- 1Department of Internal Medicine, Far Eastern Memorial Hospital, New Taipei City, Taiwan
- 2Department of Internal Medicine, National Taiwan University Hospital and College of Medicine, National Taiwan University, Taipei, Taiwan
- 3Department of Healthcare Information and Management, Ming-Chuan University, Taoyuan, Taiwan
- 4Department of Pathology, National Taiwan University Hospital, Taipei, Taiwan
- 5Department of Internal Medicine, National Taiwan University Cancer Center, National Taiwan University, Taipei, Taiwan
- 6Graduate Institute of Clinical Medicine, National Taiwan University, Taipei, Taiwan
Introduction: The MET exon 14 skipping (METex14) mutation is an important oncogenic driver in lung cancer. We performed a retrospective analysis of clinical data from lung cancer patients with the METex14 mutation to analyze their survival outcomes and associated prognostic factors.
Methods: A one-step reverse transcription-polymerase chain reaction to examine the presence of the METex14 mutation was performed using RNA samples from 1374 lung cancer patients with no detected EGFR and ALK mutations. Pathological features and immunohistochemistry (IHC) results for c-MET were analyzed in patients with METex14-positive tumors.
Results: METex14 was identified in 69 patients with lung cancer, including 53 adenocarcinoma (ADC) and 16 non-ADC patients. In comparison with patients without the METex14 mutation, lung cancer patients harboring the METex14 mutation were generally elderly individuals, never-smokers, and had poor performance scores. A higher frequency of METex14 mutations was detected in pulmonary sarcomatoid carcinoma (PSC) patients (24.3%, n = 9/37). However, stage IV PSC patients with or without the METex14 mutations showed similarly poor overall survival (OS) (p = 0.429). For all 36 METex14-positive lung ADCs, multivariate analysis showed several poor prognostic factors, including strong c-MET IHC staining (p = 0.006), initial brain metastasis (p = 0.005), and administration of only supportive care (p < 0.001). After excluding seven patients who received only supportive care, we further analyzed 29 stage IV lung ADC patients with METex14 mutations who received anti-cancer treatment. Multivariate analysis showed that pemetrexed treatment (p = 0.003), lung radiotherapy (p = 0.020), initial brain metastasis (p = 0.005), and strong c-MET IHC staining (p = 0.012) were independent prognostic factors for OS in these patients.
Conclusions: A higher frequency of METex14 mutations was detected in PSC patients. Stage IV PSC patients with or without the METex14 mutations had similarly poor overall survival. Pemetrexed-based chemotherapy, strong c-MET ICH staining, initial brain metastasis, and lung radiotherapy, may help predict survival outcomes in patients with advanced lung ADCs harboring the METex14 mutation.
1 Introduction
Acquired gene alterations in lung tumors serve as driver mutations that initiate tumorigenic and invasive abilities. Some of these mutations can be targeted by specific small-molecule inhibitors or monoclonal antibodies (1). The c-mesenchymal-epithelial transition protooncogene (MET) is an important gene that encodes the MET protein, which functions as a transmembrane receptor tyrosine kinase and may trigger tumor growth under aberrant activation (2). MET exon 14 skipping (METex14) is one of the most common gene alterations of MET, and it acts as an important oncogenic driver in lung cancer (3). The METex14 mutation results in the loss of the juxtamembrane domain of the MET protein, which regulates and prevents MET over-signaling (4). Consequently, the E3 ubiquitin ligase c-cbl fails to bind to the MET protein, reducing receptor degradation and causing overactivation of MET-mediated signaling, thereby driving oncogenesis (5).
Among patients with lung cancer, the METex14 mutation occurs in 2%-4% of those with adenocarcinomas (ADC), 1%-2% of those with squamous cell carcinoma, and 7% to 31% of the patients with pulmonary sarcomatoid carcinoma (PSC) (6–8). Several small molecules targeting and inhibiting MET tyrosine kinase have been evaluated for their efficacy in the treatment of METex14-positive non-small cell lung cancer (NSCLC). Clinical studies have demonstrated that crizotinib, a multikinase inhibitor of receptor tyrosine kinases (RTKs), reduces the tumor size in advanced NSCLC patients carrying the METex14 mutation (9). However, the phase II METROS study reported limited benefits in terms of objective response rate (ORR), progression-free survival (PFS), and overall survival (OS) (10). Capmatinib, an oral adenosine triphosphate (ATP)-competitive MET inhibitor, demonstrated anti-cancer efficacy with an ORR of 68% and a median PFS of 9.69 months in treatment-naïve patients in the phase II GEOMETRY mono-1 trial (11). Another ATP-competitive MET inhibitor, tepotinib, showed a favorable overall response rate and rapid as well as durable response in the phase II VISION study (12). Thus, both capmatinib and tepotinib are recommended as first-line treatments of choice for advanced NSCLC with METex14-positive tumors (13). Other MET-specific tyrosine kinase inhibitors (TKIs), multikinase inhibitors, and anti-MET antibodies are currently in ongoing clinical trials for the treatment of this patient population (14).
NSCLC patients carrying METex14 mutations receive conventional treatments without specific anti-MET therapy and have a poor prognosis and short OS (8, 15). Their OS is comparable to that of patients with undetected major driver mutations (16). Although METex14-positive NSCLC patients treated with selective MET TKIs reported longer OS, up to 30%-40% of these patients were reported to be non-responders (11, 17, 18). The factors associated with a poor prognosis in these patients remain unclear. Our previous study demonstrated that stage IV patients with METex14 mutations had diverse survival outcomes; some patients showed very poor survival, while others had a relatively long survival period (16). Therefore, identification of the potential factors that predict OS in these patients is important. In the present study, we performed a retrospective evaluation of clinical data from lung cancer patients with the METex14 mutation to analyze their survival outcomes and associated prognostic factors.
2 Patients and methods
2.1 Ethics statement
This study was approved by the institutional review board of National Taiwan University Hospital (NTUH), Taipei, Taiwan. Written informed consent was obtained from all patients before tumor specimen collection for clinical data acquisition and molecular analyses.
2.2 Patients
We retrospectively included patients diagnosed with lung cancer at the National Taiwan University Hospital between January 2006 and August 2020. Tumor specimens were consecutively and prospectively collected from either the primary lung tumors or distant metastatic sites by surgery, core needle biopsy, bronchial washing, endobronchial biopsy, and cell blocks of malignant pleural effusion. Only patients with lung cancer with no detected EGFR and ALK mutations were included in this study. Tumors were confirmed by mutational analysis to exclude co-major driver mutations.
2.3 Mutational studies
EGFR mutation tests were performed using a one-step reverse transcription-polymerase chain reaction (RT-PCR) with RNA samples. ALK mutations were detected by either RT-PCR or immunohistochemistry (IHC) staining using the Ventana ALK (D5F3) antibody. Patients with lung cancer with no detected EGFR and ALK mutations were examined for the METex14 mutation. The presence of other major driver mutations, including KRAS, HER2, BRAF V600E, ROS-1, and RET, was also analyzed. Tumor specimen preparation, RNA extraction, primer selection, RT-PCR conditions, and sequencing methods for all driver mutations were performed using methods described previously (16, 19). Some of the patients with ROS1 fusion and RET fusion underwent fluorescence in situ hybridization with a previously described standard protocol (19).
2.4 Acquisition of clinical and pathologic data
Demographic characteristics and clinical features of all enrolled patients were obtained from medical records. Patients who smoked less than 100 cigarettes in their lifetime were defined as nonsmokers. The Eastern Cooperative Oncology Group (ECOG) performance score (PS) was used to rank performance status (20). Distant metastases were evaluated and the number of different metastatic sites was recorded. Treatment modalities, including therapeutic surgery, chemotherapy, immunotherapy, MET TKI treatment, and local radiotherapy (RT) at the primary or metastatic sites were recorded. The endpoint of clinical analyses was OS, defined as the time from the initial diagnosis of lung cancer to death or the date of censoring at the last follow-up or loss of contact on April 30, 2022.
2.5 c-MET immunohistochemistry staining
MET protein expression was evaluated by performing c-MET IHC staining on formalin-fixed paraffin-embedded (FFPE) tissue sections of METex14-positive tumors. As described previously, 4-μm-thick FFPE sections were dewaxed, rehydrated, and reacted with a 1:50 dilution of anti-human c-MET antibody clone SP44 (Abcam, Cambridge, UK) (16). Staining was performed using an automated stainer (Ventana Benchmark; Roche Ventana, Tucson, AZ, USA) in accordance with the manufacturer’s instructions. The intensity of MET expression was scored and classified as strong (score 3+), moderate (score 2+), weak (score 1+), or absent (score 0), as described previously (16). Staining distribution patterns were recorded as diffuse, focal, or negative. Other IHC stains, including pancytokeratin (CK), thyroid transcription factor-1 (TTF-1), and vimentin, were assessed as described previously (16). A portion of the IHC data was retrieved from the medical records.
