- 1Department of Nuclear Medicine, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China
- 2Department of Nuclear Medicine, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, China
- 3Department of Nuclear Medicine and Medical Imaging, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China
- 4Department of Nuclear Medicine, Guangdong Provincial People’s Hospital, Guangzhou, Guangdong, China
Occult breast cancer is an uncommon type of breast cancer and its diagnosis is challenging. It is usually invisible on multiple imaging examines. Metastases to the rectum and inguinal lymph nodes from occult breast lobular cancer are even rarer. 68Ga-DOTA peptides can image neuroendocrine tumors by targeting specific somatostatin receptors. Besides, other tumors, including breast cancer, have been shown to express somatostatin receptors. In this case, we presented a 63-year-old woman who underwent both 18F-FDG and 68Ga-DOTATATE PET/CT due to a rectal polyp. An endoscopic excision biopsy confirmed metastatic carcinoma of suspected breast origin, but subsequent ultrasound and MRI showed no signs of malignancy in the breast and adnexa uteri. PET/CT showed obvious 68Ga-DOTATATE activity in bilateral axillary and right inguinal lymph nodes with mild 18F-FDG uptake. Final histopathology at the left axillary, right inguinal lymph nodes, and rectum indicated metastases from breast cancer while the origin remained radiologically occult. Additionally, one uterine fibroids was found with positive uptake of 68Ga-DOTATATE and negative uptake of 18F-FDG. This case suggested that 68Ga-DOTATAE PET/CT may be an effective supplement in diagnosing OBC lymph node metastases with mild 18F-FDG uptake, and it may provide a new technology for the clinical diagnosis of occult breast cancer.
Introduction
Occult breast cancer (OBC) is described as an axillary metastatic carcinoma without detection of a primary breast lesion, which is uncommon (1). The incidence of occult breast cancer is reported as being 0.3-1% of all breast cancer patients (2–4). It is thought that OBC is secondary to microinvasive breast cancer (5). Therefore, an accurate imaging examination of OBC is crucial for clinical diagnosis.
Research on imaging examination for detecting the occult breast cancer has been ongoing. The American College of Radiology recommends the use of MRI in OBC patients who do not have evidence of a primary breast lesion on the traditional radiological examination like mammogram and ultrasound (6). In a comparative analysis of MRI in 2015, contrast-enhanced mammography had equivalent if not better sensitivity (100% vs 93%) than MRI in detecting breast cancers (7). 18F-FDG PET/CT has been used in occult breast cancer, however, there are few case reports about that. Meanwhile, the imaging examination described above remains limited.
In our case, we described one case of occult breast cancer with rectum metastases who underwent both 18F-FDG and 68Ga-DOTATATE PET/CT, and enlarged lymph nodes with obvious 68Ga-DOTATATE activity and mild 18F-FDG uptake were shown at bilateral axilla and right inguinal area. It suggested that 68Ga-DOTATAE PET/CT might be an effective method for the diagnosis of occult breast cancer.
Case description
We present a case of a 63-year-old woman with a history of rectal polypectomy confirmed metastatic carcinoma for 2 months during a physical examination. Further immunohistochemical results showed that the estrogen receptor (ER), progesterone receptor (PR), and GATA Binding Protein 3 (GATA3) expression was positive, suggesting that the primary tumor may be from the female reproductive system, especially from the breast. However, the ultrasound and MRI showed no signs of malignancy in the breast and adnexa uteri, and multiple enlarged lymph nodes in the bilateral axilla and right inguinal area.
Then 18F-FDG PET/CT was performed for an unknown primary origin (Figure 1) and no abnormal activity was observed in the MIP image. The axial images of PET/CT found enlarged lymph nodes at the bilateral axillary and right inguinal with mild FDG avidity and no abnormality was seen in the bilateral breasts. Then the patient underwent 68Ga-DOTATATE PET/CT one day after 18F-FDG PET/CT (Figure 1). In the 68Ga-DOTATATE MIP image and the axial images, higher uptake of 68Ga-DOTATATE than 18F-FDG was observed in the enlarged bilateral axillary and right inguinal lymph nodes, but bilateral breasts were still negative. Moreover, a lesion with increased 68Ga-DOTATATE activity was located in the uterus with, but neither notable CT structural alterations nor 18F-FDG activity can be seen (Figure 2).

