
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 14 February 2023
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1128569
This article is part of the Research Topic Predictive Biomarkers of Immune-checkpoint Inhibitors Immunotherapies in Hepatocellular Carcinomas and Gastric Cancers View all 12 articles
 Y. Linda Wu1
Y. Linda Wu1 Grace van Hyfte2
Grace van Hyfte2 Umut Özbek2
Umut Özbek2 Marlene Reincke3
Marlene Reincke3 Anuhya Gampa4
Anuhya Gampa4 Yehia I. Mohamed5
Yehia I. Mohamed5 Naoshi Nishida6
Naoshi Nishida6 Brooke Wietharn7
Brooke Wietharn7 Suneetha Amara8
Suneetha Amara8 Pei-Chang Lee9
Pei-Chang Lee9 Bernhard Scheiner10
Bernhard Scheiner10 Lorenz Balcar10
Lorenz Balcar10 Matthias Pinter10
Matthias Pinter10 Arndt Vogel11
Arndt Vogel11 Arndt Weinmann12
Arndt Weinmann12 Anwaar Saeed7
Anwaar Saeed7 Anjana Pillai4
Anjana Pillai4 Lorenza Rimassa13,14
Lorenza Rimassa13,14 Abdul Rafeh Naqash15
Abdul Rafeh Naqash15 Mahvish Muzaffar8
Mahvish Muzaffar8 Yi-Hsiang Huang9
Yi-Hsiang Huang9 Ahmed O. Kaseb5
Ahmed O. Kaseb5 Masatoshi Kudo6
Masatoshi Kudo6 David J. Pinato16
David J. Pinato16 Celina Ang1*
Celina Ang1*Background: In patients with cirrhosis, portal hypertension increases intestinal permeability, dysbiosis, and bacterial translocation, promoting an inflammatory state that can lead to the progression of liver disease and development of hepatocellular carcinoma (HCC). We aimed to investigate whether beta blockers (BBs), which can mediate portal hypertension, conferred survival benefits in patients treated with immune checkpoint inhibitors (ICIs).
Methods: We conducted a retrospective, observational study of 578 patients with unresectable HCC treated with ICI from 2017 to 2019 at 13 institutions across three continents. BB use was defined as exposure to BBs at any time during ICI therapy. The primary objective was to assess the association of BB exposure with overall survival (OS). Secondary objectives were to evaluate the association of BB use with progression-free survival (PFS) and objective response rate (ORR) according to RECIST 1.1 criteria.
Results: In our study cohort, 203 (35%) patients used BBs at any point during ICI therapy. Of these, 51% were taking a nonselective BB. BB use was not significantly correlated with OS (hazard ratio [HR] 1.12, 95% CI 0.9-1.39, P = 0.298), PFS (HR 1.02, 95% CI 0.83-1.26, P = 0.844) or ORR (odds ratio [OR] 0.84, 95% CI 0.54-1.31, P = 0.451) in univariate or multivariate analyses. BB use was also not associated with incidence of adverse events (OR 1.38, 95% CI 0.96-1.97, P = 0.079). Specifically, nonselective BB use was not correlated with OS (HR 0.94, 95% CI 0.66-1.33, P = 0.721), PFS (HR 0.92, 0.66-1.29, P = 0.629), ORR (OR 1.20, 95% CI 0.58-2.49, P = 0.623), or rate of adverse events (OR 0.82, 95% CI 0.46-1.47, P = 0.510).
Conclusion: In this real-world population of patients with unresectable HCC treated with immunotherapy, BB use was not associated with OS, PFS or ORR.
Hepatocellular carcinoma (HCC) is a leading cause of cancer death worldwide and often diagnosed in advanced stages when cure is no longer feasible (1). For patients with advanced HCC, multikinase inhibitors such as sorafenib and lenvatinib had long been the first-line systemic therapy but offered poor outcomes and high toxicity (2). Recently, the combination of atezolizumab, a programmed death-ligand 1 (PD-L1) inhibitor, and bevacizumab was shown to improve overall survival (OS) compared to sorafenib in patients with unresectable HCC in the IMbrave150 trial (3, 4). In addition, the phase III HIMALAYA trial recently showed that the combination of durvalumab and tremelimumab had superior efficacy to sorafenib in the first-line treatment of unresectable HCC (5). Even in patients who had received multikinase inhibitors in the front line, treatment with immunotherapy on progression of disease may induce a response (6). As a result, immune checkpoint inhibitors (ICIs) have now supplanted multikinase inhibitors as standard of care front line therapy for advanced HCC. However, advanced HCC still carries a poor prognosis, and response to ICIs is limited, underscoring the need to identify markers of ICI response.
Increasingly, there is interest in understanding drug-drug interactions in the context of cancer immunotherapy. In particular, common concomitant medications such as antibiotics, steroids, antacids, metformin, and opioids that may have immunomodulatory effects have been investigated in order to examine their potential role in either enhancing ICI efficacy or contributing to toxicity (7). The disruption of the gut microbiome, through antibiotic use, for example, has been associated with decreased ICI efficacy and impaired T cell antitumor response (8, 9). In HCC, a recent study found that patients who responded to ICI had greater gut microbial diversity than non-responders, providing further evidence that the gut microbiome may impact response to ICI (10).
The interaction of the gut microbiome and ICI therapy has important implications for patients with HCC. Liver cirrhosis is well-known to underlie HCC carcinogenesis, and portal hypertension (pHTN) promotes progression of liver disease through immune activation: pHTN causes splanchnic vasodilation and pathological angiogenesis, increasing intestinal permeability and dysbiosis, which leads to bacterial translocation and induces a pro-inflammatory state (11). Both pHTN and chronic inflammation are risk factors for the development of HCC and tumor progression (12). Therefore, it is possible that the attenuation of pHTN may decrease aberrant neoangiogenesis and bacterial translocation-mediated inflammation driving HCC tumorigenesis and progression.
Beta blockers (BBs), particularly non-selective BBs, are standard prophylaxis for patients with cirrhosis and pHTN-induced varices (13). They have been shown to modulate pHTN-associated dysbiosis through a reduction in intestinal bacterial overgrowth, intestinal permeability, and bacterial translocation (14, 15). Additionally, in preclinical studies, BBs decrease tumor cell proliferation, proinflammatory cytokine load, and catecholamine-driven angiogenesis (16–18). Some clinical studies suggest that BB use is associated with lower incidence of HCC in patients with cirrhosis (19–21). One nationwide population-based study in Taiwan found that propranolol use improved OS in patients with unresectable or metastatic HCC who were treated with sorafenib, locoregional therapy, or radiotherapy (22). However, there is a paucity of data addressing the effect of beta blockade on outcomes of patients with advanced HCC in the era of immunotherapy. We aimed to evaluate whether BB use conferred survival benefits in patients treated with ICIs using real-world data.
