
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 30 March 2023
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1099168
This article is part of the Research Topic Chemoradiotherapy in Locally Advanced Rectal Cancer Patients View all 13 articles
 Tzu-Chieh Yin1,2,3
Tzu-Chieh Yin1,2,3 Po-Jung Chen3
Po-Jung Chen3 Yung-Sung Yeh4,5,6
Yung-Sung Yeh4,5,6 Ching-Chun Li1,7
Ching-Chun Li1,7 Yen-Cheng Chen3,8
Yen-Cheng Chen3,8 Wei-Chih Su3,8
Wei-Chih Su3,8 Tsung-Kun Chang3,8,9
Tsung-Kun Chang3,8,9 Ching-Wen Huang3,10
Ching-Wen Huang3,10 Chun-Ming Huang11,12,13,14
Chun-Ming Huang11,12,13,14 Hsiang-Lin Tsai3,10*
Hsiang-Lin Tsai3,10* Jaw-Yuan Wang3,8,10,11,15,16*
Jaw-Yuan Wang3,8,10,11,15,16*Background: Neoadjuvant chemoradiotherapy followed by total mesorectal excision is the standard treatment for patients with nonmetastatic locally advanced rectal cancer (LARC). However, for patients with LARC and synchronous metastasis, the optimal treatment strategy and sequence remain inconclusive. In the present study, we evaluated the efficacy and safety of concurrent radiotherapy in patients with de novo metastatic rectal cancer who received chemotherapy and targeted therapy.
Methods: We retrospectively reviewed the data of 63 patients with LARC and synchronous metastasis who received intensive therapy at the study hospital between April 2015 and November 2018. The included patients were divided into two groups: RT-CT, those who received systemic chemotherapy with targeted therapy and concurrent radiotherapy (for primary rectal cancer), and CT, those who received only systemic chemotherapy with targeted therapy.
Results: Treatment response was better in the RT-CT group than in the CT group. The rate of primary tumor resection (PTR) was higher in the RT-CT group than in the CT group (71.4% and 42.9%, respectively; P = .0286). The RT-CT group exhibited considerably longer local recurrence-free survival (P = .0453) and progression-free survival (PFS; from 13.3 to 22.5 months) than did the CT group (P = .0091); however, the groups did not differ in terms of overall survival (OS; P = .49). Adverse events were almost similar between the groups, except frequent diarrhea, the prevalence of which was higher in the RT-CT group than in the CT group (59.5% and 23.8%, respectively; P = .0075).
Conclusions: In the era of biologics, radiotherapy may increase the resectability of primary rectal tumors, reducing the risk of locoregional failure and prolonging PFS. Concurrent pelvic radiotherapy may not substantially improve OS, which is indicated by metastasis. Hence, the resection of the distant metastases may be essential for improving long-term OS. To further determine the efficacy of concurrent radiotherapy, additional prospective, randomized studies must combine preoperative pelvic radiotherapy with PTR and metastectomy to treat patients with stage IV LARC.
Approximately 704 000 new cases of rectal cancer are reported worldwide every year; of them, approximately 20% to 30% present with synchronous metastasis upon initial diagnosis (1). The liver and lungs are the most common sites of metastasis, and approximately 80% of the total cases of stage IV cancer are associated with unresectable metastatic tumor burden (2). Currently, neoadjuvant concurrent chemoradiotherapy (CCRT) followed by total mesorectal excision (TME) is the standard treatment for patients with nonmetastatic locally advanced rectal cancer (LARC). This approach results in pathological downstaging and ensures improved local control, longer disease-free survival (DFS), and tolerable toxicity (3–7). Short-course preoperative radiotherapy also reduces the risk of local failure in patients receiving TME (8, 9).
Owing to the advancement of chemotherapy and biologics, therapeutic outcomes in patients with metastatic colorectal cancer (mCRC) have improved (10–12). Highly aggressive treatment of metastatic diseases, particularly colon cancer with liver metastasis, with hepatic resection and various regional therapy improves mCRC and prolongs overall survival (OS) (13–16).
To the best of our knowledge, the optimal treatment strategy and sequence for patients with LARC with de novo metastasis have not been standardized or documented. The potential benefit of concurrent radiotherapy in this population remains unclear and may be overshadowed by the effects of multiagent systemic therapy. Thus, in the present study, we evaluated the efficacy and safety of concurrent radiotherapy in patients with stage IV LARC receiving systemic chemotherapy and targeted therapy.
