- 1Department of Urology, University of California (UC) San Diego School of Medicine, La Jolla, CA, United States
- 2Department of Urology, Fundacion Instituto Valenciano Oncologia, Valencia, Spain
- 3Department of Urology, University of Florence, Careggi Hospital, Florence, Italy
- 4Department of Urology, University Medical Centre Mannheim, Mannheim, Germany
- 5Department of Urology, Technical University of Munich, Munich, Germany
- 6Department of Urology, University of Turin-San Luigi Gonzaga Hospital, Orbassano, Italy
- 7Department of Urology, AZ Groeninge, Kortrijk, Belgium
- 8Department of Urology, Hospital 12 de Octubre, Madrid, Spain
- 9Department of Urology, Hospital Ramon y Cajal, Madrid, Spain
- 10Department of Urology, Hospital Clinic, Carrer de Villarroel, Barcelona, Spain
- 11Department of Urology, Virginia Commonwealth University (VCU) Medical Center, Richmond, VA, United States
- 12Department of Urology, UK Leuven, Leuven, Belgium
- 13Department of Urology, University of Trieste, Trieste, Italy
- 14Department of Urology, Spedali Civili Hospital, University of Brescia, Brescia, Italy
- 15Department of Urology, University “G. d’Annunzio” Chieti-Pescara, Chieti, Italy
- 16Department of Urology, Royal Bournemouth Hospital, Bournemouth, United Kingdom
Purpose: We hypothesized that two-tier re-classification of the “M” (metastasis) domain of the Tumor-Node-Metastasis (TNM) staging of Renal Cell Carcinoma (RCC) may improve staging accuracy than the current monolithic classification, as advancements in the understanding of tumor biology have led to increased recognition of the heterogeneous potential of metastatic RCC (mRCC).
Methods: Multicenter retrospective analysis of patients from the REMARCC (REgistry of MetAstatic RCC) database. Patients were stratified by number of metastases into two groups, M1 (≤3, “Oligometastatic”) and M2 (>3, “Polymetastatic”). Primary outcome was overall survival (OS). Secondary outcomes were cancer-specific survival (CSS). Cox-regression and Kaplan-Meier (KMA) analysis were utilized for outcomes, and receiver operating characteristic analysis (ROC) was utilized to assess diagnostic accuracy compared to current “M” staging.
Results: 429 patients were stratified into proposed M1 and M2 groups (M1 = 286/M2 = 143; median follow-up 19.2 months). Cox-regression revealed M2 classification as an independent risk factor for worsened all-cause mortality (HR=1.67, p=0.001) and cancer-specific mortality (HR=1.74, p<0.001). Comparing M1-oligometastatic vs. M2-polymetastatic groups, KMA revealed significantly higher 5-year OS (36% vs. 21%, p<0.001) and 5-year CSS (39% vs. 17%, p<0.001). ROC analyses comparing OS and CSS, for M1/M2 reclassification versus unitary M designation currently in use demonstrated improved c-index for OS (M1/M2 0.635 vs. unitary M 0.500) and CSS (M1/M2 0.627 vs. unitary M 0.500).
Conclusion: Subclassification of Stage “M” domain of mRCC into two clinical substage categories based on metastatic burden corresponds to distinctive tumor groups whose oncological potential varies significantly and result in improved predictive capability compared to current staging.
1 Introduction
Renal Cell Carcinoma (RCC) has been characterized by a stage migration over the last few decades as increasing proportion of patients are diagnosed with small and asymptomatic masses (1). Nonetheless 15-25% of patients diagnosed with RCC continue to present with metastatic disease, and up to 30%-35% of patients with localized disease develop recurrent metastatic disease (2). As understanding of both the heterogenous biological potential of renal neoplasms and patterns of progression has grown, revisions to the T3 and T4 of the Tumor-Node-Metastasis (TNM)/American Joint Committee on Cancer (AJCC) staging system have been successfully proposed and have correlated with improved prognostication and risk stratification (3).
Stage IV/metastatic renal cell carcinoma (mRCC) has been historically associated with a poor prognosis. Nonetheless, outcomes in metastatic disease are not uniform and are influenced by both patient and disease factors (4, 5). Existing risk stratification systems including Memorial Sloan Kettering Cancer Center (MSKCC)/Motzer criteria and International Metastatic RCC Database Consortium (IMDC)/Heng criteria staging have sought to stratify metastatic heterogeneity by incorporating clinical and laboratory data into prognostic groupings (6–8). Despite repeated validation studies and the instrumental role these prognostic indices have played in driving investigation and refining management recommendations for metastatic disease, the TNM/AJCC Staging for mRCC has remained constant. We sought to evaluate the impact of tumor burden on survival outcomes and hypothesized that subdividing Stage IV RCC into groups based on metastatic burden would rationalize metastatic staging and facilitate more accurate and individualized discussions with patients regarding prognosis.
2 Methods
2.1 Patient population
Our study is a retrospective international multi-institutional analysis utilizing the REMARCC (REgistry of MetAstatic RCC) database of patients presenting with metastatic RCC (mRCC) between 1/2006-10/2019 who underwent cytoreductive nephrectomy. Our patient population and methods have been described previously (9). All participating institutions received institutional review board approval. Patients referred with metastatic RCC underwent initial staging evaluation with CT or MRI of chest, abdomen, and pelvis with additional studies as indicated (10, 11). Determination of presence/extent of metastatic disease was made by treating clinicians based imaging and/or histologic findings at each participating institution (12, 13). Treatment decisions regarding cytoreductive nephrectomy, systemic therapy, and metastatectomy were conducted via interdisciplinary discussions between medical and urologic oncologists (14). Radiographic follow up and determination of response were conducted by RECIST (Response Evaluation Criteria in Solid Tumors) (15, 16). Patients who received cytokine or mammilian target of rapamycin (mTOR) therapy as initial therapy, and patients with non-cortical renal malignancy were excluded from analysis.
2.2 Data collection
Data were entered into institutional datasets by database managers. Collected variables include demographic data at time of diagnosis [age, sex, body mass index (BMI, Kg/M2)], baseline laboratory values [hemoglobin, lactate dehydrogenase (LDH), calcium] and clinical disease characteristics {Eastern Cooperative Oncology Group (ECOG) performance status, Karnofsky performance status, TNM stage (3), Motzer Risk Category [time from diagnosis to systemic treatment, hemoglobin concentration below lower limit of normal (3.5g/dL for men; 12.0 g/dL for women), calcium >10mg/dL, LDH >1.5 times upper limit of normal (140 U/L), and Karnofsky performance status <80%] (6), number and location of metastases}. Treatment data (cytoreductive nephrectomy, metastatectomy, systemic therapy) were collected. Survival outcomes, including progression, disease-free survival, and overall survival at last follow-up were recorded.