The study population consisted of 578 patients with unresectable HCC treated with ICI from 2017 to 2019 at 13 institutions across North America (N = 247), Europe (N = 240), and Asia (N = 91). Patients included in this study had a diagnosis of HCC in accordance with American Association for the Study of Liver Disease (23) and European Association for the Study of the Liver (24) guidelines, received systemic ICI therapy (either monotherapy or in combination), and had measurable disease according to RECIST 1.1 criteria at the start of ICI. All patients were treated according to routine clinical practice, including prescriptions for BB. The decision to start ICI therapy was made at the discretion of the treating physician based on current evidence-based practice guidelines, institutional standards, and often after multidisciplinary tumor board discussions.
Patient demographics and clinical data, including Barcelona Clinic Liver Cancer (BCLC) stage, Child-Pugh (CP) class, Eastern Cooperative Oncology Group (ECOG) performance status, alpha fetoprotein (AFP) level, presence of cirrhosis (clinically or radiologically diagnosed), etiology of liver disease, type and duration of ICI therapy, type and indication of BB use, duration of BB use, follow-up and vital status, were collected retrospectively. Baseline data were defined at the time of ICI initiation, and treatment response was evaluated through radiologic staging of the disease using computerized tomography and/or magnetic resonance imaging approximately every 9 weeks during treatment. BB use was defined as exposure at any time during ICI therapy. BBs were classified as nonselective (propranolol, nadolol, carvedilol, labetalol) and cardio-selective (metoprolol, atenolol, bisoprolol, nebivolol), and standard doses were used. Indications for BB use were evaluated and included variceal prophylaxis, cardiovascular disease, and other indications.
The primary outcome was to evaluate the association between BB use and OS, measured from the time of ICI initiation until date of death from any cause or date of last follow-up. Secondary outcomes included assessing the effect of BB use on objective response rate (ORR), defined as the proportion of patients with either radiographic complete response (CR) or partial response (PR), duration of response (DOR), defined as best response of CR, PR, or stable disease (SD), progression-free survival (PFS), measured from the time of ICI initiation until radiographic progression, and development of treatment-related adverse events (AEs) of any grade. All responses were evaluated according to RECIST 1.1 criteria. AEs were defined based on the Common Terminology Criteria for Adverse Events (CTCAE) classification, version 5.0, and identified based on investigator review of clinical notes, radiographic, and laboratory data. Evaluation of BB exposure was based on the presence of an active prescription in the medical record per clinical notes or medication records. Baseline BB use was defined as exposure within 30 days prior to ICI initiation, and concurrent BB use was defined as exposure between the dates of ICI initiation and cessation.
Patient characteristics were summarized descriptively with medians and interquartile ranges for continuous variables and frequencies and proportions for categorical variables. Categorical variables were examined across BB exposure levels utilizing either chi-square tests or Fisher’s exact tests, where appropriate, while the nonparametric Mann-Whitney U test was used for continuous variables. Univariable and multivariable Cox proportional hazard models were fitted for OS and PFS. Univariable and multivariable logistic regression models were generated to evaluate the association of the aforementioned variables with ORR, the presence of any AE, and for AEs graded 2 or higher. Covariates were selected for the multivariable models if they were found to be significant in univariable analysis. Each model is summarized with hazard ratios (HR) or odds ratios (OR) and their coinciding 95% confidence intervals (CIs). Additional subgroup analyses were performed in order to examine the interaction between each covariate and BB exposure. A forest plot was generated which summarizes the subgroup analyses with interaction term and associated p-value. For all survival analyses the proportional hazards assumption was tested and found to be satisfied. Variance inflation factors in multivariable models were below 5 to indicate an absence of multicollinearity. The level of significance was maintained at 0.05. All analyses were carried out in R version 4.1.2 (Vienna, Austria).
Baseline clinical characteristics are reported in Table 1. The majority of the cohort were male (N = 464, 80%), with a median age of 65 years (IQR: 58-70 years). Most patients (N = 406, 70%) had radiologic or pathologic evidence of cirrhosis at baseline. The causes of underlying liver disease were hepatitis C virus (N = 209, 36%), hepatitis B virus (N = 125, 22%), alcohol-related (N = 120, 21%), and nonalcoholic steatohepatitis (NASH)-associated (N = 75, 13%). Most patients had preserved liver function with CP class A disease (N = 413, 74%) and good performance status with ECOG score either 0 (N = 300, 52%) or 1 (N = 259, 45%).
At the time of initiation of ICI, 482 patients (83%) had BCLC stage C disease. The majority of patients (N = 435, 75%) treated with ICIs received a PD-1 inhibitor alone. ICI was given in the first-line in 46% of patients (N = 264), and 54% of patients (N = 312) received at least one prior systemic therapy. Many patients (N = 380, 66%) received prior locoregional therapy, with transarterial chemoembolization being the most common (N = 258, 45%). In addition, 186 patients (32%) had undergone prior surgical resection.
During a median follow-up of 30.8 months (IQR: 17.2-40.3 months), there were 360 deaths (62%) noted. A total of 541 patients could be evaluated for best radiographic response to ICI therapy per RECIST 1.1 criteria. There were 36 patients with CR (6.7%), 78 with PR (14.4%), and 216 with SD (39.9%), which correspond with an ORR of 21.1% and disease control rate (DCR) of 61.0%. At the time of analysis, the median duration of ICI therapy was 4.1 months (IQR: 1.9-9.3 months). Progression of disease was the most common cause of ICI discontinuation (N = 303, 52%).
Treatment-related AEs developed in 336 patients (58%), but only 97 patients (17%) developed grade 3 or higher events. The most common AEs were fatigue (N = 127, 22%), skin toxicity (N = 100, 17%), and liver toxicity (N = 96, 17%), with 142 (25%) experiencing other AEs, such as cytopenias, nausea, fever or infections, neuropathy, and electrolyte imbalances (Table 2). The most common grade 3 or higher AE was hepatotoxicity (N = 33, 6%), followed by fatigue (N = 13, 2.2%) and colitis (N = 13, 2.2%), with 37 patients (6%) experiencing other grade 3 or higher AEs (Table 2). A total of 37 patients (6.4%) also experienced bleeding events, 16 (43%) of which were gastrointestinal or variceal bleeding.
Two hundred and three (35%) patients had BB use at any point during ICI therapy, of which only 4 (2%) patients had used BB up to the time of ICI initiation but not concurrently with ICI. Conversely, 22 (11%) patients started BB after ICI was initiated. However, most patients (N = 177, 87%) had been on BB before the start of ICI and continued on immunotherapy. Furthermore, BBs were long-term medications for patients who had been on BBs prior to ICI, with 96% (N = 173) being prescribed for more than 4 weeks.