We retrospectively reviewed the data of 63 patients with de novo metastatic LARC who underwent intensive therapy at our institution between April 2015 and November 2018. Figure 1 illustrates the data collection process. This study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital, Taiwan (approval number: KMUHIRB-E(II)-20220041). The inclusion criteria for patient selection were as follows: diagnosis of T3 or T4 and/or N1 or N2 rectal cancer, presence of systemic metastasis, and ongoing systemic chemotherapy. Patients with synchronous secondary cancer, histological malignancy other than adenocarcinoma, or metachronous metastasis or those receiving only postoperative chemotherapy were excluded from this study. The included patients were divided into two groups: RT-CT and CT. The RT-CT group comprised patients who received systemic chemotherapy with targeted therapy and concurrent radiotherapy (for primary rectal cancer), whereas the CT group comprised patients who received only systemic chemotherapy with targeted therapy. Treatments were selected by surgeons or radiation oncologists.
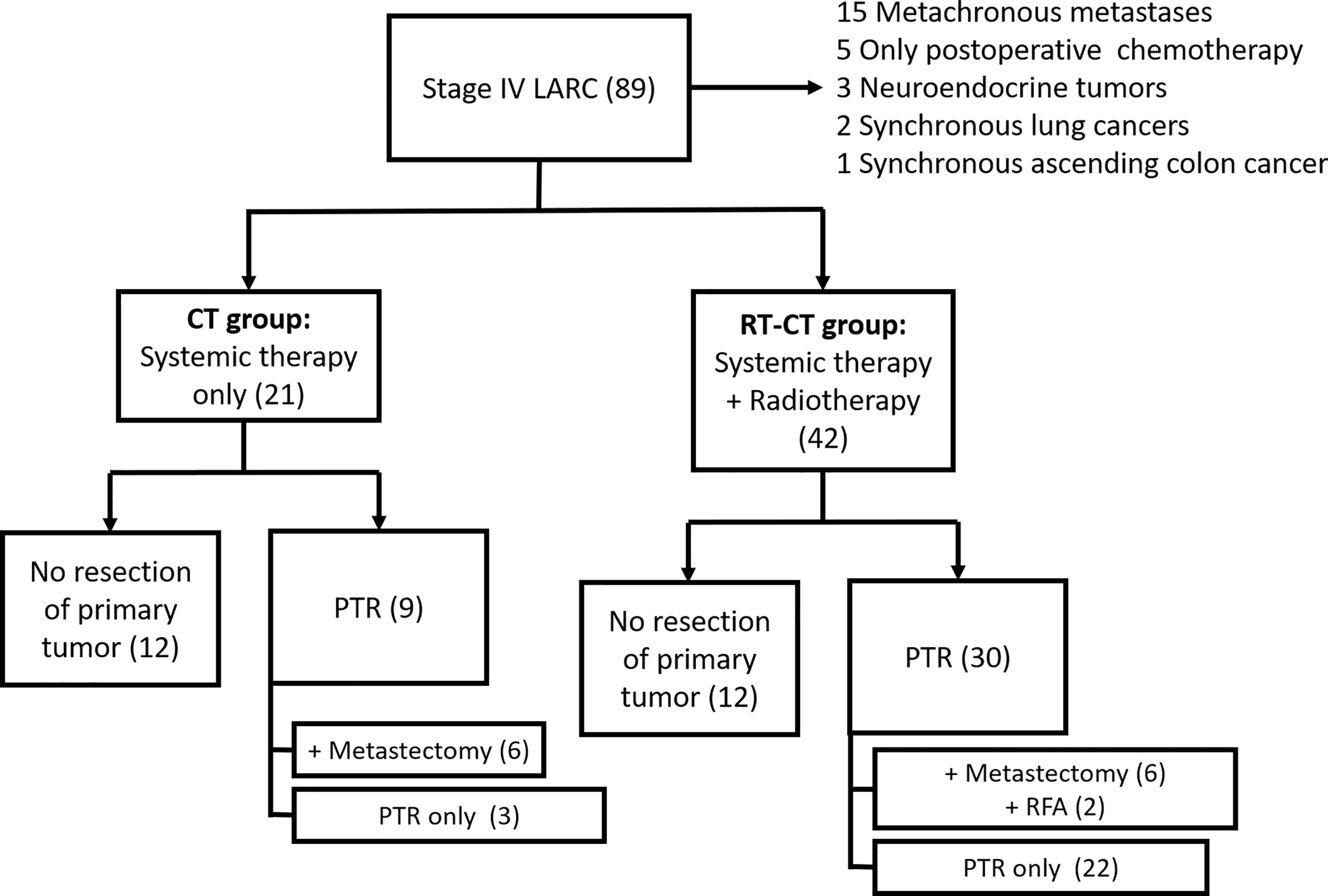
Figure 1 Consort Diagram of Data Collection Process. LARC, locally advanced rectal cancer; PTR, primary tumor resection; and RFA, radiofrequency ablation.
