
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 25 January 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1112380
This article is part of the Research Topic Cutting-edge Liver Surgery-based Modalities for Diagnosis and Treatment of Liver Tumors View all 12 articles
 Feng Tian1†
Feng Tian1† Songyao Leng1,2†
Songyao Leng1,2† Jian Chen1
Jian Chen1 Yong Cao1
Yong Cao1 Li Cao1
Li Cao1 Xiaojun Wang1
Xiaojun Wang1 Xuesong Li1
Xuesong Li1 Juan Wang3*
Juan Wang3* Shuguo Zheng1*
Shuguo Zheng1* Jianwei Li1*
Jianwei Li1*Background: Laparoscopic liver resection (LLR) for hepatocellular carcinoma (HCC) has increased. However, the long-term outcomes of LLR for HCCs should be validated further. Besides, the validity of laparoscopic minor liver resection in difficult segments (1, 4a, 7, 8) (LMLR-DS) and laparoscopic major hepatectomy (LMH) for HCCs need to be studied.
Methods: A total of 1773 HCC patients were collected: 683 received LLR and 1090 received OLR. Propensity score matching (PSM) with 1:1 ratio was used to eliminate the selection bias. Short-term and long-term outcomes were compared. In subgroup analyses, the validity of LMLR-DS or LMH for HCCs was studied.
Results: After PSM, 567 patients were in LLR or OLR group. LLR had lower intraoperative blood-loss and shorter postoperative hospital-stays than OLR. The postoperative complications were lower in LLR group (23.8% vs. 32.8%, P=0.001). The Overall survival (OS) and disease-free survival (DFS) had no significant difference between LLR and OLR groups (P=0.973, P=0.812). The cumulative 1-, 3-, and 5-year OR rates were 87.9%, 68.9%, and 57.7% for LLR group, and 85.9%, 68.8%, 58.8% for OLR group. The cumulative 1-, 3-, and 5-year DFS rates were 73.0%, 51.5%, 40.6% for LLR group, and 70.3%, 49.0%, 42.4% for OLR group. In subgroup analyses, 178 patients were in LMLR-DS or open surgery (OMLR-DS) group after PSM. LMLR-DS had lower intraoperative blood-loss and shorter postoperative hospital-stays than OMLR-DS. The postoperative complications were lower in LMLR-DS group. The OS and DFS had no difference between LMLR-DS and OMLR-DS groups. The cumulative 5-year OR and DFS rates were 61.6%, 43.9% for LMLR-DS group, and 66.5%, 47.7% for OMLR-DS group. In another subgroup analyses, 115 patients were in LMH or open major hepatectomy (OMH) group. LMH had lower blood-loss and shorter postoperative hospital-stays than OMH. The complications, OS and DFS had no significantly differences between two groups. The cumulative 5-year OR and DFS rates were 44.3%, 29.9% for LMH group, and 44.7%, 33.2% for OMH group.
Conclusions: LLR for HCCs showed better short-term outcomes and comparable long-term outcomes with OLR, even for patients who received LMLR-DS or LMH. LLR could be reliable and recommended for HCC treatment.
The Global Cancer Statistics 2020 reports that primary liver cancer is the sixth common malignancy and the third leading cause of tumor-related death (1). Hepatocellular carcinoma (HCC), which accounts for 75-85% of primary liver cancer, is a global health challenge (1, 2). Liver resection (LR) remains the mainstay of curative treatment for HCC (3, 4). LR mainly includes two types: open liver resection (OLR) and laparoscopic liver resection (LLR). OLR is the traditional and standard procedure for HCC treatment. With the development of laparoscopic technique and equipment, LLR has been progressively increasing in recent years (5–7).
Clinical researches of LLR for HCCs have always been the hot area worldwide. Advantages of LLR are reported with regard to improved short-term outcomes compared with OLR (8). However, the long-term outcome of LLR for HCCs has become an important topic of debate (9–12). Although several recent studies show that LLR has similar long-term outcomes with OLR for HCCs (13–16), it needs to be validated further in more studies with a larger number of cases. Moreover, according to Asia Pacific Consensus and Southampton Consensus Guidelines (6, 7), laparoscopic minor liver resections (LMLR) in anterolateral segments (2, 3, 4b, 5, 6), is reliable and recommended for HCC treatment, but on the other hand, laparoscopic minor liver resection in difficult segments (1, 4a, 7, 8) (LMLR-DS) and laparoscopic major hepatectomy (LMH) for HCC treatment needs to be investigated further.
The aim of this study was to compare the short-term and long-term outcomes of LLR with those of OLR for HCC in well-matched patient groups using propensity score matching (PSM) with a large number of cases at a single center. Moreover, the outcomes of LMLR-DS and LMH for HCCs were studied in subgroup analyses.
We retrospectively reviewed the data for patients who received LR for HCC in Southwest hospital, Army medical university, Chongqing, China from January 2009 to December 2017.The inclusion criteria was as follows: (1) patients aged 18-75 years; (2) liver function classified as Child-Pugh A or B; (3) the remaining liver volume was adequate with the preoperative evaluation; (4) histopathological confirmation of HCC; and (5) no extrahepatic metastasis. The exclusion criteria were as follows: (1) patients with recurrent HCC; (2) liver resection combined with abdominal organ resection other than gallbladder resection; and (3) patients with HCC who underwent prior RFA or TACE.
A total of 1773 patients with HCC were collected in this study: 683 patients in LLR group and 1090 patients in OLR group (Supplementary Table S1). Preoperative evaluations were similar in two groups and included routine blood tests, liver function, coagulation examinations, serum tumor markers, indocyanine green retention test at 15 minutes (ICG-R15), and triphasic enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI). Routine blood and hepatic function tests were performed after surgery. Abdominal ultrasonography was routinely conducted for patients before discharge. LMLR (≤ 2 segments) or LMH (≥ 3 contiguous segments) were defined according to the previous study (8).The severity of postoperative complications was graded by the Clavien–Dindo classification, and the severe complications were defned as Clavien–Dindo grade III and above (17). This study was approved by the Ethics Committee of Southwest Hospital of Army Medical University.
For LLR, either 3D or 2D laparoscopic system was used. The patient was placed in the supine position. Carbon dioxide pneumoperitoneum was established with a pressure of 11–13 mmHg. Two 12 mm ports and two 5 mm ports were applied for the operation, and one 12mm port was used for the laparoscope. Laparoscopic ultrasonography was routinely performed to confirm the positions of tumors and guide the dissection line. The Pringle maneuver was applied to control blood loss. The liver parenchyma was dissected using the ultrasonic dissector. Intraparenchymal vascular and biliary structures (≥3 mm) were ligated by titanium clips or Hem-o-lock clips. The main Glissonian pedicles or hepatic veins were transected by the laparoscopic linear stapler or Hem-o-lock clips after ligation. The specimen was placed into a sterile bag and extracted through a suprapubic incision or an upper abdominal midline incision.
For OLR, a reverse L-incision or right subcostal incision was conducted. The operating procedure was similar to LLR. CUSA or clamp crushing was used as the main method for liver parenchyma dissection.
