- Department of Dermatology, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Dermatofibrosarcoma protuberans is a rare, locally aggressive, slowly growing cutaneous fibroblastic sarcoma with a high recurrence rate and low metastatic potential. Atrophic dermatofibrosarcoma protuberans is a rare variant usually presents as atrophic plaques, easily neglected and misdiagnosed as benign lesions by patients and dermatologists. Here we report two cases of atrophic dermatofibrosarcoma protuberans, one of which was accompanied by pigment, and review other cases have been reported in the literature. Understanding the most up-to-date literature and early identification of these dermatofibrosarcoma protuberans variants can help clinicians avoid delayed diagnosis and improve prognosis.
1 Introduction
Dermatofibrosarcoma protuberans (DFSP) is a slowly growing, aggressive dermal soft tissue tumor with a high recurrence rate and low metastatic potential (1). Classic DFSP often presents as a typical protuberant nodule and is histologically characterized by monomorphic spindle cells arranged in a storiform pattern (2). Although DFSP is a rare sarcoma with an incidence of 0.8 to 4.2 cases per million persons per year, it is nevertheless one of the most common cutaneous sarcomas (3). Atrophic dermatofibrosarcoma protuberans is a histopathological variant of DFSP, which presents as an asymptomatic depressed plaque. Atypical clinical presentation and indolent behavior are easily neglected by patients and clinicians, leading to delayed diagnosis. In this report, we describe two cases of atrophic DFSP, one of which also with hyperpigmentation, and review the literature to improve our understanding of the clinical and histopathological features of this unusual variant.
2 Case reports
2.1 Case 1
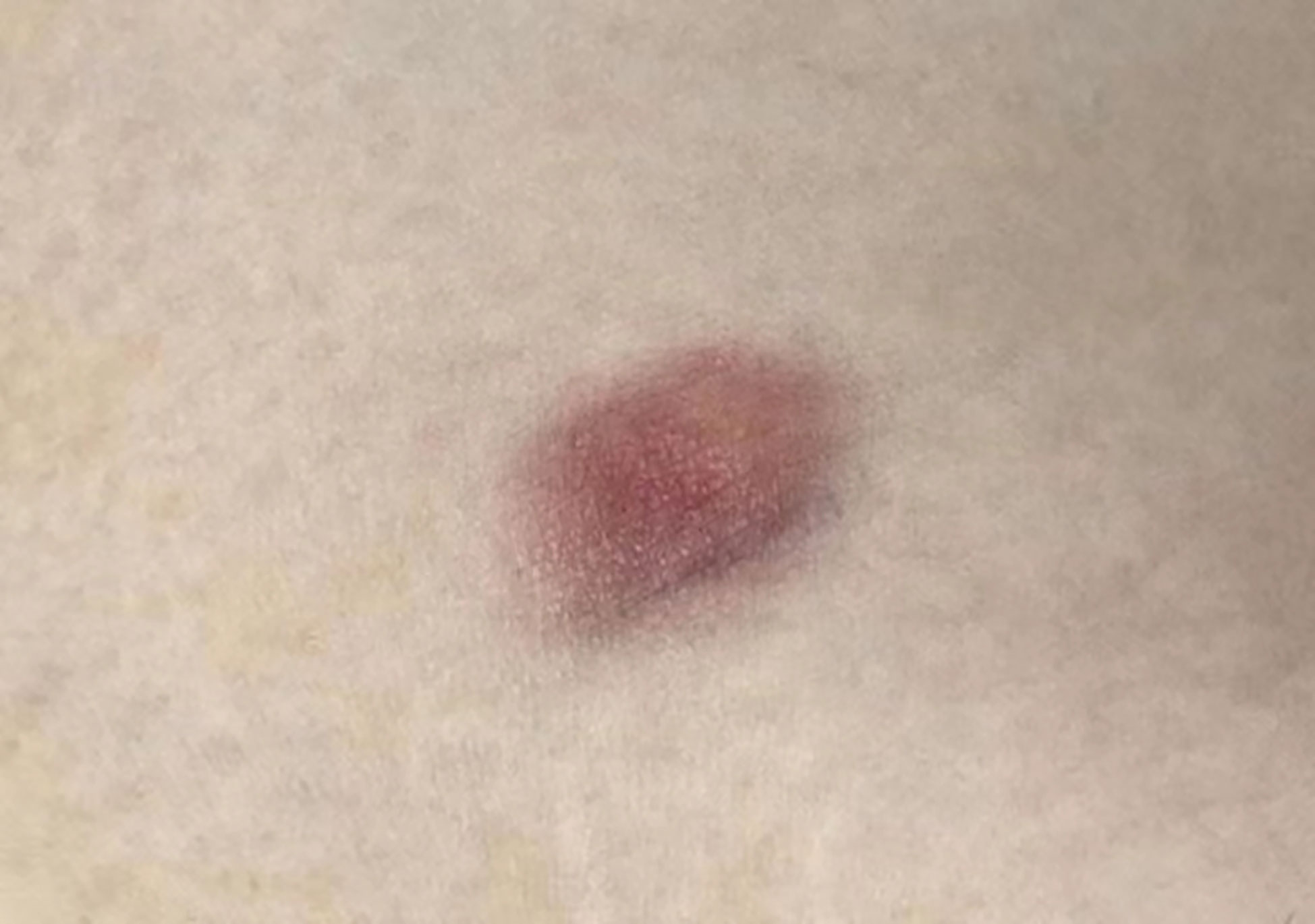
A 31-year-old woman attended our dermatology department to evaluate an erythematous plaque on her right chest that had been slowly progressing for four years. She was diagnosed as atrophic scar previously. Physical examination showed a 16×10 mm asymptomatic, smooth, atrophic plaque (Figure 1). Histopathologic examination revealed a monomorphic fibrohistiocytic spindle cell tumor arranged in a storiform pattern infiltrating the deep dermis and subcutaneous tissue forming honeycomb-like pattern (Figures 2A, B). Immunohistochemical analysis showed that cells were prominently positive for CD34 (Figure 2C) and negative for protein S-100. Next-generation sequencing (NGS) detected a fusion between exon 5 of COL1A1 and exon 2 of PDGFB. Based on these findings, the lesion was diagnosed as atrophic DFSP. The patient was treated by Mohs micrographic surgery and no recurrence was observed at the 1-year follow-up.