All patients underwent initial workups, which involved taking their medical history, physical examinations, laboratory examinations, carcinoembryonic antigen (CEA) testing, diagnostic colonoscopy, and chest to pelvic computer tomography for preoperative clinical staging. TNM classes were defined in accordance with the criteria outlined by the American Joint Commission on Cancer (AJCC)/International Union Against Cancer (17). Pelvic magnetic resonance imaging (MRI) was performed to evaluate the local status of the primary rectal tumor. To evaluate therapeutic response, MRI was performed again 8 to 10 weeks after pelvic radiotherapy and/or repeatedly performed every 3 months thereafter before primary tumor excision (PTR). Computed tomography was performed at 2- to 3-month intervals to evaluate the progression of distant metastasis and the patients’ response to systemic therapy.
The patients received biweekly systemic therapy comprising chemotherapy with 5-fluorouracil, leucovorin, and irinotecan and targeted therapy with monoclonal antibody against vascular endothelial growth factor (anti-VEGF; bevacizumab) or epidermal growth factor receptor (anti-EGFR; cetuximab or panitumumab). KRAS and NRAS mutations were detected at diagnosis. The dose of irinotecan was in accordance of UGT1A1 polymorphism and was reduced by 20% during the addition of concurrent radiotherapy (12, 18). The interval between the last dose of bevacizumab and elective surgery was at least 5 weeks, and bevacizumab was restarted at least 5 weeks postoperatively. Patients who underwent PTR subsequently received chemotherapy and targeted therapy. Long-course radiotherapy was concurrently administered with and at the beginning of systemic therapy in the RT-CT group in accordance with the procedure described in a previously published study (19). The total dose of radiation was 45 to 50.4 Gy (delivered in 25 to 30 fractions). Three-dimensional conformal or intensity- modulated radiation therapy was used for external-beam irradiation.
The response to systemic therapy and radiotherapy was evaluated on the basis of the Response Evaluation Criteria in Solid Tumors (RECIST) (20). Complete response (CR) was defined as the disappearance of all target lesions, whereas partial response (PR) was defined as a ≥30% decrease in the sum of the longest diameters of target lesions from the baseline value. Progressive disease (PD) was defined as a ≥20% increase in the sum of the longest diameters of target lesions from the value recorded at the initiation of treatment or the appearance of ≥1 new lesions. Stable disease (SD) was defined as neither PR nor PD.
The decision to perform surgery for PTR and the timing of surgery depended on the objective outcome of primary tumors and the control of distant metastases after neoadjuvant therapy. In all patients who underwent PTR, TME was performed through conventional laparotomy or minimally invasive surgery (MIS). The procedures were low anterior resection (LAR), intersphincteric resection (ISR), and abdominal perineal resection (APR). Colostomy was performed if the patients were at risk of total lumen obstruction or bowel rupture or when they underwent PTR and were at risk of anastomotic insufficiency (defunctioning stoma). Colostomy was taken down approximately 3 months after PTR (21). The options for liver-directed therapy were the surgical resection of liver metastases and radiofrequency ablation (RFA).
Postoperative and follow-up surveillance involved routine history taking, physical examinations, CEA testing, and CT at 3-month intervals. Annular colonoscopy was performed and positron emission tomography was executed (if needed). Local recurrence (LR) was defined as recurrence in the pelvic cavity or bowel lumen near an anastomosis. LR-free survival (LRFS) was defined as the interval between PTR and the first radiographic evidence of LR. Progression-free survival (PFS) was defined as the interval between the initiation of treatment and PD or the recurrence of distant metastasis. OS was defined as the between-diagnosis and all-cause death or final follow-up.
We collected data regarding the patients’ demographics and tumor characteristics, namely age, sex, TNM stage, body mass index (BMI), tumor location (distance between a tumor’s caudal margin and anal verge), tumor size, synchronous metastatic site, RAS mutation status, and presence of comorbidities. Data regarding treatment and response were biologics used, chemotherapy cycles, and RECIST findings for primary tumors and metastases. Perioperative data and surgical outcomes comprised the records of PTR, curative resection of metastases, site of metastectomy, procedures and methods performed for PTR, physical status based on the classification system of the American Society of Anesthesiologists, preservation of the anal sphincter, addition of defunctioning stoma, and nonclosure of stoma. Histopathological characteristics comprised the status of surgical margin; rate of R0 resection; rate of pathological CR (pCR); histological grading of differentiation; pathological stage of disease; number of harvested lymph nodes; lympho-vascular invasion (LVI), and perineural invasion. The tumor regression grade (TRG) was assessed using the guidelines of the AJCC (22).
Adverse events (AEs) associated with systemic therapy, radiotherapy, and surgical complications were evaluated using the US National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0; http://ctep.cancer.gov/reporting/ctc.html). AEs associated with systemic therapy were hematologic (e.g., anemia, leukopenia, and thrombocytopenia) and nonhematologic (e.g., nausea or vomiting, diarrhea, fatigue, mucositis, peripheral neuropathy, skin manifestations, alopecia, infection, abnormal liver function, and bowel perforation) events. AEs associated with radiotherapy primarily were radiation dermatitis. Surgical complications were defined as complications developed within 30 days after PTR.
