- 1Department of Medical Oncology of Respiratory, Guangxi Medical University Cancer Hospital, Nanning, Guangxi, China
- 2Medical Department, Nanjing Geneseeq Technology Inc., Nanjing, Jiangsu, China
- 3Department of Pathology, Guangxi Medical University Cancer Hospital, Nanning, Guangxi, China
- 4Department of Anesthesiology, Guangxi Medical University Cancer Hospital, Nanning, Guangxi, China
Preclinical cases suggest that EGFR tyrosine kinase inhibitors (TKIs) plus MET TKIs are a potential therapy for non-classical EGFR mutant lung cancers with MET amplification acquired resistance. Herein, we report for the first time the effectiveness of novel combination treatment regimens for patients with EGFR G719X/S768I/L861Q. Until the last follow-up assessment, two patients demonstrated improved survival after they switched to afatinib combined with savolitinib (PFS: 10 months) and furmonertinib combined with crizotinib (PFS: 6 months), respectively, that did not observed increased incidence and severity of adverse events. According to the findings of this study and literature review, various responses were observed from the combined therapy in NSCLC patients who harbored uncommon EGFR mutations and MET amplification. Furthermore, Next generation sequencing (NGS) leads to the discovery of uncommon of EGFR and reveals the co-mutations in NSCLC.
1 Introduction
Mutations of Epidermal growth factor receptor (EGFR) are one of the most prevalent oncogene drivers in non-small cell lung cancers. Up to 85 to 90% of EGFR-positive NSCLC patients carry the classical L858R and exon 19 deletions, and the remaining 10%-15% are defined as non-classical mutations (1). US Food and Drug Administration (FDA) approved therapies for non-classical EGFR mutations including G719X, S768I and L861Q, for which afatinib was deemed effective base on retrospective studies (2). MET amplification has been proven as a mechanism of acquired resistance in advanced NSCLC patients with EGFR G719X/S768I/L861Q (3, 4). A previous case report indicated that the combination of afatinib and crizotinib, the inhibitor of MET amplification, could be a promising strategy for reversing acquired resistance in patients with EGFR L861Q and MET amplification mutations (5), Nevertheless, limited evidence has demonstrated the efficacy of different combined therapy patterns in these patients. Therefore, we initiated a real-world study to investigate the distribution and therapeutic responses of NSCLC patients with EGFR G719X/S768I/L861Q and MET amplification who were treated under three different treatment patterns: afatinib plus crizotinib, afatinib plus savolitinib, furmonertinib plus crizotinib, which demonstrated significant antitumor efficacy and favorable clinical effect. In addition, there was a significant difference in progression free survival (PFS) across treatment regimens and EGFR alteration subtypes.
2 Case presentation
2.1 Case 1
A 62-year-old man with 40 pack-year smoking history was admitted to our hospital due to right chest pain (Figure 1A). The computed tomography (CT) revealed a tumor in the right lung with multiple pulmonary metastases, right clavicle involvement and right neck lymph node metastasis on November 26, 2020 (Figure 1B). Bronchoscopic biopsy and Immunohistochemical (IHC) analysis revealed adenocarcinoma with positive expression of TTF-1, CK7 and Ki67 (Figure 1C). Examination of NGS (Geneseeq Technology Inc., Nanjing China) with a panel of 139 cancer-related genes by using a biopsy tumor tissue, and it was found EGFR G719C/S768I and TP53 K320* mutations. He was diagnosed as adenocarcinoma in the upper lobe of the right lung (cT4N3M1a, stage IVA), and treated with Afatinib (30 mg once daily) combined with pemetrexed and carboplatin as the first-line treatment since Dec 25, 2020, and the best efficacy evaluation was partial response (PR). On Mar 16, 2022, chest CT showed enlarged pulmonary space-occupying lesion after 15 months of afatinib treatment. EGFR G719C/S768I, MET amplification (copy number 15), and CDK4 amplification (copy number 20) were found by comprehensive genomic profiling of the CT-guided lung puncture specimen (Table 1). MET amplification and CDK4 amplification were considered as the reasons of afatinib resistance. Given that the inhibitor of CDK4 has not been approved for lung cancer treatment, savolitinib (400 mg once daily) as the MET inhibitor were administered to the patient with the combination of afatinib (30 mg once daily). Chest CT scan 1 month later demonstrated obvious shrinkage (PR) and 2 months later it showed confirmed PR, Untill the last follow-up on 14 Jan 2023, the patient achieved 10 monthes PFS. Continuously decreasing levels of carcinoembryonic antigen (CEA) were observed. The patient recieved radiotherapy (48 Grey) during 2022.9.13-2022.10.6, due to the slow progression of leisions was observed in August. The last CT result revealed a Grade 1 radiation pneumonia. The treatment is still being continued, and the timeline of all treatment process for the patient is shown in Figure 1.
