathologists should be strict and proficient in the diagnosis and grading criteria of FL and accurately identify central cells (small, with little cytoplasm and irregularly angulated or cleaved nuclei) and centroblasts (large in size, with round or oval nuclei and 1 to 3 peripheral nucleoli), especially for cases of morphological variation. Misdiagnosis between low-grade and high-grade tumors may result in excessive or delayed treatment. Moreover, Ki-67 cannot be used as a grading factor for FL, but low-grade FL with high proliferative activity (Ki-67 ≥ 30%) is often considered to be clinically aggressive, with a risk of transformation to high-grade FL (30).
In most cases, distinguishing CHL from NHL seems to be relatively easy, but the diagnostic boundary can sometimes be difficult to discern. In this study, the misdiagnoses of CHL included benign granulomatous hyperplasia, thymoma, DLBCL, and T-NHL. Some subtypes of PTCLs, especially those with a follicular helper T-cell (TFH) phenotype, can share similar morphology and immunophenotype, including a complex inflammatory background of cell proliferation and Hodgkin/Reed-Sternberg (HRS) cells that present features of CHL phenotypes such as CD30 positivity, PAX5 weak positivity, CD45 negativity, and EBER positivity, leading to a misdiagnosis between them (31–34). However, the properties of proliferated T cells are completely different: T cells in AITL or PTCL with the TFH phenotype are malignant, while T cells in CHL are reactive. TCR rearrangement is useful for the differential diagnosis. In view of completely different treatments and prognoses, the diagnosis should be made with as much caution as possible. It should be noted that HRS cells are also observed in small B-cell lymphomas such as SLL/CLL, FL, and MCL (35). In the differential diagnosis of CHL and DLBCL, in addition to morphological differences, IHC of PAX5, CD20, CD45 (LCA), BOB1, and OCT2 can be helpful.
Castleman disease (CD) (Figure 6) should be distinguished from other reactive lymphadenectasis since a few could develop into tumors (36, 37). The clinical and pathological manifestations of CD are so heterogeneous that they are easily confused with other diseases that can cause lymphadenopathy, such as infectious diseases, autoimmune diseases, and tumors. In some difficult cases, the pathologist can communicate with clinicians and give a descriptive diagnosis that is acceptable to both parties and does not mislead clinical treatment.
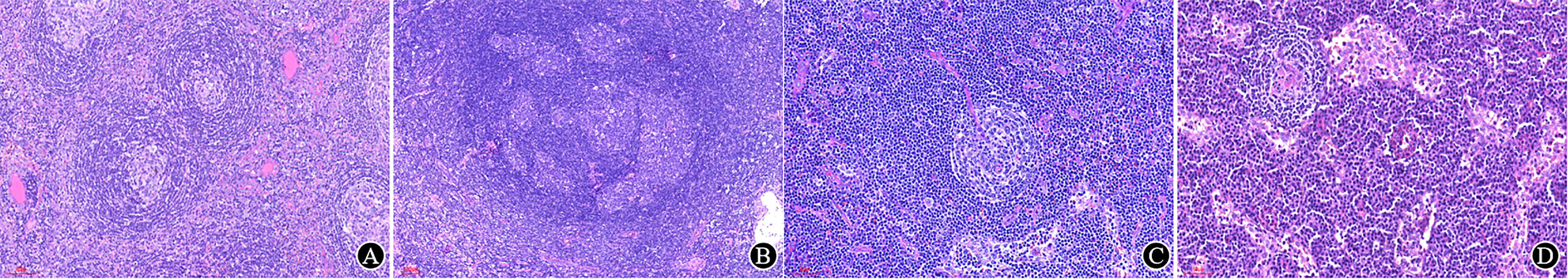
Figure 6 Typical Castleman disease. (A) Follicles with atrophic germinal centers and expanded mantle zones, forming a concentric annular structure, with an “onion-skin” look (×150); (B) Multiple germinal centers within a single mantle area (×65); (C) A remarkable hyalinized vessel grows into the germinal centers, forming a “lollipop” appearance (×200); (D) Sheets of plasma cells are seen in the interfollicular region in PC-type CD (×200).