Data were analyzed using JMP for Windows (version 16.0; SAS Institute, Cary, NC, USA). Continuous variables are presented in terms of median and interquartile region (IQR) values, and dichotomous variables are presented in terms of number and percentage values. Between-group comparisons were performed using the χ2 test for categorical variables and Student’s t test for quantitative variables. A P value of ≤.05 was considered statistically significant. Survival plots (LRFS, PFS, and OS) were constructed using the Kaplan–Meier method, and a log-rank test was used to compare the groups in terms of time-to-event distribution.
A total of 89 patients were initially identified; of them, 15 had metachronous metastasis, 5 received only postoperative chemotherapy, 3 had neuroendocrine tumors, 2 had synchronous lung cancer, and 1 had synchronous ascending colon cancer (Figure 1). After the exclusion of these patients, 63 patients remained for our analysis. Of them, 42 received systemic chemotherapy with targeted therapy and concurrent radiotherapy; they constituted the RT-CT group. The remaining 21 patients received only systemic chemotherapy with targeted therapy and constituted the CT group. In the RT-CT group, 30 patients (71.4%) underwent PTR, whereas 12 received no surgery for primary rectal tumor after radiotherapy. A total of 6 patients underwent curative resection of metastases (3 underwent partial hepatectomy for liver metastases, whereas the remaining 3 underwent lobectomy for lung metastases), and 2 patients underwent RFA for liver metastases. In the CT group, 9 (42.9%) underwent PTR, whereas 12 did not. Of the 9 patients, 6 underwent staged metastectomy (2 patients underwent partial hepatectomy, whereas 4 patients underwent lung lobectomy) after PTR. The patients were followed up until their death, final follow-up, or March 2022.
Table 1 summarizes the patients’ demographics and tumor characteristics. Not surprisingly, tumor location was more low-lying in the RT-CT group than in the CT group (P = .0011); 21.4% of the patients in the RT-CT group had a tumor location of <5 cm; this proportion was 4.8% in the CT group. KRAS or NRAS mutation was detected in 15 (35.7%) patients in the RT-CT group, which was slightly more than the proportion noted on the CT group (3 patients; 14.3%; P = .0904). The groups did not differ considerably in terms of age, sex, clinical stage, tumor size, BMI, BRAF mutation status, or the presence of comorbidities (all P >.05). The most frequent site of synchronous metastasis was the liver in the RT-CT group (27 patients; 64.3%), followed by the lungs. 12 (57.1%) patients in the CT group exhibited liver or lung metastasis.
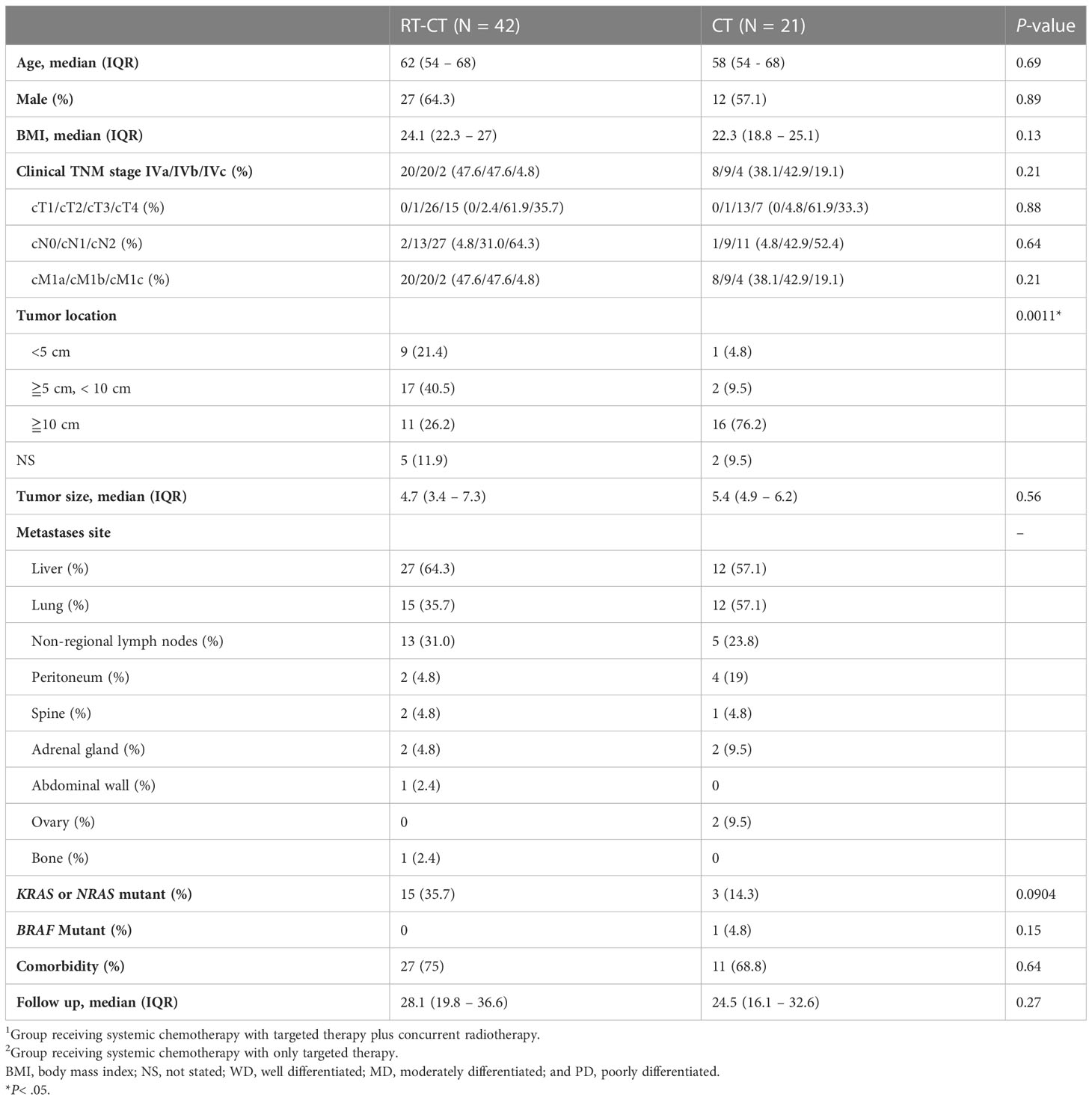
Table 1 Demographics of patients with stage IV locally advanced rectal cancer and the characteristics of their disease in the RT-CT1 and CT2 groups.
