
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Oncol. , 19 January 2023
Sec. Hematologic Malignancies
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1109866
This article is part of the Research Topic Myeloproliferative Neoplasms: Biology and Treatment View all 11 articles
Ropeginterferon alfa-2b is a novel, long-acting mono-pegylated proline-IFN-alpha-2b approved for treatment of polycythemia vera in adults, regardless of thrombotic risk level or treatment history. Clinical trial data indicate the dose and titration of ropeginterferon alfa-2b is safe and effective. However, additional studies may provide rationale for an amended, higher initial dosage and rapid titration. This article is an overview of current and upcoming studies of ropeginterferon alfa-2b in myeloproliferative neoplasms that support the exploration of an amended dosing scheme in order to optimize patient tolerability and efficacy outcomes.
Polycythemia vera (PV), essential thrombocythemia (ET), and myelofibrosis (MF) including pre-fibrotic/early primary myelofibrosis (PMF) are classical Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs) characterized by the uncontrolled clonal proliferation of hematopoietic stem or progenitor cells due to driver mutations of genes including JAK2, CALR, or MPL (1, 2). Thrombohemorrhagic complications and leukemic transformations are part of the natural history of MPNs (3, 4). Most PV patients harbor a JAK2 mutation, the vast majority being a point mutation on exon 14 (V617F) (4, 5). PV is considered an inflammatory neoplasm (4, 6) characterized by clonal erythrocytosis, often accompanied by thrombocytosis and leukocytosis, leading to a high risk of thromboembolic events (7).
Interferon (IFN) alfa-based therapies demonstrate preferential activity against neoplastic hematopoietic stem or progenitor cells and elicit complete and durable hematologic remission and molecular response, reduce PV progressive events, and shows improvement of patient myelofibrosis-free and overall survival (8–13). Ropeginterferon alfa-2b is a novel mono-pegylated IFN with pharmacokinetic properties allowing dosing once every 2 to 4 weeks (14–18). It was approved by the US Food and Drug Administration (FDA) in November 2021 and the European Medicines Agency (EMA) in February 2019 for adults with PV and is the first and only IFN approved for PV treatment (19, 20). The NCCN Guidelines place ropeginterferon alfa-2b as a treatment option for low (Category 2B) and high-risk PV patients (Category 2A) (2). The European LeukemiaNet (ELN) recommends ropeginterferon alfa-2b and pegylated IFN alfa-2a as a therapeutic option for treatment naive patients with low-risk PV requiring cytoreductive therapy (21).
The safety and efficacy of ropeginterferon alfa-2b was assessed in the pivotal phase 1/2 PEGINVERA, phase 3 PROUD-PV, and its extension CONTINUATION-PV studies (11, 12, 15, 22). Doses in these studies ranged from 50 micrograms (mcg) to 540 mcg every two weeks, leading to the recommended starting dose of 100 mcg [50 mcg for patients receiving hydroxyurea (HU)], and increasing 50 mcg every two weeks to a maximum of 500 mcg, until hematological parameters are stabilized (hematocrit <45%, platelets <400 x 109/L, WBC <10 x 109/L) (14). The dosing interval may increase to 4 weeks upon achieving hematological stability for at least one year on a stable dose (14). Since these studies were reported, additional clinical investigation into alternative dosing strategies suggest a potential role for rapid titration and higher starting doses of ropeginterferon alfa-2b.
The safety of ropeginterferon alfa-2b was also evaluated in patients with chronic hepatitis B or hepatitis C (genotypes 1 and 2) at doses ranging from 270 to 450 mcg every 2 weeks as monotherapy or in combination with ribavirin (23–27), or in COVID-19 patients in combination with standard of care at 250 mcg (28). Most reported adverse events (AEs) during ropeginterferon alfa-2b treatment were mild or moderate and toxicities ≥grade 3 were uncommon. These results suggest tolerability and safety of higher starting doses of ropeginterferon alfa-2b.
In this article, we review the clinical development of ropeginterferon alfa-2b in PV and current research extending into other related MPNs in order to closely examine a critical connection between an amended dosing schema and key disease outcome measures such as a reduction in thromboembolic risk, complete hematologic response, and reduction in the driver mutation variant allele frequency (VAF), while maintaining adequate safety and tolerability.
The PEGINVERA study was a phase 1/2 multicenter study conducted to evaluate the dosing, tolerability, and efficacy of ropeginterferon alfa-2b in PV patients (15). A diverse PV patient population was enrolled including newly diagnosed, those pretreated with HU, and those receiving HU, who were at low- or high-risk for thromboembolic events. Eight subcutaneously administered dose levels (50, 100, 150, 225, 300, 360, 450, and 540 mcg) given every 2 weeks were explored. The Phase 1 portion of the study aimed to identify the maximum tolerated dose (MTD) of ropeginterferon alfa-2b in 25 patients using a 3 + 3 dose-escalation method. No dose-limiting toxicities (DLTs) were observed and the MTD was determined to be the highest dose level that was evaluated, or 540 mcg. The Phase 2 portion of the PEGINVERA study was designed to assess efficacy in the form of complete response (CR) including hematologic remission according to modified ELN criteria (Table 1) and molecular response (MR) in 26 patients with dose titrations based on disease response and tolerability. After a median time of 5 years of treatment, results from this study showed that 27 of 42 patients (64.3%) attained a complete hematologic response (CHR) from the efficacy analysis set. It required a median time of 34 weeks of treatment for patients to achieve a CHR (22). For JAK2V617F VAF, 12/42 patients (28.6%) achieved a complete molecular response (CMR) as the best observed response. A median time of 82 weeks was required for patients to achieve a CMR and 34 weeks to achieve any MR. The study demonstrated that ropeginterferon alfa-2b treatment for up to 7 years was efficacious and well-tolerated (22).
