- 1Division of Medical Oncology, Department of Medicine, Stanford University, Stanford, CA, United States
- 2Section of Geriatric Medicine, Division of Primary Care and Population Health, Department of Medicine, Stanford University, Stanford, CA, United States
Genitourinary (GU) cancers including bladder, prostate, and kidney cancers affect older adults with a higher prevalence compared to younger adults. GU cancer treatment is associated with poorer outcomes in older adults compared to their younger counterparts. To better identify and support older adults receiving cancer care, oncologic societies recommend the use of a geriatric assessment (GA) to guide management. However, little is known about the implementation and usefulness of the GA in older adults with GU cancers. We performed a narrative review to investigate the utility of the GA in older adults with GU cancers and propose strategies to optimize the real-world use of the GA. Here, we describe a framework to incorporate GA into the routine cancer care of older adults with GU cancers and provide several implications for future research.
1 Introduction
Many genitourinary (GU) cancers occur primarily in older adults with a median age at diagnosis of 72 years for bladder cancer (1), 66 years for prostate cancer (1), and 64 years for kidney cancer (1). Older adults with GU cancers undergoing cancer therapy have overall poorer outcomes including greater impairment in health-related quality of life (2), higher rates of chemotherapy toxicity (3), and lower cancer-specific and overall survival (4–6) than their younger counterparts. However, older adults are a heterogeneous population and these outcomes in older adults may be driven by their pretreatment functional status and comorbidities, instead of chronological age alone (7, 8). Given the growing need to better identify and support older adults receiving cancer care, the American Society of Clinical Oncology (ASCO), the National Comprehensive Care Network (NCCN), and the International Society of Geriatric Oncology (SIOG) recommend the use of a geriatric assessment (GA) to guide management of older adults (9–11). In this review, we first summarize the relevant literature regarding the use of the GA in older adults with cancer and then review relevant literature for GA in GU cancers and propose settings in which the GA may best support clinical decision-making.
2 Methods
We conducted a literature search of PubMed for articles published in English up to November 21, 2022. There were three major components of the keyword and subject heading search linked with the AND operator: GU cancer terms, including bladder, prostate, and kidney cancer; older adult terms, including frailty and elder; geriatric assessment terms, including instrument, tool, geriatric domains, and implementation. We reviewed the titles and abstracts to identify research articles that described the implementation of geriatric assessment in older adults with GU cancers. We also conducted a separate manual literature review, including conference proceedings, to ensure appropriate capture of relevant studies.
3 Geriatric assessment for adults with cancer
The GA is a multidimensional holistic assessment of older adults’ medical, psychosocial, and physical functioning (12). Some form of GA can be used as a tool to assess patient fitness for surgery or radiation and to guide chemotherapy management. The gold standard is for a trained team member to perform a GA of multiple geriatric domains before chemotherapy initiation for adults age ≥ 65 years (Figure 1) (11). The results of this GA can inform a personalized care plan, which could include a myriad of interventions such as supportive care referrals, pre-habilitation for surgery, patient education, or treatment modification (9). The (Table 1) summarizes exemplar common instruments used to evaluate the GA domains in older adults with cancer. Geriatric domains commonly evaluated include risk for chemotherapy toxicity, cognition, comorbidity, nutrition, physical function, polypharmacy, and psychosocial status.
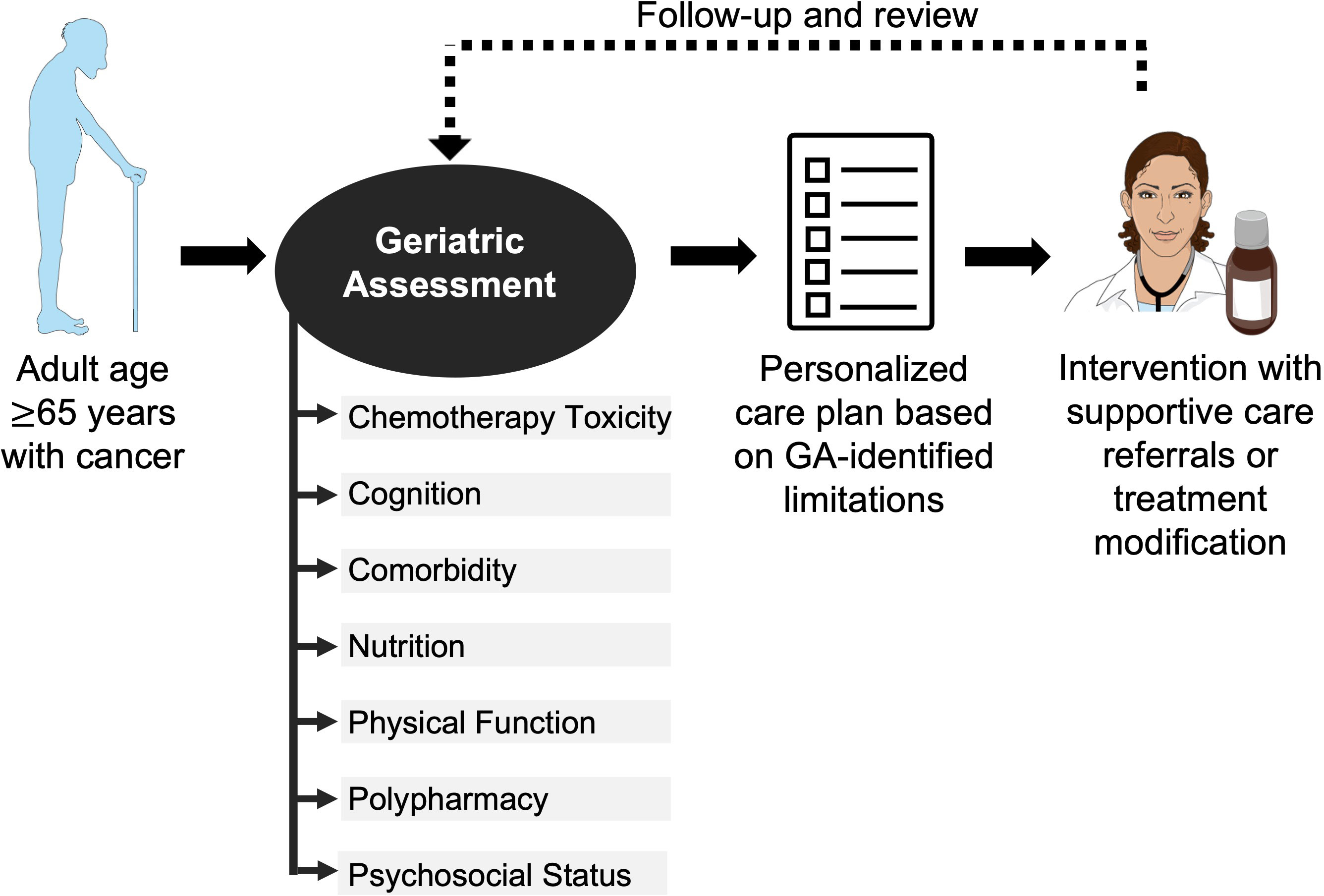
Figure 1 Use of geriatric assessment in management of older adult with cancer. The American Society of Clinical Oncology recommends all adults with cancer ≥65 years starting chemotherapy undergo a geriatric assessment of multiple domains to guide management. The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