2.6 Statistical analysis
Categorical variables were compared using the chi-squared test or Fisher’s exact test when the expected number was less than 5. Continuous variables were expressed as median values with upper and lower values. OS and univariate analyses were estimated using the Kaplan–Meier method and the log-rank test to measure all differences in survival curves. We used a Cox proportional hazard regression model for multivariate analysis of OS with the backward-stepwise method. All tests were two-sided, and differences were considered significant when p < 0.05. Analyses were performed using the IBM SPSS software for Windows (version 26.0, IBM Corp., Armonk, NY, USA).
3 Results
3.1 Clinical features of lung cancer patients with and without the METex14 mutation
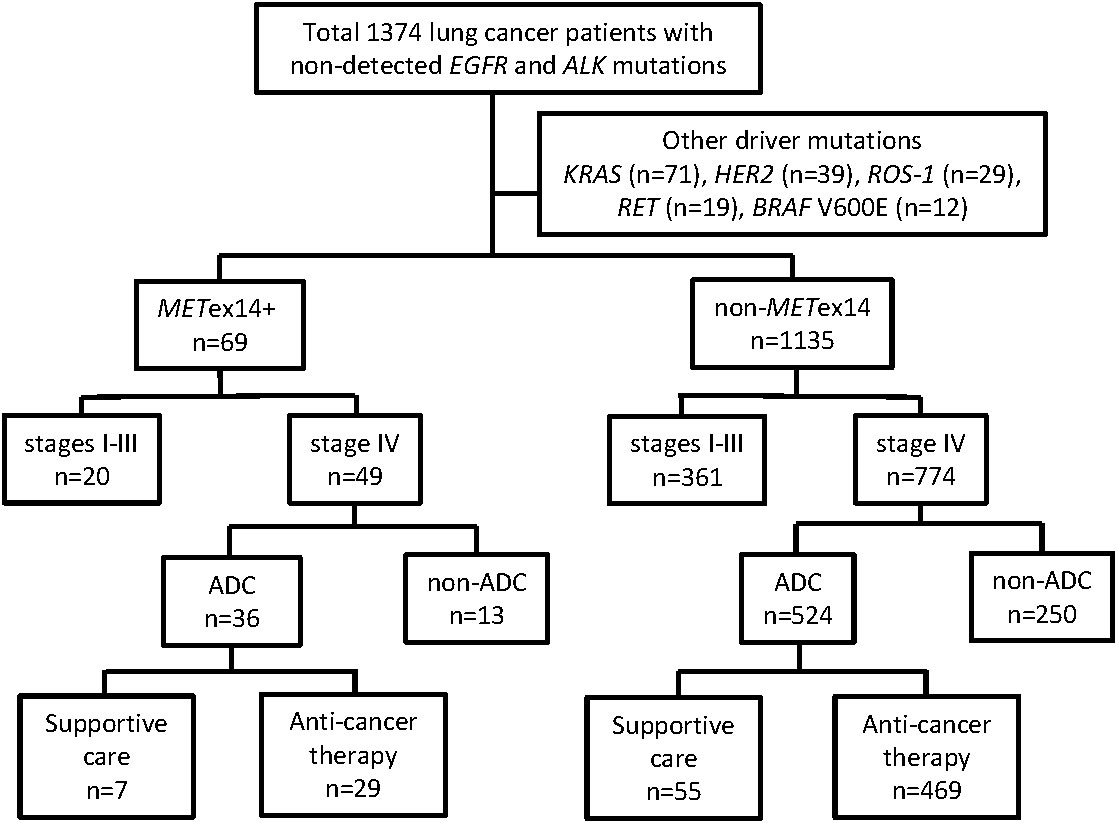
This cohort study enrolled 1374 lung cancer patients with no detected EGFR and ALK mutations (Figure 1). Among these patients, 170 had other driver mutations were excluded, including 71 with KRAS mutations, 39 with HER2 mutations, 29 with ROS-1 fusions, 19 with RET fusions, and 12 with BRAF V600E mutations (Figure 1). The METex14 mutation was identified in 69 patients, including 53 patients with ADC and 16 patients with non-ADC. Some patients with the METex14 mutation have been described in our previous report (16). In total, 1135 patients who did not show any driver mutations (METex14, EGFR, ALK, KRAS, HER2, BRAF V600E, ROS-1, or RET) were categorized into the non-METex14 group. Among the 69 patients with METex14-positive lung cancer, the median age was 74.2 years at initial diagnosis, 44 patients were male (64%), and more than half were never-smokers (41/69; 59%). The majority of the patients (48/69, 69%) had good ECOG PS scores (0-1), while 15 patients (22%) had a PS score of 2 and 6 patients had poor PS scores (3 and 4). A vast majority of the patients had stage IV disease (49/69, 71%). In comparison with the non-METex14 group, lung cancer patients harboring the METex14 mutation were generally elderly individuals (≥70 years old, p = 0.009), never-smokers (p = 0.020), had poor ECOG PS (p = 0.026), and showed different subtypes of non-ADC (p < 0.001; Table 1). The highest frequency of METex14 mutations was observed in PSCs (9/37, 24.3%), followed by ADCs (53/803, 6.6%), pleomorphic carcinomas (1/19, 5%), squamous cell carcinoma (4/107, 3.7%), NSCLC-not otherwise specified (NOS) (1/80, 1.3%), and small cell lung cancer (1/159, 0.6%; Supplementary Table 1).
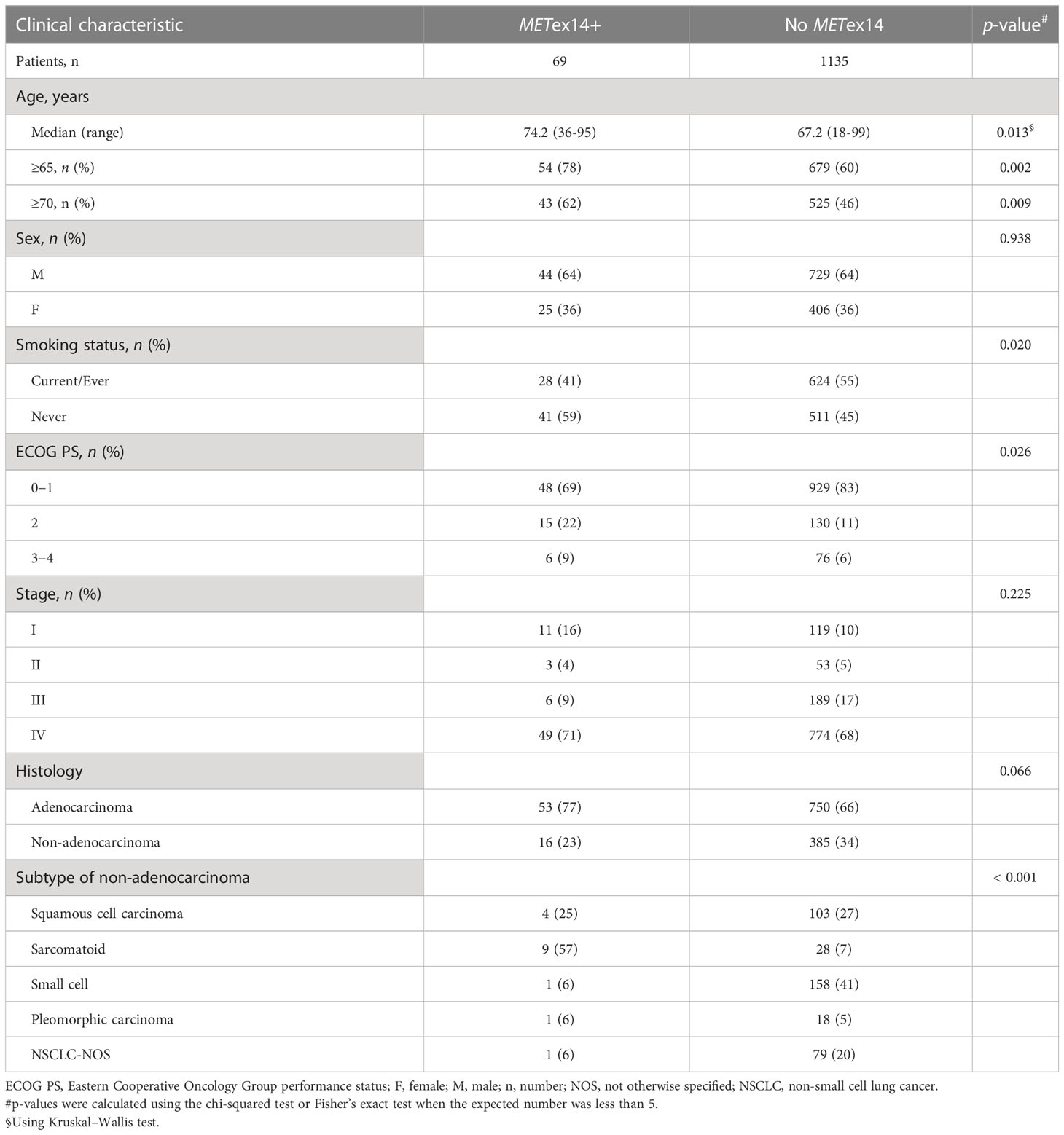
Table 1 Clinical characteristics of lung cancer patients harboring tumors with (n = 69) and without (n = 1135) the METex14 mutation.