Non-haematolymphoid neoplasms are also one of the common differential diagnoses for haematolymphoid neoplasms (30 cases in this group, accounting for 3.3% of misdiagnosed cases, 1.31% of all cases). Some poorly differentiated carcinomas, sarcoma and neuroendocrine tumors can manifest as small round cells morphologically resembling lymphoma. In this study, one lymph node biopsy demonstrated only PAX5, which was weakly positive in the T/B lineage markers, but showed CKpan, Syn and CD56 positivity in subsequent immunostains; a neuroendocrine carcinoma was subsequently diagnosed. What should be considered is that PAX5 can be expressed not only in B lymphocytes but also in some neuroendocrine carcinomas and rhabdomyosarcomas (38). It was reported that CD138, MUM-1 and CD56 can also be expressed in malignant melanoma (8), which can lead to a misdiagnosis of plasmacytoma; moreover, CD99 is expressed not only in Ewing’s sarcoma but also in precursor lymphocytes (39). There was another case of such misdiagnosis in our study. The patient attended a clinical consultation because of a mass on the upper arm, and tissue biopsy showed significant histiocytic proliferation. A histiocytic sarcoma or epithelioid sarcoma was suspected in several Class A tertiary hospitals. However, there were scattered atypical cells with CD163 and CD68 negativity against the background of a large number of proliferated histiocytes. Then, the markers CKpan, CK7 and TTF1 were stained, and a metastatic carcinoma was confirmed (Figure 7). The patient subsequently underwent a pulmonary lobectomy, and a poorly differentiated adenocarcinoma of the lung with partial large cells was indicated in the postoperative pathology. This patient was first admitted for metastatic carcinoma of the upper arm, and the biopsy showed a large number of proliferated histiocytes, which made it difficult to distinguish the mass from histiocytic sarcoma or other epithelioid sarcomas. Histiocytic sarcoma is rare. Pathologists should be cautious, should exclude the possibility of common tumors first and then should consider the rare types.
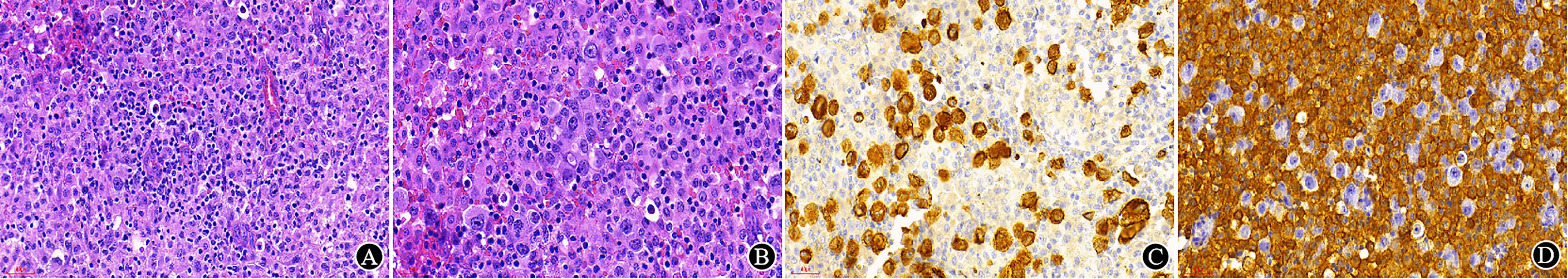
Figure 7 Misdiagnosed metastatic carcinoma. (A, B) In HE sections, there was significant histiocytic proliferation, atypical cells with large nuclei, and unremarkable epithelioid structure (A ×200, B ×300); (C) CKpan staining was positive in tumor cells (×300); (D) CD163 staining was positive in histiocytes (×300).
Finally, pathologists should try their best to give a clear diagnosis and avoid too many tendentious diagnoses.
