- 1Department of Orthopedics, Wuhan Hospital of Traditional Chinese and Western Medicine (Wuhan No.1 Hospital), Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 2Departments of Orthopedics, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 3Department of Radiation and Medical Oncology, Zhongnan Hospital, Wuhan University, Wuhan, Hubei, China
Purpose: To examine clinical outcomes of a specialized modular prosthesis used to fill a bone deficiency following removal of femoral shaft metastases.
Methods: Eighteen patients with femoral shaft metastases who underwent en bloc resection and implantation of a personalized modular prosthesis between December 2014 and December 2019 were retrospectively analyzed. Pain, limb function, and quality of life were evaluated using the visual analog scale (VAS), Musculoskeletal Tumor Society (MSTS) scale, International Society of Limb Salvage (ISOLS) scoring system, Karnofsky Performance Status (KPS) scale, and Nottingham Health Profile (NHP) scale. The Kaplan–Meier technique was used to analyze patient survival.
Results: The operation duration was 90–150 min (mean, 115 min), and the osteotomy length was 9–16 cm (mean, 11.72 cm). The patients were followed for 12–62 months (mean, 25.28 months). The VAS and NHP ratings were lower at 3, 6, and 12 months after surgery than before surgery, while the MSTS, ISOLS, and KPS scores were higher after surgery than they had been before. These differences were statistically significant (P<0.05). The survival period was between 7 and 62 months (mean, 20.89 months), and the rates of survival at 1-year and 2-year were 72.22% and 27.78%, respectively. Except for two patients with aseptic prosthesis loosening during the follow-up period, there were no problems.
Conclusion: En bloc excision and implantation of a personalized modular prosthesis can reduce pain and improve the ability of patients with femoral shaft metastases to perform daily activities, thereby improving their quality of life.
1 Introduction
The long bones of the limbs are frequently affected by bone metastases, and the femoral shaft is the most frequently affected site, accounting for 25% to 71% of long bone metastases, 25% of which lead to pathological fracture (1). Metastases in the femoral shaft can result in excruciating pain, limb impairment, and lower quality of life (2). Bone metastases weaken bones and cause pathological fractures, both of which are significant risk factors for death (3). The best treatment plan must be chosen to prevent and treat pathological fractures in patients with bone metastases (4).
There is a broad agreement that limb salvage surgery enhances the quality of life of patients with limb shaft metastases owing to recent advancements in radiotherapy, chemotherapy, surgical techniques, targeted therapy, and immunotherapy for comprehensive cancer treatment (5). Following the removal of a bone tumor, several reconstruction techniques can be used, each with advantages and disadvantages. These techniques include biological reconstruction, artificial articulation-allograft reconstruction, intramedullary needle fixation, plate screw fixation, and tumor prosthesis replacement (6, 7). The most popular reconstruction technique in limb salvage surgery is prosthesis replacement because it can quickly relieve pain and restore limb function, while having a low incidence of post-operative complications (8–10).
