- 1Daejeon Korean Medicine Hospital, Daejeon University, Daejeon, Republic of Korea
- 2East-West Cancer Center, Cheonan Korean Medicine Hospital, Daejeon University, Daejeon, Republic of Korea
- 3Liver and Immunology Research Center, Daejeon Korean Medicine Hospital of Daejeon University, Daejeon, Republic of Korea
Objectives: Primary hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related deaths, especially in Asian countries. As a practical treatment option, transarterial chemoembolization (TACE) has been well applied; however, its limited efficacy remains challenging. This study analyzed the adjuvant effects of herbal medicine on TACE to determine whether it improves clinical outcomes in patients with HCC.
Methods: A systematic review and meta-analysis was performed to compare the adjuvant effects of herbal medicine on TACE versus TACE therapy alone. We searched the literature from eight databases since January 2011.
Results: Twenty-five studies involving 2,623 participants were selected. The adjuvant therapy of herbal medicine on TACE improved the overall survival at 0.5 years (OR = 1.70; 95% CI 1.21-2.38), 1 year (OR = 2.01; 95% CI 1.65-2.46), 2 years (OR = 1.83; 95% CI 1.20-2.80), and 3 years (OR = 1.90; 95% CI 1.25-2.91). The combination therapy also increased the tumor response rate (OR = 1.84; 95% CI 1.40-2.42).
Conclusions: Despite the unsatisfactory quality of the included studies, the adjuvant therapy of herbal medicine on TACE may provide survival benefits to patients with HCC.
Systematic reviews registration: http://www.crd.york.ac.uk/PROSPERO, identifier (376691).
1 Introduction
Hepatocellular carcinoma (HCC) is the leading cause of cancer-related deaths, with an incidence of 9.3 and a mortality rate of 8.5 per 100,000 in 2018 worldwide (1). Since most HCCs are asymptomatic until they reach an advanced or late stage, HCC is difficult to diagnose and has a very poor prognosis (2). The mortality of patients with HCC has remained unchanged over the past decade (3, 4).
Adequate treatments for HCC, including surgical resection, chemotherapy, radiation therapy, radiofrequency ablation, and transarterial chemoembolization (TACE), have improved the 5-year survival rate of HCC from 9% to 18% between 2001 and 2019 (4–7). Among those therapeutics, TACE is the first-line treatment for patients with early-stage and localized HCC and causes tumor necrosis by injection of chemotherapeutic agents into the hepatic artery (8). TACE is not only applied in the early stage but is also frequently used in the unresectable and late stages for palliative care in HCC patients (9).
Approximately 80% of patients with HCC have liver fibrosis, resulting in liver cirrhosis due to chronic inflammation in the liver (10). Although TACE is a topical treatment that can minimize systemic inflammation, TACE accelerates the hepato-fibrotic changes because of its inevitable cytotoxic effects (11). Patients who receive TACE therapy sometimes suffer from complications such as acute cholecystitis, leukopenia, pulmonary embolism, hepatic abscess, bile duct injury, and gastric mucosa injury (12–14). The limitations of TACE in the clinic include not only an insufficient response but also the adverse effects listed above (15).
On the other hand, herbal medicine has been prescribed as an option for patients with hepatic inflammation and liver fibrosis in Asian countries (16, 17). In 1996, the effect of combination therapy of TACE and herbal drugs was first reported (18), and a systematic review of the beneficial outcomes of herbal medicine on TACE was published in 2013 (19). To date, the adjuvant therapy of herbal drugs on TACE for patients with HCC has been further practiced and has been continued; however, no comprehensive evaluation of the combination therapy has been conducted in the last 10 years.
Herein, we conducted a systematic review and meta-analysis to evaluate the adjuvant effect of herbal medicine on TACE in patients with HCC.
2 Materials and methods
2.1 Protocol and registration
This systematic review, including a meta-analysis, was conducted based on the PRISMA guidelines and was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (ID: 376691, http://www.crd.york.ac.uk/PROSPERO).
2.2 Search strategy
Eight databases, including the PubMed, Cochrane, ClinicalTrials.gov, EMBASE, Google Scholar, Chinese National Knowledge Infrastructure, Research Information Sharing Service, and Korean Studies Information Service System databases, were searched after January 2011 using keywords related to primary HCC, herbal medicine, TACE and overall survival. The search terms were (hepatocellular carcinoma OR hepatocellular neoplasms OR liver cancer OR liver neoplasms OR primary hepatic cancer OR intrahepatic neoplasms OR liver adenoma OR liver carcinoma OR hepatocellular adenoma OR HCC) AND (TACE OR transcatheter arterial chemoembolization OR embolization) AND (herb OR herbal medicine OR herbal decoction OR herbal drugs OR phytotherapy OR Korean medicine OR Chinese medicine).
2.3 Selection criteria
The studies that met the following criteria were included: clinical studies comparing the effects between ‘TACE combined with herbal drugs’ and ‘TACE-only’ in patients with primary HCC. There was no limit on the language, and studies that did not meet the above criteria were excluded.
2.4 Data extraction and review process
After screening the title and abstract of all the studies, the full text of the relevant articles was assessed by two reviewers. Any disagreement was resolved by discussion or consensus with the corresponding author. We conducted a systematic review on the clinical benefits of herbal medicine combined with TACE compared to TACE alone. We extracted the following data: name of the first author, patient information, sample size, herbal medicine, duration of herbal medicine, observation period, and outcome measurements (overall survival at 0.5, 1, 2, and 3 years, number of complete/partial responses, and/or Karnofsky performance status (KPS) score) of the study.
A meta-analysis was performed using odds ratios (ORs) for the overall survival rate and tumor response rate and weighted mean differences (WMDs) for the KPS score with 95% confidence intervals (CIs). Random-effect models were used due to heterogeneity. Dichotomous data are expressed as the OR with 95% CI. WMDs with the 95% CI were calculated for continuous data. The Higgins I2 test was used to assess the heterogeneity of the data (20). Statistical significance was set at P < 0.05. Review Manager 5.4.1 was used for the analysis (http://www.tech.cochrane.org/revman) (accessed on 15 July 2022) (21).