3.2 Univariate and multivariate analyses of prognostic factors for overall survival in all stage IV Adenocarcinoma patients harboring the METex14 mutation
We further focused on patients with lung cancer who were initially diagnosed with stage IV ADC, which included 36 patients harboring the METex14 mutation and 524 patients without major driver mutations (i.e., the non-METex14 group consisting of patients without detected METex14, EGFR, ALK, KRAS, HER2, BRAF V600E, ROS-1, and RET mutations) (Figure 1). We examined the prognostic role of various factors for OS, including age, sex, smoking status, pathologic features of c-MET IHC, ECOG PS, presence and number of distant metastatic sites, and provision of anti-cancer therapy or only supportive care. Univariate analyses of OS was performed using Kaplan–Meier survival analysis in 36 stage IV ADC patients, of which c-MET IHC analysis data were available for 33 patients (Table 2).
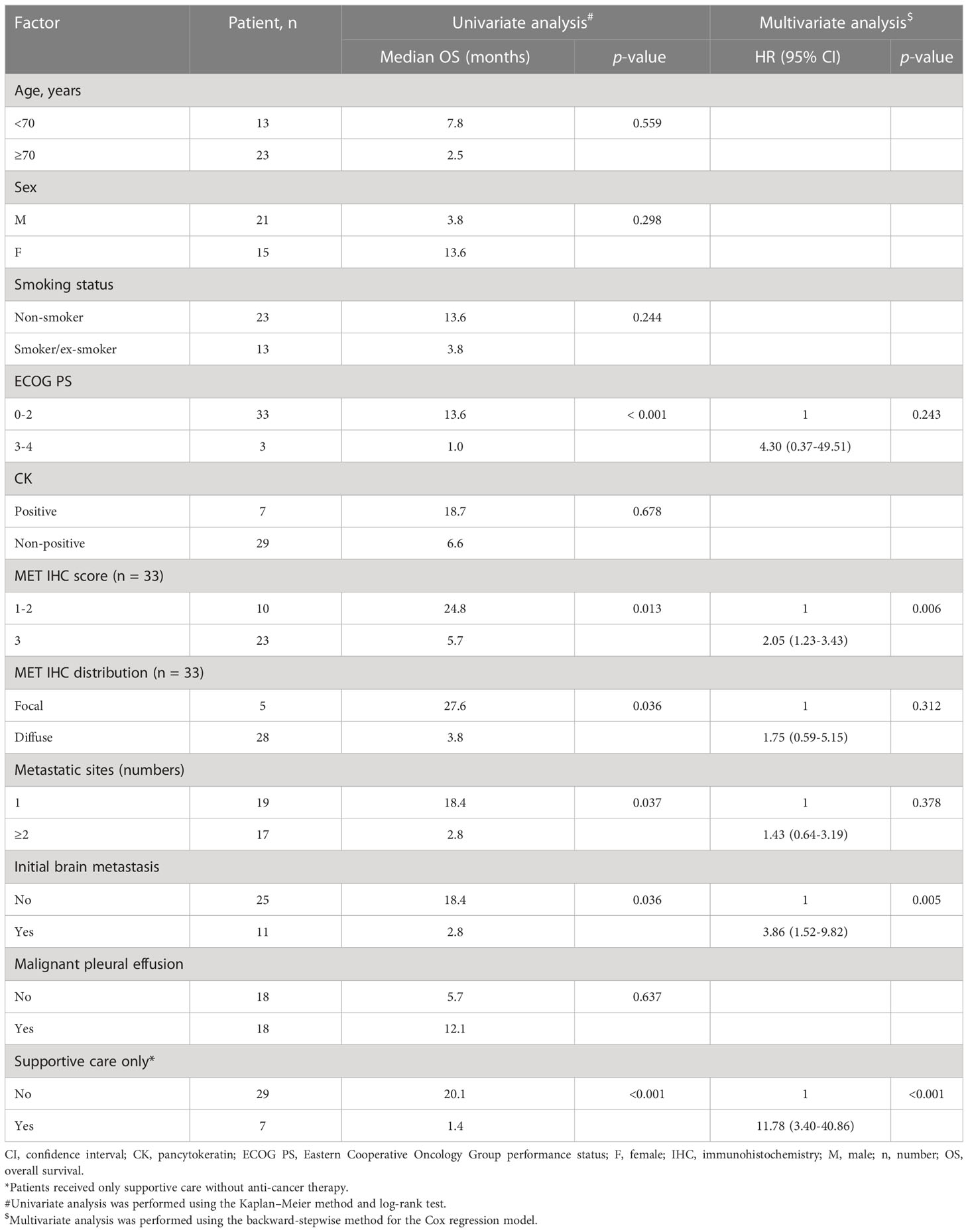
Table 2 Univariate and multivariate analyses of prognostic factors for overall survival in all patients with METex14-positive stage IV adenocarcinoma (n = 36).
Demographic factors, such as age (<70 vs. ≥70 years), sex, and smoking status, did not show statistically significant differences in relation to median OS (mOS). Pancytokeratin (CK) staining was not associated with differences in survival rates. Among the 33 tumors available for c-MET IHC, all METex14-positive tumors showed c-MET-positive expression and were categorized on the basis of staining scores (1+ ~ 2+ vs. 3+, Figure 2A). All c-MET patterns were either focal or diffuse (Figure 2C). We observed that patients with tumor samples showing strong (score 3+) c-MET IHC staining had a shorter mOS than those with weak or moderate (score 1+ ~ 2+) c-MET IHC staining (5.7 vs. 24.8 months, p = 0.013; Figure 2B). Similar findings were observed for the c-MET IHC distribution patterns; patients with tumor samples showing a diffuse pattern had shorter mOS than those with samples showing a focal pattern (3.8 vs. 27.6 months, p = 0.036; Figure 2D). Next, we evaluated the characteristics of the metastatic status for OS analysis. Patients with multiple initial metastatic sites (≥2) showed poorer survival outcomes than those with only one metastatic site (2.8 vs. 18.4 months, p = 0.037). A shorter OS was also observed in patients with metastatic brain tumors at the initial presentation (2.8 vs. 18.4 months, p = 0.036). The presence of malignant pleural effusion was not associated with survival outcomes. Among stage IV ADC patients with the METex14 mutation, seven patients received only supportive care without anti-cancer therapy and had a shorter mOS (1.4 months, 95% confidence interval [CI], 0.7-1.3) than those who received anti-cancer treatment (n = 29; mOS, 20.1 months; p < 0.001). Finally, multivariate analysis for OS revealed that a strong c-MET IHC staining score of 3+ (hazard ratio [HR]: 2.05, 95% CI: 1.23–3.43; p = 0.006), initial brain metastasis (HR: 3.86, 95% CI: 1.52–9.82; p = 0.005), and treatment with supportive care without anti-cancer therapy (HR: 11.78, 95% CI: 3.40–40.86; p < 0.001) were associated with poor survival outcomes (Table 2).