2.3 Data analysis
We a priori categorized Oligometastatic disease as ≤3 metastatic sites, while Polymetastatic disease was defined as >3 metastatic sites (17).We hypothesis tested this definition by performing serial Receiver Operating Characteristic (ROC)/area under the curve (AUC) analyses to analyze for C-index to determine optimal cut off point of metastatic burden to be most predictive of survival outcomes overall survival (OS), cancer-specific survival (CSS) and progression-free survival (PFS), and compared different cut offs of number of metastases (1 vs. >1, 2≤ vs. >2, ≤3 vs. >3, ≤4 vs. >4, ≤ vs. >5) for proposed M1 and M2 groups to the current M+ unitary group of the TNM/AJCC 8th Edition (3).
Primary outcome was all-cause mortality (ACM)/overall survival (OS) measured from date of diagnosis to date of last follow-up or death. Secondary outcomes were cancer-specific mortality (CSM)/cancer-specific survival (CSS) and progression-free survival (PFS, defined as time to radiographic progression as per RECIST criteria) (16). The cohort was divided into M1 (oligometastatic; ≤3 metastases) vs. M2 (polymetastatic; >3 metastases) groups for descriptive analyses of demographics, clinical disease characteristics, and survival outcomes.
Descriptive analyses were conducted utilizing Student’s t-test and Fisher’s exact test for continuous and categorical variables, respectively. Cox proportional hazards regression multivariable analysis was employed for analysis of ACM and CSM, and logistic regression multivariable analysis was utilized to analyze for factors associated with progression. Kaplan Meier Analysis (KMA) was performed to analyze OS, CSS, and PFS for proposed M1 and M2 groups and M+ unitary group of the TNM/AJCC 8th Edition (3). SPSS v.27 (IBM, Chicago, USA) was utilized for statistical analyses with p<0.05 considered significant.
3 Results
429 patients were analyzed (286 oligometastatic M1, 143 polymetastatic M2). Median follow-up for the overall cohort was 19.2 months (IQR: 6.82-38.4).
Figure 1 demonstrates hypothesis testing for M1/M2 cut off of ROC analyses comparing OS, CSS, and PFS for different numeric cut offs for the proposed M1/M2 reclassification (>1, >2, >3, >4, >5 metastases) versus the unitary M designation currently in use. AUC for OS was most improved for proposed M1/M2 >3 0.634 vs. unitary M 0.500. AUC for CSS was most improved for M1/M2 >3 0.626 vs. unitary M 0.500. AUC for PFS was most improved for proposed M1/M2 >1 0.623 vs. unitary M 0.500. Based on these findings, cut off of >3 was utilized as the threshold for M1/M2 subgroups.
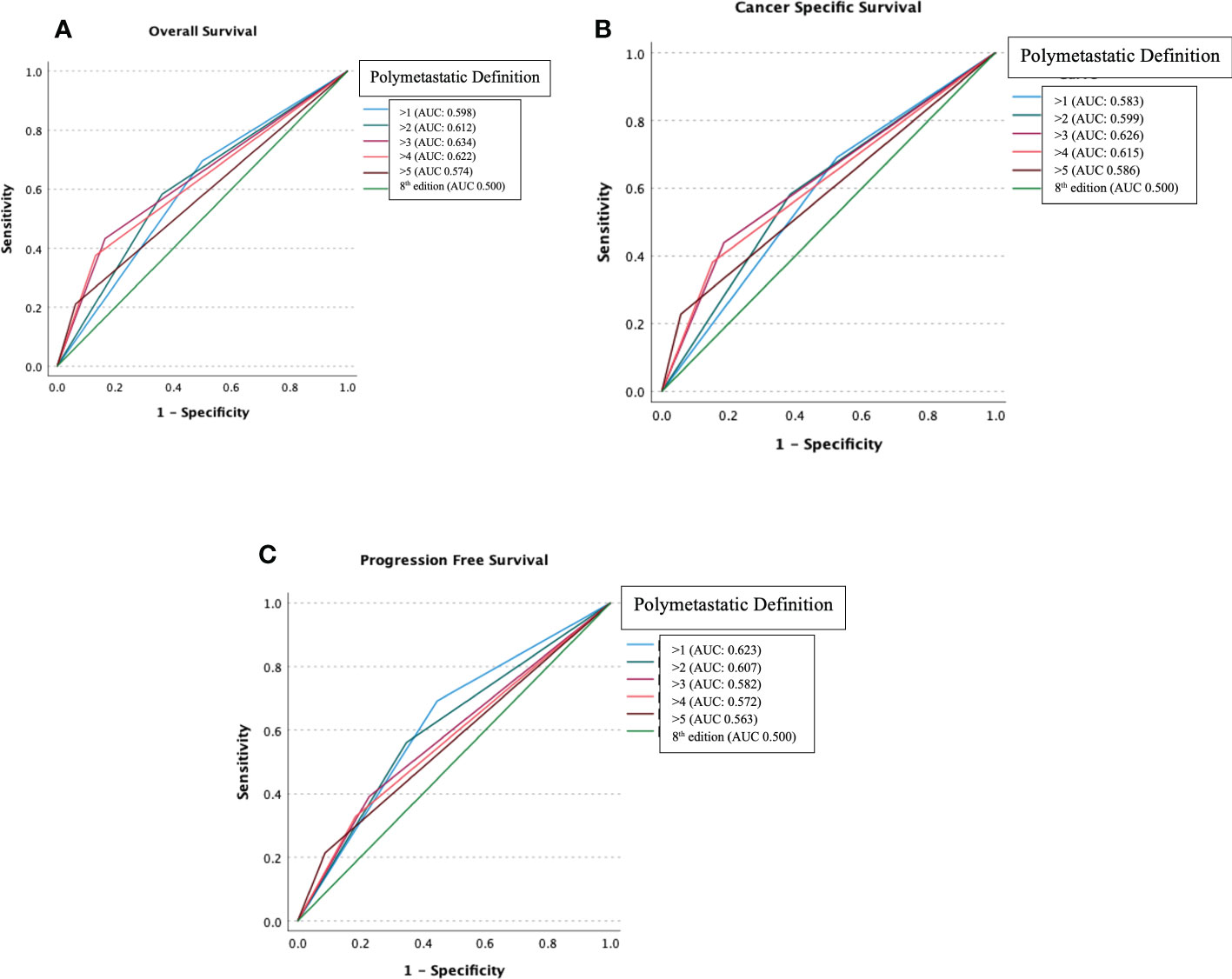
Figure 1 Receiver operating characteristic analysis comparing Overall Survival (A), Cancer-Specific Survival (B) and Progression-Free Survival (C) for different numeric cut offs for the proposed M1/M2 reclassification (>1, >2, >3, >4, >5 metastases) versus the unitary M designation.
