
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 11 April 2023
Sec. Thoracic Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1110638
The presence of anaplastic lymphoma kinase (ALK) rearrangement is reported to be related to the lack of efficacy of immune checkpoint inhibitors (ICIs). High levels of microsatellite instability (MSI-high) are important biomarkers of ICIs, particularly in colorectal cancer. The therapeutic effect of ICIs for MSI-high NSCLC is uncertain because of the rarity of these tumors. Here we report a case of ALK rearranged NSCLC with MSI-high. A 48-year-old male was diagnosed with lung adenocarcinoma, cT4N3M1a, stage IVA with ALK rearrangement, high PD-L1 expression with a tumor proportion score (TPS) of 100%, and MSI-high. The patient was treated with alectinib as the first-line therapy but progressed at five months with left atrial invasion re-expansion. The patient discontinued alectinib and was switched to pembrolizumab monotherapy. After two months, left atrial invasion significantly decreased. The patient continued pembrolizumab for a year without noticeable adverse events, and tumor shrinkage persisted. This case supports the efficacy of ICIs for MSI-high NSCLC, even in the presence of ALK rearrangement.
Anaplastic lymphoma kinase (ALK) rearrangement has been detected in 3–5% of non-small cell lung cancers (NSCLC). ALK inhibitors are effective and are considered the standard initial therapy for patients with ALK-positive NSCLCs. Unfortunately, despite the initial clinical benefit, acquired resistance to alectinib usually develops in all patients with a median progression-free survival (PFS) of approximately 2–3 years (1). If the resistance mechanism is based on “on-target,” switching to other ALK tyrosine kinase inhibitors (TKIs) has been reported to be effective however, if it is based on “off-target,” systemic therapy similar to the treatment for driver mutation-negative advanced NSCLC is recommended. However, some retrospective analyses have reported a lack of efficacy of immune checkpoint inhibitors (ICIs) in patients with ALK-positive NSCLC (2).
A high level of microsatellite instability (MSI-high) is the cause of numerous mutation accumulations at microsatellites, which are short sequence stretches spread over the entire genome. As a result of deficiencies in the DNA mismatch repair system (dMMR), errors during DNA replication, such as insertions or deletions, are not corrected. Thus, MSI is closely associated with cancer development. Patients with MSI-high solid tumors were likely to respond to ICI monotherapy. Pembrolizumab is approved for colorectal cancer with MSI-high or positive for immunohistochemistry of dMMR. However, the therapeutic effect of ICIs on MSI-high NSCLC is still unclear because of the rarity of this cancer. Moreover, the treatment of ALK-positive NSCLC with high MSI has not been established. Herein, we present a case of advanced MSI-high and ALK-positive lung adenocarcinoma. The patient had an enduring response to pembrolizumab and achieved 1-year progression-free survival (PFS).
A 48-year-old male patient was admitted to our institution with a primary complaint of a cough and back pain. The patient had a smoking history of 14 pack-years, and no other medical history or cancer. Chest computed tomography (CT) revealed a mass with a diameter of 77 mm in the lower lobe of the right lung with left atrial invasion (Figure 1B CT1), enlarged mediastinal lymph nodes (#2R, 4R, #7, and # 2L), right hilar lymph nodes, and right pleural effusion (Figure 1A). Transbronchial lung biopsy histopathological immunohistochemistry (IHC) revealed TTF-1 positive adenocarcinoma (Figures 2A–D). Based on these results, the patient was diagnosed with lung adenocarcinoma, cT4N3M1a, stage IVA. Additionally, the IHC analysis indicated that the tumor cells were positive for ALK antibody (clone D5F3, VENTANA). (Figure 2E) Molecular analysis using the Oncomine Dx Target Test Multi-CDx System (Life Technologies corporation, Frederic and Pleasanton facility, USA) and Oncomine Comprehensive Assay v3 (OCA v3; Thermo Fisher Scientific) using fresh-frozen tissues, which was performed in a lung cancer genomic screening project for individualized medicine in Japan (LC-SCRUM)revealed an EML4-ALK fusion V1 (E13:A20). The IHC analysis using programmed death-ligand 1 (PD-L1) IHC 22C3 pharmDx assay showed high PD-L1 expression with a tumor proportion score (TPS) of 100%. (Figure 2F) Additionally, analysis by electrophoresis of the PCR products revealed that the case was MSI-high. We performed immunohistochemistry for mismatch repair proteins (MMR) MLH1, MSH2, MSH6, and PMS2. The nuclei of the tumor cells were positive for MLH1 and PMS2 but negative for MSH2 and MSH6 indicating MSI status with MSH2 gene disruption (Figure 3).