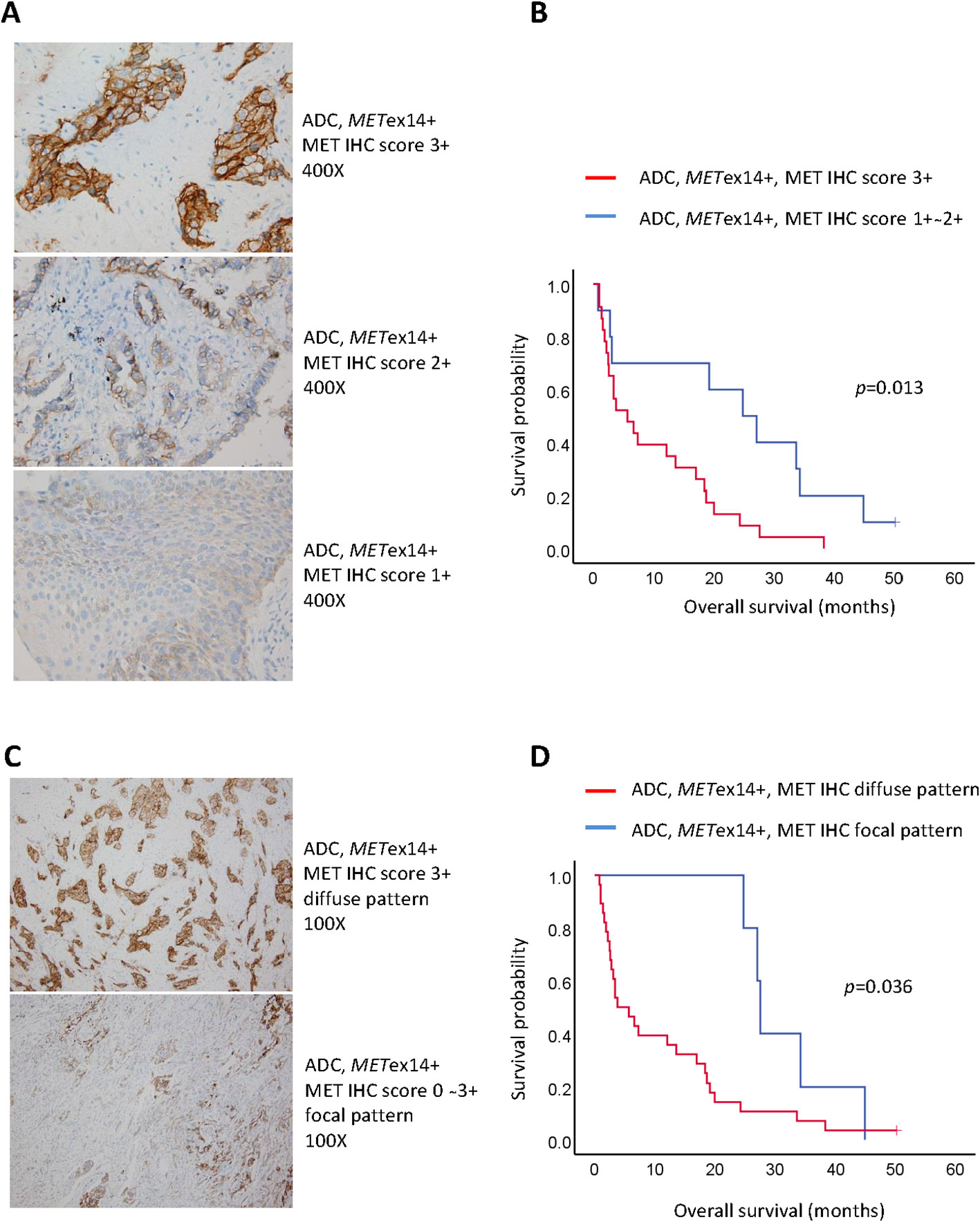
Figure 2 Pathological factors associated with poor prognosis in stage IV lung adenocarcinoma harboring METex14 (METex14+). (A) Representative figures of c-MET immunohistochemistry staining score 3+ (upper panel; original magnification: 400x), score 2+ (middle panel; original magnification: 400x), and score 1+ (lower panel; original magnification: 400x); (B) Kaplan-Meier curves of overall survival for score 1+ ~ 2+ and score 3+; (C) Representative figures of c-MET immunohistochemistry distribution patterns ― diffuse pattern (upper panel; original magnification: 100x) and focal pattern (lower panel; original magnification: 100x); (D) Kaplan-Meier curves of overall survival for diffuse and focal patterns.
3.3 Univariate and multivariate analyses of prognostic factors for overall survival in stage IV adenocarcinoma patients harboring the METex14 mutation who received anti-cancer therapy
We next aimed to determine whether patient characteristics and differences in treatment modalities would affect the OS in the 29 lung ADC patients with the METex14 mutation who received at least one anti-cancer therapy (Table 3). All the patients had an ECOG PS score of 0-2. In univariate analysis, strong c-MET ICH staining (score 3+) was consistently associated with shorter mOS than weak-to-moderate staining (score 1+ to 2+; mOS, 7.3 and 27.1 months, respectively; p = 0.015). Although the c-MET IHC distribution pattern (p = 0.068) and initial brain metastasis (p = 0.061) showed a trend, the findings did not reach statistical significance. Other characteristics, such as the number of metastatic sites and malignant pleural effusion, were not associated with survival outcomes. Nevertheless, longer survival periods were observed in some subgroups. Consistently better mOS was observed in patients who received lung radiation therapy than patients who did not receive this treatment (27.6 vs. 12.1 months, p = 0.002). Patients who received immunotherapy showed a favorable mOS (n = 5; mOS, 44.9 months) than those who did not (n = 24; mOS, 13.6 months; p = 0.032). Similarly, patients treated with pemetrexed (n = 19; mOS, 20 months) showed a more favorable mOS than those who were not (n = 10; mOS, 5.7 months; p = 0.011). Finally, patients treated with MET TKIs (n = 6; mOS, 19.2 months) showed a trend of prolonged OS in comparison with those who did not receive MET TKI (n = 23; mOS, 13.6 months; p = 0.065). Other therapeutic modalities, including lung surgery, brain RT, bone or spine RT, and chemotherapy with cisplatin doublet, gemcitabine, or taxanes, did not significantly predict OS.
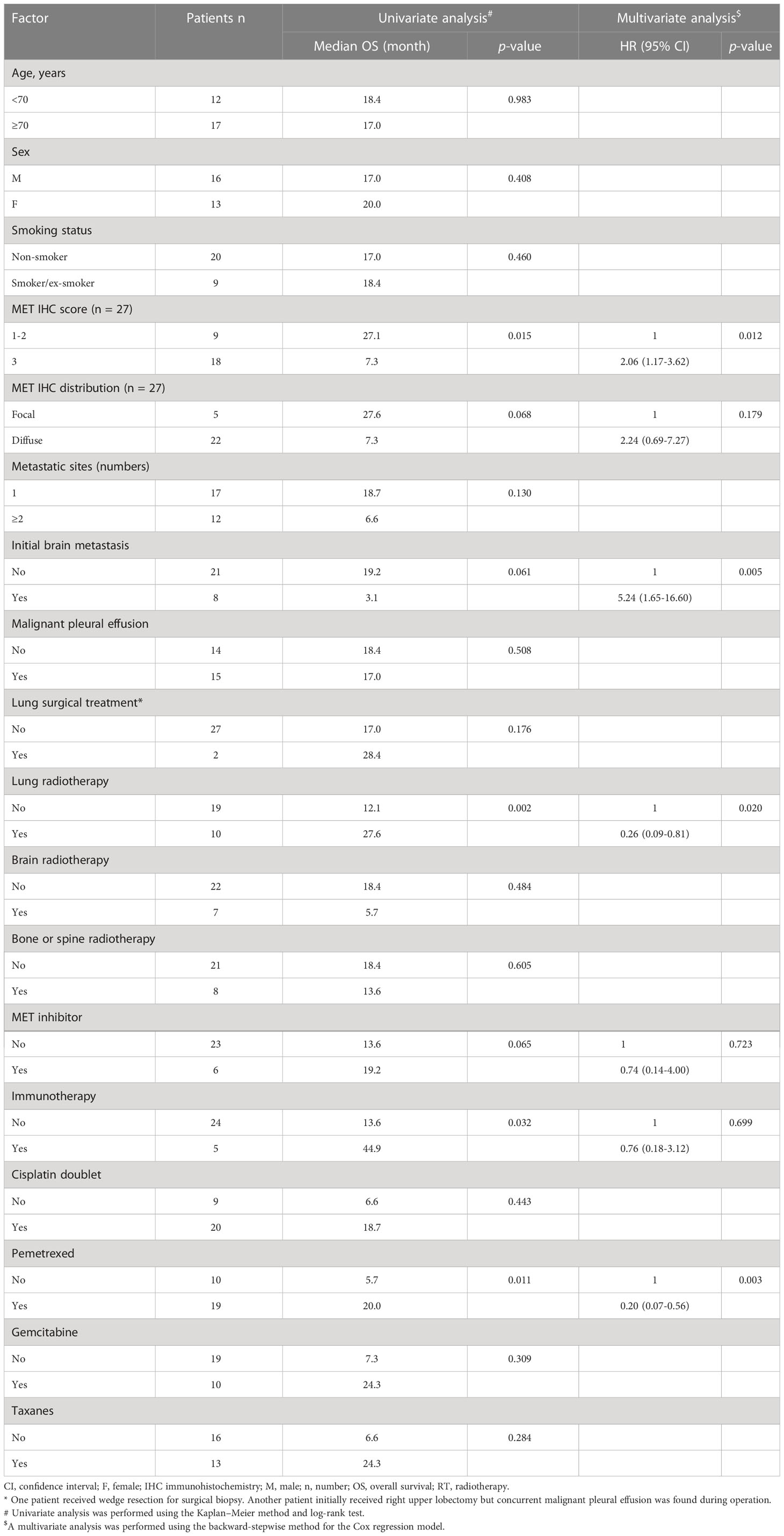
Table 3 Univariate and multivariate analyses of prognostic factors for overall survival in patients with stage IV METex14-positive adenocarcinomas who received anti-cancer treatments (n = 29).
