- 1Research Center For Biomedical Resources, Beijing You’an Hospital, Capital Medical University, Beijing, China
- 2Interventional Therapy Center For Oncology, Beijing You’an Hospital, Capital Medical University, Beijing, China
Background: The aim of this study was to investigate the association between pathologic markers and prognosis in patients with hepatocellular carcinoma who received transcatheter chemoembolization combined with locoregional ablation therapy.
Methods: This retrospective study included 111 hepatitis B virus (HBV)-associated hepatocellular carcinoma (HCC). All patients underwent transcatheter arterial chemoembolization (TACE) combined with locoregional ablation therapy, and received core needle biopsy before therapy in Beijing You ‘an Hospital affiliated to Capital Medical University from January 1, 2013 to December 31, 2016. Demographic, pathological indicators and clinical laboratory data were collected. The cumulative recurrence-free survival (RFS) and overall survival (OS) were calculated and compared by Kaplan-Meier method and Log-rank test, and Cox proportional risk model was used to screen for independent predictors of recurrence and long-term prognosis in HCC patients.
Results: There was a correlation between HBsAg expression in liver tissue and prognosis of HCC patients. Patients with negative HBsAg expression had longer 1-,3- and 5-year RFS rates than positive HBsAg expression (78.3%, 43.5%, 30.4% and 58.5%, 24.5%, 17.0%, P=0.018). Meanwhile,the postoperative 1-,3-and 5-year OS rates of HCC patients in the negative HBsAg expression group were significantly higher than those of HCC patients in the positive HBsAg expression group (100%, 89.1%, 80.4% and 100%, 75.5%, 58.5%, P=0.008).
Conclusions: The prognosis of patients with hepatocellular carcinoma with negative HBsAg expression was better than that with positive HBsAg expression. Accordingly, the expression of the liver HBsAg before combined therapy was a prognostic indicator for OS and RFS. For patients with liver HBsAg positive, follow-up should be strengthened and corresponding intervention measures should be taken to improve prognosis.
1 Introduction
There were 910,000 new cases and 830,000 deaths of hepatocellular carcinoma (HCC) worldwide, it was the sixth most common cancer globally and the third leading cause of cancer-related mortality. In China, HCC has 410,000 new cases and 390,000 deaths, ranking fifth and second in morbidity and mortality and HCC has become a health problem in China that cannot be ignored and has increased the medical burden (1).. HCC is usually developing in the context of chronic liver disease, which is mainly associated with hepatitis B virus (HBV) or hepatitis C virus (HCV) infection, alcohol intake or the metabolic syndrome. However, due to the low early diagnosis rate and high postoperative recurrence rate of HCC, the long-term prognosis of liver cancer is poor, and the 5-year survival rate is only about 12.1% (2, 3).
Due to the high sensitivity and specificity of computerized tomography(CT) or magnetic resonance imaging(MRI)for HCC detection, the diagnosis of liver cancer in most cases does not depend on pathological examination (4, 5). However, the microenvironment of liver is closely related to the occurrence and development of HCC, as it allows for a definitive diagnosis and provides prognostic information of patients (3). Ablation has similar efficacy to surgical for patients with early-stage HCC (6); and transarterial Chemoembolization (TACE) combined with ablation can effectively reduce the local blood supply of tumors and downstage tumors, and has unique advantages in preventing postoperative bleeding. TACE combined with ablation can make up for the disadvantages of TACE or ablation alone (7).
Liver microenvironment is of great significance for the occurrence and development of hepatocellular carcinoma. Therefore, this study aimed to investigate the predictive value of pathological indicators of HCC patients who received combined therapy.
2 Patients and materials
2.1 Study subjects
This is a retrospective study of 111 patients with HCC who received combination therapy from January 1, 2013 to December 31, 2016 at Beijing Youan Hospital (Beijing, China). Most of the patients were early-stage small HCC and all initial treatment patients. For the subsequent diagnosis and treatment plan and prognosis, all patients voluntarily underwent needle biopsy. Biopsy is generally performed under the guidance of ultrasound. Contraindications for percutaneous liver biopsy mainly include patients with bleeding tendency, patients with severe cardiopulmonary disease, massive ascites, severe extrahepatic obstructive jaundice, lack of consciousness, inability to cooperate, suspected hemangioma or other vascular tumors, and suspected echinococcus cyst in the liver. All patients participating in the study were required to meet the following inclusion criteria: 1) age between 18 and 75 years old; 2) the combination of TACE plus ablation is the primary treatment method; 3) Child-Pugh class A or B; 4) no other malignancies that may affect prognosis; 5) all patients underwent core needle biopsy before combined therapy; and 6) HBV-associated HCC. The exclusion criteria were described below: 1) imaging evidence of invasion of the main branches of the portal/hepatic veins; 2) presence of extrahepatic metastases; 3) severe coagulation disorders; 4) incomplete ablation; 5) secondary liver cancer; 6) co-infection with HCV; and 7) missed follow-up examinations Figure 1.