The use of the GA to guide management of older adults with cancer has been shown to increase likelihood of cancer treatment completion (40), reduce hospitalizations and emergency room visits (when paired with geriatrician co-management) (41), and improve patient-centered communication and enhance satisfaction for older adults and their caregivers (42). The pivotal clinical trial, GAP70+, demonstrated lower treatment toxicity with GA. This randomized clinical trial enrolled 718 adults ≥70 years of age with incurable solid tumors or lymphoma (15% with GU cancers) across 40 community oncology practices (43). The practices were randomized 1:1 to the intervention group where oncologists received a tailored GA summary with specific recommendations including patient education, medication review, supportive care referrals, and/or chemotherapy modification or the usual care group where no GA summary or recommendations were provided to oncologists. Fewer patients in the intervention group experienced grade 3-5 toxic effects compared with the usual care group (51% versus 71%, p<0.001), with no effect on overall survival between the groups at 6 and 12 months (43).
4 Bladder cancer
4.1 Bladder cancer in the older adult
At least 75% of bladder cancer cases are diagnosed in adults age ≥65 years (1). Age is not only a risk factor for development of bladder cancer, but also is a risk factor for increased mortality with bladder cancer (44).
The treatment approach for non-muscle invasive bladder cancers (NMIBC) is based on tumor risk stratification, with standard of care curative management ranging from single instillation of intravesical chemotherapy for low-risk tumors to 1-3 years of bacillus Calmette-Guerin intravesical instillation or consideration of radical cystectomy (RC) for high-risk tumors (45). For patients with nonmetastatic muscle invasive bladder cancer (MIBC), the standard of care curative treatment is RC preceded by neoadjuvant cisplatin-based chemotherapy for those who are cisplatin-eligible (45). However, RC is associated with high morbidity and mortality, with a 90-day complication rate up to 59% and 90-day mortality rate up to 5% (46). Organ-preservation using definitive radiation with chemotherapy is an alternative option for curative management generally reserved for patients who prefer an alternative to RC or those considered unfit for surgery (45, 47).
Metastatic bladder cancer is treated with non-curative intent with sequential systemic therapies with the goal to help patients extend and/or improve their quality of life. Standard of care first-line therapy includes platinum-based chemotherapy regimens with decision stratification based on cisplatin eligibility (45). Subsequent therapy options include immune checkpoint inhibitors targeting programmed cell death 1 (PD-1) or its ligand (PD-L1) given either as switch-maintenance therapy for those without progression of disease on platinum-based chemotherapy or as a salvage agent at the time of progression; followed by antibody-drug conjugates targeting nectin-4 (enfortumab vedotin) and trop 2 (sacituzumab govitecan). For patients with FGFR2 or FGFR3 alterations, erdafitinib, an oral tyrosine kinase inhibitor (TKI) can also be utilized as a salvage post-platinum therapy (45, 48).
4.2 Geriatric assessment in bladder cancer
Limited studies describe the implementation or usefulness of a GA in older adults with bladder cancer. One group piloted implementation of a GA in their Bladder Cancer Multidisciplinary Clinic with 94 patients and found high GA component completion rates (79-100%) with low rates of perceived burden by patients (49). The authors plan to evaluate associations between individual GA instruments, treatment decisions, clinical outcomes, and quality of life as their future work. A separate group implemented a GA in older adults ≥65 years of age with distal urinary tract cancer (25%) and prostate cancer (75%) undergoing perioperative management and found that patients with localized bladder cancer had higher pretreatment comorbidities and greater frequency of postoperative complications than patients with prostate cancer (50).