The types of BBs used were evenly divided: 51% (N = 103) of patients were on a nonselective BB and 49% (N = 100) were taking a cardio-selective BB. Of those taking a nonselective BB, the indication was predominantly for variceal prophylaxis (N = 69, 67%), followed by cardiovascular indications (N = 32, 31%), with 2 unclear indications. As expected, cardio-selective BBs were prescribed for cardiovascular indications (N = 96, 96%), except for 2 patients who had a cardio-selective BB for variceal prophylaxis and 2 more for other indications.
Overall, the baseline characteristics between patients with or without BB exposure were comparable, but there were some exceptions (Table 1). Patients who had BB exposure were more often from the United States or Europe, had a history of cirrhosis, neoplastic portal vein thrombosis (PVT), and antibiotic exposure. Patients exposed to BBs and antibiotics were most commonly treated with beta-lactams, quinolones, or cephalosporins, typically for a single week-long course for fever of unknown origin and early in the course of ICI therapy (within 30 days). The effect of antibiotic therapy on ICI outcomes in this cohort of HCC patients was previously evaluated (25). Patients without BB exposure tended to have HBV as an etiology of liver disease, CP class A disease, and a history of prior resection to treat HCC.
In univariable analysis, BB use was not significantly correlated with OS (HR 1.12, 95% CI 0.9-1.39) (Table 3). Nonselective BB type was also not associated with OS (HR 0.94, 95% CI 0.66-1.33), and these results are illustrated in Figure 1. Variables that were associated with improved OS included CP class A (HR 0.51, 95% CI 0.41-0.64) and performance status ECOG 0 (HR 0.69, 95% CI 0.56-0.84). Factors contributing to worsened OS included presence of neoplastic PVT (HR 1.93, 95% CI 1.55-2.41) and AFP > 400 (HR 1.59, 95% CI 1.29-1.96). Other baseline characteristics tested that did not have associations with OS included age, sex, cirrhosis, viral etiology of liver disease, and presence of extrahepatic metastases. Multivariable analyses identified the same independent predictors of OS, including CP class A disease (HR 0.55, 95% CI 0.44-0.70), presence of neoplastic PVT (HR 1.60, 95% CI 1.27-2.02), and AFP > 400 (HR 1.39, 95% CI 1.11-1.74), but not ECOG 0 (HR 0.81, 95% CI 0.65-1). The effect of BB exposure on OS was not affected by multiple variables evaluated in subgroup analyses (Figure 2), including age, sex, ICI monotherapy vs. combination therapy, line of therapy, cirrhosis, performance status, stage, liver function, presence of neoplastic PVT, extrahepatic metastasis, or AFP level. In particular, given the possible immunomodulatory effects of BBs, concomitant exposure to antibiotics and antacids was examined but not found to influence the effect of BB use on OS.
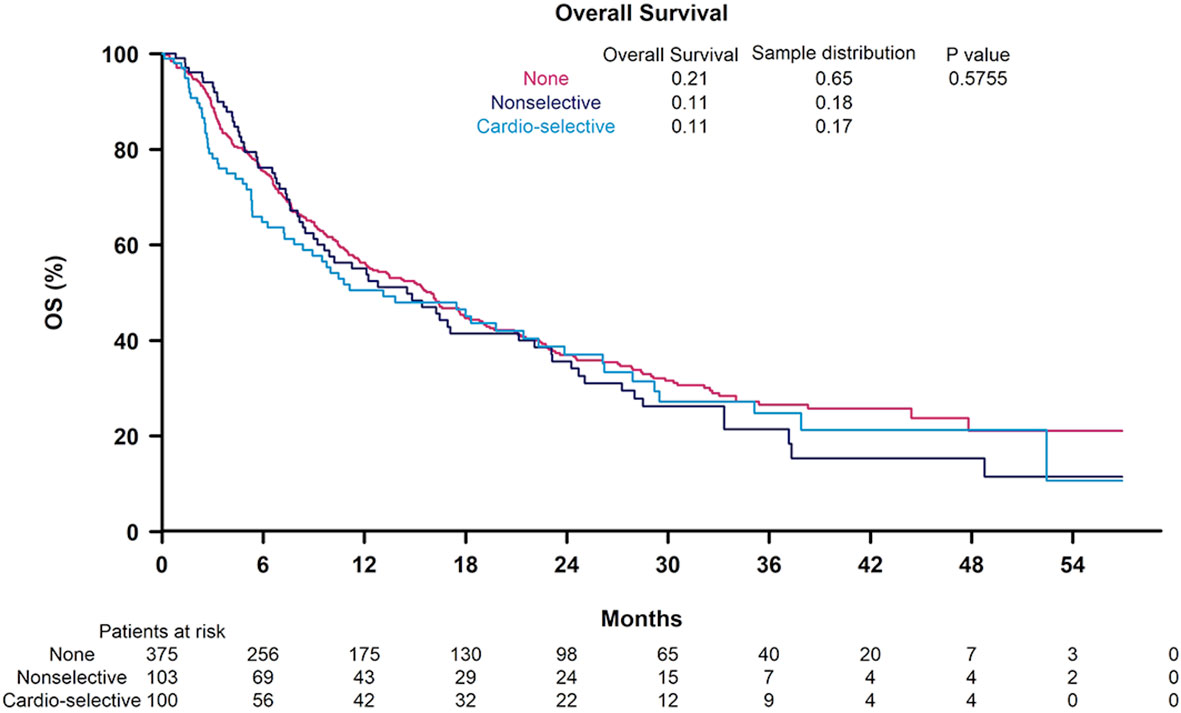
Figure 1 Kaplan-Meier curves for overall survival according to non-selective beta blocker (BB) use, cardioselective BB use, and no BB use.