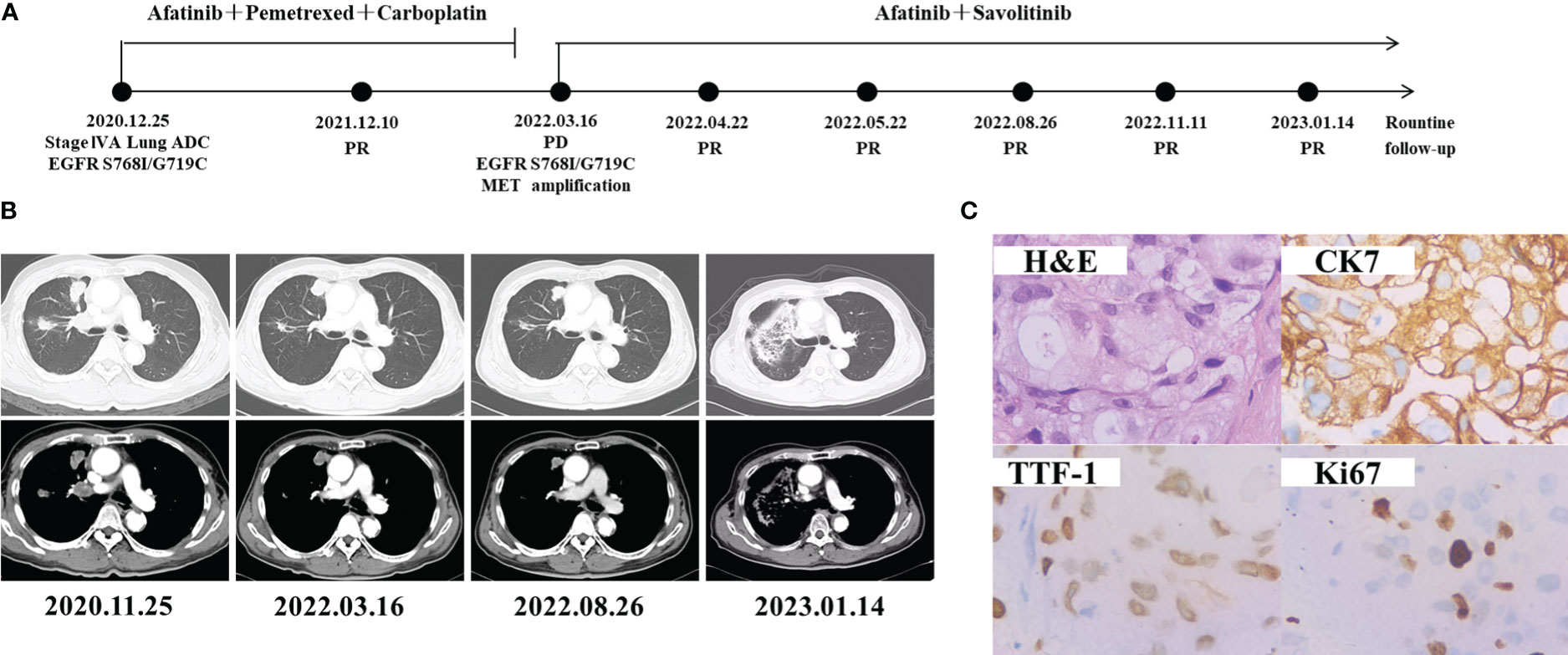
Figure 1 Schematic of treatment history of Case 1. (A) The timeline of treatment. (B) Chest CT images throughout the disease course. (C) Pathological examination of the surgical specimen. IHC testing (400×) results showed positive expression of TTF-1, CK7 and Ki67.
