- 1Department of Pharmacy, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Hubei Province Clinical Research Center for Precision Medicine for Critical Illness, Wuhan, China
- 3Department of Hematology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Dasatinib, a second-generation tyrosine kinase inhibitor, is recommended as first-line treatment for patients newly diagnosed with chronic myeloid leukemia (CML) and second-line treatment for those who are resistant or intolerant to therapy with imatinib. Dasatinib is superior to imatinib in terms of clinical response; however, the potential pulmonary toxicities associated with dasatinib, such as pulmonary arterial hypertension and pleural effusion, may limit its clinical use. Appropriate management of dasatinib-related severe events is important for improving the quality of life and prognosis of patients with CML. This review summarizes current knowledge regarding the characteristics, potential mechanisms, and clinical management of adverse reactions occurring after treatment of CML with dasatinib.
Case 1
A 62-year-old female with a pervious medical history of hypertension, type 2 diabetes mellitus, and dyslipidemia was diagnosed with chronic-phase CML. She received treatment with dasatinib 100 mg once daily. Which tests should be conducted regularly during follow-up?
Case 2
A 71-year-old male, previously diagnosed with type II diabetes, hyperlipidemia, and coronary artery disease, was diagnosed with imatinib-resistant CML. He received treatment with dasatinib 50 mg once daily. Which cardiovascular tests should be performed prior to treatment? Which cardiovascular examination should be conducted during follow-up?
1 Introduction
Chronic myeloid leukemia (CML) is a type of cancer originating from the clonal proliferation of bone marrow hematopoietic stem cells. The disease is characterized by the oncogenic Philadelphia (Ph) chromosome carrying the BCR-ABL fusion gene (1). Imatinib was the first tyrosine kinase inhibitor (TKI) approved for the treatment of patients with Ph+ CML, which significantly improved the 5-year overall survival rate from 11% to 90% (2, 3). Second-generation TKIs are mainly designed to overcome resistance to imatinib; these agents have been associated with more rapid and profound molecular responses (4, 5). Dasatinib, a second-generation TKI, is increasingly proposed as first-line treatment for patients with CML (6, 7). Nevertheless, treatment with dasatinib has been linked to some uncommon adverse events (AEs), such as pleural effusion (PE) and pulmonary arterial hypertension (PAH) (8, 9). This narrative review summarizes current knowledge regarding the characteristics, potential mechanisms, and clinical management of AEs occurring after treatment of CML with dasatinib.
2 Methods
We searched for relevant full-text articles in the PubMed database using the following terms: “dasatinib”, “tyrosine kinase inhibitor”, “chronic myeloid leukemia”, “drug-related side effects*”, “side effects*”, “adverse reactions*”, “adverse drug reactions*”, “adverse drug events*”, “adverse events*”, and “drug toxicity”. These search terms were combined with the Boolean operators (AND/OR). All relevant case reports, clinical trials, and reviews were included. Articles published in languages other than English and those for which the full text was not available were excluded.
3 Clinical characteristic of dasatinib related AEs in patients with CML
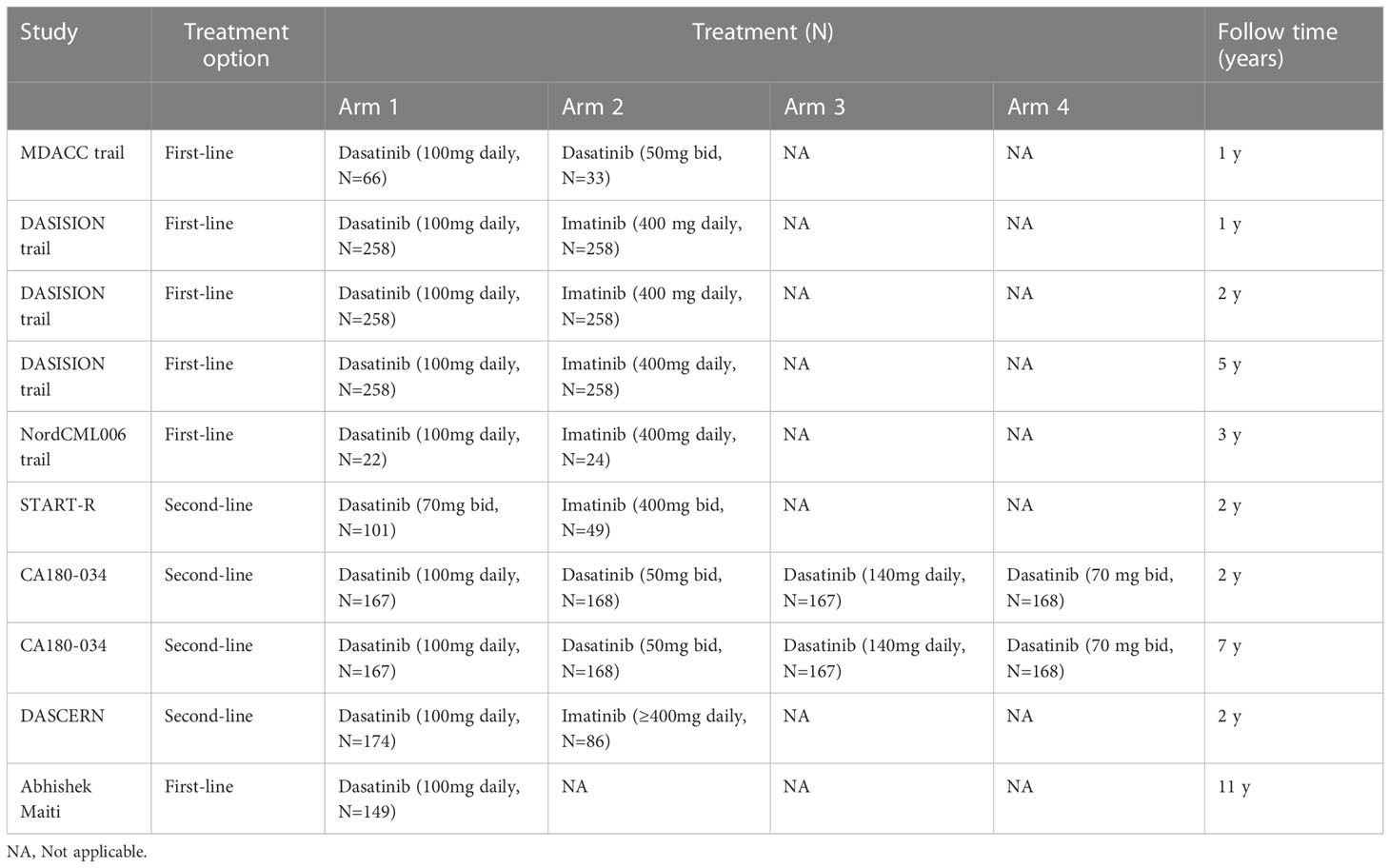
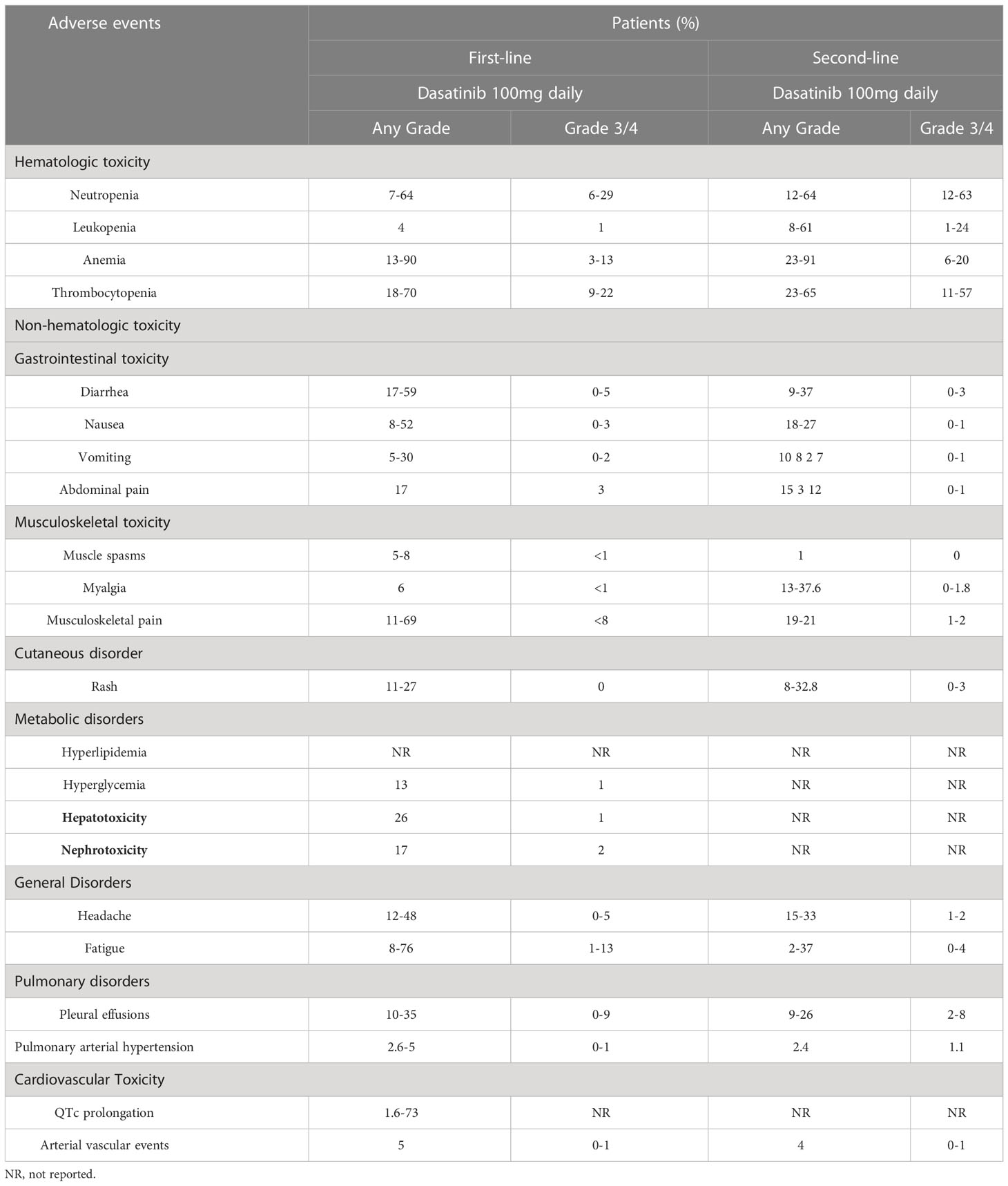
Dasatinib-related AEs are mainly attributed to hematological and non-hematological toxicities. Table 1 shows the characteristics of major clinical studies in this setting. Hematological toxicity is the most common AE associated with TKIs, and mainly manifested as leukopenia, neutropenia, anemia, and thrombocytopenia (Table 2). Serious hematological toxicity (grade 3/4) usually occurs within a few months after treatment; though rare, it is a major cause of dose reduction or treatment interruption. The administration of medication may be gradually resumed after remission. Non-hematological toxicity includes gastrointestinal toxicity, cutaneous reactions, musculoskeletal disorders, metabolic disorders, nephrotoxicity, pulmonary toxicity, and cardiovascular toxicity (Table 2). It mostly occurs at the time of treatment initiation and generally resolves without medical intervention. However, dasatinib-induced pulmonary toxicities (Table 3) require special attention, especially in the high-risk population.