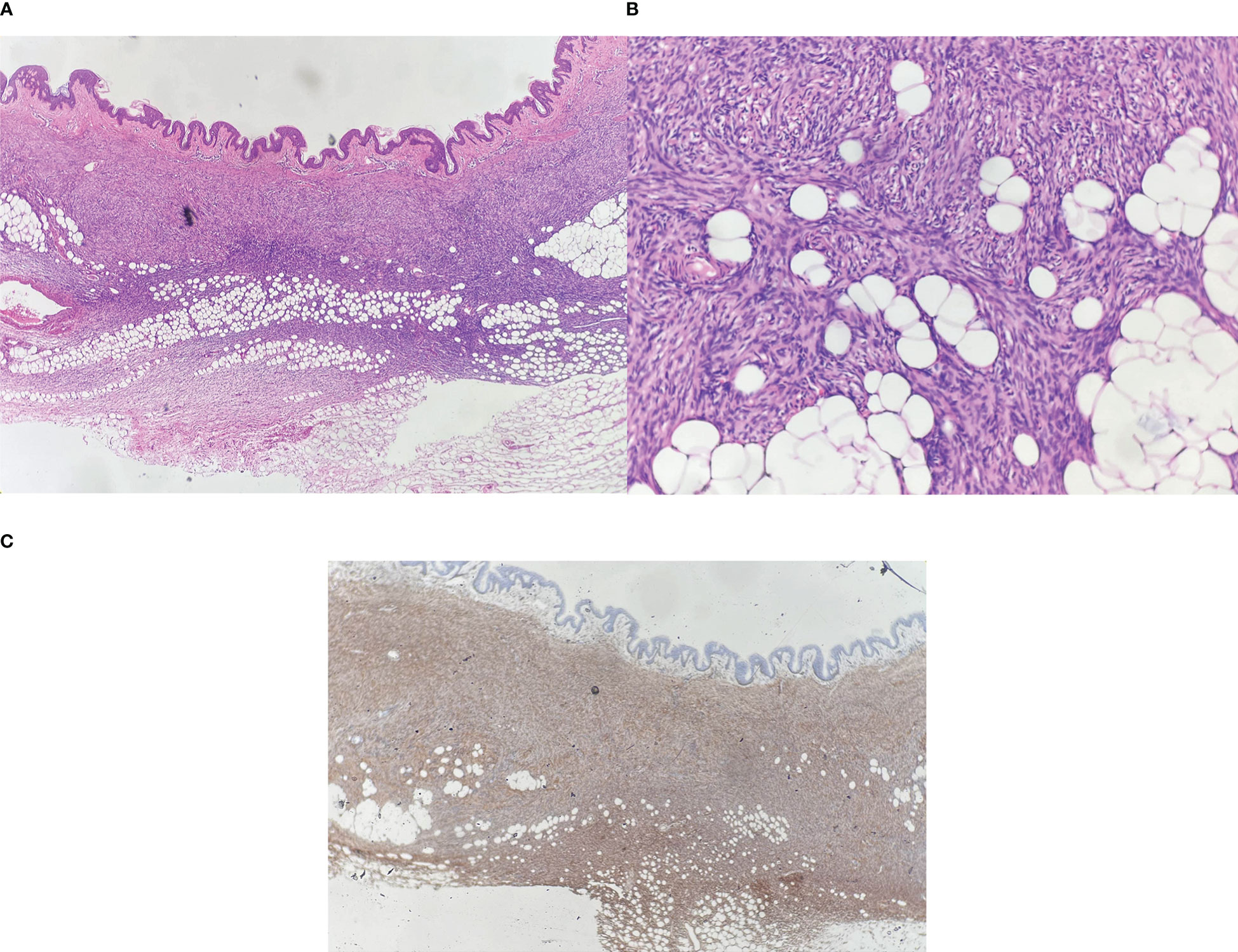
Figure 2 (A) Spindle cells infiltrate into the deep dermis and subcutaneous tissue, Hematoxylin-eosin (HE) ×40; (B) Dense proliferation of spindle cells in a storiform pattern, HE ×100; (C) Spindle cells are strongly positive for CD34, ×40.