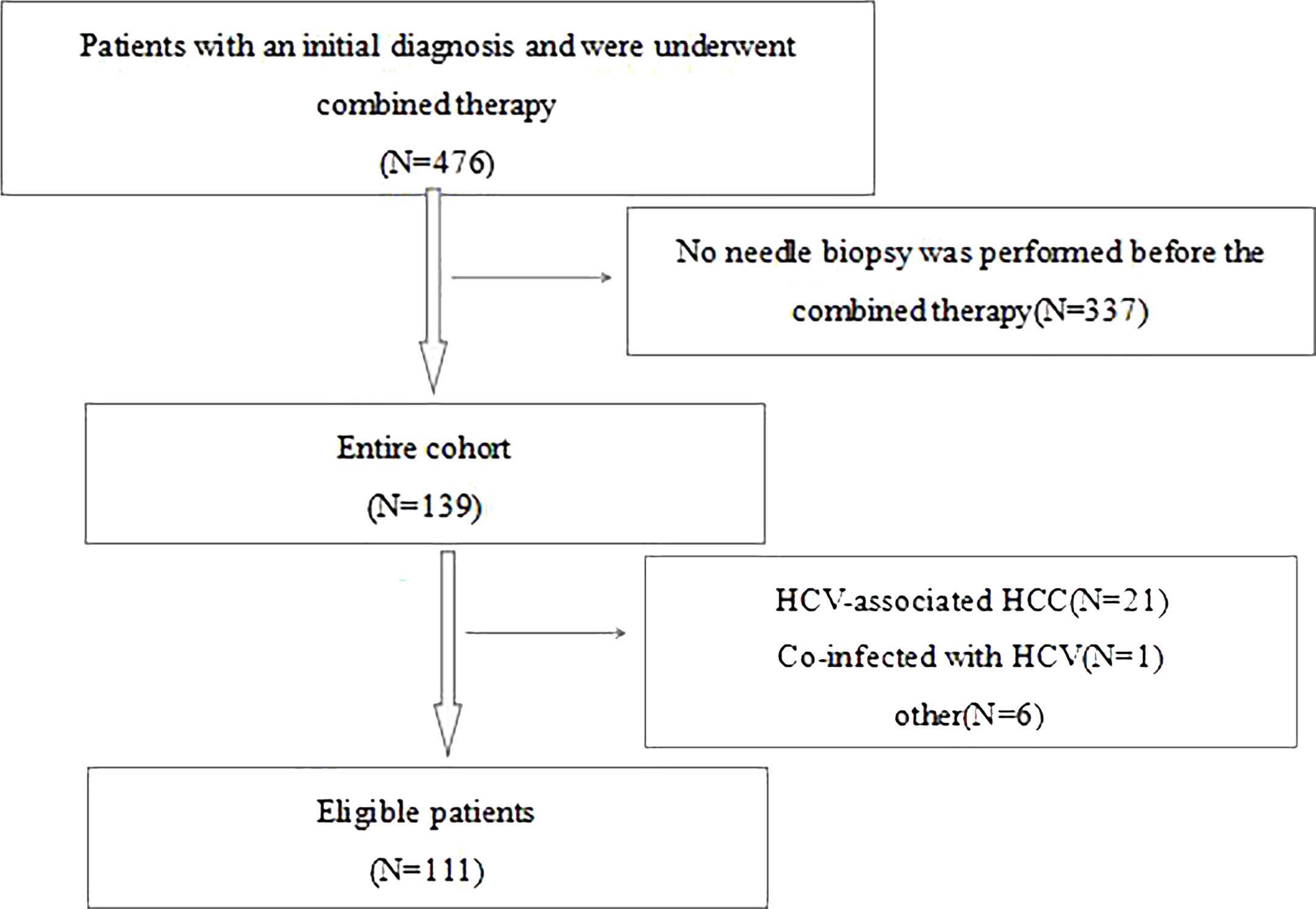
Figure 1 Flowchart of study participants. HCC, hepatocellular carcinoma; hepatitis B virus; HCV, hepatitis C virus.
Demographic information, pathological indicators and clinical laboratory data were collected: 1) demographic and etiology indicators, such as age, sex, history of hypertension and somking, serum HBsAg; 2) tumor-related indices, such as the number and size of tumors, and alpha-fetoprotein (AFP) level; 3) liver function indices, including cirrhosis, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total serum bilirubin (TBIL), serum albumin, globulin, and γ-glutamyl transpeptidase(γ-GT); 4) routine blood examinations, such as neutrophil count (NEU), lymphocyte count (LYM), platelet count (PLT); 5) blood coagulation indicators: including prothrombin time (PT), prothrombin activity (PTA), fibrinogenic (Fib); and 6) pathological indicators: Ki67, P53, HBsAg, Glypican-3 (GPC3), the degree of tumor differentiation. The study was conducted in accordance with the 1964 Declaration of Helsinki and approved by the Ethics Committee of You’an Hospital in Beijing. Information on these patients was kept confidential. As a minimal risk study, we dropped the requirement for informed consent.
2.2 Therapeutic methods
Combined therapy was performed by three qualified radiologists and hepatologists with more than 5 years of experience. The femoral artery was punctured using a modified Seldinger method, in which a microcatheter was inserted into the supplying artery of the tumor and doxorubicin (Pfizer, USA) and lipiodol (Gabcod, France) were injected. The interruption of blood flow in the tumor supplying artery was considered complete embolization.Local ablation was performed within 2 weeks after TACE. After the patient has underwent local anesthesia,the procedure is performed percutaneously by hepatologists under the guidance of triphasic computed tomography (CT) or magnetic resonance imaging (MRI). According to the number and size of tumor, overlapping ablation, multi-site ablation and fractional ablation were performed respectively. To ensure complete ablation, a safe distance of 0.5-1.0cm should be maintained around the tumor.
In order to ensure complete ablation of large tumors and multiple tumors, we use the combined treatment of TACE plus ablation, first reducing the target lesion by TACE to facilitate the subsequent complete ablation.On the one hand, TACE can block the blood supply and mark the tumor; on the other hand, TACE is beneficial for subsequent ablation; ablation therapy can further inactivate residual lesions, and ablation treatment is a minimally invasive, efficient and reproducible treatment, so for patients difficult to complete primary ablation, repeated ablation is used to achieve complete ablation.
2.3 Follow-up
Patients follow-up were performed on outpatient clinic. Follow-up consisted of physical examination, blood tests, and imaging examination, which included abdominal ultrasound every 3-6 months and ontrast-enhanced CT/MRI every 6 months. The follow-up involved a physical examination and blood tests, as well as imaging, included an abdominal ultrasound every 3–6 months and contrast-enhanced CT/MRI every 6 months. Recurrence was defined as intrahepatic local progression, distal intrahepatic recurrence and extrahepatic metastases. RFS was calculated as the time between the date of ablation and the patient’s first evidence-based recurrence or death in patients without evidence of disease recurrence, whereas OS was defined as the time between the date of ablation and tumor-related death or the date of the last visit. The cut-off date for this study is July 1, 2020. Patients were treated with radiofrequency ablation or TACE when they were found to have tumor recurrence. Contrast-enhanced CT and/or MRI is used to determine if a patient has recurrence. Recurrence is considered when a patient’s imaging shows enhancement of areas within or around the primary tumor.
2.4 Statistical analysis
Data were analyzed using SPSS 26.0 software (IBM, Armonk, NY, USA), and all figures were created by SPSS and Graphpad Prism 8.0(Graphpad software Inc). Continuous variables were expressed as the mean ± standard deviation (SD), and categorical data were presented as the frequency. Univariate and multivariate Cox regression analyses were performed to assess the independent risk factors of prognosis in HCC patients undergoing combined therapy. The RFS and OS rates were calculated with the Kaplan-Meier method, and the differences between groups were compared by using the Log-rank test. Statistical significance was considered when the P value < was 0.05.
