- 1Department of Urology, The Second Hospital of Tianjin Medical University, Tianjin, China
- 2Department of Urology, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai, Shandong, China
Background: Upper tract urothelial carcinoma (UTUC) is a rare and highly malignant urothelial tumor originating from the renal pelvis and ureter associated with poor prognosis. It has been established that 70% of ureteral tumors occur in the lower ureter. Radical nephroureterectomy (RNU) with ipsilateral bladder cuff excision is regarded as the standard treatment for UTUC. Current evidence supports the role of lymph node dissection (LND) in determining tumor staging, but no consensus has been reached on the potential survival benefits. The present study retrospectively analyzed cases of UTUC limited to the lower ureter to evaluate the survival benefits of LND during RNU.
Methods: The present study retrospectively analyzed data from patients with UTUC limited to the lower ureter from two medical centers from 2000 to 2016 and assessed the survival outcomes, including recurrence-free survival (RFS) and cancer specific survival (CSS). During subgroup analysis, we stratified by pathological tumor (pT) stages and postoperative adjuvant chemotherapy (AC).
Results: The study cohort included 297 patients separated into LND (n=111) and non-LND (n=186) groups. The two groups were comparable except for the pathological N stage. The LND group was associated with superior survival in terms of RFS (27.0% vs. 18.3%, p=0.044) and CSS (53.2 vs. 39.8%, p=0.031) compared to the non-LND group (n=186). In pT2-4 patients, the LND group was associated with better 3-year RFS (50.5% vs. 32.3%, p<0.05), 5-year RFS (29.7% vs. 12.0%, p<0.05), and overall RFS (18.7% vs. 6.0%, p<0.05) than the non-LND group. Besides, the LND group was associated with a significantly better 3-year CSS (68.1% vs. 49.6%, p=0.003), 5-year CSS (51.6% vs. 30.8%, p<0.05) and overall CSS (45.1% vs. 24.1%, p<0.05). In patients that underwent AC, the LND group had better survival benefits in terms of RFS (29.4 vs. 16.7%, p=0.023) and CSS (52.9% vs. 40.5%, p=0.038) compared to the non-LND group.
Conclusion: LND has survival benefits in patients with UTUC localized to the lower ureter, especially for≥pT2 stage UTUC and AC cohorts. Overall, the therapeutic effect of LND in UTUC cannot be replaced by AC.
1 Introduction
Urothelial carcinoma (UC) is the fourth most common male tumor and may involve different parts of the urinary system, including the renal pelvis, ureter, bladder or urethra. Upper tract urothelial carcinoma (UTUC) is a rare and highly malignant tumor originating from the renal pelvis and ureter, accounting for 5-10% of all UC cases (1). Due to the absence of clinical symptoms during the early stages and the thin muscular layer and secluded location of the upper urinary tract, UTUC is prone to invasive growth. About two-thirds of patients have advanced-stage disease upon diagnosis, resulting in poor treatment efficacy and prognosis (2).
According to the initial location, UTUC can be subdivided into four segments: renal pelvis, upper, middle and lower ureter. Current evidence suggests that the incidence of UTUC has gradually increased over the past decades, with a significant decrease in renal pelvic cancers (1.19 to 1.15) and an increase in ureteral disease (0.69 to 0.91) (3). About 70% of ureteral tumors occur in the lower ureter, 25% in the middle ureter, and 5% in the upper ureter (4). An increasing body of evidence suggests that lower ureteral tumors have the worst prognosis (5–7).
Radical nephroureterectomy (RNU) with ipsilateral bladder cuff excision is regarded as the standard treatment for UTUC (2). However, it has been reported that the prognosis for UTUC patients remains poor after RNU surgery, and the five-year survival rate is very low (8). Current evidence supports the role of lymph node dissection (LND) in determining tumor staging, but no consensus has been reached on the potential survival benefit (9). Studies from different centers have yielded inconsistent results (9–11). Accordingly, no guidelines recommend routine LND during RNU in this patient population.
Herein, we retrospectively analyzed cases of UTUC localized to the lower ureter to evaluate the survival benefits of LND during RNU.
2 Methods
2.1 Patients
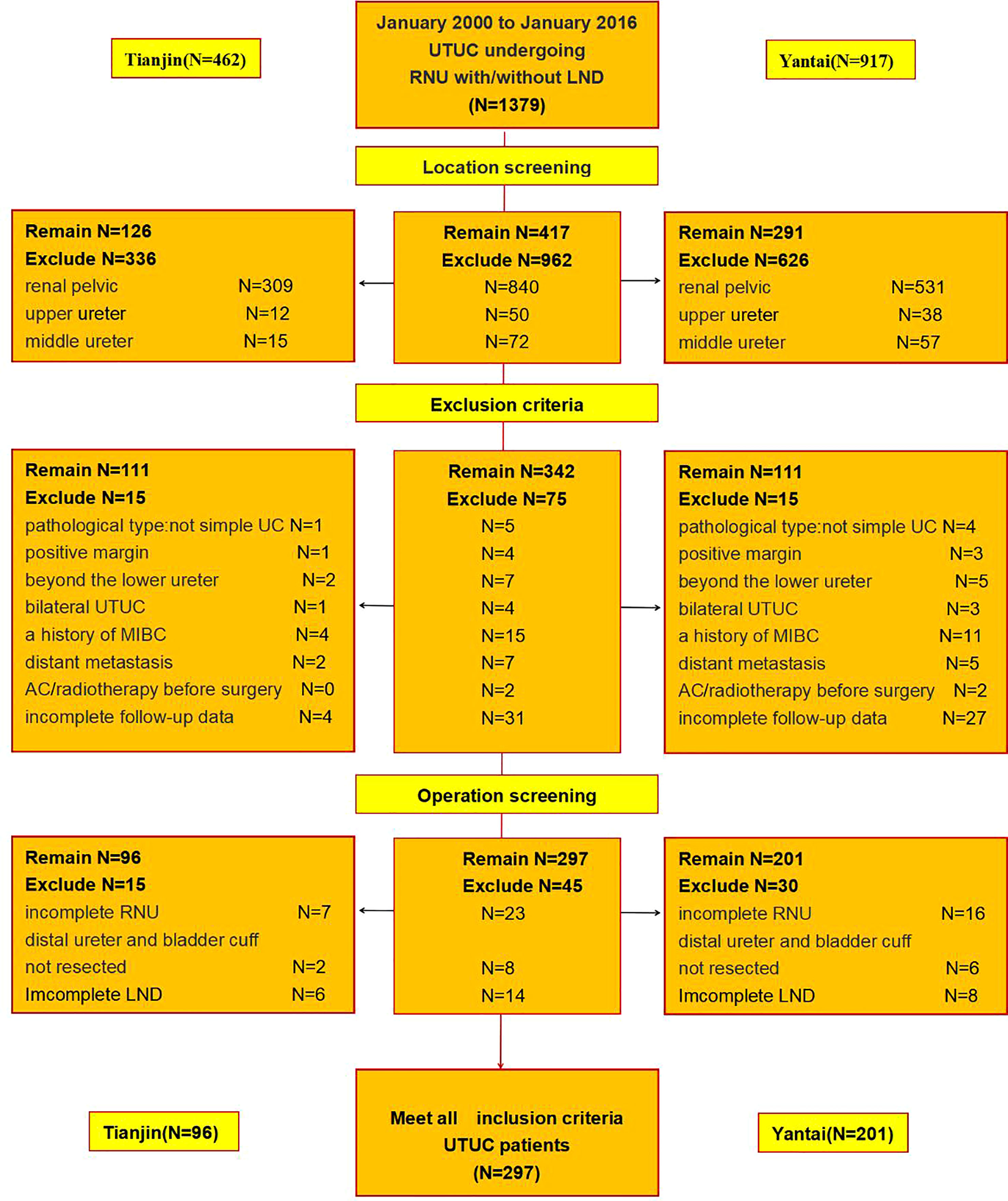
In this retrospective cohort study, we initially identified 1379 patients with UTUC that underwent open or laparoscopic RNU with/without LND at the Yantai Yuhuangding Hospital Affiliated to Qingdao University (n=917) and the Second Hospital of Tianjin Medical University (n=462) from January 2000 to January 2016.