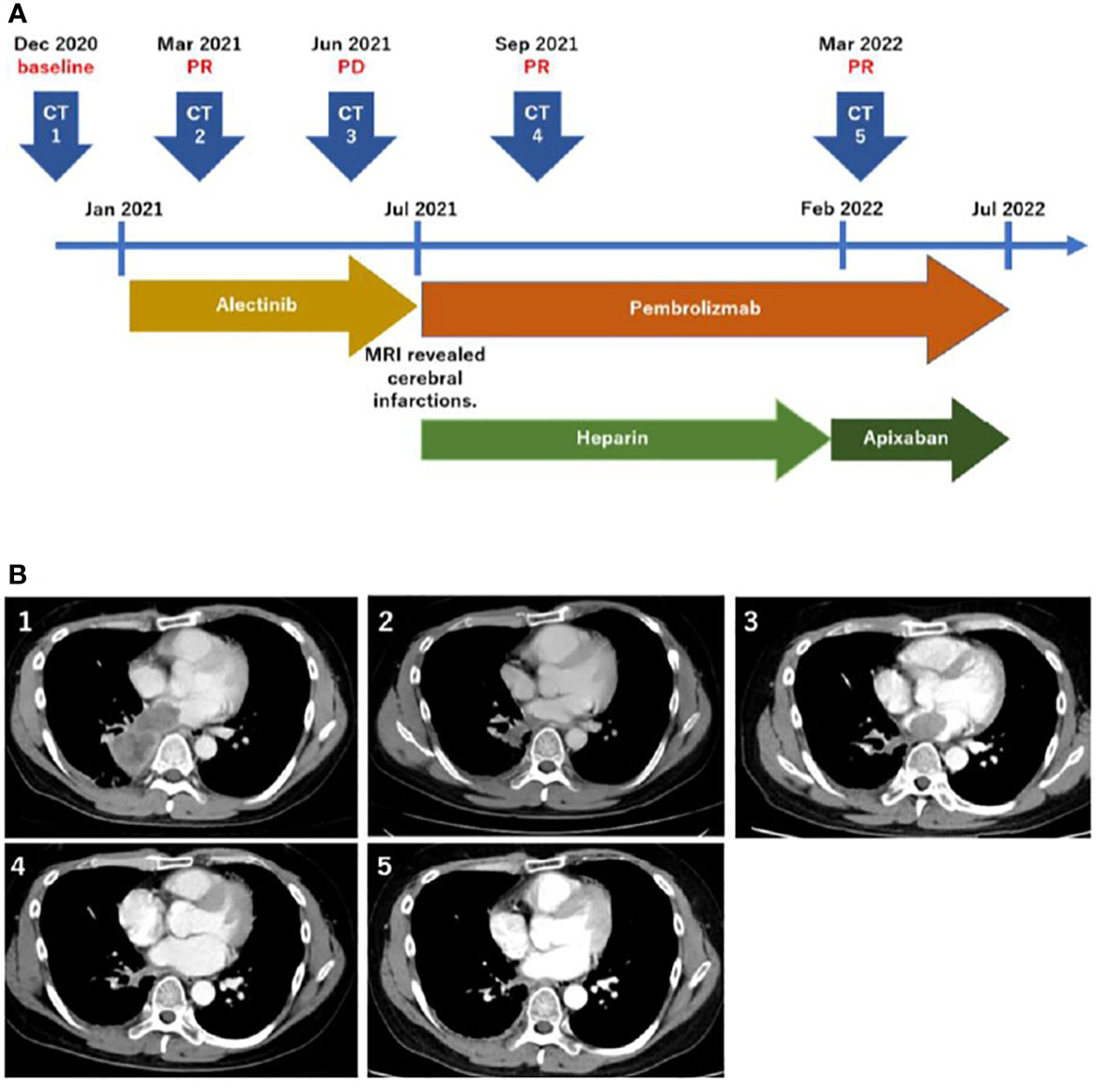
Figure 1 Timeline of the treatment and disease course (A). Computed tomography scans corresponding to the timeline (B).