3 Results
3.1 Baseline characteristics and follow-up results
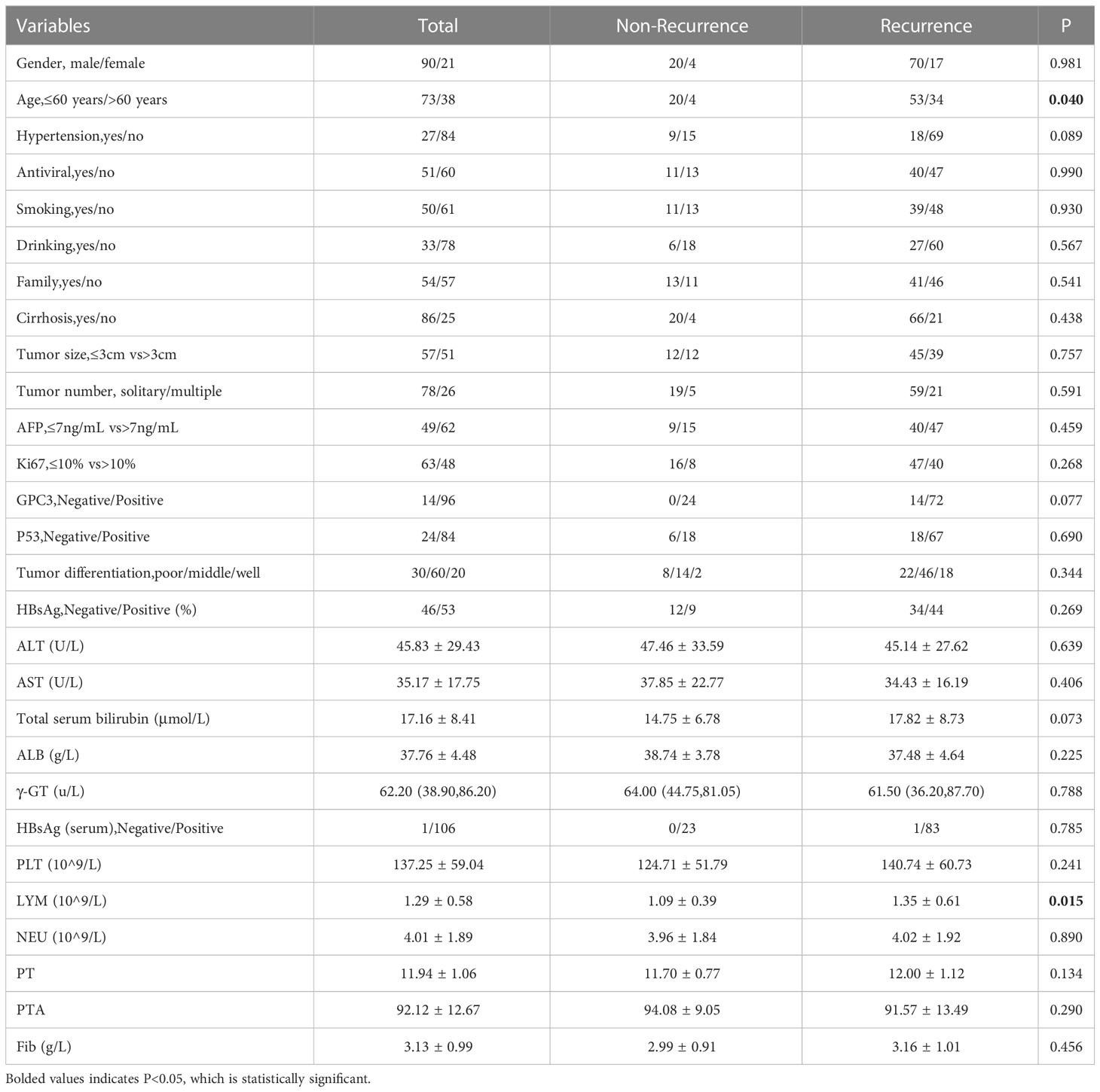
The present study consisted of 90 men (81.1%) and 21 women (18.9%) with a mean age of 56 years ± 9 years. Additionally, 27 patients (24.3%) had hypertension, 55 patients (49.5%) were received antiviral therapy before they underwent combined therapy. There were 50 patients (45.0%) who had a history of smoking and 33 patients (29.7%) with a history of drinking. By the end of follow-up, 88 (79.3%) recurred and 43 (38.7%) died. The median follow-up duration was 52.2 (36.4-64.9) months. The 1-,3- and 5-year RFS rates were 60.4% (67/111), 29.7% (33/111),and 20.7%(23/111). Moreover, the 1-, 3-, and 5-year cumulative OS rates were 100%(111/111), 73.0% (81/111) and 61.3% (68/111), respectively. There were differences in age (P=0.040) and lymphocyte count (P=0.015) between the recurrence group and the non-recurrence group (Table 1).
3.2 Indicators that correlate with RFS
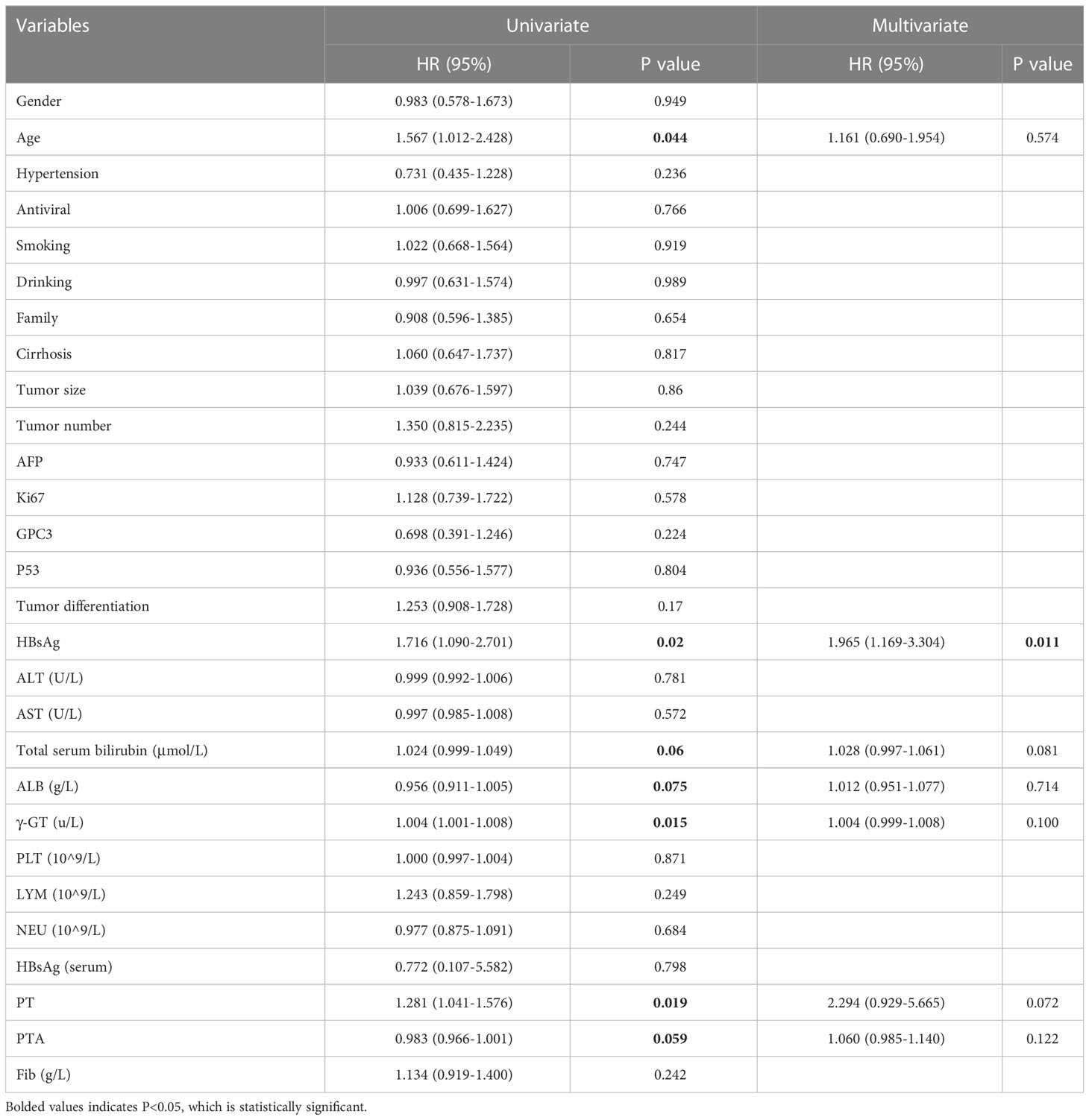