417 patients with tumors of the lower ureter were obtained after excluding 962 patients with tumors involving the renal pelvis (n=840) and upper (n=50) and middle (n=72) ureters. 75 ineligible patients were excluded based on the exclusion criteria, including 1. the pathological type is not simple urothelial carcinoma (n=5); 2. positive margins (n=4); 3. the boundary of the tumor beyond the boundary of the lower ureter(n=7); 4. bilateral ureteral tumors (n=4); 5. history of muscle-invasive bladder cancer (MIBC) (n=15); 6. distant metastasis before operation(n=7); 7. adjuvant chemotherapy or radiotherapy before surgery (n=2); 8. missing or incomplete follow-up data (n=31). Finally, we excluded cases with incomplete RNU (n=23), incomplete LND (n=14), and distal ureter and bladder cuff not resected by an open incision (n=8) (Figure 1). Ultimately, 297 patients comprised the final study population.
All patients underwent computed tomography urography (CTU) and CT angiography (CTA) or magnetic resonance hydrography of the urinary system (MRU), cystoscopy or diagnostic ureteroscopy, and urinary cytology before surgery.
The present study was conducted upon approval from the Institutional Review Board of both medical centers.
2.2 Surgical procedures
All patients underwent standard RNU with ipsilateral bladder cuff resection. RNU surgeries were performed through laparoscopic or open approach by two experienced surgical teams in each center. The distal ureter and the bladder cuff were treated by an open incision in the lower abdomen. LND was performed through this incision. LND involved ipsilateral pelvic lymph nodes, including lower 1/3 of periureter lymph tissues, common iliac lymph nodes, internal iliac lymph nodes, external iliac lymph nodes, and obturator lymph nodes.
All surgical specimens were processed according to standard pathological procedures. Two experienced anatomical pathologists reviewed all slides. Any disagreements were resolved by consensus-based discussion or by a third pathologist. All tumors were graded according to the 2004 World Health Organization/International Society of Urological Pathology (WHO/ISUP) consensus classification and staged according to TNM classification 2017 for UTUC. N0 was defined when the scope of dissection was sufficient, the number of lymph nodes was not less than 8, and all nodes were negative. Otherwise, it was excluded and regarded as incomplete LND.
Patients in both groups were routinely given intravesical instillation of 30mg piroxacin 1 week after surgery, once a week for 8 weeks, and then once a month for 6-12 months in total, to prevent tumor recurrence. Transurethral resection of bladder tumor was performed for non-muscle-invasive bladder cancer (NMIBC) postoperative recurrence.
2.3 Adjuvant treatment
None of the patients received radiation therapy after surgery. Adjuvant chemotherapy (AC) was performed mainly due to locally advanced disease (pT2-4/N0-2/M0 or pTany/N1-2/M0) after the surgeon and patients reached a consensus. The adjuvant chemotherapy scheme was based on cisplatin.
2.4 Follow-up
During follow-up, patients generally underwent cystoscopy and urinary cytology every three months for the first two years, chest and abdomen CT every 6 months and annually thereafter. A bone scan was performed when bone metastasis was suspected clinically. 31 patients were lost to follow-up at the end of the study since they could not be contacted or declined to follow-up.
2.5 Statistical analysis
The clinical and pathological characteristics of the two groups were compared using Student’s t-test for continuous variables and the chi-square test for categorical variables. Survival probabilities were estimated using the Kaplan-Meier method, and the log-rank test was applied to compare survival curves. Univariate and multivariate Cox proportional hazards regression analyses were conducted to evaluate the association between prognostic factors and survival outcomes. All statistical analyses were performed using SPSS version 21.0, and P values <0.05 were statistically significant. The primary end-points were recurrence-free survival (RFS) and cancer-specific survival (CSS). Local recurrence included cancer recurrence within the resection area regional lymph nodes, or peritoneal recurrence. Concomitant regional and distant metastases were treated as distant recurrences. RFS was measured from the date of surgery until recurrence.