Because of their positive clinical outcomes, personalized modular prostheses have recently gained recognition as a new treatment option for femoral shaft metastases (11–13). Intercalary prosthesis implantation provides the advantages of no delayed end healing and no autogenous or allogeneic bone fractures (14–16). Early post-operative functional exercise is possible because the prosthesis has good strength and can bear significant stress, provided that the post-operative limb force line is normal. Additionally, because the prosthesis may be customized, the osteotomy plane can precisely reach the area that needs to be excised, thereby reducing the chance of local recurrence. En bloc resection and intercalary prosthesis insertion take less time during surgery when the diaphysis is being repaired following large-segment osteotomy.
However, the surgical impact, functional success, and consequences of the treatment of femoral shaft metastases are not entirely obvious owing to the short duration of clinical use. This study sought to provide 18 patients with femoral shaft metastases with an effective surgical alternative by summarizing the results of en bloc resection and installation of tailored modular prostheses.
2 Materials and methods
2.1 Ethical approval and consent to participate
This retrospective study adhered to the Declaration of Helsinki and was authorized by our school’s Ethics Committee. Our ethics committee approved the process and data collection.
2.2 Inclusion and exclusion criteria
The inclusion criteria were as follows: femoral shaft metastases, an expected survival time of >3 months, an effective fixation length of the remaining bone marrow cavity at both ends after osteotomy of >5 cm, pathological fractures or a Mirels score of >9, and complete data with a follow-up period of >3 months. Patients with poor general health who could not handle anesthesia or surgery were excluded.
2.3 Patients
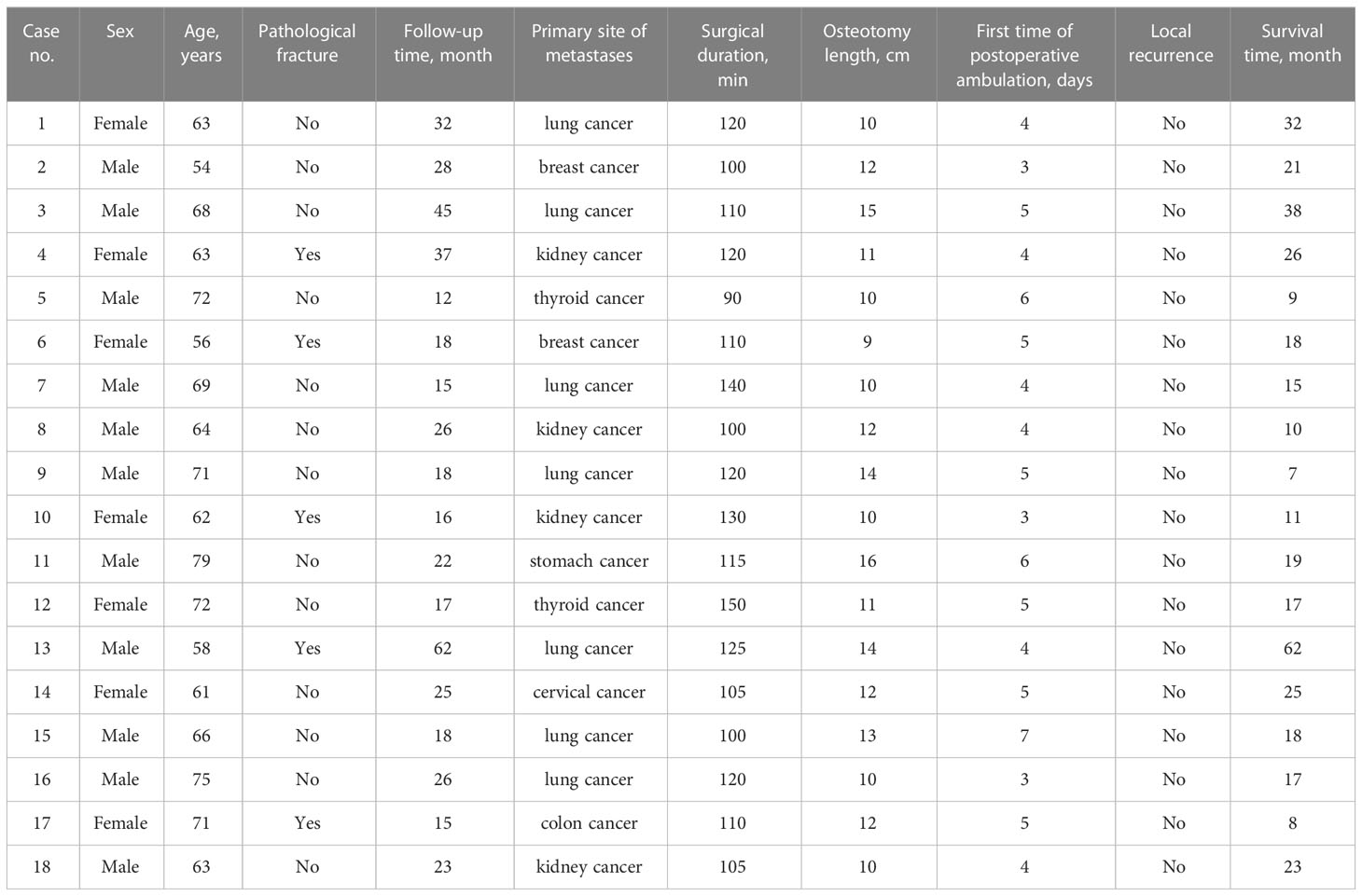
In Wuhan Hospital of Traditional Chinese and Western Medicine (Wuhan No. 1 Hospital) and Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 18 patients (of which five had pathological fractures) with femoral shaft metastases were treated between December 2014 and December 2019 by employing en bloc excision and implantation of a personalized modular prosthesis. There were 11 men and seven women aged 46–79 years (median, 65.94 years) in this group. All patients’ lower limb pain and activity restrictions led them to visit the hospital. The central segment of the femoral shaft was the location of the tumor lesions in all cases. The primary tumor types were lung cancer (n = 7), kidney cancer (n = 4), breast cancer (n = 2), thyroid cancer (n = 2), cervical cancer (n = 1), colon cancer (n = 1), and stomach cancer (n = 1).
2.4 Prosthetic design
Magnetic resonance imaging and preoperative radiography were performed to customize the modular prosthesis, which was created and produced by Beijing Lidak Technology Co., Ltd. (Beijing, China). The distal and proximal prosthesis stems, as well as the intermediate screws, were the main parts of the prosthesis, which were made of a titanium alloy (Ti6A14V). The distal and proximal prosthesis stems were grooved, and a two-fold taper connected the implanted prosthesis to the bone (Figures 1A, B).
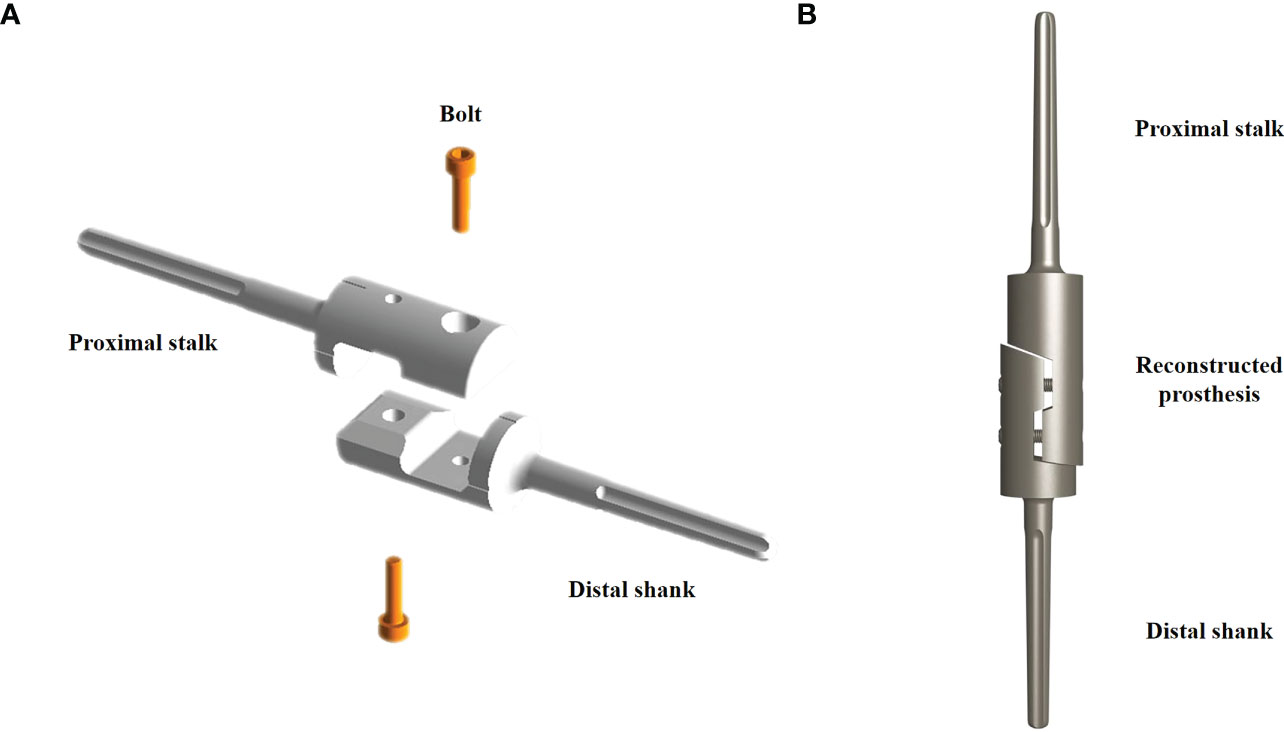
Figure 1 Schematic diagram of the customized modular prosthesis. (A) The decomposition components include the distal prosthesis stem, proximal prosthesis stem, and two intermediate screws. (B) Schematic diagram of the assembled intercalary prosthesis.