Previous studies identify risk factors for adverse outcomes after bladder cancer treatment, which we apply to create recommendations regarding GA in older adults with bladder cancer (Table 1). Older adults who underwent RC for MIBC had higher mortality risk if they had severe comorbidity as assessed by the Adult Comorbidity Evaluation 27 (ACE-27) (23) or sarcopenia as assessed by radiologist review of axial computerized tomography images (24, 34). While sarcopenia assessment can be time and labor intensive, nutritional status assessment via the Malnutrition Universal Screening Tool (MUST) (33) or Patient-Generated Subjective Global Assessment (PG-SGA) (36) could serve as a correlative marker for sarcopenia when evaluating older adults for RC (35).
Cisplatin is a foundational chemotherapy in bladder cancer, with prior studies demonstrating improved disease response rates and overall survival with cisplatin compared to carboplatin when treating metastatic urothelial carcinoma (51, 52). However, cisplatin is associated with increased renal toxicity in older adults (53, 54). It is therefore recommended to perform an assessment of risk for chemotherapy toxicity prior to treatment initiation for older adults. While this can be done using the Cancer and Aging Research Group Chemotherapy Toxicity Tool (CARG-TT) (3) or Chemotherapy Risk Assessment for High-Age Patients (CRASH) (14) tools, the Galsky criteria was developed as a consensus definition of cisplatin eligibility for use in patients with metastatic urothelial carcinoma and has been applied in clinical trials and general practice to determine whether a patient is unfit for cisplatin-based chemotherapy (15). Recent efforts defined similar consensus criteria for platinum-based chemotherapy in the current treatment era (Table 1) (16).
5 Prostate cancer
5.1 Prostate cancer in the older adult
Similar to bladder cancer, prostate cancer is a disease with much higher prevalence in older adults. The median age at prostate cancer diagnosis is 66 years, with 70% of deaths occurring in adults ≥75 years of age (1).
Expected patient survival and prostate cancer risk stratification guide treatment decisions for nonmetastatic prostate cancer, which can include active surveillance, focal ablation, radical prostatectomy (RP), external beam radiation therapy, or brachytherapy, with or without androgen deprivation therapy (ADT) (55). Like RC for bladder cancer, (RP) for prostate cancer is a highly morbid procedure with 52% of patients reporting urinary incontinence and 59% of patients reporting sexuality problems two months after surgery (56).
Metastatic prostate cancer is treated with non-curative intent, with the foundation of treatment being ADT. Men treated with ADT can have multiple deleterious effects including higher risk for metabolic syndrome and diabetes (57, 58), coronary artery disease (59), fractures (60), and cognitive dysfunction (61). In addition to ADT, further systemic treatment options include oral agents targeting testosterone axis (e.g. abiraterone, enzalutamide, etc.), cytotoxic chemotherapy (e.g. docetaxel, cabazitaxel), radioactive isotopes (Radium-223) or radionuclide therapy (Lu 177 vipivotide tetraxetan), PARP inhibitors (e.g. olaparib or rucaparib for patients with BRCA1, BRCA2, or ATM alterations) or immunotherapy (e.g. pembrolizumab for patients with MSI-high or TMB-high tumors) (55). Taxane chemotherapy in older men with prostate cancer is associated with risk of infections, thromboembolic events, and hospitalization (62).
5.2 Geriatric assessment in prostate cancer
Several studies describe the use of a GA to guide management in older adults with prostate cancer. A multicenter study implemented a GA encompassing five geriatric domains prior to initiation of docetaxel in 24 patients ≥70 years of age with metastatic castrate resistant prostate cancer (18). The study found that frail patients, defined as those who had impairment in either Instrumental Activities of Daily Living (IADL) (30), Cumulative Illness Rating Scale-Geriatric (CIRS-G) (29), Geriatric Depression Cale (GDS) (38), Mini Mental State Examination (MMSE) (22), or Lubben Social Network Scale (LSNS) (39) identified by the GA, were more likely to discontinue docetaxel chemotherapy early compared to non-frail patients (60% versus 13% early discontinuation rate, p=0.037). Two other studies prospectively administered a GA to older adults planning to undergo radiation therapy for nonmetastatic prostate cancer, which did not identify older adults at risk for significant acute radiation toxicity (63) or subsequent diminished quality of life (64).
Another team used the Geriatric 8 questionnaire (G8) (31) to screen older adults with prostate cancer for subsequent GA but did not report its utility to predict outcomes in the study population (65). In 2017, SIOG published updated guidelines for the management of older adults with prostate cancer and recommend use of the G8 to categorize older adults into one of four categories prior to selecting a specific treatment: “healthy or fit” (G8 >14), “frail” (G8 ≤ 14, but conditions are reversible based on GA), “disabled or with severe comorbidities” (nonreversible conditions based on GA), or “terminally ill” (32). Patients in the “healthy or fit” or “frail” categories were considered fit for standard prostate cancer treatments and SIOG recommended selection of treatment be based on shared-decision making. On the other hand, the authors recommended adults in the “disabled or with severe comorbidities” category have specific geriatric interventions as guided by the GA to improve fitness but did not provide explicit details on the type or frequency of intervention. As a screening tool, the G8 could be useful to quickly identify high versus low-risk older adults.
