
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 28 March 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1109643
This article is part of the Research TopicCase Reports in Surgical Oncology: 2022View all 56 articles
A correction has been applied to this article in:
Corrigendum: Case Report: Surgical treatment of a primary giant epithelioid hemangioendothelioma of the spine with total en-bloc spondylectomy
 Wanbao Ge1†
Wanbao Ge1† Yuan Qu1†
Yuan Qu1† Tingting Hou1
Tingting Hou1 Jiayin Zhang1
Jiayin Zhang1 Qiuju Li1
Qiuju Li1 Lili Yang1
Lili Yang1 Lanqing Cao2
Lanqing Cao2 Jindong Li3
Jindong Li3 Shanyong Zhang1*
Shanyong Zhang1*Background: Epithelioid hemangioendothelioma (EHE) is an extremely uncommon malignant neoplasm that originates from vascular endothelial or pre-endothelial cells. In this report, we present the case of patient who was diagnosed with a primary giant EHE of the spine and underwent treatment with total en-bloc spondylectomy (TES).
Case presentation: A 43-year-old male patient with a history of he presented to our hospital with chronic and progressive back pain. Physical examination revealed weakened sensation of acupuncture and touch on the left costal arch, while relatively normal neurological functions were preserved. Radiological examinations identified a giant destructive soft tissue lesion occupying the T8 vertebral region, with moderate destruction of the pedicle and lamina, as well as the 7th left rib. A preoperative biopsy of the 8th vertebra resulted in a diagnosis of epithelioid hemangioendothelioma(EHE). Postoperative immunohistochemical and pathological reports confirmed the presence of EHE in the left ribs and T8 ribs. The patient underwent resection of the 7th left rib and posterior pedicle screw fixation with 8 pairs of screws and a titanium mesh cage. Subsequently, thoracic en bloc spondylectomy was performed on the T8 vertebra. The patient did not receive radiation or chemotherapy following surgery. Over a period of 3 years, the patient remained free of disease and relapse.
Conclusion: The use of transarterial embolization with spherical embolic agents (TES) has been demonstrated to be a safe, effective, and reliable treatment option for hepatic epithelioid hemangioendothelioma (EHE). Nevertheless, it is crucial to conduct long-term follow-up of this patient in order to assess their clinical outcome.
Epithelioid hemangioendothelioma (EHE) is an exceedingly uncommon tumor that originates from vascular endothelial or pre-endothelial cells. This neoplasm exhibits histological properties that fall in between those of hemangiomas and high-grade angiosarcomas. Although EHE can manifest in any organ of the body, it is most frequently observed in the liver, lung, and skin, but rarely affects the spine (1, 2). Epithelioid hemangioendothelioma (EHE) has a prevalence of one in a million (3). Although mortality is generally low, some cases can lead to death due tometastases (4). Atypical localized pain is the most frequent early symptom of EHE, which serve as an indication of the disease that can later progress into a chronic illness (5). Consequently, the exclusion of other diseases in patients with primary spinal tumors can occasionally lead to an accidental diagnosis of EHE.
Despite its relatively low mortality, delayed diagnosis and treatment of EHE can result in a protracted and potentially fatal illness. Nonetheless, we report a case of primary giant EHE of the spine in which the patient underwent early wide-margin resection without postoperative adjuvant therapy. As a result, the patient achieved long-term survival free from disease and without any instrument-related complications.
A 43-year-old man presented to our hospital on foot with chronic and progressive back pain. He had been experiencing paroxysmal back pain for approximately 1 year., which was more pronounced at night and not relieved by rest. In the 15 days leading up to his admission, the back pain had intensified, accompanied by mild radiating pain and numbness in his lower limbs. The patient did not report any other physical discomfort, except for the pain, and denied any disease-related complaints. Upon further inquiry, he reported a slight weight over the past month. The patient had no significant strong family medical history of cancer or congenital diseases.
Upon physical examination, the patient exhibited weakened sensation to fine-touch and pinprick along his left costal arch, as well as grade 4 strength in his lower extremities. Pressure and percussion pain were observed over the T8 vertebra. However, the straight-leg raising test and the Babinski sign were bilaterally negative. The patient’s bilateral deep tendon reflexes for the Achilles tendon and knee jerk reflexes were normal. And no anomalies in the spinal cord or neurological conditions in the upper extremity were noted. Nonetheless, the patient exhibited evident anxiety and a strong desire for surgery.
Diagnostic tests were conducted to identify cancer cell markers, and routine laboratory tests were performed to determine the patient’s condition. The laboratory results were almost within the normal range, which increased uncertainty regarding the diagnosis of EHE, except for the elevated levels of carcinoembryonic antigen alpha-fetoprotein was elevated (16.81 ng/ml, normal: <8.78 ng/ml)and cytokeratin 19 fragment Cyra21-1 (2.74 ng/ml,normal: <2.08 ng/ml). A thoracic magnetic resonance imaging (MRI) revealed a vertebral lesion, which was assessed to determine the degree of flexibility of the vertebral spine, and a surgical intervention approach was finalized as the treatment course.
The MRI showed a giant destructive soft tissue lesion occupying the T8 vertebral region with moderate destruction of the pedicle and lamina (Figures 1A, B). Another lesion was found on the seventh left rib (Figure 1C). The preoperative medical tests, including an electrocardiogram and chest CT scan (Figure 2).