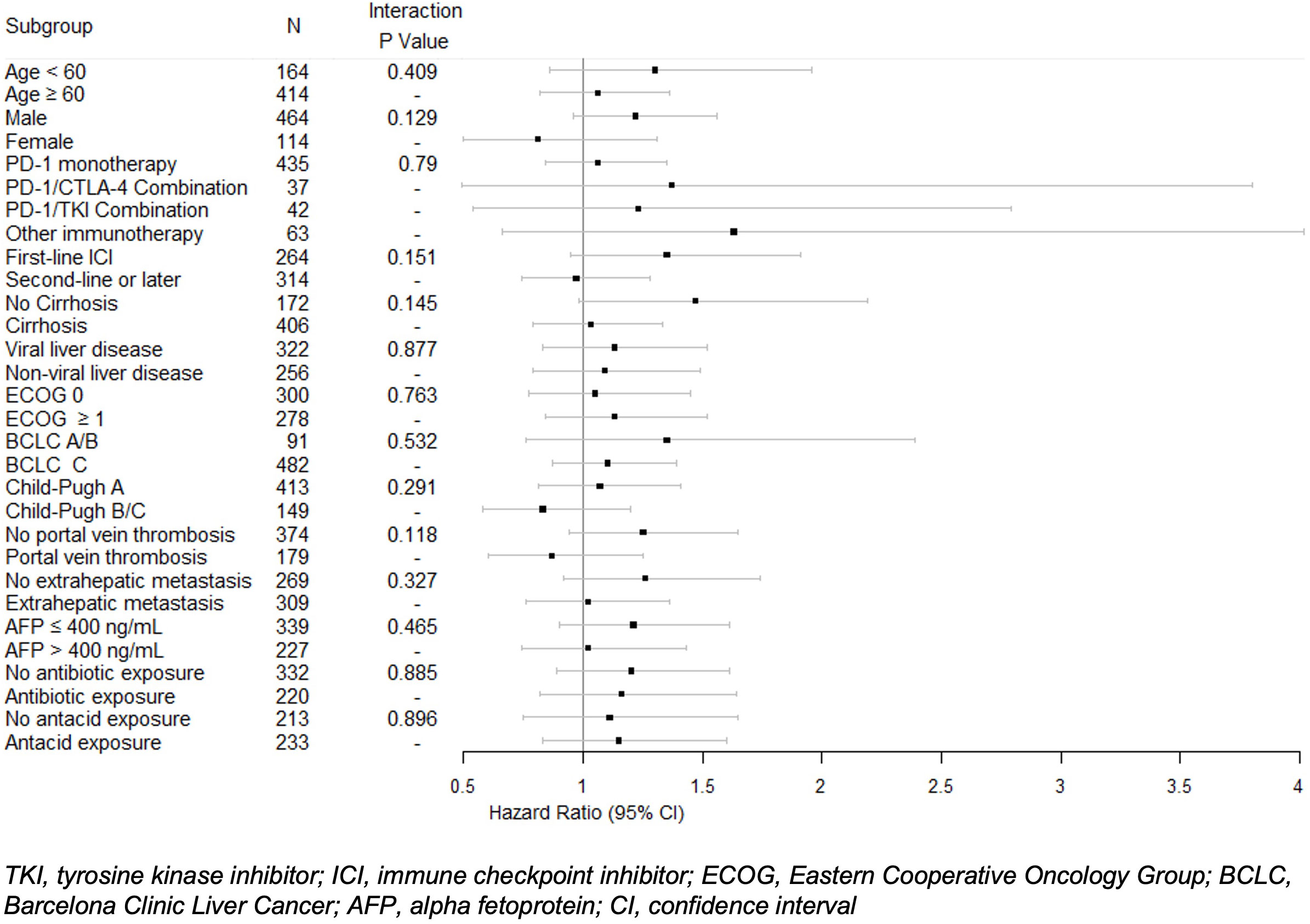
Figure 2 Univariable Cox proportional hazard model of overall survival with interactions between beta blocker exposure and subgroups. Hazard ratios and 95% confidence intervals for beta blocker exposure are shown with P values for each subgroup.
Next, the PFS was evaluated and results are tabulated in Table 4. Univariable analysis did not find a significant correlation between BB exposure and PFS (HR 1.02, 95% CI 0.83-1.26). Again, nonselective BB use was not determined to be associated with PFS (HR 0.92, 95% CI 0.66-1.29). Variables associated with PFS included CP class A disease (HR 0.71, 95% CI 0.57-0.89), presence of neoplastic PVT (HR 1.41, 95% CI 1.14-1.76), and performance status ECOG 0 (HR 0.77, 95% CI 0.63-0.94). On multivariable analyses, independent predictors of PFS were CP class A disease (HR 0.74, 95% CI 0.58-0.94) and presence of neoplastic PVT (HR 1.37, 95% CI 1.09-1.72). The cirrhosis status and CP class did not significantly affect the PFS of patients with BB exposure in subgroup analyses.
We then evaluated whether BB exposure was associated with ICI response (Table 5). Univariable analyses showed that BB use was also not significantly correlated with ORR (OR 0.84, 95% CI 0.54-1.31). Nonselective BB use did not play a role in objective response (OR 1.20, 95% 0.58-2.49). The other characteristics evaluated, including age, sex, presence of cirrhosis, viral etiology of liver disease, CP class, presence of neoplastic PVT, performance status, presence of extrahepatic metastases, and AFP level were not found to be associated with response to ICI. Subgroup analyses of the effect of BBs on ORR revealed no significant effect of cirrhosis or CP class.
Finally, the effect of BBs on development of AEs was evaluated. BB exposure was not associated with development of any AE (OR 1.38, 95% CI 0.96-1.97). No statistically significant benefit of BB exposure against bleeding events was observed (OR 1.83, 95% CI 0.94-3.58). Only 4 patients in the cohort developed ascites while treated with ICI: 2 patients who had prior BB exposure and 2 who did not. The presence of neoplastic PVT was found to increase the risk of any AE in univariable analysis (OR 1.53, 95% CI 1.05-2.22) and was an independent predictor of AE development in multivariable analysis (OR 1.56, 95% CI 1.06, 2.31) (Table 6). Conversely, patients with a viral etiology of HCC were less likely to develop any AE according to univariable analysis (OR 0.56, 95% CI 0.40-0.79) and multivariable analysis (OR 0.52, 95% CI 0.36-0.75).
In this multicenter, international observational study, we evaluated the effect of BB use in patients with advanced HCC treated with ICI and found no significant association between BB exposure and OS. No significant associations were observed between BB exposure and secondary outcomes, including PFS, ORR, and development of AEs.
The effect of BB exposure on outcomes in HCC had previously been investigated, with some evidence that BB use may improve survival in patients with HCC. One Swedish study of 2104 patients in a national cancer registry between 2006 and 2015 found that BB use, particularly nonselective BB use, was associated with a lower mortality rate, but the mortality benefit appeared to be limited to patients with localized disease (26). Similarly, a small retrospective study of 36 patients with non-metastatic HCC who had undergone surgical resection or locoregional therapy found that BB use failed to predict HCC recurrence but was associated with improved OS after these curative interventions (27). As described earlier, a Taiwanese nationwide study of 4680 patients with unresectable and metastatic HCC from 2000 to 2013 found that propranolol reduced the risk of mortality from HCC, but no significant difference in recurrence-free survival (RFS) was observed in the 867 patients with localized disease (22). However, it is also worthwhile to note that these studies were conducted before ICIs gained widespread use as front line therapy for advanced HCC.