3 Results
3.1 Characteristics of the included studies
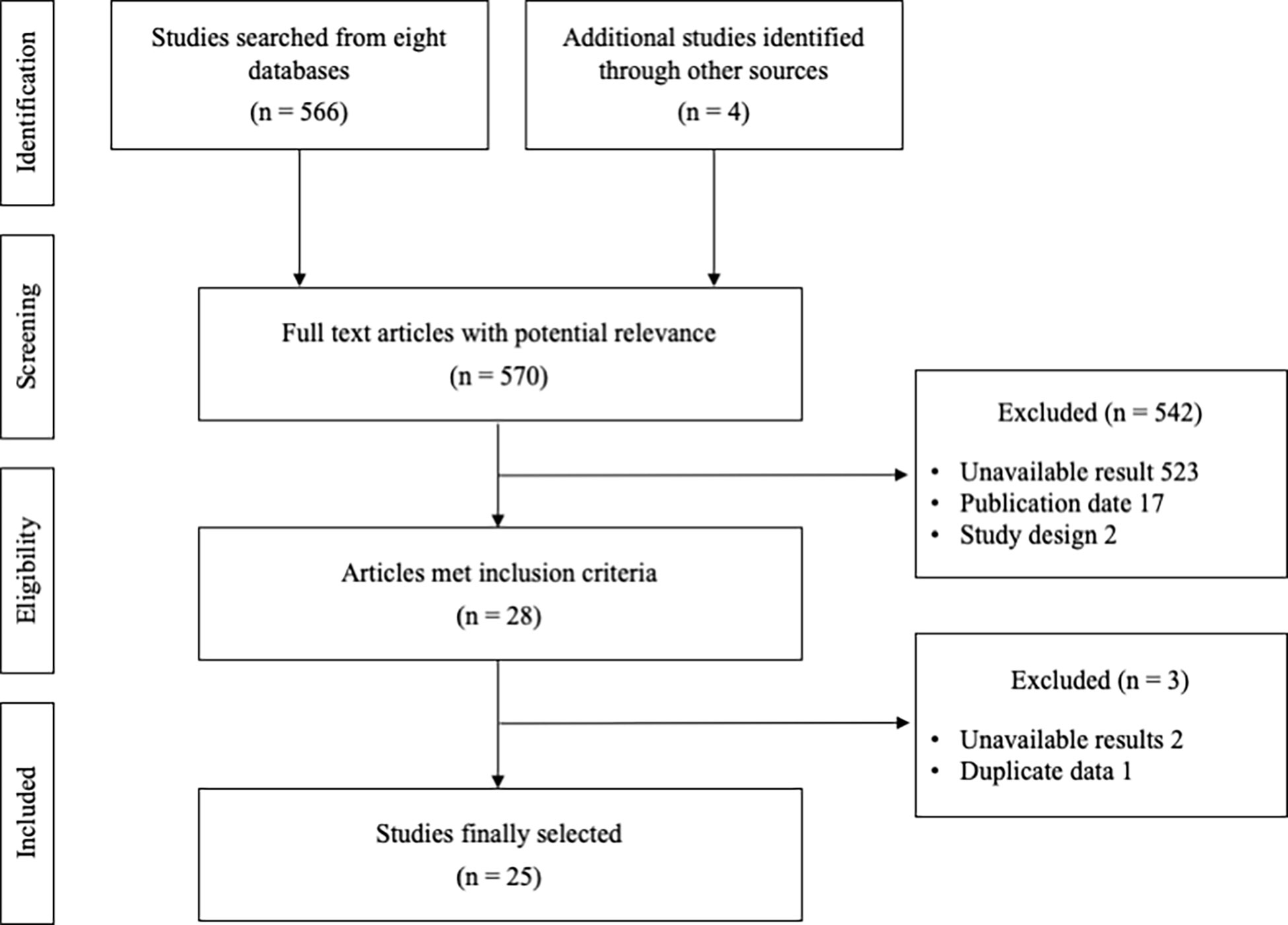
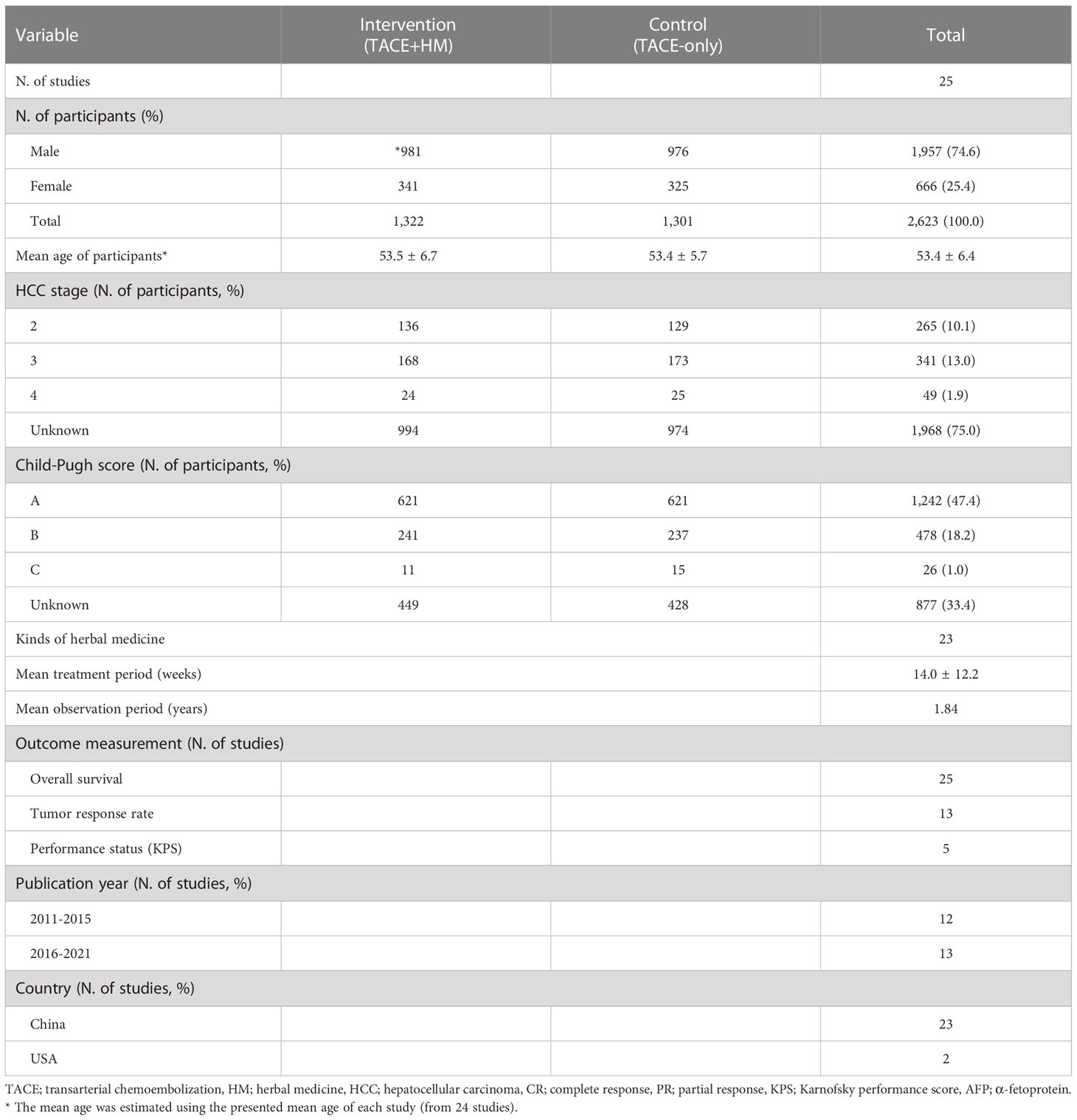
A total of 570 relevant articles were initially searched, and 25 studies were finally selected for this study (Figure 1). The total number of participants was 2,623 (male 1957, female 666), with 1,322 who took the combination therapy and 1,301 who only had TACE. The information for stage was obtained from 655 subjects, and the Child−Pugh scores were determined from 1,746 patients (Table 1). There was no significant difference in baseline characteristics between the intervention (TACE+HM) and control (TACE-only), regarding age, sex, HCC stage, Child-Pugh grade, etc. respectively (Table 1).