2.2 Case 2
A 45-year-old Chinese female non-smoker was presented to our hospital with no family history of cancer suffered from repeated coughing on Feb 25, 2021 (Figure 2A). CT of the chest revealed a density mass in the right lung and multiple metastases in the mediastinal lymph nodes, vertebral lumbalis and right adrenal (Figure 2B). The level of the CEA was 16.27 ng/mL, which was much higher than the normal range of < 5.0 ng/mL. IHC analyses revealed that the tumor cells of the right lung were negative for TTF-1 and ALK, whereas focal staining was positive for CK7 and ki67 (Figure 2C). The patient was diagnosed with lung adenocarcinoma (cT3N3M1b, stage IVB) base on these findings. To identify a more effective treatment, NGS was performed on a right lung biopsy, and detected a rare EGFR L861Q co-occurring with TP53 Y220C mutation. She was treated by osimertinib (80 mg, once a day) combined with pemetrexed and carboplatin as the first-line treatment since March 2021. However, the disease progressed after nine months as the follow-up CT scans showed enlarged lesions in middle lobe of right lung. The patient received osimertinib treatment with gemcitabine and bevacizumab for one month, but the disease progressed rapidly on December 27, 2021. The gene test result is the same as the last time, the patient was then switched to afatinib (30 mg, once a day) treatment starting from January 2022. Chest CT scan a month later demonstrated stable disease (SD) and 2 months later it showed PD. NGS profiling was performed using biopsy specimen of the lung. In addition to EGFR L861Q and TP53 Y220C mutations, newly MET amplification (copy number 11.1) was detected (Table 1). Magnetic resonance imaging (MRI) scans revealed brain metastases (Figure 2B) on April 20, 2022. She switched to furmonertinib (80 mg, once a day) plus crizotinib (250 mg, twice a day) and whole-brain radiotherapy from April 28, 2022. PR was achieved after four months when CT scans showed shrinkages of target nodules in both lung and brain. The patient remained stable with a PFS for 6 months until PD on 22 October 2022, and then docetaxel plus anlotinib was started.