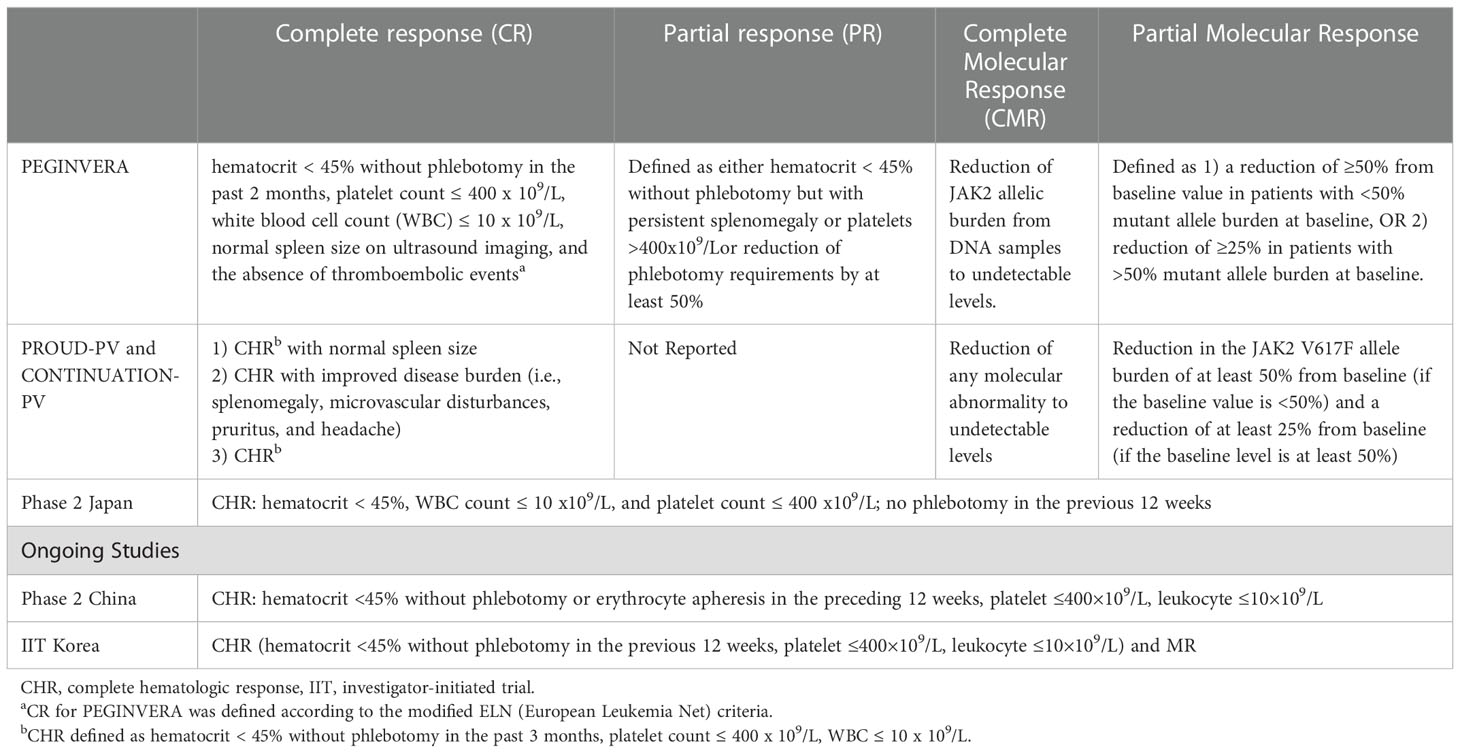
Table 1 Measured response definitions for the clinical studies of ropeginterferon alfa-2b for the PV treatment.
