- 1Department of Respiratory, Shandong Second Provincial General Hospital, Shandong University, Jinan, Shandong, China
- 2Department of Biomedical Engineering, School of Control Science and Engineering, Shandong University, Jinan, Shandong, China
- 3Department of Immunology, Guangzhou Institute of Pediatrics, Guangzhou Women and Children’s Medical Center, Guangdong Provincial Clinical Research Center for Child Health, Guangzhou Medical University, Guangzhou, China
- 4Department of Urology, First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, China
- 5Department of Urology, The Affiliated Hospital of Qingdao Binhai University, Qingdao, Shandong, China
- 6Faculty of Medicine and Health Sciences, Amran University, Amran, Yemen
Interleukin-34 (IL-34) is a cytokine that is involved in the regulation of immune cells, including macrophages, in the tumor microenvironment (TME). Macrophages are a type of immune cell that can be found in large numbers within the TME and have been shown to have a role in the suppression of immune responses in cancer. This mmune suppression can contribute to cancer development and tumors’ ability to evade the immune system. Immune checkpoint inhibitors (ICIs) are a type of cancer treatment that target proteins on immune cells that act as “checkpoints,” regulating the activity of the immune system. Examples of these proteins include programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). ICIs work by blocking the activity of these proteins, allowing the immune system to mount a stronger response against cancer cells. The combination of IL-34 inhibition with ICIs has been proposed as a potential treatment option for cancer due to the role of IL-34 in the TME and its potential involvement in resistance to ICIs. Inhibiting the activity of IL-34 or targeting its signaling pathways may help to overcome resistance to ICIs and improve the effectiveness of these therapies. This review summarizes the current state of knowledge concerning the involvement of IL-34-mediated regulation of TME and the promotion of ICI resistance. Besides, this work may shed light on whether targeting IL-34 might be exploited as a potential treatment option for cancer patients in the future. However, further research is needed to fully understand the mechanisms underlying the role of IL-34 in TME and to determine the safety and efficacy of this approach in cancer patients.
1 Introduction
IL-34 is a human protein that was discovered in 2008 by Lin and his colleagues (1). It is a hypothetical protein that may be accessed in a public database (C16orf77) and was reported as a selective protein that binds CD14+ monocytes and enhances their survival (1–3). Several reports have shown that incubating IL-34 with the extracellular domain protein of the macrophage colony-stimulating factor 1 receptor (MCSF-1R) inhibited the interaction between pure IL-34 and the monocyte in vitro. Additionally, the increase in monocyte vitality brought by IL-34 does not rely on colony-stimulating factor 1 (CSF-1), a second ligand of the CSF-1R (1, 2, 4). Although IL-34 and MCSF-1 exhibit comparable biological activity, myeloid cells respond differently to stimulation by IL-34 or MCSF-1, as evidenced by proinflammatory chemokines production and cytokines (5, 6). The interaction of MCSF-1R with IL-34 has been hypothesized to trigger the activation of autophagy and caspase signaling pathways in monocytes. This activation results in macrophage differentiation and polarization driven by IL-34 rather than CSF-1 (5). Differences in the signaling of CSF-1 and IL-34 have been attributed to differences in the affinity of MCSF-1R binding, hydrophobic/hydrophilic binding character, and differences in the binding pockets (5, 7). However, the molecular processes underlying these disparities must be investigated further in future research.
In 2010, Baudhuin et al. and colleagues published the first research on IL-34’s pathogenic role in malignant giant cell tumors (GCT) (8). GCT is a benign (noncancerous) bone tumor that is rich in osteoclasts, a cell type with a macrophage origin. Previous research suggested that CSF-1 stimulation via CSF-1R was necessary for osteoclast formation. Afterward, IL-34 was found to be abundantly expressed inside GCT and can promote mouse and human osteoclastogenesis via activating CSF-1R independently of CSF-1 (8, 9). Analysis of tumor-infiltrating immune cells also found a relation between IL-34 expression and type-2 immunosuppressive macrophages in different cancer types (4, 10–12). In patients with large B-cell lymphoma, IL-34 recruits monocytes, resulting in an increased macrophage ratio in the tissues and a poor prognosis (13) In the context of treatment failure, evidence that the TME plays a critical role in progression, metastasis, and therapeutic resistance via its interactions with cancer cells is increasing (11, 14). The tumor location of cancer cells that are resistant to treatment has a considerable number of immunosuppressive M2, which inhibits the immunological response against cancer cells (9, 15).
Blockade of immune checkpoints is an intriguing strategy that might potentially stimulate therapeutic anticancer activity. However, malignancies often develop immunological resistance to tumor antigen-specific T cells; hence, treatment outcomes in the clinic are frequently limited. Substantial evidence has shown that IL-34, a cytokine first discovered to control the function and survival of monocytes/macrophages (1, 4), is overexpressed in a wide variety of cancers, where it controls various tumor cell functions (16–18). Recent findings about understanding the activities against ICIs have shown that IL-34 helps malignancies evade the immune system, boosts immunosuppression, and reduces the efficacy of ICIs. In this review, we provide an overview of the current knowledge of the function and role of IL-34 in mediating tumor immunological resistance to ICIs in cancer.
2 Immune checkpoint inhibitors
The groundbreaking discovery of ICIs was a significant step forward in the field of immuno-oncology. Cancer cells can circumvent immunosurveillance and advance by various methods, one of which is the activation of immune checkpoint pathways, which inhibit antitumor immune responses. ICIs increase the immune-mediated clearance of tumor cells, revitalize anticancer immune responses by breaking inhibiting signaling pathways, and result in the eradication of cancer cells (19, 20).
Immune checkpoint molecules are inhibitory pathways that the immune system adopts to prevent undesirable self-immune responses (21). T lymphocytes, the main players in cell-mediated immunity, target tumor cells that express tumor-specific antigens in the TME (22). These antigens are displayed on the cell surface by major histocompatibility complex molecules, allowing T lymphocytes to recognize them (23). On the surface of activated T cells, immune inhibitory receptors are expressed including PD-1 and CTLA-4 (21). The checkpoint molecules block T-cell activation by sending a “STOP” signal to their host T cells after binding to their specific ligands. By expressing ligands, such as PD-L1, which are identified by the PD-1 T cell receptor, numerous tumor cells can evade T cell-mediated death. Blocking antibodies that interfere with these receptor–ligand interactions between tumor cells (or antigen-presenting cells) and T cells might alleviate the patch on T cells, hence releasing T cell-mediated anticancer effects. Undoubtedly, antibodies that inhibit checkpoints have been shown to be particularly effective against a wide range of cancers. In 2011, the USFDA has approved the first ICI therapy (Ipilimumab) specifically for metastatic melanoma treatment (24), leading to potential response rate regardless of surgery or targeted therapy (25). In the past few years, various studies for immune blockage mainly in renal cell carcinoma, lung cancer, pancreatic cancer (PC) and prostate cancer have yielded promising results, indicating the considerable potential for such a therapy strategy (14, 26, 27) (Figure 1). Although PD-L1 is predominantly expressed on tumor cells, it may also be expressed on antigen-presenting cells; hence, the PD-1/PD-L1 T cell inhibitory mechanism operates throughout several stages of an immune response. PD-L1 and PD-L2 are members of the B7-H1 and B7-DC subfamilies, respectively. Therefore, enhancing the anticancer efficacy of ICIs in patients with cancer and reversing the immunosuppressive phenotype are crucial.