To the best of our knowledge, our study is the first to evaluate the effect of BB use in HCC treated with immunotherapy. Since the IMbrave150 trial changed the treatment paradigm for patients with advanced HCC, it has become more important to develop prognostic markers and to understand the impact of common concomitant medications on response to treatment. The effect of BB use is of particular clinical relevance as many patients with HCC take BBs at baseline as standard of care for variceal prophylaxis and sometimes for cardiovascular indications (28). Biologically, the role of β-adrenergic signaling has been well described pre-clinically to modulate the tumor microenvironment (TME) and response to immunotherapy (29), and there is significant interest in validating these findings in the clinical setting.
Cancer cells have been shown to express β-adrenergic receptors (βARs) (30), and adrenergic signaling has been linked to tumorigenesis and cancer progression through promotion of processes such as DNA repair, oncogene activation, inflammation and immune response, angiogenesis, survival, and epithelial-mesenchymal transition (29). Mouse models have been used to mechanistically show the effect of stress on the TME in various solid tumors. For example, Bucsek et al. showed that chronic stress in mice induced by cold exposure increased intratumoral noradrenaline, which subsequently reduced intratumoral CD8+ T cell frequency and functionality (31). Conversely, the addition of propranolol reduced βAR signaling, which converted tumors to an immunologically active TME with increased CD8+ T cell frequency and effector phenotype, decreased expression of PD-1, and elevated effector CD8+ T cell to CD4+ regulatory T cell ratio, leading to increased efficacy of anti-PD-1 checkpoint blockade (31). Kokolus et al. also showed that βAR blockade enhanced the antitumor effect of anti-PD-1 checkpoint inhibitor in a murine model of melanoma (32). Recently, in mouse models of sarcoma and colon cancer, propranolol reduced tumor angiogenesis, increased T cell infiltration, and reduced myeloid-derived suppressor cell infiltration, leading to an up-regulation of PD-L1 on tumor-associated macrophages, ultimately enhancing the efficacy of anti-CTLA4 therapy (33). These preclinical studies suggest that beta blockade may improve response to ICIs.
In the clinical setting, while no study before ours has evaluated the association between BB exposure and ICIs in HCC, it has been explored in other cancers, and thus far, results have been inconclusive but largely negative. In melanoma, BBs were found to have no independent prognostic effect on RFS in a recent phase III trial of adjuvant pembrolizumab in patients with high-risk stage III resected melanoma (34). Similarly, in a retrospective study of advanced melanoma, concurrent BB use in patients treated with ICI did not affect ORR, PFS, or OS (35). In contrast, one retrospective analysis of 195 patients with metastatic melanoma treated with ICI found that nonselective BB exposure improved survival compared to no BB use and β1-selective antagonist use (32). Another retrospective study of 109 patients with non-small cell lung cancer (NSCLC) treated with ICIs demonstrated that BB use may be associated with improved PFS but not OS (36). Another study in NSCLC analyzed the effect of multiple concomitant medications with ICIs and found that baseline BB use was not associated with clinical outcomes (37). A recent meta-analysis of 13 aggregated studies in mostly melanoma, NSCLC, and renal cell carcinoma showed that concurrent BB use with immunotherapy was not significantly associated with improved survival (38). Our negative findings add to growing evidence that the effect of BBs on clinical outcomes after treatment with ICIs may be limited despite preclinical data, suggesting that multiple interdependent pathways likely modulate the TME in humans and that there is a need to account for differences in experimental and real-world observations.
The unique pathophysiology of liver cirrhosis and microbial dysbiosis also add complexity to understanding of the TME and HCC tumorigenesis, suggesting that beta blockade is unlikely to mediate these interactions in easily predictable ways. BBs have both hemodynamic and non-hemodynamic mechanisms of action. Previously, nonselective BBs have been shown to increase intestinal transit, reducing bacterial overgrowth and translocation, in patients with cirrhosis independently of their hemodynamic functions (14, 39). While dysbiosis is known to contribute to HCC tumorigenesis, the strategy for targeting the gut microbiota-liver axis is still unclear. It is also unclear how beta blockade changes the microbiome in humans. Additionally, the ways in which ICIs can affect the microbiome are not well-characterized, though the composition of gut microbiota has been shown in preclinical studies to influence immunotherapy efficacy through regulation of immune responses (40). Our study also assessed whether antacids and antibiotics modified the effect of BB use but found no survival differences. These results are consistent with prior data in HCC demonstrating that antibiotic and antacid exposure during ICI therapy did not affect OS (25, 41) but are in opposition with studies conducted in other solid tumors (42), which may indicate that the HCC microbiome has immunomodulatory effects distinct from that of other cancers. While nonselective BBs may reduce bacterial overgrowth and translocation, further studies are need to better understand whether BB use changes the HCC microbiome and how this may affect outcomes with ICI therapy.
Our study also showed that BB exposure in patients with advanced HCC treated with ICIs did not increase the development of any AEs. On the other hand, and more surprisingly, use of BB also did not reduce the number of bleeding events observed. Given the real-world population evaluated in this cohort, a limitation of our study included the inconsistent reporting of the presence or absence of esophageal varices, as not all patients underwent pretreatment esophagogastroduodenoscopy (EGD). Although the decision to perform an EGD was not standardized, it was made on a case-by-case basis by the treating physician, in line with routine clinical practice. As such, the baseline degree of pHTN could not be fully characterized in the full patient cohort. Regardless, concomitant BB use did not affect the rate of bleeding events or development of ascites, which is in turn consistent with its lack of correlation with clinical outcomes after ICI therapy. In the subgroup analysis of patients with neoplastic PVT, BB use did not confer a statistically significant survival benefit. Given the low rate of bleeding events in the cohort (6.4%), it is possible that the study was underpowered to detect any influence of BB exposure. In the overall analysis, only the presence of neoplastic PVT was identified as an independent predictor of AE development, whereas a viral etiology of HCC was linked with a reduced risk of AE. The presence of neoplastic PVT is a well-known negative prognosticator of HCC and a criterion for classification into advanced stage disease (43), and the increased risk of AE is likely reflective of worse liver function. Further, our findings that viral etiologies of HCC did not increase incidence of AE is supported by prior studies confirming the safety and tolerability of ICIs in patients with HBV and HCV in HCC and other solid tumors (44, 45). In fact, patients with viral etiologies had a lower incidence of AEs, and while the cause is not entirely clear, it is possible that patients with viral HCC are diagnosed and initiate treatment when liver disease is less advanced as these patients are more likely to receive screening and treatment for liver disease prior to diagnosis of HCC. Studies are underway to better understand the outcomes of patients with viral HCC.