The phase 3 PROUD-PV study (N=254) enrolled early-stage patients diagnosed with PV who were either HU naïve or those who had been treated with HU for <3 years (11, 12). Patients underwent stratified randomization by age, history of thrombosis, and prior use of HU, and then randomized to ropeginterferon alfa-2b or HU group. The primary endpoint was disease response rate after 12 months of therapy. Dose initiation and titration were similar to the PEGINVERA Phase 2 trial. Ropeginterferon alfa-2b treatment was started at a dose of 100 mcg or 50 mcg for patients receiving HU and the dose increased by 50 mcg every 2 weeks for the maximum dose of 500 mcg. It was estimated that the mean efficacious dose was reached after approximately 16.2 weeks (95% CI 14·8–17·6) for ropeginterferon alfa-2b and 11.4 weeks (95% CI 10·2–12·6) for HU (11). At 12 months, the PROUD-PV study failed to demonstrate superiority regarding the primary endpoint (ropeginterferon alfa-2b 21% vs. HU 28%) and secondary endpoints of CHR (ropeginterferon alfa-2b 43% vs. HU 46%) and MR (ropeginterferon alfa-2b 34% vs. HU 42%) (11). It is unknown if the difference in time to reach an effective dose or an optimal maximum dose plateau contributed to the observed outcomes.
The extension study, CONTINUATION-PV, recruited 171 patients who completed the PROUD-PV study (95 in the ropeginterferon alfa-2b group vs. 76 in control) (11). While the control group was allowed to change treatment from HU to best available treatment (BAT), 64/66 patients (97%) remained on HU treatment. The dose for ropeginterferon alfa-2b used in PROUD-PV was continued at the discretion of the investigator and administered every 2, 3, or 4 weeks. Ropeginterferon alfa-2b group showed a continuous trend of improved outcomes. At 36 months, ropeginterferon alfa-2b treatment led to CHR in 67 of 95 patients (71%) compared to 38 of 74 (51%) in the HU/BAT group (p=0·012). The ropeginterferon alfa-2b group showed a superior rate in CHR with improved disease burden than the HU/BAT group (53% versus 38%; p=0·044) (11). A consistent reduction of median JAK2V617F allele burden was observed at every timepoint starting from screening (38%) to month 60 (8%) (12).
A multicenter, 52-week single-arm study evaluated the safety and efficacy of ropeginterferon alfa-2b in 29 Japanese patients diagnosed with PV (29). This trial followed the same titration regimen as the PROUD-PV study. The primary outcome was a durable CHR at 9 and 12 months and it was achieved in 8/29 patients (28%). At 12 months, 51% of patients achieved CHR, similar to 43% at 12 months reported in PROUD-PV. One serious treatment emergent AE (TEAE) of gastroenteritis was reported but was not deemed treatment-related. All patients experienced at least one TEAE and none were grade ≥3 in severity. An extension of the study continues (NCT04655092) (30) and its results may confirm the long-term effect of ropeginterferon alfa-2b observed in the PROUD/CONTINUATION-PV study.
A phase 2b, multicenter, open-label, parallel-group, randomized study evaluated the safety and efficacy of ropeginterferon alfa-2b at a fixed dose of 100 mcg every 2 weeks with phlebotomy and aspirin compared to phlebotomy and aspirin alone in 127 patients with low-risk PV (31). The primary endpoint was the maintenance of median hematocrit <45% during a 12-month period without disease progression. Following treatment, 84% of patients in the experimental group achieved the primary end point versus 60% in the control group (CI: 7-41%; p=0.0075). No disease progression was noted in the ropeginterferon alfa-2b group compared to 8% in the control. There was no statistically significant difference between the groups in frequency of AEs of grade 3/4 observed.
Based on the hematological outcomes observed in the clinical trials for ropeginterferon alfa-2b, several international investigational studies were designed to further understand the relationship between short and long-term outcome measures and safety with an amended, and potentially optimized dosing regimen in PV patients.
An ongoing, phase 2, single-arm study in 49 Chinese PV patients who are resistant or intolerant to HU is evaluating the safety and efficacy of ropeginterferon alfa-2b when administered in a 250-350-500 mcg dosing titration regimen (32, 33). In this dosing scheme, the starting dose is 250 mcg, followed by 350 mcg at Week 2, and a target dose of 500 mcg at Week 4. Interim results reveal a 52% CHR rate at Week 24, comparable to 43% at Week 52 observed in PROUD-PV. JAK2V617F VAF decreased over time with two patients achieving a level <3%. TEAEs were reported in >95% of patients with possible treatment-related Grade ≥ 3 AEs in five patients (10.2%). This suggests that the more aggressive dosing schema results in a more rapid time to CHR.
A single-arm open-label, multicenter study in South Korea is currently evaluating the safety, efficacy, and tolerability of 250-350-500 mcg ropeginterferon alfa-2b dosing in PV patients who are either HU naïve or previously treated (34). Only 4.4% of patients at an interim data-cut required dose reductions during dose escalation, indicating good tolerability of this dosing approach. No treatment-related serious AEs were reported, and the majority of the AEs were grade 1 or 2. At the interim data-cut, 45, 20, and 6 patients were evaluable at 3, 6, and 9 months, respectively. The mean hematocrit, platelet, and WBC counts steadily decreased from baseline to Month 6 and 9. The JAK2V617F VAF showed a trend of rapid decrease with treatment from 59.7% at baseline to 42.4% at Month 3, and 37.1% at Month 6. The data suggests that ropeginterferon alfa-2b therapy with the 250-350-500 mcg dosing regimen induced hematological and molecular responses and was well-tolerated in Korean patients with PV (34).