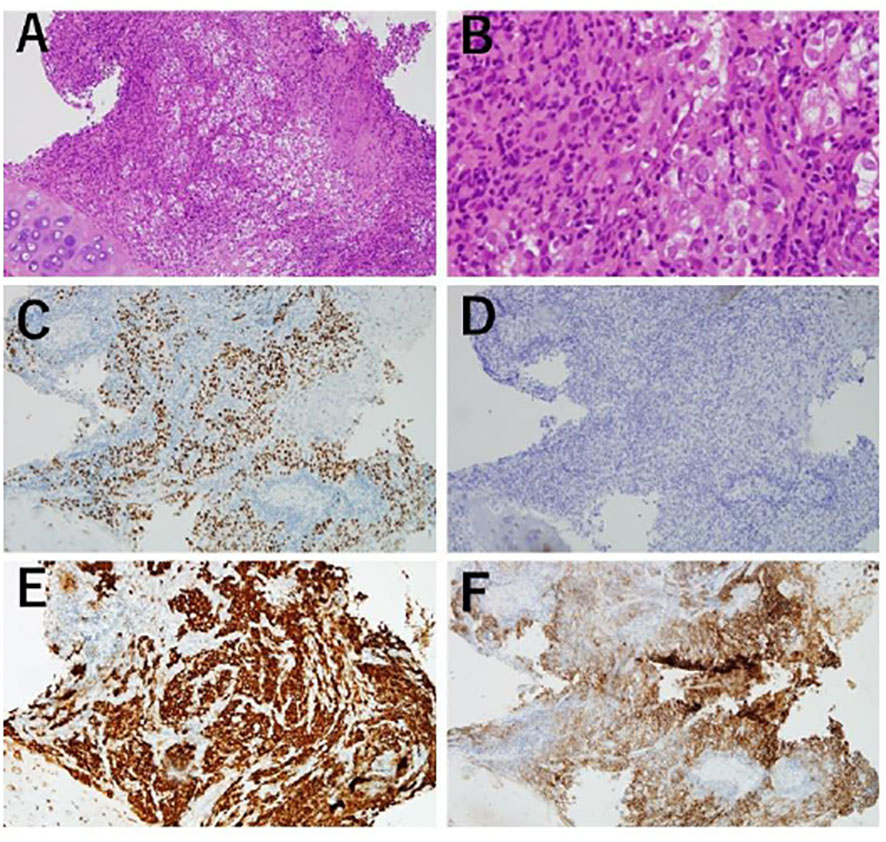
Figure 2 Transbronchiallung biopsy before treatment. (A, B) Atypical cell infiltration and lymphocyte infiltration were observed. (100x, 400x) (C) Tumor cells positive by immunohistochemistry (IHC) for TTF-1. (100x) (D) Tumor cells were negative by IHC for p40. (100x) (E) Tumor cells positive for ALK IHC (Roche-Ventana, D5F3). (100x) (F) PD-L1 IHC (Dako, 22C3), giving a tumor proportion score of 100. (100).