Previous studies identify risk factors for adverse outcomes after prostate cancer treatment, which we apply to create recommendations regarding GA in older adults with prostate cancer (Table 1). Older adults who underwent RP for nonmetastatic prostate cancer had higher postoperative complications, late urinary complications, surgery-related death, and mortality if they had high pretreatment comorbidity, defined as Charlson Comorbidity Index of ≥2 (26, 27). Docetaxel is frequently used when cytotoxic chemotherapy is indicated for metastatic prostate cancer treatment. The CARG-TT can predict chemotherapy toxicity and has been externally validated to predict ≥grade 3 toxicity in men ≥65 years of age undergoing chemotherapy or ADT for metastatic castrate-resistant prostate cancer (13). We therefore recommend assessing comorbidity for patients undergoing consideration for RP and CARG-TT for patients undergoing consideration for chemotherapy or ADT.
6 Kidney cancer
6.1 Kidney cancer in the older adult
The median age at kidney cancer diagnosis is 64 years, with older adults with metastatic kidney cancer having worse overall survival than their younger counterparts (1, 66).
The standard of care treatment approach for nonmetastatic kidney cancer is either partial (PN) or radical nephrectomy (RN) with consideration of treatment with the immune checkpoint inhibitor pembrolizumab for patients with intermediate to high-risk disease, including those with stage ≥2 tumor with nuclear grade 4 or sarcomatoid differentiation, stage ≥3 tumor, regional lymph node metastasis, or stage M1 with no evidence of disease (67). Ablative techniques are reserved for very small tumors or for patients who prefer an alternative to nephrectomy or are unfit for surgery (68, 69). RN is associated with high rates of incident chronic kidney disease in up to 69% in one retrospective study (70). Metastatic kidney cancer is treated with non-curative intent systemic therapy with immune checkpoint inhibitors, TKIs, or a combination, with treatment selection guided by the International Metastatic Renal Cell Carcinoma Database Consortium prognostic model and patient-specific characteristics (68, 71). Subsequent treatment lines include additional TKIs or mTOR inhibitors. The immune checkpoint inhibitor combination of ipilimumab targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and nivolumab targeting PD-1 is used in metastatic kidney cancer but has high rates of treatment-related toxicity with immune-related adverse events (72). On the other hand, the TKIs have high rates of bleeding and cardiovascular complications (73–75).
6.2 Geriatric assessment in kidney cancer
Studies describe the use of a GA in older adults with metastatic kidney cancer receiving TKIs. A retrospective single-institution study of 86 adults age ≥70 years treated with first line sunitinib or pazopanib for metastatic kidney cancer evaluated the ability of a pretreatment GA to predict subsequent treatment toxicity and outcomes (20). The authors used the GA to categorize patients into either a “fit”, “vulnerable” or “frail” category on the basis of five domains: IADLs, GDS, MMSE, CIRS-G, and polypharmacy. The pretreatment GA was able to differentiate patients at risk for grade 3 to 4 treatment-related toxicity, shorter progression free survival and shorter overall survival (20). However, a separate study using pretreatment GA in adults with a median age of 74 years starting sunitinib for metastatic kidney cancer found no correlation between pretreatment frailty as assessed by the GA and subsequent treatment toxicity or disease response (76). We did not identify any studies evaluating the role of GA to predict immunotherapy toxicity specifically for patients with metastatic kidney cancer. Further studies are needed in kidney cancer to explore the utility of the GA to determine future treatment toxicity.
Previous studies identify risk factors for adverse outcomes after kidney cancer treatment, which we apply to create recommendations regarding GA in older adults with kidney cancer (Table 1). Adults age ≥75 years with clinical T1 kidney tumors had worse overall survival if they had higher comorbidity based on the Charlson Comorbidity Index (28). A subsequent Medicare study of older adults with T1a kidney cancer who were nephrectomy candidates reported that compared to RN, treatment with PN was associated with greater overall survival, which was the most beneficial for patients with high pretreatment comorbidity (Charlson Comorbidity Index ≥1) on subgroup analysis (77). We therefore recommend comorbidity evaluation for patients with nonmetastatic kidney cancer who are considering either PN or RN.
7 Limitations of GA research in older adults with GU cancers
While it is encouraging to see some early studies showing implementation and utility of GA for patients with GU cancer, the studies identified have several limitations. Most studies were non-randomized, did not include control comparator arms, and had a limited sample size, which makes interpretation of patient quality of life and health outcomes with GA implementation challenging. Further, there remains limitations regarding generalizability due to substantial heterogeneity across several domains including GA instrument and delivery, patient demographics, and treatment type. Also, the GA was most frequently conducted at a single timepoint, which precluded capture of longitudinal geriatric outcomes during cancer treatment. After completion of the GA, few studies explicitly detailed the type of GA-informed supportive care intervention or treatment modification performed. This is likely because no evidence-based standard exists to guide interventions after GA, which could be an opportunity for future research. Although GAs have been successfully implemented and used to guide management of older adults with cancer, research of the GA in older adults with GU cancers remains preliminary and would benefit from further studies, including robust clinical trials.
8 Recommendations for GA use in the clinical setting
GAs have not been widely adopted into routine clinical practice with common barriers including lack of time, lack of training and knowledge, and the GA being too cumbersome (78, 79). In light of these challenges, pending prospective validation to verify feasibility and utility in the real-world setting, we propose the following recommendations:
● Use screening instruments, such as the 7-item G8 (31), to identify which older adults would most benefit from a GA.
