
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 14 July 2023
Sec. Genitourinary Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1115901
This article is part of the Research TopicCase Reports in Genitourinary Oncology : 2022View all 38 articles
Introduction: Neoadjuvant nivolumab and cabozantinib in locally advanced renal cell carcinoma in a horseshoe kidney is a novel therapeutic approach in the preoperative setting.
Methods: We report a case of a 52-year old male who presented with a large inoperable tumor of the horseshoe kidney and achieved major partial radiologic response after neoadjuvant therapy with nivolumab and cabozantinib leading to radical resection of the tumor. The patient remains tumor free on the subsequent follow-up and his renal function is only mildly decreased. The systemic treatment was complicated by hepatotoxicity leading to early nivolumab withdrawal.
Results: Currently, the combination therapy based on immune checkpoint inhibitors and tyrosine kinase inhibitors represents the treatment of choice in treatment-naïve patients with metastatic renal cell carcinoma in any prognostic group. The neoadjuvant treatment approach is being tested in prospective clinical trials and results are eagerly awaited. Renal cell carcinoma in a horseshoe kidney is an uncommon finding that is always challenging. Additionally, management guidance in this patient population is lacking. In some patients neoadjuvant therapy could be the only way to preserve kidney function. The initial treatment strategy should be individualized to patient needs aiming at the radical resection of the primary tumor as the only chance of getting the tumor under control in the long term.
Conclusion: Herein, we highlight the feasibility of neoadjuvant systemic therapy with nivolumab and cabozantinib allowing the subsequent performance of radical tumor resection with negative margins in a patient with advanced renal cell carcinoma in a horseshoe kidney, removing the primary tumor while sparing the patient from lifelong dialysis.
In recent years, the use of immune checkpoint inhibitors (ICIs) has revolutionized the therapeutic landscape of metastatic renal cell carcinoma (mRCC) (1–5). Undoubtedly, immunotherapy denotes a great opportunity to control the disease in patients from a long-term perspective. The widespread use of ICIs across various tumor types represents an unprecedented advancement in the therapy leading to cancer cell elimination. However, less advance has been made in terms of identification of patients who would derive benefit from immune targeted approach.
The current first line strategy in mRCC comprises mainly a combination of an ICI plus tyrosine-kinase inhibitor (TKI) based on prior demonstration of the benefit of these combinations. One such example is the combination of an anti-PD1 antibody nivolumab with TKI cabozantinib which has shown superior efficacy compared with sunitinib monotherapy in the first-line setting in patients with mRCC regardless of their prognosis according to the International Metastatic Database Consortium (IMDC) Risk Score for RCC, introducing a shift in the common therapeutical practice of the first-line treatment (3). Moreover, combination immunotherapy with a PD-1 inhibitor and TKIs have shown high complete and overall response rates in the metastatic setting. Nevertheless, the efficacy of the combination in the preoperative setting needs to be evaluated (6).
Localized renal cell carcinoma (RCC) can be inoperable for several reasons including solitary kidney or locally advanced disease. Data on optimal therapeutic strategy in this patient population are scarce. The potential of the neoadjuvant treatment approach in this patient population lies in downsizing the primary tumor, enabling radical tumor resection (7).
We describe a case of a patient with locally advanced inoperable RCC of a horseshoe kidney treated with neoadjuvant nivolumab and cabozantinib leading to radical resection of the primary tumor 7 months later.
A 52-year-old Caucasian male, non-smoker, with an Eastern Cooperative Oncology Group (ECOG) status 0 with no serious comorbidities presented in October 2021 with anemia and hematuria. The abdominal CT scan revealed a horseshoe kidney, a bulky tumor at the junction of the lower kidney poles affecting a large part of the right kidney. The dimensions of the tumor were 150x120x100 mm (Figures 1A, B). The tumor was located close to large abdominal vessels. The chest CT and bone scan showed no evidence of metastatic disease. Upon review of imaging during the multidisciplinary genitourinary board, it was concluded that the tumor was unresectable due to its size and proximity to the renal vasculature. Moreover, the risk of irreversible kidney injury resulting in the need of lifelong dialysis was extremely high. Ultrasound-navigated biopsy of the tumor was performed. Histology subsequently revealed grade 1 clear cell renal cell carcinoma (ccRCC). After an in-depth discussion with the patient, nephrectomy was not indicated and the patient opted to proceed with off-label systemic treatment consisting of nivolumab and cabozantinib.