Multivariate analyses for 29 stage IV ADC patients carrying the METex14 mutation were performed, and the variables with p-values less than 0.1 in the univariate analysis were included (Table 3). After adjusting for clinicopathological factors, a significantly longer OS was observed in patients who received pemetrexed (HR: 0.20; 95% CI: 0.07–0.56; p = 0.003) and those who were treated with lung radiotherapy (HR, 0.26; 95% CI: 0.09–0.81; p = 0.020). Similar to the findings for all stage IV ADC patients harboring the METex14 mutation, anti-cancer therapy with initial brain metastasis (HR: 5.24, 95% CI: 1.65–16.60; p = 0.005) and strong c-MET IHC staining (HR: 2.06, 95% CI: 1.17–3.62; p = 0.012) consistently predicted poor survival outcomes in these 29 patients.
3.4 Survival outcomes of stage IV METex14-positive lung cancer patients in comparison with those without the METex14 mutation
For the stage IV PSC cases in our cohort, the estimated mOS was 4.8 months in the seven METex14-positive patients and 3.8 months in the 23 patients without METex14, indicating a similar mOS and poor survival in both groups (p = 0.429; Figure 3A). Among the METex14-positive patients, five of the seven PSC patients were poor chemotherapy responders. Most of these patients received less than four courses of first and/or second-line cisplatin doublet-based chemotherapy with rapid progression. One patient with an ECOG PS of 4 died within 2 weeks of diagnosis who received best supportive care. Another patient received a course of pembrolizumab and was lost to follow-up. After excluding patients who received only supportive care, similar mOS was observed between METex14-positive patients (n=6; mOS, 4.8 months) and non-METex14 patients (n=18; mOS, 5.4 months, p=0.388; Supplementary Figure 1A).
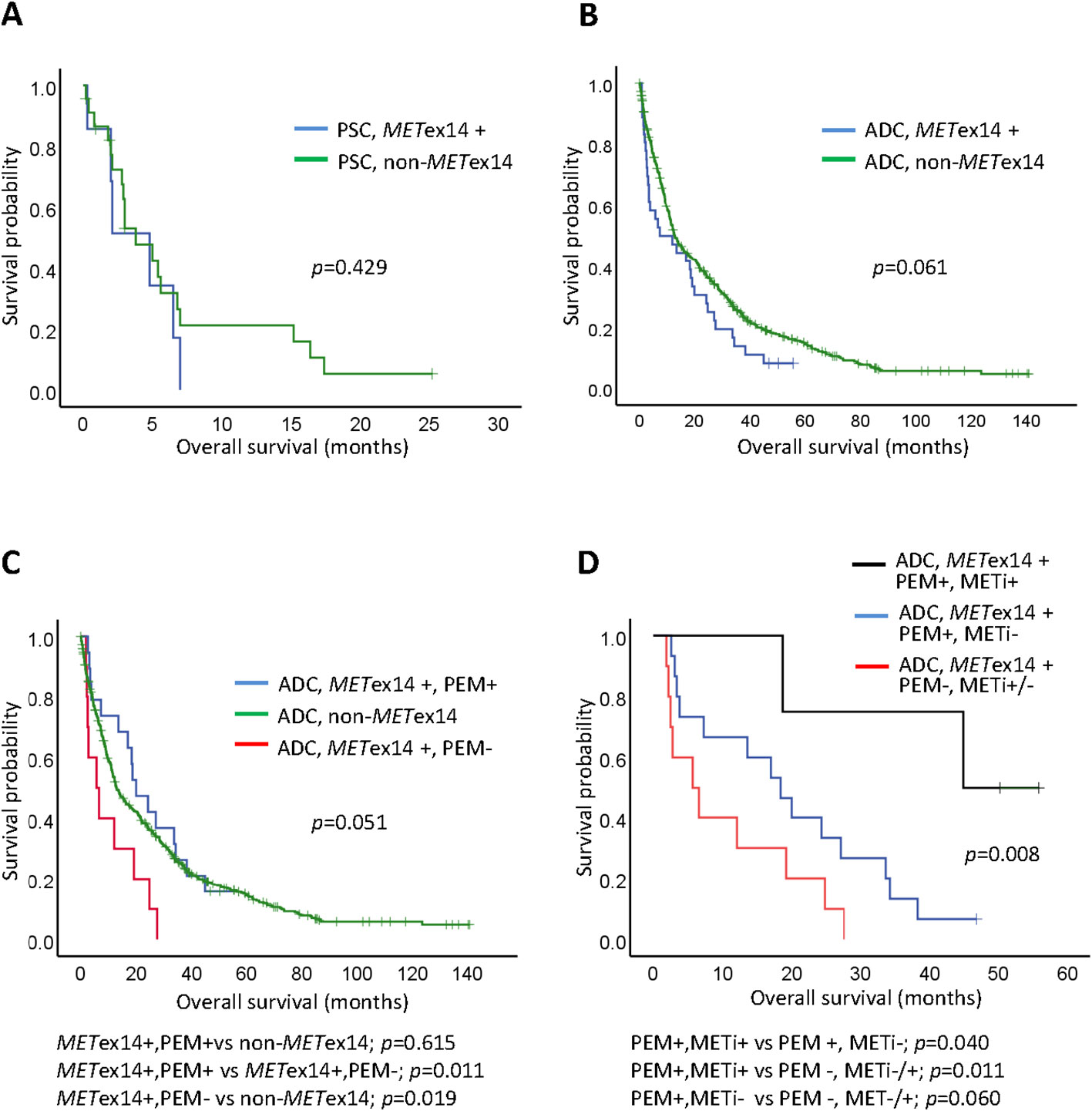
Figure 3 Kaplan-Meier curves of overall survival (OS) for Stage IV lung cancers. (A) Pulmonary sarcomatoid carcinoma (PSC) in METex14 positive (METex14+) or non-METex14 patients; (B) Adenocarcinoma (ADC) patients with METex14 (METex14+) or non-METex14 patients; (C) ADC patients with METex14 who received pemetrexed (PEM+), ADC patients with METex14 who did not received pemetrexed (PEM-), and ADC with non-METex14 patients; pairwise comparisons for p-value were shown below the panel; (D) ADC patients with METex14 who received sequential pemetrexed (PEM+) and MET inhibitor (METi+), who received pemetrexed (PEM+) without MET inhibitor (METi-), and who did not receive pemetrexed (PEM-) and with or without MET inhibitor (METi+/-); pairwise comparisons for p-value were shown below the panel.
We next evaluated and compared the survival outcomes of patients with stage IV lung ADC with and without the METex14 mutation. The mOS was 7.3 months in METex14-positive patients (n = 36; 95% CI: 0-18.9) and 12.9 months in the patients without the METex14 mutation (n = 524; 95% CI: 10.8-15.0; Figure 3B). Although the OS was shorter in patients with the METex14 mutation, this trend did not show statistical significance (p = 0.061). After excluding patients who received only supportive care, a comparable survival outcome was observed between METex14-positive patients (n=29; mOS, 18.4 months; 95% CI: 9.4-27.4) and non-METex14 patients (n=469; mOS, 15.9 months; 95% CI: 12.3-19.5 p=0.236; Supplementary Figure 1B).