2.2 Case 2
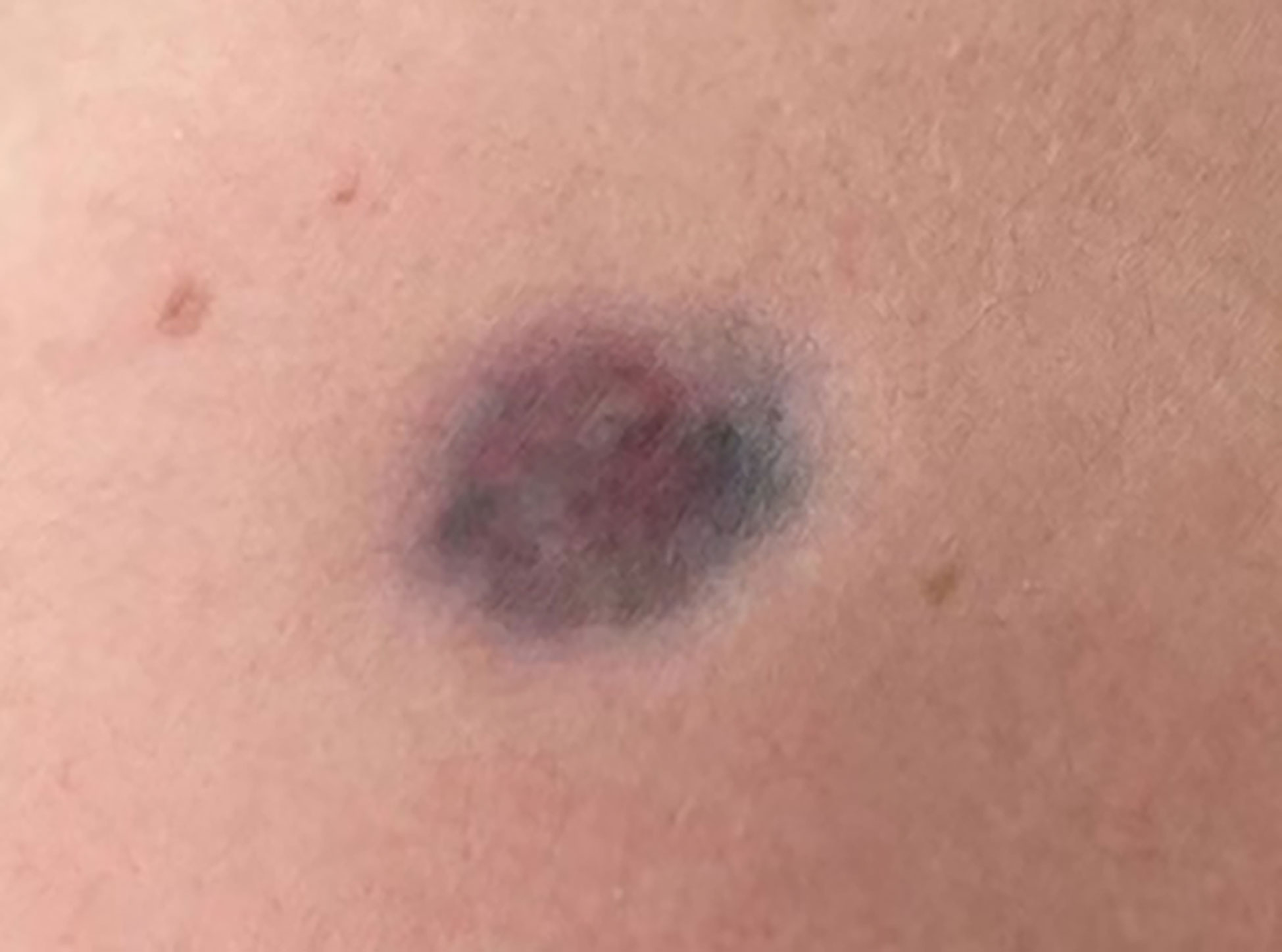
A 45-year-old man presented to our dermatology department with a 5-year history of a depressed plaque on his left shoulder. The lesion was asymptomatic and slowly enlarged. He was previously diagnosed as blue nevus and anetoderma in other clinics. Physical examination showed a 30×20 mm round erythematous-to-bluish plaque with atrophy (Figure 3). Histopathologic analysis revealed infiltration of spindle cells with slender wavy nuclei into the deep dermis, arranged in parallel or horizontally oriented fascicles. Cells containing brown pigment were scattered in the dermis (Figures 4A, B). Immunohistochemical staining showed that the spindle cells were diffusely positive for CD34 but negative for S-100 while melanin-laden dendritic cells were positive for S-100 (Figures 4C, D). NGS detected a fusion between exon 46 of COL1A1 and exon 2 of PDGFB. Based on the clinicopathologic correlation, a diagnosis of atrophic pigmented DFSP was made. Mohs micrographic surgery was performed to excise the lesion.