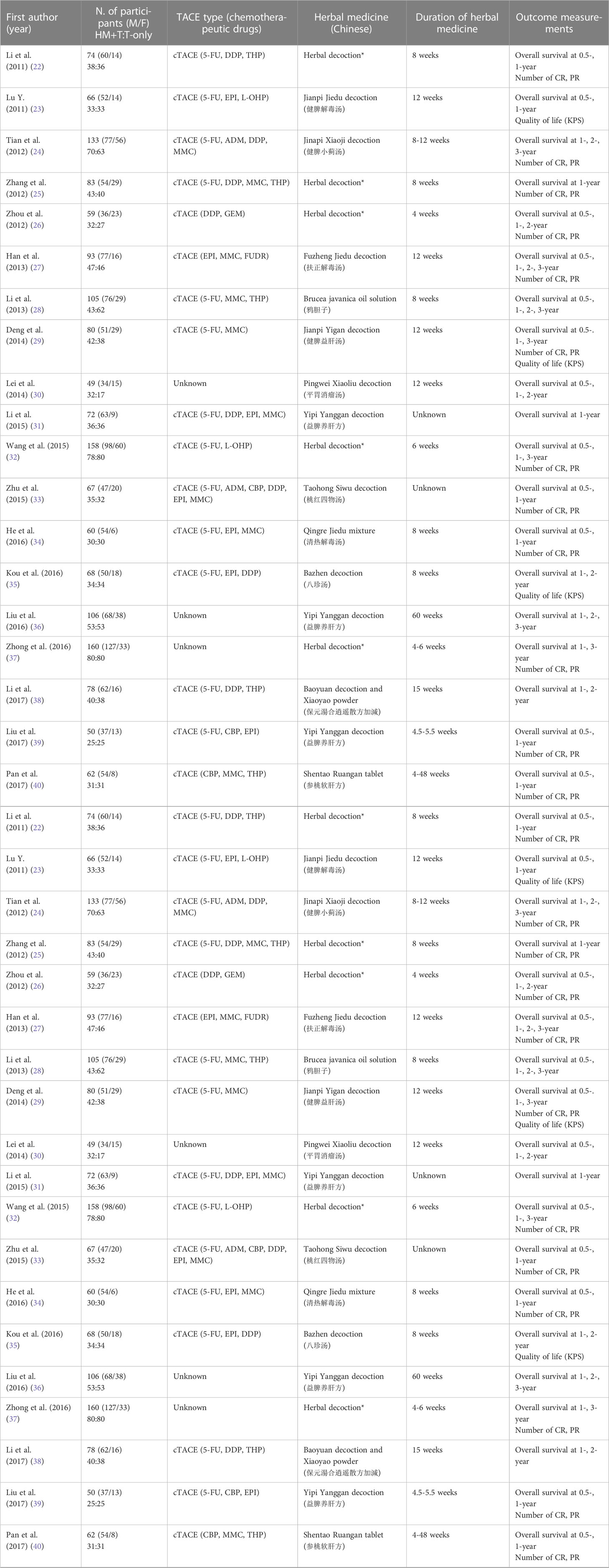
Twenty-three kinds of herbal medicines were administered for an average of 14.0 ± 12.2 weeks. The mean observation period was 1.84 years, and overall survival was evaluated as the primary measurement at four main time points (0.5, 1, 2, and/or 3 years), along with the tumor response rate and quality of life as secondary measurements (Table 1).
3.2 Herbal medicine used for combined therapy with TACE
The kinds of herbal medicine and their composition were all provided, as summarized in Supplementary Table 1. Yipi Yanggan decoction was applied in three patients, and the rest of the patients had all different kinds (Table 2). The most frequently