3 Results
3.1 Characteristics of patients
The final study cohort comprised 297 patients who underwent standard RNU and ipsilateral bladder cuff resection with (n=111, 37.4%) or without LND (n=186, 62.6%).The median follow-up time in the LND and non-LND groups was 47.8 ± 26.6 (range 4-91) and 40.2 ± 27.1 (range 4-92) months, respectively. The clinicopathological characteristics of the study cohort (LND vs. non-LND) are presented in Table 1.
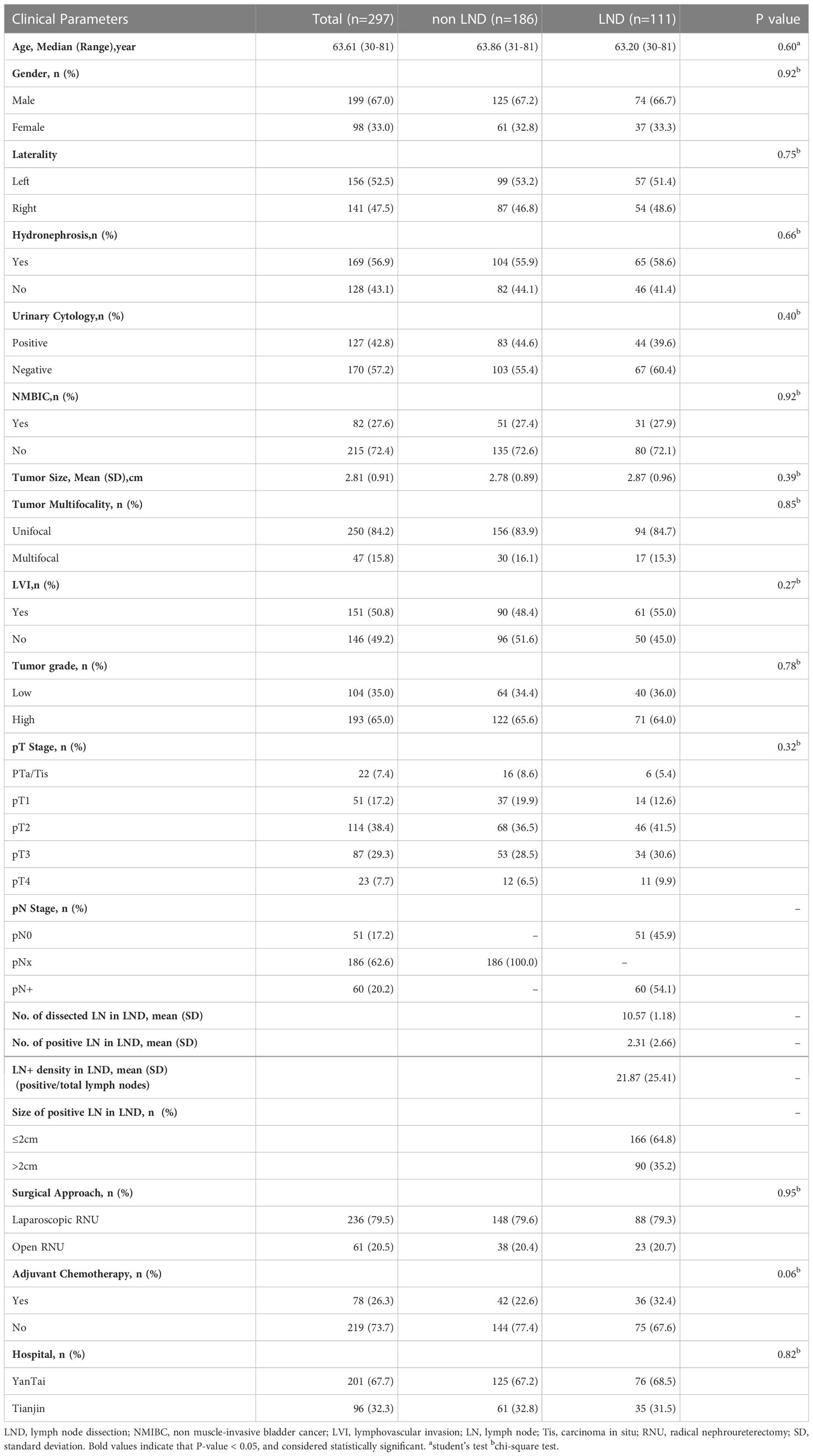
Table 1 Baseline characteristics of the study cohort and comparative analysis results according to surgical method.
Overall, the median age of the patients was 63.61 (30-81years). The male-to-female ratio was approximately 2:1. Nearly three-quarters of the patients (LND 82%, non-LND 71.5%) presented with invasive disease (≥pT2 stage), and two-thirds had high-grade features. Lymphovascular invasion (LVI) was found in about half of the patients. Of the 297 eligible patients, 201 cases were from Yantai, and 96 from Tianjin. 236 (79.5%) patients underwent laparoscopic RNU, and 61 (20.5%) patients underwent open RNU. Approximately a quarter of the patients (LND 32.4%, non-LND 22.6%) were treated with postoperative adjuvant chemotherapy. No significant differences were observed between the two groups except for the pathological N stage.
3.2 Survival outcomes in the entire cohort and prognostic factor analysis
Overall, the LND group had a better prognosis based on the RFS (27.0% vs. 18.3%, p=0.044) and CSS (53.2 vs. 39.8%, p=0.031) than the non-LND group (Figures 2A, B).