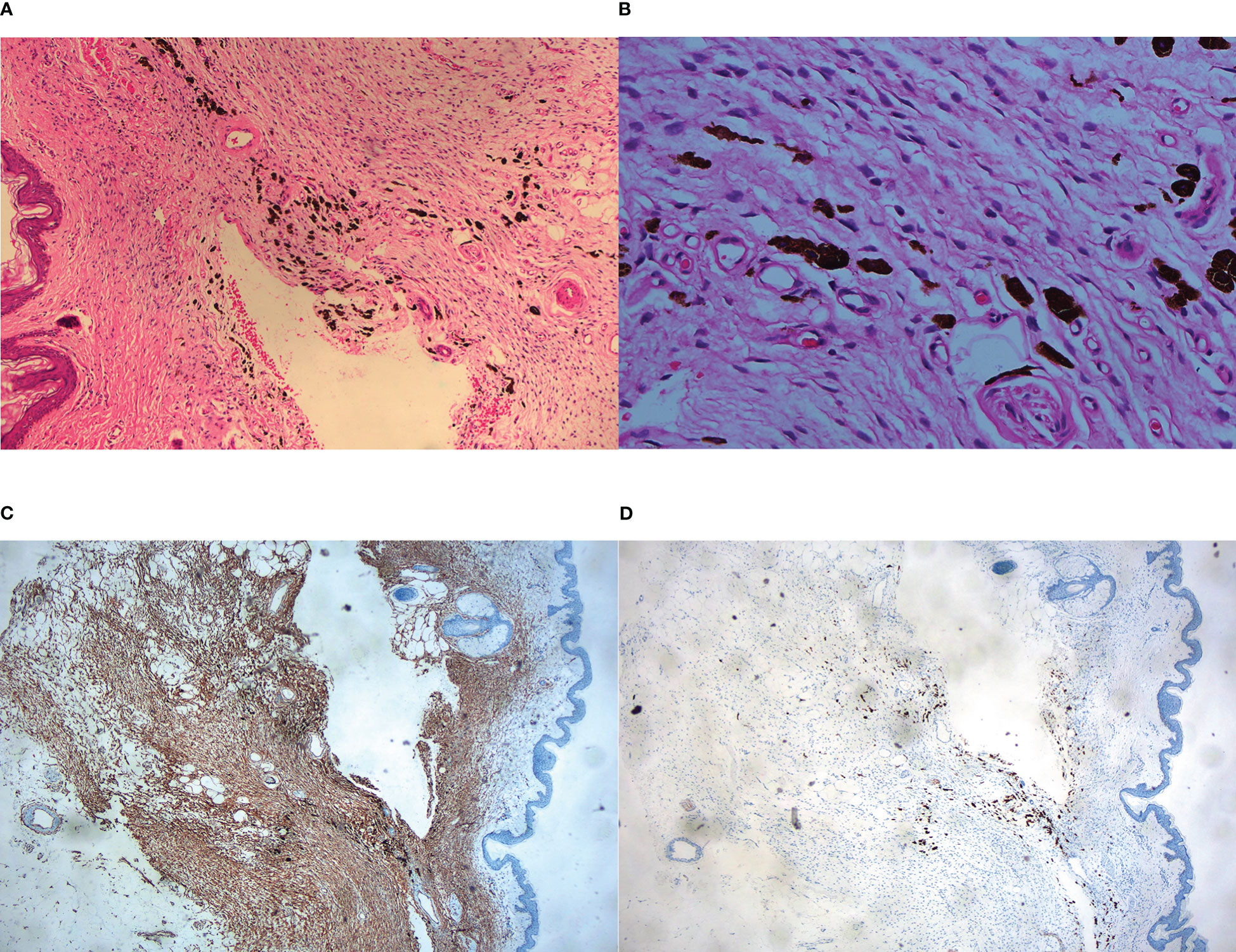
Figure 4 Histopathological examination. (A) Fascicles of spindle cells with reduced dermal thickness by about half on low-power view, HE ×40; (B) Monomorphic spindle cells with bland cytoplasm and scattered dendritic cells containing abundant melanin, HE ×200; (C) Immunohistochemistry showed CD34+ cells infiltrating into subcutis and forming honeycomb-like pattern, ×40; (D) Immunohistochemistry showed spindle cells were negative for S-100, in contrast, melanin-bearing dendritic cells were positive for S-100, ×100.
3 Discussion
Atrophic dermatofibrosarcoma protuberans is a rare variant accounting for 1.7% of all DFSP cases (4). It was first described by Lambert in 1985 (5). Unlike the classical DFSP, atrophic DFSP usually presents as a slow-growing, atrophic or sclerotic plaque, which is more easily misdiagnosed as morphea, sclerodermiform basal cell carcinoma, atrophic scar, scleroderma, anetoderma, atrophic dermatofibroma, resolving panniculitis and lipoatrophy (6). Histologically, in addition to the storiform pattern noted in classical DFSP, neoplastic spindle cells arranged in parallel or horizontally oriented fascicles are also seen in atrophic DFSP (4). On immunohistochemistry, all tumors showed diffuse and strong positivity of CD34. Due to the overlap of morphology and immunoprofile, atrophic DFSP should be differentiated from other spindle cell tumors, including dermatofibroma, solitary fibrous tumor, spindle cell/pleomorphic lipomas, neurofibroma, superficial CD34-positive fibroblastic tumor, and medallion-like dermal dendrocyte hamartoma (7).