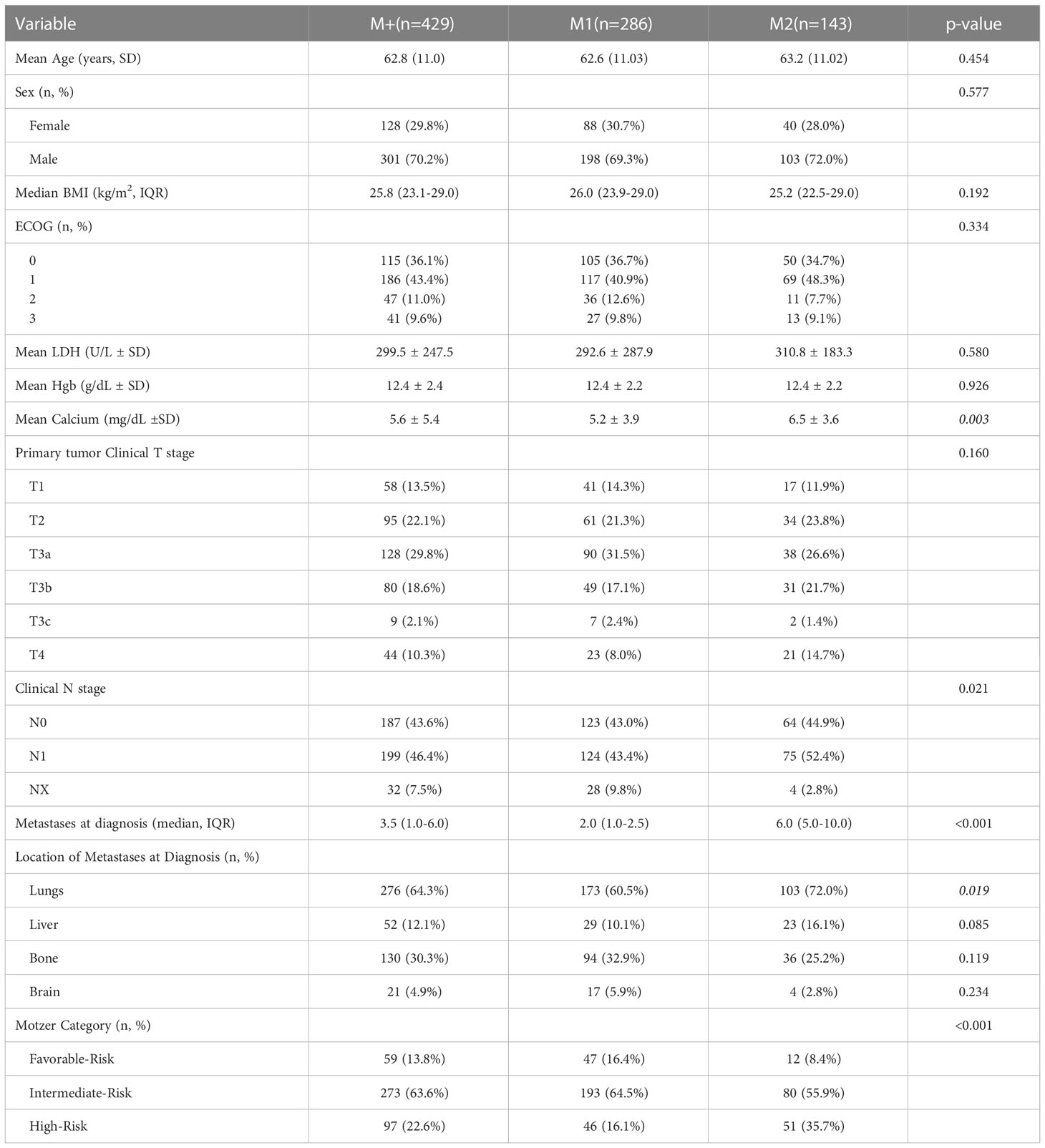
Table 1 demonstrates demographic variables and clinical disease characteristics. No differences were noted between groups with respect to age (p=0.454), sex (p=0.577), BMI (p=0.192), baseline LDH (p=0.580), baseline hemoglobin (p=0.926), and clinical stage of primary tumor (p=0.160), and ECOG (p=0.334). On the other hand, patients in M1 group had lower median number of metastases at diagnosis (M1 2.0 vs. M2 6.0, p<0.001), mean calcium (M1 5.2 vs. M2 6.5, p=0.003) and patients with high Motzer risk category (M1 16.1% vs. M2 35.7%, p<0.001).