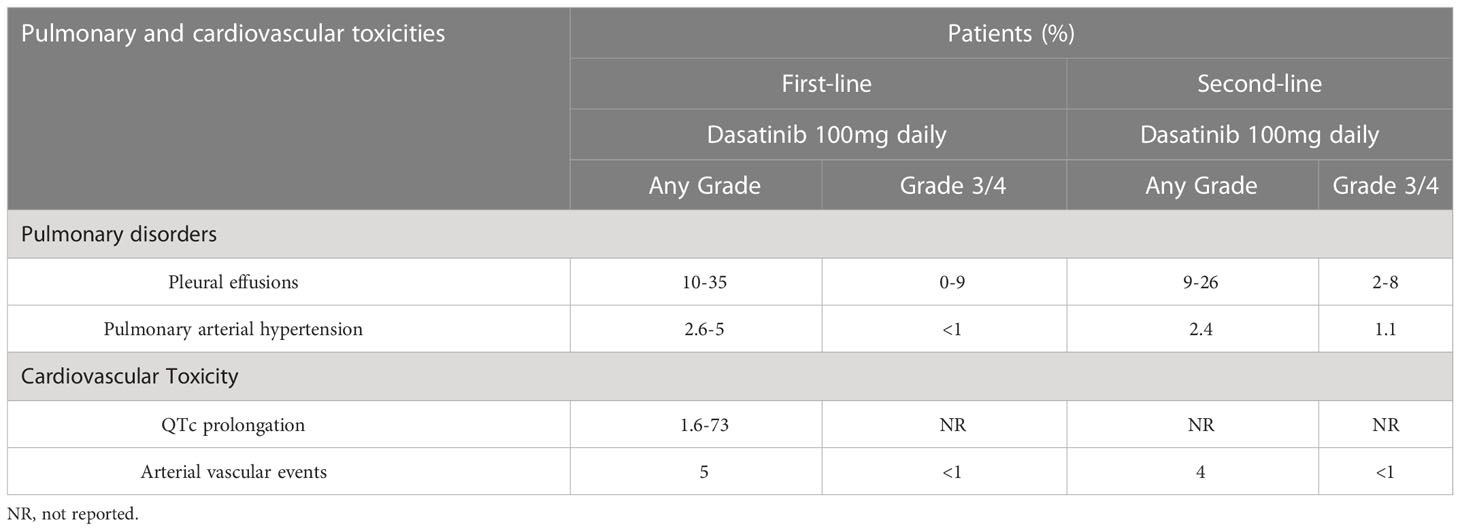
Table 3 Incidence of pulmonary disorders and cardiovascular toxicity reported in dasatinib treatment.
3.1 Hematologic toxicity, related mechanisms, and clinical management
In the MD Anderson Cancer Center (MDACC) trial, (10) dasatinib was used as first-line therapy for chronic-phase CML (CML-CP), and patients were randomized to receive 50 mg twice daily or 100 mg once daily. Most hematologic toxicities were temporary and mild; severe cases could be managed with emergency treatment disruptions and dosage reductions. The incidence rates of grade 3/4 neutropenia, anemia, and thrombocytopenia were 21% (n=13), 6% (n=4), and 10% (n=6), respectively. All grade 4 hematological toxicities (i.e., two cases of neutropenia and three cases of thrombocytopenia) occurred in the 50 mg twice daily group. The DASISION clinical trial (11) compared dasatinib (100 mg daily) with imatinib (400 mg daily). During the 12-month follow-up, most hematologic toxicities occurred within the first 4 months after treatment initiation. The incidence rates of grade 3/4 neutropenia, anemia, and thrombocytopenia were 21%, 10%, and 19% in the dasatinib group, and 7%, 10%, and 20% in the imatinib group, respectively. At 24 months, the rates of hematologic toxicity were similar to those recorded at 12 months (12). Moreover, at 5 years(13), there was no observation of new AEs. Grade 3/4 hematologic toxicity mostly occurred in the dasatinib group compared with the imatinib group (neutropenia: 29% vs. 24%; anemia: 13% vs. 9%; and thrombocytopenia: 22% vs. 14%, respectively). Maiti et al. performed a long-term follow-up study with dasatinib as initial therapy for patients with CML-CP (14). Hematological toxicities (any grade versus grade 3/4) were neutropenia (7% vs. 6%, respectively), leukopenia (4% vs. 1%, respectively), anemia (39% vs. 3%, respectively), and thrombocytopenia (27% vs. 11%, respectively). A network meta‐analysis was conducted to compare the safety profile of TKIs (i.e., ponatinib, bosutinib, dasatinib, nilotinib, imatinib, and radotinib) in patients with CML (15). The results showed that, among the examined TKIs, dasatinib was the least safe drug for CML and linked to a high risk of serious hematological toxicities. The surface under the cumulative ranking curve values for treatment with 140 mg dasatinib were 87.4%, 90.6%, 90.3%, and 97.2% for leucopenia, neutropenia, anemia, and thrombocytopenia, respectively.
There are two possible reasons associated with dasatinib-related hematologic toxicity. Firstly, Ph-negative cells were reserved by a weakened hematopoietic system compared with baseline, which is an inherent characteristic of high-risk patients. A previous study found that the development of hematologic toxicity during the first months of treatment with imatinib may be indicative of a worse long-term clinical outcome (16, 17). Secondly, dasatinib might play a role in several SRC kinase signal pathways, such as Lyn or Fyn, thereby inhibiting the downstream effect of platelet aggregation and megakaryocytopoiesis (18, 19). SRC kinases play an essential role in erythropoiesis and the survival of B cells and myeloid cells (20–22). In addition, dasatinib may induce eryptosis in human erythrocytes through Ca2+ channel loading and membrane permeabilization (23).
Most hematological toxicities are grade 1/2 and self-limiting, while grade 3/4 hematological toxicities are managed with dosage reduction or treatment discontinuation (5, 24). Therefore, at the initiation of treatment, blood testing is performed once per week during the first month, every 2 weeks in the second and third months, and every 3 months thereafter (25). In case of an absolute neutrophil count (ANC) <0.5 × 109/L or platelet count <50 × 109/L, the administration of dasatinib should be suspended. Treatment may be resumed when values for the ANC and platelet count reach ≥1 × 109/L and ≥50 × 109/L, respectively. Following the return of the ANC to the normal range within 2 weeks, dasatinib can be re-initiated at the original dose. In case of consistent decline in blood cells for >1 week after discontinuation of treatment with dasatinib, the dosage should be reduced to 70 mg or 50 mg once daily following a return within the normal range. In case of persistent neutropenia, the patient should be treated with growth factor. Although treatment with erythropoietin is effective against grade 3/4 anemia, recent guidelines do not recommend its use; instead, infusion of red blood cells is recommended (26, 27).
3.2 Gastrointestinal toxicity, related mechanisms, and management
Gastrointestinal AEs (e.g., diarrhea, nausea, vomiting, constipation, and abdominal pain) commonly occur after treatment with dasatinib. It has been reported that 30–50% of patients with CML experience gastrointestinal AEs (mostly grade 1/2) (28). In a recent clinical study of dasatinib as first-line therapy for patients with CML (14) (follow-up period: 11 years) the occurrence rate of nausea, vomiting, diarrhea, constipation, and abdominal pain was 52%, 30%, 59%, 34%, and 17%, respectively. In addition, the occurrence rate of grade 3/4 gastrointestinal AEs was <5%. Shah et al. carried out a 7-year follow-up study of treatment with dasatinib in patients with CML-CP who were resistant to imatinib or intolerant to 100 mg once daily compared with the other treatment arms (i.e., 140 mg once daily, 50 mg twice daily, and 70 mg twice daily) (CA180-034 trial) (29). The cumulative rates of gastrointestinal AEs were as follows: diarrhea (42% vs. 47%, respectively), and nausea/vomiting (27% vs. 43%, respectively); the AEs were mainly mild to moderate (grade 1/2). Furthermore, a recent meta-analysis evaluated differences in gastrointestinal AEs between different TKIs (30). The results showed that diarrhea (22.5%) was the most common symptom, followed by nausea (20.6%), and vomiting (12.9%). Also, there was significant difference in the average incidence of gastrointestinal AEs among TKIs: bostinib (52.9%), imatinib (24.2%), dasatinib (20.4%), and nilotinib (9.1%). Diarrhea was the most common gastrointestinal AE of dasatinib (28.1%). Nausea was the most common gastrointestinal AE of imatinib (33.0%) and nilotinib (13.2%). The incidence rate of grade 3/4 gastrointestinal AEs was ≤3%, except for severe diarrhea caused by bosutinib (9.5%).
A potential biological explanation of dasatinib-induced diarrhea is that the drug inhibits the stem cell factor receptor tyrosine kinase c-kit, which is highly expressed in gastrointestinal pacemaker cells (31, 32). Administration of dasatinib with food can improve symptoms of nausea and vomiting (33); treatment of nausea should be considered if the symptom persists. Supportive care, for instance, antidiarrheal agents (e.g., loperamide) with dietary modifications, should be given in case of severe or frequent diarrhea (34). Replacement with other TKIs or dose reduction, is recommended for patients with persistent severe gastrointestinal AEs and limited response to treatment (25).
3.3 Musculoskeletal toxicity, related mechanisms, and management
Dasatinib-induced musculoskeletal AEs mainly include muscle spasms, musculoskeletal pain, and myalgia. Previous studies showed that muscle spasms and myalgia were observed in 24% of patients treated with imatinib; however, these AEs occur less frequently with second-generation TKIs (35, 36). Musculoskeletal AEs were reported in 6% of patients receiving treatment with dasatinib, and the rate of grade 3/4 events was <1% (37). A long-term follow-up study showed that dasatinib-induced skeletal pain (e.g., joint pain, back pain, bone pain, limb pain, and chest wall pain) occurred in 103 patients (69%) (14). Muscle edema may be attributed to the inhibition of platelet-derived growth factor receptor beta (PDGFR-β) (38–40). However, whether PDGFR-β is associated with dasatinib-induced muscle cramps and myalgias remains unclear. Thus far, there are no drugs for the treatment of dasatinib-induced musculoskeletal AEs. Calcium supplementation can be considered to relieve muscle cramps, and bone and joint pain can be ameliorated by non-steroidal anti-inflammatory drugs (25).
3.4 Cutaneous reaction, related mechanisms, and management
Cutaneous toxicity is a common non-hematologic AE associated with TKIs. The incidence rate of cutaneous AEs in patients who received treatment with dasatinib was 11–27%(41–43). One phase I and five phase II clinical trials involving a total of 911 patients showed that the incidence of cutaneous toxicity of dasatinib was approximately 35%(44–46). In addition, the incidence of skin rash in patients with accelerated (22%) or chronic (13–27%) phase disease was higher than that recorded in patients with myeloid blast (11–14%) or lymphoid blast (15–17%) phase disease (47). Most events were grade 1/2 local and systemic erythema, macular and epidemic rash, or exfoliative rash. Of the patients, 16% had mucositis and/or stomatitis and 11% had pruritus. Dasatinib-induced rare cutaneous events were observed in individual case reports (46, 48): two patients with CML-CP who received dasatinib developed painful panniculitis. A case of possible small-vessel vasculitis by dasatinib-induced alveolitis has also been reported. However, a biopsy was not performed, and the AE resolved after intravenous administration of methyl prednisone (49).
The cutaneous toxicity of dasatinib is usually reversible, mild, and mostly self-limiting (50). For severe cases, the local use and short-term systemic administration of steroids can be considered to alleviate the symptoms. Severe or persistent manifestations may require dose reduction or temporary discontinuation of treatment. For skin-related symptoms, systemic corticosteroids should be used in conjunction with other supportive care. Following the resumption of treatment after temporary discontinuation due to serious skin events, steroids should be used at the beginning of re-introduction to prevent the recurrence of allergic reactions; the dosage can be gradually tapered after reaching the full dose. In some cases, a strategy involving the gradual increase of the dose may be required to achieve remission. Patients with fair skin should avoid direct exposure to sunlight or use sunscreen (41, 47, 50).