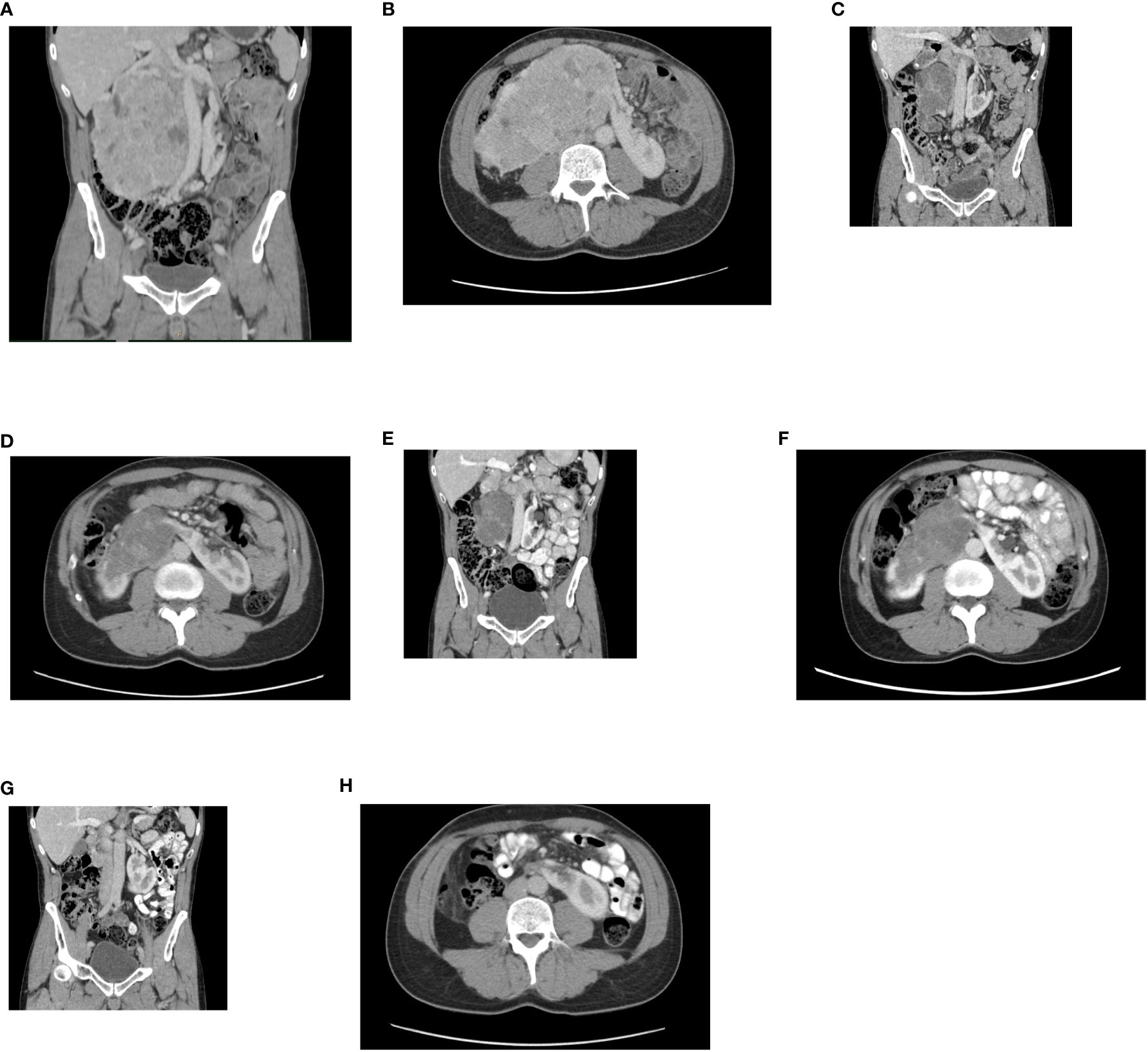
Figure 1 (A) Initial contrast-enhanced CT. A horseshoe kidney with a bulky tumor affecting a large part of the right kidney, a coronal plane. (B) An axial plane. (C) Follow-up contrast enhanced CT. Partial regression of the tumor after, 2 month from therapy initiation, a coronal plane. (D) An axial plane. (E) Follow-up contrast enhanced CT. Partial regression of the tumor, 5 months from therapy initiation, a coronal plane. (F) An axial plane. (G) Follow-up contrast enhanced CT. Complete radiographic regression of the tumor, a coronal plane. (H) An axial plane.