Molecular analysis is used to detect the COL1A1-PDGFB fusion gene, which is specifically expressed in DFSP and not related to pathological subtypes. FISH and NGS are two main molecular techniques for detecting the fusion gene. FISH is a straightforward method performed with either fusion probes or break-apart probes, which has good sensitivity and specificity. However, it doesn’t provide detailed breakpoint information on both translocation partners. NGS is another technique for detecting fusion transcripts. Previous studies have shown that NGS was more sensitive than FISH for the COL1A1-PDGFB fusion detection, especially in borderline cases (8). Additionally, NGS can effectively detect all fusion genes and fusion partners, which can make up for the shortcomings of conventional detection methods, such as missed detection, false negatives, and the inability to distinguish fusion partners. However, there are also some challenges with data analysis and interpretation, analytical validation, high-quality sample tissues, and high testing costs (9).
Surgical excision is the gold standard for the primary treatment of DFSP with wide excision and margin-controlled removal (i.e. Mohs and slow Mohs) as viable options (10, 11). For cases with recurrent, unresectable, and metastatic tumors, radiation therapy and targeted immunotherapy such as tyrosine kinase inhibitors have been shown to be effective (12). Imatinib mesylate can competitively inhibit ATP binding to the PDGF-β receptor, which slows down kinase activity, limits the growth of the tumor, and promotes apoptosis (13).
Till now, 91 cases with atrophic DFSP, including ours, have been reported (Table 1). The age ranges from 7 months to 72 years with an average of 26.7. It seems like the atrophic variant most frequently occurs during the second to fifth decades of life (n=65), which is younger compared with classic DFSP. Children and congenital cases account for 34% of all cases (n=31). In contrast to classic DFSP has no sex bias (10), atrophic DFSP shows a preference for females with a F:M ratio of 3:1. Trunk and extremities are the most frequently involved sites (n=87), which are consistent with classic DFSP (1). The tumor size ranges from 0.5 to 13cm in greatest diameter. Clinical diagnosis includes morphea, morpheaform basal cell carcinoma, anetoderma, lymphocytoma, lipoatrophy, atrophic scar, and neurofibroma. Delayed diagnosis and misdiagnosis are common. 5/8 cases of atrophic pigmented DFSP were misdiagnosed as benign lesions, including nevus of Ota, postinflammatory hyperpigmentation, hemangioma, and lipoatrophy (20, 55, 61). A high index of suspicion and a broad differential diagnosis by dermatologists is necessary.
In summary, atrophic DFSP is a diagnostic challenge to both clinicians and pathologists due to its atypical clinical presentation. Because of the initial benign and indolent behavior, many patients only seek medical care years after its onset. Here, we present a case series to further characterize its clinical and pathological features and enhance recognition of atrophic DFSP. As with these cases, atrophic lesions with no apparent cause and no symptoms should be aware of atrophic DFSP in the early stage. Histopathologic, immunohistochemical, and molecular examinations are necessary to help reduce misdiagnosis and improve prognosis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
YL analyzed the clinical data and drafted the manuscript. ZC collected the data and reviewed literature. SN managed the patient. ZW designed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
This study was supported by Innovative Research Team of High-level Local Universities in Shanghai.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin (2019) 37(4):483–8. doi: 10.1016/j.det.2019.05.006
2. Mujtaba B, Wang F, Taher A, Aslam R, Madewell JE, Spear R, et al. Dermatofibrosarcoma protuberans: Pathological and imaging review. Curr Probl Diagn Radiol (2021) 50(2):236–40. doi: 10.1067/j.cpradiol.2020.05.011
3. Kohlmeyer J, Steimle-Grauer SA, Hein R. Cutaneous sarcomas. J Dtsch Dermatol Ges (2017) 15(6):630–48. doi: 10.1111/ddg.13249
4. Xu S, Zhao L, Wang J. Atrophic dermatofibrosarcoma protuberans: a clinicopathological study of 16 cases. Pathology (2019) 51(6):615–20. doi: 10.1016/j.pathol.2019.06.002