2.5 Surgical procedure
The lateral thigh approach was used in patients who were positioned in the supine position. The length of the incision was chosen based on the degree of tumor involvement revealed on preoperative magnetic resonance imaging. Following skin and deep fascia incisions, the tumor location of the femoral shaft metastases was visible between the vastus lateralis and vastus posteris. The degree of intramedullary invasion revealed by magnetic resonance imaging was used to calculate osteotomy length and plane. The periosteum was removed at the osteotomy plane, and periosteum strippers were positioned on either side to safeguard nearby soft tissue. To complete en bloc resection, a chainsaw was used to chop the diseased bone fragment (Figures 2A, B).
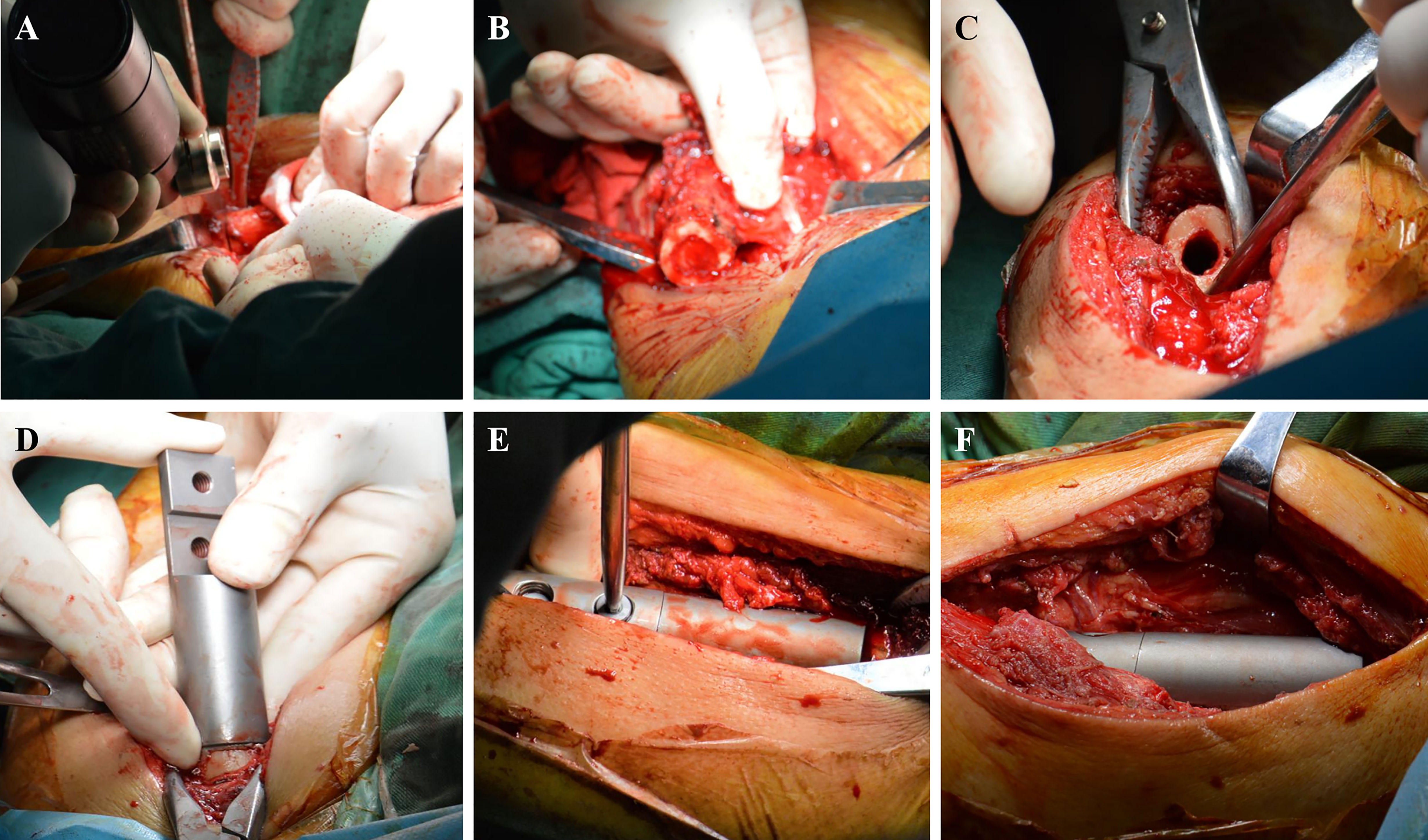
Figure 2 Surgical procedure. (A) The distal diseased bone is cut by a chainsaw. (B) The diseased bone is removed. (C) The medullary cavity is expanded. (D) Simulated prosthesis is installed. (E) The prosthesis is locked with screws. (F) Intercalary prosthesis is assembled.
The medullary cavity was completely enlarged (Figure 2C), and the prosthesis was placed (Figure 2D). The bone marrow cavity was filled with bone cement and reset according to the designated normal limb force line. The prosthesis stalk in the fixed region of the medulla was at least 5 cm long. To position the prosthesis correctly, the medullary cavity was filled with a prosthesis stem coated with bone cement (Figure 2E). Once the bone cement cooled and dried, the connecting piece was secured with two screws, and the segmental prosthesis was then attached (Figure 2F). The extracted bone was submitted to a pathologist for analysis. A negative pressure drainage tube was inserted after full hemostasis, and the surgical incision was stitched together layer-by-layer. In Figures 3, 4, two typical instances of femoral shaft metastases after en bloc excision and implantation of a specially designed modular prosthesis are shown.