The follow-up was conducted every one month within three months after operation, and then every three months within two years and three or six months afterwards. Routine investigations at each follow-up included routine blood tests, liver function, tumor markers, abdominal ultrasonography, and even CT or MRI if necessary. Overall survival (OS) was defined as the time from the surgery date to death from any cause or the last follow-up. Disease free survival (DFS) was defined as the time from the surgery date to tumor recurrence. All patients were followed up with the same protocol.
PSM with 1:1 ratio was used to eliminate the selection bias between LLR and OLR groups based on the nearest neighbor matching method without replacement. The propensity score covariates in this study included age, gender, HBV, HCV, liver cirrhosis, Child–Pugh score, American Society of Anesthesiologists score (ASA score), preoperative blood tests (ALT, TBIL, ALB, PT, Platelet count and AFP), ICG-R15, tumor location, tumor number, largest tumor diameter, type of LR, range of LR, resection tumor margin, margin status, histological grade, satellite nodule, portal vein invasion, bile duct invasion and TNM stage (Table 1). After matching, P values for the group samples were all greater than 0.05, indicating a good balance. Continuous variables were compared using a t test or Mann–Whitney test. Categorical variables were compared using the χ2 or Fisher exact test. A two-tailed P value <0.05 was considered significant. Survival curves were estimated by the Kaplan-Meier method for OS and DFS, the log-rank test was used for between-group comparisons. P values < 0.05 were considered significant. All statistical analyses were performed using SPSS software version 25.0 (IBM SPSS Inc. Chicago, IL).
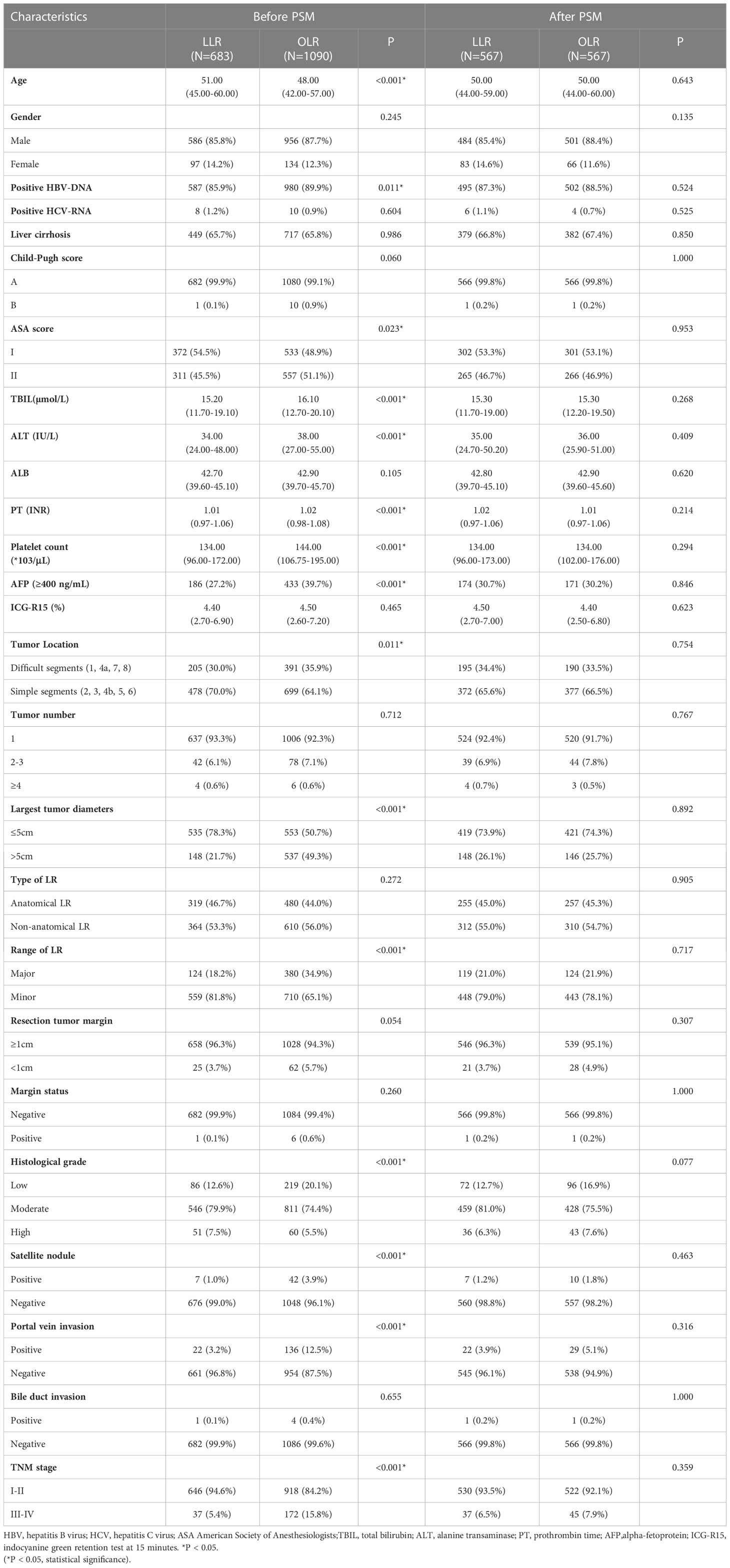
Table 1 Baseline patient characteristics between laparoscopic liver resection (LLR) and open liver resection (OLR) groups.
A total of 1773 patients with HCC were collected in this study, 683 patients received LLR and 1090 patients received OLR. The baseline characteristics were shown in Table 1. Between LLR and OLR groups, age, HBV-DNA and ASA scores were significantly different. Besides, the preoperative serum levels of TBIL, ALT, PT, Platelet count and AFP were different. Among the operative characteristics, tumor location, largest tumor diameters and the range of LR were significantly different between two groups. The characteristics of histological grade and portal vein invasion were also significantly different. Moreover, the TNM stage was different between two groups. After PSM with 1:1 ratio, there were 567 patients in each group with well-balanced baseline characteristics (Table 1).
Short-term outcomes were compared between LLR and OLR groups after PSM (Table 2). The operative time was similar between two groups. On the other hand, the operative blood loss and the rate of blood transfusion in LLR groups were significantly lower than them in OLR group (200.00 ml vs. 300.00 ml, P<0.001; 8.6% vs. 12.3%, P=0.042). The perioperative mortality was similar between two groups. However, the overall postoperative complications in LLR group were significantly lower than them in OLR group (23.8% vs. 32.8%, P=0.001). The complications mainly included seroperitoneum, hydrothorax, infection, hemorrhage, bile leak, liver failure, respiratory failure and renal failure. Moreover, the severe complications (Clavien–Dindo grade III and above) were also significantly lower in LLR groups (5.1% vs. 8.6%, P=0.019). Besides, the postoperative hospital stays were significantly shorter in LLR group (10.00 days vs. 13.00 days, P<0.001). Together, the results showed that patients in LLR group had better short-term outcomes compared with them in OLR group.