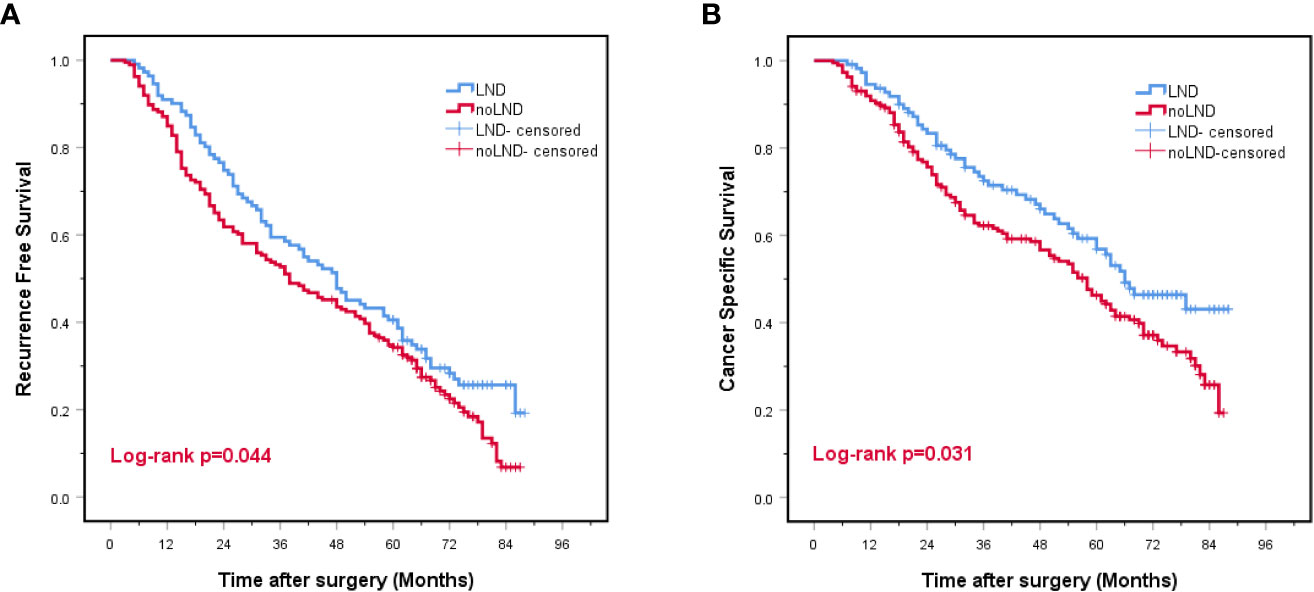
Figure 2 Kaplan-Meier curves for recurrence-free survival (A) and cancer-specific survival (B) for the entire study cohort. LND, lymph node dissection, Red font means P value < 0.05.
The univariate analysis revealed that hydronephrosis, urinary cytology, NMIBC, tumor size, tumor Multifocality, LVI, tumor grade, pT stage, pN stage and surgical method were significantly associated with RFS and CSS.
Multivariate analysis revealed that urinary cytology, tumor size, LVI, tumor grade, pT stage, pN stage, surgical method and adjuvant chemotherapy were independent risk factors for RFS. Age, urinary cytology, LVI, tumor grade, pT stage, pN stage, surgical method and adjuvant chemotherapy were independent risk factors for CSS (Table 2).

Table 2 Univariable and multivariable Cox proportional hazard models predicting recurrence-free survival and cancer-specific survival in the entire study cohort.
3.3 Subgroup analysis according to pT-stage
After stratification according to the pT-stage, there were no significant differences in RFS or CSS between the groups in patients with<pT2 stage (p>0.05) (Figures 3A, D). In contrast, in pT2-4 patients, the LND group was associated with overall RFS (18.7% vs. 6.0%, p<0.05), 3-year RFS (50.5% vs. 32.3%, p<0.05) and 5-year RFS(29.7% vs. 12.0%, p<0.05) than the non-LND group. Besides, significant improvement was observed in overall CSS (45.1% vs. 24.1%, p<0.05), 3-year CSS (68.1% vs. 49.6%, p=0.003), and 5-year CSS (51.6% vs. 30.8%, p<0.05) (Figures 3B, C, E, F).
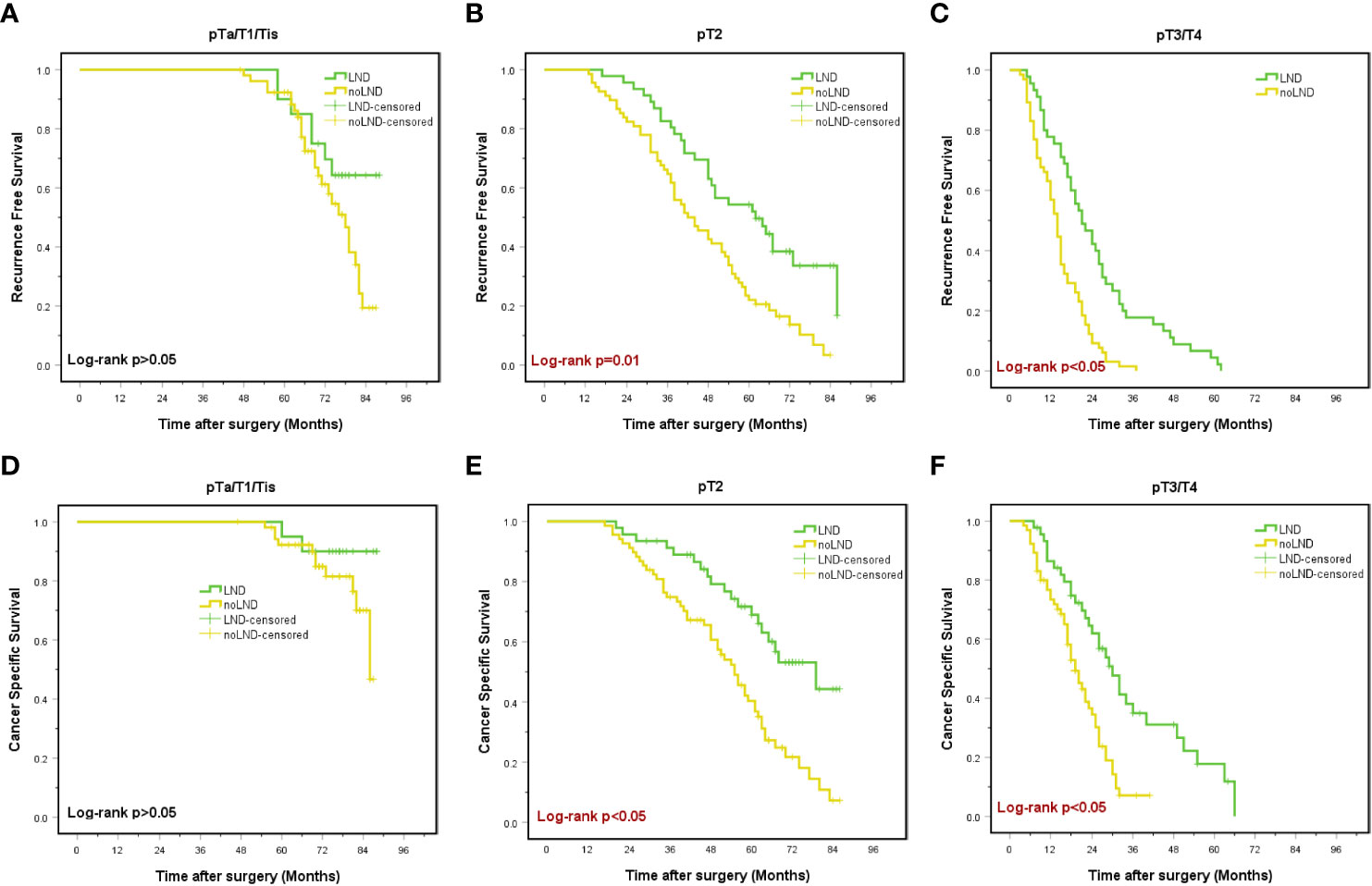
Figure 3 Kaplan-Meier curves for recurrence-free survival (A-C) and cancer-specific survival (D-F) in subgroups stratified by pathologic tumor (pT) stages. LND, lymph node dissection, Red font means P value < 0.05.