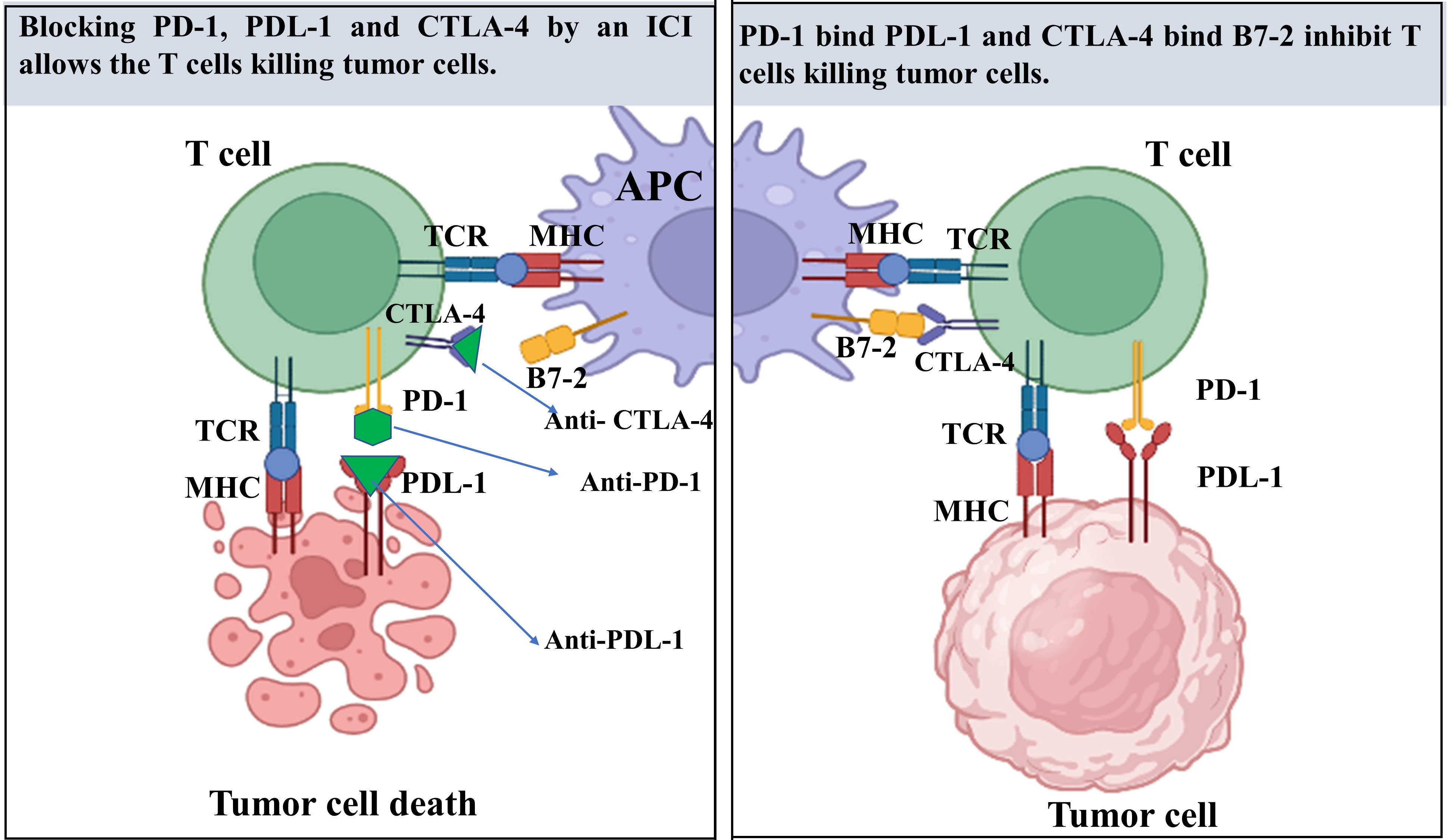
Figure 1 A review of the mechanism of immune checkpoint inhibitors (ICIs) in tumor therapy. T cells recognize antigen-presenting cells (APC), which are primarily dendritic cells (DCs), as well as tumor cells through T cell receptors (TCR). This molecule is responsible for recognizing fragments of antigen known as peptides that are presented to the cell surface of major histocompatibility complex (MHC) molecules. MHC present particular antigens at the cell surface for T-cell receptor (TCR) to becomes active. Co-stimulatory signals are required for T cell activation through the interaction between the CD28 receptor on T cells and the B7 ligands on APC. On T cells, the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), a co-inhibitory receptor, has been expressed and translocates to the cell membrane after T cell activation, where it competes with CD28 for ligand binding. Consequently, CTLA-4 functions largely during the first phases of cancer growth. in later phase, tumor cells in the tumor microenvironments (TME) release programmed death-ligand 1 (PD-L1), which binds to programmed cell death protein 1(PD-1) receptors on T cells, resulting in a co-inhibitory signal that inhibits T cell function.
During the genesis of cancer, the TME gains immunosuppression and resistance to cancer therapy. Recently, ICIs for cancer has gained popularity as a therapy following the three primary traditional tumor therapies (surgery, radiation, and chemotherapy) to combat the TME. Blocking antibodies, particularly PD-1 and CTLA-4, have shifted the paradigm of cancer therapy; however, many patients with cancer do not respond and develop resistance to ICIs. In addition to its vital function in tumors, IL-34 has been identified as a factor that biases macrophages toward immunosuppression, leading to increased tumor evasion and progression (28). ICIs alone are not sufficient for effective treatment. However, the combination of ICIs with other newly discovered targetable biomarkers can have the potential to reverse the immunosuppressive state in the TME and may represent a promising approach for future treatment strategies.
3 IL-34 and ICIs in various cancer types
The immunotherapies that target ICI molecules, e.g., CTLA 4 (29, 30), and PD-1 (31, 32), have contributed to the achievement of persistent responses in the treatment of cancer. Nevertheless, 25% of patients with melanoma that showed objective responses to ICIs acquire resistance to the therapy and suffer from the development of their disease and death (33). Numerous studies have proposed several mechanisms for immune system resistance at the cellular and molecular levels. These mechanisms include weakened T cell infiltration and activation at the TME, epigenetic alterations in cancer cells that impair IFN‐γ signaling, and local TME immunosuppression (21, 34, 35). In this regard, important roles in acquired and innate resistance to ICIs have been attributed to the infiltration of the TME with immunosuppression cells, such as M2-biased TAMs, regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs), as well as numerous inflammatory and metabolic mediators such as arginase1 (ARG1), prostaglandin E2 (PGE2), and indoleamine 2,3-dioxygenase (IDO) (36–38). In many instances, the soluble molecules secreted by the tumor cells are responsible for the formation and maintenance of the immunosuppression TME (39). In light of this, focusing on these parameters would make it possible to alleviate immunosuppression and increase immunotherapeutic responses (40, 41).
Recently, IL-34 and its receptor, CSF-1R, has drawn strong interest as crucial proteins controlling the survival, function, and proliferation of M2-biased TAMs, which are distinguished by their immunosuppressive activities (42, 43). In several cancers, notably melanoma, PC, and hepatocellular carcinoma, the CSF-1/CSF-1R axis has been associated with the emergence of resistance to PD-1/PD-L1 suppression (44–48). In addition to being activated by CSF-1, the CSF-1R receptor can also be activated differently by binding IL-34 as the second ligand (1). Although CSF-1 and IL-34 have the same receptor and have a comparable effect in vitro on myeloid cells, the two cytokines bind to distinct pockets within the extracellular immunoglobulin (Ig)-like domains of CSF-1R, resulting in various activation pathways of CSF-1R (49). An important distinction between CSF-1 and IL-34 is that IL-34 is selectively expressed in the skin and brain, whereas CSF-1 is physiologically widely dispersed throughout the body (50). Even though it is selectively expressed, IL-34 may be released by tumor cells, and it plays a crucial role in the development of tumors (1, 12, 50–54) and their resistance to chemotherapy and molecularly targeted treatment (15, 55).