We further evaluated 29 patients who had been treated with pemetrexed and/or MET TKI. The detailed duration of whole treatment regimens for patients with METex14 were shown in Supplementary Figure 2. Pairwise comparisons of the 524 patients without the METex14 mutation (non-METex14), 19 METex14-positive patients receiving pemetrexed (METex14+, PEM+), and 10 METex14-positive patients treated with chemotherapeutic agents other than pemetrexed (METex14+, PEM-) were performed (Figure 3C). As mentioned in the univariate analysis, METex14+, PEM+ patients showed better mOS than METex14+, PEM- patients (p = 0.011). No significant difference in mOS was observed between METex14+, PEM+ patients (20.0 months) and non-METex14 patients (p = 0.615). However, METex14+, PEM- ADC patients had a worse mOS (5.7 months) than the non-METex14 patients (p = 0.019; Figure 3C). Six patients received one or two lines of MET TKIs (Supplementary Table 2). Four patients treated with sequential pemetrexed with MET TKIs (at different time periods) had an mOS of NR (not reached), which was longer than that of the 15 patients who received pemetrexed without MET TKIs (mOS, 18.4 months; p = 0.040; Figure 3D), and was much better than that of the 10 patients who did not receive pemetrexed (including two patients who received MET TKIs but no pemetrexed), whose mOS was 5.7 months (p = 0.011; Figure 3D).
4 Discussion
Several clinical studies and trials have reported that NSCLC patients harboring METex14-positive tumors benefit from MET TKIs (9, 11, 13, 17, 21). However, not all patients showed clinical efficacy, and the response duration was limited. In the real world, some patients do not receive a specific MEK-TKI. In this study, we performed a multi-faceted evaluation of several prognostic factors associated with survival outcomes in a cohort of patients with lung cancer. We first successfully performed RNA-based PCR analysis and identified higher frequencies of METex14 in PSC, followed by ADC, and smaller frequencies in other lung cancer subtypes. For patients with stage IV lung ADC, we comprehensively analyzed the potential variables influencing survival outcomes. We showed that initial brain metastases and strong MET IHC staining may help predict OS. These results provide important information and shed light on the survival characteristics of lung cancer patients with METex14-positive tumors.
Previous studies reported that the overall incidence of the METex14 mutation was approximately 20%-30% in PSC and 3%-4% in ADC (6, 22). Our study reported a similar frequency of the METext14 mutation in PSC. Although pleomorphic carcinoma is categorized as a subtype of PSC in the 2015 World Health Organization (WHO) classification of lung tumors (23), we classified it as an independent subtype of lung cancer because the frequency of the METex14 mutation in pleomorphic carcinoma (5%) was quite different from that in PSC (24%). Moreover, the METex14 mutation was detected in 6.6% of lung ADC patients without EGFR and ALK mutations. Other characteristics of METex14-positive lung cancer, such as a predominance in female patients and an association with smoking, have been reported in previous studies (22, 24) but were not shown in our cohort and other studies (25, 26). The advanced age of patients with the METex14 mutation has been reported in the current and previous studies (21, 22, 25). Finally, these patients were more fragile and generally had a poorer ECOG PS than those without the METex14 mutation. As reported in previous studies, these demographic characteristics were associated with a poor OS, which may contribute to a highly aggressive subtype and short survival outcome for lung cancer patients carrying METex14-positive tumors (8, 27).
PSC is considered an aggressive subtype of lung cancer. Patients with PSCs generally show rapid progression, early metastasis, and dismal prognosis (28). The mOS of stage IV PSC patients was only 5.4 months in results from the National Cancer Database (29) and 2 months in those from the Surveillance, Epidemiology, and End Results (SEER) database (30). Patients who received anti-cancer chemotherapy still had a short mOS of only 6 months (31). A comparable and dismal survival outcome was observed in the current study; the mOS was <5 months for the advanced-stage patients with METex14-positive and METex14-negative PSC. None of the patients with METex14-positive PSC received targeted therapy, and most were not chemotherapy responders. This observation was consistent with the findings of a previous study that described the characteristics of chemoresistance and early progression in PSC (32). At present, evidence of treatment efficacy for METex14-positive PSC is inconclusive, and immune checkpoint inhibitors (ICIs) have been reported to show good efficacy in limited cases (33, 34). However, a pooled analysis of published data demonstrated that the METex14 mutation was not associated with tumor response (35). The impact of MET TKIs on OS in advanced PSC is also still unclear and variable since most reports either had small patient populations or were case reports, and described patients treated with different MET inhibitors (15, 17, 36). Lu et al. recently reported that 25 stage III or IV PSC patients with the METex14 mutation who were treated with savolitinib, a selective MET TKI, showed promising results with a response rate of 40% and a median PFS of 5.5 months (35), providing a beacon of hope for such dismal cases.
We identified several potential prognostic factors that predicted the OS in stage IV ADC patients harboring METex14 mutations. Pathological factors, including the staining score and the distribution of c-MET IHC staining, may help predict the OS. In particular, strong c-MET IHC staining with a score of 3+ was consistently associated with a short OS in both univariate and multivariate analyses for patients who received anti-cancer therapy. Awad et al. had previously reported that c-MET IHC staining in stage IV METex14-positive NSCLC was significantly stronger than that in stage I to III NSCLC with the METex14 mutation (27). The observation that strong MET expression could predict shorter OS in NSCLC (37, 38) suggests that a high MET IHC staining score may be an indicator of aggressive behavior in METex14-positive ADC (16, 39).
Among several treatment modalities, lung radiotherapy was associated with a longer mOS in both univariate and multivariate analyses. Treatment of primary lung tumors with thoracic radiotherapy has been reported to effectively ameliorate clinical respiratory symptoms, reduce tumor size, control recurrence, and prolong survival in patients with advanced NSCLC (40, 41). In our study, among 10 patients treated with lung radiotherapy, 9 patients received lung and/or mediastinal RT and other systemic therapies (chemotherapy or MET TKI) at different times and showed an mOS of 33.7 months (95% CI, 15.9-51.5), which was significantly longer than that in patients who received systemic therapy alone (mOS, 12.1 months; 95% CI, 2.1-22.0). Although MET activation plays an important role in conferring resistance to ionizing radiation by altering intracellular DNA damage response pathways in various cancer types (42), the underlying biological mechanism for prolonged OS in METex14-positive lung ADC patients receiving a combination of systemic therapy and local RT remains unclear. Radiotherapy may have diminished the resistance to systemic therapy and chemotherapy or TKI treatment may have enhanced radiosensitivity, thereby prolonging the treatment period and improving survival outcomes (43).
In multivariate analysis, initial brain metastasis was an independent risk factor for poor survival outcome in stage IV lung ADC patients harboring the METex14 mutation. The frequency of initial brain metastasis in this patient population was 30% (11 of 36), and the median OS was only 2.8 months for all 11 patients and 3.1 months for the eight patients who received anti-cancer therapy. Among them, two patients received crizotinib and six patients received brain RT plus standard chemotherapy. This mOS was inferior to that described in a previous report, which demonstrated a 6-month median OS in NSCLC with brain metastasis at initial presentation (44). The short OS in our cohort may be associated with the lack of effective treatment in most cases. Currently, the highly selective and potent MET inhibitors capmatinib and tepotinib are recommended and approved for the treatment of such patients because of their ability to cross the blood–brain barrier. A rapid partial response and impressive duration of response were reported with these inhibitors in patients with the METex14 mutation (45), and further follow-up data may be necessary to determine whether campatinib or tepotinib can prolong the OS in such patients.
Pemetrexed treatment is another strong predictor of OS for stage IV ADC patients with the METex14 mutation, which may be partly explained as a small population with MET TKI therapy in this study. However, pemetrexed–carboplatin plus pemetrexed maintenance regimen is currently used as initial chemotherapy for advanced NSCLC without targetable driver mutations (46), and may play a role in the treatment of advanced-stage ADC patients with the METex14 mutation when they are not able to receive specific MET TKIs or after MET TKI failure. Other chemotherapeutic agents, such as gemcitabine and taxanes, however, were not associated with favorable survival outcomes. Although METex14-positive ADC patients were shown to respond to platinum doublet therapy with a disease control rate of 80% in a study with small case number (47), we suggest that pemetrexed-based chemotherapy should be considered first if these patients need chemotherapy (48). Further prospective studies are needed to evaluate the role of pemetrexed in patients with advanced ADC with the METex14 mutation.