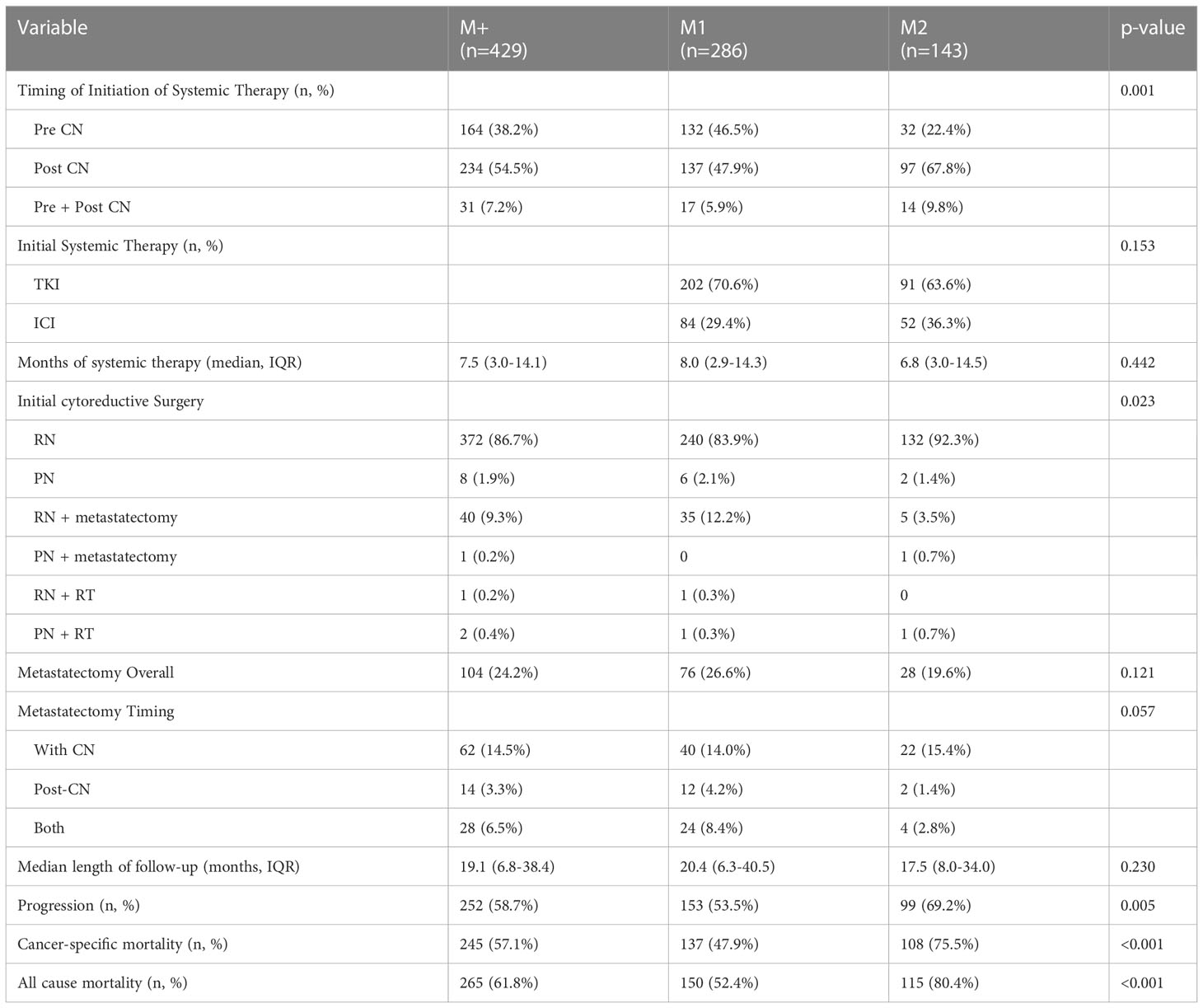
Table 2 displays therapeutic interventions, survival and oncologic outcomes. M1 patients had a higher proportion of pre-surgical systemic therapy (46.5 vs. 22.4%, p<0.001), though type of therapy (p=0.153) and use of metastasectomy (p=0.121) were not different between groups. There was no significant difference in median length of follow-up for patients (M1 20.4 months vs. M2 17.5 months, p=0.230). M2 patients had a significantly greater number of progression events (69.2% vs. 53.5%, p=0.005). In addition, cancer-specific mortality events were greater in the M2 group (75.5% vs. 47.9%, p<0.001), and all-cause mortality was also greater in the M2 group (80.4% vs. 52.4%, p<0.001).
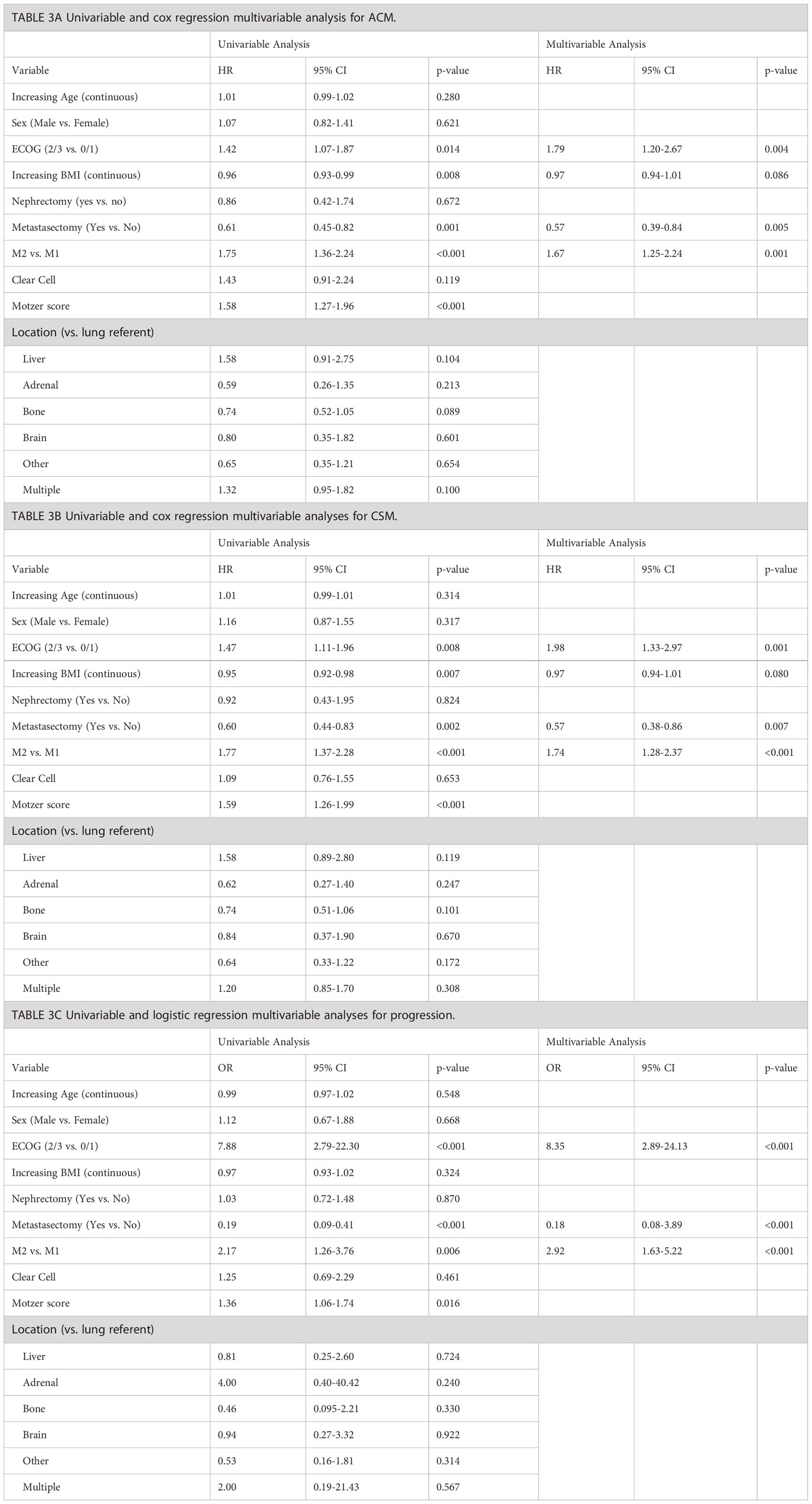
Table 3 displays univariable and multivariable analyses for ACM, CSM, and progression. ECOG 2/3 (HR=1.79, p=0.004) and M2 polymetastatic classification (HR=1.67, p=0.001) were independent risk factors for worsened ACM, while receipt of metastatectomy (HR=0.57, p=0.005) was associated with decreased ACM. Cox regression for CSM revealed ECOG 2/3 (HR=1.98, p=0.001) and M2 polymetastatic (HR=1.74, p<0.001) to be independent risk factors for worsened CSM, while metastasectomy (HR=0.57, p=0.007) was associated with decreased CSM (Table 3B). Logistic regression revealed ECOG 2/3 (OR=8.35, p<0.001) and M2 polymetastatic classification (OR=2.92 p<0.001) to be independently associated with increased risk of progression, while metastasectomy (OR=0.18, p<0.001) was associated with a decreased risk of progression. Location of metastases was not significantly associated