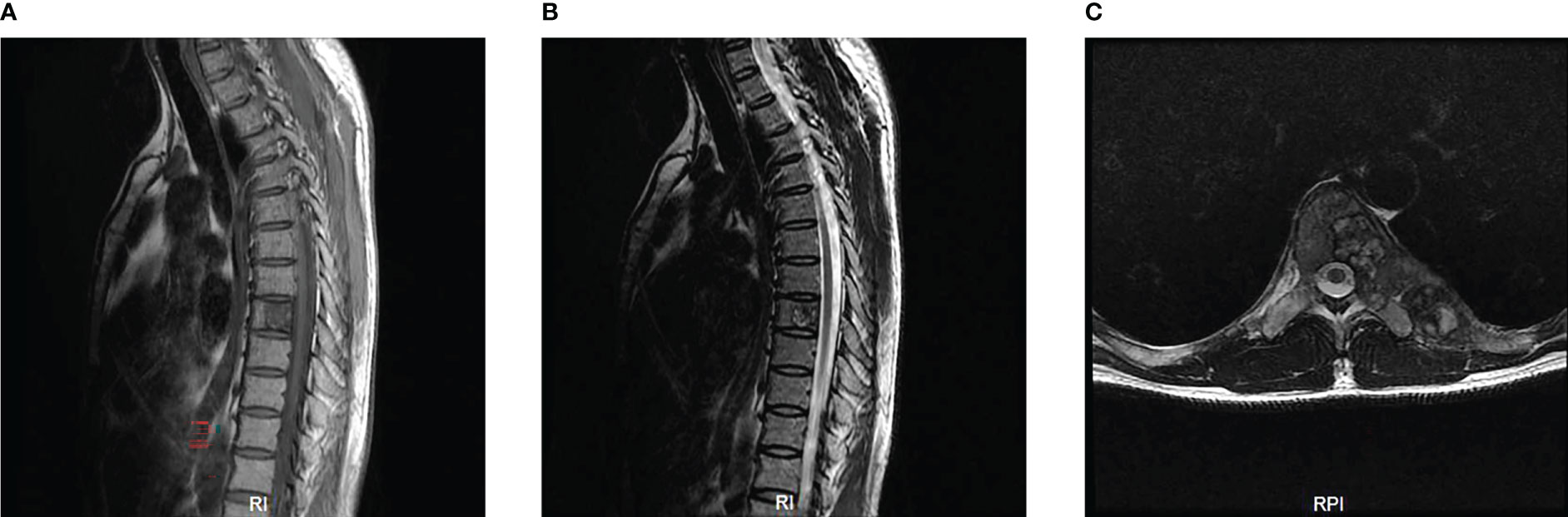
Figure 1 Preoperative MRI scan of the patient’s thoracic spine (A–C). Sagittal scan revealed the density of obvious bone destruction in the T8 on T1 and T2-weighted images (A, B). Transverse MRI scan showed a 6.5cm x 2.0cm sized and well-defined lesion occupying the T8 vertebral body, pedicle and lamina (C).
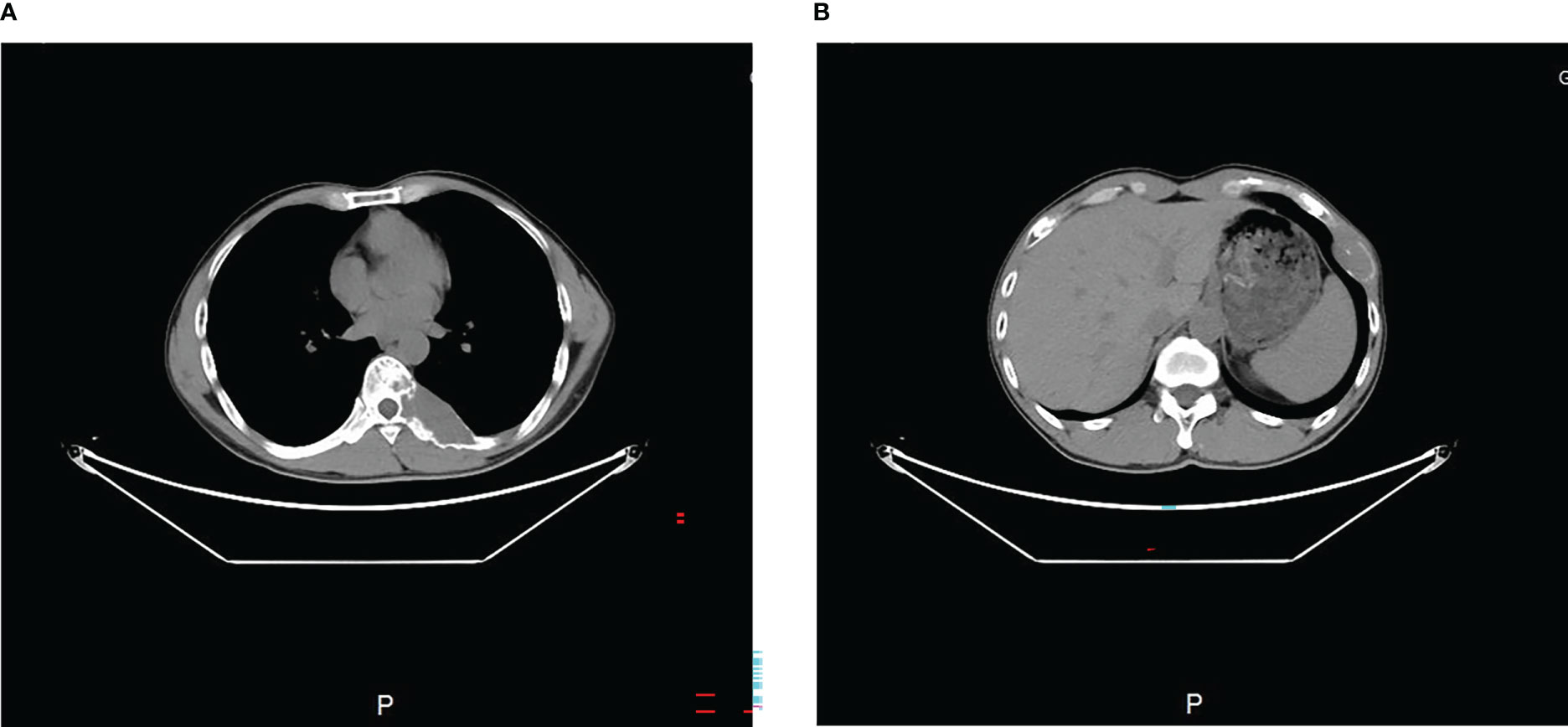
Figure 2 Preoperative transverse CT scan (A, B) showed the tumor occupied the 8th thoracic vertebral body and 7th rib.