3.5 Metabolic disorders, mechanisms, and management
Generally, hyperglycemia and hyperlipidemia are rarely caused by dasatinib in patients with CML. Lu et al. compared the incidence of metabolic-related diseases in patients with CML who received imatinib, dasatinib, and nilotinib (51). They found that dasatinib was not significantly correlated with low/high blood glucose and hypertriglyceridemia. However, some studies have reported that dasatinib can lower the levels of blood glucose. Keiko et al. observed that dasatinib significantly improved hyperglycemia in a CML patient with diabetes (52). Franklin et al. evaluated the incidence rate of type 2 diabetes mellitus and hyperlipidemia in patients with CML who received dasatinib or nilotinib. The incidence rate of type 2 diabetes mellitus (dasatinib, n=1,272; nilotinib, n=732) and hyperlipidemia (dasatinib, n=845; nilotinib, n=435) was 1.76% and 4.64%, respectively (53). A previous study showed that dasatinib could increase the levels of peroxisome proliferative activated receptor gamma coactivator 1 alpha (PGC-1α) in adipose tissue, but exerted an adverse effect on glucose intolerance in obese mice (54). However, these effects have not been investigated in human studies.
The levels of lipids and glucose should be monitored periodically during the first year of dasatinib therapy, and assessed at least once per year during follow-up. Additionally, healthcare professionals should assist patients in developing a healthy lifestyle (i.e., avoidance of tobacco use, regular exercise, and adherence to a low-fat and low-carbohydrate diet). If intervention is warranted, patients with CML should be managed according to the relevant guidelines for the treatment of hyperlipidemia and diabetes (55).
3.6 Hepatotoxicity and nephrotoxicity, related mechanisms and management
3.6.1 Hepatotoxicity
Previous studies have shown that elevation of alanine aminotransferase/aspartate aminotransferase levels may occur in 26% of patients treated with dasatinib and in <1% of severe cases(56, 57). Dasatinib-induced hyperbilirubinemia is rare in patients with CML-CP or accelerated phase disease (1%); nevertheless, it is more common in those with blast phase disease (4–5%) (58). A recent meta-analysis showed that bosutinib, nilotinib, or ponatinib were associated with a higher risk of hepatotoxicity than imatinib; however, dasatinib was not linked to a significantly increased risk (59). The mechanism of dasatinib-related hepatotoxicity remains unclear. Direct and indirect mitochondrial toxicity may play an important role in the mechanism underlying the hepatotoxicity induced by dasatinib and bonatinib (60). In addition, an in vitro study investigating drug-induced hepatotoxicity showed that treatment with TKIs upregulated bile acid synthesis and further altered bile acid uptake and excretion (61).
3.6.2 Nephrotoxicity
Although preclinical and clinical trials failed to link dasatinib to the occurrence of severe nephrotoxicity, some rare cases of nephrotoxicity have been found in clinical practice. An analysis of the recently updated US Food and Drug Administration (FDA) Adverse Reporting System database indicated that dasatinib was related with a high risk of nephrotoxicity, especially protein glomerular disease, versus other TKIs (62). Although, three cases of acute renal failure have been reported, they were not definitively linked to dasatinib (63). A previous study showed that the incidence of proteinuria in patients treated with dasatinib was 18%, and grade 3/4 events were rare (64). To our knowledge, thus far, nine cases of nephrotic syndrome caused by dasatinib have been reported (three children, six adults) (65–72).
Biopsy specimens obtained from patients with dasatinib-related nephrotic proteinuria revealed pinocytosis; electron microscopy showed that these cases were characterized by reduced podocyte foot processes (67–70, 72). Podocytes are essential for the establishment of the glomerular blood urine filtration barrier, and damage to these cells leads to proteinuria (73). Nephrotic proteinuria associated with dasatinib may be attributed to the inhibition of vascular endothelial-derived growth factor (VEGF) production (74–76). VEGF is produced in podocytes and binds to VEGFR-2, thereby controlling the cytoskeleton and interstitial membrane of these cells(77). Therefore, the glomerular damage induced by dasatinib may be similar to that caused by other VEGF inhibitors. However, a recent study showed that dasatinib-associated nephrotoxicity was mainly related with its direct action on podocytes, rather than inhibition of VEGF (62). A previous study found that, following treatment with dasatinib, podocytes showed obvious molecular changes in adhesions, the actin cytoskeleton, and morphology (62, 78). Accumulating evidence confirms that dasatinib-induced glomerular toxicity directly affects the structural integrity of the podocyte cytoskeleton. This results in decreased cell elasticity and disruption of the key function of the podocyte cytoskeleton as a structural member of the filtration barrier (62, 79, 80).
Liver and renal function should be evaluated before initiating treatment with dasatinib. Moreover, routine testing should be performed during treatment. Dasatinib-induced hepatotoxicity and nephrotoxicity is usually reversible and self-limiting. Sustained significant or severe hepatotoxicity and nephrotoxicity may require more extensive evaluation. Such cases might be managed with dose reduction, temporary discontinuation of treatment, or replacement of dasatinib with other TKIs. In case of severe liver or renal injury, a specialist should be contacted for active treatment (81).
3.7 Pulmonary disorders, related mechanisms, and management
Pulmonary toxicities are unique AEs of dasatinib; the most commonly reported pulmonary toxicities are PE, PH, pulmonary edema, interstitial lung disease, pneumonia, chylothorax, etc. (82–85). Most of these AEs are clinically significant; hence, following the occurrence of these reactions, treatment discontinuation and additional medical intervention are required. Dasatinib-induced PE and PAH are discussed in more detail in the following sections.
3.7.1 PE
PE has been observed after treatment with all BCR-ABL1 TKIs, and dasatinib has been linked to the highest incidence (≤35%) (86–88). The frequency of occurrence, PE-related risk factors, and outcomes were assessed in two phase 3 clinical trials [i.e., DASISION (11) and 034/Dose optimization (29)] and in a pooled population (n=2,712) of 11 trials (12, 13, 29, 44, 45, 89–93) involving patients with CML and Ph+ acute lymphoblastic leukemia who received dasatinib therapy. PE was found in 6–9% and 5–15% of patients per year in the DASISION and 034/Dose optimization trials, respectively. During the minimum follow-up of 5 and 7 years, PE occurred in 28% and 33% of patients in the DASISION(13) and 034/Dose optimization trials (29), respectively. In a long-term follow-up study of 149 patients with CML who received dasatinib (14), the incidence of PE was 26%; 3% of cases experienced grade 3/4 events. Annually, PE occurred in 15% of patients in the first year of treatment, 2–5% in the following 3 years, and approximately 1–2% thereafter. Shah et al. reported that PE occurred in 6% of CML patients treated with dasatinib 50 mg daily (89). However, PE was not correlated with the overall response to dasatinib, progression-free survival, or overall survival in patients with CML (94). Furthermore, risk factors for PE were the initial dosage (140 mg vs. 100 mg), twice-daily regimen, hypercholesterolemia, hypertension, other pulmonary diseases, and a medical history of cardiovascular events or autoimmune diseases (7, 82, 95). Moreover, the accelerated and blast phases of disease were closely associated with the development of PE, especially in patients who received high-dose dasatinib (94). In addition, the most important risk factor for the development of PE in clinical studies is patient age, which may be involved with exposure to dasatinib (96–98). Notably, dasatinib-related PE was not found to be correlated with fluid retention (7).
The pathogenesis of dasatinib-related PE may be attributed to the inhibition of PDGFR expression in pericytes, which are involved in the regulation of angiogenesis (89). Moreover, it may be linked to the inhibition of Yes and SRC kinases, which are associated with pleural epithelial stability through the regulation of cell adhesion (99). Previous studies demonstrated that dasatinib was associated with lymphocytosis. Regarding the pharmacodynamic mechanism, dasatinib affects the ability to maintain pulmonary endothelial integrity in a dose-dependent manner by generating mitochondrial oxidative stress, inducing endothelial cell apoptosis, and impairing vascular permeability (100, 101). Recently, it has been proposed that the development of dasatinib-related PE is associated with changes in intercellular connectivity and the production of stress fibers and reactive oxygen species in the cytoplasm (102, 103).
Treatment interruption, dose reduction, and supportive care are recommended for the management of PE (94, 104, 105). Firstly, for the CML patients at high risk of developing PE, and respiratory failure and prior or concomitant pleuro-pulmonary disorders in primary care, one of the TKIs other than dasatinib should be chosen. In addition, initial treatment with half-dose dasatinib (50 mg/day) was suggested to be a safe option for patients with newly diagnosed CP-CML, which accompanied with more favorable clinical response and toxicity profile compared with the DASISION trial at a longer follow-up (106). Iurlo et al. (105) retrospectively collected 853 CML patients receiving dasatinib in both first-line and second-line of therapy. The results indicated that 70.4% of the PE events were observed in patients received 100 mg/day, and patients who have reached the MMR or DMR, dose reduction before PE development may help to reduce the incidence rate of PE. Furthermore, therapeutic drug monitoring (TDM)-guided dose adjustment may have potential benefits for dasatinib treatment in patients with CML. Rousselot et al. (97) found that TDM-guided dasatinib dose optimization was feasible and resulted in a significant reduction of PE events in the long-term treatment, without impairing MMR rate. Therefore, individual characteristics of the CML patients, comorbidities, toxicity profile, and physician-clinical center experience are among the critical factors to be comprehensively taken into account while deciding on the proper and personalized first-line dasatinib therapy (107).