Figure 1 No abnormal activity was observed in the 18F-FDG MIP image (A). Enlarged lymph nodes were found at bilateral axillary and right inguinal region with mild FDG avidity (SUVmax, 2.1) on 18F-FDG PET/CT (B–D fused PET/CT; white arrows) and no abnormality was seen in the bilateral breasts. In the 68Ga-DOTATATE MIP image (E) and the axial images (F–H fused PET/CT), higher 68Ga-DOTATATE uptake than 18F-FDG was observed in the enlarged bilateral axillary and right inguinal lymph nodes (SUVmax, 8.4; white arrows), and bilateral breasts were still negative.
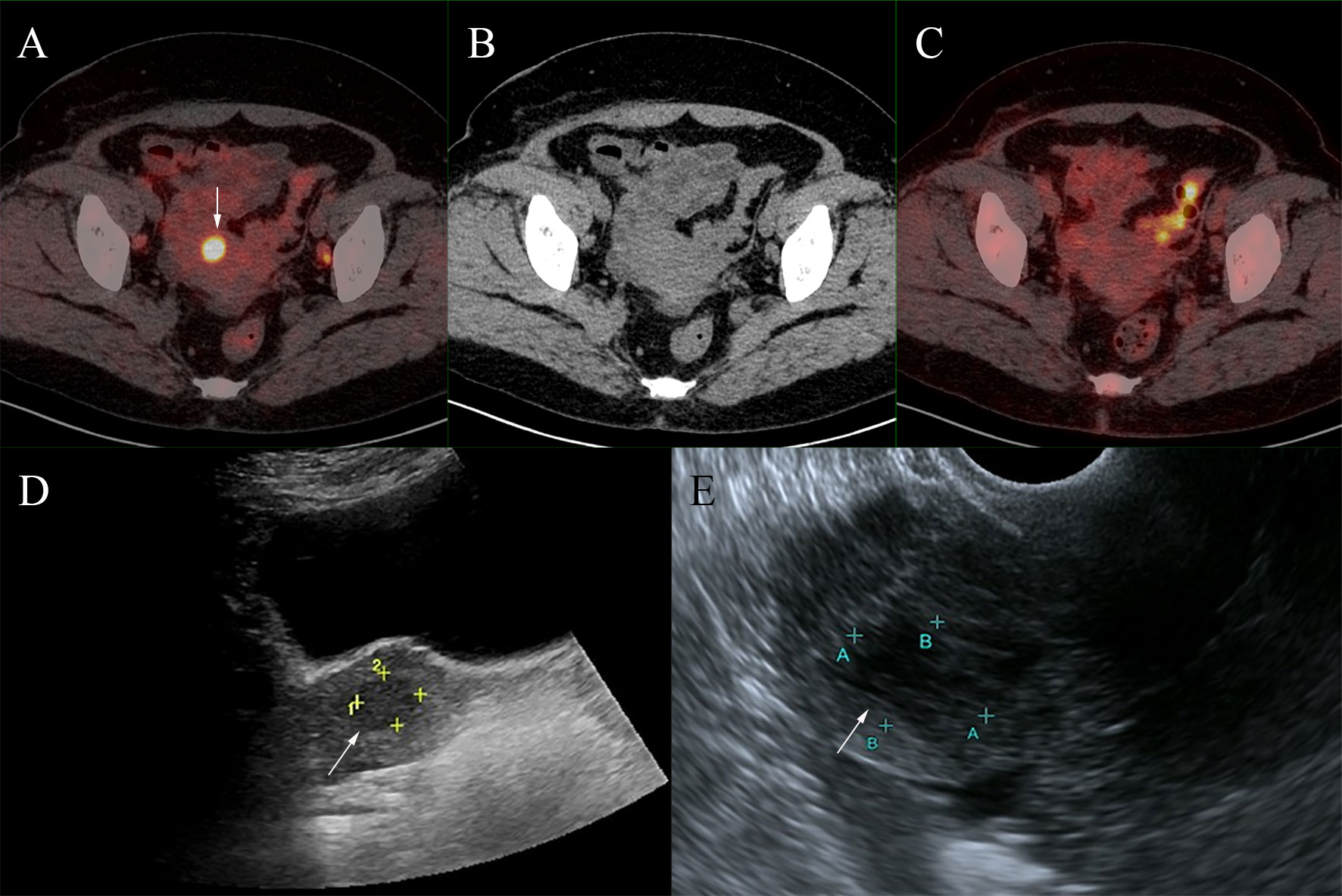
Figure 2 A foci of increased 68Ga-DOTATATE activity (A, fused PET/CT) was located in the uterus (SUVmax,11.0; white arrows), but neither notable CT structural alterations nor 18F-FDG activity was seen (B, CT; C, fused PET/CT). This lesion was found to be uterine fibroids after ultrasound examination (D, transabdominal ultrasonography; E, transvaginal ultrasonography).