Moreover, during univariable and multivariable Cox regression analysis in pT2-4 patients, LND was an independent predictor of better RFS (HR 0.255; 95% CI 0.142-0.456; p<0.05) and CSS (HR 0.271; 95% CI 0.137-0.537; p<0.05) than the non-LND group (Table 3).
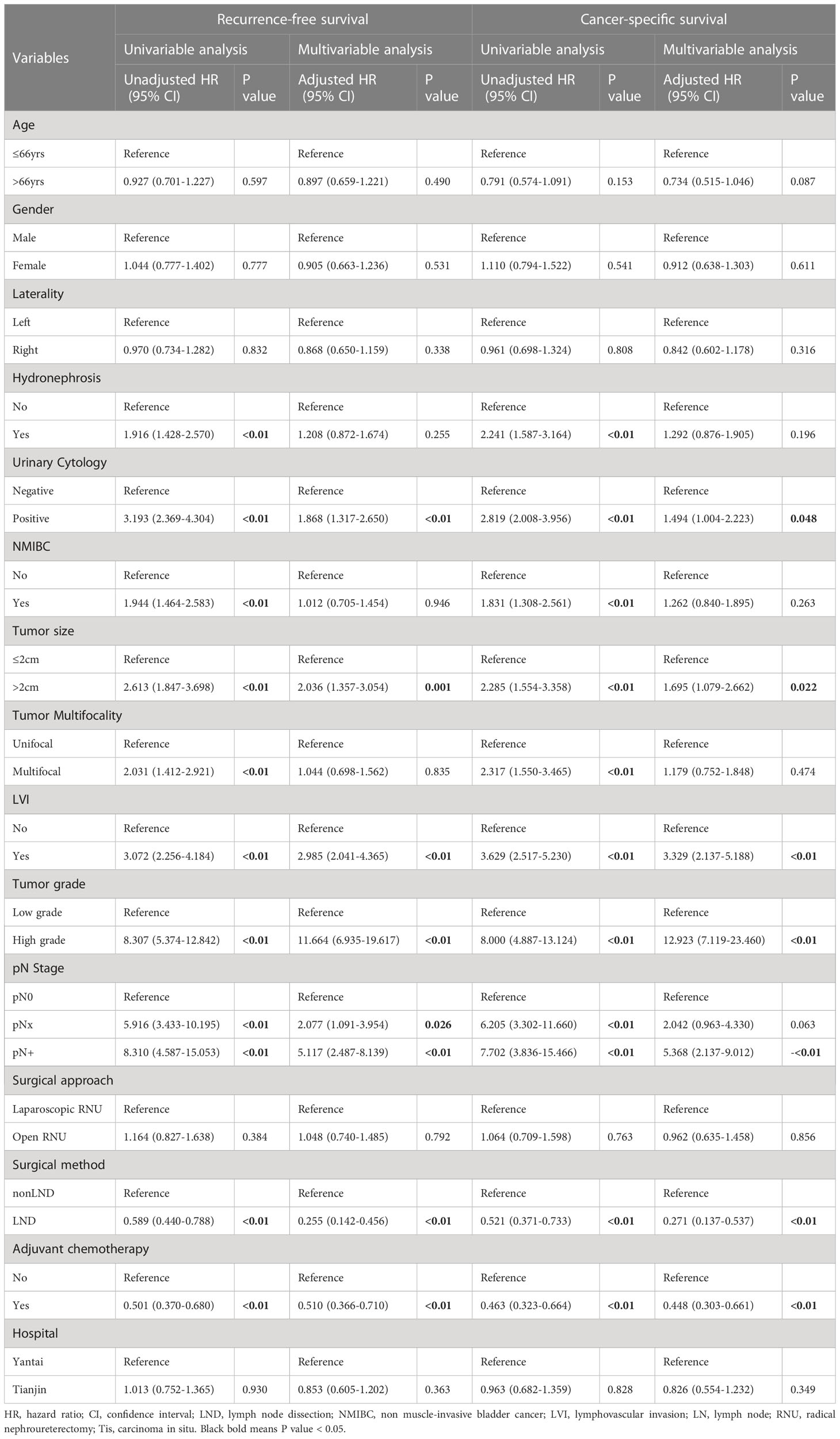
Table 3 Univariable and multivariable Cox proportional hazard models predicting recurrence-free survival and cancer-specific survival in patients diagnosed with pT2-4 stage.