5. Lambert WC, Abramovits W, Gonzalez-Sevra A, Souchon E, Schwartz RA, Little WP Jr. Dermatofibrosarcoma non-protuberans: description and report of five cases of a morpheaform variant of dermatofibrosarcoma. J Surg Oncol (1985) 28(1):7–11. doi: 10.1002/jso.2930280104
6. Acosta AE, Vélez CS. Dermatofibrosarcoma protuberans. Curr Treat Options Oncol (2017) 18(9):56. doi: 10.1007/s11864-017-0498-5
7. Thway K, Noujaim J, Jones RL, Fisher C. Dermatofibrosarcoma protuberans: pathology, genetics, and potential therapeutic strategies. Ann Diagn Pathol (2016) 25:64–71. doi: 10.1016/j.anndiagpath.2016.09.013
8. Zhu R, Yan J, Li B, Tan F, Yan W, Shen J, et al. Determination of COL1A1-PDGFB breakpoints by next-generation sequencing in the molecular diagnosis of dermatofibrosarcoma protuberans. Exp Mol Pathol (2021) 122:104672. doi: 10.1016/j.yexmp.2021.104672
9. Cheng YW, Meyer A, Jakubowski MA, Keenan SO, Brock JE, Azzato EM, et al. Gene fusion identification using anchor-based multiplex PCR and next-generation sequencing. J Appl Lab Med (2021) 6(4):917–30. doi: 10.1093/jalm/jfaa230
10. Saiag P, Grob J-J, Lebbe C, Malvehy J, del Marmol V, Pehamberger H, et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur J Cancer (2015) 51(17):2604–8. doi: 10.1016/j.ejca.2015.06.108
11. Durack A, Gran S, Gardiner MD, Jain A, Craythorne E, Proby CM, et al. A 10-year review of surgical management of dermatofibrosarcoma protuberans. Br J Dermatol (2021) 184(4):731–9. doi: 10.1111/bjd.19346
12. Badhey AK, Tikhtman R, Tang AL. Management of dermatofibrosarcoma protuberans. Curr Opin Otolaryngol Head Neck Surg (2021) 29(4):278–82. doi: 10.1097/MOO.0000000000000721
13. Rutkowski P, Van Glabbeke M, Rankin CJ, Ruka W, Rubin BP, Debiec-Rychter M, et al. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials. J Clin Oncol (2010) 28(10):1772–9. doi: 10.1200/JCO.2009.25.7899
14. Sei Y, Kimura A, Kobayashi H, Ishikura N, Yasuda Y. Dermatofibrosarcoma protuberans representing a depressed lesion. Skin Res (1986) 28(4):638–43. doi: 10.11340/SKINRESEARCH1959.28.638
15. Page EH, Assaad DM. Atrophic dermatofibroma and dermatofibrosarcoma protuberans. J Am Acad Dermatol (1987) 17(6):947–50. doi: 10.1016/S0190-9622(87)70283-7
16. McKee PH, Fletcher CD. Dermatofibrosarcoma protuberans presenting in infancy and childhood. J Cutan Pathol (1991) 18(4):241–6. doi: 10.1111/j.1600-0560.1991.tb01230.x
17. Ashack RJ, Tejada E, Parker C, Hanke CW. A localized atrophic plaque on the back. dermatofibrosarcoma protuberans (DFSP) (Atrophic variant). Arch Dermatol (1992) 128(4):549, 52. doi: 10.1001/archderm.1992.01680140133020
18. Annessi G, Cimitan A, Girolomoni G, Giannetti A. Congenital dermatofibrosarcoma protuberans. Pediatr Dermatol (1993) 10(1):40–2. doi: 10.1111/j.1525-1470.1993.tb00011.x
19. Zelger BW, Ofner D, Zelger BG. Atrophic variants of dermatofibroma and dermatofibrosarcoma protuberans. Histopathology (1995) 26(6):519–27. doi: 10.1111/j.1365-2559.1995.tb00270.x
20. Chuan MT, Tsai TF, Wu MC, Wong TH. Atrophic pigmented dermatofibrosarcoma presenting as infraorbital hyperpigmentation. Dermatology (1997) 194(1):65–7. doi: 10.1159/000246061
21. Bouyssou-Gauthier ML, Labrousse F, Longis B, Bedane C, Bernard P, Bonnetblanc JM. Dermatofibrosarcoma protuberans in childhood. Pediatr Dermatol (1997) 14(6):463–5. doi: 10.1111/j.1525-1470.1997.tb00691.x
22. Kobayashi T, Hasegawa Y, Konohana A, Nakamura N. A case of bednar tumor. immunohistochemical positivity for CD34. Dermatology (1997) 195(1):57–9. doi: 10.1159/000245689
23. Fujimoto M, Kikuchi K, Okochi H, Furue M. Atrophic dermatofibrosarcoma protuberans: a case report and review of the literature. Dermatology (1998) 196(4):422–4. doi: 10.1159/000017936
24. Davis DA, Sánchez RL. Atrophic and plaquelike dermatofibrosarcoma protuberans. Am J dermatopathol (1998) 20(5):498–501. doi: 10.1097/00000372-199810000-00014
25. Martin L, Combemale P, Dupin M, Chouvet B, Kanitakis J, Bouyssou-Gauthier ML, et al. The atrophic variant of dermatofibrosarcoma protuberans in childhood: a report of six cases. Br J Dermatol (1998) 139(4):719–25. doi: 10.1046/j.1365-2133.1998.02476.x
26. See AC, Kossard SS, Murrell DF. Guess what. dermatofibrosarcoma protuberans presenting as an atrophic red plaque. Eur J Dermatol (2001) 11(2):147–9.