ECLIPSE-PV (NCT05481151) is a phase 3b study to assess the efficacy, safety, and tolerability of ropeginterferon alfa-2b in North American adult PV patients utilizing the 250-350-500 mcg regimen (35, 36). It is hypothesized that the response rates of PROUD/CONTINUATION-PV will be observed in a shorter period of time (36).
Decades of clinical research conducted with IFN alfa support its role as a treatment option across MPNs (37–40). While ropeginterferon alfa-2b is not currently approved to treat ET or MF, the existing IFN alfa data support further clinical evaluation of ropeginterferon alfa-2b for other MPN diagnoses (Table 2).
The ongoing global phase 3, multicenter, randomized, controlled SURPASS-ET trial (NCT04285086) (41), and the single-arm EXCEED-ET trial (NCT05482971) in US and Canada (47), are currently evaluating the 250-350-500 mcg dosing scheme. Previous exploratory clinical research and meta-analysis already indicated that interferon therapy could be a safe and effective treatment for ET (42). This Phase 3 trial aims to provide pivotal data to support the approval of ropeginterferon alfa-2b for the treatment of ET.
A phase 2 study evaluated the efficacy of ropeginterferon alfa-2b in 25 patients with pre-fibrotic PMF (43). Ropeginterferon alfa-2b was administered at doses between 50 to 200 mcg every 4 weeks during the 24-month treatment period in the treatment-naïve group. Clinical improvements were observed, and no patient showed disease progression at 2 years. Two patients withdrew from the study one year after starting ropeginterferon alfa-2b treatment due to psychological related AEs (43). A second study recruited 8 MF patients (2 early and 6 intermediate/high-risk) who received 50 mcg with dose titration to 300 mcg every two weeks (44). Preliminary clinical improvement regarding spleen size and symptom scores was observed. One patient discontinued therapy due to dizziness and atrial fibrillation (44).
An ongoing, phase 2 investigator-initiated trial assessing the efficacy and safety of the 250-350-500 mcg dosing titration of ropeginterferon alfa-2b in early MF (NCT04988815), includes 56 patients with pre-fibrotic/early MF, overt PMF, post-PV MF or post-ET MF, and low/intermediate - 1 risk category according to dynamic international prognostic scoring system (DIPSS) (46). Interim results indicate 71% of treated patients at Week 12 and 67% at Week 24 achieved clinico-hematologic complete response. Of 39 patients harboring JAK2 V617F, 36 reported reductions in VAF at 24 weeks with a reduction >50% in three treated patients and undetectable levels in one patient. No progression to overt MF or blast phase disease was observed. No treatment discontinuation, new safety signals, or deaths were reported thus far. The treatment options for patients with early MF including pre-fibrotic PMF have been very limited. The data from this study suggest that ropeginterferon alfa-2b potentially provides an effective treatment option for patients with early MF.
Further, a compassionate use program (CUP) recruited HU and/or anagrelide-resistant or intolerant MPN patients and administered ropeginterferon alfa-2b mostly using the 250-350-500 mcg dosing regimen in Taiwan (45). Published data from an initial cohort of nine patients show tolerability and substantial efficacy. Additional data in 20 MPN patients (14 PV, 4 ET, 1 post-ET MF, 1 pre-fibrotic PMF) further demonstrate tolerability and efficacy (45). At Week 52, eight of 11 response-evaluable PV patients (72.7%) achieved CHR and two of three ET patients (66.7%) achieved CHR. The median time to CHR was 27 and 24 weeks for PV and ET, respectively.
Overall, ropeginterferon alfa-2b treatment of MPNs at the 250-350-500 mcg dosing regimen appears to lead to notable and rapidly occurring clinical efficacy or activities with tolerability. The drug-related adverse events were generally well-tolerated and manageable, indicating a favorable benefit-risk profile.
The response outcomes observed in multiple clinical trials of ropeginterferon alfa-2b confirm clinical benefits in MPN patients. Emerging data utilizing the 250-350-500 mcg regimen suggest that a higher initial dose with faster dose titration may lead to earlier complete hematological remission. Although a direct comparison is not possible in the absence of head-to-head data, treatment with this regimen in PV for 6 months led to CHR rates comparable to those observed at 12 months in the studies utilizing the low starting dose and slower titration schema.
Support for a higher starting dose of ropeginterferon alfa-2b comes from the outcomes noted in the PROUD-PV and the study by Edahiro et al. compared to the outcomes from the emerging data of the 250-350-500 mcg ropeginterferon alfa-2b titration regimen from several recent sources including a CUP in Taiwan (46, 48), a Phase 2 study in China (32, 33), an IIT study in Korea (34). For example, the study by Edahiro et al. followed the slow titration regimen, whereas the 14 patients with HU and/or anagrelide resistance or intolerance enrolled in the CUP study followed the 250-350-500 mcg titration regimen, and the median time to response was 52 weeks vs 27 weeks. At 52 weeks, patients from Edahiro et al. study had a CHR rate of 51% compared to 73% in the CUP study. Interim results from the Chinese Phase II study in HU-resistant or intolerant PV patients showed a CHR rate of 52% at 24 weeks (32). The CHR rate at 24 weeks (6 months) was even notably higher than the rate of 43% observed at 12 months in the PROUD-PV study. The data from the Korean IIT study in HU naïve or pre-treated PV patients also indicate higher hematologic and molecular responses at 6 months (34).