This is the first study investigating the effect of BB exposure in patients with advanced HCC treated with immunotherapy using real-world data. Although other studies of BB use in patients receiving immunotherapy for solid tumors have produced inconsistent results, our findings add to the body of evidence that BB use is not associated with improved survival outcomes. Overall, our international, multicenter cohort offers broad generalizability and is reflective of a diverse, real-world population, including patients with more advanced liver disease (CP classes B and C) who are typically excluded from clinical trial participation. Collection of detailed patient characteristics also allowed us to control for multiple possible confounding factors, such as the patient’s age, performance status, liver function, disease stage, and HCC risk factor.
However, the study also had several limitations, including those inherent to retrospective cohort studies that require validation in prospective studies. Patients taking BBs at baseline likely have increased comorbid conditions, including cirrhosis and cardiovascular disease, that may increase their risk of mortality. We accounted for these possible confounders by controlling for baseline liver function and performance status. However, the OS evaluated in this study was only reflective of all-cause mortality, and the specific cause of death was often unavailable or inconsistently documented in the medical record. While the effect of other potentially confounding concomitant medications such as antibiotics and antacids were assessed, other medications such as aspirin, statins, metformin, and steroids that may affect survival outcomes in HCC were not examined in this study (46–49). In addition, due to the observational design, the definition of BB exposure did not include dose and duration, and BB adherence could not be confirmed based on review of medical records alone. The number of patients in our study may also have been too small to detect an association between BB use and OS, particularly when subdivided by BB type and duration. Finally, our cohort does not have a large proportion of patients treated with the IMbrave150 regimen consisting of atezolizumab and bevacizumab that has now become standard of care for advanced HCC, and prior studies in colon cancer suggest a favorable effect of BB use in bevacizumab-containing therapy (50). However, studies are currently underway to assess the prognostic impact of concomitant medications on treatment outcomes with this combination.
In conclusion, in our retrospective cohort of patients with unresectable HCC treated with ICI, no statistically significant differences in OS, PFS, or ORR were observed between patients who used BBs and those who did not. Concomitant BB use was safe and did not increase the risk of AEs. Further prospective and larger observational studies, as well as mechanistic studies, are needed to elucidate the effect of beta blockade on HCC and its interaction with the microbiome and immune activation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board (IRB) at Imperial College London, whose review was accepted by all participating institutions’ IRBs. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
YLW and CA designed the study. YLW conducted the investigation, analysis, interpretation of data, and wrote the original draft. GvH and UÖ contributed to data analysis and methodology. MR, AG, YM, NN, BW, SA, P-CL, BS, LB, MP, AV, AW, AS, AP, LR, AN, MM, YHH, AK, MK, and DP contributed to the investigation and data curation. DP and CA supervised the study. All authors contributed to the article and approved the submitted version.
DP is supported by grant funding from the Wellcome Trust Strategic Fund (PS3416) and has received direct project funding by the NIHR Imperial Biomedical Research Center and ITMAT Push for Impact Grant Scheme 2019. Research reported in this publication was supported in part by the National Cancer Institute (Support Grant P30CA196521-01 awarded to the Tisch Cancer Institute of the Icahn School of Medicine at Mount Sinai) and used the Biostatistics Shared Resource Facility. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors would like to acknowledge the infrastructure provided by the Tisch Cancer Institute and the Icahn School of Medicine at Mount Sinai. DJP acknowledges infrastructural support by the Cancer Research UK Imperial Centre and the Imperial Experimental Cancer Medicine Centre and the Imperial College Tissue Bank.
UÖ is affiliated with Eli Lilly and Company. MR received lecture fees from Falk Foundation e.V. AV received consulting fees from Amgen, AstraZeneca, Baxalta, Bayer, BTG, EISA, Ipsen, Lilly, Novartis, Pierre Fabre, and Roche; travel fees from Bayer, Ipsen, and Roche; and research funding from Novartis. AS received lecture fees from Daiichi Sankyo/AstraZeneca; consulting fees from AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo/ AstraZeneca, Exelixis, Five Prime Therapeutics, and Pfizer; and institutional funding from Actuate Therapeutics, Astellas Pharma, AstraZeneca/MedImmune, Bristol-Myers Squibb, Clovis Oncology, Daiichi Sankyo/UCB Japan; Exelixis, Fiver Prime Therapeutics, KAHR Medical, Merck Sharp & Dohme, and Seattle Genetics. AK received consulting fees from AstraZeneca, Bayer, Bristol-Myers Squibb, Eisai, Exelixis, Genentech/Roche, and Merck; travel fees from from Bayer/Onyx, Bristol-Myers Squibb, Exelixis, and Merck; and institutional research funding from Adaptimmune, Bayer/Onyx, Bristol-Myers Squibb, Genentech, Hengrui Pharmaceutical, and Merck. AP received consulting fees for Eisai Inc, Exelixis, AstraZeneca, Replimune and Genentech. LR received consulting fees from Amgen, ArQule, AstraZeneca, Basilea, Bayer, Bristol-Myers Squibb, Celgene, Eisai, Exelixis, Genenta Science, Hengrui Therapeutics, Incyte, Ipsen, IQvia, Lilly, Merck