27. Marini M, Saponaro A, Magariños G, de Baldrich A, Lynch P, Remorino L. Congenital atrophic dermatofibrosarcoma protuberans. Int J Dermatol (2001) 40(7):448–50. doi: 10.1046/j.1365-4362.2001.01217.x
28. Teixeira F, Devlin M, Hung N, Yun K. An atrophic plaque on the chest. Aust Fam Physician (2002) 31(4):359–60.
29. Cavuşoğlu T, Yavuzer R, Tuncer S. Dermatofibrosarcoma protuberans of the breast. Aesthetic Plast Surg (2003) 27(2):104–6. doi: 10.1007/s00266-003-0107-9
30. Young CR 3rd, Albertini MJ. Atrophic dermatofibrosarcoma protuberans: case report, review, and proposed molecular mechanisms. J Am Acad Dermatol (2003) 49(4):761–4. doi: 10.1067/S0190-9622(03)00793-X
31. Sheehan DJ, Madkan V, Strickling WA, Peterson CM. Atrophic dermatofibrosarcoma protuberans: a case report and reappraisal of the literature. Cutis (2004) 74(4):237–42.
32. Wu JK, Malik MM, Egan CA. Atrophic dermatofibrosarcoma protuberans: an uncommon and misleading variant. Australas J Dermatol (2004) 45(3):175–7. doi: 10.1111/j.1440-0960.2004.00083.x
33. Sathyanarayana BD. Childhood onset dermatofibrosarcoma protuberans. Indian J Dermatol Venereol Leprol (2004) 70(5):310–2.
34. Sinovich V, Hollowood K, Burge S. Atrophic dermatofibrosarcoma protuberans. Australas J Dermatol (2005) 46(2):114–7. doi: 10.1111/j.1440-0960.2005.00156.x
35. Hirashima N, Misago N, Shinogi T, Inoue T, Miura Y, Narisawa Y. Atrophic dermatofibrosarcoma protuberans with diffuse eosinophilic infiltrate. J Dermatol (2006) 33(7):486–8. doi: 10.1111/j.1346-8138.2006.00114.x
36. Kostrzewa E, Beylot-Barry M, Vergier B, Pedeutour F, Beylot C. [Childhood-onset multifocal atrophic dermatofibrosarcoma]. Ann Dermatol Venereol (2006) 133(4):359–61. doi: 10.1016/S0151-9638(06)70915-2
37. Hanabusa M, Kamo R, Harada T, Ishii M. Dermatofibrosarcoma protuberans with atrophic appearance at early stage of the tumor. J Dermatol (2007) 34(5):336–9. doi: 10.1111/j.1346-8138.2007.00283.x
38. Llombart B, Sanmartin O, Requena C, Monteagudo C, Botella-Estrada R, Nagore E, et al. Atrophic dermatofibrosarcoma protuberans with the fusion gene COL1A1-PDGFB. J Eur Acad Dermatol Venereol (2008) 22(3):371–4. doi: 10.1111/j.1468-3083.2007.02327.x
39. Feramisco J, Larsen F, Weitzul S, Cockerell C, Ghali F. Congenital atrophic dermatofibrosarcoma protuberans in a 7-month-old boy treated with mohs micrographic surgery. Pediatr Dermatol (2008) 25(4):455–9. doi: 10.1111/j.1525-1470.2008.00718.x
40. Gerlini G, Mariotti G, Urso C, Brandani P, Reali UM, Borgognoni L. Dermatofibrosarcoma protuberans in childhood: two case reports and review of the literature. Pediatr Hematol Oncol (2008) 25(6):559–66. doi: 10.1080/08880010802235066
41. Marque M, Bessis D, Pedeutour F, Viseux V, Guillot B, Fraitag-Spinner S. Medallion-like dermal dendrocyte hamartoma: the main diagnostic pitfall is congenital atrophic dermatofibrosarcoma. Br J Dermatol (2009) 160(1):190–3. doi: 10.1111/j.1365-2133.2008.08896.x
42. Bakry O, Attia A. Atrophic dermatofibrosarcoma protuberans. J Dermatol Case Rep (2012) 6(1):14–7. doi: 10.3315/jdcr.2012.1089
43. Qiao J, Patel KU, López-Terrada D, Fang H. Atrophic dermatofibrosarcoma protuberans: report of a case demonstrated by detecting COL1A1-PDGFB rearrangement. Diagn Pathol (2012) 7:166. doi: 10.1186/1746-1596-7-166
44. Al-Balbeesi AO. Atrophic dermatofibrosarcoma protuberans and enlargement with pregnancy: Case report and literature review. J Saudi Soc Dermatol Dermatol Surg (2012) 16:21–3. doi: 10.1016/j.jssdds.2011.09.001
45. de Morais OO, de Araújo LC, Gomes CM, Coutinho AS, Souza FA, Costa IM. Congenital dermatofibrosarcoma protuberans. Cutis (2012) 90(6):285–8.