The combination therapy with nivolumab (240 mg intravenously every two weeks) and cabozantinib 40 mg orally daily was started in December 2021. In January 2022, 6 weeks after therapy initiation, the patient developed grade 3 hepatotoxicity according to Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 manifested by elevation of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) with bilirubin levels within normal limits. Other potential causes of hepatotoxicity including infections or obstruction were ruled out. The therapy was stopped immediately and corticosteroid administration with prednisolone 1mg/kg orally was initiated with close monitoring of liver enzymes and other related parameters. Within a week of therapy, slow lowering of corticosteroid dose was possible because of a decrease in AST and ALT levels to grade 1 suggesting immune-related toxicity. In February 2022, the restaging CT scan was performed showing tumor size reduction to 90x60x100 mm, i.e. partial regression by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 was achieved (Figures 1C, D). Since the surgical intervention was not feasible yet, it was decided to continue with systemic treatment for additional 3 months. However, nivolumab had to be permanently discontinued due to the history of grade 3 hepatotoxicity, and single agent cabozantinib was administered since March 2022. The standard cabozantinib dose of 60 mg daily had to be reduced to 40 mg daily because of hand-foot syndrome. In May 2022, the CT scan showed an additional decrease in the tumor size (90x55x95mm) with no evidence of distant metastases. The most marked effect of the therapy was noticed at the start of the treatment, with only mild reduction of the tumor in the last 3 months (Figures 1E, F). Taking this into consideration, the multidisciplinary tumor board team decided to proceed with radical tumor resection. In June 2022, after one-month washout of cabozantinib, the radical resection, including a right heminephrectomy of the horseshoe kidney was performed successfully without any postoperative complications. A significant decrease in estimated glomerular filtration (GF) (>25%) was noted, but the value of GF remained above 50 ml/min (chronic kidney disease stage 3a). Final histological analysis showed regressively changed (ccRCC) (Figures 2A–C), pathologic TNM classification was pT2a with negative margins evaluated as a major partial response.