Indeed, the risk of a thromboembolic event is highest immediately before or after establishing the diagnosis of PV (3, 49–51). A potential risk of thrombosis may not be adequately addressed by the low starting dose and slow titration regimen as time to hematologic response is delayed. Furthermore, it is widely known that high JAK2V617F VAF poses greater risk of myelofibrotic transformation (52–54), and that high JAK2V617F VAF is associated with high leukocyte count (55, 56) which has also been implicated as an independent risk factor for thromboembolic events (57, 58). Results from ongoing clinical studies utilizing the 250-250-500 dosing regimen will provide key insights into the correlation between dosage and outcome response and rates of thromboembolic complications. Thromboembolic complications have not been reported during the intra-patient dose escalations with the 250-250-500 dosing regimen from the existing data. Given that it has only three step dose escalations with a higher starting dose, it is reasonable to believe that the dosing regimen might potentially minimize the risk of thrombosis and hemorrhage associated with an under-dosing during dose titrations. Although a thromboembolic risk associated with an under-dosing during the slow dose titrations in PV patients could potentially be managed with phlebotomies, the 250-350-500 dosing regimen of ropeginterferon alfa-2b provides a treatment option of leveraging the risk with rapid induction of hematologic remission and molecular response associated with VAF decreases of drive mutations such as JAK2V617F in broader MPN patients. Therefore, the importance of an optimal dosing and titration schedule for ropeginterferon alfa-2b may need to be further explored to better understand its potential impact on critical efficacy and tolerability outcomes in PV or other MPNs.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors thank Jewell Jessup, PhD and Megan Flynn, PhD for providing considerate comments and editorial assistance.
JM: Research support provided to the institution from Incyte, Novartis, Merck, CTI Bio, Roche, Kartos, Abbvie, Geron, BMS, and PharmaEssentia; Consulting fees from Incyte, Novartis, Roche, CTI Bio, Morphosys, BMS, Galecto, Karyopharm, Kartos, Abbvie, Sierra Onc, GSK, and PharmaEssentia. AQ, RU, LY, TA work for and are employees of PharmaEssentia Corporation.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Barbui T, Thiele J, Gisslinger H, Kvasnicka HM, Vannucchi AM, Guglielmelli P, et al. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J (2018) 8(2):15. doi: 10.1038/s41408-018-0054-y
2. NCCN. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Myeloproliferative neoplasms. version 3. Plymouth Meeting, PA, USA (2022). https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1477.
3. Kc D, Falchi L, Verstovsek S. The underappreciated risk of thrombosis and bleeding in patients with myelofibrosis: a review. Ann Hematol (2017) 96(10):1595–604. doi: 10.1007/s00277-017-3099-2
4. Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood (2013) 122(13):2176–84. doi: 10.1182/blood-2013-03-460154
5. Spivak JL. How I treat polycythemia vera. Blood (2019) 134(4):341–52. doi: 10.1182/blood.2018834044
6. Hasselbalch HC, Holmström MO. Perspectives on interferon-alpha in the treatment of polycythemia vera and related myeloproliferative neoplasms: minimal residual disease and cure? Semin Immunopathol (2019) 41(1):5–19. doi: 10.1007/s00281-018-0700-2
7. McMullin MF, Wilkins BS, Harrison CN. Management of polycythaemia vera: a critical review of current data. Br J Haematol (2016) 172(3):337–49. doi: 10.1111/bjh.13812
8. Kiladjian J-J, Cassinat B, Chevret S, Turlure P, Cambier N, Roussel M, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood (2008) 112:3065–72. doi: 10.1182/blood-2008-03-143537
9. Tashi T, Swierczek S, Kim SJ, Salama ME, Song J, Heikal N, et al. Pegylated interferon Alfa-2a and hydroxyurea in polycythemia vera and essential thrombocythemia: differential cellular and molecular responses. Leukemia (2018) 32:1830–3. doi: 10.1038/s41375-018-0080-6
10. Abu-Zeinah G, Krichevsky S, Cruz T, Hoberman G, Jaber D, Savage N, et al. Interferon-alpha for treating polycythemia vera yields improved myelofibrosis-free and overall survival. Leukemia (2021) 35(9):2592–601. doi: 10.1038/s41375-021-01183-8
11. Gisslinger H, Klade C, Georgiev P, Krochmalczyk D, Gercheva-Kyuchukova L, Egyed M, et al. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): a randomised, non-inferiority, phase 3 trial and its extension study. Lancet Haematol (2020) 7(3):e196–208. doi: 10.1016/S2352-3026(19)30236-4
12. Kiladjian JJ, Klade C, Georgiev P, Krochmalczyk D, Gercheva-Kyuchukova L, Egyed M, et al. Long-term outcomes of polycythemia vera patients treated with ropeginterferon Alfa-2b. Leukemia (2022) 36(5):1408–11. doi: 10.1038/s41375-022-01528-x
13. Verger E, Soret-Dulphy J, Maslah N, Roy L, Rey J, Ghrieb Z, et al. Ropeginterferon alpha-2b targets JAK2V617F-positive polycythemia vera cells in vitro and in vivo. Blood Cancer J (2018) 8(10):94. doi: 10.1038/s41408-018-0133-0
14. PharmaEssentia USA. BESREMI (ropeginterferon alfa-2b-njft). prescribing information (2021). Available at: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=9583405d-53a0-49dc-88eb-5e6384ebabcb&type=display.