Consistent with its immunosuppressive properties, IL-34 expression in tumors is related to lower cellular frequencies (CD4+, CD8+ T cells, and M1-biased macrophage) and molecular (various chemokines and cytokines) effector frequencies in the TME (11, 56). Using a neutralizing antibody against IL-34 improved ICI’s therapeutic advantages in combinational therapy models involving a patient-derived xenograft model (57). Therefore, cancer therapy may gain a potentially game-changing new option if IL-34 inhibitors can be used to limit protumorigenic effects and ICI resistance (Table 1 and Figure 2).
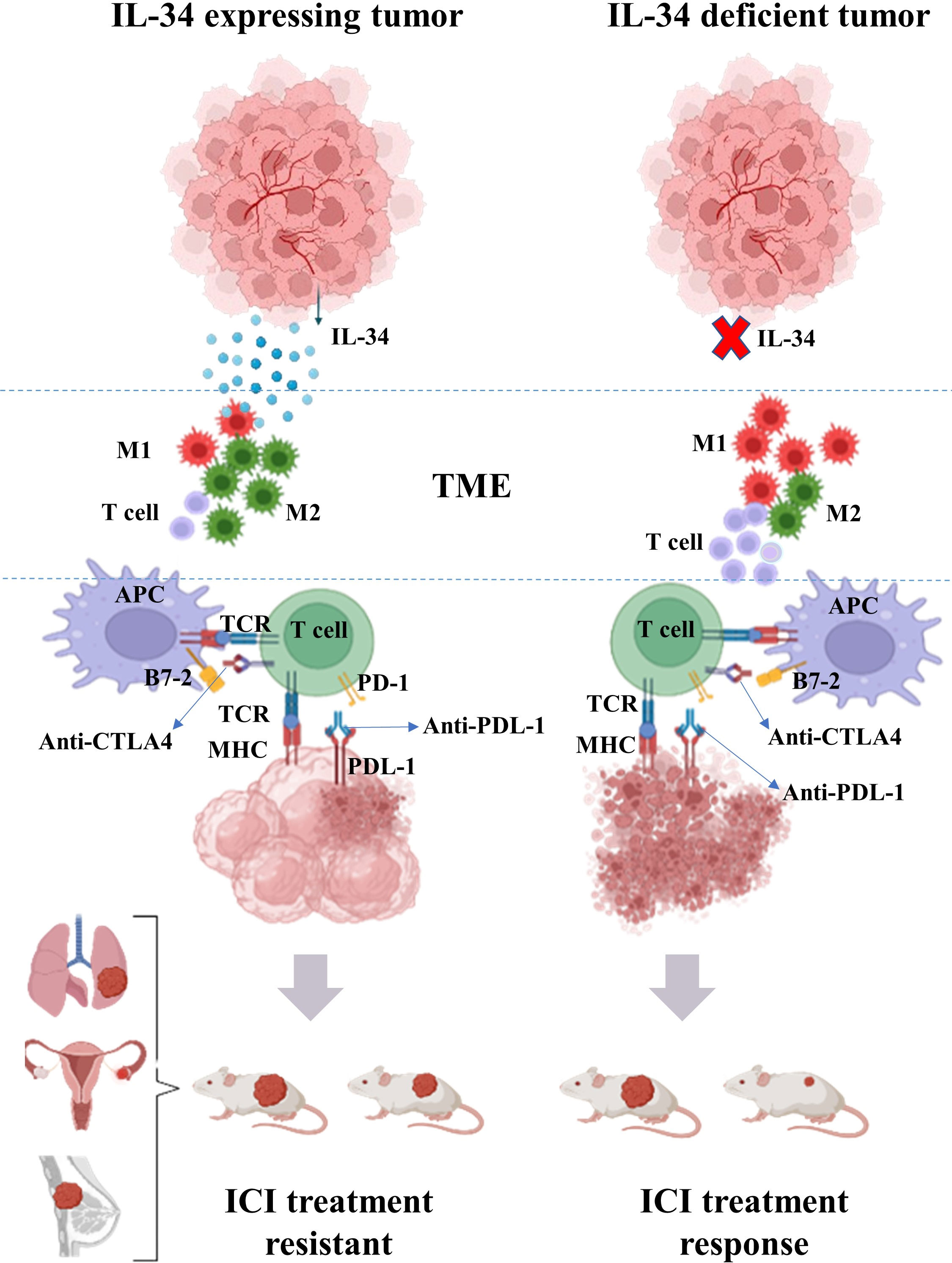
Figure 2 Overview of the effect of IL-34 on the efficacy of immune checkpoint inhibitors (ICIs) in the treatment of cancer. Tumor-derived IL-34 can modulate the tumor microenvironments (TME) leading to lower frequency of T cell and increases in the ratio of M2-biased macrophages to M1-biased macrophages in TME. Conjunction with an ICI to treat malignancies, a neutralizing antibody directed against IL-34 increase the therapeutic benefits of the inhibitor.
3.1 Ovarian cancer
Ovarian cancer is the third most frequent gynecological malignancy globally, yet it has the greatest fatality rate among these tumors. Immunotherapy response rates for patients with ovarian cancer remain to be modest, although ICI therapy is developing quickly (58, 59). In ovarian cancer, to assess the effects of IL-34 on ICI therapy, Hama et al. (60) conducted their study with the mouse ovarian tumor cell line-HM-1, which produces a considerable amount of IL-34. Only IL-34KO HM-1 tumor reacted to PD-1 blockage exhibiting reduced tumor sizes than the control IgG therapy. Furthermore, PD-1 blockade had a minor effect in a mock HM-1, suggesting that the blockade of PD-1 is most effective in ovarian cancer without IL-34 (60). Moreover, the IL-34KO HM-1 tumor exhibited a large infiltrating of CD4+ T and CD8+ cells; these data indicate the increase in T cell infiltration in the IL-34KO HM-1 tumor is not dependent on PD-1 inhibition. In addition, quantitative PCR analysis demonstrated that IL-34KO HM-1 tumors had elevated levels of IFN-γ and tumor necrosis factor-alpha (TNF-α) (60). These results suggest that IL-34 excreted by cancer cells may inhibit antitumor T cell-mediated immunity, which appears to have influenced the effectiveness of PD-1 blocking treatment.
The production of T cell chemoattractants Cxcl11, Cxcl10, and Cxcl9 is stimulated by IFN-γ (61). Cxcl9 expression considerably increased in tumors of IL-34KO HM-1, which had been treated with an anti-PD-1 antibody (60). By interacting with its receptor Cxcr3, Cxcl9 has been demonstrated to enhance the activity of T cell killers considerably (62), which may explain why PD-1 inhibition was more effective against IL-34KO HM-1 tumors.
To assess in vivo the therapeutic advantages of IL-34 blockage in combination with ICIs, IL-34-expressed mock HM-1 cells were implanted into B6C3F1 mice; subsequently treated with anti-PD-1, a combination of anti-PD-1 and IL-34 antibodies, or IgG as a control. Compared with the control group, PD-1 inhibition alone had a negligible effect on tumor growth 14 days after therapy. By contrast, PD-1 inhibition combined with an anti-IL-34 antibody remarkably suppressed tumor growth and weight (60). Comparing the TME cellular components in each group revealed that inhibiting IL-34 increased CD8+ T cell infiltration but had no effect on CD4+ T cells. Additionally, combined therapy demonstrated a small reduction in CD11b+F4/80+ cells (60). All these results interpret the broad therapeutic effect of IL-34 blockage inside the TME. In the same context, several recent studies have shown that the blockage of CSF-1R increases the antitumor response to anti-PD-1 therapy in a wide range of tumor types including ovarian cancer (63–66). Comparing the anticancer effectiveness of IL-34 blockage to that of CSF-1R blockage in vivo under the same conditions, reveal a similar effect when paired with anti-PD-1 therapy (48, 67–71). Overall, these findings suggest that blocking IL-34 or its receptor, CSF-1R, can potentially increase the effectiveness of anti-PD-1 treatment.