Previous studies have recommended preoperative embolization to generally “shrink” the tumor (5). Therefore, vascular embolization was planned and performed. Preoperative angiography showed abundant blood supply in the tumor (Figure 3). To decrease intraoperative blood flow volume and maintain vital signs, four segmental arteries were embolized 12 hours preoperatively. EHE was ultimately diagnosed by a standard biopsy to determine the therapy course. Neuroelectromyography testing was not performed and an IV infusion of cephalosporin was administered 30 minutes before surgery to prevent infection. Tranexamic acid was not administered for prophylactic hemostasis. In consultation with a thoracic surgeon and an anesthesiologist, resection of the 7th rib and total en-bloc spondylectomy (TES) were performed sequentially. The patient was positioned right lateral decubitus and the rib was resected. During costectomy, a mass measuring 4.5 × 11 cm could be recognized, and no implant nodules, hydrothorax, or visible abnormality in the lungs were found around the 7th rib (Figure 4A). The patient was then transferred to prone location of TES. During TES, the pedicles of T6, T7, T9, and T10 were fixed with routine screws first (Screws are made of titanium alloy. Lengths are T6:35mm, T7:35mm, T9:35mm, T10:40mm, respectively. Diameters are T6:40mm, T7:40mm, T9:40mm, T10:45mm, respectively). C-arm fluoroscopy showed satisfactory positioning of the pedicle screws bilaterally. The left 8th vertebral body was exposed, and a giant tumor invading the vertebral body and the costovertebral joint was resected (Figure 4B). An appropriate titanium mesh cage was implanted and the incision was closed with drains. The whole surgical procedure was successful, and the intraoperative blood loss was only about 1000 ml. The proper placement of the surgical implants was immediately checked by X-ray.
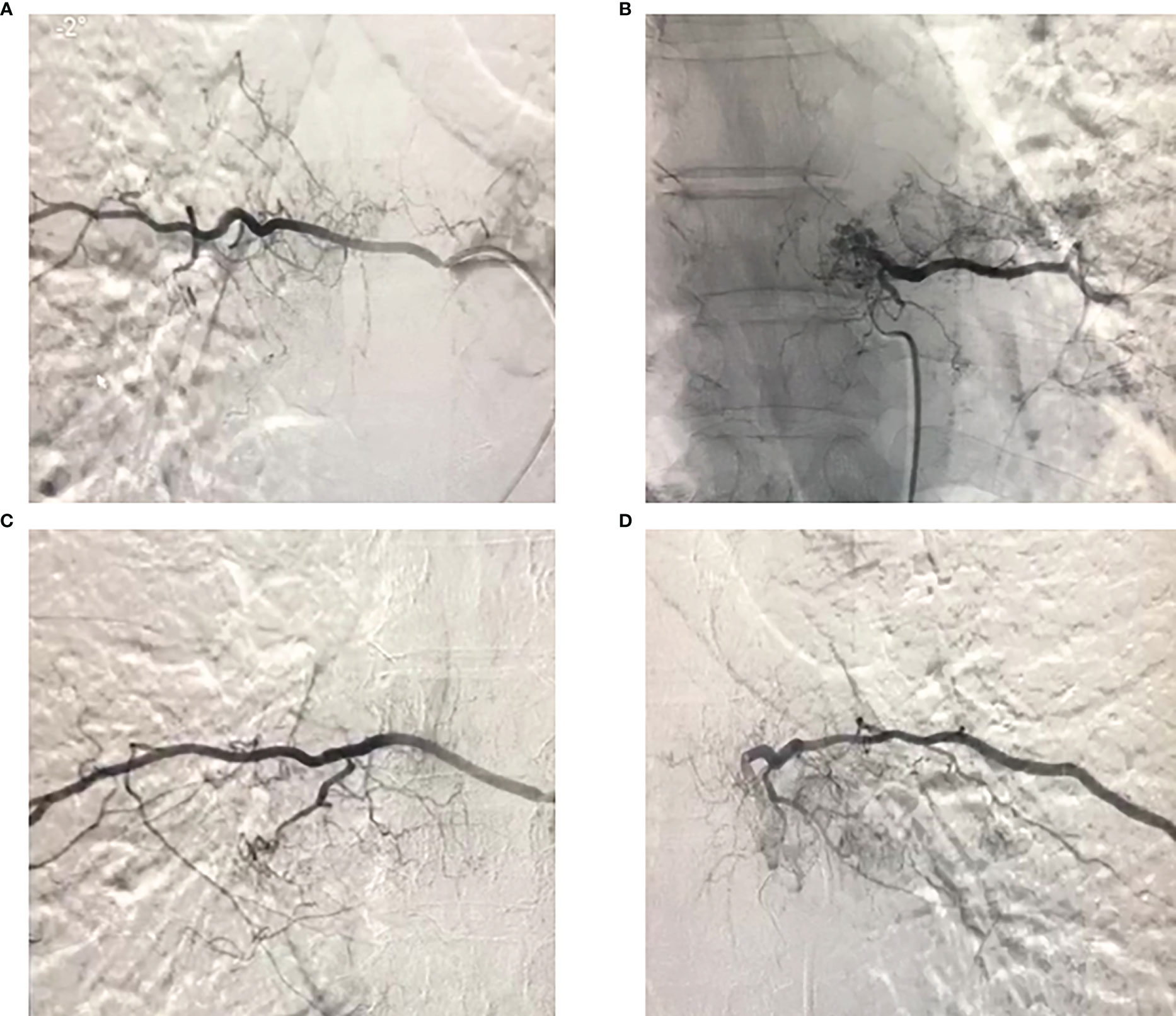
Figure 3 Preoperative angiography of the 7th and 8th thoracic vertebral segment arteries showed that the tumor had a rich blood supply, and embolization was performed.