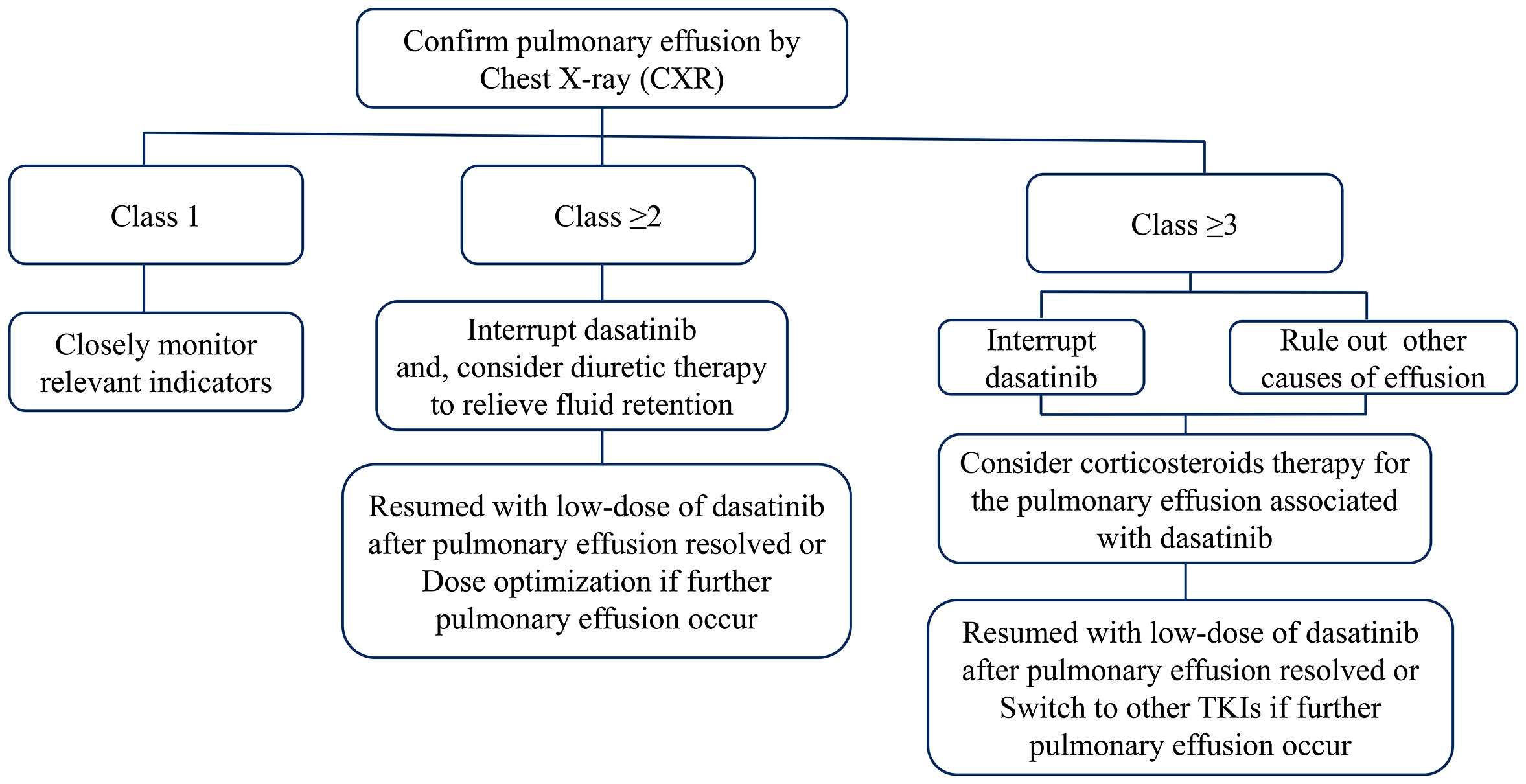
Early identification of PE by regular physical examination and chest radiography is crucial to avoid the development of more severe conditions. Intervention is not required for patients with grade 1 PE. For asymptomatic patients with grade ≥2 PE, the administration of dasatinib should be interrupted, and diuretic therapy could be initiated if fluid retention occurs. Dasatinib therapy can be resumed after the effusion has subsided. The dosage should also be reconsidered for the prevention of further episodes. For symptomatic patients with grade ≥2 PE, and asymptomatic patients with grade ≥3 PE, dasatinib therapy should be discontinued, and aggressive treatment with corticosteroids (40 mg daily for 4 days) should be initiated. Therapeutic thoracentesis should also be performed in severe cases. Dasatinib can be re-introduced following the resolution of the effusion. However, switching to other TKIs is recommended for the management of PE recurrence in severe cases (Figure 1).
Another approach for the management of PE depends on the severity and effusion volume. Cortes et al. (103) defined PE as: “small effusion” (effusion volume <500 mL with a blunting view of the costophrenic angle), “medium effusion” (opacity above the costophrenic angle), and “large effusion” (>30–50% effusion in half of the thorax). Asymptomatic patients with “small effusion” should be followed up regularly with chest X-ray (CXR) monitoring every 3 months in the first year and twice annually in the second year. For symptomatic patients, the frequency of CXR monitoring should be increased; dose reduction may be considered based on the treatment response, and CXR monitoring should be performed 1 month later. In case of persistent PE severity, CXR monitoring should be performed as described above. When the symptoms persist or worsen, the management is similar to that for medium/large effusions. Following the first episode of medium/large effusion, dasatinib should be immediately discontinued, and therapeutic thoracentesis should be performed. This should be followed by CXR monitoring every 2–4 weeks to assess the effusion volume until complete resolution. According to the clinical response of patients, dasatinib can be re-administered at a lower dose. Following the occurrence of more than two episodes, it is recommended to replace dasatinib with other TKIs.
3.7.2 Pulmonary arterial hypertension
PAH is a life-threatening condition associated with long-term dasatinib therapy, especially in patients with PE (108, 109). In the absence of timely treatment, PAH may lead to right ventricular failure. Dasatinib-related reversible PAH was first reported in 2009(110). In 2012, nine cases of severe dasatinib-related PAH were described (111). According to the study conducted by Montani et.al, the estimated minimum annual incidence of dasatinib-related PAH in France is 0.45% (8). However, in a multicenter Australian study involving 212 patients who received dasatinib therapy, the estimated incidence of PH based on echocardiography was 5%; however, these cases were not confirmed by right-heart catheterization (112). As of December 31, 2017, the World Health Organization Pharmacovigilance Database (Vigibase®) included >440 cases of PAH associated with protein kinase inhibitors (8). In registry data, the overall incidence of dasatinib-related PH was <1%(113). In the DASISION trial (11), PH was suspected in 5% of patients based on echocardiography; nevertheless, only one patient underwent right-heart catheterization, which did not confirm the presence of PH. In the CA180-034 study (dasatinib as second-line therapy [n=670] in a 7-year follow-up) (29), PH of any grade was reported in 16 patients (2.4%); among those, grade 3/4 PH was detected in seven patients (1.1%). Of note, PAH was confirmed through right-heart catheterization in one patient. Furthermore, in a long-term follow-up study of dasatinib as initial therapy for patients with CML (n=149)(14), four patients with PE developed grade ≤2 PH, as detected by echocardiography and right-heart catheterization (two cases each).
The majority of patients who experienced PAH were female with history or present PE receiving long-term treatment with dasatinib(114, 115). Dasatinib-induced PAH mechanisms still remain unclear. It has been shown that dasatinib therapy increases the production of mitochondrial reactive oxygen species in a dose-dependent manner, further leading to endothelial apoptosis, pulmonary endothelial dysfunction, and PH (116). In addition, previous study indicated that chronic exposure to dasatinib may attenuate hypoxic pulmonary vasoconstriction responses and thus increase susceptibility to PAH, and seems to be independent of Src-inhibition (117–119). However, another reported mechanism underlying the effects of dasatinib is associated with SRC family kinases and PDGF pathways (120). SRC family kinases are responsible for cell proliferation in smooth muscle and reduce pulmonary arterial muscle tone. Inhibition of SRC family kinases leads to cell apoptosis and increased vascular resistance. Several studies have suggested that other signal pathways also contribute to endothelial dysfunction, leading to dasatinib-related PAH (99, 117, 121). Animal studies confirmed that dasatinib increased the biological activities of endothelial dysfunction markers (e.g., soluble vascular cell adhesion molecule 1 [VCAM-1], soluble intercellular adhesion molecule 1 [ICAM-1], and soluble E-selectin), leading to minimization of hypoxic vasoconstriction and impairment of endoplasmic reticulum function (117).
Given the relatively low incidence rate of dasatinib-related symptomatic PAH, systematic screening in asymptomatic patients is not readily feasible in the clinical setting. Transthoracic echocardiography is the preferred imaging technique for baseline risk stratification as it provides quantitative assessment of left and right ventricular function, chamber dilation, left ventricular hypertrophy, regional wall motion abnormalities, diastolic function, PAH, and pericardial disease, which may influence the therapeutic decision. Echocardiography could be performed as a reference baseline before initiating treatment with dasatinib to determine pre-existing PH. Following the occurrence of unexplained dyspnea with or without symptoms of right-heart dysfunction in the process of treatment, the first step is to evaluate the possibility of PH through echocardiography. Subsequently, examination through pulmonary function testing, CXR monitoring, and cardiac biomarker levels (e.g., troponin or N terminal pro-brain natriuretic peptide precursor) should be carried out to comprehensively assess the existence of other underlying conditions (e.g., PE, pneumonia, interstitial or obstructive pulmonary disease, ischemic heart disease, or left side heart failure). According to the current PAH guidelines, right-heart catheterization is recommended if echocardiography indicates an increased tricuspid regurgitation velocity >3.4 m/s, or <3.4 m/s with secondary PH signs (e.g., ventricular septum flattening or right ventricular dilation). High-resolution computed tomography or ventilatory perfusion scans should be performed to exclude other underlying causes of PH, such as pulmonary parenchymal disease or chronic thromboembolism. Notably, right-heart catheterization is essential for the accurate diagnosis and characterization of the hemodynamic profile. This is because dasatinib-induced PH may result in bilateral PE that could erroneously indicate post-capillary PH since it can be masked by left-heart disease. Patients treated with dasatinib may also have post-capillary PH due to elevated left cardiac pressure or increased PAH by overactivated circulation as a result of anemia or infection. Post-capillary PH is accompanied by high cardiac output volume and normal pulmonary vascular resistance(122, 123).
Treatment with dasatinib can be continued after confirmation of post-capillary PH or the hyperdynamic status through right-heart catheterization. However, following the confirmation of PAH, dasatinib should be immediately discontinued. For low-risk patients with mild symptoms, no right-heart failure, and low-risk hemodynamics, interruption of dasatinib therapy without intervention for PAH could be considered. Patients with moderate- or high-risk characteristics (e.g., severe symptoms, severe hemodynamic disturbances, or clinical symptoms of right-heart failure) should be proactively treated according to the guidelines for PAH (124, 125). Currently available medications for the treatment of PAH include phosphodiesterase type 5 inhibitor agents (e.g., sildenafil, tadalafil), soluble guanosine cyclase stimulants (e.g., riociguat), endothelin receptor antagonists (e.g., bosentan, macitentan), prostacyclin analogues (e.g., epoprostenol, traprost), and oral prostacyclin agonists (e.g., selexipag). Previous studies have reported that a combination of endothelin receptor antagonist and phosphodiesterase type 5 inhibitor can effectively treat TKI-induced PAH (108). Short-term re-assessment should be performed in all patients within 3–4 months of whether PAH related treatment is initiated. Given the high incidence of persistent PAH, invasive hemodynamic re-assessment should be performed wherever possible. Selection of the appropriate TKI depends on the severity of PAH and the stage of the underlying CML. Considering the likelihood of PAH deterioration or recurrence in this situation, patients should be closely monitored.
3.8 Cardiovascular toxicity, mechanisms, and management
3.8.1 Corrected QT interval prolongation
Only one patient (0.4%) had QTc >500 ms in the DASISION trial; this finding may be attributed to the exclusion of patients with severe or even controlled cardiovascular disease (13). It has been reported that dasatinib moderately prolongs the QTc interval according to Fridericia (QTcF) by an average of +3 to +6 ms, and is less frequently associated with QTcF prolongation >500 ms (0.7%) or QTcF increase >60 ms (2.9%). However, there was no correlation between cumulative exposure and QTcF prolongation (126). An extensive meta-analysis of studies conducted up to 2018 indicated significant differences in the incidence of QTc prolongation induced by different TKI agents. QTc interval prolongation was more commonly observed with dasatinib (range: 1.6–73%) versus other TKIs (127). In addition, the US prescribing information for dasatinib advises caution in patients with increased risk of QT interval prolongation (128).