3.4 Subgroup analysis stratified by AC
In patients that underwent AC, we found the LND group had a better prognosis in terms of RFS (29.4 vs. 16.7%, p=0.023) and CSS (52.9% vs. 40.5%, p=0.038) than the non-LND group (Figures 4A, B).
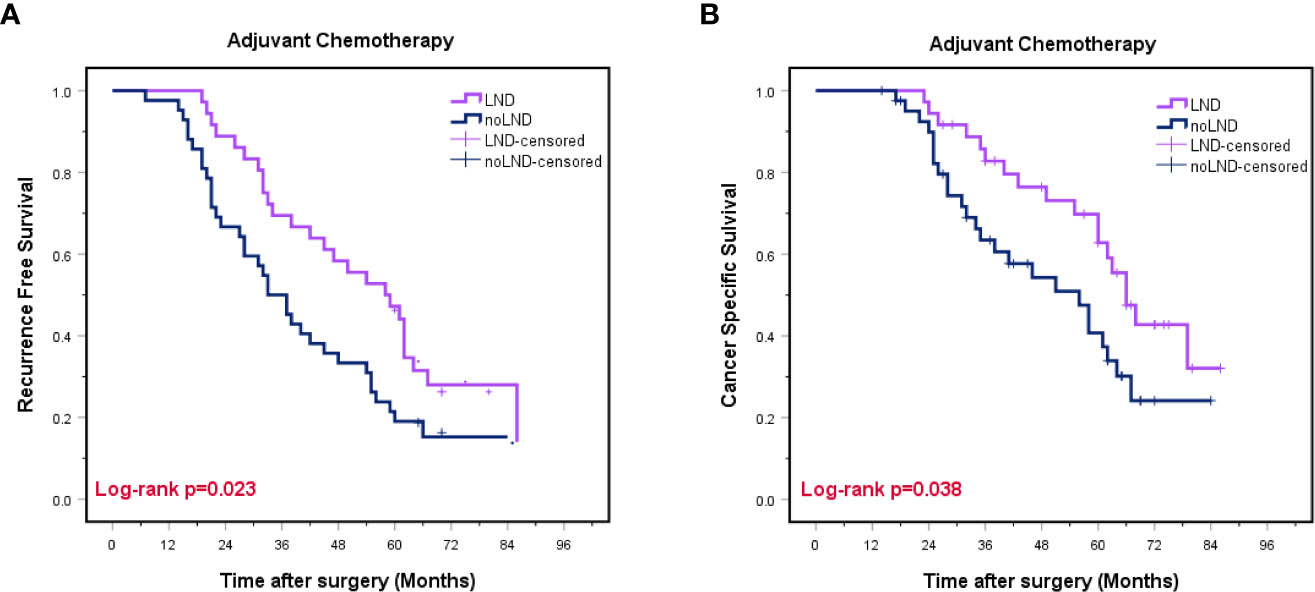
Figure 4 Kaplan-Meier curves for recurrence-free survival (A) and cancer-specific survival (B) in subgroups stratified by adjuvant chemotherapy. LND, lymph node dissection, Red font means P value < 0.05.
Univariable and multivariable Cox regression analysis in patients with AC showed that LND was an independent predictor of better RFS (HR 0.172; 95% CI 0.063-0.467; p=0.001) and CSS (HR 0.129; 95% CI 0.033-0.506; p=0.003) compared to the non-LND group (Table 4).
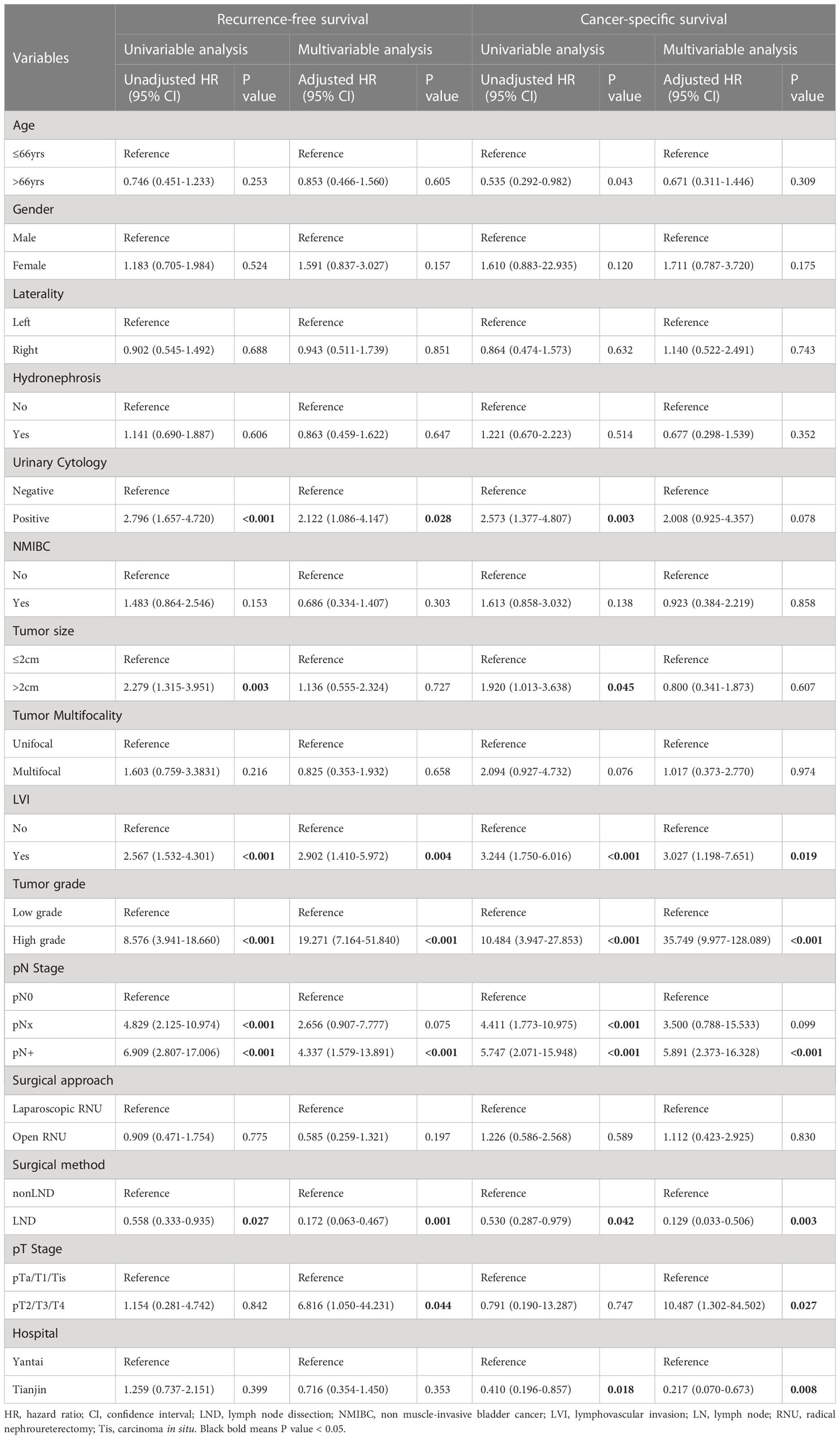
Table 4 Univariable and multivariable Cox proportional hazard models predicting recurrence-free survival and cancer-specific survival in patients who underwent postoperative adjuvant chemotherapy.