In both groups, most patients received bevacizumab (Table 2). In the RT-CT group, 26 patients (61.9%) received bevacizumab, and 14 (33.3%) received cetuximab. A total of 13 (61.9%) and 7 (33.3%) patients in the CT group received bevacizumab and cetuximab, respectively. The RT-CT and CT groups received 14 (median; IQR, 9 to 16) and 12 (IQR, 9 to 13) cycles of chemotherapy, respectively. The groups did not differ substantially in terms biologics used or systemic therapy cycles (both P >.05). A total of 12 patients (28.6%) in the RT-CT group were at a risk of total lumen obstruction before or during treatment; loop colostomy was performed to avoid such a situation. In the CT group, 11 (52.4%) patients underwent loop colostomy. The RT-CT group exhibited no increased tendency of acute bowel obstruction after the addition of concurrent radiotherapy to their systemic therapy regimen (P = .0663). The response rate (CR + PR) of primary rectal tumor was significantly higher in the RT-CT group than in the CT group (73.8% and 47.6%, respectively; P = .0398). The disease control rate (CR + PR + SD) of distant metastases was similar between the RT-CT and CT groups (88.1% and 85.7%, respectively; P = .63); distant metastasis remained at-least stable during the first-line therapy in 37 patients in the RT-CT group and 18 patients in the CT group (P = .63).
The proportion of patients who underwent PTR was significantly higher in the RT- CT group than in the CT group (P = .0286; Table 3). A total of 30 (71.4%) patients in the RT-CT group underwent PTR after receiving concurrent radiotherapy with systemic therapy, whereas 9 patients (42.9%) in the CT group underwent PTR after receiving systemic therapy. In the RT-CT group, 24 (80%), 4 (13.3%), and 2 (6.7%) patients underwent LAR, ISR, and APR, respectively. All patients in the CT group received LAR. MIS was performed in 16 (53.4%) and 7 (77.8%) patients in the RT-CT and CT groups, respectively; the groups did not differ in terms of surgical method (P = .34). The rates of anal preservation in the RT-CT and CT groups were 93.3% and 100%, respectively. Defunctioning stoma was created during PTR performed in 13 patients (43.3%) in the RT-CT group and 1 patient (11.1%) in the CT group. This was expected because the number of patients with low-lying rectal cancer was higher in the RT-CT group than in the CT group. Metastectomy or liver-directed local therapy (RFA) was performed in 8 patients (19.1%) in the RT-CT group; of them, 3 underwent partial hepatectomy, 2 underwent RFA, and 3 underwent lung lobectomy. Curative resection of metastases was performed in 6 patients (28.6%) in the CT group; of them, 2 underwent partial hepatectomy, and 4 underwent lung lobectomy. In both groups, metastectomy was performed in a staged manner; the number patients who underwent metastectomy didn’t vary significantly between the groups (P = .40).