Correlations between demographic and pathological indicators and clinical laboratory data and RFS were assessed with univariate and multifactorial analyses. The univariate analysis demonstrated that RFS was significantly associated with age, intrahepatic HBsAg, albumin, total serum bilirubin, PT and PTA. The multivariate analysis showed that intrahepatic HBsAg expression (HR: 1.965; 95%CI: 1.169-3.304) was an independent predictor of HCC recurrence (P<0.05) (Table 2)
3.3 Indicators that correlate with OS
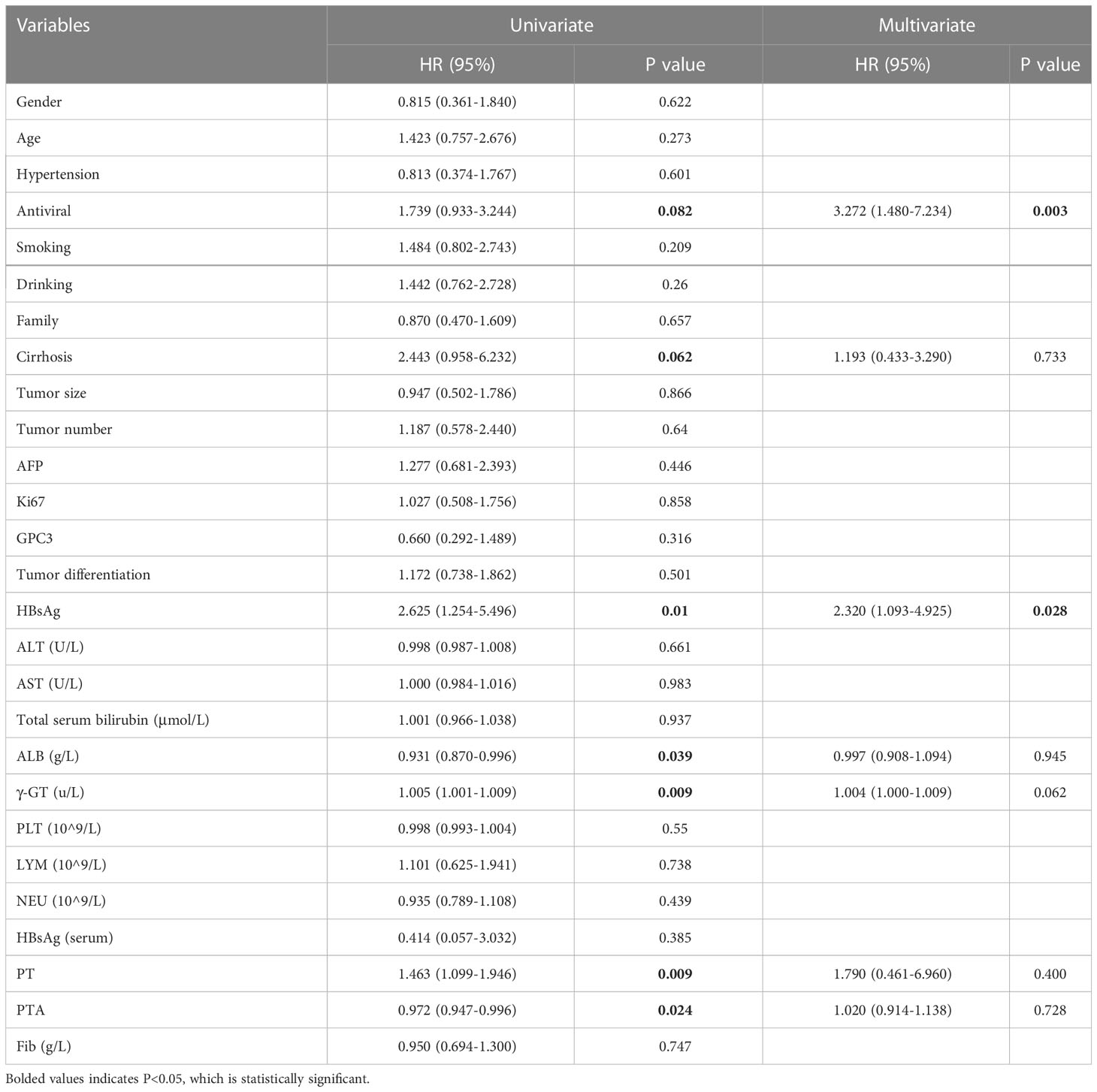
Univariate and multivariate analyses were performed to assess the relationship between demographic, pathological indicators and clinical laboratory data and OS. Univariate analysis showed that OS was significantly correlated with cirrhosis, antiviral, intrahepatic HBsAg expression, GGT, PT and PTA. The multivariate analysis showed that intrahepatic HBsAg expression (HR:2.320;95%CI:1.093~4.925)and antiviral(HR:3.272;95%CI:1.480~7.234) were an independent predictor of HCC survival status (P<0.05) (Table 3).