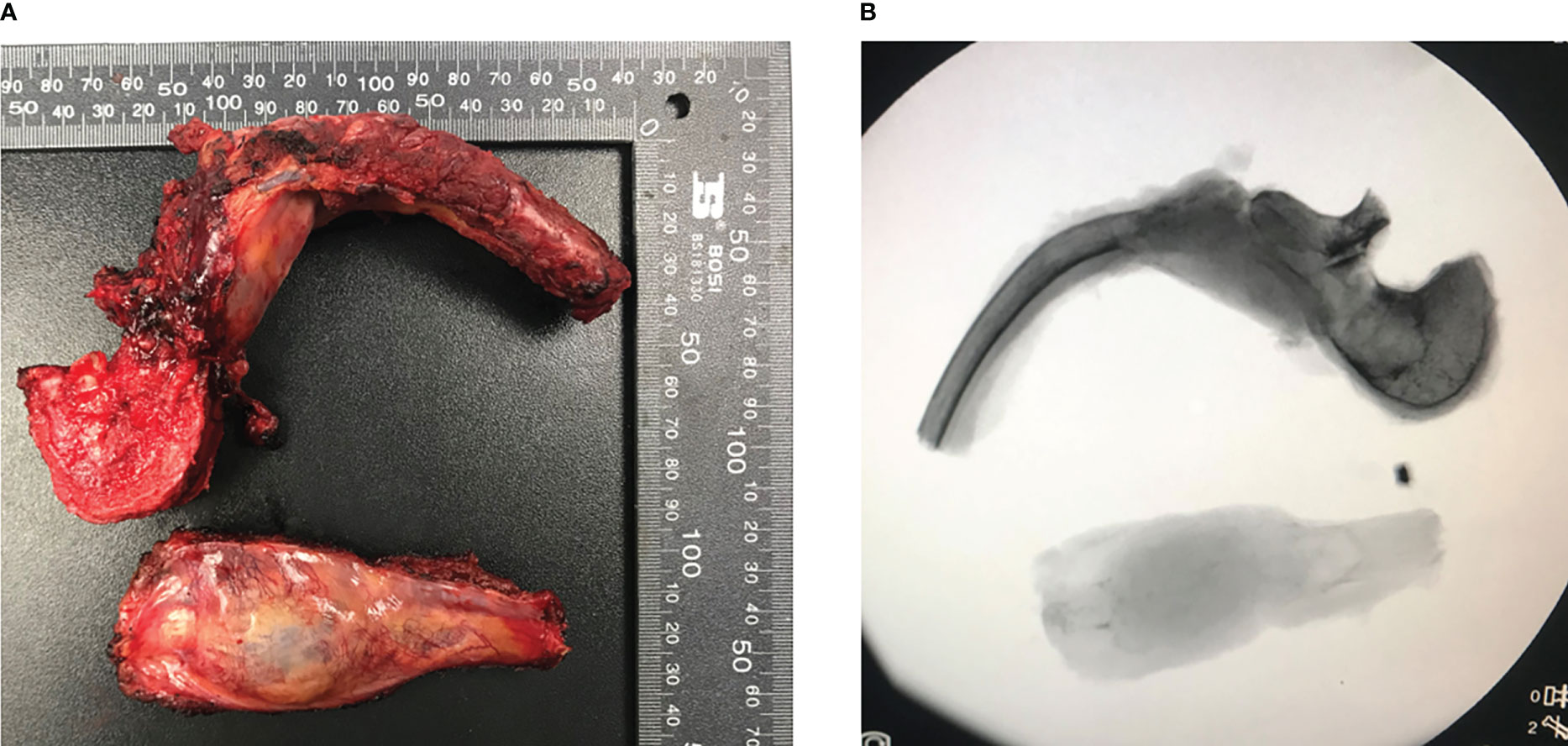
Figure 4 En-bloc excision tumor specimen in T8 and part of 7th rib of the patient and corresponding perspective image (A, B). Both images showed the integrity of tumor and the lesion was completely removed.
Postoperative pathology reports confirmed the diagnosis of EHE. Microscopic examination revealed that mostly eosinophilic endothelial cells constituted the tumor, without any well-formed vessels (Figure 5). Immunohistochemical staining was performed for friend leukemia integration 1 (FLI-1), CD31, and CD34 expression. Combined CD31 and FLI-1 staining suggested the diagnosis of EHE (Figure 6). In this case, the tumor sample was also positive for endothelial markers such as smooth muscle alpha-actin. Moreover, cytokeratin (AE1/AE3), indicating epithelial origin, was positive, with 8% of the cells being Ki-67-positive.