This study showed that the misdiagnosis rate of haematolymphoid neoplasms is still high in China and involves various types of misdiagnosis and complicated causes. The hematopathology is more challenging than that in other subspecialties of pathology. Therefore, pathologists should not only be proficient in morphology and immunohistochemistry but must also remain updated regarding advancements in technology; this, integrated the clinical information and ancillary data formulating a diagnosis as accurate as possible based on the latest WHO classification to ensure timely, standardized, precise and individualized treatment for patients. Meanwhile, the systematic specialized training of pathologists in hematology is recommended to enhance their cognition in this specialty and improve the diagnosis level of hematopathology in China.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by ethical committee of Beijing GoBroad Boren Hospital. The patients/participants provided their written informed consent to participate in this study.
JD wrote the article, collected the materials. XZ performed the statistical analysis. LY performed the experiments. ZG and CZ reviewed the cases. LG designed the paper and revised the paper. All authors contributed to the article and approved the submitted version.
We thank the two expert hematopathologists, Professor Zifen Gao and Chunju Zhou, for their contributions to this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haemtopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC Press (2017).
2. Lester JF, Dojcinov SD, Attanoos RL, O'Brien CJ, Maughan TS, Toy ET, et al. The clinical impact of expert pathological review on lymphoma management: a regional experience. Br J Haematol (2003) 123(3):463–8. doi: 10.1046/j.1365-2141.2003.04629.x
3. Proctor IE, McNamara C, Rodriguez-Justo M, Isaacson PG, Ramsay A. Importance of expert central review in the diagnosis of lymphoid malignancies in a regional cancer network. J Clin Oncol (2011) 29(11):1431–5. doi: 10.1200/JCO.2010.31.2223
4. Matasar MJ, Shi W, Silberstien J, Lin O, Busam KJ, Teruya- Feldstein J, et al. Expert second-opinion pathology review of lymphoma in the era of the world health organization classification. Ann Oncol (2012) 23(1):159–66. doi: 10.1093/annonc/mdr029
5. Bowen JM, Perry AM, Laurini JA, Smith LM, Klinetobe K, Bast M, et al. Lymphoma diagnosis at an academic centre: Rate of revision and impact on patient care. Br J Haematol (2014) 166(2):202–8. doi: 10.1111/bjh.12880
6. Laurent C, Baron M, Amara N, Haioun C, Dandoit M, Maynadié M , et al. Impact of expert pathologic review of lymphoma diagnosis: Study of patients from the French lymphopath network. J Clin Oncol (2017) 35(18):2008–17. doi: 10.1200/JCO.2016.71.2083
7. Jaffe ES, Arber DA, Campo E, Harris NL, Quintanilla-Martinez L. Hematopathology. 2nd ed. Philadelphia, PA: Elsevier (2017).
8. Li X. Pitfalls in the pathological diagnosis of lymphoma. Chin Clin Oncol (2015) 4(1):3. doi: 10.3978/j.issn.2304-3865.2014.11.04
9. Wilkins BS. Pitfalls in lymphoma pathology: Avoiding errors in diagnosis of lymphoid tissues. J Clin Pathol (2011) 64(6):466–76. doi: 10.1136/jcp.2010.080846
10. Niitsu N, Okamoto M, Tamaru JI, Yoshino T, Nakamura N, Nakamura S, et al. Clinicopathologic characteristics and treatment outcome of the addition of rituximab to chemotherapy for CD5-positive in comparison with CD5-negative diffuse large b-cell lymphoma. Ann Oncol (2010) 21(10):2069–74. doi: 10.1093/annonc/mdq057
11. Bene MC, Castoldi G, Knapp W, Ludwig WD, Matutes E, Orfao A, et al. Proposals for the immunological classification of acute leukemias. European group for the immunological characterization of leukemias (EGIL). Leukemia (1995) 9(10):1783–6.