used herbs were Atractylodes macrocephala Koidz. (17 times), Wolfiporia extensa (15 times), Curcuma longa L. (12 times), Bupleurum falcatum L. (12 times), Astragalus propinquus Schischk (11 times) (Supplementary Table 2).
3.3 Benefits in overall survival (primary measurement)
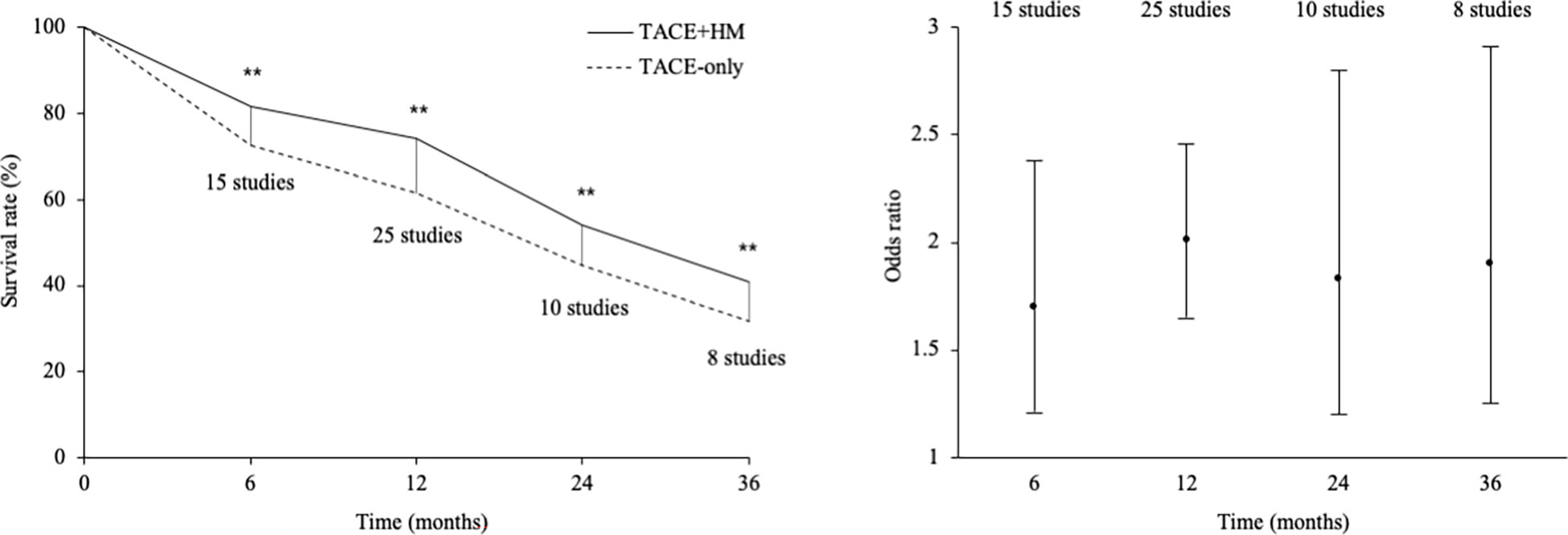
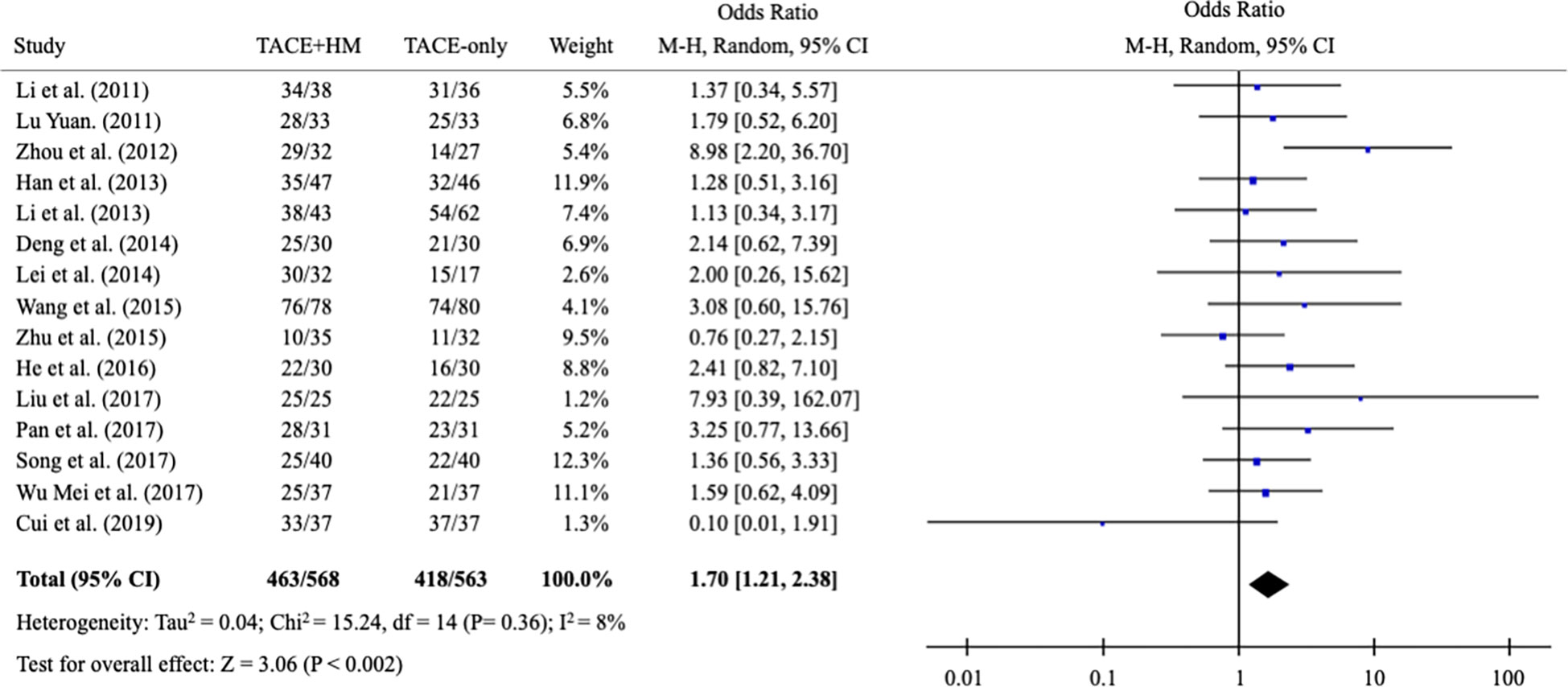
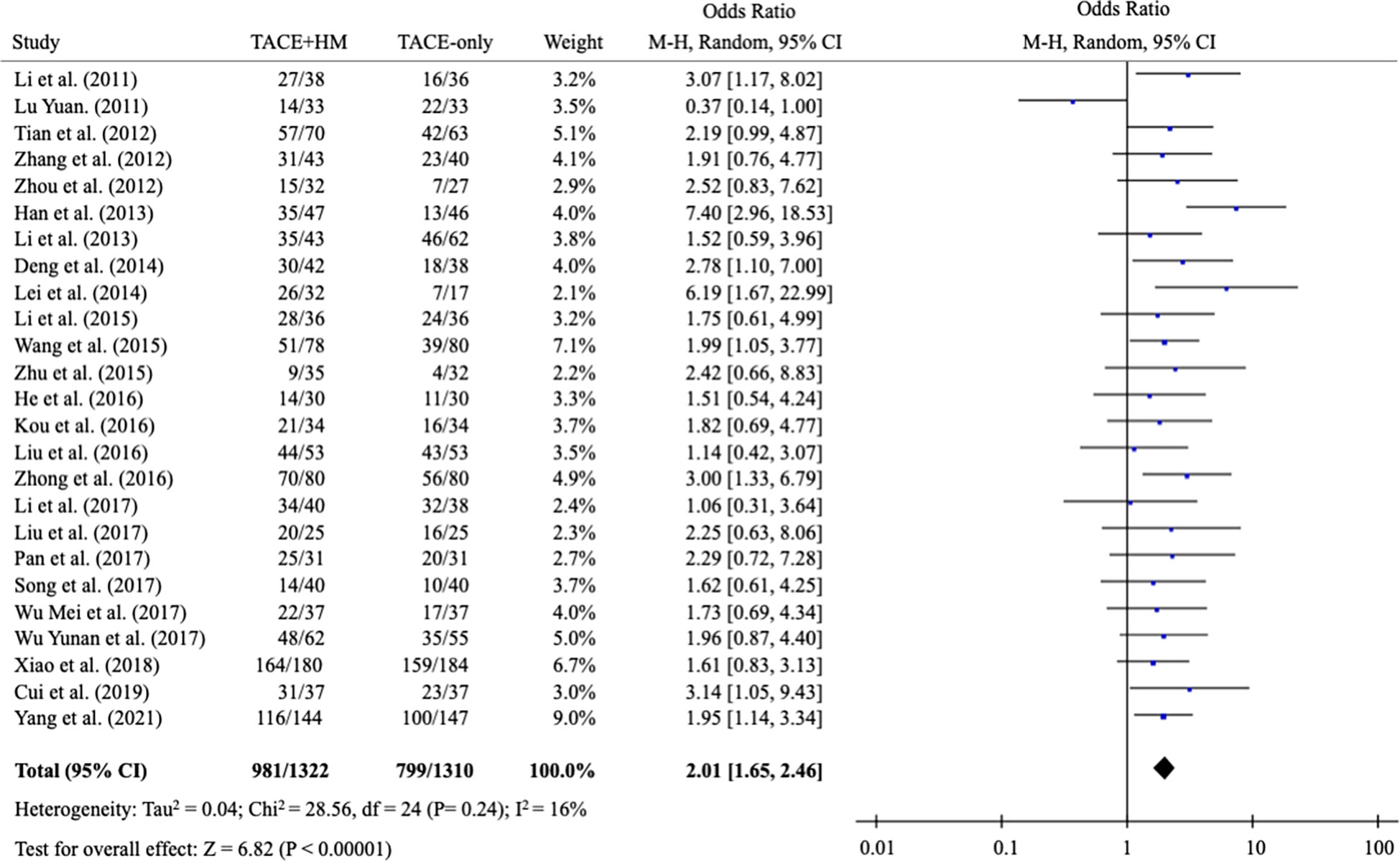
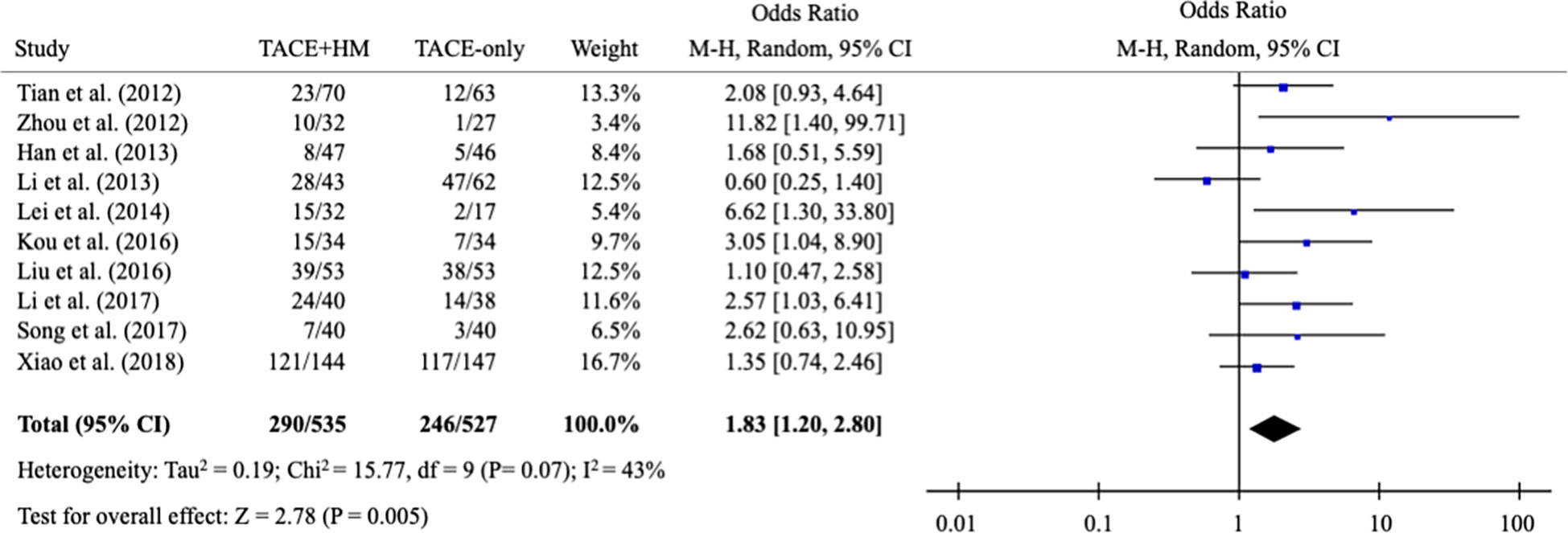
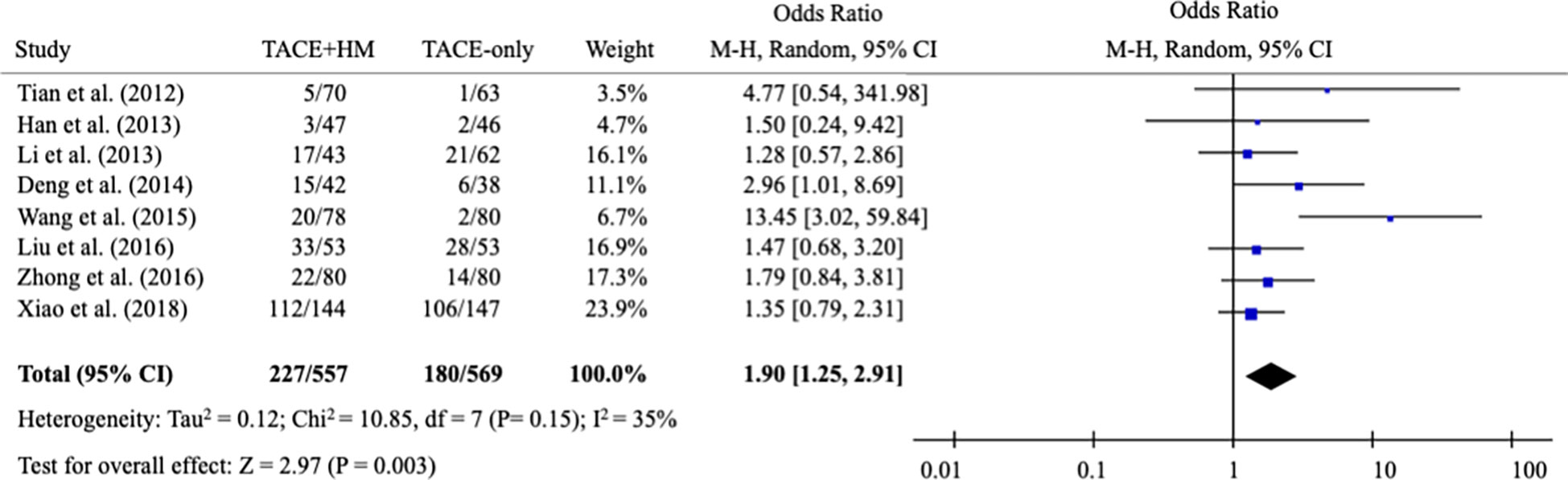
From the meta-analysis of the overall survival rate, the combination therapy showed a significant improvement in the survival rate at all measured points (Figure 2); OR = 1.70 at 0.5 years (95% CI 1.21-2.38; P < 0.002, 15 studies, 1,131 participants) (Figure 3), OR = 2.01 at 1 year (95% CI 1.65-2.46; P < 0.00001, 25 studies, 2,623 participants) (Figure 4), OR = 1.83 at 2 years (95% CI 1.20-2.80; P = 0.005, 10 studies, 1,062 participants) (Figure 5), and OR = 1.90 at 3 years (95% CI 1.25-2.91; P = 0.003, 8 studies, 1,126 participants) (Figure 6).
3.4 Benefits in tumor response rate and quality of life (secondary measurement)
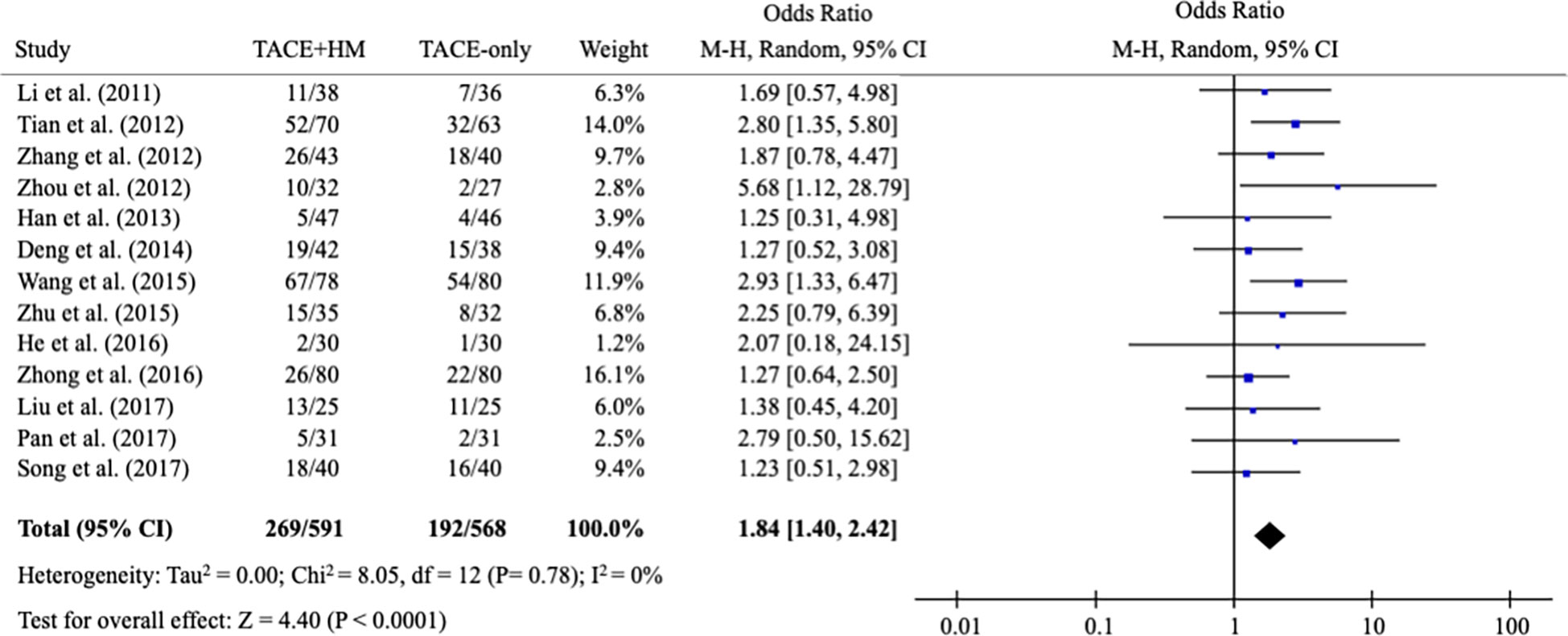
As secondary measurements, the meta-analysis for the response rate of treatment was significantly increased in the combination group as the OR = 1.84 (95% CI 1.40-2.42; P < 0.0001) from 13 studies (n=1,159) (Figure 7).