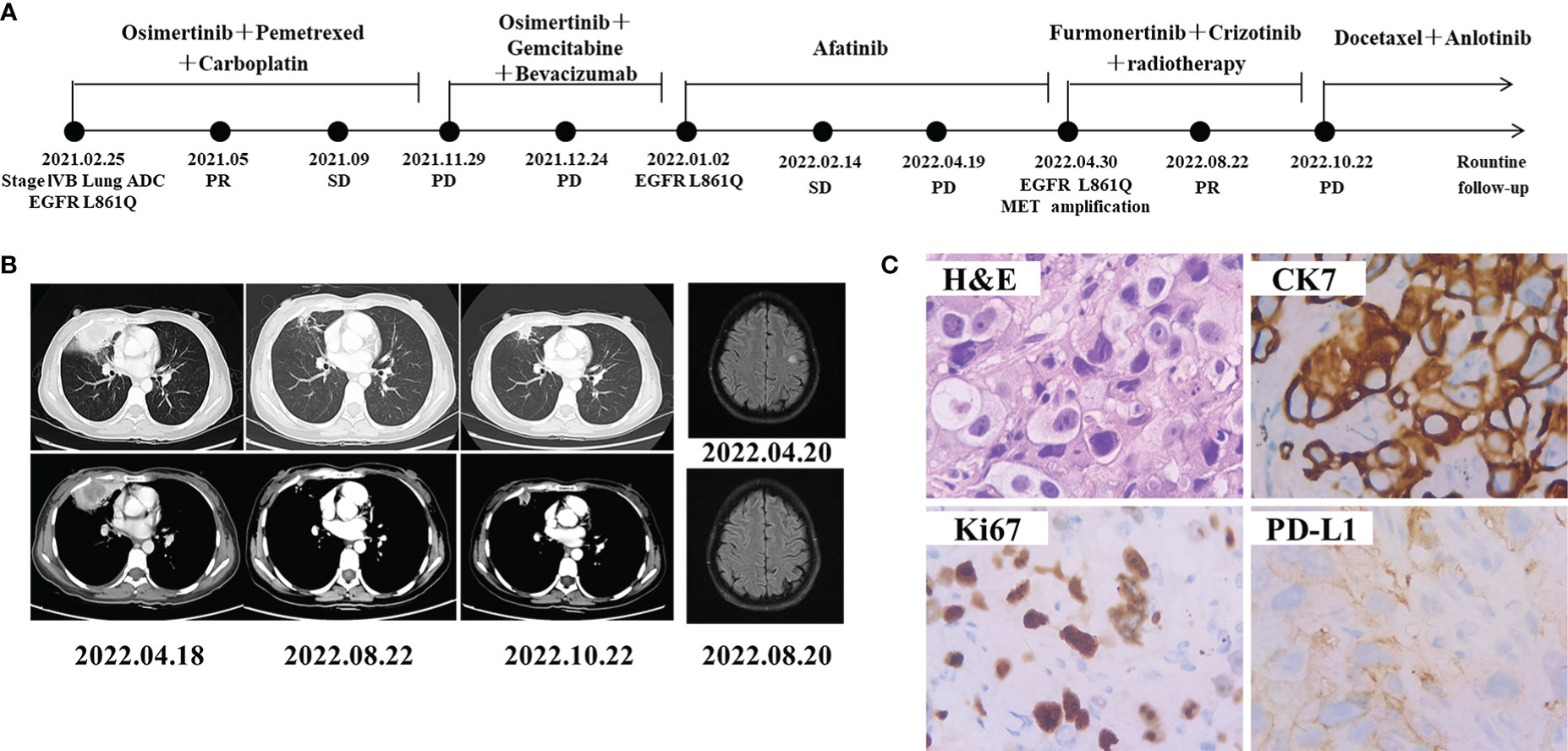
Figure 2 Schematic of treatment history of Case 2. (A) The timeline of treatment. (B) Chest CT images and brain MRI throughout the disease course. (C) Pathological examination of the surgical specimen. IHC testing (400×) results showed positive expression of CK7, PD-L1 and Ki67.
2.3 Case 3
A 67-year-old Chinese male with no history of smoking visited our hospital in August 2017 with the chief complaint of dyspnea and pain in chest (Figure 3A). CT revealed a massive shadow in the upper lobe of the left lung, as well as metastases in both lungs and mediastinal lymph nodes (Figure 3B). Multiple bone metastases were also observed on a bone emission computed tomography (ECT) scan. CEA and cancer antigen 125 (CA-125) were detected at high levels of 100 ng/mL and 125.4 U/mL. The IHC analysis showed TTF-1(+), NapsinA (+), and ALK (−), Ki67 positive rate 40%, indicating lung adenocarcinoma (Figure 3C). The EGFR G719A mutation was detected by PCR. The patient was diagnosed with lung adenocarcinoma (cT1N2M1b, stage IVA) base on the findings above. He started to received afatinib (40 mg, once a day) since August 27, 2017. Chest CT scan 1month later demonstrated obvious shrinkage (PR) and 8 months later it showed confirmed PR (Figure 3A). The lung lesion was re-evaluated using CT scans on January 24, 2019, seven months following afatinib monotherapy, which revealed an increased tumor size. Comprehensive genomic profiling of the plasma identified EGFR G719A, MET amplification(copy number 8.4), and TP53 V73Gfs*75 were found (Table 1). On March 7, 2019, he started to adopt the combination therapy of afatinib (40mg once a day) and crizotinib (250mg once a day). After 2 weeks treatment, CT revealed shrinkage of lesion in the left lung, and showed SD response. Unfortunately, the patient died due to no specific cause in April 2019.