3.8.2 Arterial vascular events
In October 2011, the US FDA issued a warning regarding the cardiopulmonary risks associated with dasatinib. It was recommended that patients using dasatinib should be monitored for signs and symptoms of cardiopulmonary disease before and during treatment with dasatinib (55). The 5-year follow-up of the DASISION trial(13) indicated an approximately 5% risk of an ischemic event in all patients, and two patients experienced transient ischemic attacks. A long-term follow-up study (14) showed that 13 patients (9%) had an arterial thrombotic event. These included nine cardiovascular events, comprising myocardial infarction (n=3), coronary artery disease (n=4), and chest pain (n=2); three cerebrovascular events (stroke or transient ischemic attack); and two cases of peripheral artery disease, including carotid stenosis (n=1) and peripheral artery disease together with coronary artery disease (n=1). The CA180-034 study (29) revealed an overall low incidence of ischemic events of all grades. The rate of cardiovascular ischemic events (e.g., myocardial infarction, angina, or coronary artery disease) was similar between the 100 mg daily group and all other dose groups (4% each). Main cardiac ischemic events reported in the 100 mg daily and the other dose groups were myocardial infarction (2% vs. 1%, respectively), angina (1% vs. 2%, respectively), and coronary artery disease (1% vs. <1%, respectively); the majority of ischemic events were grade 3/4. Furthermore, one patient experienced fatal myocardial infarction. Of the patients, 1% reported peripheral vascular events (all grades) in the other dosage groups (there were no events recorded in the 100 mg daily group), while <1% of patients reported grade 3/4 events. Cerebrovascular events (all grades) occurred in 3% and 1% of patients in the 100 mg daily and other dosage groups, respectively. Only 1% of the cerebrovascular events that occurred in the 100 mg daily group were grade 3/4. In contrast, all AEs recorded in other dosage groups were grade 3/4. A case/non-case study using AE reports registered in the US FDA Adverse Event Reporting System database compare the risk of cardiovascular events associated with TKIs. The results showed that dasatinib was associated with the highest incidence of heart failure and PH (129).
Clinical trials of dasatinib have yielded inconsistent results regarding the severity of cardiotoxicity. A statistical assessment showed that dasatinib did not significantly increase the risk of developing cardiovascular ischemia events in patients receiving the drug versus those with a similar condition (130). An analysis pooled the data obtained from the DASISION, READY, and 11 Phase I and II trials. The results demonstrated that the occurrence rate of ischemic events was 2–4%; however, most patients had a medical history or high-risk factors of atherosclerosis (128).
The mechanisms leading to cardiotoxicity are postulated to include mitochondrial dysfunction. Will et al. examined the effects of imatinib, dasatinib, sunitinib, and sorafenib on ATP content in H9c2 cells grown and respiratory capacity of isolated rat heart mitochondria. They found that only sorafenib directly impaired mitochondrial function at clinically relevant concentrations. For the other three kinase inhibitors lacking direct mitochondrial effects, altered kinase and other signaling pathways, are a more reasonable explanation for their cardiotoxicity (131). It is likely that toxicity is due to receptor kinase binding both on- and off-target. Five tyrosine kinase inhibitors (bosutinib, dasatinib, imatinib, nilotinib, and ponatinib) were evaluated in a neonatal rat myocyte model for their relative ability to induce myocyte damage (132). Results demonstrated that a lack of target selectivity was correlated with myocyte damage, but a correlation also existed with the strength of on-target Kd (dissociation constant).
Dexrazoxane is an anthracycline widely used as a cardiac protective agent; research has shown that this agent is ineffective in preventing dasatinib-induced damage to myocytes (133). Furthermore, treatment with dasatinib increased the accumulation of doxorubicin in myocytes, leading to damage. The mechanism underlying this effect may be associated with the ability of the drug to bind to one or more ATP-binding cassette-type efflux transporters(133, 134). Treatment with dasatinib also reduced the levels of phosphorylated extracellular signal-regulated kinase (ERK) in myocytes most likely by inhibiting RAF. It is well established that RAF/MEK/ERK is a pro-survival pathway. Thus, inhibition of this pathway by dasatinib suggests that this mechanism is involved in cardiovascular toxicity induced by this drug (133). Endothelial dysfunction was suggested to play an important role in dasatinib-induced cardiovascular toxicity (135–137). Moreover, Alsaad et al. found that dasatinib could induce hypertrophic markers in rat cardiomyocyte H9c2 cells through the aryl hydrocarbon receptor (AHR)-independent pathway (138), and Xu et al. revealed that dasatinib-induced cardiotoxicity acted via leading cardiomyocytes to High-mobility group box 1 (HMGB1)-mediated necroptosis(139), thereby suggesting other mechanisms may associated with dasatinib-induced cardiovascular toxicity.
Despite the low incidence of overall toxicity after treatment with dasatinib, such events can be life-threatening. According to 2022 ESC Guidelines on cardio-oncology (140), prior to the initiation of treatment with dasatinib, baseline cardiovascular risk assessment (including physical examination, blood pressure measurement, electrocardiogram, lipid profile, and HbA1c measurement) is recommended. In addition, baseline echocardiography is recommended in patients scheduled to receive dasatinib.
During the treatment, the use of drugs that may prolong the QT interval and strong cytochrome P450 family 3 subfamily A member 4 (CYP3A4) inhibitors that potentially increase drug accumulation and lead to life-threating cardiotoxicity should be avoided in patients treated with dasatinib. Extra caution should be exercised in patients receiving combination therapy with dasatinib and other cardiotoxic agents. Echocardiography may be considered every 6–12 months in patients who require long-term (>12 months) dasatinib therapy, and monitoring frequency should be considered every 3 months during the first year in high- and very high-risk patients. Importantly, clinicians should monitor the patients for any signs or symptoms of water and sodium retention, and intervene in time to reduce cardiac load and avoid cardiac insufficiency. Owing to the different effects of dasatinib on vascular diseases, the status of cardiovascular and metabolic conditions (e.g., hypertension, hyperlipidemia, and diabetes) should be evaluated multiple times after the initiation of treatment. The ankle-brachial index is a sensitive and specific method for the detection of systematic atherosclerosis. This index provides informative and noninvasive diagnosis for patients who initiate treatment with a specific TKI. When the ankle-brachial index value is beyond the normal range, duplex ultrasound must be conducted to identify the involved artery and atherosclerotic plaque; if necessary, angiography should be performed. Real-life data support the use of reduced doses of dasatinib to maintain optimal responses in patients initially treated with a full dose, with the aim of minimizing dose-dependent cardiovascular toxicity (119). However, following the occurrence of serious adverse cardiovascular reactions, dasatinib should be immediately discontinued. Cardiovascular events should be actively treated through collaboration with cardiologists, vascular medicine experts, or cardiac oncologists (141, 142).
4 Conclusion
Elucidation of the characteristics and potential mechanisms underlying the occurrence of dasatinib-associated AEs is crucial for the prevention, monitoring, and treatment of severe events. This would improve the clinical application of dasatinib and the quality of life of patients. The development of dasatinib-related AEs is linked to the therapeutic targets; hence, the distinction of therapeutic effects and toxicity is a major challenge for future research. Additional basic and clinical studies are warranted to gain deeper insight into the mechanisms underlying the dasatinib-induced toxicities. Such investigations may provide new directions for the discovery of novel BCR/ABL1 TKIs.
Author contributions
FC: Writing - Data Curation, Original Draft, Data Analysis. QX: Data Curation, Writing - Original Draft. QL: Data Curation, Writing- Original Draft. ZC: Data Curation. WL: Conceptualization, Visualization, Revised Draft.FZ: Project administration, Revised Draft. All authors contributed to the article and approved thesubmitted version.
Funding
This work was supported by Key R&D Program of Hubei Province (No.2020BCA060).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cortes J, Pavlovsky C, Saußele S. Chronic myeloid leukaemia. Lancet (2021) 398(10314):1914–26. doi: 10.1016/s0140-6736(21)01204-6
2. Bedard PL, Hyman DM, Davids MS, Siu LL. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet (2020) 395(10229):1078–88. doi: 10.1016/s0140-6736(20)30164-1
3. Cohen P, Cross D, Jänne PA. Kinase drug discovery 20 years after imatinib: progress and future directions. Nat Rev Drug Discovery (2021) 20(7):551–69. doi: 10.1038/s41573-021-00195-4
4. García-Gutiérrez V, Breccia M, Jabbour E, Mauro M, Cortes JE. A clinician perspective on the treatment of chronic myeloid leukemia in the chronic phase. J Hematol Oncol (2022) 15(1):90. doi: 10.1186/s13045-022-01309-0
5. Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2022 update on diagnosis, therapy, and monitoring. Am J Hematol (2022) 97(9):1236–56. doi: 10.1002/ajh.26642
6. Keating GM. Dasatinib: A review in chronic myeloid leukaemia and ph+ acute lymphoblastic leukaemia. Drugs (2017) 77(1):85–96. doi: 10.1007/s40265-016-0677-x
7. Khoury HJ, Guilhot F, Hughes TP, Kim DW, Cortes JE. Dasatinib treatment for Philadelphia chromosome-positive leukemias: practical considerations. Cancer (2009) 115(7):1381–94. doi: 10.1002/cncr.24155
8. Weatherald J, Bondeelle L, Chaumais MC, Guignabert C, Savale L, Jaïs X, et al. Pulmonary complications of bcr-abl tyrosine kinase inhibitors. Eur Respir J (2020) 56(4):2000279. doi: 10.1183/13993003.00279-2020
9. Nekoukar Z, Moghimi M, Salehifar E. A narrative review on adverse effects of dasatinib with a focus on pharmacotherapy of dasatinib-induced pulmonary toxicities. Blood Res (2021) 56(4):229–42. doi: 10.5045/br.2021.2021117
10. Pemmaraju N, Kantarjian HM, Luthra R, O'Brien S, Cortes JE. Results of a phase II trial of dasatinib as frontline therapy for chronic myeloid leukemia (CML) in chronic phase (CP). Blood (2011) 118(21):1700–0. doi: 10.1182/blood.V118.21.1700.1700
11. Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med (2010) 362(24):2260–70. doi: 10.1056/NEJMoa1002315
12. Kantarjian HM, Shah NP, Cortes JE, Baccarani M, Agarwal MB, Undurraga MS, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood (2012) 119(5):1123–9. doi: 10.1182/blood-2011-08-376087
13. Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boqué C, et al. Final 5-year study results of DASISION: The dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. J Clin Oncol (2016) 34(20):2333–40. doi: 10.1200/jco.2015.64.8899
14. Maiti A, Cortes JE, Patel KP, Masarova L, Borthakur G, Ravandi F, et al. Long-term results of frontline dasatinib in chronic myeloid leukemia. Cancer (2020) 126(7):1502–11. doi: 10.1002/cncr.32627
15. Fachi MM, Tonin FS, Leonart LP, Aguiar KS, Lenzi L, Figueiredo BC, et al. Comparative efficacy and safety of tyrosine kinase inhibitors for chronic myeloid leukaemia: A systematic review and network meta-analysis. Eur J Cancer (2018) 104:9–20. doi: 10.1016/j.ejca.2018.08.016