The present study had several limitations. Patient recruitment and data collection were retrospective, resulting in an inherent selection bias. Moreover, because of the relative rarity of METex14 in lung cancer, the imbalance in the number of mutation-positive and mutation-negative patients limited the viability of the analyses. In addition, intrinsic analysis of OS for stage IV ADC disease with the METex14 mutation may have been affected by the limited number of cases in each group. Finally, the type, combination, and sequence of chemotherapy, MET TKI, and immunotherapy varied among the study patients. Nevertheless, the present study provided crucial insights into the characteristics, associated factors, and survival outcomes of lung cancer patients with the METex14 mutation. Further large-scale prospective studies focusing on these prognostic factors are necessary to overcome these limitations.
5 Conclusion
In both lung ADC and PSC, patients with and without the METex14 mutation showed comparable survival outcomes. A higher frequency of METex14 mutations was detected in PSC patients and these patients had poor overall survival. In lung ADC, pemetrexed-based chemotherapy (with or without MET TKI), strong c-MET ICH staining, brain metastasis at initial presentation, and lung radiotherapy were independent prognostic factors associated with survival outcomes. These findings provide information that can be expected to be important in clinical settings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the National Taiwan University Hospital, Taipei, Taiwan (201509070RINA and 201103013RC). The patients/participants provided their written informed consent to participate in this study.
Author contributions
C-HG and J-YS designed the study and analyzed the data. M-SH contributed to pathological analysis. Y-LC and Y-NL performed genetic mutation analysis. C-HG wrote the manuscript. C-HG, M-SH, Y-NL, S-GW, and J-YS edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the Far Eastern Memorial Hospital-National Taiwan University Hospital Joint Research Program grant (No. 110-FTN24), Taiwan.
Acknowledgments
The authors sincerely thank Department of Medical Research, National Taiwan University Hospital, for providing laboratory facilities.
Conflict of interest
J-YS has served as an advisory board member for Amgen, AstraZeneca, Roche, Pfizer, Novartis, Merck Sharp, Dohme, Takeda, CStone Pharmaceuticals, Janssen, and Bristol-Myers Squibb; received speaking honoraria from ACT Genomics, Amgen, Chugai Pharma, CStone Pharmaceuticals, Bayer, AstraZeneca, Eli Lilly, Boehringer Ingelheim, Genconn Biotech, Roche, Novartis, TTY Biopharm, Pfizer, Orient EuroPharma, MundiPharma, Takeda, Janssen, Merck Sharp, Dohme, and Bristol-Myers Squibb; and received a grant from F. Hoffmann-La Roche Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1113696/full#supplementary-material
References
1. Waarts MR, Stonestrom AJ, Park YC, Levine RL. Targeting mutations in cancer. J Clin Invest (2022) 132(8):e154943. doi: 10.1172/JCI154943
2. Sadiq AA, Salgia R. Met as a possible target for non-Small-Cell lung cancer. J Clin Oncol (2013) 31(8):1089–96. doi: 10.1200/JCO.2012.43.9422
3. Awad MM. Impaired c-met receptor degradation mediated by met exon 14 mutations in non-Small-Cell lung cancer. J Clin Oncol (2016) 34(8):879–81. doi: 10.1200/JCO.2015.64.2777
4. Socinski MA, Pennell NA, Davies KD. Met exon 14 skipping mutations in non-Small-Cell lung cancer: An overview of biology, clinical outcomes, and testing considerations. JCO Precis Oncol (2021) 5:PO.20.00516. doi: 10.1200/PO.20.00516
5. DaviDavies KD, Merrick DT. Skipping expected mechanisms of met-mediated oncogenesis. J Thorac Oncol (2020) 15(1):9–11. doi: 10.1016/j.jtho.2019.11.003
6. Vuong HG, Ho ATN, Altibi AMA, Nakazawa T, Katoh R, Kondo T. Clinicopathological implications of met exon 14 mutations in non-small cell lung cancer - a systematic review and meta-analysis. Lung Cancer (2018) 123:76–82. doi: 10.1016/j.lungcan.2018.07.006
7. Liu XW, Chen XR, Rong YM, Lyu N, Xu CW, Wang F, et al. Met exon 14 skipping mutation, amplification and overexpression in pulmonary sarcomatoid carcinoma: A multi-center study. Trans Oncol (2020) 13(12):100868. doi: 10.1016/j.tranon.2020.100868
8. Tong JH, Yeung SF, Chan AW, Chung LY, Chau SL, Lung RW, et al. Met amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res (2016) 22(12):3048–56. doi: 10.1158/1078-0432.CCR-15-2061
9. Paik PK, Drilon A, Fan PD, Yu H, Rekhtman N, Ginsberg MS, et al. Response to met inhibitors in patients with stage iv lung adenocarcinomas harboring met mutations causing exon 14 skipping. Cancer Discovery (2015) 5(8):842–9. doi: 10.1158/2159-8290.CD-14-1467
10. Landi L, Chiari R, Tiseo M, D'Inca F, Dazzi C, Chella A, et al. Crizotinib in met-deregulated or Ros1-rearranged pretreated non-small cell lung cancer (Metros): A phase ii, prospective, multicenter, two-arms trial. Clin Cancer Res (2019) 25(24):7312–9. doi: 10.1158/1078-0432.CCR-19-0994
11. Wolf J, Seto T, Han JY, Reguart N, Garon EB, Groen HJM, et al. Capmatinib in met exon 14-mutated or met-amplified non-Small-Cell lung cancer. New Engl J Med (2020) 383(10):944–57. doi: 10.1056/NEJMoa2002787
12. Paik PK, Felip E, Veillon R, Sakai H, Cortot AB, Garassino MC, et al. Tepotinib in non-Small-Cell lung cancer with met exon 14 skipping mutations. New Engl J Med (2020) 383(10):931–43. doi: 10.1056/NEJMoa2004407
13. Davies KD, Ritterhouse LL, Snow AN, Sidiropoulos N. Met exon 14 skipping mutations: Essential considerations for current management of non-small cell lung cancer. J Mol diagn JMD (2022) 24(8):841-43. doi: 10.1016/j.jmoldx.2022.04.005
14. Friedlaender A, Drilon A, Banna GL, Peters S, Addeo A. The meteoric rise of met in lung cancer. Cancer (2020) 126(22):4826–37. doi: 10.1002/cncr.33159
15. Awad MM, Leonardi GC, Kravets S, Dahlberg SE, Drilon A, Noonan SA, et al. Impact of met inhibitors on survival among patients with non-small cell lung cancer harboring met exon 14 mutations: A retrospective analysis. Lung Cancer (2019) 133:96–102. doi: 10.1016/j.lungcan.2019.05.011
16. Gow CH, Hsieh MS, Wu SG, Shih JY. A comprehensive analysis of clinical outcomes in lung cancer patients harboring a met exon 14 skipping mutation compared to other driver mutations in an East Asian population. Lung Cancer (2017) 103:82–9. doi: 10.1016/j.lungcan.2016.12.001
17. Illini O, Fabikan H, Swalduz A, Vikstrom A, Krenbek D, Schumacher M, et al. Real-world experience with capmatinib in met exon 14-mutated non-small cell lung cancer (Recap): A retrospective analysis from an early access program. Ther Adv Med Oncol (2022) 14:17588359221103206. doi: 10.1177/17588359221103206
18. Guo R, Offin M, Brannon AR, Chang J, Chow A, Delasos L, et al. Met exon 14-altered lung cancers and met inhibitor resistance. Clin Cancer Res (2021) 27(3):799–806. doi: 10.1158/1078-0432.CCR-20-2861
19. Gow CH, Hsieh MS, Lin YT, Liu YN, Shih JY. Validation of immunohistochemistry for the detection of braf V600e-mutated lung adenocarcinomas. Cancers (2019) 11(6):866. doi: 10.3390/cancers11060866
20. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol (1982) 5(6):649–55. doi: 10.1097/00000421-198212000-00014
21. Schrock AB, Frampton GM, Suh J, Chalmers ZR, Rosenzweig M, Erlich RL, et al. Characterization of 298 patients with lung cancer harboring met exon 14 skipping alterations. J Thorac Oncol (2016) 11(9):1493–502. doi: 10.1016/j.jtho.2016.06.004