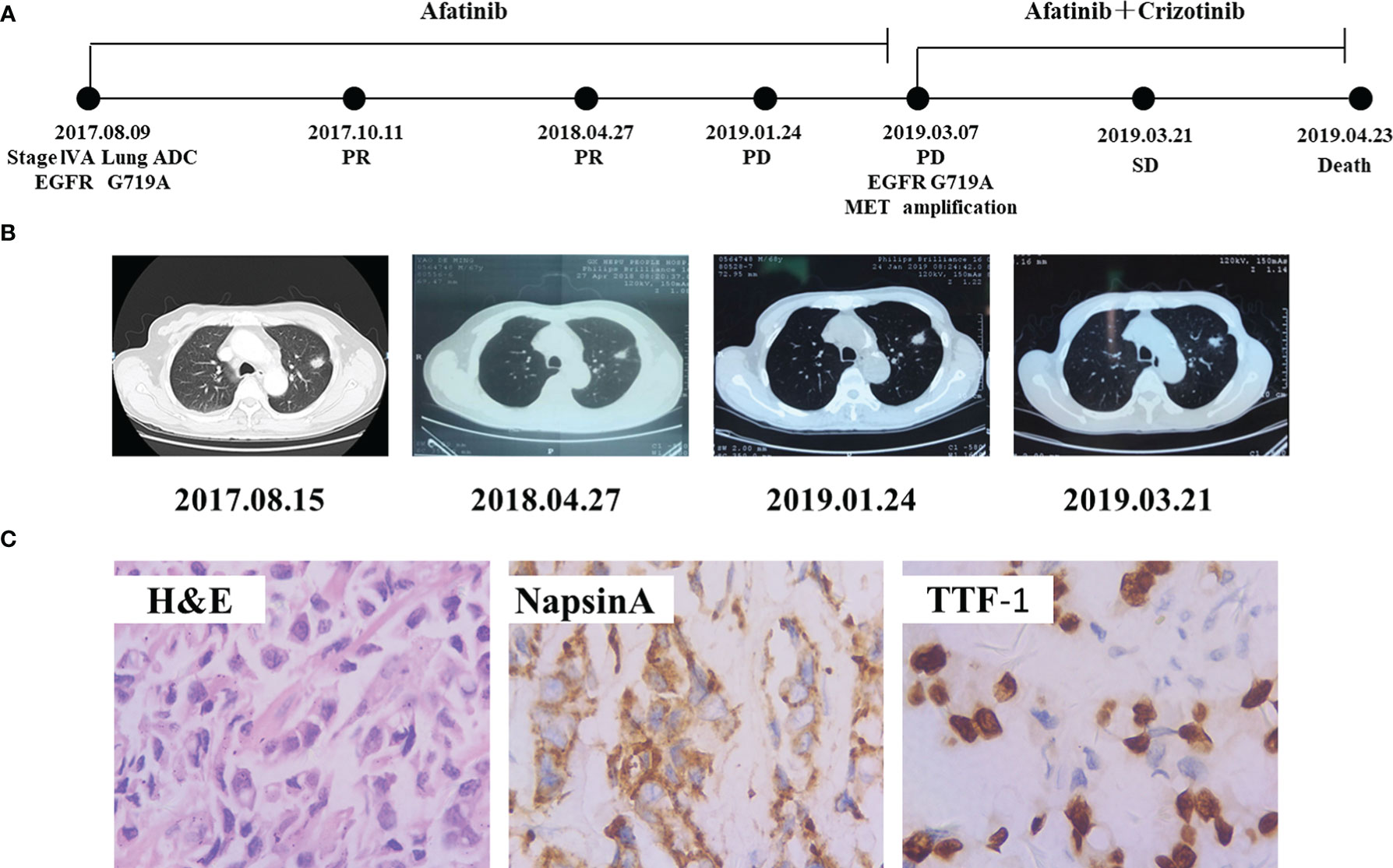
Figure 3 Schematic of treatment history of Case 3. (A) The timeline of treatment. (B) Chest CT images throughout the disease course. (C) Pathological examination of the surgical specimen. IHC testing (400×) results showed positive expression of Napsin A and TTF-1.
3 Discussion
With the fast development of NGS and innovative medications, we may have started to avoid the exclusion of patients with uncommon or complex mutations who may benefit from anti-EGFR therapy. However, there are still some patients with non-classic EGFR mutation acquired resistance to EGFR-TKI. Some studies have suggested that MET amplification may be one of the main resistance mechanisms of afatinib in patients with EGFR G719X/S768I/L861Q (4). Abnormal activation of MET, which recruiting the MET receptor kinase to phosphorylate HER3 and activate the PI3K-Akt survival pathway, and thus, the PI3K-Akt pathway could be successfully blocked by inhibiting EGFR and MET simultaneously (6). The phase 1b TATTON study demonstrated that the combination of osimertinib and savolitinib had acceptable safety profile and promising antitumor activity in patients with MET amplification, classical EGFR mutations, advanced NSCLC, who had disease progression on a previous EGFR-TKI (7), illustrating that our understanding of single gene-targeted therapy has switched to associated genes and combined therapy. Nevertheless, evidence on the effectiveness of combined therapy among patients with non-classical EGFR mutations after acquiring MET amplification resistance are limited (Table 2).