16. Sneed TB, Kantarjian HM, Talpaz M, O'Brien S, Rios MB, Bekele BN, et al. The significance of myelosuppression during therapy with imatinib mesylate in patients with chronic myelogenous leukemia in chronic phase. Cancer (2004) 100(1):116–21. doi: 10.1002/cncr.11863
17. Deininger MW, Manley P. What do kinase inhibition profiles tell us about tyrosine kinase inhibitors used for the treatment of CML? Leuk Res (2012) 36(3):253–61. doi: 10.1016/j.leukres.2011.09.018
18. Quintás-Cardama A, Han X, Kantarjian H, Cortes J. Tyrosine kinase inhibitor-induced platelet dysfunction in patients with chronic myeloid leukemia. Blood (2009) 114(2):261–3. doi: 10.1182/blood-2008-09-180604
19. Gratacap MP, Martin V, Valéra MC, Allart S, Garcia C, Sié P, et al. The new tyrosine-kinase inhibitor and anticancer drug dasatinib reversibly affects platelet activation in vitro and in vivo. Blood (2009) 114(9):1884–92. doi: 10.1182/blood-2009-02-205328
20. Satterthwaite AB, Lowell CA, Khan WN, Sideras P, Alt FW, Witte ON. Independent and opposing roles for btk and lyn in b and myeloid signaling pathways. J Exp Med (1998) 188(5):833–44. doi: 10.1084/jem.188.5.833
21. Saijo K, Schmedt C, Su IH, Karasuyama H, Lowell CA, Reth M, et al. Essential role of src-family protein tyrosine kinases in NF-kappaB activation during b cell development. Nat Immunol (2003) 4(3):274–9. doi: 10.1038/ni893
22. Harder KW, Quilici C, Naik E, Inglese M, Kountouri N, Turner A, et al. Perturbed myelo/erythropoiesis in Lyn-deficient mice is similar to that in mice lacking the inhibitory phosphatases SHP-1 and SHIP-1. Blood (2004) 104(13):3901–10. doi: 10.1182/blood-2003-12-4396
23. Chan WY, Lau PM, Yeung KW, Kong SK. The second generation tyrosine kinase inhibitor dasatinib induced eryptosis in human erythrocytes-an in vitro study. Toxicol Lett (2018) 295:10–21. doi: 10.1016/j.toxlet.2018.05.030
24. Rabian F, Lengline E, Rea D. Towards a personalized treatment of patients with chronic myeloid leukemia. Curr Hematol Malig Rep (2019) 14(6):492–500. doi: 10.1007/s11899-019-00546-4
25. Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia (2020) 34(4):966–84. doi: 10.1038/s41375-020-0776-2
26. Deininger MW, Shah NP, Altman JK, Berman E, Bhatia R, Bhatnagar B, et al. Chronic myeloid leukemia, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2020) 18(10):1385–415. doi: 10.6004/jnccn.2020.0047
27. Shimada A. Hematological malignancies and molecular targeting therapy. Eur J Pharmacol (2019) 862:172641. doi: 10.1016/j.ejphar.2019.172641
28. Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin (2016) 66(4):337–50. doi: 10.3322/caac.21342
29. Shah NP, Rousselot P, Schiffer C, Rea D, Cortes JE, Milone J, et al. Dasatinib in imatinib-resistant or -intolerant chronic-phase, chronic myeloid leukemia patients: 7-year follow-up of study CA180-034. Am J Hematol (2016) 91(9):869–74. doi: 10.1002/ajh.24423
30. Mohanavelu P, Mutnick M, Mehra N, White B, Kudrimoti S, Hernandez Kluesner K, et al. Meta-analysis of gastrointestinal adverse events from tyrosine kinase inhibitors for chronic myeloid leukemia. Cancers (Basel) (2021) 13(7):1643. doi: 10.3390/cancers13071643
31. Galanis A, Levis M. Inhibition of c-kit by tyrosine kinase inhibitors. Haematologica (2015) 100(3):e77–79. doi: 10.3324/haematol.2014.117028
32. Abbaspour Babaei M, Kamalidehghan B, Saleem M, Huri HZ, Ahmadipour F. Receptor tyrosine kinase (c-kit) inhibitors: a potential therapeutic target in cancer cells. Drug Des Devel Ther (2016) 10:2443–59. doi: 10.2147/dddt.S89114
33. Brave M, Goodman V, Kaminskas E, Farrell A, Timmer W, Pope S, et al. Sprycel for chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia resistant to or intolerant of imatinib mesylate. Clin Cancer Res (2008) 14(2):352–9. doi: 10.1158/1078-0432.Ccr-07-4175
34. Hartmann JT, Haap M, Kopp HG, Lipp HP. Tyrosine kinase inhibitors - a review on pharmacology, metabolism and side effects. Curr Drug Metab (2009) 10(5):470–81. doi: 10.2174/138920009788897975
35. Stagno F, Stella S, Spitaleri A, Pennisi MS, Di Raimondo F, Vigneri P. Imatinib mesylate in chronic myeloid leukemia: frontline treatment and long-term outcomes. Expert Rev Anticancer Ther (2016) 16(3):273–8. doi: 10.1586/14737140.2016.1151356
36. Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. The New England journal of medicine (2006) 355(23):2408–2417. doi: 10.1056/NEJMoa062867
37. Breccia M, Alimena G. Occurrence and current management of side effects in chronic myeloid leukemia patients treated frontline with tyrosine kinase inhibitors. Leuk Res (2013) 37(6):713–20. doi: 10.1016/j.leukres.2013.01.021
38. Virakul S, Dalm VA, Paridaens D, van den Bosch WA, Hirankarn N, van Hagen PM, et al. The tyrosine kinase inhibitor dasatinib effectively blocks PDGF-induced orbital fibroblast activation. Graefes Arch Clin Exp Ophthalmol (2014) 252(7):1101–9. doi: 10.1007/s00417-014-2674-7
39. Fiedler J, Etzel N, Brenner RE. To go or not to go: Migration of human mesenchymal progenitor cells stimulated by isoforms of PDGF. J Cell Biochem (2004) 93(5):990–8. doi: 10.1002/jcb.20219
40. Chaudhary LR, Hruska KA. The cell survival signal akt is differentially activated by PDGF-BB, EGF, and FGF-2 in osteoblastic cells. J Cell Biochem (2001) 81(2):304–11. doi: 10.1002/1097-4644(20010501)81:2<304::AID-JCB1045>3.0.CO;2-U
41. Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol (2015) 72(2):203–218; quiz 219-220. doi: 10.1016/j.jaad.2014.07.032
42. Karaatmaca B, Aytac S, Sahiner UM, Sekerel BE, Soyer O. Successful oral desensitization with dasatinib in delayed cutaneous hypersensitivity reactions. Ann Allergy Asthma Immunol (2019) 123(2):216–7. doi: 10.1016/j.anai.2019.05.011
43. Delgado L, Giraudier S, Ortonne N, Zehou O, Cordonnier C, Hulin A, et al. Adverse cutaneous reactions to the new second-generation tyrosine kinase inhibitors (dasatinib, nilotinib) in chronic myeloid leukemia. J Am Acad Dermatol (2013) 69(5):839–40. doi: 10.1016/j.jaad.2013.07.025
44. Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med (2006) 354(24):2531–41. doi: 10.1056/NEJMoa055229
45. Hochhaus A, Kantarjian HM, Baccarani M, Lipton JH, Apperley JF, Druker BJ, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood (2007) 109(6):2303–9. doi: 10.1182/blood-2006-09-047266
46. Assouline S, Laneuville P, Gambacorti-Passerini C. Panniculitis during dasatinib therapy for imatinib-resistant chronic myelogenous leukemia. N Engl J Med (2006) 354(24):2623–4. doi: 10.1056/NEJMc053425
47. Amitay-Laish I, Stemmer SM, Lacouture ME. Adverse cutaneous reactions secondary to tyrosine kinase inhibitors including imatinib mesylate, nilotinib, and dasatinib. Dermatol Ther (2011) 24(4):386–95. doi: 10.1111/j.1529-8019.2011.01431.x
48. Patel AB, Solomon AR, Mauro MJ, Ehst BD. Unique cutaneous reaction to second- and third-generation tyrosine kinase inhibitors for chronic myeloid leukemia. Dermatology (2016) 232(1):122–5. doi: 10.1159/000437383
49. Radaelli F, Bramanti S, Fantini NN, Fabio G, Greco I, Lambertenghi-Deliliers G. Dasatinib-related alveolar pneumonia responsive to corticosteroids. Leuk Lymphoma (2006) 47(6):1180–1. doi: 10.1080/10428190600555868
50. Brazzelli V, Grasso V, Borroni G. Imatinib, dasatinib and nilotinib: a review of adverse cutaneous reactions with emphasis on our clinical experience. J Eur Acad Dermatol Venereol (2013) 27(12):1471–80. doi: 10.1111/jdv.12172
51. Yu L, Liu J, Huang X, Jiang Q. Adverse effects of dasatinib on glucose-lipid metabolism in patients with chronic myeloid leukaemia in the chronic phase. Sci Rep (2019) 9(1):17601. doi: 10.1038/s41598-019-54033-0
52. Ono K, Suzushima H, Watanabe Y, Kikukawa Y, Shimomura T, Furukawa N, et al. Rapid amelioration of hyperglycemia facilitated by dasatinib in a chronic myeloid leukemia patient with type 2 diabetes mellitus. Intern Med (2012) 51(19):2763–6. doi: 10.2169/internalmedicine.51.8314
53. Franklin M, Burns L, Perez S, Yerragolam D, Makenbaeva D. Incidence of type 2 diabetes mellitus and hyperlipidemia in patients prescribed dasatinib or nilotinib as first- or second-line therapy for chronic myelogenous leukemia in the US. Curr Med Res Opin (2018) 34(2):353–60. doi: 10.1080/03007995.2017.1399870
54. Sylow L, Long JZ, Lokurkar IA, Zeng X, Richter EA, Spiegelman BM. The cancer drug dasatinib increases PGC-1α in adipose tissue but has adverse effects on glucose tolerance in obese mice. Endocrinology (2016) 157(11):4184–91. doi: 10.1210/en.2016-1398
55. Medeiros BC, Possick J, Fradley M. Cardiovascular, pulmonary, and metabolic toxicities complicating tyrosine kinase inhibitor therapy in chronic myeloid leukemia: Strategies for monitoring, detecting, and managing. Blood Rev (2018) 32(4):289–99. doi: 10.1016/j.blre.2018.01.004
56. Bonvin A, Mesnil A, Nicolini FE, Cotte L, Michallet M, Descotes J, et al. Dasatinib-induced acute hepatitis. Leuk Lymphoma (2008) 49(8):1630–2. doi: 10.1080/10428190802136384
57. Shah RR, Morganroth J, Shah DR. Hepatotoxicity of tyrosine kinase inhibitors: clinical and regulatory perspectives. Drug Saf (2013) 36(7):491–503. doi: 10.1007/s40264-013-0048-4
58. Mauro MJ, Deininger MW. Management of drug toxicities in chronic myeloid leukaemia. Best Pract Res Clin Haematol (2009) 22(3):409–29. doi: 10.1016/j.beha.2009.06.001
59. Wang Z, Wang X, Wang Z, Feng Y, Jia Y, Jiang L, et al. Comparison of hepatotoxicity associated with new BCR-ABL tyrosine kinase inhibitors vs imatinib among patients with chronic myeloid leukemia: A systematic review and meta-analysis. JAMA Netw Open (2021) 4(7):e2120165. doi: 10.1001/jamanetworkopen.2021.20165
60. Mingard C, Paech F, Bouitbir J, Krähenbühl S. Mechanisms of toxicity associated with six tyrosine kinase inhibitors in human hepatocyte cell lines. J Appl Toxicol (2018) 38(3):418–31. doi: 10.1002/jat.3551
61. Saran C, Sundqvist L, Ho H, Niskanen J, Honkakoski P, Brouwer KLR. Novel bile acid-dependent mechanisms of hepatotoxicity associated with tyrosine kinase inhibitors. J Pharmacol Exp Ther (2022) 380(2):114–25. doi: 10.1124/jpet.121.000828
62. Calizo RC, Bhattacharya S, van Hasselt JGC, Wei C, Wong JS, Wiener RJ, et al. Disruption of podocyte cytoskeletal biomechanics by dasatinib leads to nephrotoxicity. Nat Commun (2019) 10(1):2061. doi: 10.1038/s41467-019-09936-x
63. Abbas A, Mirza MM, Ganti AK, Tendulkar K. Renal toxicities of targeted therapies. Target Oncol (2015) 10(4):487–99. doi: 10.1007/s11523-015-0368-7
64. Demetri GD, Lo Russo P, MacPherson IR, Wang D, Morgan JA, Brunton VG, et al. Phase I dose-escalation and pharmacokinetic study of dasatinib in patients with advanced solid tumors. Clin Cancer Res (2009) 15(19):6232–40. doi: 10.1158/1078-0432.Ccr-09-0224
65. ElShaer A, Almasry M, Alawar M, Masoud H, El Kinge AR. Dasatinib-induced nephrotic syndrome: A case report. Cureus (2021) 13(12):e20330. doi: 10.7759/cureus.20330
66. Hirano T, Hashimoto M, Korogi Y, Tsuji T, Miyanaka K, Yamasaki H, et al. Dasatinib-induced nephrotic syndrome. Leuk Lymphoma (2016) 57(3):726–7. doi: 10.3109/10428194.2015.1075020
67. Ruebner RL, Copelovitch L, Evageliou NF, Denburg MR, Belasco JB, Kaplan BS. Nephrotic syndrome associated with tyrosine kinase inhibitors for pediatric malignancy: case series and review of the literature. Pediatr Nephrol (2014) 29(5):863–9. doi: 10.1007/s00467-013-2696-0
68. Lim YT, Kim YJ, Park YH, Hah JO, Lee JM. A case of dasatinib-induced nephrotic syndrome in a child with Philadelphia chromosome positive acute lymphoblastic leukemia. Yonsei Med J (2016) 57(2):532–3. doi: 10.3349/ymj.2016.57.2.532
69. Ochiai S, Sato Y, Minakawa A, Fukuda A, Fujimoto S. Dasatinib-induced nephrotic syndrome in a patient with chronic myelogenous leukemia: a case report. BMC Nephrol (2019) 20(1):87. doi: 10.1186/s12882-019-1273-6
70. Wallace E, Lyndon W, Chumley P, Jaimes EA, Fatima H. Dasatinib-induced nephrotic-range proteinuria. Am J Kidney Dis (2013) 61(6):1026–31. doi: 10.1053/j.ajkd.2013.01.022
71. Luca MD, Carmosino I, Stefanizzi C, Campanelli M, De AF, Cesini L, et al. Nephrotic proteinuria developed under dasatinib treatment in a patient with chronic myeloid leukemia. In: A case report and review of the literature. Ann Hematol Oncol (2016) 3(8): 1106.