Table 4 summarizes the histopathological characteristics of primary tumors. The status of resection margin in terms of distal resection margin and circumferential resection margin (CRM) was similar between the groups. A total of 2 patients in the RT-CT group and 1 patient in the CT group exhibited positive CRM. The rate of R0 resection in the RT-CT and CT groups was 93.3% and 88.9%, respectively. In the RT-CT group, 4 patients exhibited pCR (13.3%) after concurrent radiotherapy and TME; this number was 1 in the CT group (P = .67). TRGs 0, 1, 2, and 3 were detected in, respectively, 4 (13.3%), 7 (23.3%), 14 (46.7%), and 5 (16.7%) patients in the RT-CT group and 1 (12.5), 1 (12.5%), 3 (37.5%), and 3 (37.5) patients in the CT group (P = .65). After preoperative radiotherapy, tumor size markedly reduced with a median size of 2.5 cm compared with 3.5 cm without radiotherapy (P = .0105). Regarding pathological stages, the groups did not vary significantly in terms of ypT stage (P = .64). However, significant between-group differences were noted in terms of ypN stage (P = .0197); the proportion of patients with ypN2 stage tumor was higher in the CT group (33.3%) than in the RT-CT group (6.7%). The number of harvested lymph nodes was lower in the RT-CT group (median number, 7) than in the CT group (median number, 16; P = .0365).
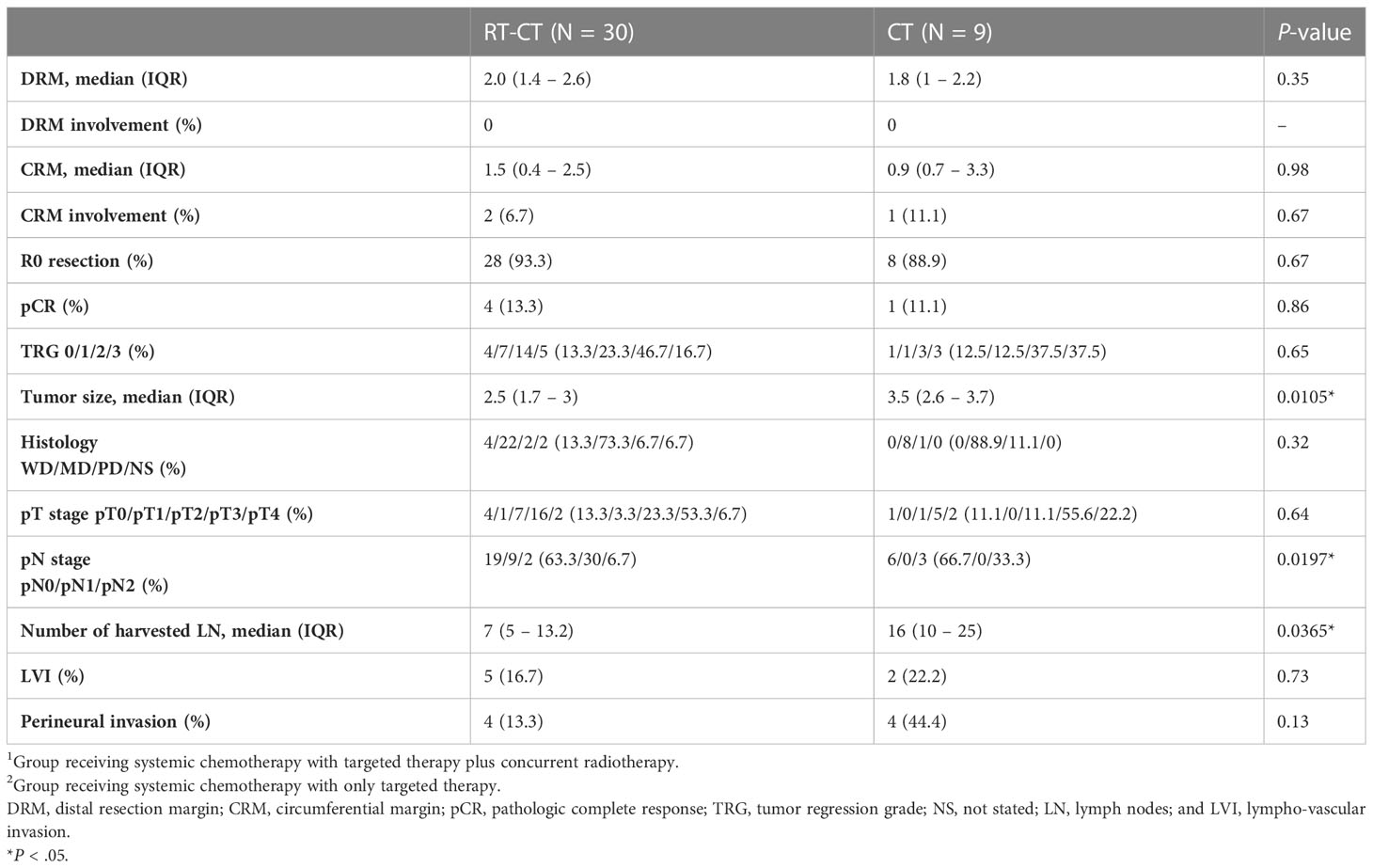
Table 4 Comparison between the RT-CT and CT groups in terms of the histopathologic characteristics of resected primary tumors.