4 Discussion
Due to the rarity of UTUC, most published studies have been limited to small samples with a lack of standardized and prospective randomized trials. The clinical significance of LND during RUN surgery remains subject to debate. During clinical practice, surgeons rarely choose LND due to the burden of surgery (12, 13). According to a study based on the US National Cancer Database, less than 20% of RNUs performed involved LND (14). However, with the development of laparoscopic technology, there is an increasing consensus that LND does not increase the risk of perioperative complications (15, 16). Accordingly, it is necessary to further explore the prognostic significance of LND in UTUC.
In 2017, the European Association of Urology (EAU) guidelines group for non-muscle- invasive bladder cancer published a systematic review, which documented the survival benefit of LND in ≥pT2 patients of the renal pelvis. However, the benefits of LAD in ureteral tumors remains uncertain (17). To our knowledge, few studies have hitherto been conducted on UTUC localized to the ureter. It has been established that ureteral UTUC localized to the lower ureter is the most predominant and malignant emphasizing the importance of stratifying patients according to tumor location. Accordingly, the present analysis was innovatively conducted on UTUC cases localized to the lower ureter.
According to the latest European Association of urology guidelines, hydronephrosis and tumor multifocality are high-risk factors for UTUC. Although univariate analysis demonstrated that hydronephrosis and tumor multifocality are the risk factors of prognosis in this study, only pathological T stage and AC were independent risk factors during multivariate analysis. Therefore, we conducted a survival analysis after stratification according to pathological T stage and AC. LND was associated with survival benefits for cases with ≥pT2 stage. In addition, during subgroup analysis, the AC cohort showed that the LND group had better survival benefits. Taken together, our findings suggest that AC cannot replace the therapeutic effect of LND in UTUC.
Fan et al. (18) showed that LND could bring survival benefits to patients diagnosed with clinically node-negative UTUC, especially those with T2-4 disease. Ikeda et al. (19) also showed that LND improved OS and CSS in pT3 or later stages. However, unlike the present study, they concluded that LND is not beneficial for patients with pT2. Besides, Austin et al. (20) showed that extended LND at any clinical stage did not significantly impact OS in cN+ patients with UTUC (20). However, it should be borne in mind that cN+ UTUV is a systemic disease that cannot be controlled by removing the local nodes.
Several limitations found in our study should be acknowledged. Data analyzed in the present study were retrospectively collected from two institutions in China, and the cohort size was relatively small. Further large prospective RCTs are encouraged to substantiate our results. Besides, data on other factors, such as comorbidities (e.g., smoking) and postoperative complications, were not collected. Tobacco is widely acknowledged as an exogenous risk factor for developing UTUC. Indeed, being a smoker at diagnosis increases the risk for disease recurrence and mortality after RNU. The presence of post-operative complications delays AC, increasing the risk for disease recurrence and mortality after RNU. Besides, it has been reported that for tumors of the distal ureter, pelvic dissection could capture only about 75% of lymph node metastases (11). Accordingly, only pelvic lymph node dissection was selected for the present study, which affected the reliability of our findings to a certain extent, emphasizing the need for further studies. Another limitation is the limited sample size. Based on the limited number of samples, it is difficult to conduct subgroup analysis according to the number, size and location of pelvic vessels.
5 Conclusion
In conclusion, we provided hitherto undocumented evidence that LND brings survival benefits to patients with UTUC localized to the lower ureter, especially those with ≥pT2 stage and AC cohort. In addition, AC cannot replace the therapeutic effect of LND in UTUC.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was exempt by Institutional Review Board approval.