● Evaluate comorbid conditions and nutritional status for older adults considering surgery.
● Assess risk for chemotherapy toxicity, physical function, polypharmacy, and psychosocial status for high-risk older adults considering chemotherapy.
● Screen for cognitive decline in older adults considering ADT or chemotherapy.
● The results of this focused GA can be applied to inform fitness for surgery and systemic therapy, guide referrals to supportive care services, and allow for patient-centered shared-decision making.
9 Conclusions
Geriatric assessment is a valuable clinical tool with the potential to better personalize care delivery for patients with cancer. Patients with GU cancers may be a particularly suitable population for GA implementation, given the high prevalence of older adults and the variable treatment considerations in both the localized and metastatic setting based on patient fitness and treatment tolerance. Through this narrative review, we describe the usefulness of the GA in older adults with GU cancers and propose strategies to optimize the real-world use of the GA. As we identify in this review, the literature regarding GA implementation often comes from studies including patients with multiple cancer types, which may not capture the nuance of treatment decisions in patients with specific cancers. Furthermore, there remains a paucity of evidence of the use of GA for specific treatment decisions outside of systemic chemotherapy. Ideally the GA tools would be tested in clinical trials in settings where they may inform specific interventions (e.g. surgery versus radiation for bladder or prostate cancer). Future studies should evaluate GA in specific tumor types and clinical settings with the ultimate goal to improve care delivery for older adults with GU cancers.
Author contributions
All authors contributed to this study. All authors read and approved the final manuscript. Conceptualization: SS and AK. Literature review: SS, JM, and AK. Preparation of manuscript: SS, JM, and AK. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Stanford Cancer Center Clinical Innovation Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Stanford Cancer Center.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AK has research collaborations with Tempus labs and Natera; uncompensated consulting with Janssen and uncompensated advisory board participation with Seagen/Astellas, all unrelated to this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Kurian CJ, Leader AE, Thong MSY, Keith SW, Zeigler-Johnson CM. Examining relationships between age at diagnosis and health-related quality of life outcomes in prostate cancer survivors. BMC Public Health (2018) 18(1):1–9. doi: 10.1186/S12889-018-5976-6/TABLES/3
3. Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol (2011) 29(25):3457–65. doi: 10.1200/JCO.2011.34.7625
4. Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol (2011) 29(2):235–41. doi: 10.1200/JCO.2010.30.2075
5. Gómez Pérez L, Budía Alba A, Delgado Oliva FJ, Ruiz Cerdá JL, Bonillo García MA, Jiménez Cruz JF. Renal cancer in elderly: clinical and histopathological findings. Actas Urol. Esp. (2006) 30(2):139–44. doi: 10.1016/s0210-4806(06)73415-5
6. Fonteyne V, Ost P, Bellmunt J, Droz JP, Mongiat-Artus P, Inman B, et al. Curative treatment for muscle invasive bladder cancer in elderly patients: A systematic review. Eur Urol. (2018) 73(1):40–50. doi: 10.1016/j.eururo.2017.03.019
7. Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A. Functional versus chronological age: Geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol (2018) 19(6):e305–16. doi: 10.1016/S1470-2045(18)30348-6
8. Swaminathan D, Swaminathan V. Geriatric oncology: Problems with under-treatment within this population. Cancer Biol Med (2015) 12(4):275–83. doi: 10.7497/j.issn.2095-3941.2015.0081
9. Mohile SG, Dale W, Somerfield MR, Schonberg MA, Boyd CM, Burhenn PS, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol (2018) 36(22):2326–47. doi: 10.1200/JCO.2018.78.8687
10. Dotan E, Walter LC, Browner IS, Clifton K, Cohen HJ, Extermann M, et al. NCCN guidelines® insights: Older adult oncology, version 1.2021. J Natl Compr Canc. Netw (2021) 19(9):1006–19. doi: 10.6004/jnccn.2021.0043
11. Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen MLG, Extermann M, et al. International society of geriatric oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol (2014) 32(24):2595–603. doi: 10.1200/JCO.2013.54.8347
12. Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: A meta-analysis of controlled trials. Lancet (1993) 342(8878):1032–6. doi: 10.1016/0140-6736(93)92884-v