Figures 2A–F display Kaplan-Meier Survival Analyses (KMA) for overall survival (OS), cancer specific survival (CSS), and progression-free survival (PFS). Figure 2A demonstrates KMA for OS for the entire unitary M+ cohort utilizing the current classification. 5-year OS was 28%. Figure 2B demonstrates KMA for OS utilizing proposed M1 and M2 subgroups. 5-year OS was significantly higher for M1-oligometastatic vs. M2-polymetastatic (36% vs. 21%, p<0.001; Figure 2A). Figure 2C demonstrates KMA for CSS for entire M+ cohort, which revealed 5-year CSS of 30%. Figure 2D demonstrates KMA for CSS utilizing proposed M1 and M2 subgroups. 5-year CSS was higher for M1-oligometastatic vs. M2-polymetastatic (39% vs. 17%, p<0.001) groups. Figure 2E demonstrated KMA for PFS of the entire M+ cohort, which revealed median PFS of 5.54 months, and Figure 2F demonstrates KMA for PFS utilizing proposed M1 and M2 subgroups, which demonstrated significantly longer median time to progression for M1 vs. M2 groups (8.0 months vs. 4.6 months, p=0.025).
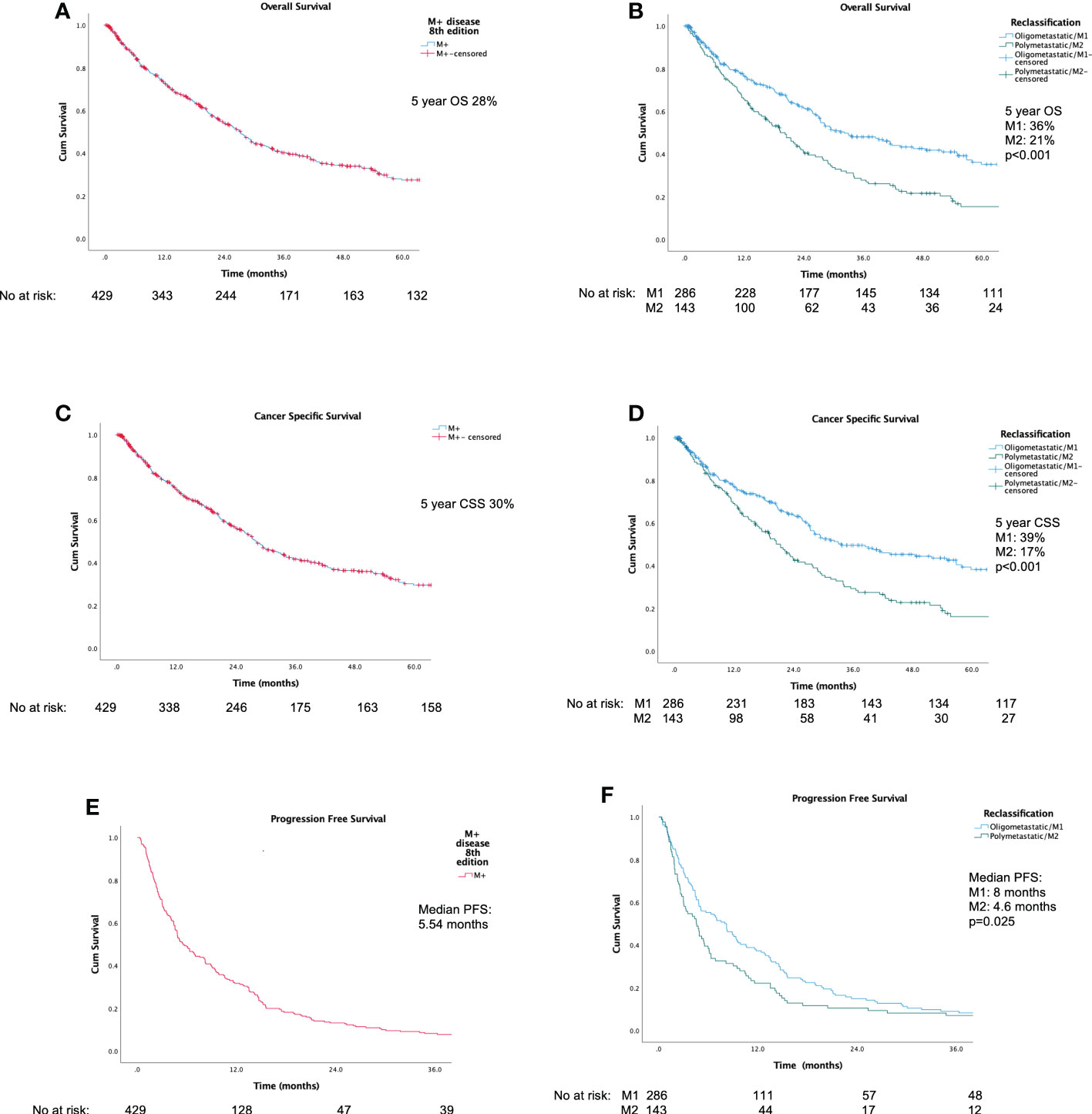
Figure 2 Kaplan-Meier survival analyses: (A) Overall Survival for current unitary M+ classification; (B) Overall Survival for proposed M1/M2 Subgroups; (C) Cancer-Specific Survival for current unitary M+ classification; (D) Cancer-Specific Survival for M1/M2 Subgroups; (E) Progression-Free Survival for current unitary M+ classification; (F) Progression-Free Survival for M1/M2 Subgroups.