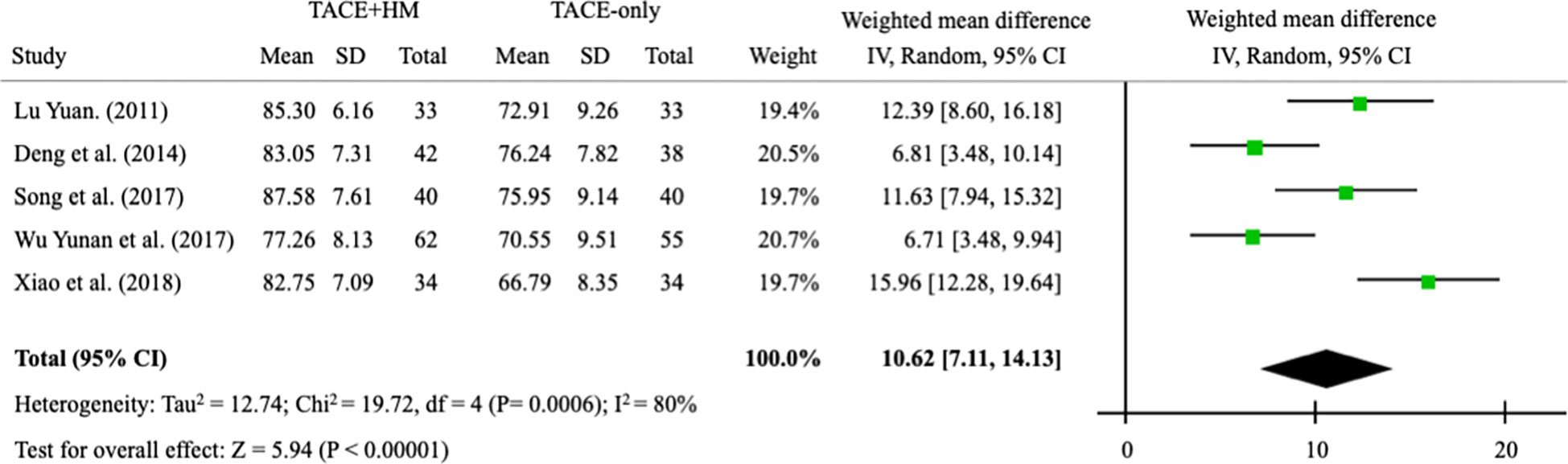
Quality of life measured by the KPS score was significantly improved by combination therapy, with an WMD = 10.62 (95% CI 7.11–14.13; P < 0.00001) from 5 studies (n = 411) (Figure 8).
4 Discussion
The well-known risk factors for HCC are infection with hepatitis B virus (HBV) or hepatitis C virus (HCV), excessive alcohol consumption, and nonalcoholic fatty liver disease (NAFLD) (47–49). In Asian countries, including China, 70~80% of HCC cases are known to be caused by HBV infections (50), while most of our data for meta-analysis did not describe the causes of HCC. As it is known, there is a male-predominance of HCC patients, and our data showed a 3.0-fold higher number of male patients than female patients (Table 1).
From 25 studies containing 2,623 participants (1,322 herbal interventions), we found a 1.29-fold survival benefit compared to the TACE-only group (primary endpoint) (Figure 2). The present results may indicate that the add-on therapy on TACE obtained a positive clinical outcome on survival gain. In fact, TACE therapy is usually coadapted with chemotherapies (51). A study reported a 1.6-fold improvement in the survival rate in patients with HCC with Child−Pugh score A using an adjuvant therapy of sorafenib with TACE (52), and that study’s data was slightly superior to our data. In our study, the majority of the participants for whom the stage information was provided (only 25% of the total participants) were stages II and III, and the majority of the patients (from 50% of participants) were Child−Pugh score A. TACE had significant survival advantages compared to supportive care (21.2 vs. 14.5 months for 4 years of observation time) for patients with late-stage HCC (53); thus, the evaluation of the adjuvant effects of herbal medicine are necessary. As for the types of TACE, novel treatment of chemoembolization with drug-eluting beads (DEB-TACE) has been introduced to reduce drawbacks of conventional TACE (c-TACE) and to improve the overall results (54). However, 21 studies except 4 RCTs not described the types of TACE used cTACE in this systematic review (Table 2).
Patients with HCC suffer from various symptoms, such as abdominal pain, diarrhea, nausea, vomiting, jaundice, cholangitis, and fever (55). Surgical resection or TACE can cause pain or discomfort and deteriorate the quality of life (56). In our results, the adjuvant treatment of herbal drugs improved the quality of life after treatment by 10.6 out of 100 points compared to the TACE-alone group. This finding is similar to the result of one article that reported quality of life improvements of 10.0 out of 100 points in three-dimensional conformal radiotherapy, which is typically used for adjuvant therapy with TACE (57). Our study supported that herbal medicine could improve the quality of life by relieving symptoms when combined with TACE. On the other hand, hepatic fibrosis is a crucial factor in determining the prognosis of HCC patients, and hepatic fibrosis progresses gradually and leads to fatal outcomes (58). However, there is no optimal therapeutic for liver fibrosis to date (59). Herbal medicines have been investigated as potential treatments for liver fibrosis due to their anti-inflammatory and antiviral properties (60). For example, Chunggan syrup (CGX), a standardized herbal formula in Korea, improved liver fibrosis, as assessed by the decreases in liver stiffness measurement score, in a clinical trial (61). In another trial, oxymatrin, extracted from Sophora alopecuraides L., showed a significant antifibrotic effect (with a total effective rate of 48% vs. 4% compared to placebo after 24 weeks of administration) (62). These antifibrotic actions may contribute to survival benefits in patients with HCC treated by TACE. Besides antifibrotic properties, there would be other mechanisms corresponding to adjuvant effects of herbal drugs on TACE, we however currently cannot identify them from present data.
In this review, mostly different kinds of herbal medicine were used in 25 studies, except for in 3 of the patients (Supplementary Table 1), and the compositions of these therapies were also diverse (Supplementary Table 2). The heterogeneity of the herbal medicines was the main limitation of this study, which makes it difficult to clarify the interaction between herbal medicine and TACE, and their corresponding mechanisms. Other limitations would include the unsatisfactory initial data from relatively poorly designed clinical trials and the possibility of publication bias due to only a very few studies reporting negative outcomes. To strengthen the clinical evidence for the adjuvant efficacy of herbal medicine on TACE therapy to treat HCC patients, further strictly designed clinical trials should be performed that have standardized herbal remedies. Herbal drugs have been adopted worldwide, but concerns regarding their safety have arisen (63). Regarding the adverse effects of combination therapy on HCC, the present data did not show any notable frequency compared to only TACE therapy.