13. Alibhai SMH, Breunis H, Hansen AR, Gregg R, Warde P, Timilshina N, et al. Examining the ability of the cancer and aging research group tool to predict toxicity in older men receiving chemotherapy or androgen-receptor-targeted therapy for metastatic castration-resistant prostate cancer. Cancer (2021) 127(14):2587–94. doi: 10.1002/cncr.33523
14. Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, et al. Predicting the risk of chemotherapy toxicity in older patients: The chemotherapy risk assessment scale for high-age patients (CRASH) score. Cancer (2012) 118(13):3377–86. doi: 10.1002/cncr.26646
15. Galsky MD, Hahn NM, Rosenberg J, Sonpavde G, Hutson T, Oh WK, et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J Clin Oncol (2011) 29(17):2432–8. doi: 10.1200/JCO.2011.34.8433
16. Gupta S, Bellmunt J, Plimack ER, Sonpavde GP, Grivas P, Apolo AB, et al. Defining “platinum-ineligible” patients with metastatic urothelial cancer (mUC). J Clin Oncol (2022) 40(16_suppl):4577–7. doi: 10.1200/JCO.2022.40.16_suppl.4577
17. Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: A cognitive “vital signs” measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry (2000) 15(11):1021–7. doi: 10.1002/1099-1166(200011)15:11<1021::aid-gps234>3.0.co;2-6
18. della Pepa C, Cavaliere C, Rossetti S, di Napoli M, Cecere SC, Crispo A, et al. Predictive comprehensive geriatric assessment in elderly prostate cancer patients: The prospective observational scoop trial results. Anticancer Drugs (2017) 28(1):104–9. doi: 10.1097/CAD.0000000000000428
19. de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl J Med (2020) 382(22):2091–102. doi: 10.1056/NEJMoa1911440
20. Pierantoni F, Basso U, Maruzzo M, Lamberti E, Bimbatti D, Tierno G, et al. Comprehensive geriatric assessment is an independent prognostic factor in older patients with metastatic renal cell cancer treated with first-line sunitinib or pazopanib: A single center experience. J Geriatr Oncol (2021) 12(2):290–7. doi: 10.1016/j.jgo.2020.09.009
21. Tsoi KKF, Chan JYC, Hirai HW, Wong SYS, Kwok TCY. Cognitive tests to detect dementia: A systematic review and meta-analysis. JAMA Intern Med (2015) 175(9):1450–8. doi: 10.1001/jamainternmed.2015.2152
22. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res (1975) 12(3):189–98. doi: 10.1016/0022-3956(75)90026-6
23. Piccirillo JF. Prognostic Importance of Comorbidity in a Hospital-Based Cancer Registry. JAMA (2004) 291(20):2441. doi: 10.1001/jama.291.20.2441
24. Fairey A, Chetner M, Metcalfe J, Moore R, Todd G, Rourke K, et al. Associations among age, comorbidity and clinical outcomes after radical cystectomy: Results from the Alberta urology institute radical cystectomy database. J Urol. (2008) 180(1):128–34. doi: 10.1016/j.juro.2008.03.057
25. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis (1987) 40(5):373–83. doi: 10.1016/0021-9681(87)90171-8
26. Tewari A, Johnson CC, Divine G, Crawford ED, Gamito EJ, Demers R, et al. Long-term survival probability in men with clinically localized prostate cancer: A case-control, propensity modeling study stratified by race, age, treatment and comorbidities. J Urol. (2004) 171(4):1513–9. doi: 10.1097/01.ju.0000117975.40782.95
27. Begg CB, Riedel ER, Bach PB, Kattan MW, Schrag D, Warren JL, et al. Variations in morbidity after radical prostatectomy. N. Engl J Med (2002) 346(15):1138–44. doi: 10.1056/NEJMsa011788
28. Lane BR, Abouassaly R, Gao T, Weight CJ, Hernandez AV, Larson BT, et al. Active treatment of localized renal tumors may not impact overall survival in patients aged 75 years or older. Cancer (2010) 116(13):3119–26. doi: 10.1002/cncr.25184
29. Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, et al. Rating chronic medical illness burden in geropsychiatric practice and research: Application of the cumulative illness rating scale. Psychiatry Res (1992) 41(3):237–48. doi: 10.1016/0165-1781(92)90005-n
30. Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol. (1981) 36(4):428–34. doi: 10.1093/geronj/36.4.428
31. Bellera CA, Rainfray M, Mathoulin-Pélissier S, Mertens C, Delva F, Fonck M, et al. Screening older cancer patients: First evaluation of the G-8 geriatric screening tool. Ann Oncol (2012) 23(8):2166–72. doi: 10.1093/annonc/mdr587
32. Droz JP, Albrand G, Gillessen S, Hughes S, Mottet N, Oudard S, et al. Management of prostate cancer in elderly patients: Recommendations of a task force of the international society of geriatric oncology. Eur Urol. (2017) 72(4):521–31. doi: 10.1016/J.EURURO.2016.12.025
33. Elia M. Screening for malnutrition: A multidisciplinary responsibility. development and use of the malnutrition universal screening tool (‘MUST’) for adults (2003). Available at: https://www.bapen.org.uk/pdfs/must/must-report.pdf (Accessed November 20, 2022).