Author contributions
YC: study concepts and designs, data acquisition, statistical analysis, and drafted the manuscript. YL: data acquisition, data analysis and interpretation, and manuscript editing. JW: data analysis, manuscript review. CQ: study concepts and designs, data acquisition, data analysis and interpretation, manuscript review. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from National Natural Science Foundation of China (No. 8197101267), Yantai Science and Technology Plan Project (No. 2019YD018) and the young talents program of Yantai Yuhuangding Hospital (No. kj0261).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
LND, Lymphadenectomy/lymph node dissection; RNU, radical nephroureterectomy; UTUC, upper tract urothelial carcinoma; LVI, lymphovascular invasion; RFS, recurrence-free survival; CSS, cancer specific survival; AC, adjuvant chemotherapy; pT, pathological tumor; UC, urothelial carcinoma; MIBC, muscle-invasive bladder cancer; EAU, European Association of Urology; CTU, computed tomography urography; CTA, CT angiography; MRU, magnetic resonance hydrography of the urinary system; NMIBC, non-muscle-invasive bladder cancer.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin (2019) 69:7–34. doi: 10.3322/caac.21551
2. Rouprêt M, Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, et al. European Association of urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol. (2021) 79(1):62–79. doi: 10.1016/j.eururo.2020.05.042
3. Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973-2005. BJU Int (2011) 107(7):1059–64. doi: 10.1111/j.1464-410X.2010.09675.x
4. Anderström C, Johansson SL, Pettersson S, Wahlqvist L. Carcinoma of the ureter: A clinicopathologic study of 49 cases. J Urol. (1989) 142(2 Pt 1):280–3. doi: 10.1016/s0022-5347(17)38730-x
5. Ouzzane A, Colin P, Xylinas E, Pignot G, Ariane MM, Saint F, et al. Ureteral and multifocal tumours have worse prognosis than renal pelvic tumours in urothelial carcinoma of the upper urinary tract treated by nephroureterectomy. Eur Urol. (2011) 60(6):1258–65. doi: 10.1016/j.eururo.2011.05.049
6. Hurel S, Rouprêt M, Seisen T, Comperat E, Phé V, Droupy S, et al. Influence of preoperative factors on the oncologic outcome for upper urinary tract urothelial carcinoma after radical nephroureterectomy. World J Urol. (2015) 33(3):335–41. doi: 10.1007/s00345-014-1311-8
7. Inamoto T, Matsuyama H, Komura K, Ibuki N, Fujimoto K, Shiina H, et al. Tumor location based segmentation in upper-tract urothelial carcinoma impacts on the urothelial recurrence-free survival: A multi-institutional database study. Curr Urol. (2020) 14(4):183–190. doi: 10.1159/000499240
8. Fairey AS, Kassouf W, Estey E, Tanguay S, Rendon R, Bell D, et al. Comparison of oncological outcomes for open and laparoscopic radical nephroureterectomy: Results from the Canadian upper tract collaboration. BJU Int (2013) 112(6):791–7. doi: 10.1111/j.1464-410X.2012.11474.x
9. Roscigno M, Shariat SF, Margulis V, Karakiewicz P, Remzi M, Kikuchi E, et al. Impact of lymph node dissection on cancer specific survival in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy. J Urol. (2009) 181(6):2482–9. doi: 10.1016/j.juro.2009.02.021
10. Roscigno M, Brausi M, Heidenreich A, Lotan Y, Margulis V, Shariat SF, et al. Lymphadenectomy at the time of nephroureterectomy for upper tract urothelial cancer. Eur Urol. (2011) 60(4):776–83. doi: 10.1016/j.eururo.2011.07.009
11. Goltzman ME, Gogoj A, Ristau BT. The role of lymphadenectomy at the time of radical nephroureterectomy for upper tract urothelial carcinoma. Transl Androl Urol. (2020) 9(4):1860–7. doi: 10.21037/tau.2019.11.29
12. Roupret M, Babjuk M, Burger M, Compérat E, Cowan N, Gontero P, et al. Urothelial carcinoma of the upper urinary tract. In: EAU- ESTRO-ESUR-SIOG guidelines, 2017 edn. Arnhem: EAU Guidelines Office (2017). p. 16–7.
13. Pearce SM, Pariser JJ, Patel SG, Steinberg GD, Shalhav AL, Smith ND. The effect of surgical approach on performance of lymphadenectomy and perioperative morbidity for radical nephroureterectomy. Urol Oncol (2016) 34(3):121e115–121. doi: 10.1016/j.urolonc.2015.09.008
14. Zareba P, Rosenzweig B, Winer AG, Coleman JA. Association between lymph node yield and survival among patients undergoing radical nephroureterectomy for urothelial carcinoma of the upper tract. Cancer (2017) 123:1741–50. doi: 10.1002/cncr.30516
15. Chan VW, Wong CH, Yuan Y, Teoh JY. Lymph node dissection for upper urothelial tract carcinoma: A systematic review. Arab J Urol. (2021) 19:37–45. doi: 10.1080/2090598X.2020.1791563
16. Ishiyama Y, Kondo T, Kubota S, Shimada K, Yoshida K, Takagi T, et al. Therapeutic benefit of lymphadenectomy for older patients with urothelial carcinoma of the upper urinary tract: A propensity score matching study. Jpn J Clin Oncol (2021) 51(5):802–9. doi: 10.1093/jjco/hyaa256
17. Dominguez-Escrig JL, Peyronnet B, Seisen T, Bruins HM, Yuan CY, Babjuk M, et al. Potential benefit of lymph node dissection during radical nephroureterectomy for upper tract urothelial carcinoma: A systematic review by the European association of urology guidelines panel on non-muscle-invasive bladder cancer. Eur Urol Focus (2019) 5(2):224–41. doi: 10.1016/j.euf.2017.09.015
18. Dong F, Xu T, Wang X, Shen Y, Zhang X, Chen S, et al. Lymph node dissection could bring survival benefits to patients diagnosed with clinically node-negative upper urinary tract urothelial cancer: a population -based, propensity score-matched study. Int J Clin Oncol (2019) 24(3):296–305. doi: 10.1007/s10147-018-1356-6
19. Ikeda M, Matsumoto K, Sakaguchi K, Ishii D, Tabata KI, Kurosawa K, et al. Effect of lymphadenectomy during radical nephroureterectomy in locally advanced upper tract urothelial carcinoma. Clin Genitourin Canc. (2017) 15:556–62. doi: 10.1016/j.clgc.2017.04.004
Keywords: upper tract urothelial carcinoma, lymphadenectomy, nephroureterectomy, recurrence-free survival, cancer-specific survival
Citation: Cui Y, Lu Y, Wu J and Quan C (2023) Benefits of lymphadenectomy for upper tract urothelial carcinoma only located in the lower ureter: a bicentre retrospective cohort study. Front. Oncol. 13:1115830. doi: 10.3389/fonc.2023.1115830
Received: 04 December 2022; Accepted: 21 March 2023;
Published: 14 April 2023.
Edited by:
Trushar Patel, University of South Florida, United StatesCopyright © 2023 Cui, Lu, Wu and Quan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changyi Quan, jjkkll8532788@sina.com; Jitao Wu, wjturology@163.com
†These authors have contributed equally to this work and share first authorship
 Yupeng Cui
Yupeng Cui Youyi Lu
Youyi Lu Jitao Wu2*
Jitao Wu2*