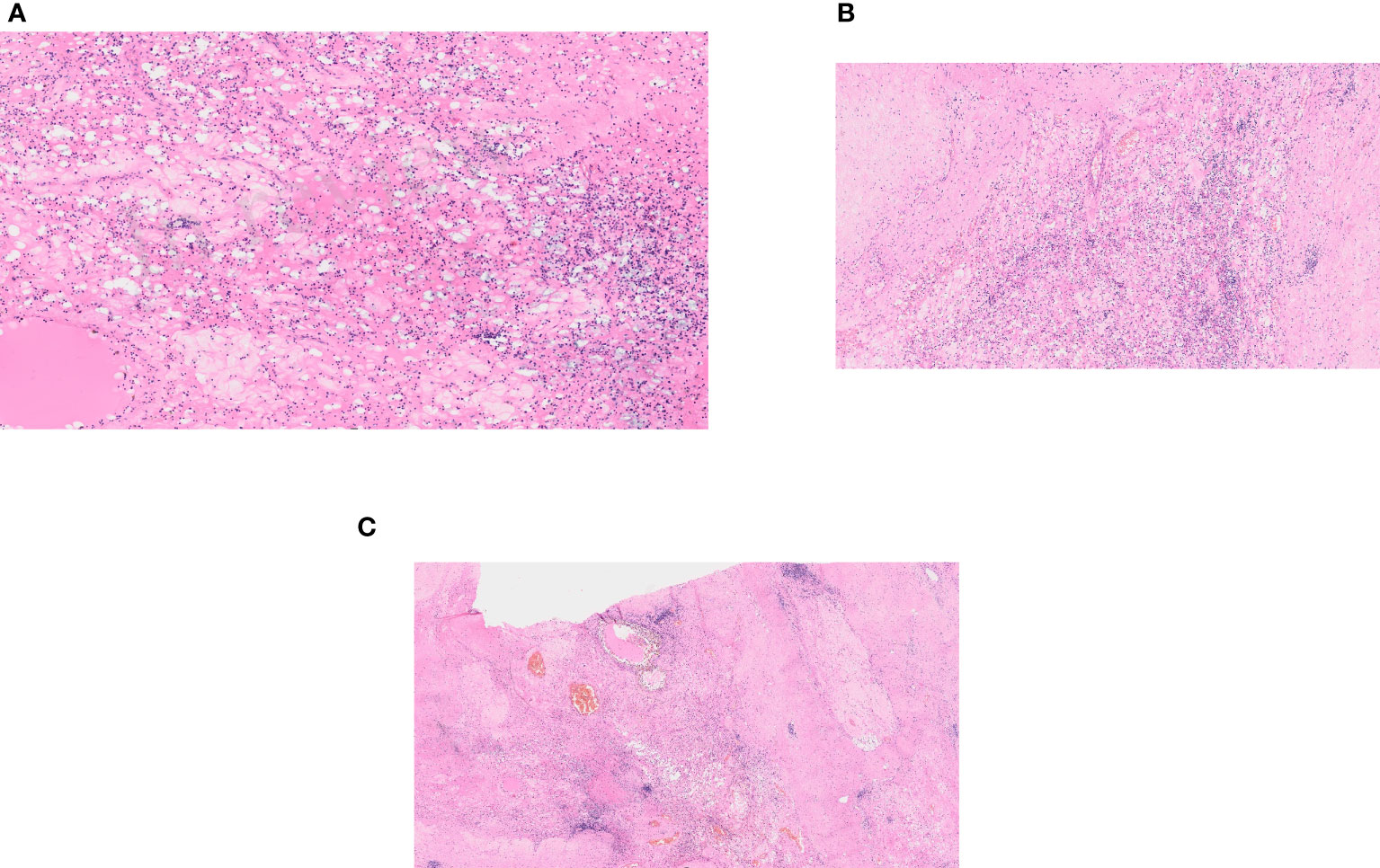
Figure 2 Histological examination. (A) A regressively changed clear cell renal cell carcinoma. (B) A regressively changed clear cell renal cell carcinoma in detail. (C) A regressively changed clear cell renal cell carcinoma, hyalinosis and stromal edema.
In September 2022, a follow up CT scan confirmed complete radiographic remission, and no further systemic therapy was offered after the operation (Figures 1G, H). The patient remains asymptomatic and disease-free 20 months after the initial diagnosis. All relevant data are depicted in a timeline (Figure 3).
We report here an excellent outcome of the combination of nivolumab plus cabozantinib given in the neoadjuvant setting in a patient with locally advanced inoperable RCC in a horseshoe kidney. The therapy led to significant tumor shrinkage and subsequent successful surgical resection of the tumor with durable remission. To the best of our knowledge, this is the first report of neoadjuvant nivolumab plus cabozantinib, enabling radical tumor resection due to therapy-induced downsizing of the primary tumor, reported in the literature.
Neoadjuvant therapy remains under investigation and no systemic therapy in localized or locally advanced RCC has been FDA-approved so far. The successful use of a preoperative treatment approach to facilitate surgical resection using multiple tyrosine kinase inhibitors (MTKIs) was reported only in anecdotal case reports (8, 9). In several phase 2 trials addressing the neoadjuvant strategy low complete response rate has been typically observed (10–12). Neoadjuvant cabozantinib in patients with unresectable locally advanced RCC facilitating nephrectomy has also been reported (13–15). Combination therapy of nivolumab and cabozantinib yielded the greatest reduction in the primary tumor size in a retrospective study (16).