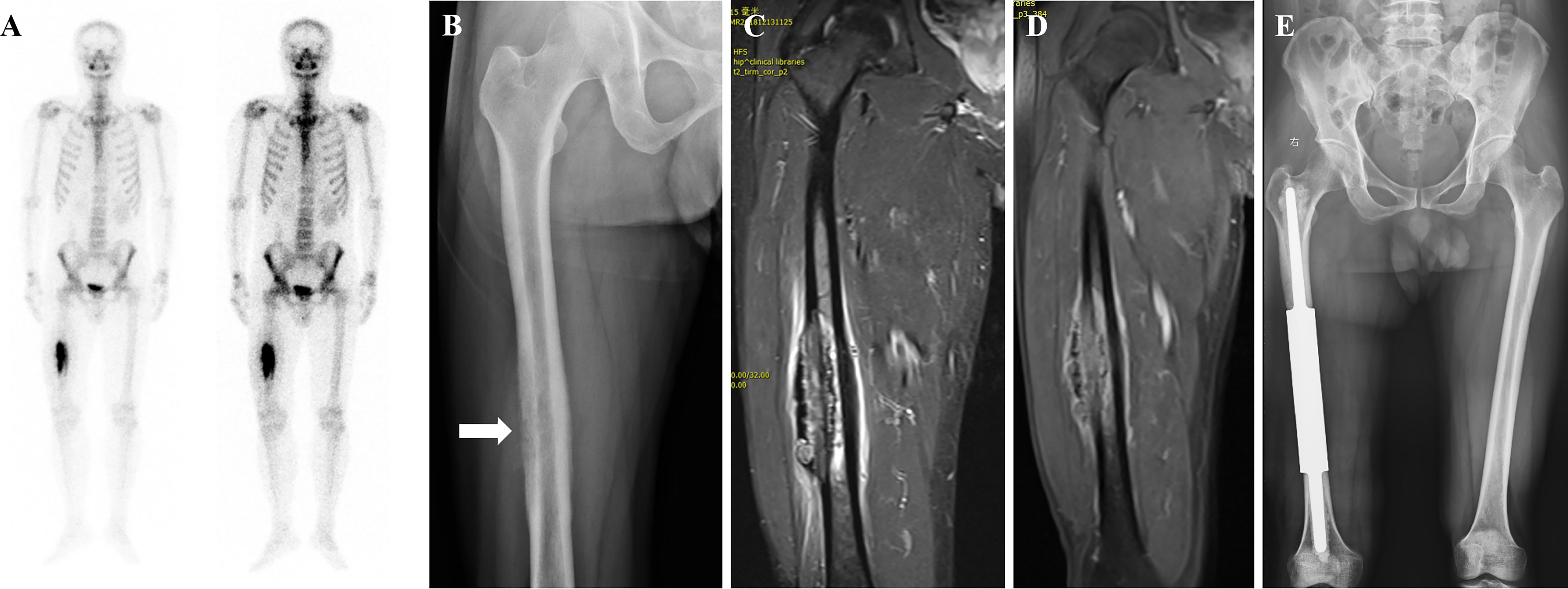
Figure 3 A patient with isolated metastasis of the right femoral shaft. (A) Emission Computed Tomography showing an isolated metastatic lesion in the right femoral shaft with active metabolism. (B) Radiograph showing osteolytic destruction of the right femoral shaft. (C, D) Magnetic resonance image showing decreased T1-weighted image signal and increased T2-weighted image signal, consistent with the diagnosis of osteolytic bone metastases. (E) Post-operative radiograph of customized modular prosthesis implantation.
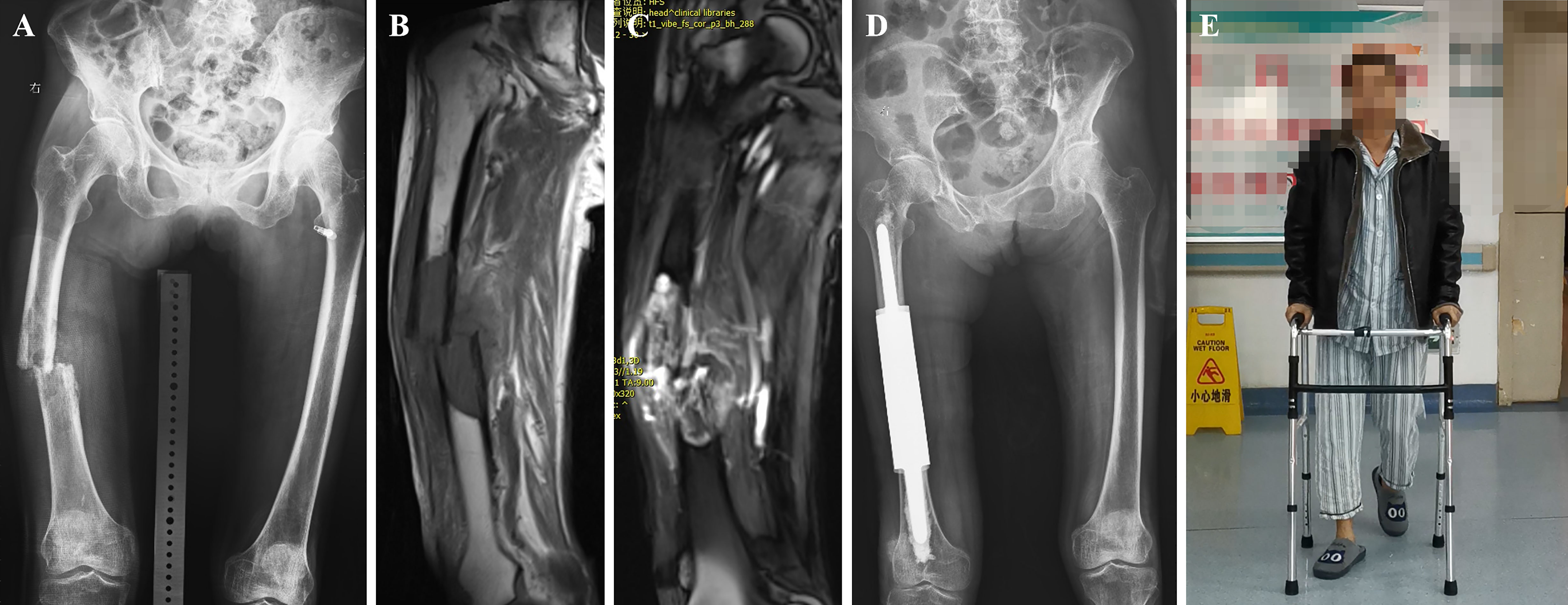
Figure 4 A case of metastatic lesion of the right femoral shaft with pathological fracture. (A) Radiograph showing osteolytic destruction of the right femoral shaft. (B, C) Long T1 and T2 signal shadows in the medullary cavity, local nodular changes, swelling of the surrounding muscle group, and increased signal. (D) Post-operative radiograph of customized modular prosthesis implantation. (E) Functional photo of the patient on the third postoperative day.