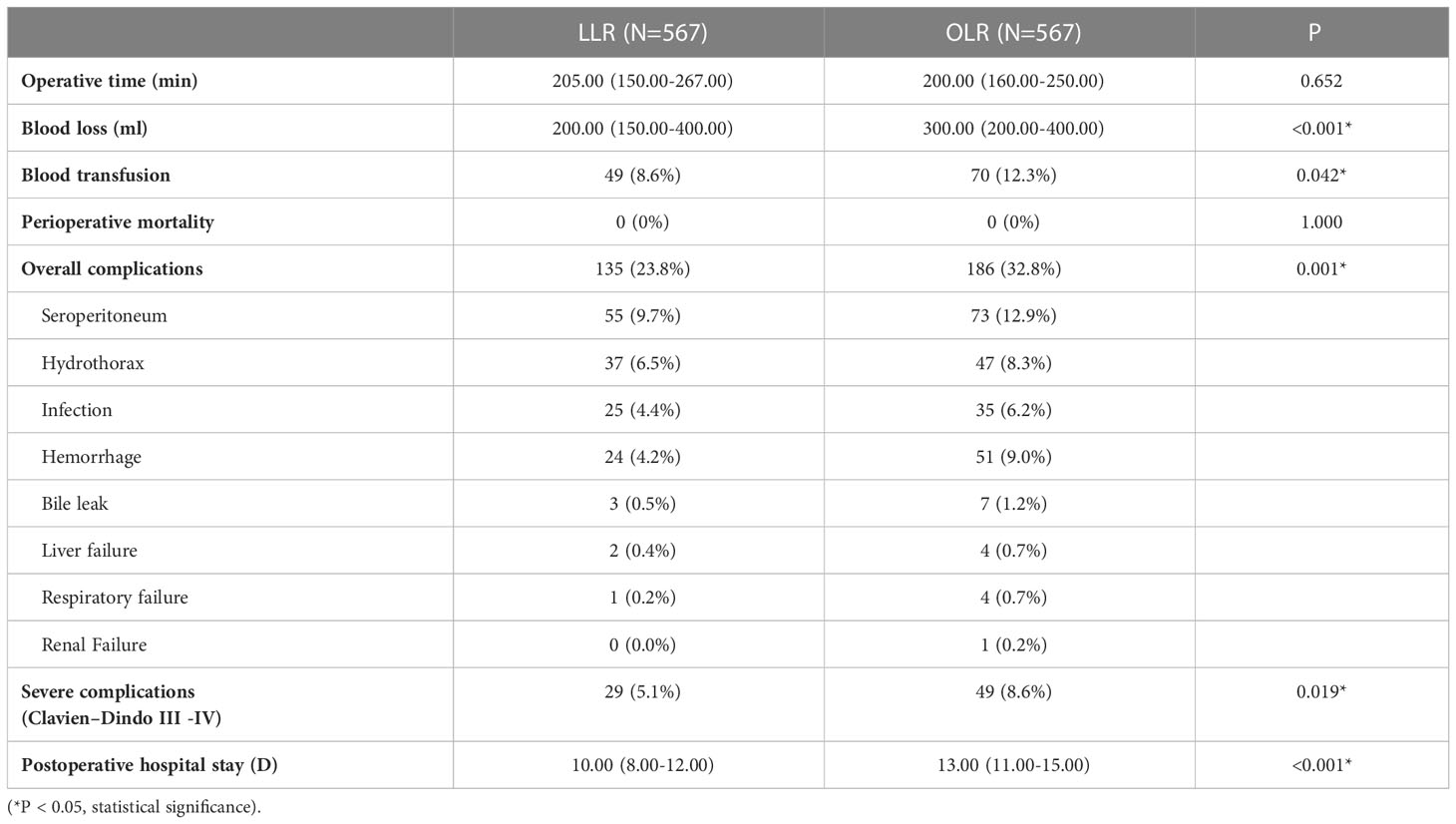
Table 2 Operative details and postoperative outcomes between laparoscopic liver resection (LLR) and open liver resection (OLR) groups after propensity score matching.
The long-term outcomes were compared between LLR and OLR groups after PSM. The OS and DFS curves were presented in Figure 1. Survival analysis showed that the OS had no significant difference between LLR and OLR groups (Figure 1, P=0.973). The cumulative 1-, 3-, and 5-year OS rates were 87.9%, 68.9%, and 57.7% for patients in LLR group, and were 85.9%, 68.8%, and 58.8% for patients in OLR group, respectively. Consistently, the DFS had no difference between LLR and OLR groups (Figure 1, P=0.812). The cumulative 1-, 3-, and 5-year DFS rates were 73.0%, 51.5%, and 40.6% for patients in LLR group, and were 70.3%, 49.0%, and 42.4% for patients in OLR group, respectively. Together, the results showed that patients in LLR group had comparable long-term outcomes with them in OLR group.
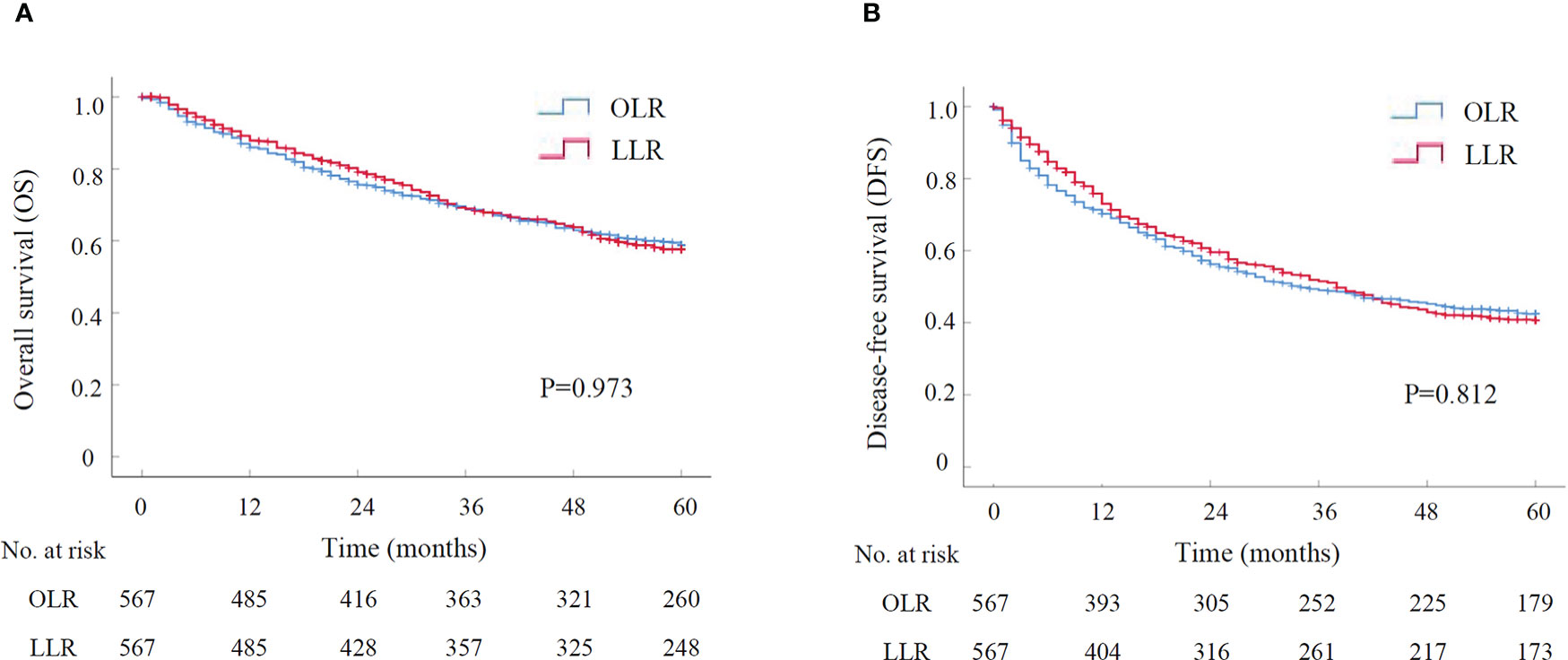
Figure 1 The survival curve of LLR versus OLR for HCCs after PSM by Kaplan-Meier analysis (N=567 for each group). (A) Overall survival; (B) Disease-free survival. LLR, laparoscopic liver resection; OLR, open liver resection; PSM, propensity score matching.