Antitumor response promoted by ICIs via PD-1/PD-L1 inhibition potentially induces a long-term effect by eliminating of metastatic clones. Concerning immunotherapy in the preoperative setting, in 2021 Gorin et al. published data on high-risk nonmetastatic RCC patients given preoperative nivolumab (only 3 doses of nivolumab, 240 mg, administered intravenously) with only 1 patient out of 17 having pathological response with immune-related features in the removed kidney (17). The PROSPER RCC trial presented at ESMO 2022 did not show any improvement in recurrence free survival in patients with a high risk of recurrence in the arm with perioperative nivolumab (10 doses of nivolumab, 480 mg, 1 dose administered prior to surgery followed by 9 doses postoperatively) (18). Results from further clinical trials currently underway, including dual-agent immunotherapy, may elucidate the optimal therapeutic strategy in this patient population (19, 20). In Checkmate-9ER, the overall response rate in the whole population was 55.7%, however, efficacy data of locally advanced patients from the trial have not been published yet (3).
The therapy preference should always be carefully considered and discussed in patients with a solitary kidney or significantly impaired renal function where kidney function preservation is at risk. Regarding the large inoperable tumor mass in this case, we decided to initiate the combination strategy of nivolumab with cabozantinib to attempt downsizing the tumor. One of the critical moments of the whole process is finding a strategy that would preserve maximum of functional renal parenchyma, and failure to do this adversely affects the patient´s prognosis
The tumor size reduction was rapid at the beginning of the therapy when the combination of both drugs was given. The tumor diameter decreased by 30% after 2 months of cabozantinib therapy with nivolumab, while an additional 4% decrease was obtained during single-agent cabozantinib therapy. The pathologist described the tumor as 85x87x50 mm in size, but only a small portion of the tumor consisted of viable tumor cells. The discrepancy between the radiological and actual tumor size has been previously described as viable tumor cells might represent only a small proportion of the remaining mass (21).
Management guidance on tumors arising from a horseshoe kidney remains anecdotal owing to the rarity of this presentation. Only case reports or small case series provide clinical data on therapeutic management specific to this particular presentation. The variable anatomy of a horseshoe kidney, aberrant vasculature with accessory arteries and branches arising from arteries other than the aorta, and the complexity of the tumor renders surgery highly demanding (22). In order to optimize the final treatment outcome, preoperative planning should always precede.
Various histopathological subtypes of renal abnormalities have been described in association with renal tumors in a horseshoe kidney, hence a tumor biopsy preceding the operation is warranted.
In the current case, no complications associated with the surgery or wound healing disturbances were noted. Wound healing problems resulting from TKI therapy have been previously published (23), as were fibrotic changes induced by ICIs and discovered during the surgery (24). A combination of both approaches may become a challenge for the operating surgeon.
Not only efficacy but also safety is of concern when we manage a patient with localized disease considering a future surgical procedure. Identifying the optimal therapy at the very beginning is crucial as novel potent therapies emerge. The patient in the present case experienced grade 3 hepatotoxicity leading to ICI termination followed by single agent TKI. In the Checkmate-9ER trial 5.6% patients discontinued both agents and nearly 20% patients permanently stopped the combination therapy and continued with single agent therapy due to adverse events (3). Toxicity may develop later during the course of therapy such as the case of a RCC patient who developed hypophysitis 9 months after ICI therapy initiation that manifested during the postoperative course following cytoreductive nephrectomy (25). Adverse events induced by ICIs may be unpredictable and patients whenever received immunotherapy should be carefully followed for potential side effects.
The present report demonstrates the potential of neoadjuvant nivolumab and cabozantinib therapy to downsize an initially inoperable RCC leading to subsequent radical surgical resection. The neoadjuvant approach with nivolumab and cabozantinib may be a viable option for patients with initially inoperable RCC, including patients with a solitary kidney.
The original contributions presented in the case report are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the case report in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the individual for the publication of any potentiall identifiable images or data included in the article.