3.2 Colon and breast cancer
Colon cancer is the most common cancer worldwide and one of the top three malignancies in the USA in terms of occurrence and fatality (72). However, breast cancer remains the most common cancer-related cause of burden disease for women, affecting one out of 20 worldwide and up to one in every eight in developed countries (4, 73). Many studies have reported that IL-134 overexpression is conversely correlated with poor survival and prognosis in patients with colon and breast cancer (43). Concerning cancer immunotherapy, CT26 colon cancer cells are sensitive to ICIs, such as anti-CTLA-4 and anti-PD-1 antibodies (74, 75). However, the efficacy of ICIs in this cell line is dramatically diminished by IL-34 overexpression (60, 76, 77). To determine how tumor cell-derived IL-34 blocks the ICIs in colon and breast cancer, researchers used CRISPR-Cas9 technology to create IL-34-deficient CT26 and 4T1 cell lines, respectively. The IL-34KO CT26 cell line was induced to express IL-34 and generate an IL-34-overexpressed cell line (IL-34OE CT26). The tumor cells were subsequently transplanted into Syngenic BALB/c mice, which were then treated with either anti-PD-1 or IgG as control. The IL-34KO tumors responded better to anti-PD-1 antibody therapy, as evidenced by reduced tumor sizes in comparison to the control IgG; however the favorable effects of PD-1 blocking were canceled out by IL-34, which is produced by tumor cells (60).
Next-generation sequencing and gene ontology analysis determined which set of genes was responsible for the greatest antitumor effectiveness within the study group. The group that has been infected with IL-34KO CT26 and treated with anti-PD-1 exhibited a greater number of immune cell response clusters. These immune cell response clusters included the signaling pathway of T cell receptors, antigen presentation and processing, and cytokine–cytokine receptor interactions. Moreover, many genes related to an accumulation of T cells (Cd8a, Cd3e, Cd4), inflammations (Cxcl9, Tnf, Ifng), and a subset of M1-macrophage (Ciita, Nos2, Cd86) have elevated in anti-PD-1 antibody-treated IL-34KO CT26 tumor. By contrast, gene expressions of the M2-macrophage subset (Arg1, Mrc1, and Chi3l3) were lower in IL-34KO CT26 tumors treated with anti-PD-1 than in IL-34OE CT26 tumors (60). In this context, molecular evidence suggests that IL-34KO inhibits the progression of tumors by inducing an elevation in the M1 subset and a reduction in the M2 subset markers.
In the therapeutic models’ cellular components of the TME, IL-34 was shown to alter the cellular profiles and the inflammatory state of the TME in the tumor (10, 56, 78). An IL-34KO CT26 tumor cell model was conducted to determine if tumor-derived IL-34 contributes to TME M1- and M2-predominant macrophage populations. The results showed that the number of tumor-infiltrating Nos2+ M1-biased macrophages increased whereas the number of Arg1+ M2-biased macrophages decreased (10, 51, 60). CSF-1R inhibition causes a substantial decrease in tumor-associated macrophages (79, 80). In the same context when treated with a PD-1 inhibitor, the 4T1 and CT26 tumor cell models demonstrated a considerably increased production of inflammatory and pro-inflammatory cytokines. Additionally, the number of M1-biased macrophages that infiltrated the tumor were shown to be higher in the IL-34KO 4T1 tumor (60). These results imply that IL-34 produced by tumors may inhibit the growth of M1-biased macrophages and inhibit the formation of the anti-inflammatory milieu. To understand further the correlation between IL-34 and ICIs resistance, the combination of anti-PD-1 and anti-CTLA-4 with or without anti-IL-34 antibodies was added to IL-34OE CT26 tumors. The combined therapy of CTLA-4 and anti-PD-1 without anti-IL-34 antibodies resulted in considerable suppression of tumors, whereas anti-CTLA-4 and anti-PD-1 combined with anti-IL-34 antibodies resulted in a more dramatic suppression of tumors (60). Overall, these findings suggest that IL-34 inhibits the efficiency of ICIs not only by competing with accumulations of T-cells but also by changing the inflammatory state of TME through an imbalance of polarization in the M1 and M2 macrophage populations. Additionally, inhibiting IL-34 with particular antibodies inverts ICI resistance by restoring the inflammatory circuitry inside the TME.
3.3 Lung cancer
Lung cancer is a common leading cause of death among patients with cancer worldwide. The mortality rate of lung cancer remains high despite breakthroughs in treatment during the previous decade, including targeted medications, diagnostics, staging, surgical procedures, and chemotherapy and radiation (81). The expression of IL-34 and lung cancer progression is still under debate; some previous studies have reported that IL-34 overexpression is related to the poor survival and prognosis of patients with lung cancer. By contrast, Zhendong et al. found that the loss expression of IL-34 correlated with poor prognosis of patients with lung adenocarcinoma (LUAD) (56, 82, 83). Recently, ICIs have shown promise in treating majority of lung cancer subtypes, including nonsmall cell lung cancer (adenocarcinoma, squamous cell carcinoma, and large cell carcinoma) (84, 85). LUAD is a major type of lung cancer with a five-year overall survival rate of fewer than 15% because majority of patients are detected at a late stage during their initial visit (86). In immunotherapy, IL-34 blockade has potential therapeutic effects in LUAD. In clinical settings, the patient-derived xenograft (PDX) model of human primary lung cancer tissues was injected into HuNSG mice. The humanized PDX mice model revealed that PD-L1 and IL-34 were highly expressed in LUAD, which was then treated with anti-PD-1 antibodies alone or anti-PD-1 antibodies and IL-34 antibodies in combination. Consequently, PD-1 blockage alone had an insufficient response, whereas the combination of both antibodies considerably inhibited tumor development (60). This result shows that the combined treatment was successful in releasing a powerful anticancer effect. Moreover, a substantial amount of immune cell infiltration was observed in the tumor that responded to combination treatment (60). Therefore, IL-34 contributes to the development of ICI resistance in human cancer, and inhibiting IL-34 can restore the therapeutic effect of ICIs.
3.4 Melanoma
Melanoma is the most lethal type of skin cancer, and its prevalence has increased dramatically in recent decades (87, 88). Several studies have recently revealed specific biomarkers that may lead to aggressive behavior in malignant melanoma cells. Giricz, et al. found that high IL-34 expression was associated with resistance and poor prognosis in patients with malignant melanoma (55). In the treatment of melanoma, the use of ICIs that target immune checkpoint molecules has contributed to the achievement of sustained responses (89). Nevertheless, after a median follow-up of 21 months, 25% of patients with melanoma who demonstrated objective responses to PD-1 blockade acquired resistance and suffered disease progression (90). Immune resistance mechanisms in melanoma have mostly remained unexplored. Previous studies in melanoma tissues found a link between genetic changes and acquired ICI resistance (91–94) such as the lack of beta-2-microglobulin (95) or anomalies in the IFN pathways (96). Under normal conditions, IL-34 expression is restricted to the brain and the skin, where it regulates the development, biology, and function of Microglia and Langerhans cells, respectively (97, 98).