A subgroup analysis was performed to assess the outcomes of LMLR-DS for HCCs compared with OMLR-DS. After PSM with 1:1 ratio, there were 178 patients in LMLR-DS or OMLR-DS group, and all baseline characteristics were well-balanced (Supplementary Table S2). The operative time and the rate of blood transfusion were similar in two groups. While, the operative blood loss in LMLR-DS group was significantly lower than it in OMLR-DS group (200.00 ml vs. 300.00 ml, P<0.001). There was no perioperative death in both two groups. However, the overall and severe postoperative complications were significantly lower in LMLR-DS group compared with them in OMLR-DS group (27.5% vs. 39.9%, P=0.014; 3.9% vs. 11.2%, P=0.009; Table 3). The postoperative stays were significantly shorter in LMLR-DS group (10.00 days vs. 13.50 days, P<0.001). For long-term survival analysis, the OS had no significant difference between LMLR-DS and OMLR-DS groups (P=0.476, Figure 2). The cumulative 1-, 3-, and 5-year OS rates were 92.6%, 76.0%, and 61.6% for patients in LMLR-DS group, and were 90.9%, 78.2%, and 66.5% for patients in OMLR-DS group, respectively. Consistently, the DFS had no difference between LMLR-DS and OMLR-DS groups (Figure 2, P=0.536). The cumulative 1-, 3-, and 5-year DFS rates were 76.7%, 51.2%, and 43.9% for patients in LMLR-DS group, and were 75.7%, 54.1%, and 47.7% for patients in OMLR-DS group, respectively. Together, the results showed that LMLR-DS showed better short-term outcomes and similar long-term outocomes with OMLR-DS for HCCs.
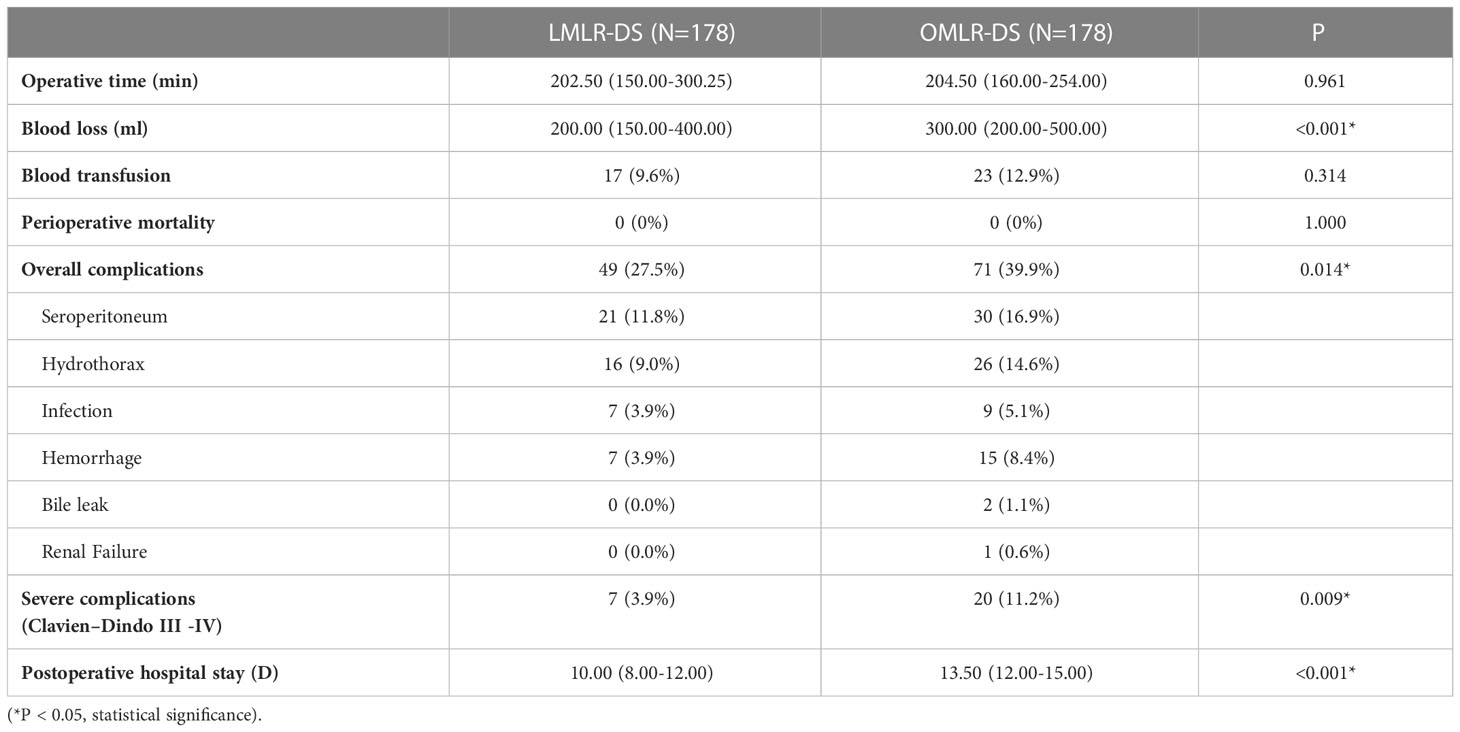
Table 3 Operative details and postoperative outcomes between laparoscopic minor liver resection in difficult segments (LMLR-DS) and open minor liver resection in difficult segments (OMLR-DS) groups after propensity score matching.