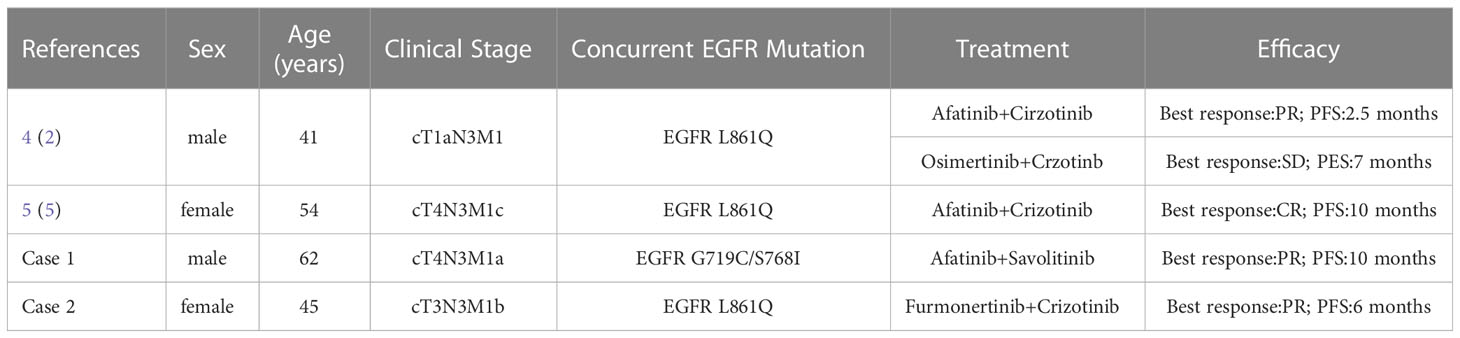
Table 2 Reported combined therapy responses in lung cancer with EGFR G719X/S768I/L861Q and acquired MET amplification.
In our study, case 1 was treated with afatinib plus savolitinib to learn whether combined modality had a significant influence in the patient harboring MET amplification resistance with EGFR G719C/S768I mutations. Intriguingly, it could induce tumor remission with a PFS of more than 10 months. A case reported that an advanced NSCLC patient was diagnosed with acquired resistance of MET amplification (9.54 copy numbers) in the context of EGFR L861Q, and the combination therapy of afatinib plus crizotinib led to a 10 months PFS (5). A recent study also described a patient with concomitant EGFR L861Q and MET amplification at the time of initial diagnosis, who achieved PR after treating with afatinib and crizotinib with a PFS of 2.5 months, then he changed to osimertinib plus crizotinib due to brain metastases, and maintained SD for 6 months (4). Based on the computational modeling, furmonertinib might confer favorable activity to S768I and L861Q (8). Actually our study suggested that furmonertinib plus crizotinib showed potent efficacy in Case 2 with EGFR L861Q and MET amplification, she attained a 6 months PFS. In contrast, MET amplification in Case 3 with baseline EGFR G719A was found to be a driver of acquired resistance to afatinib. Treated by afatinib combined with crizotinib, the patient achieved disease control for only 1 months, yet he only experienced grade 1 heart rates drop. Patients harboring EGFR L861Q mutation maybe respond better than EGFR G719A to the combination therapy of afatinib and crizotinib. Last year, furmonertinib and savolitinib had granted approval in China for the NSCLC patients with classical EGFR mutation and MET exon14-skipping respectively (9, 10). These drugs are extensively utilized in China. Our cases provided additional treatment strategies for this subset of patients with EGFR G719X/L861Q/S768I and acquired MET amplification.
4 Conclusion
In line with our study, the acquired resistance mechanisms mediated by MET amplification, could be suppressed by unreported combination strategies involving targeted therapies, even when the concomitant non-classical EGFR mutation were G719C, S768I and L861Q. If our inference is validated by more cases, In this way, more favorable survival outcomes would be observed from combined therapy. Due to the heterogeneity and low prevalence of non-classical EGFR alterations, as well as the paucity of large-scale randomized clinical trials, clinical outcomes of diverse treatment modalities for NSCLC patient harboring non-classical EGFR alterations have not been fully elucidated. To guide precision therapy, further study is required to evaluate which treatment modality is the most effective for non-classical EGFR alterations. Of note, disease monitoring via NGS sequencing is crucial for targeted therapy in lung adenocarcinoma.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethical Committee of Guangxi Medical University Cancer Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YZ and CS were involved in the diagnostic flow and patient follow-up, LS wrote this article, and the manuscript was edited by CL. Collection and assembly of data: WL, ZL, HW, RN, QY and WJ. All authors contributed to the article and approved the submitted version.