The most recent data in a case-control study found that a patient with refractory melanoma exhibited elevated IL-34 expression (77). These findings are consistent with previous studies that reported a correlation between IL-34 expression and tumor metastasis, progression, and resistance to therapy (8, 12, 51, 99, 100). Tissue from a patient with Nivolumab-resistant metastatic melanoma showed a remarkable increase in expression of IL-34 compared with primary site melanoma tissues, and this upsurge in expression was demonstrated to be connected with greater frequencies of CD163, an M2 macrophage marker (77). This result is consistent with previous studies that provided evidence that IL-34 plays a key role in the induction of M2-polarized macrophages, which have immunosuppressive effects (101). Tumor-derived IL-34 enhances tumor-associated M2-polarized macrophages that exhibit immunosuppression and promote tumor growth (15). Compared with primary melanoma, elevated IL-34 expression is associated with increased CD163+ (an M2-polarization marker) macrophages in refractory metastatic melanoma (15). According to the Human Protein Atlas (http://www.proteinatlas.org ) (102), higher IL-34 expression is related with poor prognosis in melanoma, as shown by the Kaplan–Meier analysis of overall survival (77). As a result, many studies are now using experimental animal models to determine whether or not inhibiting IL-34 in IL-34-producing melanomas may help overcome the therapeutic resistance issues.
4 Future perspectives
Widespread cancer types may benefit from the immunoregulatory activities of IL-34. An increase in tumorigenesis is associated with the overexpression of IL-34, which has been demonstrated to play a role in the resistance, progression, and overall survival of patients. Despite the many advantages of ICIs, several challenges about ICI resistance have not been addressed. IL-34 may be responsible for inhibiting the therapeutic benefits of ICIs, according to new findings. To assess ICIs effectively, having a comprehensive understanding of the potential benefits and risks of IL-34 dysregulation in the TME is vital. Therefore, clinical trials based on robust preclinical evidence, as well as extensive translational research to explain the optimal dose and administration, potential side effects with safety, and the mechanisms of IL-34’s effects in combination with ICIs, are essential to further treatment approaches for various types of cancer.
5 Conclusion
Since its discovery, IL-34 has been the subject of studies regarding how it affects immune responses. Furthermore, IL-34 and its functional receptors activate intracellular pathways that are involved in the development and progression of many different types of cancer. Here, studies suggesting that IL-34 may play a role in the development of therapeutic resistance to tumor-ICI therapies were summarized. IL-34 limits the effectiveness of ICIs not only by interfering with T-cell attraction but also by altering the inflammatory situation of the TME through polarization imbalances of the M1 and M2 macrophage populations. The combination of IL-34 inhibition with ICIs has raised its profile as a potentially essential treatment option for patients with cancer in the future.
Author contributions
The review was designed and revised by FA, MS, YAA, Al-Azab M, AA, MA and AR. FA and MS conducted the literature collection. FA and YAA draw the figures and CZ provided guidance and revised this manuscript. Correspondence MS and CZ. Funding and correspondence; CZ. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Natural Science Foundation of China, Grant/Award Number: JJH20211227.
Acknowledgments
Thanks are due to the individual who helped in reviewing and editing the content. Also, we thank the protein atlas database; https://www.proteinatlas.org/ ENSG00000157368-IL34 accessed 10 September 2022.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
TCR T Cell Receptor; MHC Major Histocompatibility Complex; CTLA-4 Cytotoxic T-Lymphocyte-Associated pro-tein 4; PD-1 Programmed Death 1; FDA U.S. Food and Drug Administration; APC Antigen Presenting Cells; T cell T lymphocyte; B cell B lymphocyte; NSCLC None Small Cell Lung Cancer; MCSF-1 Macrophage colony-stimulating factor 1; MCSF-1R Macrophage colony-stimulating factor 1 receptor; CTLA-4 Cytotoxic T-lymphocyte-associated protein 4; PD-1 Programmed cell death protein 1; ICIs Immune Checkpoint Inhibitors; ICB Blockade of immune checkpoints; TME Tumor microenvironment; MHC Major histocompatibility complex; IFN‐γ Interferon‐gamma; TAMs Tumor-associated Macrophages; MDSCs Myeloid-derived suppressor cells; Tregs Regulatory T cells; ARG1 Inflammatory mediators such as arginase1; IDO Indoleamine 2,3-dioxygenase; PGE2 Prostaglandin E2; TNF-α Tumor necrosis factor-alpha.; OS Overall Survival; LUAD Lung Adenocarcinoma; PDX Patient Derived Xenografts.
References
1. Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. (2008) 320(5877):807–11. doi: 10.1126/science.1154370
2. Ma X, Lin WY, Chen Y, Stawicki S, Mukhyala K, Wu Y, et al. Structural basis for the dual recognition of helical cytokines IL-34 and CSF-1 by CSF-1R. Structure. (2012) 20(4):676–87. doi: 10.1016/j.str.2012.02.010
3. Sjöstedt E, Zhong W, Fagerberg L, Karlsson M, Mitsios N, Adori C, et al. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science (2020) 367(6482). doi: 10.1126/science.aay5947
4. Monteleone G, Maresca C, Colella M, Pacifico T, Congiu D, Troncone E, et al. Targeting IL-34/MCSF-1R axis in colon cancer. Front Immunol (2022) 13:917955. doi: 10.3389/fimmu.2022.917955
5. Boulakirba S, Pfeifer A, Mhaidly R, Obba S, Goulard M, Schmitt T, et al. IL-34 and CSF-1 display an equivalent macrophage differentiation ability but a different polarization potential. Sci Rep (2018) 8(1):1–11. doi: 10.1038/s41598-017-18433-4
6. Nakamichi Y, Udagawa N, Takahashi N. IL-34 and CSF-1: similarities and differences. J Bone mineral Metab (2013) 31(5):486–95. doi: 10.1007/s00774-013-0476-3
7. Liu H, Leo C, Chen X, Wong BR, Williams LT, Lin H, et al. The mechanism of shared but distinct CSF-1R signaling by the non-homologous cytokines IL-34 and CSF-1. Biochim Biophys Acta (BBA)-Proteins Proteomics. (2012) 1824(7):938–45. doi: 10.1016/j.bbapap.2012.04.012
8. Baud'Huin M, Renault R, Charrier C, Riet A, Moreau A, Brion R, et al. Interleukin-34 is expressed by giant cell tumours of bone and plays a key role in RANKL-induced osteoclastogenesis. J pathology. (2010) 221(1):77–86. doi: 10.1002/path.2684
9. Otsuka R, Wada H, Seino K-i eds. IL-34, the rationale for its expression in physiological and pathological conditions. In: Seminars in immunology. 54, 101517. Academic Press: Elsevier.
10. Zins K, Heller G, Mayerhofer M, Schreiber M, Abraham D. Differential prognostic impact of interleukin-34 mRNA expression and infiltrating immune cell composition in intrinsic breast cancer subtypes. Oncotarget (2018) 9(33):23126. doi: 10.18632/oncotarget.25226
11. Wang Z, Wang F, Ding X-Y, Li T-E, Wang H-Y, Gao Y-H, et al. Hippo/YAP signaling choreographs the tumor immune microenvironment to promote triple negative breast cancer progression via TAZ/IL-34 axis. Cancer Letters. (2022) 527:174–90. doi: 10.1016/j.canlet.2021.12.016
12. Zhou SL, Hu ZQ, Zhou ZJ, Dai Z, Wang Z, Cao Y, et al. miR-28-5p-IL-34-macrophage feedback loop modulates hepatocellular carcinoma metastasis. Hepatology. (2016) 63(5):1560–75. doi: 10.1002/hep.28445
13. Noyori O, Komohara Y, Nasser H, Hiyoshi M, Ma C, Pan C, et al. Expression of IL-34 correlates with macrophage infiltration and prognosis of diffuse large b-cell lymphoma. Clin Transl Immunol (2019) 8(8):e1074. doi: 10.1002/cti2.1074
14. Wang S, Li Y, Xing C, Ding C, Zhang H, Chen L, et al. Tumor microenvironment in chemoresistance, metastasis and immunotherapy of pancreatic cancer. Am J Cancer Res (2020) 10(7):1937–53.