12. Chen YP, Jones D, Chen TY, Chang KC. Epstein-Barr Virus present in T cells or b cells shows differential effects on hemophagocytic symptoms associated with outcome in T-cell lymphomas. Leuk Lymphoma (2014) 55(9):2038–47. doi: 10.3109/10428194.2013.861068
13. Charles NJ, Boyer DF. Mixed-phenotype acute leukemia: Diagnostic criteria and pitfalls. Arch Pathol Lab Med (2017) 141(11):1462–8. doi: 10.5858/arpa.2017-0218-RA
14. Porwit A, Béné MC. Multiparameter flow cytometry applications in the diagnosis of mixed phenotype acute leukemia. Cytometry B Clin Cytom (2019) 96(3):183–94. doi: 10.1002/cyto.b.21783
15. Alexander TB, Orgel E. Mixed phenotype acute leukemia: Current approaches to diagnosis and treatment. Curr Oncol Rep (2021) 23(2):22. doi: 10.1007/s11912-020-01010-w
16. Khan M, Siddiqi R, Naqvi K. An update on classification, genetics, and clinical approach to mixed phenotype acute leukemia (MPAL). Ann Hematol (2018) 97(6):945–53. doi: 10.1007/s00277-018-3297-6
17. Meng X, Min Q, Wang JY. B cell lymphoma. Adv Exp Med Biol (2020) 1254:161–81. doi: 10.1007/978-981-15-3532-1_12
18. Debliquis A, Voirin J, Harzallah I, Maurer M, Lerintiu F, Drénou B, et al. Cytomorphology and flow cytometry of brain biopsy rinse fluid enables faster and multidisciplinary diagnosis of large b-cell lymphoma of the central nervous system. Cytometry B Clin Cytom (2018) 94(1):182–8. doi: 10.1002/cyto.b.21403
19. van der Meulen M, Bromberg JEC, Lam KH, Dammers R, Langerak AW, Doorduijn JK, et al. Flow cytometry shows added value in diagnosing lymphoma in brain biopsies. Cytometry B Clin Cytom (2018) 94(6):928–34. doi: 10.1002/cyto.b.21641
20. Langerak AW, Groenen PJ, Bruggemann M, Beldjord K, Bellan C, Bonello L, et al. EuroClonality/BIOMED-2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations. Leukemia (2012) 26(10):2159–71. doi: 10.1038/leu.2012.246
21. Heimann P, Dewispelaere L. Indications of next-generation sequencing in non-hodgkin's lymphoma. Curr Opin Oncol (2020) 32(5):391–7. doi: 10.1097/CCO.0000000000000666
22. Nakamura S. Helicobacter pylori and gastric mucosa-associated lymphoid tissue lymphoma: Recent progress in pathogenesis and management. World J Gastroenterol (2013) 19(45):8181. doi: 10.3748/wjg.v19.i45.8181
23. Zhu W, He QY, Lu C, Fu CY, Zhou JH, Liu S, et al. Detection of immunoglobulin and T-cell receptor gene rearrangements in angioimmunoblastic T-cell lymphoma. Int J Clin Exp Pathol (2018) 11(5):2642–53.