34. Psutka SP, Carrasco A, Schmit GD, Moynagh MR, Boorjian SA, Frank I, et al. Sarcopenia in patients with bladder cancer undergoing radical cystectomy: Impact on cancer-specific and all-cause mortality. Cancer (2014) 120(18):2910–8. doi: 10.1002/cncr.28798
35. Bellanti F, lo Buglio A, Quiete S, Pellegrino G, Dobrakowski M, Kasperczyk A, et al. Comparison of three nutritional screening tools with the new glim criteria for malnutrition and association with sarcopenia in hospitalized older patients. J Clin Med (2020) 9(6):1898. doi: 10.3390/jcm9061898
36. Bauer J, Capra S, Ferguson M. Use of the scored patient-generated subjective global assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr (2002) 56(8):779–85. doi: 10.1038/sj.ejcn.1601412
37. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? a systematic review of definitions. BMC Geriatr. (2017) 17(1):230. doi: 10.1186/s12877-017-0621-2
38. Sheikh JI, Yesavage JA. Geriatric depression scale (GDS): Recent evidence and development of a shorter version. Clin Gerontol. (1986) 5(1-2):165–73. doi: 10.1300/J018v05n01_09
39. Lubben JE. Assessing social networks among elderly populations. Fam. Community Health (1988) 11(3):42–52. doi: 10.1097/00003727-198811000-00008
40. Kalsi T, Babic-Illman G, Ross PJ, Maisey NR, Hughes S, Fields P, et al. The impact of comprehensive geriatric assessment interventions on tolerance to chemotherapy in older people. Br J Cancer. (2015) 112(9):1435–44. doi: 10.1038/bjc.2015.120
41. Soo WK, King M, Pope A, Parente P, Darzins P, Davis ID. Integrated geriatric assessment and treatment (INTEGERATE) in older people with cancer planned for systemic anticancer therapy. J Clin Oncol (2020) 38(15_suppl):12011–1. doi: 10.1200/JCO.2020.38.15_SUPPL.12011
42. Mohile SG, Epstein RM, Hurria A, Heckler CE, Canin B, Culakova E, et al. Communication with older patients with cancer using geriatric assessment: A cluster-randomized clinical trial from the national cancer institute community oncology research program. JAMA Oncol (2020) 6(2):196–204. doi: 10.1001/jamaoncol.2019.4728
43. Mohile SG, Mohamed MR, Xu H, Culakova E, Loh KP, Magnuson A, et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): A cluster-randomised study. Lancet (2021) 6736(21):1–11. doi: 10.1016/s0140-6736(21)01789-x
44. Abdollah F, Gandaglia G, Thuret R, Schmitges J, Tian Z, Jeldres C, et al. Incidence, survival and mortality rates of stage-specific bladder cancer in united states: A trend analysis. Cancer Epidemiol. (2013) 37(3):219–25. doi: 10.1016/j.canep.2013.02.002
45. Powles T, Bellmunt J, Comperat E, de Santis M, Huddart R, Loriot Y, et al. Bladder cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol (2022) 33(3):244–58. doi: 10.1016/j.annonc.2021.11.012
46. Maibom SL, Joensen UN, Poulsen AM, Kehlet H, Brasso K, Røder MA. Short-term morbidity and mortality following radical cystectomy: A systematic review. BMJ Open (2021) 11(4):e043266. doi: 10.1136/bmjopen-2020-043266
47. Mak RH, Hunt D, Shipley WU, Efstathiou JA, Tester WJ, Hagan MP, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: A pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol (2014) 32(34):3801–9. doi: 10.1200/JCO.2014.57.5548
48. Tagawa ST, Balar AV, Petrylak DP, Kalebasty AR, Loriot Y, Fléchon A, et al. TROPHY-U-01: A phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol (2021) 39(22):2474–85. doi: 10.1200/JCO.20.03489
49. Psutka SP, Gore JL, Holt SK, Dwyer E, Schade G, Grivas P, et al. Prospective evaluation of a comprehensive geriatric assessment (CGA) in multidisciplinary bladder cancer care: Feasibility and impact on decisional conflict. (2022) 40(6_suppl):479–9. doi: 10.1200/JCO.2022.40.6_SUPPL.479
50. Zangl Q, Wirth J, Karl A, Stief C, Zwissler B, von Dossow V. Value of geriatric assessment in patients with genitourinary carcinoma. Oncology (2021) 35(9):620–7. doi: 10.46883/ONC.2021.3510.0620
51. Galsky MD, Chen GJ, Oh WK, Bellmunt J, Roth BJ, Petrioli R, et al. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Ann Oncol (2012) 23(2):406–10. doi: 10.1093/annonc/mdr156
52. Huang SY, Wu CC, Hsieh MC, Rau KM, Chiang PH, Sung MT, et al. Comparative study of the safety and efficacy of first-line cisplatin and carboplatin chemotherapy in elderly patients with metastatic urothelial carcinoma. Oncology (2020) 98(3):146–53. doi: 10.1159/000504393
53. Duan Z, Cai G, Li J, Chen X. Cisplatin-induced renal toxicity in elderly people. Ther Adv Med Oncol (2020) 12:1758835920923430. doi: 10.1177/1758835920923430
54. Duan ZY, Liu JQ, Yin P, Li JJ, Cai GY, Chen XM. Impact of aging on the risk of platinum-related renal toxicity: A systematic review and meta-analysis. Cancer Treat Rev (2018) 69:243–53. doi: 10.1016/j.ctrv.2018.07.002
55. Schaeffer E, Srinivas S. NCCN guidelines version 4.2022 prostate cancer. J NCCN (2022) 20(12):1288–98. doi: 10.6004/jnccn.2022.0063
56. Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N. Engl J Med (2008) 358(12):1250–61. doi: 10.1056/NEJMoa074311
57. Kintzel PE, Chase SL, Schultz LM, O’Rourke TJ. Increased risk of metabolic syndrome, diabetes mellitus, and cardiovascular disease in men receiving androgen deprivation therapy for prostate cancer. Pharmacotherapy (2008) 28(12):1511–22. doi: 10.1592/phco.28.12.1511