2.6 Post-operative treatment
A negative pressure drainage tube was typically installed for 48 h and withdrawn when the daily discharge dropped below 50 mL. Analgesia, anticoagulant treatment, and postoperative infection control were frequently administered. A variety of post-operative systemic therapies, including radiotherapy, chemotherapy, hormone therapy, biotherapy, and immunotherapy, were used, depending on the systemic health of the patient and the features of the underlying metastatic tumor. Bisphosphonates or denosumab were administered for the management of bone pain and prevention of skeletal-related events.
2.7 Outcome assessment
The amount of intraoperative blood loss, surgery time, wound healing time, postoperative infection, internal fixation loosening or fracture, and re-fracture were recorded. After surgery, distant metastasis and local recurrence in the affected limb were routinely monitored.
Pre and post surgery (at 3, 6, and 12 months), the severity of pain was assessed using the visual analog scale (VAS), with a high score denoting severe discomfort (17). Lower limb function was assessed using the Musculoskeletal Tumor Society (MSTS) functional score, with a total score of 30; a high score indicates good function of the affected limb (18). A high score implies good limb function in the International Society of Limb Salvage (ISOLS) rating system (19). The Karnofsky Performance Status (KPS) scale was used to evaluate functional status; a high score indicates good functional health (20). The Nottingham Health Profile (NHP) scale was used to measure quality of life; a low score suggests minimal functional impairment and a good quality of life (21).
2.8 Statistical analysis
SPSS (version 21.0; IBM Corp., Armonk, NY, USA) was used for the statistical analysis. Using the paired sample t-test, the VAS pain, functional, and quality of life scores were compared. Statistical significance was set at P<0.05.
3 Results
Table 1 lists the traits of the study participants. Each patient underwent an effective surgery and had stable vital signs throughout the procedure. Following surgery, post-operative pathology findings revealed bone metastases despite total removal of all tumors. The osteotomies ranged in length from 9 to 16 cm (mean, 11.72 cm), and the surgical duration ranged from 90 to 150 min (mean, 115.00 min). Patients were monitored for 12–62 months (mean, 25.28 months). No issues emerged during the observation period, except for two patients’ aseptic prostheses becoming looser.
The VAS and NHP scores decreased at 3, 6, and 12 months after surgery; however, the MSTS, ISOLS, and KPS scores increased, and the changes were statistically significant (P<0.05) (Table 2). The survival period was between 7 and 62 months (mean, 20.89 months), and the 1-year and 2-year survival rates were 72.22% and 27.78%, respectively (Figure 5).
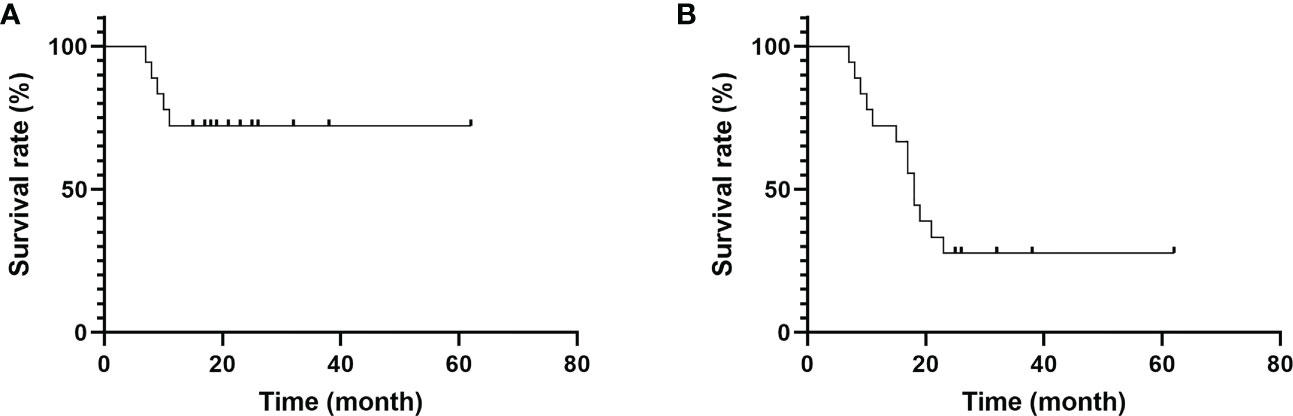
Figure 5 Survival time of patients. (A) The 1-year survival rate was 72.22%. (B) The 2-year survival rate was 27.78%.
4 Discussion
Patients with local tumor control, close pathological fractures, or failure of preventive internal fixation are candidates for whole-segment excision of primary or metastatic long-shaft malignancies (10, 22). Following a major resection of a diaphysis tumor, reconstructive techniques include the placement of massive allografts or autografts, replantation of inactive tumor bone, distraction osteogenesis, and insertion of segmental prostheses (23–27). Large allograft segments are immobilized during allograft implantation using intramedullary nails or steel plates (28). If the transplant is successful, no future revision surgery is required because the bone may permanently fuse with the allograft. Additionally, the transplanted allograft bone may cling to the repaired soft tissue, improving the post-operative stability (29). However, allografts have several major disadvantages, including allograft or residual bone fracture, graft rejection, non-union or poor matching between the allograft and autologous bone, and allograft non-union (30, 31). Additionally, even without a rejection reaction, the transplanted autologous bone may be unable to support weight for a considerable amount of time following the procedure, severely impairing the quality of life and reducing the function of the damaged limb (32). This approach cannot be used to reconstruct a significant backbone defect and carries the risk of graft breakage (33). After receiving inactivation treatment, the tumor tissue from the bone that constitutes the tumor segment is removed and replanted in its original location, restoring the continuity of the limb (34). However, it has drawbacks such as wound non-union, infection, fracture non-union, and replanted bone fracture, which have resulted in this kind of surgical method to be gradually abandoned (35). Distraction osteogenesis is a lengthy treatment that does not promote functional recovery or post-operative radiotherapy, carries the potential risk of needle tract infection, and is inappropriate for patients with metastatic disease (36).
The broad resection and repair of diaphysis tumors have recently used intercalary prosthesis implantation owing to the rapid development of biomaterials, biomechanics, iconography, internal fixation technology, and other procedures (10, 37, 38). Intercalary prosthesis implantation is clearly superior to intramedullary needle fixation, allogeneic bone transplantation, external fixation, and other techniques in terms of resisting extrusion, bending, and twisting (6). In a previous investigation, intercalary prosthesis implantation did not cause graft fracture or fracture healing after autologous and allogeneic bone transplantation (39). Functional exercise can be guaranteed in the early post-operative period, and normal function of the affected limb can be restored relatively sooner as the prosthesis has enough strength to bear stress similar to normal bone tissue, provided that the post-operative anatomical force line of the limb is normal (6). The osteotomy plane can precisely reach the area that needs to be excised because of the prosthesis’s ability to be customized, thereby lowering the local recurrence rate (40). In the case of diaphysis repair after large-segment osteotomy, the duration of the intercalary prosthesis implantation procedure is similarly reduced. These are well-known advantages of using an intercalary prosthesis over other types of restorations. These findings show that installing a tailored modular prosthesis has the added benefits of less trauma and less procedure time.