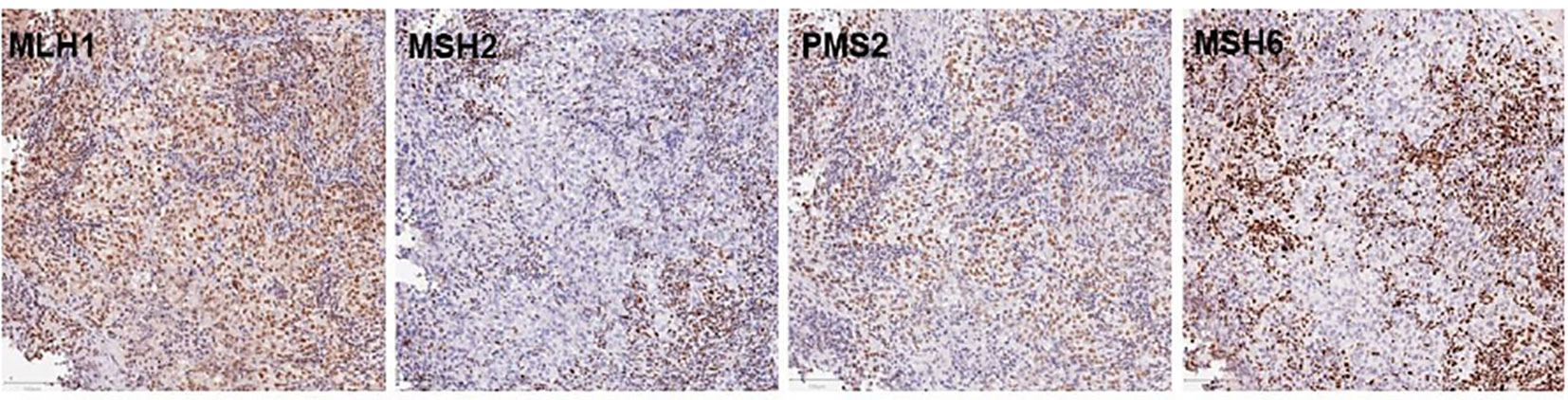
Figure 3 Immunohistochemistry for MMR proteins MLH1, MSH2, PSM2, and MSH6. The nuclei of tumor cell were clearly positive for MLH1 and PMS2 staining together with the surrounding non-neoplastic cell nuclei, largely of infiltrating lymphocytes. In contrast. MSH2 and MSH6 were negative in tumor cells with positive staining for non-neoplastic cell nuclei, serving as internal positive control for staining. The bars in the lower left corner indicate 100mm.
The patient was treated with alectinib at the Japanese-approved dose of 300 mg twice a day. The tumor size decreased two months after the initiation of alectinib therapy (Figure 1B CT2), and he continued its intake as prescribed without any adverse events. However, five months after alectinib treatment, the patient experienced pain in the left fingertips and was diagnosed with peripheral arterial occlusive disease. Brain magnetic resonance imaging (MRI) revealed multiple cerebral infarctions, and laboratory tests demonstrated elevated D-dimer (21.6 μg/ml) levels. Additionally, the CT scan showed left atrial invasion progression (Figure 1B CT3). The patient was then put on Heparin therapy for the treatment of diagnosed cancer-associated thromboembolism. Subsequently, alectinib was discontinued and the patient was switched to pembrolizumab IV therapy at 200 mg every three weeks. After evidence of ALK inhibitor resistance, the combined therapy with cytotoxic chemotherapy and pembrolizumab is one of the preferred treatments, regardless of PD-L1 expression. However, because this patient exhibited MSI-high, pembrolizumab monotherapy can be considered as an alternative. After discussing with the patient, he decided to receive pembrolizumab monotherapy. The left atrial invasion was significantly decreased two months after pembrolizumab therapy was initiated (Figure 1B CT4). Subsequently, D-dimer levels also returned to normal levels. The patient continued pembrolizumab therapy for a year with no adverse events, and continued tumor shrinkage (Figure 1B CT5). Heparin was switched to Apixaban, which the patient continues to use (Figure 1A).