3.4 Analysis of clinical and prognostic data based on intrahepatic HBsAg expression
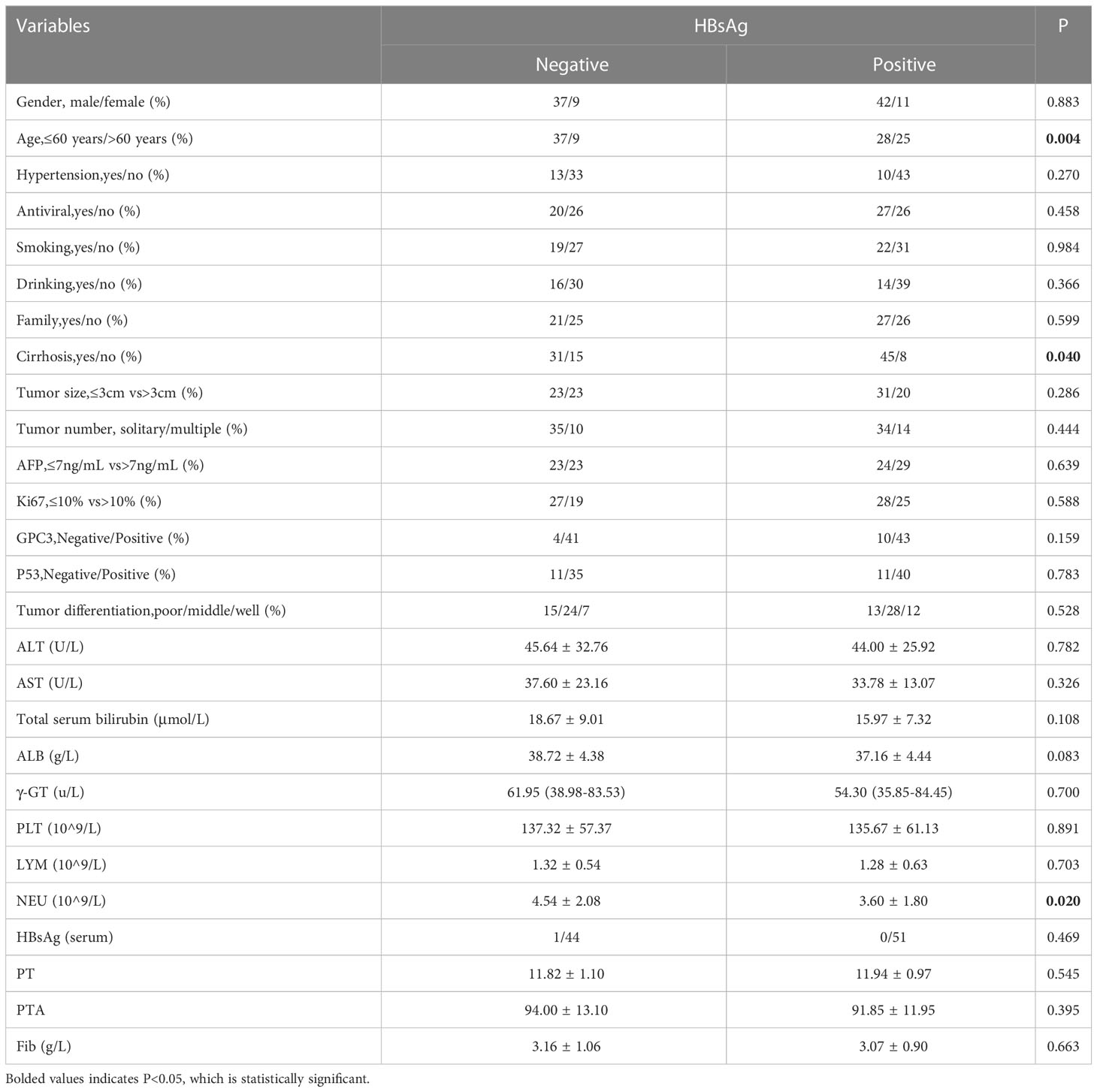
To investigate the influence of intrahepatic HBsAg expression on patient prognosis, patients were divided into two groups according to the expression of intrahepatic HBsAg; one group was positive intrahepatic HBsAg expression, and the other one was negative intrahepatic HBsAg expression. Statistical analysis showed that the age (P=0.004), cirrhosis (P=0.040) and neutrophil count (P=0.020) were significant difference between the two group (Table 4; Figure 2).
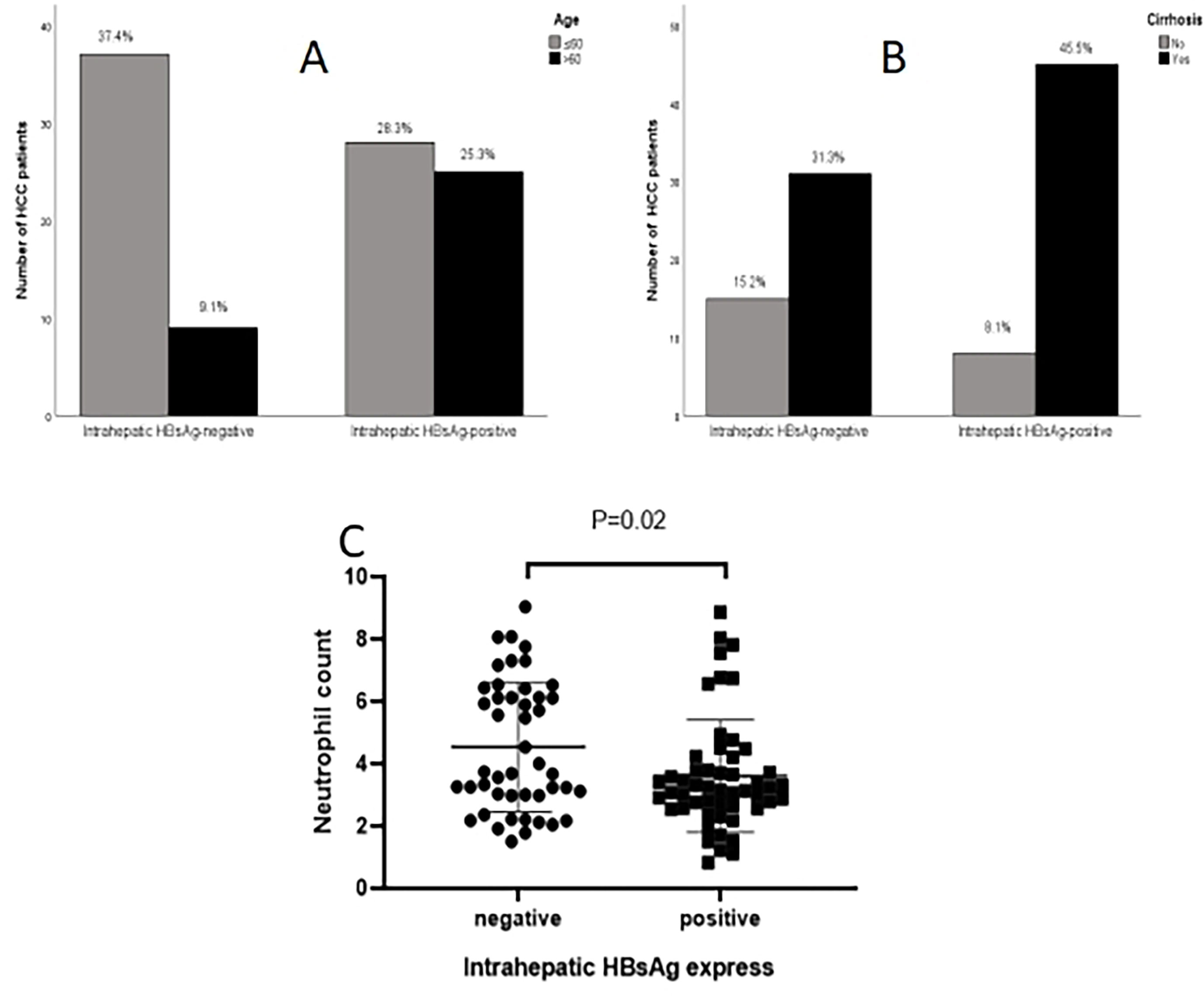
Figure 2 Comparison of clinical data based on intrahepatic HBsAg. (A) Age; (B) Cirrhosis; (C, D) neutrophil count.