4 Discussion
Utilizing an international, multi-center cohort, we present the first proposal for a reclassification of the “M” domain in TNM staging. Our findings suggest existence of two distinct subgroups driven by extent of metastatic burden, with the optimal cut off point being >3 metastases as demonstrated by ROC/AUC analyses, and is superior in its predictive ability for survival outcomes compared to the current unitary “M” domain classification. Furthermore, as evidenced in our multivariable analyses, we demonstrated that increased number of metastases was independently associated with worsened overall and cancer specific survival, which was consistent with survival estimates noted in the Kaplan-Meier analyses. While further validation is requisite, our data suggest that stratification by tumor burden may more rationally and accurately guide patient counseling and management strategy in mRCC.
Multiple studies have examined the impact of disease burden to explain heterogeneous outcomes in mRCC. In a study of 124 patients with mRCC, Iocavelli et al. found a direct relationship between tumor burden, progression-free survival, and overall survival, with each 1 cm increase in tumor burden increasing risk of progression and risk of death by 4.5% and 5%, respectively (p<0.001) (18). Number of metastatic sites has also been demonstrated to be a surrogate for disease burden (18, 19).Atzpodien studied 425 patients with mRCC and found that greater metastatic burden, defined as greater than 3 metastatic sites, was independently associated with worsened overall survival (HR=1.4, p=0.01) (20). In a previous analysis of the REMARCC database, Marchioni et al. similarly determined greater than 3 metastatic was associated with worsened overall survival (HR=1.29, p=0.040) (21). Sharma et al. analyzed 105 patients with mRCC who underwent cytoreductive nephrectomy and noted that >2 metastatic sites was independently associated with worsened OS (HR=2.09, p=0.006) (22). As demonstrated by our ROC/AUC analyses, while increasing number of metastases is indeed associated with worsened outcomes, further sub-classification of >3 distinct metastases elucidates significant differences for both overall and cancer specific survival.
The American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) system serves as the universally established staging method for renal cell carcinoma (23). Staging reclassifications in RCC have been proposed and adopted as understanding of tumor biology and heterogeneity has increased, with 8 editions since inception in 1977 (8, 23). Recent editions have been notable for modifications of parameters for AJCC Stage 3 and T3 RCC. In a study of 697 patients with pT3 and pT4 RCC, Thompson et al. reclassified patients into 4 prognostic groups based on direct adrenal invasion, perinephric fat invasion, and tumor thrombus level with improved prognostic accuracy (reclassification c-index 0.61 vs. 0.55) (24). Such proposals ultimately paved the way for reclassification of T3 from tumor extension into major veins, perinephric tissues, or adrenal gland in the 6th edition to tumor extension into major veins or perinephric tissues but not ipsilateral adrenal gland in the 7th edition (8). More recently, pT3a RCC has been further modified to include collecting system invasion in addition to renal vein/venous branches, renal sinus fat and perinephric fat between the 7th and 8th edition (25). To our knowledge, ours is the first attempt at reclassification of the “M” stage of the current AJCC TNM system. Our findings challenge the monolithic paradigm of “M” in TNM staging, and suggest that a reclassification of the “M” domain based on number of metastatic disease sites improves prognostic accuracy at least as well as prior modifications to Stage III disease.
Motzer (Memorial Sloan Kettering) and Heng (International Metastatic RCC Database Consortium) scores have been widely utilized and validated as prognostic schema for mRCC in contemporary cohorts (6, 7). Motzer/MSKCC scoring system is comprised of performance status, pre-operative laboratory values, and time to systemic therapy (26). Heng/IMDC criteria similarly employs performance status and time to systemic therapy, but replaces pre-operative LDH and with neutrophil and platelet couts (7). Multiple studies have sought to quantify the predictive capacity of both Heng and Motzer scores. Bamias et al. reported an AUC of 0.661 for overall survival based on Motzer in a study of 109 mRCC patients (27). Utilizing a cohort of 89 patients, Assi et al. yielded an AUC of 0.631 for overall survival for Heng criteria (28). Similarly, in a study of overall survival of 106 patients treated with sunitinib, Kwon demonstrated an AUC of 0.670 and 0.653 for Heng and Motzer, respectively (29). In a study of 628 patients with mRCC who underwent bevacizumab plus interferon treatment, Karakiewicz et al. revealed an AUC of 0.518 for progression free survival based on Motzer score (30). Analyzing Motzer criteria in our cohort, we found AUC values of 0.597, 0.588, and 0.596 for overall, cancer specific, and progression free survival, respectively. The previously demonstrated predictive capacities of Motzer and Heng scores are similar to our AUC of 0.635 for overall survival and 0.627 for cancer specific survival, as well as our AUC of 0.582 for progression free survival, suggesting that our proposed M1/M2 reclassification schema can accurately prognosticate mRCC survival outcomes in a manner similar to existing Motzer/Heng criteria. While our proposed reclassification does not aim to replace Motzer and Heng criteria, it nonetheless offers a streamlined framework within the TNM criteria in which to classify risk and counsel patients.
Our study is limited by the inherent limitations of a retrospective design. Additionally, variation in diagnostic protocols, data collection and follow-up protocols between participating centers may introduce further confounding and limitation. We acknowledge that our findings did not demonstrate location of metastasis to be associated with worsened outcomes as has been previously demonstrated in mRCC and other genitourinary malignancies which may be a result of sampling bias (31–33). Nonetheless, AUC demonstrated robust predictive ability comparable to existing risk stratification criteria for oncologic outcomes in metastatic RCC, and the diverse nature of this international registry and the robustness of the findings lend support to the validity and applicability of our findings. Our work should be regarded as hypothesis forming and as such validation by external series is requisite. Our findings suggest that subclassification of the M domain with a cut-off point of 3 metastases may optimally result into two distinct categories of outcomes based and number of metastatic sites, and presents an alternative to rationally contextualize risk, guide patient counseling, and drive further investigation.