Due to unknown primary origin, this patient underwent ultrasound-guided percutaneous biopsy for the 68Ga-DOTATATE-avid left axilla and right inguinal lymph nodes, and the pathological results revealed metastatic invasive lobular breast carcinoma (Figure 3). Interestingly, the histopathology from the left axillary lymph node showed “No lymph node tissue seen”. Finally, the patient was diagnosed with occult breast cancer with metastases to the lymph nodes and rectum.
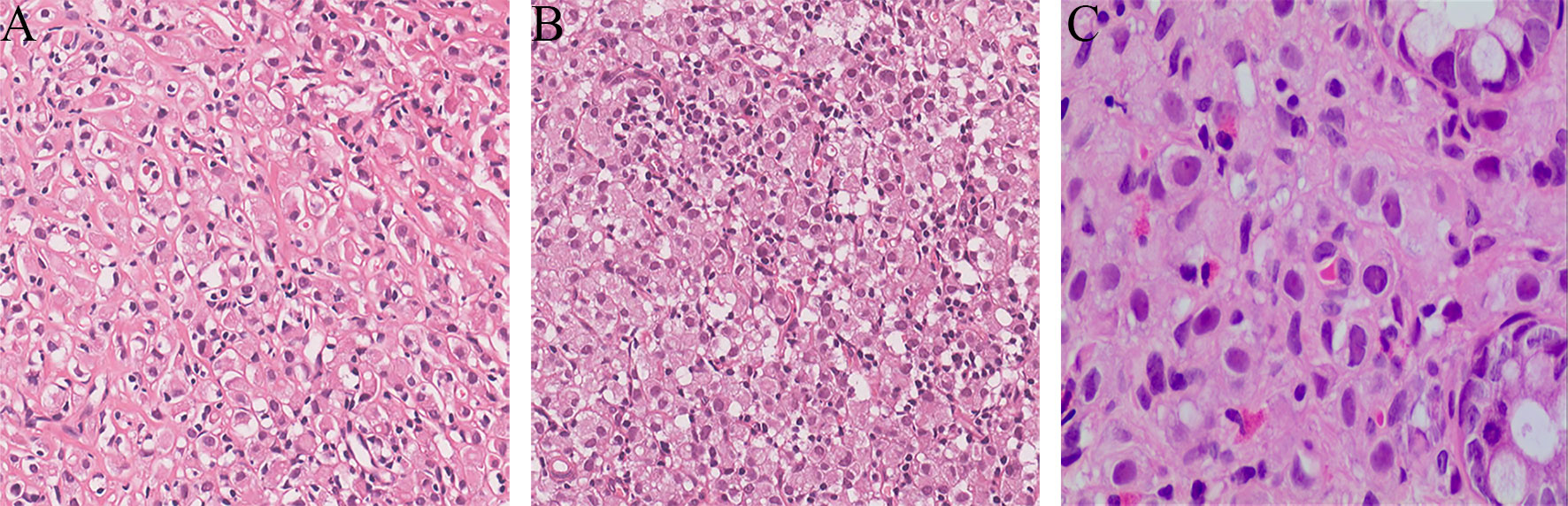
Figure 3 Ultrasound-guided percutaneous biopsy for the 68Ga-DOTATATE-avid left axilla and right inguinal lymph nodes. The pathological results revealed metastatic invasive lobular breast carcinoma (A, left axillary lymph node, HE×200; B, right inguinal lymph node, HE×200). The rectal polyp confirmed metastatic carcinoma of suspected breast origin (C, rectum polyp, HE×200).
Discussion
Occult breast cancer is defined as an axillary metastatic carcinoma without detection of a primary breast lesion in a patient, and without a history of prior breast cancer, without clinical, radiological, or pathological evidence of a primary lesion in the breast. According to previous literature and case reports, the most common sites of pathological diagnosis or metastasis were the axilla, bone, and orbit. Meanwhile, some uncommon sites had also been reported, including gastrointestinal tract, liver, lung, thyroid, and brain (8–10). OBC patients with distant metastatic disease have a much worse prognosis, with a 5-year survival of 14.3% (8). Thus, it is of great significance to ascertain whether there is distant metastasis in patients of OBC.