AZ: writing and revision of the manuscript, collected data. HS: conceptualization, supervision, writing and revision of the manuscript. AK: collected data, revision of the manuscript. TT: revision of the manuscript. VS: revision of the manuscript. BM: revision of the manuscript. All authors contributed to the article and approved the submitted version.
This manuscript was supported by the Czech Science Foundation (IGA LF 2022 003) project No. SPP 911104101/31.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Motzer R, Rini BI, McDermott DF, Frontera OA, Hammers HJ, Carducci MA, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol (2019) 20(10):1370–85. doi: 10.1016/S1470-2045(19)30413-9
2. Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. New Engl J Med (2019) 380(12):1103–15. doi: 10.1056/NEJMoa1816047
3. Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. New Engl J Med (2021) 384(9):829–41. doi: 10.1056/NEJMoa2026982
4. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. New Engl J Med (2019) 380(12):1116–27. doi: 10.1056/NEJMoa1816714
5. Motzer RJ, Porta C, Eto M, Powles T, Grunwald V, Hutson TE, et al. Phase 3 trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) or everolimus (EVE) versus sunitinib (SUN) monotherapy as a firstline treatment for patients (pts) with advanced renal cell carcinoma (RCC) (CLEAR study). J Clin Oncol (2021) 39(6). doi: 10.1200/JCO.2021.39.6_suppl.269
6. Bindayi A, Hamilton ZA, McDonald ML, Yim K, Millard F, McKay RR, et al. Neoadjuvant therapy for localized and locally advanced renal cell carcinoma. Urol Oncol (2018) 36(1):31–7. doi: 10.1016/j.urolonc.2017.07.015
7. Watson DD, Farha NM, Kallail KJ, Dakhil S, Farha AJ. From radical to partial nephrectomy in the setting of solitary functioning kidney: neoadjuvant treatment of renal cell carcinoma. Rev Urol (2020) 22(3):126–9.
8. Bigot P, Fardoun T, Bernhard JC, Xylinas E, Berger J, Rouprêt M, et al. Neoadjuvant targeted molecular therapies in patients undergoing nephrectomy and inferior vena cava thrombectomy: is it useful? World J Urol (2014) 32(1):109–14. doi: 10.1007/s00345-013-1088-1
9. Robert G, Gabbay G, Bram R, Wallerand H, Deminière C, Cornelis F, et al. Case study of the month. complete histologic remission after sunitinib neoadjuvant therapy in T3b renal cell carcinoma. Eur Urol (2009) 55(6):1477–80. doi: 10.1016/j.eururo.2008.12.036
10. Borregales LD, Adibi M, Thomas AZ, Wood CG, Karam JA. The role of neoadjuvant therapy in the management of locally advanced renal cell carcinoma. Ther Adv Urol (2016) 8(2):130–41. doi: 10.1177/1756287215612962
11. Rini BI, Garcia J, Elson P, Wood L, Shah S, Stephenson A, et al. The effect of sunitinib on primary renal cell carcinoma and facilitation of subsequent surgery. J Urol (2012) 187(5):1548–54. doi: 10.1016/j.juro.2011.12.075
12. Karam JA, Devine CE, Urbauer DL, Lozano M, Maity T, Ahrar K, et al. Phase 2 trial of neoadjuvant axitinib in patients with locally advanced nonmetastatic clear cell renal cell carcinoma. Eur Urol (2014) 66(5):874–80. doi: 10.1016/j.eururo.2014.01.035
13. Bilen MA, Jiang JF, Jansen CS, Brown JT, Harik LR, Sekhar A, et al. Neoadjuvant cabozantinib in an unresectable locally advanced renal cell carcinoma patient leads to downsizing of tumor enabling surgical resection: a case report. Front Oncol (2020) 10:622134. doi: 10.3389/fonc.2020.622134