In conclusion, this systematic review and meta-analysis showed survival benefits in patients with HCC by combined treatment with herbal medicine and TACE. The adjuvant effect of herbal drugs on TACE needs to be further evaluated by well-designed RCTs in the future.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
H-MO: wrote the main manuscript text, and conducted statistical analysis; E-JK, H-RB: contributed to the data collection and manuscript preparation including revision process; J-HC, C-GS: supervised the manuscript; N-HL: supervised the manuscript, and directed final version of all contents. All authors reviewed and approved this manuscript.
Funding
This work was supported by grants from the National Research Foundation of Korea (NRF) funded by the Korean government (2017M3A9E4065193, 2020R1F1A1069711).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1106827/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Motola-Kuba D, Zamora-Valdés D, Uribe M, Méndez-Sánchez N. Hepatocellular carcinoma. an overview. Ann Hepatol (2006) 5:16–24. doi: 10.1016/S1665-2681(19)32034-4
3. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin (2008) 58:71–96. doi: 10.3322/CA.2007.0010
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin (2019) 69:7–34. doi: 10.3322/caac.21551
5. Ryder SD. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut (2003) 52:1111–8. doi: 10.1136/gut.52.suppl_3.iii1
6. N'Kontchou G, Mahamoudi A, Aout M, Ganne-Carrié N, Grando V, Coderc E, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology (2009) 50:1475–83. doi: 10.1002/hep.23181
7. Golabi P, Fazel S, Otgonsuren M, Sayiner M, Locklear CT, Younossi ZM. Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Med (Baltimore) (2017) 96:e5904. doi: 10.1097/MD.0000000000005904
8. Tsurusaki M, Murakami T. Surgical and locoregional therapy of HCC: TACE. Liver Cancer (2015) 4:165–75. doi: 10.1159/000367739
9. Geschwind JF, Kudo M, Marrero JA, Venook AP, Chen XP, Bronowicki JP, et al. TACE treatment in patients with sorafenib-treated unresectable hepatocellular carcinoma in clinical practice: final analysis of GIDEON. Radiology (2016) 279:630–40. doi: 10.1148/radiol.2015150667
10. Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology (2009) 49:851–9. doi: 10.1002/hep.22734
11. Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology (2010) 52:762–73. doi: 10.1002/hep.23725
12. Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet (2002) 359:1734–9. doi: 10.1016/S0140-6736(02)08649-X
13. Tasneem A, Abbas Z, Luck N, Hassan S, Faiq S. Adverse events following transarterial chemoembolization for hepatocellular carcinoma and factors predicting such events. J Pak Med Assoc (2013) 6:239–44.
14. Kim SI, Jin YJ, Cho SG, Shin WY, Kim JM, Lee JW. Duodenal perforation and esophageal ischemia following transarterial chemoembolization for hepatocellular carcinoma. Medicine (2016) 95:e3987. doi: 10.1097/MD.0000000000003987
15. Lencioni R, Baere T, Soulen MC Rilling WS, Geschwind JFH. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology (2016) 64:106–16. doi: 10.1002/hep.28453
16. Zhao CQ, Zhou Y, Ping J, Xu LM. Traditional Chinese medicine for treatment of liver diseases: progress, challenges and opportunities. J Integr Med (2014) 12:401–8. doi: 10.1016/S2095-4964(14)60039-X
17. Park CR, Lee G, Son CG, Cho JH, Lee NH. Recovery from hepatitis a after Korean medicine-based treatment: a case report. Integr Med Res (2019) 8:257–60. doi: 10.1016/j.imr.2019.11.001
18. Zhang SY, Geng NY, Liu YE, Jiang W, Jiang WF. Clinical study of hepatic artery infusion chemotherapy combined with AC-III injection in the treatment of hepatocellular carcinoma. Inf Tradit Chin Med (1996) 4:29–31.
19. Cheung F, Wang X, Wang N, Yuen MF, Ziea TC, Tong Y, et al. Chinese Medicines as an adjuvant therapy for unresectable hepatocellular carcinoma during transarterial chemoembolization: a meta-analysis of randomized controlled trials. Evidence-Based Complementary Altern Med (2013) 2013:1–25. doi: 10.1155/2013/487919
20. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. John Wiley Sons (2019). doi: 10.1002/9780470712184
21. RevMan 5 download. Cochrane: Cochrane Training. Available at: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-5-download.
22. Li Y, Liang H, Zhang C. Clinical observation of traditional Chinese medicine combined with hepatic arterial chemoembolization in the treatment of primary liver cancer. Acta Univ Medicinalis Nanjing (2011) 31:742–5.
23. Lu Y. Clinical study of in treating primary liver cancer with jian pi jie Du prescription and its mechanism. (China: Nanjing University) (2011). Doctoral Dissertation.
24. Tian Y, Li H, Du H. Study on curative effects of the integration of traditional Chinese medicine and Western medicine for the treatment of liver cancer. China J Chin Med (2012) 27:1246–7.
25. Zhang N, Chen G, Shi Q. Clinical observation of transcatheter hepatic arterial chemoembolization combined with Chinese herbal medicine in treating primary liver cancer. J New Chin Med (2012) 44:72–5.
26. Zhou Y. Observation on the curative effect of integrated traditional Chinese and western medicine in the treatment of advanced liver cancer. Guangming J Chin Med (2012) 5:979–80.
27. Han KQ, Xie GQ, Chen J, He TL, Qian ZP, Gu W, et al. Clinical study on the efficacy of transcatheter arterial chemoembolization with fuzhengjiedu prescription of traditional Chinese medicine on hepatocellular carcinoma in advanced stage. Chin J Integrated Tradit Western Med (2013) 21:57–60.
28. Li J, Qi CH. Clinical efficacy of transcatheter artery chemoembolization combined traditional Chinese medicine for the treatment of primary liver cancer. Chin J Integr Med (2013) 11:627–9.
29. Deng L, Peng G, Jiang Y, Mo J, Shangling Y. Observation of curative effect of percutaneous transarterial chemoembolization combined with jianpi yigan prescription in the treatment of senile primary liver cancer. Shandong Med J (2014) 54:59–61.
30. Lei Q. Clinical observation on treating 32 cases of hepatocellular carcinoma with the pingwei xiaoliu decoction. Clin J Chin Med (2014) 6:106–7.
31. Li J, Guo S, Xi Q, Yazhu L, Shuguang Y, Yi H, et al. Efficacy observation of yipi yanggan decoction combined with hepatic arterial chemoembolization in the treatment of primary liver cancer. Shaanxi J Tradit Chin Med (2015) 36:944–5.
32. Wang YQ, Qi ZP, Zhao WL. Clinical research of liver cancer treated with Chinese medicine and transarterial chemoembolization. World J Integrated Tradit Western Med (2015) 10:1723–5.
33. Zhu X. Oral administration of Chinese herbs combined with interventional operation for treating 35 cases with primary liver cancer. Henan Tradit Chin Med (2015) 35:291–3.
34. He TL, Ma LL, Xie GQ, Chen J, Guo XD, Zhang XM. Qingre jiedu mixture with transcatheter arterial chemoembolization in treating advanced hepatocellular carcinoma. Shanghai J Tradit Chin Med (2016) 50:1007–334.