Several case reports have shown that primary breast cancer can demonstrate avidity to 68Ga-DOTA peptides (11–13). Andrew et al. reported that 68Ga-DOTATATE was as sensitive as 18F-FDG in staging of primary ER+/PR+ breast cancers (8/10 vs. 8/10) (14). Another case report showed a primary invasive lobular breast carcinoma lesion with 68Ga-DOTATATE activity, which was not 18F-FDG-avid (11). About 50% of breast tumors express SSR, 68Ga-DOTA peptide PET/CT examination can incidentally detect breast tumors (15–17). However, the report about 68Ga-DOTATAE PET/CT detection of occult breast cancer remain rare. In our case, the primary breast cancer remained radiologically insidious, however, bilateral axillary and right inguinal lymph nodes metastases showed significantly increased 68Ga-DOTATATE uptake and better tumor-to-background ratio than 18F-FDG. Our case suggested that 68Ga-DOTATAE PET/CT maybe an effective supplement in diagnosing OBC lymph node metastases with mild 18F-FDG uptake.
One previous study suggested that occult breast cancer may originate from ectopic breast tissue presented in axillary lymph nodes, and emphasized that the immunohistochemical subtype in OBC comprised various types similar to primary breast cancer (18). In our case, histopathology from left axillary lymph node metastases showed “No lymph node tissue seen’’, and this may be the origin of OBC. Perhaps 68Ga-DOTATAE PET/CT is as useful in the diagnosis of the origin of OBC as it is in the detection of OBC metastatic lesions.
Furthermore, the 68Ga-DOTATATE-avid lesion located in the uterus was found to be uterine fibroids by further ultrasound examination, corroborating the finding of a recent case report (19). The false-positive results of 68Ga-DOTATATE may lead to diagnostic challenges and require further differentiation.
Conclusions
Our case report provides preliminary evidence that 68Ga-DOTATATE PET/CT may be a helpful clinically problem-solving imaging modality in diagnosing occult breast cancer, especially in diagnosing metastatic lesions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
TH, RZ, JH, BW and ZZ: manuscript writing. LL and YZ: pathological review. TH, RZ, BZ, SH, ZZ, and PH: manuscript revision. All authors contributed to the article and approved the submitted version.
Funding
This research supported in part by the National Natural Science Foundation of China (No.81901772), the Natural Science Foundation of Guangdong Province, China (No. 2019A1515011893), the State Key Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia & The First People’s Hospital of Kashi Fund (No.SKL-HIDCA-2020-KS2), Guangzhou Basic and Applied Basic Research Foundation (No.202102080400).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ofri A, Moore K. Occult breast cancer: Where are we at? Breast. (2020) 54:211–5. doi: 10.1016/j.breast.2020.10.012
2. Foroudi F, Tiver KW. Occult breast carcinoma presenting as axillary metastases. Int J Radiat Oncol Biol Phys (2000) 47(1):143–7. doi: 10.1016/s0360-3016(99)00542-8
3. Walker GV, Smith GL, Perkins GH, Oh JL, Woodward W, Yu TK, et al. Population-based analysis of occult primary breast cancer with axillary lymph node metastasis. Cancer. (2010) 116(17):4000–6. doi: 10.1002/cncr.25197
4. Kemeny MM, Rivera DE, Terz JJ, Benfield JR. Occult primary adenocarcinoma with axillary metastases. Ann Surg Oncol (1986) 152(1):43–7. doi: 10.1016/0002-9610(86)90135-2
5. Hessler LK, Molitoris JK, Rosenblatt PY, Bellavance EC, Nichols EM, Tkaczuk KHR, et al. Factors influencing management and outcome in patients with occult breast cancer with axillary lymph node involvement: Analysis of the national cancer database. Ann Surg Oncol (2017) 24(10):2907–14. doi: 10.1245/s10434-017-5928-x