Kaplan-meier analysis confirmed that positive intrahepatic HBsAg expression was a negative predictor of RFS and OS. The cumulative 1-, 3-, and 5-year RFS rates for patients with negative HBsAg expression after combined therapy were 78.3%, 43.5% and 30.4%; while for patients with positive HBsAg expression were 58.5%, 24.5% and 17.0%, respectively (P=0.018).(Figure 3) Meanwhile, the cumulative 1-, 3-, and 5-year OS rates for patients with negative HBsAg expression after combined therapy the were 100%, 89.1% and 80.4%, while, for patients with positive HBsAg expression were 100%, 75.5% and 58.5%, respectively (P=0.008) (Figure 4).
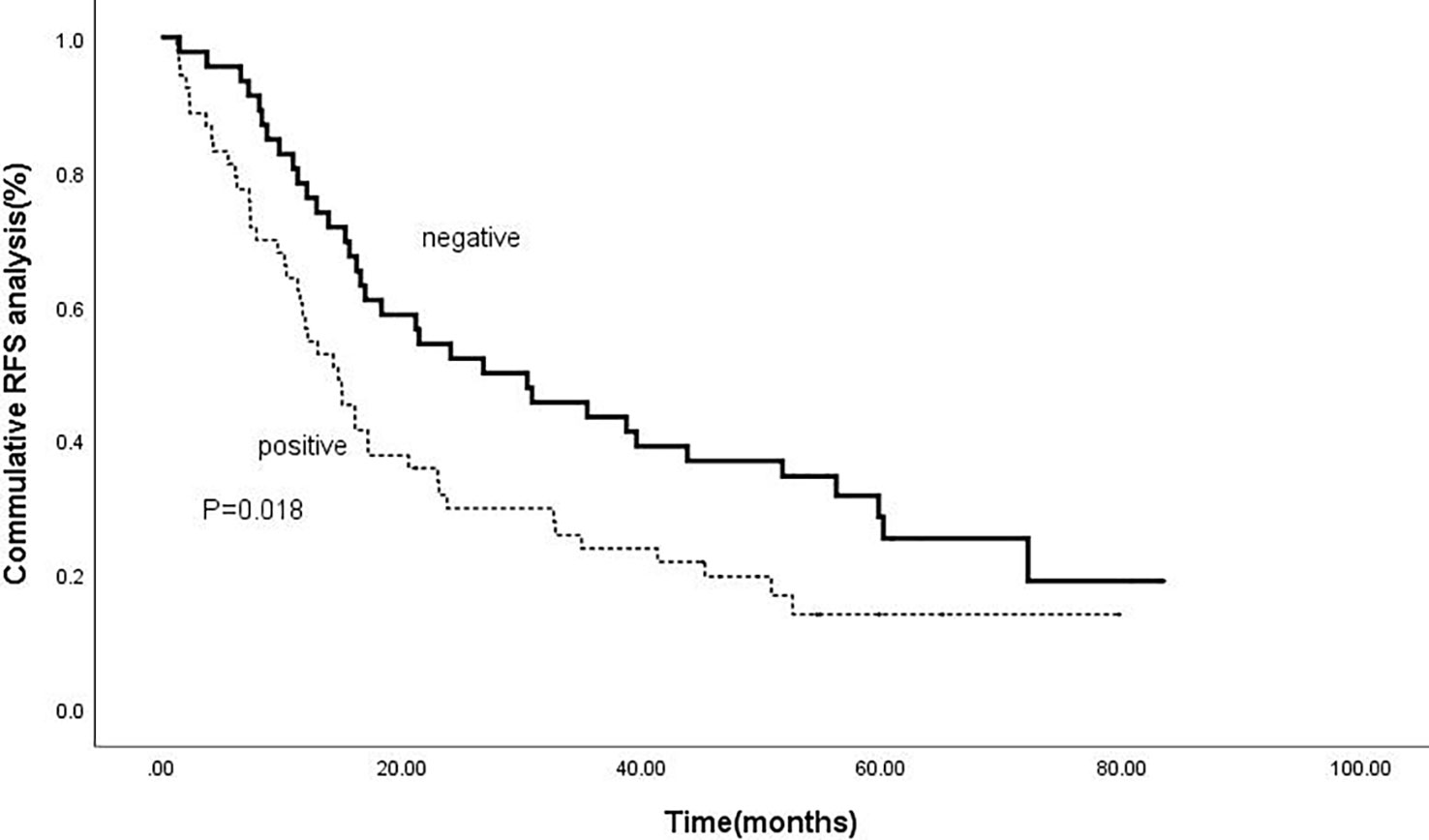
Figure 3 Comparison of RFS based on intrahepatic HBsAg. The cumulative 1-, 3-, and 5-year RFS rates for patients with negative HBsAg expression after combined therapy were 78.3%, 43.5% and 30.4%; while for patients with positive HBsAg expression were 58.5%, 24.5% and 17.0%, respectively (P=0.018).