15. Baghdadi M, Wada H, Nakanishi S, Abe H, Han N, Putra WE, et al. Chemotherapy-induced IL34 enhances immunosuppression by tumor-associated macrophages and mediates survival of chemoresistant lung cancer CellsAn importance of IL34 in cancer chemoresistance. Cancer Res (2016) 76(20):6030–42. doi: 10.1158/0008-5472.CAN-16-1170
16. Lin W, Xu D, Austin CD, Caplazi P, Senger K, Sun Y, et al. Function of CSF1 and IL34 in macrophage homeostasis, inflammation, and cancer. Front Immunol (2019) 10:2019. doi: 10.3389/fimmu.2019.02019
17. Freuchet A, Salama A, Remy S, Guillonneau C, Anegon I. IL-34 and CSF-1, deciphering similarities and differences at steady state and in diseases. J Leukocyte Biol (2021) 110(4):771–96. doi: 10.1002/JLB.3RU1120-773R
18. Franzè E, Marafini I, Troncone E, Salvatori S, Monteleone G. Interleukin-34 promotes tumorigenic signals for colon cancer cells. Cell Death discovery. (2021) 7(1):1–7. doi: 10.1038/s41420-021-00636-4
19. Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA network Open (2019) 2(5):e192535–e. doi: 10.1001/jamanetworkopen.2019.2535
20. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med (2018) 50(12):1–11. doi: 10.1038/s12276-018-0191-1
21. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. (2012) 12(4):252–64. doi: 10.1038/nrc3239
22. Rivoltini L, Carrabba M, Huber V, Castelli C, Novellino L, Dalerba P, et al. Immunity to cancer: Attack and escape in T lymphocyte–tumor cell interaction. Immunol Rev (2002) 188(1):97–113. doi: 10.1034/j.1600-065X.2002.18809.x
23. Renkvist N, Castelli C, Robbins PF, Parmiani G. A listing of human tumor antigens recognized by T cells. Cancer Immunol Immunother (2001) 50(1):3–15. doi: 10.1007/s002620000169
24. Lipson EJ, Drake CG. Ipilimumab: An anti-CTLA-4 antibody for metastatic MelanomaIpilimumab for metastatic melanoma. Clin Cancer Res (2011) 17(22):6958–62. doi: 10.1158/1078-0432.CCR-11-1595
25. Paddock LE, Lu SE, Bandera EV, Rhoads GG, Fine J, Paine S, et al. Skin self-examination and long-term melanoma survival. Melanoma Res (2016) 26(4):401–8. doi: 10.1097/CMR.0000000000000255
26. Sharon E, Streicher H, Goncalves P, Chen HX. Immune checkpoint inhibitors in clinical trials. Chin J cancer. (2014) 33(9):434. doi: 10.5732/cjc.014.10122
27. Morrison AH, Byrne KT, Vonderheide RH. Immunotherapy and prevention of pancreatic cancer. Trends Cancer. (2018) 4(6):418–28. doi: 10.1016/j.trecan.2018.04.001
28. Al-Shaebi F, Wenzhang L, Hezam K, Almezgagi M, Wei L. Recent insights of the role and signalling pathways of interleukin-34 in liver diseases. Int Immunopharmacology. (2020) 89:107023. doi: 10.1016/j.intimp.2020.107023
29. Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol (2015) 33(17):1889. doi: 10.1200/JCO.2014.56.2736
30. Eroglu Z, Kim DW, Wang X, Camacho LH, Chmielowski B, Seja E, et al. Long term survival with cytotoxic T lymphocyte-associated antigen 4 blockade using tremelimumab. Eur J Cancer. (2015) 51(17):2689–97. doi: 10.1016/j.ejca.2015.08.012
31. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. New Engl J Med (2015) 372(4):320–30. doi: 10.1056/NEJMoa1412082
32. Hamid O, Robert C, Daud A, Hodi FS, Hwu W-J, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti–PD-1) in melanoma. New Engl J Med (2013) 369(2):134–44. doi: 10.1056/NEJMoa1305133
33. Schoenfeld AJ, Hellmann MD. Acquired resistance to immune checkpoint inhibitors. Cancer Cell (2020) 37(4):443–55. doi: 10.1016/j.ccell.2020.03.017
34. O'Donnell JS, Long GV, Scolyer RA, Teng MW, Smyth MJ. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat Rev (2017) 52:71–81. doi: 10.1016/j.ctrv.2016.11.007
35. Ribas A. Adaptive immune resistance: How cancer protects from immune AttackAdaptive immune resistance. Cancer discovery. (2015) 5(9):915–9. doi: 10.1158/2159-8290.CD-15-0563
36. Ugel S, De Sanctis F, Mandruzzato S, Bronte V. Tumor-induced myeloid deviation: When myeloid-derived suppressor cells meet tumor-associated macrophages. J Clin Invest (2015) 125(9):3365–76. doi: 10.1172/JCI80006
37. Prima V, Kaliberova LN, Kaliberov S, Curiel DT, Kusmartsev S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc Natl Acad Sci (2017) 114(5):1117–22. doi: 10.1073/pnas.1612920114
38. Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol (2016) 37(3):208–20. doi: 10.1016/j.it.2016.01.004
39. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med (2018) 24(5):541–50. doi: 10.1038/s41591-018-0014-x
40. Ghirelli C, Hagemann T. Targeting immunosuppression for cancer therapy. J Clin Invest (2013) 123(6):2355–7. doi: 10.1172/JCI69999
41. Pitt J, Marabelle A, Eggermont A, Soria J-C, Kroemer G, Zitvogel L. Targeting the tumor microenvironment: Removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol (2016) 27(8):1482–92. doi: 10.1093/annonc/mdw168
42. Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. (2014) 41(1):49–61. doi: 10.1016/j.immuni.2014.06.010
43. Poudel M, Kim G, Bhattarai PY, Kim JY, Choi HS. Interleukin-34-CSF1R signaling axis promotes epithelial cell transformation and breast tumorigenesis. Int J Mol Sci (2021) 22(5). doi: 10.3390/ijms22052711
44. Zhu Y, Yang J, Xu D, Gao X-M, Zhang Z, Hsu JL, et al. Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade. Gut. (2019) 68(9):1653–66. doi: 10.1136/gutjnl-2019-318419
45. Quaranta V, Rainer C, Nielsen SR, Raymant ML, Ahmed MS, Engle DD, et al. Macrophage-derived granulin drives resistance to immune checkpoint inhibition in metastatic pancreatic CancerGranulin drives anti–PD-1 therapy resistance in PDAC. Cancer Res (2018) 78(15):4253–69. doi: 10.1158/0008-5472.CAN-17-3876
46. Neubert NJ, Schmittnaegel M, Bordry N, Nassiri S, Wald N, Martignier C, et al. T Cell–induced CSF1 promotes melanoma resistance to PD1 blockade. Sci Trans Med (2018) 10(436):eaan3311. doi: 10.1126/scitranslmed.aan3311
47. Gyori D, Lim EL, Grant FM, Spensberger D, Roychoudhuri R, Shuttleworth SJ, et al. Compensation between CSF1R+ macrophages and Foxp3+ treg cells drives resistance to tumor immunotherapy. JCI Insight (2018) 3(11). doi: 10.1172/jci.insight.120631
48. Cannarile MA, Weisser M, Jacob W, Jegg A-M, Ries CH, Rüttinger D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J immunotherapy cancer. (2017) 5(1):1–13. doi: 10.1186/s40425-017-0257-y
49. Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res (2006) 66(11):5527–36. doi: 10.1158/0008-5472.CAN-05-4128
50. Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of langerhans cells and microglia. Nat Immunol (2012) 13(8):753–60. doi: 10.1038/ni.2360
51. Ségaliny AI, Mohamadi A, Dizier B, Lokajczyk A, Brion R, Lanel R, et al. Interleukin-34 promotes tumor progression and metastatic process in osteosarcoma through induction of angiogenesis and macrophage recruitment. Int J cancer. (2015) 137(1):73–85. doi: 10.1002/ijc.29376
52. Franzè E, De Simone V, Dinallo V, Rizzo A, Caprioli F, Colantoni A, et al. OC. 01.4: interleukin-34 sustains pro-tumorigenic signals in colon cancer tissue. Digestive Liver Disease. (2017) 2(49):e78. doi: 10.18632/oncotarget.23289
53. Baghdadi M, Endo H, Takano A, Ishikawa K, Kameda Y, Wada H, et al. High co-expression of IL-34 and m-CSF correlates with tumor progression and poor survival in lung cancers. Sci Rep (2018) 8(1):1–10. doi: 10.1038/s41598-017-18796-8
54. Baghdadi M, Ishikawa K, Nakanishi S, Murata T, Umeyama Y, Kobayashi T, et al. A role for IL-34 in osteolytic disease of multiple myeloma. Blood advances. (2019) 3(4):541–51. doi: 10.1182/bloodadvances.2018020008
55. Giricz O, Mo Y, Dahlman KB, Cotto-Rios XM, Vardabasso C, Nguyen H, et al. The RUNX1/IL-34/CSF-1R axis is an autocrinally regulated modulator of resistance to BRAF-V600E inhibition in melanoma. JCI Insight (2018) 3(14). doi: 10.1172/jci.insight.120422
56. Wang Z, Zhu J, Wang T, Zhou H, Wang J, Huang Z, et al. Loss of IL-34 expression indicates poor prognosis in patients with lung adenocarcinoma. Front Oncol (2021) 11:639724. doi: 10.3389/fonc.2021.639724
57. Han N, Jang HY, Hama N, Kobayashi T, Otsuka R, Wada H, et al. An optimized protocol for patient-derived xenograft in humanized mice to evaluate the role of IL-34 in immunotherapeutic resistance. STAR Protoc (2021) 2(2):100460. doi: 10.1016/j.xpro.2021.100460
58. Yang C, Xia BR, Zhang ZC, Zhang YJ, Lou G, Jin WL. Immunotherapy for ovarian cancer: Adjuvant, combination, and neoadjuvant. Front Immunol (2020) 11:577869. doi: 10.3389/fimmu.2020.577869
59. Kuroki L, Guntupalli SR. Treatment of epithelial ovarian cancer. BMJ (2020) 371:m3773. doi: 10.1136/bmj.m3773
60. Hama N, Kobayashi T, Han N, Kitagawa F, Kajihara N, Otsuka R, et al. Interleukin-34 limits the therapeutic effects of immune checkpoint blockade. Iscience. (2020) 23(10):101584. doi: 10.1016/j.isci.2020.101584
61. Tokunaga R, Zhang W, Naseem M, Puccini A, Berger MD, Soni S, et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation–a target for novel cancer therapy. Cancer Treat Rev (2018) 63:40–7. doi: 10.1016/j.ctrv.2017.11.007
62. Chow MT, Ozga AJ, Servis RL, Frederick DT, Lo JA, Fisher DE, et al. Intratumoral activity of the CXCR3 chemokine system is required for the efficacy of anti-PD-1 therapy. Immunity. (2019) 50(6):1498–512.e5. doi: 10.1016/j.immuni.2019.04.010
63. Mantovani A, Allavena P, Marchesi F, Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discovery (2022) 21, 1–22. doi: 10.1038/s41573-022-00520-5
64. El-Gamal MI, Al-Ameen SK, Al-Koumi DM, Hamad MG, Jalal NA, Oh CH. Recent advances of colony-stimulating factor-1 receptor (CSF-1R) kinase and its inhibitors. J Med Chem (2018) 61(13):5450–66. doi: 10.1021/acs.jmedchem.7b00873
65. Xu J, Escamilla J, Mok S, David J, Priceman S, West B, et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res (2013) 73(9):2782–94. doi: 10.1158/0008-5472.CAN-12-3981
66. Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell (2014) 25(6):846–59. doi: 10.1016/j.ccr.2014.05.016
67. Mok S, Koya RC, Tsui C, Xu J, Robert L, Wu L, et al. Inhibition of CSF-1 receptor improves the antitumor efficacy of adoptive cell transfer ImmunotherapyCSF-1R blockade improves immunotherapy. Cancer Res (2014) 74(1):153–61. doi: 10.1158/0008-5472.CAN-13-1816
68. Kumar V, Donthireddy L, Marvel D, Condamine T, Wang F, Lavilla-Alonso S, et al. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell (2017) 32(5):654–68.e5. doi: 10.1016/j.ccell.2017.10.005
69. Magkouta SF, Vaitsi PC, Pappas AG, Iliopoulou M, Kosti CN, Psarra K, et al. CSF1/CSF1R axis blockade limits mesothelioma and enhances efficiency of anti-PDL1 immunotherapy. Cancers. (2021) 13(11):2546. doi: 10.3390/cancers13112546
70. Holmgaard RB, Brachfeld A, Gasmi B, Jones DR, Mattar M, Doman T, et al. Timing of CSF-1/CSF-1R signaling blockade is critical to improving responses to CTLA-4 based immunotherapy. Oncoimmunology. (2016) 5(7):e1151595. doi: 10.1080/2162402X.2016.1151595
71. Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer ModelsCSF1R blockade improves checkpoint immunotherapy. Cancer Res (2014) 74(18):5057–69. doi: 10.1158/0008-5472.CAN-13-3723
72. Jia SN, Han YB, Yang R, Yang ZC. Chemokines in colon cancer progression. Semin Cancer Biol (2022) 86(Pt 3):400–7. doi: 10.1016/j.semcancer.2022.02.007
73. Britt KL, Cuzick J, Phillips KA. Key steps for effective breast cancer prevention. Nat Rev Cancer. (2020) 20(8):417–36. doi: 10.1038/s41568-020-0266-x
74. Fu C, Zhu X, Xu P, Li Y. Pharmacological inhibition of USP7 promotes antitumor immunity and contributes to colon cancer therapy. OncoTargets Ther (2019) 12:609. doi: 10.2147/OTT.S182806
75. Jure-Kunkel M, Masters G, Girit E, Dito G, Lee F, Hunt JT, et al. Synergy between chemotherapeutic agents and CTLA-4 blockade in preclinical tumor models. Cancer Immunology Immunother (2013) 62(9):1533–45. doi: 10.1007/s00262-013-1451-5
76. Naimi A, Mohammed RN, Raji A, Chupradit S, Yumashev AV, Suksatan W, et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Communication Signaling (2022) 20(1):1–31. doi: 10.1186/s12964-022-00854-y
77. Han N, Baghdadi M, Ishikawa K, Endo H, Kobayashi T, Wada H, et al. Enhanced IL-34 expression in nivolumab-resistant metastatic melanoma. Inflammation Regeneration. (2018) 38(1):1–5. doi: 10.1186/s41232-018-0060-2
78. Ge Y, Huang M, Yao Y-m. Immunomodulation of interleukin-34 and its potential significance as a disease biomarker and therapeutic target. Int J Biol Sci (2019) 15(9):1835. doi: 10.7150/ijbs.35070