72. Koinuma K, Sakairi T, Watanabe Y, IIzuka A, Watanabe M, Hamatani H, et al, et al. A case of long-term dasatinib-induced proteinuria and glomerular injury. CEN Case Rep (2020) 9(4):359–64. doi: 10.1007/s13730-020-00484-8
73. Kostovska I, Trajkovska KT, Topuzovska S, Cekovska S, Labudovic D, Kostovski O, et al. Nephrinuria and podocytopathies. Adv Clin Chem (2022) 108:1–36. doi: 10.1016/bs.acc.2021.08.001
74. Jhaveri KD, Wanchoo R, Sakhiya V, Ross DW, Fishbane S. Adverse renal effects of novel molecular oncologic targeted therapies: A narrative review. Kidney Int Rep (2017) 2(1):108–23. doi: 10.1016/j.ekir.2016.09.055
75. Muller-Hansma AHG, van der Lugt J, Zwaan CM. Nephrotic syndrome under treatment with dasatinib: be aware of a possible adverse drug reaction. Neth J Med (2017) 75(10):428–31.
76. Liang W, Kujawski M, Wu J, Lu J, Herrmann A, Loera S, et al. Antitumor activity of targeting SRC kinases in endothelial and myeloid cell compartments of the tumor microenvironment. Clin Cancer Res (2010) 16(3):924–35. doi: 10.1158/1078-0432.Ccr-09-1486
77. Advani A. Vascular endothelial growth factor and the kidney: something of the marvellous. Curr Opin Nephrol Hypertens (2014) 23(1):87–92. doi: 10.1097/01.mnh.0000437329.41546.a9
78. Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, et al. Cofilin phosphorylation by LIM-kinase 1 and its role in rac-mediated actin reorganization. Nature (1998) 393(6687):809–12. doi: 10.1038/31735
79. Falkenberg CV, Azeloglu EU, Stothers M, Deerinck TJ, Chen Y, He JC, et al. Fragility of foot process morphology in kidney podocytes arises from chaotic spatial propagation of cytoskeletal instability. PloS Comput Biol (2017) 13(3):e1005433. doi: 10.1371/journal.pcbi.1005433
80. Embry AE, Liu Z, Henderson JM, Byfield FJ, Liu L, Yoon J, et al. Similar biophysical abnormalities in glomeruli and podocytes from two distinct models. J Am Soc Nephrol (2018) 29(5):1501–12. doi: 10.1681/asn.2017050475
81. Piscitani L, Sirolli V, Di Liberato L, Morroni M, Bonomini M. Nephrotoxicity associated with novel anticancer agents (Aflibercept, dasatinib, nivolumab): Case series and nephrological considerations. Int J Mol Sci (2020) 21(14): 4878. doi: 10.3390/ijms21144878
82. Bergeron A, Réa D, Levy V, Picard C, Meignin V, Tamburini J, et al. Lung abnormalities after dasatinib treatment for chronic myeloid leukemia: a case series. Am J Respir Crit Care Med (2007) 176(8):814–8. doi: 10.1164/rccm.200705-715CR
83. Chen B, Wu Z, Wang Q, Li W, Cheng D. Dasatinib-induced chylothorax: report of a case and review of the literature. Invest New Drugs (2020) 38(5):1627–32. doi: 10.1007/s10637-020-00932-3
84. Montani D, Bergot E, Günther S, Savale L, Bergeron A, Bourdin A, et al. Pulmonary arterial hypertension in patients treated by dasatinib. Circulation (2012) 125(17):2128–37. doi: 10.1161/circulationaha.111.079921
85. Ameri A, Kantarjian H, Burton E, O'Brien S, Ravandi F, Borthakur G, et al. Low risk of infectious events in patients (Pts) with chronic myeloid leukemia (CML) in chronic phase (CP) treated with dasatinib. Blood (2009) 114(22): 3291. doi: 10.1182/blood.V114.22.3291.3291
86. Brixey AG, Light RW. Pleural effusions due to dasatinib. Curr Opin Pulm Med (2010) 16(4):351–6. doi: 10.1097/MCP.0b013e328338c486
87. Caldemeyer L, Dugan M, Edwards J, Akard L. Long-term side effects of tyrosine kinase inhibitors in chronic myeloid leukemia. Curr Hematol Malig Rep (2016) 11(2):71–9. doi: 10.1007/s11899-016-0309-2
88. Quintás-Cardama A, Kantarjian H, O'Brien S, Borthakur G, Bruzzi J, Munden R, et al. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol (2007) 25(25):3908–14. doi: 10.1200/jco.2007.12.0329
89. Shah NP, Kantarjian HM, Kim DW, Réa D, Dorlhiac-Llacer PE, Milone JH, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol (2008) 26(19):3204–12. doi: 10.1200/jco.2007.14.9260
90. Shah NP, Kim DW, Kantarjian H, Rousselot P, Llacer PED, Enrico A, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica (2010) 95(2):232. doi: 10.3324/haematol.2009.011452
91. Saglio G, le Coutre P, Cortes JE, Mayer J, Rowlings P, Subar M, et al. Safety and tolerability of dasatinib in patients with chronic myeloid leukemia (CML) and philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL): Pooled analysis of over 2400 patients. Haematologica (2014) 2014 99(suppl):abstr P884.
92. Guilhot F, Apperley J, Kim DW, Bullorsky EO, Baccarani M, Roboz GJ, et al. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phase. Blood (2007) 109(10):4143–50. doi: 10.1182/blood-2006-09-046839
93. Kantarjian H, Pasquini R, Hamerschlak N, Rousselot P, Shah N. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: A randomized phase 2 trial. Blood (2007) 109(12):5143–50. doi: 10.1182/blood-2006-11-056028
94. Hughes TP, Laneuville P, Rousselot P, Snyder DS, Rea D, Shah NP, et al. Incidence, outcomes, and risk factors of pleural effusion in patients receiving dasatinib therapy for Philadelphia chromosome-positive leukemia. Haematologica (2019) 104(1):93–101. doi: 10.3324/haematol.2018.188987
95. de Lavallade H, Punnialingam S, Milojkovic D, Bua M, Khorashad JS, Gabriel IH, et al. Pleural effusions in patients with chronic myeloid leukaemia treated with dasatinib may have an immune-mediated pathogenesis. Br J Haematol (2008) 141(5):745–7. doi: 10.1111/j.1365-2141.2008.07108.x
96. Larson RA, Yin OQ, Hochhaus A, Saglio G, Clark RE, Nakamae H, et al. Population pharmacokinetic and exposure-response analysis of nilotinib in patients with newly diagnosed ph+ chronic myeloid leukemia in chronic phase. Eur J Clin Pharmacol (2012) 68(5):723–33. doi: 10.1007/s00228-011-1200-7
97. Rousselot P, Mollica L, Guilhot J, Guerci A, Nicolini FE, Etienne G, et al. Dasatinib dose optimisation based on therapeutic drug monitoring reduces pleural effusion rates in chronic myeloid leukaemia patients. Br J Haematol (2021) 194(2):393–402. doi: 10.1111/bjh.17654
98. He S, Bian J, Shao Q, Zhang Y, Hao X, Luo X, et al. Therapeutic drug monitoring and individualized medicine of dasatinib: Focus on clinical pharmacokinetics and pharmacodynamics. Front Pharmacol (2021) 12:797881. doi: 10.3389/fphar.2021.797881
99. Fazakas C, Nagaraj C, Zabini D, Végh AG, Marsh LM, Wilhelm I, et al. Rho-kinase inhibition ameliorates dasatinib-induced endothelial dysfunction and pulmonary hypertension. Front Physiol (2018) 9:537. doi: 10.3389/fphys.2018.00537
100. Dasgupta SK, Le A, Vijayan KV, Thiagarajan P. Dasatinib inhibits actin fiber reorganization and promotes endothelial cell permeability through RhoA-ROCK pathway. Cancer Med (2017) 6(4):809–18. doi: 10.1002/cam4.1019
101. Dahlén T, Edgren G, Ljungman P, Flygt H, Richter J, Olsson-Strömberg U, et al. Adverse outcomes in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: Follow-up of patients diagnosed 2002-2017 in a complete coverage and nationwide agnostic register study. Am J Hematol (2022) 97(4):421–30. doi: 10.1002/ajh.26463
102. Phan C, Jutant EM, Tu L, Thuillet R, Seferian A, Montani D, et al. Dasatinib increases endothelial permeability leading to pleural effusion. Eur Respir J (2018) 51(1):1701096. doi: 10.1183/13993003.01096-2017
103. Cortes JE, Jimenez CA, Mauro MJ, Geyer A, Pinilla-Ibarz J, Smith BD. Pleural effusion in dasatinib-treated patients with chronic myeloid leukemia in chronic phase: Identification and management. Clin Lymphoma Myeloma Leuk (2017) 17(2):78–82. doi: 10.1016/j.clml.2016.09.012
104. Latagliata R, Breccia M, Fava C, Stagno F, Tiribelli M, Luciano L, et al. Incidence, risk factors and management of pleural effusions during dasatinib treatment in unselected elderly patients with chronic myelogenous leukaemia. Hematol Oncol (2013) 31(2):103–9. doi: 10.1002/hon.2020
105. Iurlo A, Galimberti S, Abruzzese E, Annunziata M, Bonifacio M, Latagliata R, et al. Pleural effusion and molecular response in dasatinib-treated chronic myeloid leukemia patients in a real-life Italian multicenter series. Ann Hematol (2018) 97(1):95–100. doi: 10.1007/s00277-017-3144-1
106. Naqvi K, Jabbour E, Skinner J, Anderson K, Dellasala S, Yilmaz M, et al. Long-term follow-up of lower dose dasatinib (50 mg daily) as frontline therapy in newly diagnosed chronic-phase chronic myeloid leukemia. Cancer (2020) 126(1):67–75. doi: 10.1002/cncr.32504
107. Ciftciler R, Haznedaroglu IC. Tailored tyrosine kinase inhibitor (TKI) treatment of chronic myeloid leukemia (CML) based on current evidence. Eur Rev Med Pharmacol Sci (2021) 25(24):7787–98. doi: 10.26355/eurrev_202112_27625
108. Coons JC, Pogue K, Kolodziej AR, Hirsch GA, George MP. Pulmonary arterial hypertension: a pharmacotherapeutic update. Curr Cardiol Rep (2019) 21(11):141. doi: 10.1007/s11886-019-1235-4
109. Weatherald J, Chaumais MC, Savale L, Jaïs X, Seferian A, Canuet M, et al. Long-term outcomes of dasatinib-induced pulmonary arterial hypertension: a population-based study. Eur Respir J (2017) 50(1):1700217. doi: 10.1183/13993003.00217-2017