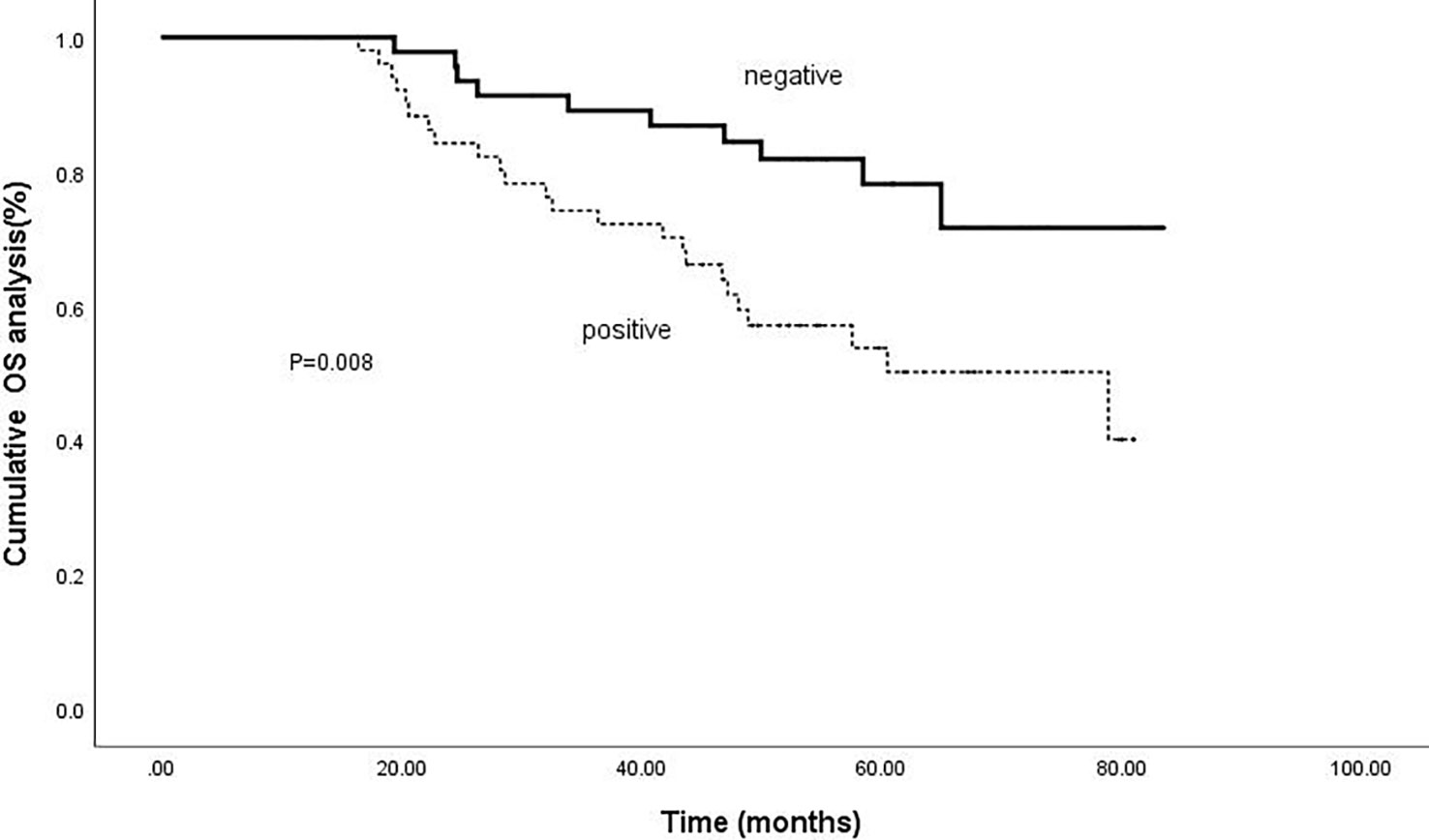
Figure 4 Comparison of OS based on intrahepatic HBsAg. The cumulative 1-, 3-, and 5-year OS rates for patients with negative HBsAg expression after combined therapy the were 100%, 89.1% and 80.4%; for patients with positive HBsAg expression were 100%, 75.5% and 58.5%, respectively (P=0.008).
4 Discussion
The incidence of HCC is very high and increasing in developing countries, especially in China. And its incidence and mortality rates continue to increase (8). It has been reported that even in patients with early-stage (BCLC-0/A), the 5-year recurrence rate is 50-85% (9–13). As a result, HCC has increased the burden of medical care in our country and become a serious health problem (14).
The results of this study suggest that antiviral treatment was an independent risk factor for OS. A number of previously conducted studies indicated that HBV reactivation is the main risk factor for liver cancer recurrence (15–17), postoperative antiviral therapy can reduce viral load and liver inflammation, improve liver function and the prognosis of patients, and reduce HCC recurrence and mortality. The current research showed that antiviral treatment can effectively improve the outcomes of patients who received surgical and ablation therapy while improve the survival rate of patients (18).
HBV infection to the progression of cancer is a multi-step process (19), HBsAg promotes the proliferation of HCC cells through the activation of the Src/pi3k/Akt pathway (20). Chronic inflammation caused by chronic viral infection leads to changes in the liver microenvironment and increases increase the risk of HCC development. The results of this study confirm that intrahepatic HBsAg positive expression is an independent risk factor for predicting OS and RFS in patients who received combined therapy. The study also showed that the OS and RFS of negative express patients were better than those with positive HBsAg expression.
The results of this study showed there was no correlation between HBsAg in serum and HBsAg in liver tissues. This is consistent with the results of the studies reported in previous articles (21). However, the results of the present study indicated that serum HBsAg expression is associated with intrahepatic HBsAg expression, the paper also suggested that not all serum HBsAg expression was consistent with intrahepatic HBsAg expression (22), the negative correlations observed between low liver HBsAg levels and the increased expression of T- and B cell-activated genes may point toward a limited HBsAg-induced immune exhaustion and enhanced immune control and/or a higher proportion of leukocytes in the livers of these patients. Therefore, for patients with positive intrahepatic HBsAg expression, follow-up strategies should be enhanced to monitor tumor progression more closely and to help physicians take timely interventions to decrease the recurrence rate and improve the long-term prognosis of patients.
The prognosis of HCC patients is still poor. Thus, it is crucial to explore the biological indicators that can predict patients’ prognosis and make the corresponding clinical decisions according to the patients’ situation. In addition, this study is a single-center study while also a small sample study, thus required more centers and a larger sample sizes participation in the validation.
5 Conclusions
Intrahepatic HBsAg positive expression is associated with poor prognosis of HCC patients who underwent combined therapy. Accordingly, for patients with positive HBsAg express in liver tissue, corresponding clinical decisions should be made to improve patients the long-term prognosis of patients.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The data used to support the findings are available from the corresponding author upon request. Requests to access these datasets should be directed to emhhbmd5aEBjY211LmVkdS5jbg==.