After intercalary prosthesis implantation, problems include prosthesis loosening, prosthesis wear, and prosthesis fracture, with prosthesis loosening being the most significant (41). Deviation of the limb force line is caused by loosening of the prosthesis, which can negatively impact the quality of life and necessitate reoperation. When the residual diaphysis or prosthesis cavity stalk becomes shorter following osteotomy, resulting in uneven tension on the prosthesis, prosthesis loosening may develop. Otherwise, it would be impossible to use bone cement to secure the prosthesis (42). In the case of a short prosthesis cavity stalk, some researchers have inserted an external cortical plate for better fixation to prevent prosthesis loosening; however, its long-term effects are yet to be determined (10). Despite the high prevalence of prosthesis loosening following surgery, few patients require reoperation for this complication (16, 43). Huang et al. described 16 cases of femoral metastatic tumors with pathological fractures treated with intercalary prosthesis implantation, one of which developed aseptic loosening 7 months following surgery (10). Sewell et al. reported 18 cases of tibial cancer treated with intercalary prosthesis implantation, four of which exhibited aseptic loosening. The authors considered that a stronger rotational force, larger medullary void in the metaphysis, and problematic distribution of bone cement contributed to easy loosening of the prosthesis (42). In our investigation, no complications occurred throughout the follow-up period, except for aseptic prosthesis loosening in two patients; however, revision surgery was not performed because the patients’ function was satisfactory.
In determining the success or failure of a surgery, post-operative function is an essential factor. Several biomechanical investigations (11) have proven that intercalary prostheses perform better than conventional fastening systems under various types of loading (four-point bending, torsion, and compression). Intercalary prosthetic repair is advantageous for patients with metastatic diaphyseal malignancies because of the advantages of instant stability, preservation of surrounding joints, and early return of function, according to research employing intercalary prostheses (9, 12, 16, 37, 40, 41). The MSTS score is used to evaluate the functional status of the musculoskeletal system of the skeleton after tumor removal and repair. Obtaining an adequate knowledge of surgical efficacy requires both subjective and objective post-operative evaluations. Ahlmann et al. retrospectively evaluated the clinical efficacy of intercalary prosthesis implantation in six patients with diaphyseal bone tumors, with a mean follow-up period of 21.6 months, and reported an average MTST score of 27 points, indicating that 90% of the functional status was restored (40). Abudu et al. reported the clinical outcomes in 13 cases of tibial and femoral diaphyseal tumors treated with intercalary prosthesis implantation; at the most recent follow-up, 84% of the patients’ function had been restored (44). The average post-operative MSTS score after intercalary prosthesis implantation for humeral malignancies, as reported by McGrath et al. (43), suggested 77% restoration of the patients’ functional status. In our study, the MSTS scores at 3, 6, and 12 months post-operatively were, 27.56 ± 1.98, 28.28 ± 1.56, and 28.75 ± 2.13, respectively. The three-dimensional printed prosthesis has a stronger bone integration effect and is worth looking forward to. The host bone is closely embedded with the prosthesis to achieve immediate stability, the microporous layer on the surface of the prosthesis is fused with the host bone, enabling long-term stability of the prosthesis (8, 9).
In the treatment of bone metastases, multimodal therapy is emphasized to prevent the progression of pain and skeletal-related events, and individualized treatment has become the direction of future development (45, 46). A multidisciplinary team of professionals in the diagnosis and treatment of bone tumors should select the most appropriate treatment strategy based on the patient’s unique condition, pathological type, metastasis, life expectancy, and family financial standing (47). In our study, the median survival time was 20.89 months, while the rate of complications was only 11.11%; the lower complication rate is more appropriate for patients with bone metastases who have a limited survival time.
5 Conclusion
For the treatment of femoral shaft metastases, en bloc resection and customized modular prosthesis implantation can reduce pain, improve limb function, and improve the quality of life. However, owing to the lack of a control group and the small sample size in our study, their efficacy should be tested further. Additionally, owing to the great variation in patients and primary tumors, it is difficult to generalize accurate and reliable universal principles and conclusions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the institutional review board of our hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
FP and ZZ performed the study and wrote the manuscript. FP and ZZ made substantial contributions to conception and design of the study. ZS and YY were responsible for the design of the study. YY and WW analyzed the study data. JF and FC assisted with the statistical analysis. YY and FC critically revised the manuscript, provided final approval of the version to be published and made substantial contributions to conception and design. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by The National Natural Science Foundation of China (grant no. 82274559, 81904231).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Trompeter A. Management of metastatic bone disease (MBD). Injury (2022) 53(12):3869–71. doi: 10.1016/j.injury.2022.09.054