14. de Velasco G, Carril-Ajuria L, Guerrero-Ramos F, Alonso-Gordoa T, Rodríguez-Moreno JF, Carretero A, et al. A case series of advanced renal cell carcinoma patients treated with neoadjuvant cabozantinib prior to cytoreductive nephrectomy within the phase 2 CABOPRE trial. Oncotarget. (2020) 11(47):4457–62. doi: 10.18632/oncotarget.27807
15. Roy AM, Briggler A, Tippit D, Dawson K, Verma R. Neoadjuvant cabozantinib in renal-cell carcinoma: a brief review. Clin Genitourin Cancer (2020) 18(6):e688–e91. doi: 10.1016/j.clgc.2020.04.003
16. Jurascheck L, Yang YH, Jackson-Spence F, Toms C, Sng CC, Flanders L, et al. 1467P optimal neoadjuvant treatment choice for localised renal cell carcinoma. Ann Oncol (2022) 33:S1217. doi: 10.1016/j.annonc.2022.07.1570
17. Gorin MA, Patel HD, Rowe SP, Hahn NM, Hammers HJ, Pons A, et al. Neoadjuvant nivolumab in patients with high-risk nonmetastatic renal cell carcinoma. Eur Urol Oncol (2021) 5(1):113–7. doi: 10.1016/j.euo.2021.04.002
18. Allaf M, Kim SE, Harshman LC, McDermott DF, Master VA, Signoretti S, et al. LBA67 phase III randomized study comparing perioperative nivolumab (nivo) versus observation in patients (Pts) with renal cell carcinoma (RCC) undergoing nephrectomy (PROSPER, ECOG-ACRIN EA8143), a national clinical trials network trial. Ann Oncol (2022) 33:S1432–S3. doi: 10.1016/j.annonc.2022.08.072
19. Ghali F, Patel SH, Derweesh IH. Current status of immunotherapy for localized and locally advanced renal cell carcinoma. J Oncol (2019) 2019:7309205. doi: 10.1155/2019/7309205
20. Martini A, Fallara G, Pellegrino F, Cirulli GO, Larcher A, Necchi A, et al. Neoadjuvant and adjuvant immunotherapy in renal cell carcinoma. World J Urol (2021) 39(5):1369–76. doi: 10.1007/s00345-020-03550-z
21. Cottrell TR, Thompson ED, Forde PM, Stein JE, Duffield AS, Anagnostou V, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol (2018) 29(8):1853–60. doi: 10.1093/annonc/mdy218
22. Roussel E, Tasso G, Campi R, Kriegmair MC, Kara Ö, Klatte T, et al. Surgical management and outcomes of renal tumors arising from horseshoe kidneys: results from an international multicenter collaboration. Eur Urol (2021) 79(1):133–40. doi: 10.1016/j.eururo.2020.09.012
23. Chapin BF, Delacroix SE Jr., Culp SH, Nogueras Gonzalez GM, Tannir NM, Jonasch E, et al. Safety of presurgical targeted therapy in the setting of metastatic renal cell carcinoma. Eur Urol (2011) 60(5):964–71. doi: 10.1016/j.eururo.2011.05.032
24. Pignot G, Thiery-Vuillemin A, Walz J, Lang H, Bigot P, Werle P, et al. Nephrectomy after complete response to immune checkpoint inhibitors for metastatic renal cell carcinoma: a new surgical challenge? Eur Urol (2020) 77(6):761–3. doi: 10.1016/j.eururo.2019.12.018
Keywords: renal carcinoma, neoadjuvant, immunotherapy, cabozantinib, nivolumab, complete response, horseshoe kidney, case report
Citation: Zemankova A, Studentova H, Kopova A, Tichy T, Student V and Melichar B (2023) Neoadjuvant nivolumab and cabozantinib in advanced renal cell carcinoma in a horseshoe kidney – how to achieve a safe and radical resection? a case report and review of the literature. Front. Oncol. 13:1115901. doi: 10.3389/fonc.2023.1115901
Received: 04 December 2022; Accepted: 28 June 2023;
Published: 14 July 2023.
Edited by:
Maribel Acién, Miguel Hernández University of Elche, SpainReviewed by:
Xun Lin, Pfizer, United StatesCopyright © 2023 Zemankova, Studentova, Kopova, Tichy, Student and Melichar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hana Studentova, aGFuYS5zdHVkZW50b3ZhQGZub2wuY3o=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.