22. Coleman N, Harbery A, Heuss S, Vivanco I, Popat S. Targeting un-met needs in advanced non-small cell lung cancer. Lung Cancer (2022) 164:56–68. doi: 10.1016/j.lungcan.2021.12.016
23. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 world health organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol (2015) 10(9):1243–60. doi: 10.1097/JTO.0000000000000630
24. Zheng D, Wang R, Ye T, Yu S, Hu H, Shen X, et al. Met exon 14 skipping defines a unique molecular class of non-small cell lung cancer. Oncotarget (2016) 7(27):41691–702. doi: 10.18632/oncotarget.9541
25. Digumarthy SR, Mendoza DP, Zhang EW, Lennerz JK, Heist RS. Clinicopathologic and imaging features of non-Small-Cell lung cancer with met exon 14 skipping mutations. Cancers (2019) 11(12):2033. doi: 10.3390/cancers11122033
26. Champagnac A, Bringuier PP, Barritault M, Isaac S, Watkin E, Forest F, et al. Frequency of met exon 14 skipping mutations in non-small cell lung cancer according to technical approach in routine diagnosis: Results from a real-life cohort of 2,369 patients. J Thorac Dis (2020) 12(5):2172–8. doi: 10.21037/jtd.2020.04.21
27. Awad MM, Oxnard GR, Jackman DM, Savukoski DO, Hall D, Shivdasani P, et al. Met exon 14 mutations in non-Small-Cell lung cancer are associated with advanced age and stage-dependent met genomic amplification and c-met overexpression. J Clin Oncol (2016) 34(7):721–30. doi: 10.1200/JCO.2015.63.4600
28. Brazel D, Zhang S, Nagasaka M. Spotlight on tepotinib and capmatinib for non-small cell lung cancer with met exon 14 skipping mutation. Lung Cancer (2022) 13:33–45. doi: 10.2147/LCTT.S360574
29. Steuer CE, Behera M, Liu Y, Fu C, Gillespie TW, Saba NF, et al. Pulmonary sarcomatoid carcinoma: An analysis of the national cancer data base. Clin Lung Cancer (2017) 18(3):286–92. doi: 10.1016/j.cllc.2016.11.016
30. Chen M, Yang Q, Xu Z, Luo B, Li F, Yu Y, et al. Survival analysis and prediction model for pulmonary sarcomatoid carcinoma based on seer database. Front Oncol (2021) 11:630885. doi: 10.3389/fonc.2021.630885
31. Xiao C, Yang X, Hao J, Guo C, Pu Q, Liu L. Clinicopathological features and prognostic analysis of metastatic pulmonary sarcomatoid carcinoma: A seer analysis. J Thorac Dis (2021) 13(2):893–905. doi: 10.21037/jtd-20-2826
32. Giroux Leprieur E, Antoine M, Vieira T, Duruisseaux M, Poulot V, Rabbe N, et al. Clinical and molecular features in patients with advanced non-Small-Cell lung carcinoma refractory to first-line platinum-based chemotherapy. Lung Cancer (2013) 79(2):167–72. doi: 10.1016/j.lungcan.2012.10.010
33. Xu L, Tao NN, Liang B, Li DW, Li HC, Su LL. Use of pd-1 inhibitor tislelizumab in the treatment of advanced pulmonary sarcomatoid carcinoma: A case report. Thorac Cancer (2022) 13(3):502–5. doi: 10.1111/1759-7714.14290
34. Taniguchi H, Takemoto S, Ozasa M, Honda N, Suyama T, Umeyama Y, et al. Remarkable response to pembrolizumab with platinum-doublet in pd-L1-Low pulmonary sarcomatoid carcinoma: A case report. Thorac Cancer (2021) 12(7):1126–30. doi: 10.1111/1759-7714.13890
35. Lu S, Fang J, Li X, Cao L, Zhou J, Guo Q, et al. Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-Small-Cell lung cancers harbouring met exon 14 skipping alterations: A multicentre, single-arm, open-label, phase 2 study. Lancet Respir Med (2021) 9(10):1154–64. doi: 10.1016/S2213-2600(21)00084-9
36. Gu L, Wei X, Zhang Z, Heng W. Treatment response to immunotherapy after crizotinib resistance in a patient with pulmonary sarcomatoid carcinoma harboring met exon 14 skipping mutation: A case report. Clin Med Insights Oncol (2022) 16:11795549211067185. doi: 10.1177/11795549211067185
37. Pyo JS, Kang G, Cho WJ, Choi SB. Clinicopathological significance and concordance analysis of c-met immunohistochemistry in non-small cell lung cancers: A meta-analysis. Pathol Res Pract (2016) 212(8):710–6. doi: 10.1016/j.prp.2016.05.006
38. Yin W, Guo M, Tang Z, Toruner GA, Cheng J, Medeiros LJ, et al. Met expression level in lung adenocarcinoma loosely correlates with met copy number Gain/Amplification and is a poor predictor of patient outcome. Cancers (2022) 14(10):2433. doi: 10.3390/cancers14102433
39. Canadas I, Rojo F, Taus A, Arpi O, Arumi-Uria M, Pijuan L, et al. Targeting epithelial-to-Mesenchymal transition with met inhibitors reverts chemoresistance in small cell lung cancer. Clin Cancer Res (2014) 20(4):938–50. doi: 10.1158/1078-0432.CCR-13-1330
40. Su SF, Hu YX, Ouyang WW, Lu B, Ma Z, Li QS, et al. Overall survival and toxicities regarding thoracic three-dimensional radiotherapy with concurrent chemotherapy for stage iv non-small cell lung cancer: Results of a prospective single-center study. BMC Cancer (2013) 13:474. doi: 10.1186/1471-2407-13-474
41. Higginson DS, Chen RC, Tracton G, Morris DE, Halle J, Rosenman JG, et al. The impact of local and regional disease extent on overall survival in patients with advanced stage Iiib/Iv non-small cell lung carcinoma. Int J Radiat oncol biol Phys (2012) 84(3):e385–92. doi: 10.1016/j.ijrobp.2012.04.045
42. Bhardwaj V, Cascone T, Cortez MA, Amini A, Evans J, Komaki RU, et al. Modulation of c-met signaling and cellular sensitivity to radiation: Potential implications for therapy. Cancer (2013) 119(10):1768–75. doi: 10.1002/cncr.27965
43. Cui J, Li L, Yuan S. The value of radiotherapy for advanced non-small cell lung cancer with oncogene driver-mutation. Front Oncol (2022) 12:863715. doi: 10.3389/fonc.2022.863715
44. Waqar SN, Samson PP, Robinson CG, Bradley J, Devarakonda S, Du L, et al. Non-Small-Cell lung cancer with brain metastasis at presentation. Clin Lung Cancer (2018) 19(4):e373–e9. doi: 10.1016/j.cllc.2018.01.007
45. Paik PK, Goyal RK, Cai B, Price M, Davis K, Derrien Ansquer V, et al. Real-world assessment of clinical outcomes in nsclc patients with met exon 14 skipping mutation and brain metastases (Bm) treated with capmatinib. J Clin Oncol (2022) 40(16_suppl):e21171–e. doi: 10.1200/JCO.2022.40.16_suppl.e21171
46. Zukin M, Barrios CH, Pereira JR, Ribeiro Rde A, Beato CA, do Nascimento YN, et al. Randomized phase iii trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-Small-Cell lung cancer and Eastern cooperative oncology group performance status of 2. J Clin Oncol (2013) 31(23):2849–53. doi: 10.1200/JCO.2012.48.1911
47. Wong SK, Alex D, Bosdet I, Hughesman C, Karsan A, Yip S, et al. Met exon 14 skipping mutation positive non-small cell lung cancer: Response to systemic therapy. Lung Cancer (2021) 154:142–5. doi: 10.1016/j.lungcan.2021.02.030
Keywords: adenocarcinoma, immunohistochemistry, MET exon 14 skipping, pulmonary sarcomatoid carcinoma, overall survival
Citation: Gow C-H, Hsieh M-S, Chen Y-L, Liu Y-N, Wu S-G and Shih J-Y (2023) Survival outcomes and prognostic factors of lung cancer patients with the MET exon 14 skipping mutation: A single-center real-world study. Front. Oncol. 13:1113696. doi: 10.3389/fonc.2023.1113696
Received: 01 December 2022; Accepted: 23 February 2023;
Published: 09 March 2023.
Edited by:
Shinkichi Takamori, National Hospital Organization Kyushu Cancer Center, JapanReviewed by:
Ying Chen, Kunming Medical University, ChinaLei Lei, University of Chinese Academy of Sciences, China
Copyright © 2023 Gow, Hsieh, Chen, Liu, Wu and Shih. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin-Yuan Shih, anlzaGloQG50dS5lZHUudHc=
 Chien-Hung Gow
Chien-Hung Gow Min-Shu Hsieh
Min-Shu Hsieh Yi-Lin Chen2
Yi-Lin Chen2 Shang-Gin Wu
Shang-Gin Wu Jin-Yuan Shih
Jin-Yuan Shih