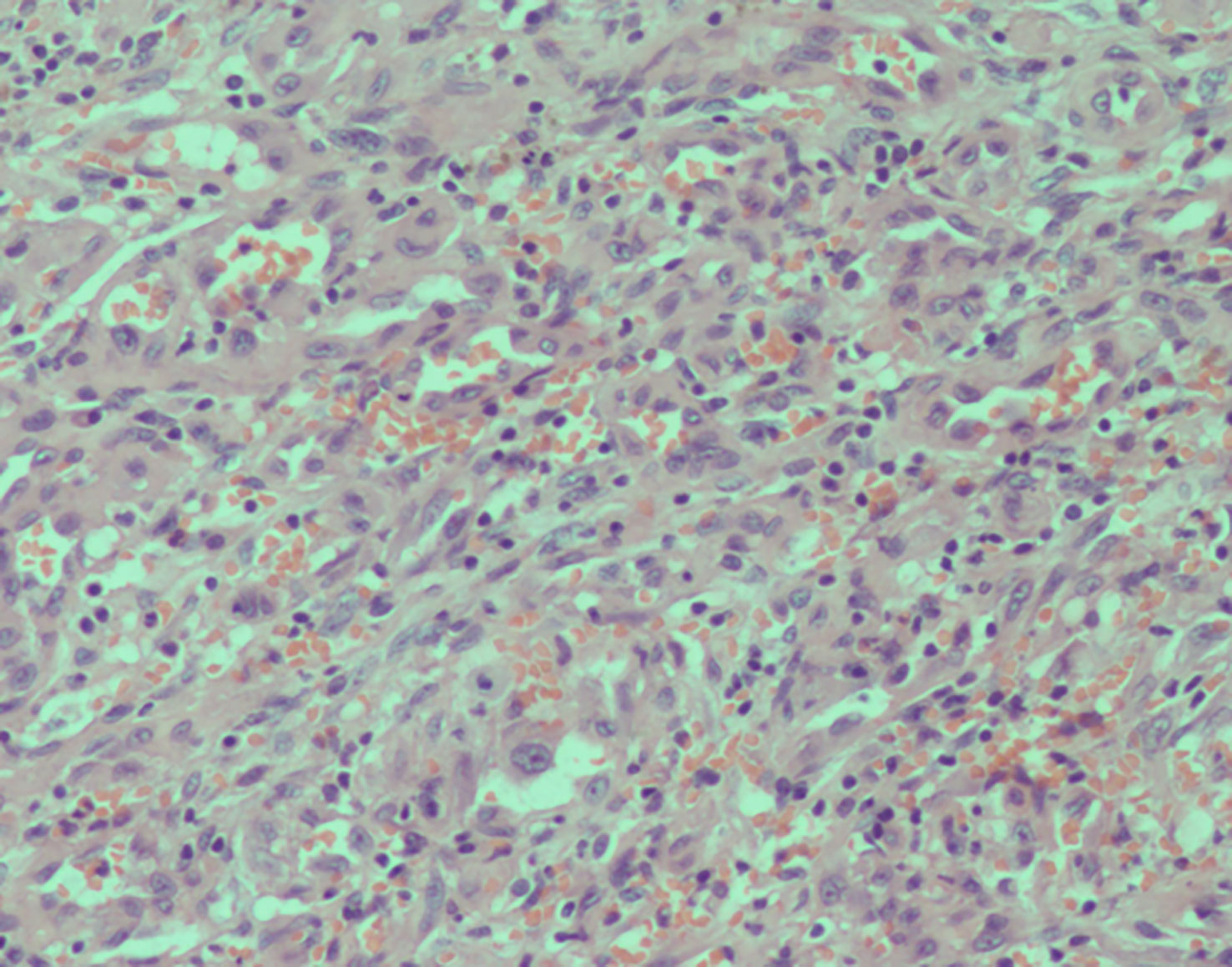
Figure 5 Histopathology of the resected mass. High-power magnification (200×) shows cords and groups of epithelioid cells in a dense without well-formed vascular structures.
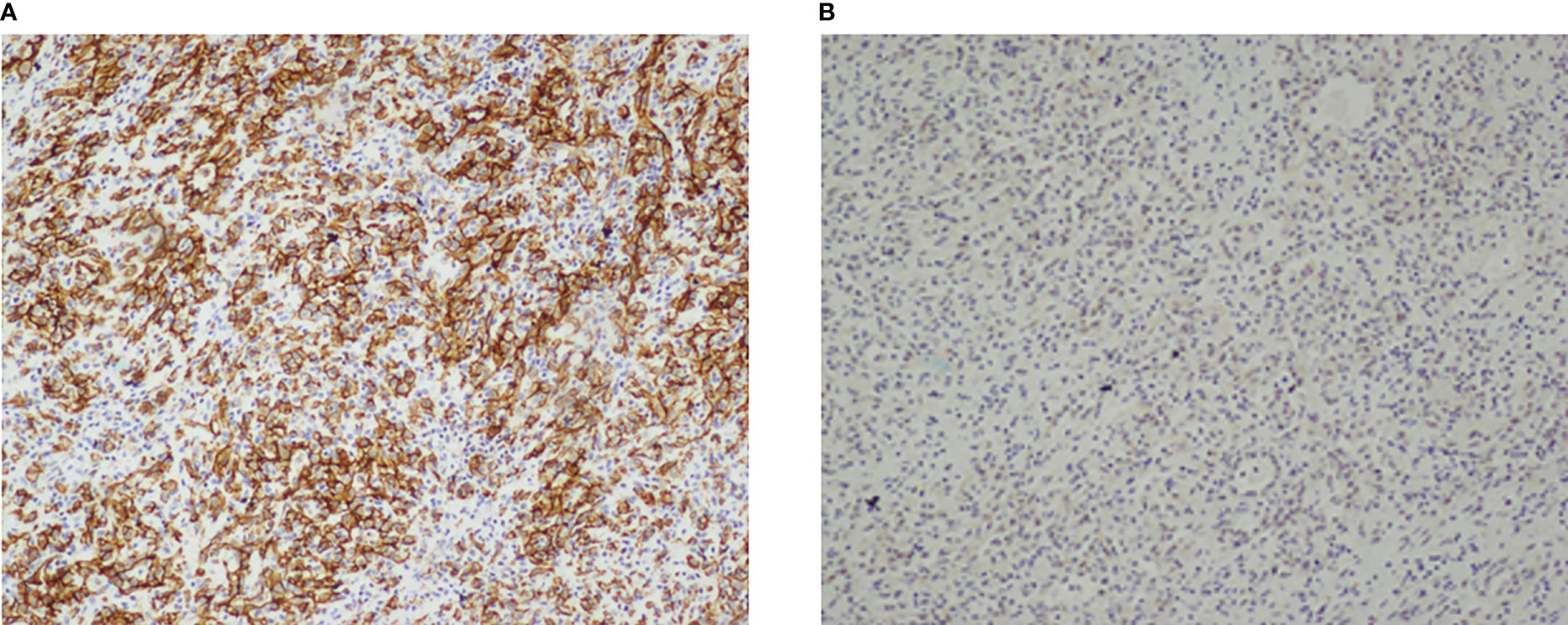
Figure 6 Immunohistochemical stain of the resected mass. (A, B) Tumor cells expressed CD31 (A) and FIL-1 (B). Both immunohistochemical stain were in 100×magnification.