6. Lewin J. Comparison of contrast-enhanced mammography and contrast-enhanced breast MR imaging. Magn Reson Imaging Clin N Am (2018) 26(2):259–63. doi: 10.1016/j.mric.2017.12.005
7. Łuczyńska E, Heinze-Paluchowska S, Hendrick E, Dyczek S, Ryś J, Herman K, et al. Comparison between breast MRI and contrast-enhanced spectral mammography. Med Sci Monit (2015) 21:1358–67. doi: 10.12659/MSM.893018
8. Hotta M, Khurana M, Sung M, O'Connor VV. Understanding the biology of occult breast cancer: Examination of 31 cases reveals aggressive behavior. Am Surg (2020) 86(3):e116–8. doi: 10.1177/000313482008600304
9. Xu W, Meng T, Shang Q, Pang Y, Chen H. Uncommon metastases from occult breast cancer revealed by 18 f-FDG and 68 Ga-FAPI PET/CT. Clin Nucl Med (2022) 47(8):751–3. doi: 10.1097/RLU.0000000000004193
10. Zhang K, Yu Y, Zang Y, Xu H, Lv B, Wang Q. Case report: Unique ultrasound feature of thyroid metastases in occult breast cancer. Front Oncol (2022) 12:970286. doi: 10.3389/fonc.2022.970286
11. Guirguis MS, Adrada BE, Surasi DS, Dryden MJ. 68Ga-DOTATATE uptake in primary breast cancer. Clin Nucl Med (2021) 46(3):248–9. doi: 10.1097/RLU.0000000000003421
12. Dalm SU, Melis M, Emmering J, Kwekkeboom DJ, de Jong M. Breast cancer imaging using radiolabelled somatostatin analogues. Nucl Med Biol (2016) 43:559–65. doi: 10.1016/j.nucmedbio.2016.05.012
13. Yamaga LYI, Wagner J, Funari MBG. 68Ga-DOTATATE PET/CT in nonneuroendocrine tumors: A pictorial essay. Clin Nucl Med (2017) 42(6):e313–6. doi: 10.1097/RLU.0000000000001620
14. Nguyen A, Fullard K, Sheehan-Dare G, Tang R, Chan L, Ho B, et al. Diagnostic value of 68 Ga-DOTATATE PET-CT imaging for staging of ER+ /PR+ HER2- breast cancer patients with metastatic disease: Comparison with conventional imaging with bone scan, diagnostic CT and 18 f-FDG PET-CT in a prospective pilot trial. J Med Imaging Radiat Oncol (2022) 66(6):731–7. doi: 10.1111/1754-9485.13342
15. Reubi JC, Krenning E, Lamberts SW, Kvols L. In vitro detection of somatostatin receptors in human tumors. Metabolism. (1992) 41(9 Suppl 2):104–10. doi: 10.1016/0026-0495(92)90042-9
16. Ferone D, Semino C, Boschetti M, Cascini GL, Minuto F, Lastoria S. Initial staging of lymphoma with octreotide and other receptor imaging agents. Semin Nucl Med (2005) 35(3):176–85. doi: 10.1053/j.semnuclmed.2005.03.001
17. Elgeti F, Amthauer H, Denecke T, Steffen I, Heuck F, Stelter L, et al. Incidental detection of breast cancer by 68Ga-DOTATOC-PET/CT in women suffering from neuroendocrine tumours. Nuklearmedizin. (2008) 47(6):261–5. doi: 10.3413/nukmed-0185
18. Terada M, Adachi Y, Sawaki M, Hattori M, Yoshimura A, Naomi G, et al. Occult breast cancer may originate from ectopic breast tissue present in axillary lymph nodes. Breast Cancer Res Treat (2018) 172(1):1–7. doi: 10.1007/s10549-018-4898-4
Keywords: 68Ga-DOTATATE, 18F-FDG, PET/CT, occult breast cancer, lymph node metastases
Citation: Hu T, Zhang R, Zhang B, He S, Liu L, Zou Y, Huang J, Wang B, Hu P and Zhang Z (2023) Case report: Uncommon multiple metastases from occult breast cancer revealed by 68Ga-DOTATATE PET/CT. Front. Oncol. 13:1106890. doi: 10.3389/fonc.2023.1106890
Received: 24 November 2022; Accepted: 06 February 2023;
Published: 22 February 2023.
Edited by:
Zhang Sheng Jian, Fudan University, ChinaReviewed by:
Hubing Wu, Southern Medical University, ChinaXiaoran Li, Xuanwu Hospital, Capital Medical University, China
Copyright © 2023 Hu, Zhang, Zhang, He, Liu, Zou, Huang, Wang, Hu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Hu, aHVwaW5nQG1haWwuc3lzdS5lZHUuY24=; Zhanwen Zhang, emh6aGFudzdAbWFpbC5zeXN1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Tianyuan Hu1†
Tianyuan Hu1† Bing Zhang
Bing Zhang Zhanwen Zhang
Zhanwen Zhang