Table 5 summarizes the AEs associated with concurrent radiotherapy and systemic therapy. Anemia was identified to be the most common hematologic AE in both the RT- CT (90.5%) and CT (95.2%) groups. In the RT-CT group, the most prevalent nonhematologic AEs were diarrhea (25 patients; 59.5%) and fatigue (18 patients; 42.9%). In the CT group, the leading AEs were nausea/vomiting and fatigue, which were observed in 10 (47.6%) patients. In the CT group, diarrhea (any grade) was noted in only 5 (23.8%) patients, which was significantly less than in the RT-CT group (P = .0075). Grade III or IV AEs were not frequently detected. Leukopenia and infectious complications were prominent AEs observed in 7 (16.7%) and 5 (11.9%) patients in the RT-CT group, respectively. 3 (14.3%) patients in the CT group developed leukopenia during the treatment course. Radiation dermatitis was observed in 13 (31%) patients in the RT-CT group. Notably, spontaneous rectal perforation developed during or shortly after preoperative radiotherapy in 3 patients (7.1%), and they immediately underwent loop colostomy. Of them, only 1 underwent subsequent PTR. In patients who received concurrent radiotherapy and underwent PTR, infectious complications and postoperative anastomotic leakage were noted in 3 (10%) and 2 (6.7%) patients despite the creation of defunctioning stoma during PTR. Bevacizumab was the monoclonal antibody used in systemic therapy in all the 3 patients of spontaneous rectal perforation and 2 patients of postoperative anastomotic leakage.
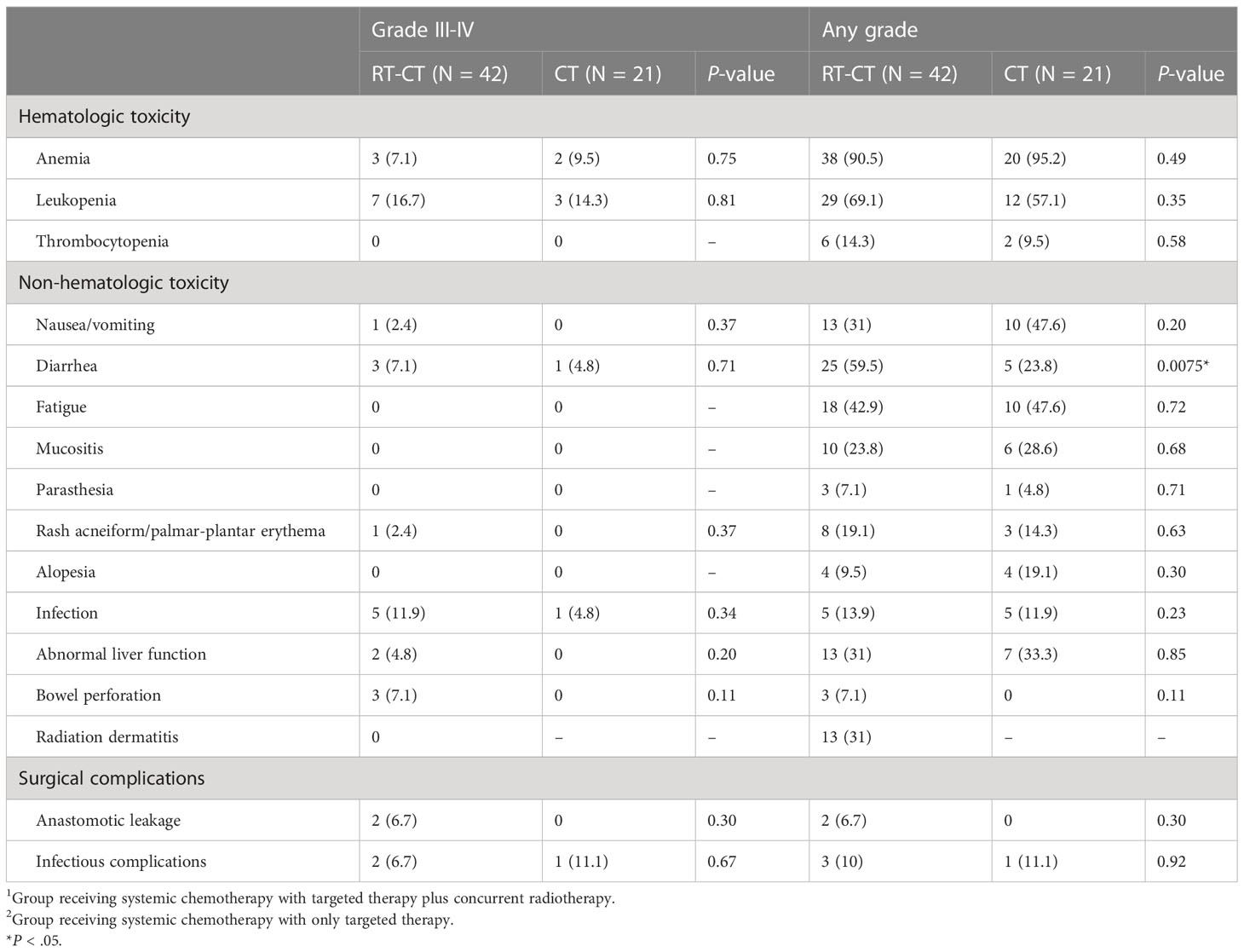
Table 5 Adverse effects related to systemic therapy, radiotherapy, and surgical complications in the RT-CT and CT groups.