Funding
This work were supported by the Promoting Project of Basic Capacity for Young and Middle-aged University Teachers in Guangxi, China (no.2021KY0098), and Guangxi Medical and health key discipline construction project.
Conflict of interest
Authors LS and CL was employed by Nanjing Geneseeq Technology Inc.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shen YC, Tseng GC, Tu CY, Chen WC, Liao WC, Chen WC, et al. Comparing the effects of afatinib with gefifitinib or erlotinib in patients with advanced-stage lung adenocarcinoma harboring non-classical epidermal growth factor receptor mutations. Lung Cancer (2017) 110:56–62. doi: 10.1016/j.lungcan.2017.06.007
2. Harvey RD, Adams VR, Beardslee T, Medina P. Afatinib for the treatment of EGFR mutation-positive NSCLC: A review of clinical findings. J Oncol Pharm Pract (2020) 26(6):1461–74. doi: 10.1177/1078155220931926
3. Yang JC, Sequist LV, Geater SL, Tsai CM, Mok TS, Schuler M, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: A combined post-hoc analysis of LUX-lung 2, LUX-lung 3, and LUX-lung 6. Lancet Oncol (2015) 16(7):830–8. doi: 10.1016/S1470-2045(15)00026-1
4. Pang LL, Gan JD, Tan JR, Huang YH, Liao J, Liang WT, et al. Efficacy and potential resistance mechanisms of afatinib in advanced non-small cell lung cancer patients with EGFR G719X/L861Q/S768I. Cancer. (2022) 128(21):3804–14. doi: 10.1002/cncr.34451
5. Long Y, Zhang K, Li Y, Yu M, Zhu J, Huang M. Durable complete response after afatinib and crizotinib in an advanced non-small cell lung cancer patient with EGFR L861Q mutation and acquired MET amplification: A case report. Ann Palliat Med (2020) 9(5):3609–13. doi: 10.21037/apm-19-482
6. Lee M, Jain P, Wang F, Ma PC, Borczuk A, Halmos B. MET alterations and their impact on the future of non-small cell lung cancer (NSCLC) targeted therapies. Expert Opin Ther Targets (2021) 25(4):249–68. doi: 10.1080/14728222.2021.1925648
7. Sequist LV, Han JY, Ahn MJ, Cho BC, Yu H, Kim SW, et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, NonSmall-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: Interim results from a multicentre, open-label, phase 1b study. Lancet Oncol (2020) 21(3):373–86. doi: 10.1016/S1470-2045(19)30785-5
8. Xu H, Yang G, Liu R, Yang Y, Li W, Li J, et al. EGFR uncommon alterations in advanced non-small cell lung cancer and structural insights into sensitivity to diverse tyrosine kinase inhibitors. Front Pharmacol (2022) 13:976731. doi: 10.3389/fphar.2022.976731
Keywords: uncommon mutation, EGFR G719X/S768I/L861Q, resistance, MET amplification, non-small cell lung cancer (NSCLC)
Citation: Zhao Y, Su C, Shi L, Luo W, Liu Z, Liang C, Wang H, Ning R, Yu Q and Jiang W (2023) Case Report: The effective treatment of patients in advanced no-small cell lung cancer patients with EGFR G719X/S768I/L861Q and acquired MET amplification: A case series and literature review. Front. Oncol. 13:1126325. doi: 10.3389/fonc.2023.1126325
Received: 17 December 2022; Accepted: 09 February 2023;
Published: 22 February 2023.
Edited by:
Matiullah Khan, AIMST University, MalaysiaReviewed by:
Christos Chouaid, Hospital Center Intercommunal De Créteil, FranceFrancesco Pepe, University of Naples Federico II, Italy
Copyright © 2023 Zhao, Su, Shi, Luo, Liu, Liang, Wang, Ning, Yu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qitao Yu, eXF0MTc4QDE2My5jb20=; Wei Jiang, d2VpY3kyMDE2QDE2My5jb20=
†These authors have contributed equally to this work
 Yun Zhao1†
Yun Zhao1† Cuiyun Su
Cuiyun Su Lina Shi
Lina Shi Wenqi Luo
Wenqi Luo Zhen Liu
Zhen Liu Huilin Wang
Huilin Wang Wei Jiang
Wei Jiang