Alectinib, a highly selective inhibitor of ALK, is one of the recommended first-line treatments for ALK-positive NSCLC. The median PFS with first-line alectinib was 34.8 months in the ALEX study, however, some patients acquired resistance to alectinib earlier (1). High expression of PD-L1 and smoking history are associated with acquisition of resistance. A retrospective study for ALK-positive NSCLC patients who received second-generation ALK-TKIs including alectinib, showed that median PFS was shorter in patients with high PD-L1 expression (median PFS in patients with PD-L1 TPS of 0% vs 1–49% vs ≥ 50% were 27.43 months vs 30.63 months vs 9.50 months, respectively, P = 0.001) (3). In this case, early tumor progression at only six months was observed despite the initial tumor shrinkage with alectinib, which was consistent with previous reports of poor response to alectinib, as the patient was a smoker and had high PD-L1 expression.
For patients with driver-negative NSCLC with high PD-L1 expression (≥50%), ICIs with or without cytotoxic chemotherapy is recommended. However, ICIs are generally less efficacious in patients with mutations such as EGFR, ALK, and ROS-1 (4). The efficacy of ICIs in patients with EGFR -positive NSCLC with high PD-L1 expression has been reported, however the efficacy of this group of drug for patients with ALK rearrangement is unknown (2, 5). S. Baldacci et al. reported the first case of ALK-positive NSCLC with complete and prolonged response to ICI monotherapy (6). In this study, the patient’s first-line treatment was ceritinib, an ALK-TKI, lasted for only five months with tumor progression. Subsequently, two months after initiation of nivolumab therapy, a complete response was observed. Early resistance to ALK-TKIs and the efficacy of ICIs were similar to those in our case. The case reported by Baldacci et al. also showed high PD-L1 expression (100%), which could be a reason for these common therapeutic effects. This case and ours raise hopes for the potential use of ICIs in patients with ALK-positive NSCLC having high PD-L1 expression.
In addition to high PD-L1 expression, the present case showed MSI-high. To the best of our knowledge, there are no data on ICIs in patients with MSI-high NSCLC harboring driver mutation. Our case report potentially provides information about a better medication for these patients. These ICIs are effective in patients with MSI-high colorectal cancer and other MSI-high solid tumors, however, the therapeutic effect of MSI-high NSCLC is unclear due its low frequency. The frequency of MSI-high in NSCLC is reported to be only 0.19 – 2.0% (7–9). We performed immunohistochemistry, uses anti-human antibody clones against MSH6, PMS2, MLH1 and MSH2, for mismatch repair proteins (MMR). This case was negative for MSH2 and MSH6. M.E. Salem et al. examined 1057 MSI-high tumors and reported MMR mutations occur in less than half of MSI-high tumors. They found loss of MSH2/MSH6 co-expression is associated with a higher tumor mutation burden compared to loss of MLH1/PMS2 (10). The one limitation of this case is that the patient was not tested for Lynch syndrome, which is caused by germline alterations in MMR genes. Nevertheless, the possibility of Lynch syndrome-associated NSCLC was low because NSCLC is not one of the recognized Lynch syndrome-associated tumors and he had no other malignancy.
Notably, MSI-high NSCLC often has some mutations, which are reported in 68 – 100% of cases (7, 8). Warth et al. analyzed 480 cases of lung adenocarcinoma and found that 4 of them had high MSI, and all of them had mutations in EGFR (n=2), KRAS (n=1), or BRAF (n=1) (8). A cohort of 526 Brazilian patients with NSCLC found only one case of high MSI with TP53 mutations (7). In 12485 Chinese lung cancer cases, 67 were MSI-high. Of these, 46 had mutations, most of which were EGFR (n=26) and the others were KRAS (n=5), RET (n=2), PIK3CA (n=8), and FGFR2/3 (n=5) (9). These results suggest that driver mutations are frequent in cases with high MSI. This case report provides new insights into the treatment of MSI-high NSCLC with driver mutations.