79. MacDonald KP, Palmer JS, Cronau S, Seppanen E, Olver S, Raffelt NC, et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue-and tumor-associated macrophages but does not inhibit inflammation. Blood J Am Soc Hematology. (2010) 116(19):3955–63. doi: 10.1182/blood-2010-02-266296
80. Wang X, Zhang J, Hu B, Qian F. High expression of CSF-1R predicts poor prognosis and CSF-1R(high) tumor-associated macrophages inhibit anti-tumor immunity in colon adenocarcinoma. Front Oncol (2022) 12:850767. doi: 10.3389/fonc.2022.850767
81. Antoine L, Nathan D, Laure M, Briac C, Jean-François M, Corinne B. Compliance with night-time overcorrection bracing in adolescent idiopathic scoliosis: Result from a cohort follow-up. Med Eng physics. (2020) 77:137–41. doi: 10.1016/j.medengphy.2020.01.003
82. Baghdadi M, Endo H, Takano A, Ishikawa K, Kameda Y, Wada H, et al. High co-expression of IL-34 and m-CSF correlates with tumor progression and poor survival in lung cancers. Sci Rep (2018) 8(1):418. doi: 10.1038/s41598-017-18796-8
83. Wang B, Xu W, Tan M, Xiao Y, Yang H, Xia TS. Integrative genomic analyses of a novel cytokine, interleukin-34 and its potential role in cancer prediction. Int J Mol Med (2015) 35(1):92–102. doi: 10.3892/ijmm.2014.2001
84. Gridelli C, Rossi A, Carbone DP, Guarize J, Karachaliou N, Mok T, et al. Non-small-cell lung cancer. Nat Rev Dis primers. (2015) 1(1):1–16. doi: 10.1038/nrdp.2015.9
85. Ruiz-Cordero R, Devine WP. Targeted therapy and checkpoint immunotherapy in lung cancer. Surg Pathol Clinics. (2020) 13(1):17–33. doi: 10.1016/j.path.2019.11.002
86. Denisenko TV, Budkevich IN, Zhivotovsky B. Cell death-based treatment of lung adenocarcinoma. Cell Death disease. (2018) 9(2):1–14. doi: 10.1038/s41419-017-0063-y
87. Marzagalli M, Ebelt ND, Manuel ER eds. Unraveling the crosstalk between melanoma and immune cells in the tumor microenvironment. In: Seminars in cancer biology. 59, 236–250. Academic Press: Elsevier.
88. Pasquali S, Hadjinicolaou AV, Sileni VC, Rossi CR, Mocellin S. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Systematic Rev (2018) 2. doi: 10.1002/14651858.CD011123.pub2
89. Safi M, Jin C, Aldanakh A, Feng P, Qin H, Alradhi M, et al. Immune checkpoint inhibitor (ICI) genes and aging in malignant melanoma patients: a clinicogenomic TCGA study. BMC Cancer. (2022) 22(1):978. doi: 10.1186/s12885-022-09860-2
90. Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. Jama. (2016) 315(15):1600–9. doi: 10.1001/jama.2016.4059
91. Ascierto ML, Makohon-Moore A, Lipson EJ, Taube JM, McMiller TL, Berger AE, et al. Transcriptional mechanisms of resistance to anti–PD-1 TherapyTranscriptional mechanisms of resistance to anti–PD-1. Clin Cancer Res (2017) 23(12):3168–80. doi: 10.1158/1078-0432.CCR-17-0270
92. Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. New Engl J Med (2016) 375(9):819–29. doi: 10.1056/NEJMoa1604958
93. Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, et al. Primary resistance to PD-1 blockade mediated by JAK1/2 MutationsPrimary resistance to PD-1 blockade. Cancer discovery. (2017) 7(2):188–201. doi: 10.1158/2159-8290.CD-16-1223
94. Sucker A, Zhao F, Real B, Heeke C, Bielefeld N, Maβen S, et al. Genetic evolution of T-cell resistance in the course of melanoma ProgressionGenetic evolution of T-cell resistance in melanoma. Clin Cancer Res (2014) 20(24):6593–604. doi: 10.1158/1078-0432.CCR-14-0567
95. Restifo NP, Marincola FM, Kawakami Y, Taubenberger J, Yannelli JR, Rosenberg SA. Loss of functional beta2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. JNCI: J Natl Cancer Institute. (1996) 88(2):100–8. doi: 10.1093/jnci/88.2.100
96. Dunn GP, Sheehan KC, Old LJ, Schreiber RD. IFN unresponsiveness in LNCaP cells due to the lack of JAK1 gene expression. Cancer Res (2005) 65(8):3447–53. doi: 10.1158/0008-5472.CAN-04-4316
97. Guillonneau C, Bézie S, Anegon I. Immunoregulatory properties of the cytokine IL-34. Cell Mol Life Sci (2017) 74(14):2569–86. doi: 10.1007/s00018-017-2482-4
98. Baghdadi M, Endo H, Tanaka Y, Wada H, Seino K-i. Interleukin 34, from pathogenesis to clinical applications. Cytokine. (2017) 99:139–47. doi: 10.1016/j.cyto.2017.08.020
99. Raggi C, Correnti M, Sica A, Andersen JB, Cardinale V, Alvaro D, et al. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J Hepatology. (2017) 66(1):102–15. doi: 10.1016/j.jhep.2016.08.012
100. Rietkötter E, Bleckmann A, Bayerlová M, Menck K, Chuang H-N, Wenske B, et al. Anti-CSF-1 treatment is effective to prevent carcinoma invasion induced by monocyte-derived cells but scarcely by microglia. Oncotarget. (2015) 6(17):15482. doi: 10.18632/oncotarget.3855
101. Foucher ED, Blanchard S, Preisser L, Garo E, Ifrah N, Guardiola P, et al. IL-34 induces the differentiation of human monocytes into immunosuppressive macrophages. antagonistic effects GM-CSF IFNγ. PloS One (2013) 8(2):e56045. doi: 10.1371/journal.pone.0056045
Keywords: immune checkpoint inhibitors (ICIs), interluikin-34 (IL-34), tumor microenvironment, programmed cell death protein 1 (PD-1), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), cancer therapy
Citation: Alshaebi F, Safi M, Algabri YA, Al-Azab M, Aldanakh A, Alradhi M, Reem A and Zhang C (2023) Interleukin-34 and immune checkpoint inhibitors: Unified weapons against cancer. Front. Oncol. 13:1099696. doi: 10.3389/fonc.2023.1099696
Received: 24 November 2022; Accepted: 09 January 2023;
Published: 31 January 2023.
Edited by:
Shicheng Guo, University of Wisconsin-Madison, United StatesReviewed by:
Hanyu Zhang, Peking Union Medical College, ChinaXiaoxuan Wang, Houston Methodist Research Institute, United States
Bojie Zhang, Regeneron Pharmaceuticals, Inc., United States
Zhaohua Hou, Memorial Sloan Kettering Cancer Center, United States
Copyright © 2023 Alshaebi, Safi, Algabri, Al-Azab, Aldanakh, Alradhi, Reem and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammed Safi, bWhvc2FmaTg2QGdtYWlsLmNvbQ==; Caiqing Zhang, ZnJlZXpjcTExQHNkdS5lZHUuY24=
†These authors have contributed equally to this work
 Fadhl Alshaebi
Fadhl Alshaebi Mohammed Safi
Mohammed Safi Yousif A. Algabri
Yousif A. Algabri Mahmoud Al-Azab3
Mahmoud Al-Azab3 Alariqi Reem
Alariqi Reem Caiqing Zhang
Caiqing Zhang