15. Gisslinger H, Zagrijtschuk O, Buxhofer-Ausch V, Thaler J, Schloegl E, Gastl GA, et al. Ropeginterferon alfa-2b, a novel IFNα-2b, induces high response rates with low toxicity in patients with polycythemia vera. Blood (2015) 126(15):1762–9. doi: 10.1182/blood-2015-04-637280
16. Miyachi N, Zagrijtschuk O, Kang L, Yonezu K, Qin A. Pharmacokinetics and pharmacodynamics of ropeginterferon alfa-2b in healthy japanese and caucasian subjects after single subcutaneous administration. Clin Drug Investig (2021) 41:391–404. doi: 10.1007/s40261-021-01026-5
17. Huang Y-W, Qin A, Fang J, Wang T-F, Tsai C-W, Ling K-C, et al. Novel long-acting ropeginterferon alfa-2b: Pharmacokinetics, pharmacodynamics and safety in a phase I clinical trial. Br J Clin Pharmacol (2022) 88:2396–407. doi: 10.1111/bcp.15176
18. Huang Y-W, Tsai C-Y, Tsai C-W, Wang W, Zhang J, Qin A, et al. Pharmacokinetics and pharmacodynamics of novel long acting ropeginterferon alfa-2b in healthy chinese subjects. Adv Ther (2021) 38:4756–70. doi: 10.1007/s12325-021-01863-y
19. US FDA. FDA Approves treatment for rare blood disease (2021). Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-treatment-rare-blood-disease.
20. European Medicines Agency. Besremi: ropeginterferon alfa-2b . Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/besremi#authorisation-details-section (Accessed November 16, 2022).
21. Marchetti M, Vannucchi AM, Griesshammer M, Harrison C, Koschmieder S, Gisslinger H, et al. Appropriate management of polycythaemia vera with cytoreductive drug therapy: European LeukemiaNet 2021 recommendations. Lancet Haematol (2022) 9(4):e301–11. doi: 10.1016/S2352-3026(22)00046-1
22. Gisslinger H, Buxhofer-Ausch V, Thaler J, Forjan E, Willenbacher E, Wolf D, et al. Long-term efficacy and safety of ropeginterferon alfa-2b in patients with polycythemia vera — final phase I/II Peginvera study results. Blood (2018) 132(Supplement 1):3030. doi: 10.1182/blood-2018-99-118584
23. Huang YW, Hsu CW, Lu SN, Yu ML, Su CW, Su WW, et al. Ropeginterferon alfa-2b every 2 weeks as a novel pegylated interferon for patients with chronic hepatitis b. Hepatol Int (2020) 14(6):997–1008. doi: 10.1007/s12072-020-10098-y
24. Hsu SJ, Yu ML, Su CW, Peng CY, Chien RN, Lin HH, et al. Ropeginterferon Alfa-2b administered every two weeks for patients with genotype 2 chronic hepatitis c. J Formos Med Assoc (2021) 120(3):956–64. doi: 10.1016/j.jfma.2020.09.018
25. Lin HH, Hsu SJ, Lu SN, Chuang WL, Hsu CW, Chien RN, et al. Ropeginterferon alfa-2b in patients with genotype 1 chronic hepatitis c: Pharmacokinetics, safety, and preliminary efficacy. JGH Open (2021) 5(8):929–40. doi: 10.1002/jgh3.12613
26. Chen C-Y, Chuang W-L, Qin A, Zhang WH, Zhu LY, Zhang GQ, et al. A phase 3 clinical trial validating the potency and safety of an innovative, extra-long-acting interferon in chronic hepatitis c. JGH Open (2022) 6(11):782–91. doi: 10.1002/jgh3.12825
27. Qin A, Huang YW, Chen PJ. A validated new-generation pegylated interferon therapy for chronic hepatitis B and possibly D. Curr Trends Gastroenerology Hepatology. (2022) 4(1):343–5. doi: 10.32474/CTGH.2022.04.000178
28. Chen KY, Lee KY, Qin A, Luo CS, Yeh YK, Zheng JQ, et al. Clinical eExperience with ropeginterferon alfa-2b in the off-label use for the treatment of COVID-19 patients in Taiwan. Adv Ther (2022) 39(2):910–22. doi: 10.1007/s12325-021-01998-y
29. Edahiro Y, Ohishi K, Gotoh A, Takenaka K, Shibayama H, Shimizu T, et al. Efficacy and safety of ropeginterferon alfa-2b in Japanese patients with polycythemia vera: An open-label, single-arm, phase 2 study [published correction appears in int J hematol. 2022 Oct;116(4):642-643]. Int J Hematol (2022) 116(2):215–27. doi: 10.1007/s12185-022-03341-9
30. PharmaEssentia Japan K.K. Extension study of P1101 after completion of phase 2 study in PV patients or phase 3 study in ET patients. Available at: https://clinicaltrials.gov/ct2/show/NCT04655092 (Accessed November 3, 2022).