We have described a case of advanced lung adenocarcinoma with ALK rearrangement, high PD-L1 expression, and MSI-high, in which shrinkage persisted for a year with patient on pembrolizumab therapy. This case supports the efficacy of ICI for MSI-high lung cancer, even in the presence of ALK rearrangement.
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
SM and NS collected the clinical information, diagnostic information, therapeutic information, and images of the patients. NS wrote the manuscript. SM wrote some sections of manuscript. TY and NS collected the pathological images of the patients. YM performed immunohistochemistry for mismatch repair proteins. SM, TY, HS revised the manuscript. All authors contributed to the article and approved the submitted version.
We would like to thank the staff at the National Cancer Center East and those involved in the LC-SCRUM studies.
SM reported personal fees from AstraZeneca, Chugai Pharmaceutical, Boehringer Ingelheim, Taiho Pharmaceutical, and Ono Pharmaceutical.
HS reports grants from Chugai Pharmaceutical and AstraZeneca, and personal fees from Ono Pharmaceutical, Nippon Boehringer Ingelheim, MSD, and Novartis Pharma.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al. Alectinib versus crizotinib in untreated ALK-positive non-Small-Cell lung cancer. N Engl J Med (2017) 377(9):829–38.
2. Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol (2019) 30(8):1321–8.
3. Li M, Hou X, Chen J, Zhang B, Wang N, Han H, et al. ALK fusion variant 3a/b, concomitant mutations, and high PD-L1 expression were associated with unfavorable clinical response to second-generation ALK TKIs in patients with advanced ALK-rearranged non-small cell lung cancer (GASTO 1061). Lung Cancer (2022) 165:54–62.
4. Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: A retrospective analysis. Clin Cancer Res (2016) 22(18):4585–93.
5. Oya Y, Kuroda H, Nakada T, Takahashi Y, Sakakura N, Hida T. Efficacy of immune checkpoint inhibitor monotherapy for advanced non-Small-Cell lung cancer with ALK rearrangement. Int J Mol Sci (2020) 21(7):2623.
6. Baldacci S, Gregoire V, Patrucco E, Chiarle R, Jamme P, Wasielewski E, et al. Complete and prolonged response to anti-PD1 therapy in an ALK rearranged lung adenocarcinoma. Lung Cancer (2020) 146:366–9.
7. De Marchi P, Berardinelli GN, Cavagna RO, Pinto IA, da Silva FAF, Duval da Silva V, et al. Microsatellite instability is rare in the admixed Brazilian population of non-small cell lung cancer: A cohort of 526 cases. Pathobiology. (2022) 89(2):101–6.
8. Warth A, Korner S, Penzel R, Muley T, Dienemann H, Schirmacher P, et al. Microsatellite instability in pulmonary adenocarcinomas: a comprehensive study of 480 cases. Virchows Arch (2016) 468(3):313–9.
9. Su L, Liu X, Huang X, Huang M. Correlation of microsatellite-instable (MSI) status and genomic characteristics in 12,000 Chinese lung cancer patients. J Clin Oncol (2021) 39(15_suppl):e21019–e.
Keywords: non-small cell lung cancer, anaplastic lymphoma kinase rearrangement, immune checkpoint inhibitor, microsatellite instability, case report, programmed death ligand (PD-L) 1, adenocarcinoma, advanced lung adenocarcinoma
Citation: Shigeta N, Murakami S, Yokose T, Miyagi Y and Saito H (2023) Case report: anaplastic lymphoma kinase (ALK) rearranged adenocarcinoma with high level of microsatellite instability response to pembrolizumab. Front. Oncol. 13:1110638. doi: 10.3389/fonc.2023.1110638
Received: 29 November 2022; Accepted: 27 March 2023;
Published: 11 April 2023.
Edited by:
Anurag Mehta, Rajiv Gandhi Cancer Institute and Research Centre, IndiaReviewed by:
Tanja Mesti, Institute of Oncology Ljubljana, SloveniaCopyright © 2023 Shigeta, Murakami, Yokose, Miyagi and Saito. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuji Murakami, bXVyYWthbWlzQGtjY2guanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.