During a recent postoperative follow-up after the surgery, there was no indication of any tumor progression or the emergence of any new symptoms (Figure 7). However, the patient and their family have politely declined radiation treatment. Currently, the patient’s back pain has been relieved, and the sensory function, including fine-touch and pinprick sensation, of the left costal arch has returned to normal.
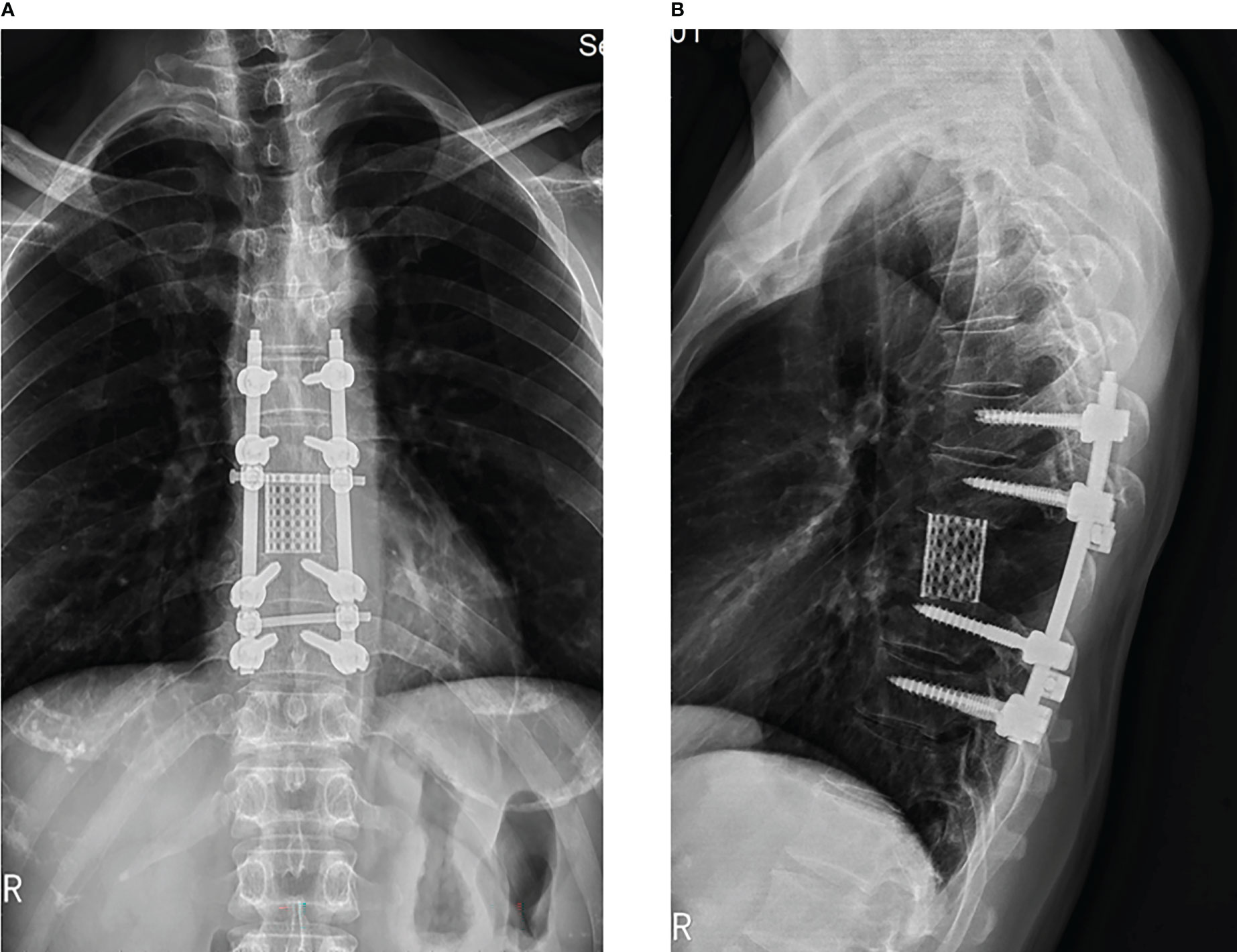
Figure 7 3 months follow-up after the surgery, x-ray display of thoracic spine showing transpedicular screw and a titanium mesh were used in the reconstruction of the stability of the spine in patient. (A: orthotopic position, B: side position).
Primary spinal EHE is a rare type of malignancy, accounting for only 1% of all malignant bone tumors (6). EHE is characterized by polygonal or round epithelioid endothelial cells with abundant eosinophilic hyaline cytoplasm and vesicular nuclei. Due to its local aggressiveness and metastatic potential, EHE is considered to have histological features that fall between those of hemangioma and high-grade angiosarcoma. Typically, most patients in the early stages of EHE development are asymptomatic, and the accidental discovery of the tumor during routine physical examinations is a common way to achieve early diagnosis. Although patients may not place importance on the most common symptom of atypical local pain, EHE can still lead to complications and mortality (Table 1). However, our study demonstrated significant therapeutic effects during the follow-up period.