58. Basaria S, Muller DC, Carducci MA, Egan J, Dobs AS. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer (2006) 106(3):581–8. doi: 10.1002/cncr.21642
59. Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: A meta-analysis. Am J Med (2006) 119(10):812–9. doi: 10.1016/j.amjmed.2006.02.031
60. Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N. Engl J Med (2005) 352(2):154–64. doi: 10.1056/NEJMoa041943
61. Jayadevappa R, Chhatre S, Malkowicz SB, Parikh RB, Guzzo T, Wein AJ. Association between androgen deprivation therapy use and diagnosis of dementia in men with prostate cancer. JAMA Netw Open (2019) 2(7):e196562. doi: 10.1001/jamanetworkopen.2019.6562
62. Kongsted P, Svane IM, Lindberg H, Sengeløv L. Predictors of chemotherapy-induced toxicity and treatment outcomes in elderly versus younger patients with metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. (2016) 14(6):e559–68. doi: 10.1016/j.clgc.2016.03.018
63. Osborne GEC, Appleyard SA, Gilbert DC, Jones CI, Lorimer C, Villanueva M, et al. Comprehensive geriatric assessment in men aged 70 years or older with localised prostate cancer undergoing radical radiotherapy. Clin Oncol (R. Coll Radiol) (2017) 29(9):609–16. doi: 10.1016/j.clon.2017.05.003
64. Goineau A, Campion L, d’Aillières B, Vié B, Ghesquière A, Béra G, et al. Comprehensive geriatric assessment and quality of life after localized prostate cancer radiotherapy in elderly patients. PloS One (2018) 13(4):e0194173. doi: 10.1371/JOURNAL.PONE.0194173
65. Méry B, Vallard A, Espenel S, Badie N, Thiermant M, Lambert V, et al. Prostate cancer of elderly patients: Place and role of geriatric assessment. Prog Urol. (2016) 26(9):524–31. doi: 10.1016/j.purol.2016.07.002
66. Rao A, Wiggins C, Lauer RC. Survival outcomes for advanced kidney cancer patients in the era of targeted therapies. Ann Transl Med (2018) 6(9):8–8. doi: 10.21037/ATM.2018.04.44
67. Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Chang Y, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. New Engl J Med (2021) 385(8):683–94. doi: 10.1056/NEJMoa2106391
68. Motzer RJ, Jonasch E, Agarwal N, Alva A, Baine M, Beckermann K, et al. NCCN guidelines version 1.2023 kidney cancer. J NCCN (2022) 20(1):67–81. doi: 10.6004/jnccn.2022.0001
69. Quivy A, Daste A, Harbaoui A, Duc S, Bernhard JC, Gross-Goupil M, et al. Optimal management of renal cell carcinoma in the elderly: A review. Clin Interv. Aging. (2013) 8:433–42. doi: 10.2147/CIA.S30765
70. Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj G, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: A retrospective cohort study. Lancet Oncol (2006) 7(9):735–40. doi: 10.1016/S1470-2045(06)70803-8
71. Heng DYC, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, et al. External validation and comparison with other models of the international metastatic renal-cell carcinoma database consortium prognostic model: A population-based study. Lancet Oncol (2013) 14(2):141–8. doi: 10.1016/S1470-2045(12)70559-4
72. Xing P, Zhang F, Wang G, Xu Y, Li C, Wang S, et al. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: a systematic review and meta-analysis. J Immunother Cancer. (2019) 7(1):341. doi: 10.1186/s40425-019-0779-6
73. Je Y, Schutz FAB, Choueiri TK. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: A systematic review and meta-analysis of clinical trials. Lancet Oncol (2009) 10(10):967–74. doi: 10.1016/S1470-2045(09)70222-0
74. Hall PS, Harshman LC, Srinivas S, Witteles RM. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail (2013) 1(1):72–8. doi: 10.1016/j.jchf.2012.09.001
75. Ghatalia P, Morgan CJ, Je Y, Nguyen PL, Trinh QD, Choueiri TK, et al. Congestive heart failure with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol Hematol (2015) 94(2):228–37. doi: 10.1016/j.critrevonc.2014.12.008
76. Brunello A, Basso U, Sacco C, Sava T, de Vivo R, Camerini A, et al. Safety and activity of sunitinib in elderly patients (≥70 years) with metastatic renal cell carcinoma: A multicenter study. Ann Oncol (2013) 24(2):336–42. doi: 10.1093/ANNONC/MDS431
77. Tan HJ, Norton EC, Ye Z, Hafez KS, Gore JL, Miller DC. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA (2012) 307(15):1629–35. doi: 10.1001/jama.2012.475
78. Dale W, Williams GRR, MacKenzie A, Soto-Perez-de-Celis E, Maggiore RJ, Merrill JK, et al. How is geriatric assessment used in clinical practice for older adults with cancer? a survey of cancer providers by the American society of clinical oncology. JCO Oncol Pract (2021) 17(6):336–44. doi: 10.1200/op.20.00442
Keywords: geriatric assessment, older adult, bladder cancer, kidney cancer, prostate cancer
Citation: Singhal S, Marwell JG and Khaki AR (2023) Geriatric assessment in the older adult with genitourinary cancer: A narrative review. Front. Oncol. 13:1124309. doi: 10.3389/fonc.2023.1124309
Received: 15 December 2022; Accepted: 17 January 2023;
Published: 02 February 2023.
Edited by:
Vadim S. Koshkin, University of California San Francisco, United StatesReviewed by:
Philipp Ivanyi, Hannover Medical School, GermanyCopyright © 2023 Singhal, Marwell and Khaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Surbhi Singhal, c2luZzA0NDFAZ21haWwuY29t
 Surbhi Singhal
Surbhi Singhal Julianna G. Marwell
Julianna G. Marwell Ali Raza Khaki
Ali Raza Khaki