46. Wen P, Yu R, Wang L. Atrophic dermatofibrosarcoma protuberans: a case report. Int J Dermatol (2013) 52(4):463–5. doi: 10.1111/j.1365-4632.2011.05309.x
47. Han XY, Wei HQ, Pan Q, Liu J. [Atrophic dermatofibrosarcoma protuberans: report of a case]. Zhonghua Bing Li Xue Za Zhi (2013) 42(1):52–3. doi: 10.3760/cma.j.issn.0529-5807.2013.01.014
48. Güngör S, Büyükbabani N, Büyük M, Tarıkçı N, Kocatürk E. Atrophic dermatofibrosarcoma protuberans: are there specific dermatoscopic features? J Dtsch Dermatol Ges (2014) 12(5):425–7. doi: 10.1111/ddg.12308
49. Akay BN, Unlu E, Erdem C, Heper AO. Dermatoscopic findings of atrophic dermatofibrosarcoma protuberans. Dermatol Pract Concept (2015) 5(1):71–3. doi: 10.5826/dpc.0501a12
50. Xu WJ, Wang JS. Atrophic dermatofibrosarcoma protuberans with the fusion gene COL1A1-PDGFB detected by RT-PCR using only a single primer pair. Int J Clin Exp Pathol (2015) 8(6):7457–63.
51. Makino M, Sasaoka S, Nakanishi G, Makino E, Fujimoto W. Congenital atrophic dermatofibrosarcoma protuberans detected by COL1A1-PDGFB rearrangement. Diagn Pathol (2016) 11:24. doi: 10.1186/s13000-016-0474-6
52. Taura M, Wada M, Kataoka Y, Ueda Y, Takenaka H, Katoh N, et al. Case of pigmented dermatofibrosarcoma protuberans with atrophic change. J Dermatol (2016) 43(10):1231–2. doi: 10.1111/1346-8138.13366
53. Liu Y, Zhang B, Han X, Ma L. Pediatric atrophic dermatofibrosarcoma protuberans. Pediatr Investig (2017) 1(1):50–2. doi: 10.1002/ped4.12008
54. Aragão S, Leite E, Cardoso AEC, Houly RLS. An unusual variant of atrophic dermatofibrosarcoma protuberans. Bras Dermatol (2018) 93(2):282–4. doi: 10.1590/abd1806-4841.20187049
55. Zhang Y, Chen H, Sun J. Two childhood cases of pigmented dermatofibrosarcoma protuberans with atrophic change. Eur J Dermatol (2018) 28(2):225–6. doi: 10.1684/ejd.2017.3198
56. Saigusa R, Miyagawa T, Toyama S, Omatsu J, Miyagaki T, Masui Y, et al. Dermatofibrosarcoma protuberans presenting as a Large atrophic plaque on the chest. Acta Derm Venereol (2018) 98(1):155–6. doi: 10.2340/00015555-2800
57. Fagan KK, Sanchez AT, Davis LS. Childhood onset atrophic plaque on the chest of a woman with history of acral lentiginous melanoma. Int J Dermatol (2019) 58(6):669–71. doi: 10.1111/ijd.14376
58. Kibbi N, Wang D, Wang WL, Galan A, Leffell DJ, Christensen SR, et al. Dermatofibrosarcoma protuberans in pregnancy: a case series and review of the literature. Int J Dermatol (2021) 60(9):1114–9. doi: 10.1111/ijd.15497
59. Chow ML, Sennett R, Hinds B, Brian Jiang SI. Large Atrophic plaque on the chest: Answer. Am J dermatopathol (2021) 43(4):308–9. doi: 10.1097/DAD.0000000000001791
60. Bai J, Liu B, Liu T, Qiao J, Fang H. Atrophic pigmented dermatofibrosarcoma protuberans: A case report and literature review. Front Oncol (2021) 11:669754. doi: 10.3389/fonc.2021.669754
61. Lin P, Yang Z, Tu P, Li H. Atrophic pigmented dermatofibrosarcoma protuberans misdiagnosed as hyperpigmentation. Indian J Dermatol Venereol Leprol (2021) 87(5):693–5. doi: 10.25259/IJDVL_713_20
62. Wang P, Xiong JX, Chen AJ, Cai T. A case of atrophic dermatofibrosarcoma protuberans. Ann Dermatol (2022) 34(5):387–8. doi: 10.5021/ad.20.144
Keywords: dermatofibrosarcoma protuberans, atrophic, molecular diagnosis, case report, literature review
Citation: Li Y, Chen Z, Nie S and Wu Z (2023) Atrophic dermatofibrosarcoma protuberans: Two case reports and literature review. Front. Oncol. 13:1100398. doi: 10.3389/fonc.2023.1100398
Received: 16 November 2022; Accepted: 27 January 2023;
Published: 09 February 2023.
Edited by:
Vladimir Spiegelman, Penn State Milton S. Hershey Medical Center, United StatesCopyright © 2023 Li, Chen, Nie and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhouwei Wu, emhvdXdlaS53dUBzaGdoLmNu
†These authors have contributed equally to this work
 Yiting Li
Yiting Li Zile Chen
Zile Chen Shu Nie
Shu Nie Zhouwei Wu
Zhouwei Wu