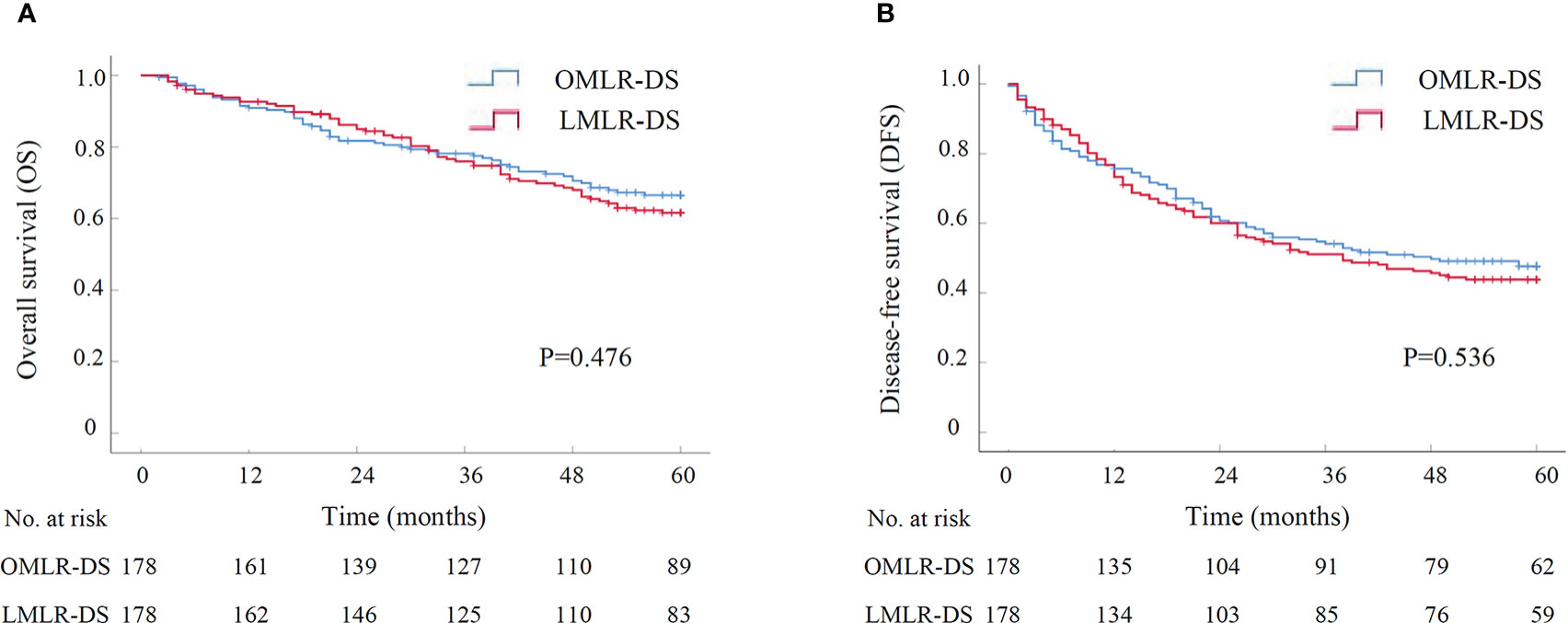
Figure 2 The survival curve of LMLR-DS versus OMLR-DS for HCCs after PSM by Kaplan-Meier analysis (N=178 for each group). (A) Overall survival; (B) Disease-free survival. LMLR-DS, laparoscopic minor liver resection in difficult segments (1, 4a, 7 and 8); OMLR-DS, open minor liver resection in difficult segments; PSM, propensity score matching.
Another subgroup analysis was performed to assess short-term and long-term outcomes of LMH for HCCs compared with OMH. There were 115 patients in either LMH or OMH group after PSM, and all baseline characteristics were well-balanced (Supplementary Table S3). The operative time was similar in two groups. However, the operative blood loss and the rate of blood transfusion in LMH group were significantly lower than them in OMH group (300.00ml vs. 400.00ml, P=0.003; 13.0% vs. 24.3%, P=0.028; Table 4). There was no perioperative death in both two groups. Besides, the overall and severe postoperative complications had no significant difference between two groups (Table 4). The postoperative hospital stays were significantly shorter in LMH group (11.00 days vs. 14.00 days, P<0.001). For long-term survival analyses, the OS had no significant difference between LMH and OMH groups (P=0.939, Figure 3). The cumulative 1-, 3-, and 5-year OS rates were 70.8%, 53.9%, and 44.3% for patients in LMH group, and were 75.5%, 49.2%, and 44.7% for patients in OMH group, respectively. Consistently, the DFS had no difference between two groups (P=0.681, Figure 3). The cumulative 1-, 3-, and 5-year DFS rates were 54.4%, 42.2%, and 29.9% for patients in LMH group, and were 55.7%, 33.5%, and 33.2% for patients in OMH group, respectively. Together, the results showed that LHM for HCCs had comparable short-term and long-term outcomes with OHM.
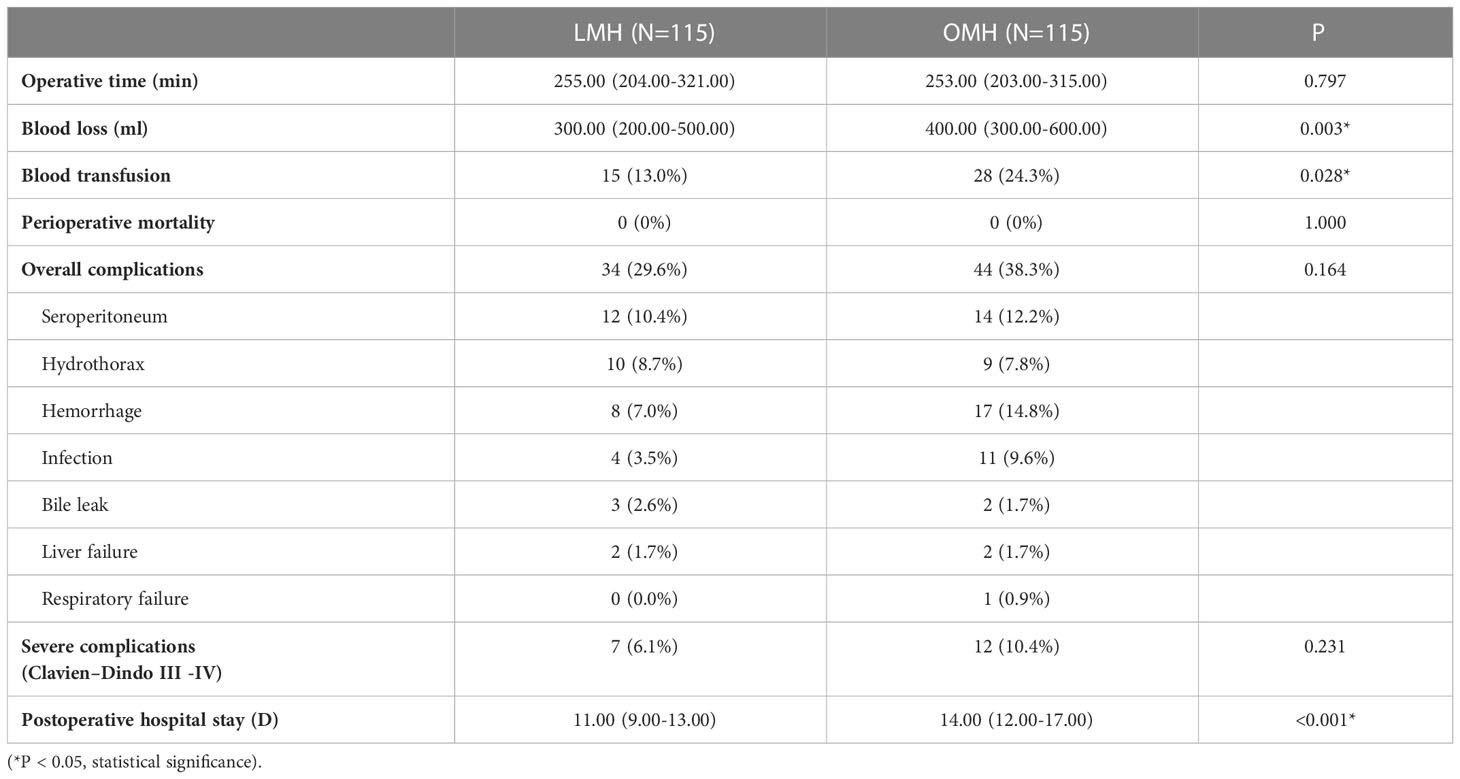
Table 4 Operative details and postoperative outcomes between laparoscopic major hepatectomy (LMH) and open major hepatectomy (OMH) groups after propensity score matching.