Pathological examination is considered the gold standard for EHE diagnosis. In this case, we performed immunohistochemistry, and the examination results were consistent with diagnosis of EHE. However, with advancements in medical technology, new approaches such as molecular genetic testing can be applied to further clarify the diagnosis of EHE. A specific recurrent WWTR1-CAMTA1 gene fusion has been detected in the majority of EHE patients, and this genetic hallmark can be used as a new diagnostic indicator of EHE (11). Additionally, the detection of the WWTR1-CAMTA1 gene fusion can distinguish EHE from other benign or malignant vascular tumors (11, 12). Some recent studies have suggested that an alternate YAP1-TFE3 fusion, found in a subset of cases, may also be meaningful in EHE diagnosis. However, it remains unclear whether the YAP1-TFE3 gene fusion should be used to identify EHE (12). In the present case, preoperative puncture biopsy, clinical symptoms, and imaging features suggested that the patient required surgical intervention. Therefore, we performed TES, which resulted in satisfactory clinical results. We also recommended that the patient and his family undergo genetic testing. However, although we suggested genetic testing, the family members refused, and we believe that this did not affect the patient’s treatment and prognosis.
There are no standard treatment courses available for the management of spinal EHE. Preoperative embolization and surgical resection, followed by postoperative radiotherapy and/or chemotherapy, are the alternative options. Surgical resection is generally considered the optimal treatment approach (13). However, en-bloc resection of spinal EHE can be challenging due to the highly vascular nature of the tumor and the risk of intraoperative bleeding. The extent of surgical can range from wide margin to marginal or intralesional resection. Several clinical studies suggest that wide resection (i.e., TES), should be performed to achieve favorable outcomes (14). Luzzati et al. reported the largest case series of ten spinal EHE patients and found that patients who received relatively broad or marginal resection had better prognoses (15). While TES is currently considered the preferred choice to control spinal EHE progression, further analysis of large volumes of clinical outcomes and substantial experimental data is needed to establish the ultimate conclusion. In our case, we successfully performed a wide resection and achieved a clear resection margin.
In addition to surgery, systemic therapy, including chemotherapy and radiotherapy, may also benefit patients with spinal epithelioid hemangioendothelioma (EHE) According to a few reports, radiotherapy for partially excised spinal EHE lesions can be effective (8). Moreover, some clinical centers use radiation (45–50 Gy) as a postoperative systemic treatment to achieve long-term local control and pain relief (16). While systemic therapy can provide physiological support for patients who refuse other treatments (17, 18), surgery remains the optimal treatment choice. However, there is currently no evidence to support a strong correlation between chemotherapy or radiotherapy and good prognosis in patients with spinal EHE. In our case, the patient declined radiation or chemotherapy treatment, and we followed the patient for 3 years without observing tumor recurrence. We plan to extend the follow-up period if necessary. Our patient, who received only extensive resection surgery with favorable outcomes, may provide insight for developing a standardized treatment plan for EHE in the future. In contrast, expectant observation may be an alternative approach for unresectable tumors or those that may lead to severe complications if resected.
Regarding prognosis, patients with multifocality, pleural involvement, lymph node invasion, or remote metastases generally have considerably poor outcomes. Conversely, patients without these adverse factors have an average 5-year survival rate of >70% (9). In this case, only one lesion was presented, and there was no evidence of multifocality, pleural involvement, lymph node involvement, or metastasis. According to Rosenbaum et al. (9), satisfactory clinical outcomes can be achieved after standard surgical treatment and 3 years of tumor-free survival. However, according to recent research, the WWTR1-CAMTA1 gene fusion may indicate that other genetic anomalies can lead to more aggressive biological behavior (9). Simultaneously, the 5-year survival for WWTR1-CAMTA1 fusion is 59%, compared to 86% for YAP1-TFE3 fusion (9). Some asymptomatic patients may also have a good prognosis through careful monitoring, as spontaneous regressions have been reported (7). Unfortunately, our patient declined the proposal for genetic testing. We believe that genetic detection is significant for the prognosis of EHE patients and for EHE prevention in their families. Screening for the gene mutation causing EHE in other family members can enable early diagnosis and prompt treatment, particularly for asymptomatic family members, to prevent distant metastases and poor prognosis. The prognosis of EHE varies depending on anatomical site. EHE of soft tissues can be indolent. However, EHE arising in the lung or bone have a worse prognosis than soft tissue tumors, and patients often present with metastatic disease. Additionally, in the recently identified YAP1-TFE3 subset of cases, the metastatic rate may be higher. A traditional risk stratification is based on mitotic activity and tumor size. During the one-year follow-up, the patient reported remission of back pain and no recurrence of pain.
This case report has several limitations that should be acknowledged. The follow-up period is less than 5 years, and the study involves only one patient. Therefore, to accurately evaluate the therapeutic effect of TES, multicenter, large-sample, long-follow-up randomized controlled trials are necessary. Additionally, the patient did not undergo genetic testing, which limits the ability to make definitive conclusions regarding the impact of genetic anomalies on the clinical outcomes of EHE. As such, genetic counseling and education should be offered to patients and their family members for prevention, early diagnosis, and treatment.
TES has been demonstrated to be safe, effective, and reliable for the treatment of EHE, and in this case, we were able to successfully diagnose and treat the patient. Nonetheless, the patient requires long-term follow-up to assess the clinical outcome and to determine if any recurrence or metastasis occurs. This is especially important given that EHE is known to have a variable clinical course and prognosis, which can be influenced by a range of factors such as genetic anomalies, tumor location, and disease stage. Long-term follow-up will also provide additional insight into the efficacy and safety of TES as a treatment modality for EHE. This study was reported in agreement with principles of the CARE guidelines (19).
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Data Curation: Writing - Original Draft: WG, YQ. Writing - Review and Editing: SZ. Visualization: TH, JZ, LY. Supervision: LC. Project administration: QL, JL. All authors contributed to the article and approved the submitted version.
The authors would like to acknowledge the financial support from the Natural Science Foundation of Science and Technology Department of Jilin Province (20200201551JC).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
EHE, epithelioid hemangioendothelioma; TES, total en-bloc spondylectomy; MRI, magnetic resonance imaging; CT, computed tomography; FLI-1, friend leukemia integration 1 transcription factor; WWTR1, WW domain-containing transcription regulator 1; CAMTA1, calmodulin-binding transcription activator 1.