5 Conclusion
Stratification of the “M” domain in mRCC into two clinical substage categories based on metastatic burden with a cut-off point of 3 metastases more accurately predicts survival outcomes, capturing lower and higher risk subsets of mRCC eclipsed by the current TNM staging, and offering a simplified alternative to more complex prognostic schemas.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed toaWRlcndlZXNoQGdtYWlsLmNvbQ==.
Ethics statement
The studies involving human participants were reviewed and approved by National Review Board. The patients/participants provided their written informed consent to participate in this study.
Author contributions
MFM performed data collection, data analysis, and was the primary manuscript writer/editor. MCM and AMi performed data collection and manuscript writing/editing. MK, MH, FP, SB, EL, VH, MD, AV, ER, FC, CP, MMa, JA, CS, FL, JR, RC, AMa, TA, EC, MMu, GG, NP, MA, and AA were involved in data collection and data management. TK, RA, and RM contributed in protocol development and manuscript writing/editing. Finally, ID lead the team through project development and manuscript writing/editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Stephen Weissman Kidney Cancer Research Fund.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kane CJ, Mallin K, Ritchey J, Cooperberg MR, Carroll PR. Renal cell cancer stage migration: analysis of the national cancer data base. Cancer (2008) 113(1):78. doi: 10.1002/cncr.23518
2. Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet (2009) 373:1119. doi: 10.1016/S0140-6736(09)60229-4
3. Amin MB, Edge S, Greene F, Compton CC, Gershenwald JE, Brookland RK, et al. AJCC cancer staging manual. 8th edition. New York: Springer-Verlag (2017) 67(2):93–9. doi: 10.3322/caac.21388
4. Panian J, Lin X, Simantov R, Derweesh I, Choueiri TK, McKay RR. The impact of age and gender on outcomes of patients with advanced renal cell carcinoma treated with targeted therapy. Clin Genitourin Cancer (2020) 18(5):e598–e609. doi: 10.1016/j.clgc.2020.03.010
5. Zhang G, Zhu Y, Dong D, Gu W, Zhang H, Sun L, et al. Clinical outcome of advanced and metastatic renal cell carcinoma treated with targeted therapy: is there a difference between young and old patients? Onco Targets Ther (2014) 7:2043–52. doi: 10.2147/OTT.S70012
6. Motzer RJ, Bacik J, Schwartz LH, Reuter V, Russo P, Marion S, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol (2004) 22(3):454–63. doi: 10.1200/JCO.2004.06.132
7. Heng DYC, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, et al. External validation and comparison with other models of the international metastatic renal-cell carcinoma database consortium prognostic model: a population-based study. Lancet Oncol (2013) 14(2):141–8. doi: 10.1016/S1470-2045(12)70559-4
8. Swami U, Nussenzveig RH, Haaland B, Agarwal N. Revisiting AJCC TNM staging for renal cell carcinoma: quest for improvement. Ann Transl Med (2019) 7(Suppl 1):S18. doi: 10.21037/atm.2019.01.50
9. Roussel E, Campi R, Larcher A, Verbiest A, Antonelli A, Palumbo C, et al. Rates and predictors of perioperative complications in cytoreductive nephrectomy: Analysis of the registry for metastatic renal cell carcinoma. Eur Urol Oncol (2020) 3(4):523–9. doi: 10.1016/j.euo.2020.04.006
10. Vig SVL, Zan E, Kang SK. Imaging for metastatic renal cell carcinoma. Urol Clin North Am (2020) 47:281. doi: 10.1016/j.ucl.2020.04.005
11. Bossé D, Lin X, Simantov R, Lalani AA, Derweesh I, Chang SL, et al. Response of primary renal cell carcinoma to systemic therapy. Eur Urol (2019) 76(6):852–60. doi: 10.1016/j.eururo.2019.08.035
12. Motzer RJ, Jonasch E, Boyle S, Carlo MI, Manley B, Agarwal N, et al. NCCN guidelines insights: Kidney cancer, version 1.2021. J Natl Compr Canc Netw (2020) 18(9):1160–70. doi: 10.6004/jnccn.2020.0043
13. Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European Association of urology guidelines on renal cell carcinoma. the 2019 update. Eur Urol (2019) 82(4):399–410. doi: 10.1016/j.eururo.2019.02.011
14. Stroup SP, Raheem OA, Palazzi KL, Liss MA, Mehrazin R, Kopp RP, et al. Does timing of cytoreductive nephrectomy impact patient survival with metastatic renal cell carcinoma in the tyrosine kinase inhibitor era? a multi-institutional study. Urology (2014) 81(4):805–11. doi: 10.1016/j.urology.2012.10.054
15. Schwartz LH, Mazumdar M, Wang L, Smith A, Marion S, Panicek DM, et al. Response assessment classification in patients with advanced renal cell carcinoma treated on clinical trials. Cancer (2003) 98(8):1611–9. doi: 10.1002/cncr.11712
16. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the united states, national cancer institute of Canada. J Natl Cancer Inst (2000) 92(3):205–16. doi: 10.1093/jnci/92.3.205
17. Reyes DK, Pienta KJ. The biology and treatment of oligometastatic cancer. Oncotarget (2015) 6:8491. doi: 10.18632/oncotarget.3455
18. Iacovelli R, Lanoy E, Albiges L, Escudier B. Tumour burden is an independent prognostic factor in metastatic renal cell carcinoma. BJU Int (2012) 110:1747. doi: 10.1111/j.1464-410X.2012.11518.x
19. Mekhail TM, Abou-Jawde RM, Boumehri G, Malhi S, Wood L, Elson P, et al. Validation and extension of the memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol (2005) 23(4):832–41. doi: 10.1200/JCO.2005.05.179