2. Kask G, Nieminen J, Parry MC, van Iterson V, Pakarinen TK, Ratasvuori M, et al. Revision rate of reconstructions in surgically treated diaphyseal metastases of bone. Eur J Surg Oncol (2019) 45(12):2424–30. doi: 10.1016/j.ejso.2019.05.021
3. Casadei R, Drago G, Di Pressa F, Donati D. Humeral metastasis of renal cancer: Surgical options and review of literature. Orthop Traumatol Surg Res (2018) 104(4):533–8. doi: 10.1016/j.otsr.2018.03.009
4. Wedin R, Hansen BH, Laitinen M, Trovik C, Zaikova O, Bergh P, et al. Complications and survival after surgical treatment of 214 metastatic lesions of the humerus. J Shoulder Elbow Surg (2012) 21(8):1049–55. doi: 10.1016/j.jse.2011.06.019
5. Ricard MM, Stavropoulos NA, Nooh A, Ste-Marie N, Goulding K, Turcotte R. Intramedullary nailing versus plate osteosynthesis for humeral shaft metastatic lesions. Cureus (2021) 13(3):e13788. doi: 10.7759/cureus.13788
6. Zhao LM, Tian DM, Wei Y, Zhang JH, Di ZL, He ZY, et al. Biomechanical analysis of a novel intercalary prosthesis for humeral diaphyseal segmental defect reconstruction. Orthop Surg (2018) 10(1):23–31. doi: 10.1111/os.12368
7. Sakellariou VI, Mavrogenis AF, Babis GC, Soucacos PN, Magnissalis EA, Papagelopoulos PJ. Comparison of four reconstructive methods for diaphyseal defects of the humerus after tumor resection. J Appl Biomech (2012) 28(5):568–78. doi: 10.1123/jab.28.5.568
8. Pu F, Wu W, Jing D, Yu Y, Peng Y, Liu J, et al. Three-dimensional-printed titanium prostheses with bone trabeculae enable mechanical-biological reconstruction after resection of bone tumours. Biomater Transl (2022) 3(2):134–41. doi: 10.12336/biomatertransl.2022.02.005
9. Pu F, Zhang Z, Wang B, Liu J, Shao Z. En bloc resection and intercalary prosthesis implantation for the treatment of humeral diaphyseal bone metastases. Int Orthop (2021) 45(1):281–8. doi: 10.1007/s00264-020-04845-x
10. Huang HC, Hu YC, Lun DX, Miao J, Wang F, Yang XG, et al. Outcomes of intercalary prosthetic reconstruction for pathological diaphyseal femoral fractures secondary to metastatic tumors. Orthop Surg (2017) 9(2):221–8. doi: 10.1111/os.12327
11. McLynn RP, Ondeck NT, Grauer JN, Lindskog DM. What is the adverse event profile after prophylactic treatment of femoral shaft or distal femur metastases? Clin Orthop Relat Res (2018) 476(12):2381–8. doi: 10.1097/CORR.0000000000000489
12. Windhager R. CORR insights(®): Outcomes of a modular intercalary endoprosthesis as treatment for segmental defects of the femur, tibia, and humerus. Clin Orthop Relat Res (2016) 474(2):549–50. doi: 10.1007/s11999-015-4635-9
13. Lun DX, Hu YC, Yang XG, Wang F, Xu ZW. Short-term outcomes of reconstruction subsequent to intercalary resection of femoral diaphyseal metastatic tumor with pathological fracture: Comparison between segmental allograft and intercalary prosthesis. Oncol Lett (2018) 15(3):3508–17. doi: 10.3892/ol.2018.7804
14. Pitera T, Guzik G, Biega P, Tarczyńska M, Gawęda K. Assessment of physical performance and early treatment outcomes after implantation of modular prostheses of femoral and tibial shaft. Ortop Traumatol Rehabil (2019) 21(5):349–58. doi: 10.5604/01.3001.0013.5783
15. Benevenia J, Kirchner R, Patterson F, Beebe K, Wirtz DC, Rivero S, et al. Outcomes of a modular intercalary endoprosthesis as treatment for segmental defects of the femur, tibia, and humerus. Clin Orthop Relat Res (2016) 474(2):539–48. doi: 10.1007/s11999-015-4588-z
16. Ruggieri P, Mavrogenis AF, Bianchi G, Sakellariou VI, Mercuri M, Papagelopoulos PJ. Outcome of the intramedullary diaphyseal segmental defect fixation system for bone tumors. J Surg Oncol (2011) 104(1):83–90. doi: 10.1002/jso.21893
17. Sung YT, Wu JS. The visual analogue scale for rating, ranking and paired-comparison (VAS-RRP): A new technique for psychological measurement. Behav Res Methods (2018) 50(4):1694–715. doi: 10.3758/s13428-018-1041-8
18. Xu L, Li X, Wang Z, Xiong J, Wang S. Functional evaluation for patients with lower extremity sarcoma: application of the Chinese version of musculoskeletal tumor society scoring system. Health Qual Life Outcomes (2017) 15(1):107. doi: 10.1186/s12955-017-0685-x
19. Jones CW, Shatrov J, Jagiello JM, Millington S, Hong A, Boyle R, et al. Clinical, functional and radiological outcomes of extracorporeal irradiation in limb salvage surgery for bone tumours. Bone Joint J (2017) 99-B(12):1681–8. doi: 10.1302/0301-620X.99B12.BJJ-2016-0462.R2
20. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol (1984) 2(3):187–93. doi: 10.1200/JCO.1984.2.3.187
21. Chung YL, Mitchell HL, Houssien DA, Al-Mahrouki H, Carr AJ, Scott DL. A comparative study of outcome in myositis and other musculoskeletal disorders assessed using the Nottingham health profile. Clin Exp Rheumatol (2001) 19(4):447–50.
22. Szczerba P, Guzik G, Bohatyrewicz A, Kotrych D. Bone diaphysis metastases, the ways and results of surgical treatment saving the joints. Ortop Traumatol Rehabil (2019) 21(2):107–15. doi: 10.5604/01.3001.0013.1914
23. Aponte-Tinao L, Farfalli GL, Ritacco LE, Ayerza MA, Muscolo DL. Intercalary femur allografts are an acceptable alternative after tumor resection. Clin Orthop Relat Res (2012) 470(3):728–34. doi: 10.1007/s11999-011-1952-5
24. Li J, Wang Z, Guo Z, Chen GJ, Fu J, Pei GX. The use of allograft shell with intramedullary vascularized fibula graft for intercalary reconstruction after diaphyseal resection for lower extremity bony malignancy. J Surg Oncol (2010) 102(5):368–74. doi: 10.1002/jso.21620
25. Vijayan S, Bartlett W, Lee R, Ostler P, Blunn GW, Cannon SR, et al. Use of irradiated autologous bone in joint sparing endoprosthetic femoral replacement tumor surgery. Indian J Orthop (2011) 45(2):161–7. doi: 10.4103/0019-5413.77137