31. Barbui T, Vannucchi AM, De Stefano V, Masciulli A, Carobbio A, Ferrari A, et al. Ropeginterferon alfa-2b versus phlebotomy in low-risk patients with polycythaemia vera (Low-PV study): a multicentre, randomised phase 2 trial [published correction appears in lancet haematol. 2021 Mar;8(3):e170]. Lancet Haematol (2021) 8(3):e175–84. doi: 10.1016/S2352-3026(20)30373-2
32. Jin J, Zhang L, Shao Z, Phase II. Open-label, multicenter, single-arm study investigating the efficacy and safety of ropeginterferon alfa-2b in Chinese patients with polycythemia vera resistance or intolerant to hydroxyurea (HU) [ASH 2022 abstract 3050] . Available at: https://ash.confex.com/ash/2022/webprogram/Paper165789.html (Accessed November 9, 2022).
33. PharmaEssentia. A study to access efficacy and safety of P1101 in Chinese PV patients who are iIntolerant or rResistance to HU . Available at: https://clinicaltrials.gov/ct2/show/NCT05485948 (Accessed November 3, 2022).
34. Lee SE, Yoon SS, Yang DH, et al. Study to assess molecular response of P1101 therapy in patients with polycythemia vera and elevated hematocrit [ASH 2022 abstract 3046] . Available at: https://ash.confex.com/ash/2022/webprogram/Paper168687.html (Accessed November 9, 2022).
35. PharmaEssentia. A study to assess efficacy, safety, and tolerability of P1101 in adult patients with PV . Available at: https://clinicaltrials.gov/ct2/show/NCT05481151 (Accessed November 3, 2022).
36. Mascarenhas J, Zagrijtschuk O, Qin A, et al. A pPhase 3b, single-arm, multicenter study to assess the efficacy, safety, and tolerability of ropeginterferon alfa-2b-njft (P1101) in adult patients with polycythemia vera [ASH 2022 abstract 3004] . Available at: https://ash.confex.com/ash/2022/webprogram/Paper162693.html (Accessed November 9, 2022).
37. Stauffer Larsen T, Iversen KF, Hansen E, Mathiasen AB, Marcher C, Frederiksen M, et al. Long term molecular responses in a cohort of Danish patients with essential thrombocythemia, polycythemia vera and myelofibrosis treated with recombinant interferon alpha. Leuk Res (2013) 37(9):1041–5. doi: 10.1016/j.leukres.2013.06.012
38. Quintás-Cardama A, Abdel-Wahab O, Manshouri T, Kilpivaara O, Cortes J, Roupie AL, et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon α-2a. Blood (2013) 122(6):893–901. doi: 10.1182/blood-2012-07-442012
39. Masarova L, Patel KP, Newberry KJ, Cortes J, Borthakur G, Konopleva M, et al. Pegylated interferon alfa-2a in patients with essential thrombocythaemia or polycythaemia vera: a post-hoc, median 83 month follow-up of an open-label, phase 2 trial [published correction appears in lancet haematol. 2017 Jun;4(6):e257]. Lancet Haematol (2017) 4(4):e165–75. doi: 10.1016/S2352-3026(17)30030-3
40. Yacoub A, Mascarenhas J, Kosiorek H, Prchal JT, Berenzon D, Baer MR, et al. Pegylated interferon alfa-2a for polycythemia vera or essential thrombocythemia resistant or intolerant to hydroxyurea. Blood (2019) 134(18):1498–509. doi: 10.1182/blood.2019000428
41. Verstovsek S, Komatsu N, Gill H, Jin J, Lee SE, Hou HA, et al. SURPASS-ET: phase III study of ropeginterferon alfa-2b versus anagrelide as second-line therapy in essential thrombocythemia. Future Oncol (2022) 18(27):2999–3009. doi: 10.2217/fon-2022-0596
42. Bewersdorf JP, Giri S, Wang R, Podoltsev N, Williams RT, Tallman MS, et al. Interferon alpha therapy in essential thrombocythemia and polycythemia vera – a systematic review and meta-analysis. Leukemia (2021) 35(6):1643–60. doi: 10.1038/s41375-020-01020-4
43. Gisslinger H, Gisslinger B, Schalling M, Krejcy K, Widmann RS, Kralovics R, et al. Effect of ropeginterferon alfa-2b in prefibrotic primary myelofibrosis. Blood (2018) 132(Supplement 1):3029. doi: 10.1182/blood-2018-99-119268
44. Palmer J, Kosiorek HE, Zimmerman C, Zagrijtschuk O, Camoriano J, Mesa RA. Clinical benefit of ropeginterferon alfa 2b (P1101) in patients with myelofibrosis. Blood (2018) 132(Supplement 1):5475. doi: 10.1182/blood-2018-99-115521
45. Huang CE, Wu TY, Hsu CC, Chen YJ, Tsou HY, Li CP, et al. Real-world experience with ropeginterferon-alpha 2b (Besremi) in Philadelphia-negative myeloproliferative neoplasms. J Formos Med Assoc (2021) 120(2):863–73. doi: 10.1016/j.jfma.2020.08.021
46. Gill H, Au L, Yim R, et al. Efficacy and safety of ropeginterferon alfa-2b for pre-fibrotic primary myelofibrosis and DIPSS low/intermediate-1 risk myelofibrosis [ASH 2022 abstract 629] . Available at: https://ash.confex.com/ash/2022/webprogram/Paper169613.html (Accessed November 9, 2022).