1. Mentzel T, Beham A, Calonje E, Katenkamp D, Fletcher C. Epithelioid hemangioendothelioma of skin and soft tissues: Clinicopathologic and immunohistochemical study of 30 cases. Am J Surg Pathol (1997) 21:363–74. doi: 10.1097/00000478-199704000-00001
2. Mendlick MR, Nelson M, Pickering D, Johansson SL, Seemayer TA, Neff JR, et al. Translocation t(1;3)(p36.3;q25) is a nonrandom aberration in epithelioid hemangioendothelioma. Am J Surg Pathol (2001) 25:684–7. doi: 10.1097/00000478-200105000-00019
3. Sardaro A, Bardoscia L, Petruzzelli M, Portaluri M. Epithelioid hemangioendothelioma: An overview and update on a rare vascular tumor. Oncol Rev (2014) 8:259. doi: 10.4081/oncol.2014.259
4. Deyrup A, Tighiouart M, Montag A, Weiss S. Epithelioid hemangioendothelioma of soft tissue: A proposal for risk stratification based on 49 cases. Am J Surg Pathol (2008) 32:924–7. doi: 10.1097/PAS.0b013e31815bf8e6
5. Munier O, Muckensturm B, Fesneau M, Wachter T. Epithelioid hemangioendothelioma of the spine: A case report. Cancer Radiother (2017) 21:222–5. doi: 10.1016/j.canrad.2016.11.006
6. Kerry G, Marx O, Kraus D, Vogel M, Kaiser A, Ruedinger C, et al. Multifocal epithelioid hemangioendothelioma derived from the spine region: Case report and literature review. Case Rep Oncol (2012) 5:91–8. doi: 10.1159/000336947
7. Kitaichi M, Nagai S, Nishimura K, Itoh H, Asamoto H, Izumi T, et al. Pulmonary epithelioid haemangioendothelioma in 21 patients, including three with partial spontaneous regression. Eur Respir J (1998) 12:89–96. doi: 10.1183/09031936.98.12010089
8. Aflatoon K, Staals E, Bertoni F, Bacchini P, Donati D, Fabbri N, et al. Hemangioendothelioma of the spine. Clin Orthop Relat Res (2004) 418:191–7. doi: 10.1097/00003086-200401000-00031
9. Rosenbaum E, Jadeja B, Xu B, Zhang L, Agaram NP, Travis W, et al. Prognostic stratification of clinical and molecular epithelioid hemangioendothelioma subsets. Modern Pathol (2020) 33:591–602. doi: 10.1038/s41379-019-0368-8
10. Shiba S, Imaoka H, Shioji K, et al. Clinical characteristics of Japanese patients with epithelioid hemangioendothelioma: A multicenter retrospective study. BMC Cancer (2018) 18:993. doi: 10.1186/s12885-018-4934-0
11. Flucke U, Vogels R, de Saint Aubain Somerhausen N, et al. Epithelioid hemangioendothelioma: Clinicopathologic, immunhistochemical, and molecular genetic analysis of 39 cases. Diagn Pathol (2014) 9:131. doi: 10.1186/1746-1596-9-131
12. Doyle L, Fletcher C, Hornick J. Nuclear expression of CAMTA1 distinguishes epithelioid hemangioendothelioma from histologic mimics. Am J Surg Pathol (2016) 40:94–102. doi: 10.1007/s00586-011-1798-2
13. Ma J, Wang L, Mo W, Yang X, Xiao J. Epithelioid hemangioendotheliomas of the spine: Clinical characters with middle and long-term follow-up under surgical treatments. Eur Spine J (2011) 20:1371–6. doi: 10.1007/s00586-011-1798-2
14. Lee S, Lee J. Surgical treatment of asymptomatic epithelioid hemangioendothelioma originating from the superior vena cava: A case report. Medicine (2020) 99:e19859. doi: 10.1097/MD.0000000000019859
15. Luzzati A, Gagliano F, Perrucchini G, Scotto G, Zoccali C. Epithelioid hemangioendothelioma of the spine: Results at seven years of average follow-up in a series of 10 cases surgically treated and a review of literature. Eur Spine J (2015) 24:2156–64. doi: 10.1007/s00586-014-3510-9
16. O’Shea B, Kim J. Epithelioid hemangioma of the spine: Two cases. Radiol Case Rep (2014) 9:984. doi: 10.2484/rcr.v9i4.984
17. Agulnik M, Yarber JL, Okuno SH, von Mehren M, Jovanovic BD, Brockstein BE, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol (2013) 24:257–63. doi: 10.1093/annonc/mds237
18. Chevreau C, Le Cesne A, Ray-Coquard I, Italiano A, Cioffi A, Isambert N, et al. Sorafenib in patients with progressive epithelioid hemangioendothelioma: A phase 2 study by the French sarcoma group (GSF/GETO). Cancer (2013) 119:2639–44. doi: 10.1002/cncr.28109
Keywords: epithelioid hemangioendothelioma, total en-bloc spondylectomy, vertebral lesion, vascular neoplasm, giant
Citation: Ge W, Qu Y, Hou T, Zhang J, Li Q, Yang L, Cao L, Li J and Zhang S (2023) Case report: Surgical treatment of a primary giant epithelioid hemangioendothelioma of the spine with total en-bloc spondylectomy. Front. Oncol. 13:1109643. doi: 10.3389/fonc.2023.1109643
Received: 01 December 2022; Accepted: 08 March 2023;
Published: 28 March 2023.
Edited by:
Zhaolun Cai, Sichuan University, ChinaReviewed by:
Yang Yang, First Affiliated Hospital of Sun Yat-sen University, ChinaCopyright © 2023 Ge, Qu, Hou, Zhang, Li, Yang, Cao, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shanyong Zhang, anpoYW5nc2hhbnlvbmdAeWVhaC5uZXQ=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.