26. Puri A, Byregowda S, Gulia A, Patil V, Crasto S, Laskar S. Reconstructing diaphyseal tumors using radiated (50 Gy) autogenous tumor bone graft. J Surg Oncol (2018) 118(1):138–43. doi: 10.1002/jso.25092
27. Lesensky J, Prince DE. Distraction osteogenesis reconstruction of large segmental bone defects after primary tumor resection: pitfalls and benefits. Eur J Orthop Surg Traumatol (2017) 27(6):715–27. doi: 10.1007/s00590-017-1998-5
28. Zekry KM, Yamamoto N, Hayashi K, Takeuchi A, Alkhooly AZA, Abd-Elfattah AS, et al. Reconstruction of intercalary bone defect after resection of malignant bone tumor. J Orthop Surg (Hong Kong) (2019) 27(1):2309499019832970. doi: 10.1177/2309499019832970
29. Weichman KE, Dec W, Morris CD, Mehrara BJ, Disa JJ. Lower extremity osseous oncologic reconstruction with composite microsurgical free fibula inside massive bony allograft. Plast Reconstr Surg (2015) 136(2):396–403. doi: 10.1097/PRS.0000000000001463
30. Pesenti S, Peltier E, Pomero V, Authier G, Roscigni L, Viehweger E, et al. Knee function after limb salvage surgery for malignant bone tumor: Comparison of megaprosthesis and distal femur allograft with epiphysis sparing. Int Orthop (2018) 42(2):427–36. doi: 10.1007/s00264-017-3608-x
31. Aurégan JC, Pietton R, Bégué T, Anract P, Biau D. Effect of anatomic site and irradiation on the rates of revision and infection of allograft-prosthesis composites after resection of a primary bone tumor: A meta-analysis. Arch Orthop Trauma Surg (2016) 136(10):1371–80. doi: 10.1007/s00402-016-2549-y
32. Yi C, Zheng J, Li R, Lan Y, He M, Lai J, et al. Preliminary proposal: A classification system for reconstruction with autologous femoral head after periacetabular tumors resection. J Orthop Surg Res (2021) 16(1):119. doi: 10.1186/s13018-021-02275-y
33. Lawal YZ, Garba ES, Ogirima MO, Dahiru IL, Maitama MI, Abubakar K, et al. Use of non-vascularized autologous fibula strut graft in the treatment of segmental bone loss. Ann Afr Med (20181) 10(1):25–8. doi: 10.4103/1596-3519.76571
34. Li Y, Yang Y, Huang Z, Shan H, Xu H, Niu X. Bone defect reconstruction with autologous bone inactivated with liquid nitrogen after resection of primary limb malignant tumors: An observational study. Med (Baltimore) (2020) 99(24):e20442. doi: 10.1097/MD.0000000000020442
35. Yu XC, Xu SF, Xu M, Liu XP, Song RX, Fu ZH. Alcohol-inactivated autograft replantation with joint preservation in the management of osteosarcoma of the distal femur: A preliminary study. Oncol Res Treat (2014) 37(10):554–60. doi: 10.1159/000367799
36. Verdiyev V, Verdiyev F. Application of distraction osteogenesis in managing bone cysts. J Orthop Traumatol (2014) 15(2):103–9. doi: 10.1007/s10195-013-0272-9
37. Zhao J, Yu XC, Xu M, Zheng K, Hu YC, Wang F, et al. Intercalary prosthetic reconstruction for pathologic diaphyseal humeral fractures due to metastatic tumors: Outcomes and improvements. J Shoulder Elbow Surg (2018) 27(11):2013–20. doi: 10.1016/j.jse.2018.03.027
38. Zheng K, Yu XC, Hu YC, Shao ZW, Xu M, Wang BC, et al. Outcome of segmental prosthesis reconstruction for diaphyseal bone tumors: A multi-center retrospective study. BMC Cancer (2019) 19(1):638. doi: 10.1186/s12885-019-5865-0
39. Ma XL, Wang DB, Ma JX, Wang Y, Sun L, Lu B, et al. Custom-made prosthesis for reconstruction after radical resection for chondrosarcoma of manubrium. Orthop Surg (2018) 10(3):272–5. doi: 10.1111/os.12388
40. Ahlmann ER, Menendez LR. Intercalary endoprosthetic reconstruction for diaphyseal bone tumours. J Bone Joint Surg Br (2006) 88(11):1487–91. doi: 10.1302/0301-620X.88B11.18038
41. Damron TA, Leerapun T, Hugate RR, Shives TC, Sim FH. Does the second-generation intercalary humeral spacer improve on the first? Clin Orthop Relat Res (2008) 466(6):1309–17. doi: 10.1007/s11999-008-0246-z
42. Sewell MD, Hanna SA, McGrath A, Aston WJ, Blunn GW, Pollock RC, et al. Intercalary diaphyseal endoprosthetic reconstruction for malignant tibial bone tumours. J Bone Joint Surg Br (2011) 93(8):1111–7. doi: 10.1302/0301-620X.93B8.25750
43. McGrath A, Sewell MD, Hanna SA, et al. Custom endoprosthetic reconstruction for malignant bone disease in the humeral diaphysis. Acta Orthop Belg (2011) 77(2):171–9.
44. Abudu A, Carter SR, Grimer RJ. The outcome and functional results of diaphyseal endoprostheses after tumour excision. J Bone Joint Surg Br (1996) 78(4):652–7. doi: 10.1302/0301-620X.78B4.0780652
45. Chen F, Pu F. Safety of denosumab versus zoledronic acid in patients with bone metastases: A meta-analysis of randomized controlled trials. Oncol Res Treat (2016) 39(7-8):453–9. doi: 10.1159/000447372
46. Zhang Z, Pu F, Shao Z. The skeletal-related events of denosumab versus zoledronic acid in patients with bone metastases: A meta-analysis of randomized controlled trials. J Bone Oncol (2017) 9:21–4. doi: 10.1016/j.jbo.2017.09.003
Keywords: femoral shaft, bone metastases, en bloc resection, customized modular prosthesis, surgical treatment
Citation: Pu F, Yu Y, Shao Z, Wu W, Feng J, Chen F and Zhang Z (2023) Clinical efficacy of customized modular prosthesis in the treatment of femoral shaft metastases. Front. Oncol. 13:1115898. doi: 10.3389/fonc.2023.1115898
Received: 04 December 2022; Accepted: 17 March 2023;
Published: 06 April 2023.
Reviewed by:
Zhiwen Luo, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaJunyi Liao, The First Affiliated Hospital of Chongqing Medical University, China
Copyright © 2023 Pu, Yu, Shao, Wu, Feng, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Feng, ZmVuZ2ppbmd3aHl5eUAxNjMuY29t; Fengxia Chen, ZmVuZ3hpYWNoZW5Ad2h1LmVkdS5jbg==; Zhicai Zhang, emhpY2FpemhhbmdAaHVzdC5lZHUuY24=
†These authors have contributed equally to this work
 Feifei Pu
Feifei Pu Yihan Yu
Yihan Yu Zengwu Shao
Zengwu Shao Wei Wu
Wei Wu Jing Feng1*
Jing Feng1* Fengxia Chen
Fengxia Chen