47. PharmaEssentia. A study designed to assess the efficacy, safety, and tolerability of P1101 after the 12 month core treatment period in patients with ET. Available at: https://clinicaltrials.gov/ct2/show/NCT05482971?term=Pharmaessentia&draw=2&rank=6 (Accessed November 3, 2022).
48. Chen CC, Kuo MC, Su YJ, Huang CE, Hsu CC, Wu YY, et al. Compassionate use of ropeginterferon to treat myeloproliferative neoplasms in Taiwan. HemaSphere (2019) 3(S1):999. doi: 10.1097/01.HS9.0000567388.41296.09
49. Gruppo Italiano Studio Politicitemia. Polycythemia vera: The natural history of 1213 patients followed for 20 years. Gruppo italiano studio policitemia. Ann Intern Med (1995) 123(9):656–64. doi: 10.7326/0003-4819-123-9-199511010-00003
50. Marchioli R, Finazzi G, Landolfi R, Kutti J, Gisslinger H, Patrono C, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol (2005) 23(10):2224–32. doi: 10.1200/JCO.2005.07.062
51. Pemmaraju N, Gerds AT, Yu J, Parasuraman S, Shah A, Xi A, et al. Thrombotic events and mortality risk in patients with newly diagnosed polycythemia vera or essential thrombocythemia. Leuk Res (2022) 115:106809. doi: 10.1016/j.leukres.2022.106809
52. Alvarez-Larrán A, Bellosillo B, Pereira A, Kerguelen A, Hernández-Boluda JC, Martínez-Avilés L, et al. JAK2V617F monitoring in polycythemia vera and essential thrombocythemia: clinical usefulness for predicting myelofibrotic transformation and thrombotic events. Am J Hematol (2014) 89(5):517–23. doi: 10.1002/ajh.23676
53. Shirane S, Araki M, Morishita S, Edahiro Y, Sunami Y, Hironaka Y, et al. Consequences of the JAK2V617F allele burden for the prediction of transformation into myelofibrosis from polycythemia vera and essential thrombocythemia. Int J Hematol (2015) 101(2):148–53. doi: 10.1007/s12185-014-1721-9
54. Bertozzi I, Bogoni G, Biagetti G, Duner E, Lombardi AM, Fabris F, et al. Thromboses and hemorrhages are common in MPN patients with high JAK2V617F allele burden. Ann Hematol (2017) 96(8):1297–302. doi: 10.1007/s00277-017-3040-8
55. Passamonti F, Rumi E, Pietra D, Elena C, Boveri E, Arcaini L, et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia (2010) 24(9):1574–9. doi: 10.1038/leu.2010.148
56. Zhao S, Zhang X, Xu Y, Feng Y, Sheng W, Cen J, et al. Impact of JAK2V617F mutation burden on disease phenotype in Chinese patients with JAK2V617F-positive polycythemia vera (PV) and essential thrombocythemia (ET). Int J Med Sci (2016) 13(1):85–91. doi: 10.7150/ijms.10539
57. Tefferi A, Guglielmelli P, Lasho TL, Coltro G, Finke CM, Loscocco GG, et al. Mutation-enhanced international prognostic systems for essential thrombocythaemia and polycythaemia vera. Br J Haematol (2020) 189(2):291–302. doi: 10.1111/bjh.16380
Keywords: ropeginterferon alfa-2b, myeloproliferative neoplasm, polycythemia vera, alternative dosing strategy, clinical study, interferon, pegylated interferon
Citation: Qin A, Urbanski RW, Yu L, Ahmed T and Mascarenhas J (2023) An alternative dosing strategy for ropeginterferon alfa-2b may help improve outcomes in myeloproliferative neoplasms: An overview of previous and ongoing studies with perspectives on the future. Front. Oncol. 13:1109866. doi: 10.3389/fonc.2023.1109866
Received: 28 November 2022; Accepted: 04 January 2023;
Published: 19 January 2023.
Edited by:
Gabriela Baerlocher, University of Bern, SwitzerlandReviewed by:
Cristina Bucelli, Hematology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, ItalyCopyright © 2023 Qin, Urbanski, Yu, Ahmed and Mascarenhas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John Mascarenhas, am9obi5tYXNjYXJlbmhhc0Btc3NtLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.