110. Rasheed W, Flaim B, Seymour JF. Reversible severe pulmonary hypertension secondary to dasatinib in a patient with chronic myeloid leukemia. Leuk Res (2009) 33(6):861–4. doi: 10.1016/j.leukres.2008.09.026
111. Ding PN, Lord SJ, Gebski V, Links M, Bray V, Gralla RJ, et al. Risk of treatment-related toxicities from EGFR tyrosine kinase inhibitors: A meta-analysis of clinical trials of gefitinib, erlotinib, and afatinib in advanced EGFR-mutated non-small cell lung cancer. J Thorac Oncol (2017) 12(4):633–43. doi: 10.1016/j.jtho.2016.11.2236
112. Mattei D, Feola M, Orzan F, Mordini N, Rapezzi D, Gallamini A. Reversible dasatinib-induced pulmonary arterial hypertension and right ventricle failure in a previously allografted CML patient. Bone Marrow Transplant (2009) 43(12):967–8. doi: 10.1038/bmt.2008.415
113. Hlavaty A, Roustit M, Montani D, Chaumais MC, Guignabert C, Humbert M, et al. Identifying new drugs associated with pulmonary arterial hypertension: A WHO pharmacovigilance database disproportionality analysis. Br J Clin Pharmacol (2022) 88(12):5227–5237. doi: 10.1111/bcp.15436
114. Orlikow E, Weatherald J, Hirani N. Dasatinib-induced pulmonary arterial hypertension. Can J Cardiol (2019) 35(11):1604.e1601–1604.e1603. doi: 10.1016/j.cjca.2019.08.002
115. Shah NP, Wallis N, Farber HW, Mauro MJ, Wolf RA, Mattei D, et al. Clinical features of pulmonary arterial hypertension in patients receiving dasatinib. Am J Hematol (2015) 90(11):1060–4. doi: 10.1002/ajh.24174
116. Jose A, Rafei H, Ahari J. Combination targeted pulmonary hypertension therapy in the resolution of dasatinib-associated pulmonary arterial hypertension. Pulm Circ (2017) 7(4):803–7. doi: 10.1177/2045893217716659
117. Guignabert C, Phan C, Seferian A, Huertas A, Tu L, Thuillet R, et al. Dasatinib induces lung vascular toxicity and predisposes to pulmonary hypertension. J Clin Invest (2016) 126(9):3207–18. doi: 10.1172/jci86249
118. Özgür Yurttaş N, Eşkazan AE. Dasatinib-induced pulmonary arterial hypertension. Br J Clin Pharmacol (2018) 84(5):835–45. doi: 10.1111/bcp.13508
119. Santoro M, Mancuso S, Accurso V, Di Lisi D, Novo G, Siragusa S. Cardiovascular issues in tyrosine kinase inhibitors treatments for chronic myeloid leukemia: A review. Front Physiol (2021) 12:675811. doi: 10.3389/fphys.2021.675811
120. Cornet L, Khouri C, Roustit M, Guignabert C, Chaumais MC, Humbert M, et al. Pulmonary arterial hypertension associated with protein kinase inhibitors: a pharmacovigilance-pharmacodynamic study. Eur Respir J (2019) 53(5):1802472. doi: 10.1183/13993003.02472-2018
121. Wang R, Pan J, Han J, Gong M, Liu L, Zhang Y, et al. Melatonin attenuates dasatinib-aggravated hypoxic pulmonary hypertension via inhibiting pulmonary vascular remodeling. Front Cardiovasc Med (2022) 9:790921. doi: 10.3389/fcvm.2022.790921
122. Weatherald J, Savale L, Humbert M. Medical management of pulmonary hypertension with unclear and/or multifactorial mechanisms (Group 5): Is there a role for pulmonary arterial hypertension medications? Curr Hypertens Rep (2017) 19(11):86. doi: 10.1007/s11906-017-0783-5
123. Beshay S, Sahay S, Humbert M. Evaluation and management of pulmonary arterial hypertension. Respir Med (2020) 171:106099. doi: 10.1016/j.rmed.2020.106099
124. Vazquez ZGS, Klinger JR. Guidelines for the treatment of pulmonary arterial hypertension. Lung (2020) 198(4):581–96. doi: 10.1007/s00408-020-00375-w
125. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERS): Endorsed by: Association for European paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT). Eur Heart J (2016) 37(1):67–119. doi: 10.1093/eurheartj/ehv317
126. Izumi-Nakaseko H, Fujiyoshi M, Hagiwara-Nagasawa M, Goto A, Chiba K, Kambayashi R, et al. Dasatinib can impair left ventricular mechanical function but may lack proarrhythmic effect: A proposal of non-clinical guidance for predicting clinical cardiovascular adverse events of tyrosine kinase inhibitors. Cardiovasc Toxicol (2020) 20(1):58–70. doi: 10.1007/s12012-019-09538-5
127. Abu Rmilah AA, Lin G, Begna KH, Friedman PA, Herrmann J. Risk of QTc prolongation among cancer patients treated with tyrosine kinase inhibitors. Int J Cancer (2020) 147(11):3160–7. doi: 10.1002/ijc.33119
128. Chaar M, Kamta J, Ait-Oudhia S. Mechanisms, monitoring, and management of tyrosine kinase inhibitors-associated cardiovascular toxicities. Onco Targets Ther (2018) 11:6227–37. doi: 10.2147/ott.S170138
129. Cirmi S, El Abd A, Letinier L, Navarra M, Salvo F. Cardiovascular toxicity of tyrosine kinase inhibitors used in chronic myeloid leukemia: An analysis of the FDA adverse event reporting system database (FAERS). Cancers (Basel) (2020) 12(4):826. doi: 10.3390/cancers12040826
130. Barber MC, Mauro MJ, Moslehi J. Cardiovascular care of patients with chronic myeloid leukemia (CML) on tyrosine kinase inhibitor (TKI) therapy. Hematol Am Soc Hematol Educ Program (2017) 2017(1):110–4. doi: 10.1182/asheducation-2017.1.110
131. Will Y, Dykens JA, Nadanaciva S, Hirakawa B, Jamieson J, Marroquin LD, et al. Effect of the multitargeted tyrosine kinase inhibitors imatinib, dasatinib, sunitinib, and sorafenib on mitochondrial function in isolated rat heart mitochondria and H9c2 cells. Toxicol Sci (2008) 106(1):153–61. doi: 10.1093/toxsci/kfn157
132. Hasinoff BB, Patel D, Wu X. The myocyte-damaging effects of the BCR-ABL1-Targeted tyrosine kinase inhibitors increase with potency and decrease with specificity. Cardiovasc Toxicol (2017) 17(3):297–306. doi: 10.1007/s12012-016-9386-7
133. Hasinoff BB, Patel D. Mechanisms of the cardiac myocyte-damaging effects of dasatinib. Cardiovasc Toxicol (2020) 20(4):380–9. doi: 10.1007/s12012-020-09565-7
134. Pichot CS, Hartig SM, Xia L, Arvanitis C, Monisvais D, Lee FY, et al. Dasatinib synergizes with doxorubicin to block growth, migration, and invasion of breast cancer cells. Br J Cancer (2009) 101(1):38–47. doi: 10.1038/sj.bjc.6605101
135. Alkebsi L, Wang X, Ohkawara H, Fukatsu M, Mori H, Ikezoe T. Dasatinib induces endothelial-to-mesenchymal transition in human vascular-endothelial cells: counteracted by cotreatment with bosutinib. Int J Hematol (2021) 113(3):441–55. doi: 10.1007/s12185-020-03034-1
136. Haguet H, Bouvy C, Delvigne AS, Modaffari E, Wannez A, Sonveaux P, et al. The risk of arterial thrombosis in patients with chronic myeloid leukemia treated with second and third generation BCR-ABL tyrosine kinase inhibitors may be explained by their impact on endothelial cells: An in-vitro study. Front Pharmacol (2020) 11:1007. doi: 10.3389/fphar.2020.01007
137. Kreutzman A, Colom-Fernández B, Jiménez AM, Ilander M, Cuesta-Mateos C, Pérez-García Y, et al. Dasatinib reversibly disrupts endothelial vascular integrity by increasing non-muscle myosin II contractility in a ROCK-dependent manner. Clin Cancer Res (2017) 23(21):6697–707. doi: 10.1158/1078-0432.Ccr-16-0667
138. Alsaad AMS. Dasatinib induces gene expression of CYP1A1, CYP1B1, and cardiac hypertrophy markers (BNP, β-MHC) in rat cardiomyocyte H9c2 cells. Toxicol Mech Methods (2018) 28(9):678–84. doi: 10.1080/15376516.2018.1497746
139. Xu Z, Jin Y, Yan H, Gao Z, Xu B, Yang B, et al. High-mobility group box 1 protein-mediated necroptosis contributes to dasatinib-induced cardiotoxicity. Toxicol Lett (2018) 296:39–47. doi: 10.1016/j.toxlet.2018.08.003
140. Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European hematology association (EHA), the European society for therapeutic radiology and oncology (ESTRO) and the international cardio-oncology society (IC-OS). Eur Heart J (2022) 43(41):4229–361. doi: 10.1093/eurheartj/ehac244
141. Aghel N, Delgado DH, Lipton JH. Cardiovascular toxicities of BCR-ABL tyrosine kinase inhibitors in chronic myeloid leukemia: preventive strategies and cardiovascular surveillance. Vasc Health Risk Manag (2017) 13:293–303. doi: 10.2147/vhrm.S108874
Keywords: dasatinib, chronic myeloid leukemia, adverse reactions, pharmacotherapy, pleural effusion
Citation: Cheng F, Xu Q, Li Q, Cui Z, Li W and Zeng F (2023) Adverse reactions after treatment with dasatinib in chronic myeloid leukemia: Characteristics, potential mechanisms, and clinical management strategies. Front. Oncol. 13:1113462. doi: 10.3389/fonc.2023.1113462
Received: 01 December 2022; Accepted: 23 January 2023;
Published: 06 February 2023.
Edited by:
Mario Annunziata, Hospital Antonio Cardarelli, ItalyReviewed by:
Ibrahim C. Haznedaroglu, Hacettepe Univrsity Hospital, TürkiyeDaniela Di Lisi, Azienda Ospedaliera Universitaria Policlinico Paolo Giaccone, Italy
Copyright © 2023 Cheng, Xu, Li, Cui, Li and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiming Li, bGVlOTM3QDEyNi5jb20=; Fang Zeng, ZmFuY3l6ZW5nQDEyNi5jb20=
†These authors have contributed equally to this work
 Fang Cheng
Fang Cheng Qiling Xu
Qiling Xu Qiang Li1,2
Qiang Li1,2 Weiming Li
Weiming Li Fang Zeng
Fang Zeng