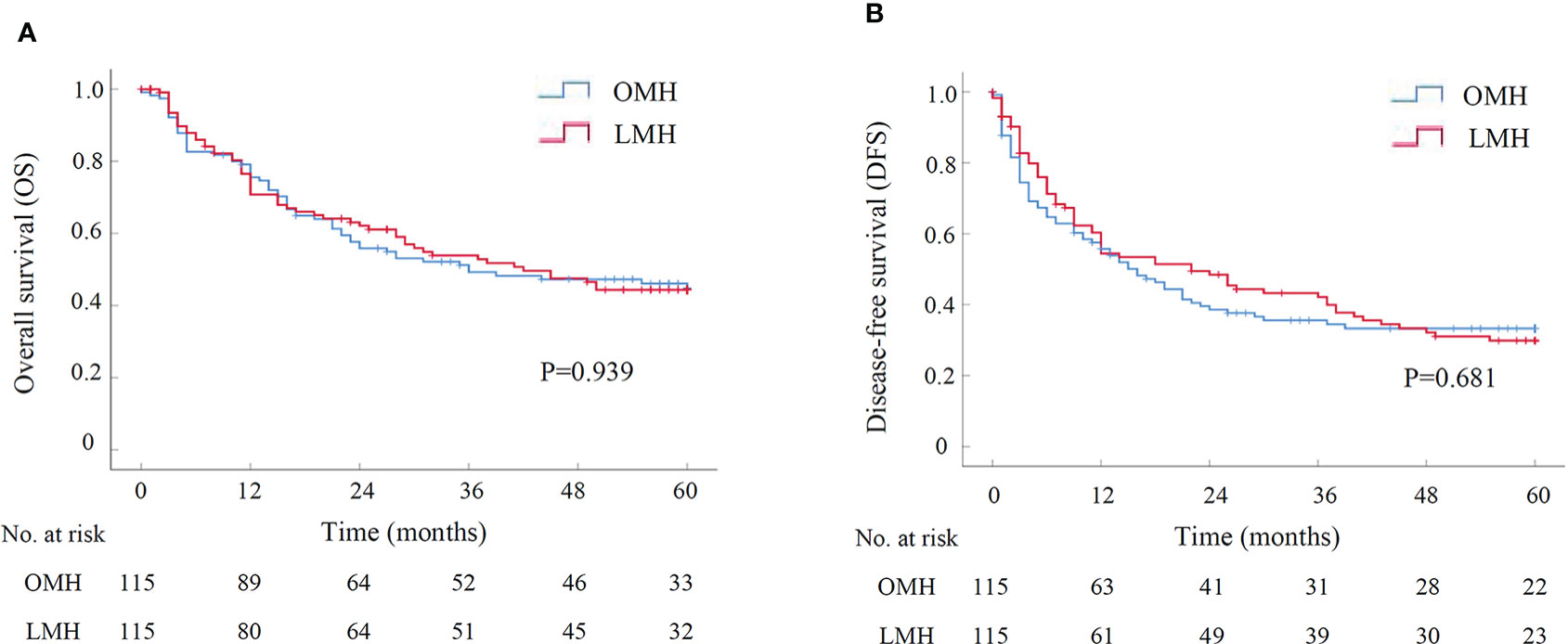
Figure 3 The survival curve of LMH versus OMH for HCCs after PSM by Kaplan-Meier analysis (N=115 for each group). (A) Overall survival; (B) Disease-free survival. LMH, laparoscopic major hepatectomy; OMH, open major hepatectomy; PSM, propensity score matching.
As a minimally invasive technique, the application of LLR has increased rapidly. Initially, LMLR was mainly applied for HCCs located in anterolateral segments (18, 19). With the development of laparoscopic equipment and techniques, LMLR was gradually applied in difficult segments and LMH were also gradually applied for HCCs. However, the oncological adequacy of LLR for HCCs, especially LMLR-DS and LMH, has become an important topic of debate. Although several reports of the successful outcomes of LMLR-DS and LMH for HCCs have been published (20–23), most of them were performed with small number of cases, and the long-term outcomes were not available in most studies. Our present study included a large number of HCC patients who received LLR. The data of a relative long follow-up period were also included. Besides, we employed PSM to decrease the inter-group baseline differences. Moreover, subgroup analysis of either LMLR-DS or LMH for HCCs was performed, respectively. In these respects, our study was meaningful and convincing.
The Asia Pacific Consensus and Southampton Consensus Guidelines indicate that more evidence is needed to support the growth of LMLR-DS and LMH for HCCs (6, 7). One major problems of LMLR in segment 7 or 8 is the limited visualization (24), and several measures might be useful for overcoming this limitation in our experience: (1) the patient lied down with a cushion underneath the right back; (2) the right and cephalic sides of the operating table were raised; (3) the whole liver ligaments were separated and then the assistant pushed the right liver toward the left anterior inferior direction; (4) a water balloon was pulled under the right diaphragm to elevate the right posterosuperior segment. For LMLR in segment 1, the operative approach has always been an important issue. Three surgical approaches are described: left side, right side and trans-parenchymal approach (25, 26). Left side approach is commonly used for HCC in Spiegel’s lobe. The most common approach for HCC in caudate process is from the right side. Our previous study showed that the trans-parenchymal approach might be suitable for selected HCC originating in the paracaval portion (27). Another major problem of LMLR-DS is the intraoperative bleeding. As the difficult segments (1, 4a, 7, 8) were close to the main hepatic veins, skilled suture techniques are necessary for control of hepatic vein bleeding. 3D laparoscopic system, which offers the surgeon binocular vision and depth perception, might be benefit for suture compared with 2D system (28). Together, by using the above measures, LMLR-DS for HCCs could be successfully performed in most cases.
Most of the HCCs were combined with liver cirrhosis (29). LMH for HCCs is technically more demanding because of the increased risk of intraoperative bleeding and postoperative liver failure, especially in cirrhotic patients (22, 30). Some measures might be benefit for its performance in our experience. Firstly, Preoperative evaluation of liver function, Child-Pugh Grade A and ICG-R15 less than 10%, and remaining liver volume that accounts for >40% might be the prerequisites for HCC patients with liver cirrhosis to receive LMH (31, 32). Secondly, priority of Glissonian pedicle ligation could help to control the intraoperative bleeding (33), and Laennec’s approach is a recently reported easy and reliable measure to isolate the Glissonean pedicles (34). The Laennec’s approach might be more easily performed under the laparoscopic system because of the visual amplification compared with open surgery. Thirdly, the anterior approach of major hepatectomy, with benefit of reducing intraoperative bleeding compared with conventional approach, might be more easily performed under the laparoscopic system (35, 36). Fourthly, Pringle maneuver and low central venous pressure (LCVP, less than 5 cmH2O) was used to reduce intraoperative bleeding (37–39). Through the above measures, LMH for HCCs, especially with liver cirrhosis, could be successfully performed in most cases.