20. Atzpodien J, Royston P, Wandert T, Reitz M;, DGCIN -- German Cooperative Renal Carcinoma Chemo-Immunotherapy Trials Group. Metastatic renal carcinoma comprehensive prognostic system. Br J Cancer (2003) 88(3):348–53. doi: 10.1038/sj.bjc.6600768
21. Marchioni M, Kriegmair M, Heck M, Amiel T, Porpiglia F, Ceccucci E, et al. Development of a novel risk score to select the optimal candidate for cytoreductive nephrectomy among patients with metastatic renal cell carcinoma. results from a multi-institutional registry (REMARCC). Eur Urol Oncol (2021) 4(2):256–63. doi: 10.1016/j.euo.2020.12.010
22. Sharma P, Zargar-Shoshtari K, Cracciolo JT, Fishman M, Poch MA, Pow- Sang J, et al. Sarcopenia as a predictor of overall survival after cytoreductive nephrectomy for metastatic renal cell carcinoma. Urol Oncol (2015) 33(8):339.e17–23. doi: 10.1016/j.urolonc.2015.01.011
23. Compérat E. Changes of tumour-Node-Metastasis staging in 2017: Concepts and evolutions in the European and American continents. Eur Urol (2018) 73:570. doi: 10.1016/j.eururo.2018.01.003
24. Thompson RH, Cheville JC, Lohse CM, Webster WS, Zincke H, Kwon ED, et al. Reclassification of patients with pT3 and pT4 renal cell carcinoma improves prognostic accuracy. Cancer (2005) 104(1):53–60. doi: 10.1002/cncr.21125
25. Paner GP, Stadler WM, Hansel DE, Montironi R, Lin D, Amin M, et al. Updates in the eighth edition of the tumor-Node-Metastasis staging classification for urologic cancers. Eur Urol (2018) 73:560. doi: 10.1016/j.eururo.2017.12.018
26. Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol (2002) 20(1):289–96. doi: 10.1200/JCO.2002.20.1.289
27. Bamias A, Karadimou A, Lampaki S, Lainakis G, Malettou L, Timotheadou E, et al. Prognostic stratification of patients with advanced renal cell carcinoma treated with sunitinib: comparison with the memorial Sloan-Kettering prognostic factors model. BMC Cancer (2010) 10:45. doi: 10.1186/1471-2407-10-45
28. Assi HI, Patenaude F, Toumishey E, Ross L, Abdelsalam M, Reiman T, et al. A simple prognostic model for overall survival in metastatic renal cell carcinoma. Can Urol Assoc J (2016) 10(3-4):113–9. doi: 10.5489/cuaj.3351
29. Kwon WA, Cho IC, Yu A, Nam BH, Joung JY, Seo HK, et al. Validation of the MSKCC and heng risk criteria models for predicting survival in patients with metastatic renal cell carcinoma treated with sunitinib. Ann Surg Oncol (2013) 20(13):4397–404. doi: 10.1245/s10434-013-3290-1
30. Karakiewicz PI, Sun M, Bellmunt J, Sneller V, Escudier B. Prediction of progression-free survival rates after bevacizumab plus interferon versus interferon alone in patients with metastatic renal cell carcinoma: comparison of a nomogram to the motzer criteria. Eur Urol (2011) 60:48. doi: 10.1016/j.eururo.2010.12.011
31. Wei H, Miao J, Cui J, Zheng W, Chen X, Zhang Q, et al. The prognosis and clinicopathological features of different distant metastases patterns in renal cell carcinoma: analysis based on the SEER database. Sci Rep (2021) 11(1):17822. doi: 10.1038/s41598-021-97365-6
32. Cheaib JG, Claus LE, Patel HD, Kates MR, Matoso A, Hahn NM, et al. Site of metastatic recurrence impacts prognosis in patients with high-grade upper tract urothelial carcinoma. Urol Oncol (2021) 39(1):74. doi: 10.1016/j.urolonc.2020.09.029
Keywords: carcinoma, renal cell, neoplasm metastasis, neoplasm staging, nephrectomy, survival analysis, TNM staging system
Citation: Meagher MF, Mir MC, Minervini A, Kriegmair M, Heck M, Porpiglia F, Van Bruwaene S, Linares E, Hevia V, D’Anna M, Veccia A, Roussel E, Claps F, Palumbo C, Marchioni M, Afari J, Saitta C, Liu F, Rubio J, Campi R, Mari A, Amiel T, Checcucci E, Musquera M, Guruli G, Pavan N, Albersen M, Antonelli A, Klatte T, Autorino R, McKay RR and Derweesh IH (2023) Proposal for a Two-Tier Re-classification of Stage IV/M1 domain of Renal Cell Carcinoma into M1 (“Oligometastatic”) and M2 (“Polymetastatic”) subdomains: Analysis of the Registry for Metastatic Renal Cell Carcinoma (REMARCC). Front. Oncol. 13:1113246. doi: 10.3389/fonc.2023.1113246
Received: 01 December 2022; Accepted: 12 January 2023;
Published: 29 March 2023.
Edited by:
Hiten D Patel, Northwestern University, United StatesReviewed by:
Xun Lin, Pfizer, United StatesDmitry Enikeev, I.M. Sechenov First Moscow State Medical University, Russia
Copyright © 2023 Meagher, Mir, Minervini, Kriegmair, Heck, Porpiglia, Van Bruwaene, Linares, Hevia, D’Anna, Veccia, Roussel, Claps, Palumbo, Marchioni, Afari, Saitta, Liu, Rubio, Campi, Mari, Amiel, Checcucci, Musquera, Guruli, Pavan, Albersen, Antonelli, Klatte, Autorino, McKay and Derweesh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ithaar H. Derweesh, aWRlcndlZXNoQGdtYWlsLmNvbQ==
 Margaret F. Meagher1
Margaret F. Meagher1 Maria C. Mir
Maria C. Mir Andrea Minervini
Andrea Minervini Maximilian Kriegmair
Maximilian Kriegmair Matthias Heck
Matthias Heck Francesco Porpiglia
Francesco Porpiglia Siska Van Bruwaene
Siska Van Bruwaene Alessandro Veccia
Alessandro Veccia Eduard Roussel
Eduard Roussel Francesco Claps
Francesco Claps Carlotta Palumbo
Carlotta Palumbo Michele Marchioni
Michele Marchioni Riccardo Campi
Riccardo Campi Andrea Mari
Andrea Mari Enrico Checcucci
Enrico Checcucci Mireia Musquera
Mireia Musquera Nicola Pavan
Nicola Pavan Riccardo Autorino
Riccardo Autorino Ithaar H. Derweesh
Ithaar H. Derweesh