35. Kou X, Hao M, Xie X. Clinical observation on bazhen decoction used for primary liver cancer patients after TACE treatment. J Modern Oncol (2016) 24:2920–2.
36. Liu H, Lu W. Application value of traditional Chinese and Western medicine in the treatment of postoperative recurrence of liver cancer. Pract J Cancer (2016) 31:493–5.
37. Zhong C, Hu M, Huang J, Jian W, Huidong L, Rongping G. Clinical study of strengthening spleen and removing blood stasis with traditional Chinese medicine combined with TACE in the treatment of postoperative recurrence of liver cancer. J New Chin Med (2016) 48:208–10.
38. Li L, Xu G. Clinical observation of applying TACE combined with traditional Chinese medicine in the treatment of primary liver cancer during medium and advanced stage. J Sichuan Tradit Chin Med (2017) 35:104–7.
39. Liu Y, Xi Q, Yan R, Jingtao L, Chunrong S, Ran N, et al. Clinical evaluation of primary liver cancer by decoction of strengthening spleen and supplementing liver combined with TACE and 3DCRT. Liaoning J Tradit Chin Med (2017) 44:975–8.
40. Pan Z. The clinical study of shen Tao ruan gan tablet combined with TACE in the treatment of advanced hepatocarcinama. (China: Guangzhou University) (2017). Master Dissertation.
41. Song Y, Zhang J, Zhang S, Guoyu W, Jing N, Saifei H. Clinical research of wenyang jiedu formula with TACE in the treatment of advanced primary hepatic carcinoma. J Yunnan Univ Tradit Chin Med (2017) 40:22–5.
42. Wu M, Yueqiu G, Bin Z, Yundong L, Tao Z. Clinical observation of xiaoliu powder combined with palliative therapy in the treatment of advanced primary hepatocarcinoma. Shanghai J Tradit Chin Med (2017) 51:77–9.
43. Wu Y, Zhang D, Sun K. Clinical effect of bielong ruangan decoction combined with transcatheter arterial chemoembolization in treatment of hepatitis b virus-related primary liver cancer. J Clin Hepatol (2017) 33:2152–7.
44. Xiao FZ, Xia LL, Feng S, Fan J, Ling CQ. Traditional herbal medicine prevents postoperative recurrence of small hepatocellular carcinoma: A randomized controlled study. Cancer (2018) 123:2161–8.
45. Cui J, Hao P, Cao Y. Efficacy of traditional Chinese medicine combined with hepatic arterial chemoembolization in the treatment of advanced primary liver cancer. psychol Mon (2019) 2:99–100.
46. Yang X, Feng Y, Liu Y, Ye X, Ji X, Sun L, et al. Fuzheng jiedu xiaoji formulation inhibits hepatocellular carcinoma progression in patients by targeting the AKT/CyclinD1/p21/p27 pathway. Phytomedicine (2021) 87:153575. doi: 10.1016/j.phymed.2021.153575
47. Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens–part b: biological agents. Lancet Oncol (2009) 10:321–2. doi: 10.1016/S1470-2045(09)70096-8
48. Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer (2015) 112:580–93. doi: 10.1038/bjc.2014.579
49. Jinjuvadia R, Patel S, Liangpunsakul S. The association between metabolic syndrome and hepatocellular carcinoma: systemic review and meta-analysis. J Clin Gastroenterol (2014) 48:172–7. doi: 10.1097/MCG.0b013e3182a030c4
50. Al-Mahtab M, Uddin H, Akbar SMF. Epidemiology and risk factors of hepatocellular carcinoma in Asia. J Gastroenterol Hepatol Res (2014) 3:1019–23.
51. Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology (2016) 150:835–53. doi: 10.1053/j.gastro.2015.12.041
52. Kok VC, Chen YC, Chen YY, Su YC, Ku MC, Kuo JT, et al. Sorafenib with transarterial chemoembolization achieves improved survival vs. sorafenib alone in advanced hepatocellular carcinoma: a nationwide population-based cohort study. Cancers (2019) 11:985. doi: 10.3390/cancers11070985
53. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology (2003) 37:429–42. doi: 10.1053/jhep.2003.50047
54. Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol (2007) 46(3):474–81. doi: 10.1016/j.jhep.2006.10.020
55. El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology (2008) 134:1752–63. doi: 10.1053/j.gastro.2008.02.090
56. Chie WC, Yu F, Li M, Baccaglini L, Blazeby JM, Hsiao CF, et al. Quality of life changes in patients undergoing treatment for hepatocellular carcinoma. Qual Life Res (2015) 24:2499–506. doi: 10.1007/s11136-015-0985-8
57. Xie L, Qian Z, Xu J. Clinical intervention effect of TACE combined with 3DCRT in patients with primary liver cancer. Am J Trans Res (2021) 13:7960.
58. Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology (2004) 127:S35–50. doi: 10.1053/j.gastro.2004.09.014
59. Altamirano-Barrera A, Barranco-Fragoso B, Méndez-Sánchez N. Management strategies for liver fibrosis. Ann Hepatol (2017) 16:48–56. doi: 10.5604/16652681.1226814
60. Luk JM, Wang X, Liu P, Wong KF, Chan KL, Tong Y, et al. Traditional Chinese herbal medicines for treatment of liver fibrosis and cancer: from laboratory discovery to clinical evaluation. Liver Int (2007) 27:879–90. doi: 10.1111/j.1478-3231.2007.01527.x
61. Joung JY, Kim HG, Lee JS, Cho JH, Ahn YC, Lee DS, et al. Anti-hepatofibrotic effects of CGX, a standardized herbal formula: A multicenter randomized clinical trial. Biomed Pharmacother (2020) 126:110105. doi: 10.1016/j.biopha.2020.110105
62. Mao YM, Zeng MD, Lu LG, Wan MB, Li CZ, Chen CW, et al. Capsule oxymatrine in treatment of hepatic fibrosis due to chronic viral hepatitis: a randomized, double blind, placebo-controlled, multicenter clinical study. World J Gastroenterol (2004) 10:3269. doi: 10.3748/wjg.v10.i22.3269
Keywords: herbal medicine (HM), transarterial chemoembolization, hepatocellular carcinoma, overall survival (OS), systematic review & meta-analysis
Citation: Oh H-M, Kim E-J, Bae H-R, Cho J-H, Son C-G and Lee N-H (2023) Adjuvant effect of herbal medicine on transarterial chemoembolization in patients with hepatocellular carcinoma: A systematic review and meta-analysis. Front. Oncol. 13:1106827. doi: 10.3389/fonc.2023.1106827
Received: 24 November 2022; Accepted: 27 January 2023;
Published: 09 February 2023.
Edited by:
Irene Cacciola, University of Messina, ItalyReviewed by:
Davi dos Santos Romao, Hospital Sirio Libanes, BrazilJiacheng Liu, Huazhong University of Science and Technology, China
Copyright © 2023 Oh, Kim, Bae, Cho, Son and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chang-Gue Son, Y2tzb25AZGp1LmFjLmty; Nam-Hun Lee, bmhsZWVAZGp1LmFjLmty
†These authors have contributed equally to this work and share first authorship
 Hyeon-Muk Oh
Hyeon-Muk Oh Eun-Ji Kim
Eun-Ji Kim Hye-Ri Bae2
Hye-Ri Bae2 Jung-Hyo Cho
Jung-Hyo Cho Chang-Gue Son
Chang-Gue Son Nam-Hun Lee
Nam-Hun Lee