In our study, the short-term outcomes of LLR for HCCs were analyzed. In general, LLR had significantly less intraoperative blood loss and less blood transfusion compared with OLR. The control of hepatic vein bleeding is the major issue for LR. Although LCVP was performed in both LLR and OLR, the combination of LCVP and high pneumoperitoneum pressure (HPP, 13mmHg) might be more useful for control of hepatic vein bleeding in LLR. With the visual magnification of laparoscopic system, the parenchyma dissection in LLR might be more precise compared with it in OLR, which might be benefit for the decrease of intraoperative bleeding. Besides, the overall and severe postoperative complications after LLR were less than them after OLR. Because of the lower rate of complications, the postoperative hospital stays after LLR were shorter than it after OLR. Moreover, subgroup analyses confirmed that LMLR-DS had better short-term outcomes than OMLR-DS for HCCs. Patients after LHM also had lower operative blood loss and shorter postoperative hospital stays than OHM. Taken together, LLR could be a safe measure for HCC treatment.
The most important point of this study was to assess the long-term outcomes of LLR for HCCs. In general, LLR had comparable OS and DFS with OLR for HCCs. In subgroup analyses, LMLR-DS had similar OS and DFS with OMLR-DS for HCCs, and consistently, the OS and DFS had no differences between LMH and OMH groups. Taken together, considering that LLR had comparable long-term outcomes with OLR, LLR could be a reliable measure for HCC treatment. Thus, the indications of laparoscopic approach for HCCs in our center were mainly consistent with those of open approach. Besides, several points should be noticed for improving the long-term outcomes of either LLR or OLR for HCCs, which need to be validated further. Firstly, a wide-margin LR (≥1cm) might improve the long-term outcomes for patients with HCC (40, 41). Secondly, anatomic LR might be superior to non-anatomic LR regarding the long-term outcomes for HCCs (42, 43). Thirdly, the anterior approach for major hepatectomy with large HCC might have better long-term outcomes compared with the conventional approach (44).
Although our study included a large number of patients who received LLR for HCC, it still had limitations because that it was a retrospective study. Hence, a well-designed prospective study will be needed to affirm the validity of LLR for HCCs.
Our results indicated that LLR for HCCs showed better short-term outcomes and comparable long-term outcomes with OLR, even for patients who received LMLR in difficult segments (1, 4a, 7 and 8) or LMH. Thus, LLR could be reliable and recommended for HCC treatment.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ethics Committee of Southwest Hospital of Army Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JL, SZ, FT, SL and JW participated in research design, data analysis, and writing of the manuscript. JC, YC, LC, XW and XL participated in data collection and analysis. All authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1112380/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers (2021) 7(1):6. doi: 10.1038/s41572-021-00245-6
3. European Association for the Study of the Liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol (2018) 69(1):182–236. doi: 10.1016/j.jhep.2018.03.019
4. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology (2018) 68(2):723–50. doi: 10.1002/hep.2991
5. Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D, Dagher I, et al. The international position on laparoscopic liver surgery: The Louisville statement, 2008. Ann Surg (2009) 250(5):825–30. doi: 10.1097/sla.0b013e3181b3b2d8
6. Cheung TT, Han HS, She WH, Chen KH, Chow PKH, Yoong BK, et al. The Asia pacific consensus statement on laparoscopic liver resection for hepatocellular carcinoma: A report from the 7th Asia-pacific primary liver cancer expert meeting held in Hong Kong. Liver Cancer (2018) 7(1):28–39. doi: 10.1159/000481834
7. Abu Hilal M, Aldrighetti L, Dagher I, Edwin B, Troisi RI, Alikhanov R, et al. The Southampton consensus guidelines for laparoscopic liver surgery: From indication to implementation. Ann Surg (2018) 268(1):11–8. doi: 10.1097/SLA.0000000000002524
8. Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G. Comparative short-term benefits of laparoscopic liver resection: 9000 cases and climbing. Ann Surg (2016) 263(4):761–77. doi: 10.1097/SLA.0000000000001413
9. Cheung TT, Poon RT, Yuen WK, Chok KS, Jenkins CR, Chan SC, et al. Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: A single-center experience. Ann Surg (2013) 257(3):506–11. doi: 10.1097/SLA.0b013e31827b947a
10. Cheung TT, Dai WC, Tsang SH, Chan AC, Chok KS, Chan SC, et al. Pure laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma in 110 patients with liver cirrhosis: A propensity analysis at a single center. Ann Surg (2016) 264(4):612–20. doi: 10.1097/SLA.0000000000001848
11. Moris D, Burkhart RA, Beal EW, Pawlik TM. Laparoscopic hepatectomy for hepatocellular carcinoma: Are oncologic outcomes truly superior to an open approach? Hepatobiliary Surg Nutr (2017) 6(3):200–2. doi: 10.21037/hbsn.2017.03.09
12. Cheung TT. Superior oncological outcome in laparoscopic hepatectomy for hepatocellular carcinoma, hype or hope? Hepatobiliary Surg Nutr (2017) 6(6):437–8. doi: 10.21037/hbsn.2017.09.01
13. Ciria R, Gomez-Luque I, Ocana S, Cipriani F, Halls M, Briceno J, et al. Systematic review and meta-analysis comparing the short- and long-term outcomes for laparoscopic and open liver resections for hepatocellular carcinoma: Updated results from the European guidelines meeting on laparoscopic liver surgery, Southampton, UK, 2017. Ann Surg Oncol (2019) 26(1):252–63. doi: 10.1245/s10434-018-6926-3
14. Yoon YI, Kim KH, Cho HD, Kwon JH, Jung DH, Park GC, et al. Long-term perioperative outcomes of pure laparoscopic liver resection versus open liver resection for hepatocellular carcinoma: A retrospective study. Surg Endosc (2020) 34(2):796–805. doi: 10.1007/s00464-019-06831-w
15. Troisi RI, Berardi G, Morise Z, Cipriani F, Ariizumi S, Sposito C, et al. Laparoscopic and open liver resection for hepatocellular carcinoma with child-pugh b cirrhosis: Multicentre propensity score-matched study. Br J Surg (2021) 108(2):196–204. doi: 10.1093/bjs/znaa041
16. Wang Q, Li HJ, Dai XM, Xiang ZQ, Zhu Z. Laparoscopic versus open liver resection for hepatocellular carcinoma in elderly patients: Systematic review and meta-analysis of propensity-score matched studies. Int J Surg (2022) 105:106821. doi: 10.1016/j.ijsu.2022.106821
17. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
18. Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg (2009) 250(5):831–41. doi: 10.1097/SLA.0b013e3181b0c4df
19. Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. conclusions of the Barcelona-2000 EASL conference. European association for the study of the liver. J Hepatol (2001) 35(3):421–30. doi: 10.1016/s0168-8278(01)00130-1
20. Xiao L, Xiang LJ, Li JW, Chen J, Fan YD, Zheng SG. Laparoscopic versus open liver resection for hepatocellular carcinoma in posterosuperior segments. Surg Endosc (2015) 29(10):2994–3001. doi: 10.1007/s00464-015-4214-x
21. D'Hondt M, Tamby E, Boscart I, Turcotte S, Parmentier I, Pottel H, et al. Laparoscopic versus open parenchymal preserving liver resections in the posterosuperior segments: A case-matched study. Surg Endosc (2018) 32(3):1478–85. doi: 10.1007/s00464-017-5835-z
22. Xu HW, Liu F, Li HY, Wei YG, Li B. Outcomes following laparoscopic versus open major hepatectomy for hepatocellular carcinoma in patients with cirrhosis: A propensity score-matched analysis. Surg Endosc (2018) 32(2):712–9. doi: 10.1007/s00464-017-5727-2
23. Delvecchio A, Conticchio M, Ratti F, Gelli M, Anelli FM, Laurent A, et al. Laparoscopic major hepatectomy for hepatocellular carcinoma in elderly patients: A multicentric propensity scorebased analysis. Surg Endosc (2021) 35(7):3642–52. doi: 10.1007/s00464-020-07843-7
24. Ishizawa T, Gumbs AA, Kokudo N, Gayet B. Laparoscopic segmentectomy of the liver: From segment I to VIII. Ann Surg (2012) 256(6):959–64. doi: 10.1097/SLA.0b013e31825ffed3
25. Ruzzenente A, Ciangherotti A, Aldrighetti L, Ettorre GM, De Carlis L, Ferrero A, et al. Technical feasibility and short-term outcomes of laparoscopic isolated caudate lobe resection: An IgoMILS (Italian group of minimally invasive liver surgery) registry-based study. Surg Endosc (2022) 36(2):1490–9. doi: 10.1007/s00464-021-08434-w
26. Chaib E, Ribeiro MA Jr, Silva Fde S, Saad WA, Cecconello I. Caudate lobectomy: Tumor location, topographic classification, and technique using right- and left-sided approaches to the liver. Am J Surg (2008) 196(2):245–51. doi: 10.1016/j.amjsurg.2007.11.020
27. Sun TG, Wang XJ, Cao L, Li JW, Chen J, Li XS, et al. Laparoscopic anterior hepatic transection for resecting lesions originating in the paracaval portion of the caudate lobe. Surg Endosc (2021) 35(9):5352–8. doi: 10.1007/s00464-021-08455-5
28. Au KP, Chan MY, Chu KW, Kwan CLY, Ma KW, She WH, et al. Impact of three-dimensional (3D) visualization on laparoscopic hepatectomy for hepatocellular carcinoma. Ann Surg Oncol (2022) 29(11):6731–44. doi: 10.1245/s10434-022-11716-9
29. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet (2018) 391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2
30. Komatsu S, Brustia R, Goumard C, Perdigao F, Soubrane O, Scatton O. Laparoscopic versus open major hepatectomy for hepatocellular carcinoma: A matched pair analysis. Surg Endosc (2016) 30(5):1965–74. doi: 10.1007/s00464-015-4422-4
31. Chan A, Kow A, Hibi T, Di Benedetto F, Serrablo A. Liver resection in cirrhotic liver: Are there any limits? Int J Surg (2020) 82S:109–14. doi: 10.1016/j.ijsu.2020.06.050
32. Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer (2020) 9(6):682–720. doi: 10.1159/000509424
33. Yamamoto M, Ariizumi SI. Glissonean pedicle approach in liver surgery. Ann Gastroenterol Surg (2018) 2(2):124–8. doi: 10.1002/ags3.12062
34. Hu W, Zhang G, Chen M, Zhong C, Li M, Sun X, et al. Laennec's approach for laparoscopic anatomical hemihepatectomy. World J Surg Oncol (2021) 19(1):295. doi: 10.1186/s12957-021-02404-1
35. Hasegawa Y, Nitta H, Takahara T, Katagiri H, Kanno S, Umemura A, et al. Anterior approach for pure laparoscopic donor right hepatectomy. Surg Endosc (2020) 34(10):4677–8. doi: 10.1007/s00464-020-07649-7
36. Peng Y, Li B, Xu H, Guo S, Wei Y, Liu F. Is the anterior approach suitable for laparoscopic right hemihepatectomy in patients with large HCC (5-10 cm)? a propensity score analysis. Surg Endosc (2022) 36(8):6024–34. doi: 10.1007/s00464-022-09119-8
37. Piardi T, Lhuaire M, Memeo R, Pessaux P, Kianmanesh R, Sommacale D. Laparoscopic Pringle maneuver: How we do it? Hepatobiliary Surg Nutr (2016) 5(4):345–9. doi: 10.21037/hbsn.2015.11.01
38. Cai J, Zheng J, Xie Y, Kirih MA, Jiang G, Liang Y, et al. A novel simple intra-corporeal Pringle maneuver for laparoscopic hemihepatectomy: How we do it. Surg Endosc (2020) 34(6):2807–13. doi: 10.1007/s00464-020-07513-8
39. Liu TS, Shen QH, Zhou XY, Shen X, Lai L, Hou XM, et al. Application of controlled low central venous pressure during hepatectomy: A systematic review and meta-analysis. J Clin Anesth (2021) 75:110467. doi: 10.1016/j.jclinane.2021.110467
40. Yang P, Si A, Yang J, Cheng Z, Wang K, Li J, et al. A wide-margin liver resection improves long-term outcomes for patients with HBV-related hepatocellular carcinoma with microvascular invasion. Surgery (2019) 165(4):721–30. doi: 10.1016/j.surg.2018.09.016
41. Liu L, Shui Y, Yu Q, Guo Y, Zhang L, Zhou X, et al. Narrow-margin hepatectomy resulted in higher recurrence and lower overall survival for R0 resection hepatocellular carcinoma. Front Oncol (2020) 10:610636. doi: 10.3389/fonc.2020.610636
42. Shindoh J, Makuuchi M, Matsuyama Y, Mise Y, Arita J, Sakamoto Y, et al. Complete removal of the tumor-bearing portal territory decreases local tumor recurrence and improves disease-specific survival of patients with hepatocellular carcinoma. J Hepatol (2016) 64(3):594–600. doi: 10.1016/j.jhep.2015.10.015
43. Jiao S, Li G, Zhang D, Xu Y, Liu J. Anatomic versus non-anatomic resection for hepatocellular carcinoma, do we have an answer? a meta-analysis. Int J Surg (2020) 80:243–55. doi: 10.1016/j.ijsu.2020.05.008
Keywords: hepatocellar carcinoma, laparoscopic liver resection, open liver resection, laparoscopic minor liver resection in difficult segments, laparoscopic major hepatectomy, prognosis
Citation: Tian F, Leng S, Chen J, Cao Y, Cao L, Wang X, Li X, Wang J, Zheng S and Li J (2023) Long-term outcomes of laparoscopic liver resection versus open liver resection for hepatocellular carcinoma: A single-center 10-year experience. Front. Oncol. 13:1112380. doi: 10.3389/fonc.2023.1112380
Received: 30 November 2022; Accepted: 10 January 2023;
Published: 25 January 2023.
Edited by:
Haoming Zhou, Nanjing Medical University, ChinaReviewed by:
Lianxin Liu, First Affiliated Hospital of Harbin Medical University, ChinaCopyright © 2023 Tian, Leng, Chen, Cao, Cao, Wang, Li, Wang, Zheng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianwei Li, bGppYW53ZWkxMDE1QHllYWgubmV0; Shuguo Zheng, c2h1Z3VvemhAMTYzLmNvbQ==; Juan Wang, MTUxMTE5NzYzOTlAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.