24. Zhou XG, Xie JL, Jin Y, Zheng YY. Clinical manifestations of lymphoma and its significance in pathological diagnosis. Chin J Pathol (2012) 41(01):57–8. doi: 10.3760/cma.j.issn.0529-5807.2012.01.017
25. Alnughmush A, Fakih RE, Mohammed S, Aljurf M. Pediatric-type follicular lymphoma: A short review. Int J Hematol Oncol (2022) 11(4):IJH41. doi: 10.2217/ijh-2022-0003
26. Attarbaschi A, Abla O, Arias Padilla L, Beishuizen A, Burke GAA, Brugières L, et al. Rare non-Hodgkin lymphoma of childhood and adolescence: A consensus diagnostic and therapeutic approach to pediatric-type follicular lymphoma, marginal zone lymphoma, and nonanaplastic peripheral T-cell lymphoma. Pediatr Blood Cancer (2020) 67(8):e28416. doi: 10.1002/pbc.28416
27. Slack GW, Gascoyne RD. MYC and aggressive b-cell lymphomas. Adv Anat Pathol (2011) 18(3):219–28. doi: 10.1097/PAP.0b013e3182169948
28. Ahmadi SE, Rahimi S, Zarandi B, Chegeni R, Safa M. MYC: A multipurpose oncogene with prognostic and therapeutic implications in blood malignancies. J Hematol Oncol (2021) 14(1):121. doi: 10.1186/s13045-021-01111-4
29. Freedman A, Jacobsen E. Follicular lymphoma: 2020 update on diagnosis and management. Am J Hematol (2020) 95(3):316–27. doi: 10.1002/ajh.25696
30. Wang SA, Wang L, Hochberg EP, Muzikansky A, Harris NL, Hasserjian RP, et al. Low histologic grade follicular lymphoma with high proliferation index: Morphologic and clinical features. Am J Surg Pathol (2005) 29(11):1490–6. doi: 10.1097/01.pas.0000172191.87176.3b
31. Laforga JB, Gasent JM, Vaquero M. Potential misdiagnosis of angioimmunoblastic T-cell lymphoma with hodgkin's lymphoma: A case report. Acta Cytol (2010) 54(5 Suppl):840–4.
32. Szablewski V, Dereure O, Rene C, Tempier A, Durand L, Alame M, et al. Cutaneous localization of angioimmunoblastic T-cell lymphoma may masquerade as b-cell lymphoma or classical Hodgkin lymphoma: A histologic diagnostic pitfall. J Cutan Pathol (2019) 46(2):102–10. doi: 10.1111/cup.13382
33. Abukhiran I, Syrbu SI, Holman CJ. Markers of follicular helper T cells are occasionally expressed in T-cell or histiocyte-rich Large b-cell lymphoma, classic Hodgkin lymphoma, and atypical paracortical hyperplasia a diagnostic pitfall for T-cell lymphomas of T follicular helper origin. Am J Clin Pathol (2021) 156(3):409–26. doi: 10.1093/ajcp/aqaa249
34. Wang HW, Balakrishna JP, Pittaluga S, Jaffe ES. Diagnosis of Hodgkin lymphoma in the modern era. Br J Haematol (2019) 184(1):45–59. doi: 10.1111/bjh.15614
35. Parente P, Zanelli M, Sanguedolce F, Mastracci L, Graziano P. Hodgkin Reed-Sternberg-Like cells in non-Hodgkin lymphoma. Diagn (Basel) (2020) 10(12:1019. doi: 10.3390/diagnostics10121019
36. Dispenzieri A, Fajgenbaum DC. Overview of castleman disease. Blood (2020) 135(16):1353–64. doi: 10.1182/blood.2019000931
37. Larroche C, Cacoub P, Soulier J, Oksenhendler E, Clauvel JP, Piette JC, et al. Castleman's disease and lymphoma: Report of eight cases in HIV-negative patients and literature review. Am J Hematol (2002) 69(2):119–26. doi: 10.1002/ajh.10022
38. Morgenstern DA, Gibson S, Sebire NJ, Anderson J. PAX5 expression in rhabdomyosarcoma. Am J Surg Pathol (2009) 33(10):1575–7. doi: 10.1097/PAS.0b013e3181abe137
Keywords: Haematolymphoid, lymphoma, pathology, misdiagnosis, diagnostic pitfalls, expert review, accurate diagnosis
Citation: Deng J, Zuo X, Yang L, Gao Z, Zhou C and Guo L (2023) Misdiagnosis analysis of 2291 cases of haematolymphoid neoplasms. Front. Oncol. 13:1128636. doi: 10.3389/fonc.2023.1128636
Received: 21 December 2022; Accepted: 15 March 2023;
Published: 26 April 2023.
Edited by:
Onder Alpdogan, Thomas Jefferson University, United StatesReviewed by:
Gerhard C. Hildebrandt, University of Missouri, United StatesCopyright © 2023 Deng, Zuo, Yang, Gao, Zhou and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ligai Guo, cGF5eWJsa0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.