Ethics statement
The study has been approved by the ethics committee of the Beijing You’an Hospital affiliated to Capital Medical University. As a minimum risk study that was in accordance with the Helsinki protocol, the requirement for patients’ informed consent was waived by the same ethics committee that approved the study (Beijing You’an Hospital affiliated to Capital Medical University), and all methods were carried out in accordance with relevant guidelines and regulations. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
YZ conceived and designed the protocol. JZ and YZ collected the data. BL and QW wrote the manuscript. TM and KL analyzed the data. WG and CY critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by a grant Beijing Municipal Natural Science Foundation (7191004), Capital health development project (2020-1-2182 and 2020-2-1153), Beijing Key Laboratory (BZ0373), Key medical professional development plan of Beijing municipal administration of hospitals (ZYLX201711), and Beijing Municipal Administration of Hospitals’ Incubating Program (PX2018059).
Acknowledgments
The authors highly appreciate all the patients who were involved in the present study and our team from Beijing You’an Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Villanueva A. Hepatocellular carcinoma. N Engl J Med (2019) 380(15):1450–62. doi: 10.1056/NEJMra1713263
3. Calderaro J, Ziol M, Paradis V, Zucman-Rossi J. Molecular and histological correlations in liver cancer. J Hepatol (2019) 71(3):616–30. doi: 10.1016/j.jhep.2019.06.001
4. Vogel A, Cervantes A., Chau I., Daniele B., Llovet J. M., Meyer T., et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2018) 29(Suppl 4):iv238–55. doi: 10.1093/annonc/mdy308
5. European Association for the Study of the Liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol (2018) 69(1):182–236. doi: 10.1016/j.jhep.2018.03.019
6. Chen Z, Xie H, Hu M, Huang T, Hu Y, Sang N, et al. Recent progress in treatment of hepatocellular carcinoma. Am J Cancer Res (2020) 10(9):2993–3036.
7. Xu Z, Xie H, Zhou L, Chen X, Zheng S. The combination strategy of transarterial chemoembolization and radiofrequency ablation or microwave ablation against hepatocellular carcinoma. Anal Cell Pathol (Amst) (2019) 2019:8619096. doi: 10.1155/2019/8619096
8. Bruix J, Boix L, Sala M, Llovet JM. Focus on hepatocellular carcinoma. Cancer Cell (2004) 5(3):215–9. doi: 10.1016/S1535-6108(04)00058-3
9. Bruix J, Rei M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology (2016) 150(4):835–53. doi: 10.1053/j.gastro.2015.12.041
10. Sun P, Yang X, He RQ, Hu QG, Song ZF, Xiong J, et al. Antiviral therapy after curative treatment of hepatitis B/C virus-related hepatocellular carcinoma: A systematic review of randomized trials. Hepatol Res (2014) 44(3):259–69. doi: 10.1111/hepr.12115
11. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet (2003) 362(9399):1907–17. doi: 10.1016/S0140-6736(03)14964-1
12. Kudo M. Adjuvant therapy after curative treatment for hepatocellular carcinoma. Oncology (2011) 81 Suppl 1:50–5. doi: 10.1159/000333259
13. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology (2011) 53(3):1020–2. doi: 10.1002/hep.24199
14. Liu CJ, Ma XW, Zhang XJ, Shen SQ. Pri-miR-34b/c rs4938723 polymorphism is associated with hepatocellular carcinoma risk: a case-control study in a Chinese population. Int J Mol Epidemiol Genet (2017) 8(1):1–7.
15. Jang JW, Kwon JH, You CR, Kim JD, Woo HY, Bae SH, et al. Risk of HBV reactivation according to viral status and treatment intensity in patients with hepatocellular carcinoma. Antivir Ther (2011) 16(7):969–77. doi: 10.3851/IMP1840
16. Ko CJ, Chien SY, Chou CT, Chen LS, Chen ML, Chen YL. Factors affecting prognosis of small hepatocellular carcinoma in Taiwanese patients following hepatic resection. Can J Gastroenterol (2011) 25(9):485–91. doi: 10.1155/2011/790528
17. Ou DP, Yang L. Y., Huang G. W., Tao Y. M., Ding X., Chang Z. G.. Clinical analysis of the risk factors for recurrence of HCC and its relationship with HBV. World J Gastroenterol (2005) 11(14):2061–6. doi: 10.3748/wjg.v11.i14.2061
18. Qi W, Zhang Q, Xu Y, Wang X, Yu F, Zhang Y, et al. Peg-interferon and nucleos(t)ide analogue combination at inception of antiviral therapy improves both anti-HBV efficacy and long-term survival among HBV DNA-positive hepatocellular carcinoma patients after hepatectomy/ablation. J Viral Hepat (2020) 27(4):387–96. doi: 10.1111/jvh.13236
19. Xu L, Xiao H, Xu M, Zhou C, Yi L, Liang H. Glioma-derived T cell immunoglobulin- and mucin domain-containing molecule-4 (TIM4) contributes to tumor tolerance. J Biol Chem (2011) 286(42):36694–9. doi: 10.1074/jbc.M111.292540
20. Liu H, Xu J, Zhou L, Yun X, Chen L, Wang S, et al. Hepatitis b virus large surface antigen promotes liver carcinogenesis by activating the Src/PI3K/Akt pathway. Cancer Res (2011) 71(24):7547–57. doi: 10.1158/0008-5472.CAN-11-2260
21. Guner R, Karahocagil M, Buyukberber M, Kandemir O, Ural O, Usluer G, et al. Correlation between intrahepatic hepatitis b virus cccDNA levels and other activity markers in patients with HBeAg-negative chronic hepatitis b infection. Eur J Gastroenterol Hepatol (2011) 23(12):1185–91. doi: 10.1097/MEG.0b013e32834ba13a
Keywords: prognosis, HBsAg, interventional therapy, hepatocellular carcinoma, pathology
Citation: Liu B, Wang Q, Mei T, Zheng J, Gao W, Yuan C, Li K and Zhang Y (2023) Effect of HBsAg expression in liver tissue on prognosis of hepatocellular carcinoma after minimally invasive interventional therapy. Front. Oncol. 13:1106333. doi: 10.3389/fonc.2023.1106333
Received: 23 November 2022; Accepted: 21 February 2023;
Published: 09 March 2023.
Edited by:
Tommaso Maria Manzia, University of Rome Tor Vergata, ItalyReviewed by:
Shuanggang Chen, Yuebei People’s Hospital, ChinaHao Xing, Second Military Medical University, China
Copyright © 2023 Liu, Wang, Mei, Zheng, Gao, Yuan, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonghong Zhang, emhhbmd5aEBjY211LmVkdS5jbg==
†These authors have contributed equally to this work
 Biyu Liu
Biyu Liu Qi Wang
Qi Wang Tingting Mei1
Tingting Mei1 Chunwang Yuan
Chunwang Yuan Kang Li
Kang Li Yonghong Zhang
Yonghong Zhang