Sharp & Dohme, Nerviano Medical Sciences, Roche, Sanofi, Servier, Taiho Oncology, and Zymeworks; lecture fees from AbbVie, Amgen, Bayer, Eisai, Gilead, Incyte, Ipsen, Lilly, Merck Serono, Roche, Sanofi; travel fees from AstraZeneca; and institutional research funding from Agios, ARMO BioSciences, AstraZeneca, BeiGene, Eisai, Exelixis, FibroGen, Incyte, Ipsen, Lilly, Merck Sharp & Dohme, Nerviano Medical Sciences, Roche, and Zymeworks. Y-HH received lecture fees from Bayer, Bristol-Myers Squibb, Eisai, Gilead Sciences, Lilly, MSD, and Roche; and consulting fees from Bayer, Bristol-Myers Squibb, Eisai, Gilead Sciences, Lilly, MSD, and Roche. DP received lecture fees from ViiV Healthcare, Bayer, Falk Pharma and Roche; travel expenses from Bristol-Myers Squibb, Bayer, and MSD Oncology; consulting fees for AstraZeneca, Da Volterra, EISAI, H3 Biomedicine, Ipsen, Mina Therapeutics, and Roche; and institutional research funding from Bristol-Myers Squibb, GlaxoSmithKline, and MSD Oncology.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Llovet J, Villanueva A, Lachenmayer A, Finn R. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol (2015) 12(7):408–24. doi: 10.1038/nrclinonc.2015.103
3. Finn R, Qin S, Ikeda M, Galle P, Ducreux M, Kim T, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
4. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol (2022) 76(4):862–73. doi: 10.1016/j.jhep.2021.11.030
5. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evidence. (2022) 1(8):EVIDoa2100070. doi: 10.1056/EVIDoa2100070
6. Yau T, Kang Y-K, Kim T-Y, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol (2020) 6(11):e204564–e. doi: 10.1001/jamaoncol.2020.4564
7. Hussain N, Naeem M, Pinato DJ. Concomitant medications and immune checkpoint inhibitor therapy for cancer: causation or association? Hum Vaccin Immunother (2021) 17(1):55–61. doi: 10.1080/21645515.2020.1769398
8. Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol (2019) 5(12):1774–8. doi: 10.1001/jamaoncol.2019.2785
9. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science (2018) 359(6371):91–7. doi: 10.1126/science.aan3706
10. Zheng Y, Wang T, Tu X, Huang Y, Zhang H, Tan D, et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer (2019) 7(1):193. doi: 10.1186/s40425-019-0650-9
11. Simbrunner B, Mandorfer M, Trauner M, Reiberger T. Gut-liver axis signaling in portal hypertension. World J Gastroenterol (2019) 25(39):5897–917. doi: 10.3748/wjg.v25.i39.5897
12. Allaire M, Rudler M, Thabut D. Portal hypertension and hepatocellular carcinoma: Des liaisons dangereuses…. Liver Int (2021) 41(8):1734–43. doi: 10.1111/liv.14977
13. Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med (2010) 362(9):823–32. doi: 10.1056/NEJMra0901512
14. Pérez-Paramo M, Muñoz J, Albillos A, Freile I, Portero F, Santos M, et al. Effect of propranolol on the factors promoting bacterial translocation in cirrhotic rats with ascites. Hepatology (2000) 31(1):43–8. doi: 10.1002/hep.510310109
15. Senzolo M, Fries W, Buda A, Pizzuti D, Nadal E, Sturniolo GC, et al. Oral propranolol decreases intestinal permeability in patients with cirrhosis: another protective mechanism against bleeding? Am J Gastroenterol (2009) 104(12):3115–6. doi: 10.1038/ajg.2009.457
16. Wang F, Liu H, Wang F, Xu R, Wang P, Tang F, et al. Propranolol suppresses the proliferation and induces the apoptosis of liver cancer cells. Mol Med Rep (2018) 17(4):5213–21. doi: 10.3892/mmr.2018.8476
17. Darnaud M, Faivre J, Moniaux N. Targeting gut flora to prevent progression of hepatocellular carcinoma. J Hepatol (2013) 58(2):385–7. doi: 10.1016/j.jhep.2012.08.019
18. Liao X, Che X, Zhao W, Zhang D, Bi T, Wang G. The β-adrenoceptor antagonist, propranolol, induces human gastric cancer cell apoptosis and cell cycle arrest via inhibiting nuclear factor κB signaling. Oncol Rep (2010) 24(6):1669–76. doi: 10.3892/or_00001032
19. Herrera I, Pascual S, Zapater P, Carnicer F, Bellot P, María Palazón J. The use of β-blockers is associated with a lower risk of developing hepatocellular carcinoma in patients with cirrhosis. Eur J Gastroenterol Hepatol (2016) 28(10):1194–7. doi: 10.1097/MEG.0000000000000677
20. Thiele M, Albillos A, Abazi R, Wiest R, Gluud LL, Krag A. Non-selective beta-blockers may reduce risk of hepatocellular carcinoma: a meta-analysis of randomized trials. Liver Int (2015) 35(8):2009–16. doi: 10.1111/liv.12782
21. Wijarnpreecha K, Li F, Xiang Y, Xu X, Zhu C, Maroufy V, et al. Nonselective beta-blockers are associated with a lower risk of hepatocellular carcinoma among cirrhotic patients in the united states. Alimentary Pharmacol Ther (2021) 54(4):481–92. doi: 10.1111/apt.16490
22. Chang PY, Chung CH, Chang WC, Lin CS, Lin HH, Dai MS, et al. The effect of propranolol on the prognosis of hepatocellular carcinoma: A nationwide population-based study. PloS One (2019) 14(5):e0216828. doi: 10.1371/journal.pone.0216828
23. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology (2018) 67(1):358–80. doi: 10.1002/hep.29086
24. Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul J-L, et al. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol (2018) 69(1):182–236. doi: 10.1016/j.jhep.2018.03.019
25. Fessas P, Naeem M, Pinter M, Marron TU, Szafron D, Balcar L, et al. Early antibiotic exposure is not detrimental to therapeutic effect from immunotherapy in hepatocellular carcinoma. Liver Cancer (2021) 10(6):583–92. doi: 10.1159/000519108
26. Udumyan R, Montgomery S, Duberg AS, Fang F, Valdimarsdottir U, Ekbom A, et al. Beta-adrenergic receptor blockers and liver cancer mortality in a national cohort of hepatocellular carcinoma patients. Scand J Gastroenterol (2020) 55(5):597–605. doi: 10.1080/00365521.2020.1762919
27. Yu D, Holmes S, Uhanova J, Lipschitz J, McKay A, Gerald MY. Predicting hepatocellular carcinoma recurrence and survival. Hepatogastroenterology (2014) 61(131):776–83.
28. Villanueva C, Albillos A, Genescà J, Garcia-Pagan JC, Calleja JL, Aracil C, et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): A randomised, double-blind, placebo-controlled, multicentre trial. Lancet (2019) 393(10181):1597–608. doi: 10.1016/S0140-6736(18)31875-0
29. Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer (2015) 15(9):563–72. doi: 10.1038/nrc3978
30. Rains SL, Amaya CN, Bryan BA. Beta-adrenergic receptors are expressed across diverse cancers. Oncoscience (2017) 4(7-8):95–105. doi: 10.18632/oncoscience.357
31. Bucsek MJ, Qiao G, MacDonald CR, Giridharan T, Evans L, Niedzwecki B, et al. β-adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8(+) T cells and undermines checkpoint inhibitor therapy. Cancer Res (2017) 77(20):5639–51. doi: 10.1158/0008-5472.CAN-17-0546
32. Kokolus KM, Zhang Y, Sivik JM, Schmeck C, Zhu J, Repasky EA, et al. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology (2018) 7(3):e1405205. doi: 10.1080/2162402X.2017.1405205
33. Fjæstad KY, Rømer AMA, Goitea V, Johansen AZ, Thorseth ML, Carretta M, et al. Blockade of beta-adrenergic receptors reduces cancer growth and enhances the response to anti-CTLA4 therapy by modulating the tumor microenvironment. Oncogene (2022) 41(9):1364–75. doi: 10.1038/s41388-021-02170-0
34. Kennedy OJ, Kicinski M, Valpione S, Gandini S, Suciu S, Blank CU, et al. Prognostic and predictive value of β-blockers in the EORTC 1325/KEYNOTE-054 phase III trial of pembrolizumab versus placebo in resected high-risk stage III melanoma. Eur J Cancer (2022) 165:97–112. doi: 10.1016/j.ejca.2022.01.017
35. Wang DY, McQuade JL, Rai RR, Park JJ, Zhao S, Ye F, et al. The impact of nonsteroidal anti-inflammatory drugs, beta blockers, and metformin on the efficacy of anti-PD-1 therapy in advanced melanoma. Oncologist (2020) 25(3):e602–e5. doi: 10.1634/theoncologist.2019-0518
36. Oh MS, Guzner A, Wainwright DA, Mohindra NA, Chae YK, Behdad A, et al. The impact of beta blockers on survival outcomes in patients with non-small-cell lung cancer treated with immune checkpoint inhibitors. Clin Lung Cancer (2021) 22(1):e57–62. doi: 10.1016/j.cllc.2020.07.016
37. Cortellini A, Di Maio M, Nigro O, Leonetti A, Cortinovis DL, Aerts JG, et al. Differential influence of antibiotic therapy and other medications on oncological outcomes of patients with non-small cell lung cancer treated with first-line pembrolizumab versus cytotoxic chemotherapy. J Immunother Cancer (2021) 9(4):e002421. doi: 10.1136/jitc-2021-002421
38. Zhang Y, Chen H, Chen S, Li Z, Chen J, Li W. The effect of concomitant use of statins, NSAIDs, low-dose aspirin, metformin and beta-blockers on outcomes in patients receiving immune checkpoint inhibitors: a systematic review and meta-analysis. Oncoimmunology (2021) 10(1):1957605. doi: 10.1080/2162402X.2021.1957605
39. Senzolo M, Cholongitas E, Burra P, Leandro G, Thalheimer U, Patch D, et al. Beta-blockers protect against spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. Liver Int (2009) 29(8):1189–93. doi: 10.1111/j.1478-3231.2009.02038.x
40. Dai Z, Zhang J, Wu Q, Fang H, Shi C, Li Z, et al. Intestinal microbiota: a new force in cancer immunotherapy. Cell Commun Signal (2020) 18(1):90. doi: 10.1186/s12964-020-00599-6
41. Jun T, Ozbek U, Dharmapuri S, Hardy-Abeloos C, Zhu H, Lin JY, et al. Antacid exposure and immunotherapy outcomes among patients with advanced hepatocellular carcinoma. Ther Adv Med Oncol (2021) 13:17588359211010937. doi: 10.1177/17588359211010937
42. Spakowicz D, Hoyd R, Muniak M, Husain M, Bassett JS, Wang L, et al. Inferring the role of the microbiome on survival in patients treated with immune checkpoint inhibitors: causal modeling, timing, and classes of concomitant medications. BMC Cancer (2020) 20(1):383. doi: 10.1186/s12885-020-06882-6
43. Mähringer-Kunz A, Steinle V, Düber C, Weinmann A, Koch S, Schmidtmann I, et al. Extent of portal vein tumour thrombosis in patients with hepatocellular carcinoma: The more, the worse? Liver Int (2019) 39(2):324–31. doi: 10.1111/liv.13988
44. Lee PC, Chao Y, Chen MH, Lan KH, Lee IC, Hou MC, et al. Risk of HBV reactivation in patients with immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J Immunother Cancer (2020) 8(2):e001072. doi: 10.1136/jitc-2020-001072
45. Shah NJ, Al-Shbool G, Blackburn M, Cook M, Belouali A, Liu SV, et al. Safety and efficacy of immune checkpoint inhibitors (ICIs) in cancer patients with HIV, hepatitis b, or hepatitis c viral infection. J Immunother Cancer (2019) 7(1):353. doi: 10.1186/s40425-019-0771-1
46. Ricciotti E, Wangensteen KJ, FitzGerald GA. Aspirin in hepatocellular carcinoma. Cancer Res (2021) 81(14):3751–61. doi: 10.1158/0008-5472.CAN-21-0758
47. Wang J, Li X. Impact of statin use on the risk and prognosis of hepatocellular carcinoma: a meta-analysis. Eur J Gastroenterol Hepatol (2021) 33(12):1603–9. doi: 10.1097/MEG.0000000000002040
48. Zhou J, Ke Y, Lei X, Wu T, Li Y, Bao T, et al. Meta-analysis: The efficacy of metformin and other anti-hyperglycemic agents in prolonging the survival of hepatocellular carcinoma patients with type 2 diabetes. Ann Hepatol (2020) 19(3):320–8. doi: 10.1016/j.aohep.2019.11.008
49. Cortellini A, Tucci M, Adamo V, Stucci LS, Russo A, Tanda ET, et al. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J Immunother Cancer (2020) 8(2):e001361. doi: 10.1136/jitc-2020-001361
50. Fiala O, Ostasov P, Sorejs O, Liska V, Buchler T, Poprach A, et al. Incidental use of beta-blockers is associated with outcome of metastatic colorectal cancer patients treated with bevacizumab-based therapy: A single-institution retrospective analysis of 514 patients. Cancers (Basel) (2019) 11(12):1856. doi: 10.3390/cancers11121856
Keywords: hepatocellular carcinoma, immune checkpoint inhibitors, cancer immunotherapy, beta-adrenergic blockade, beta blocker
Citation: Wu YL, van Hyfte G, Özbek U, Reincke M, Gampa A, Mohamed YI, Nishida N, Wietharn B, Amara S, Lee P-C, Scheiner B, Balcar L, Pinter M, Vogel A, Weinmann A, Saeed A, Pillai A, Rimassa L, Naqash AR, Muzaffar M, Huang Y-H, Kaseb AO, Kudo M, Pinato DJ and Ang C (2023) Outcomes of beta blocker use in advanced hepatocellular carcinoma treated with immune checkpoint inhibitors. Front. Oncol. 13:1128569. doi: 10.3389/fonc.2023.1128569
Received: 20 December 2022; Accepted: 30 January 2023;
Published: 14 February 2023.
Edited by:
Changchang Jia, Third Affiliated Hospital of Sun Yat-sen University, ChinaReviewed by:
Lorenza Di Marco, University of Modena and Reggio Emilia, ItalyCopyright © 2023 Wu, van Hyfte, Özbek, Reincke, Gampa, Mohamed, Nishida, Wietharn, Amara, Lee, Scheiner, Balcar, Pinter, Vogel, Weinmann, Saeed, Pillai, Rimassa, Naqash, Muzaffar, Huang, Kaseb, Kudo, Pinato and Ang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Celina Ang, Y2VsaW5hLmFuZ0Btc3NtLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.