The median follow-up duration was 27 (range, 6.7 to 89.2) months. The 24-month LRFS rates of the RT-CT and CT groups were 82.6% and 50%, respectively (Figure 2A). In patients with stage IV LARC who underwent PTR, LRFS was significantly better (P = .0453) in those who received concurrent radiotherapy than in those who did not. The median PFS of the RT-CT group was 22.5 months, which was significantly better than that of the CT group (13.3 months; P = .0091; Figure 2B). However, the 2 groups did not differ significantly in terms of OS (RT-CT group, 31.5 months; CT group, 30.6 months; P = .49; Figure 2C).
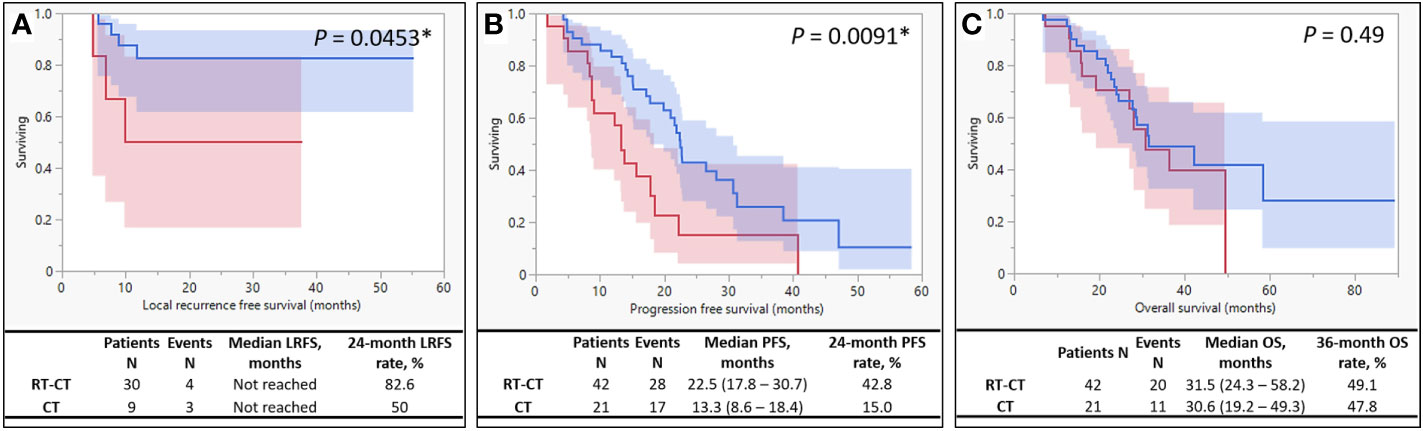
Figure 2 Kaplan–Meier Survival Curves. The survival curve of the RT-CT (systemic chemotherapy with targeted therapy plus pelvic radiotherapy) group is indicated by blue, and that of the CT (systemic chemotherapy with targeted therapy alone) group is indicated by red. (A) Local recurrence-free survival, (B) progression-free survival, and (C) overall survival.
Our findings indicate that patients with relatively low-lying rectal tumors exhibit a high tendency of receiving radiotherapy in addition to systemic therapy even in stage IV of the disease. Although the 2 groups in our study varied in terms of metastatic tumor sites and load, they exhibited similarity in terms of M stage. The addition of concurrent radiotherapy enhanced tumor response. Consistent with the findings of studies on LARC (23) and locally advanced colon cancer (24), in our study, a prolonged interval between preoperative radiotherapy and surgery did not increase the risk of disease progression; this assertion is based on the fact that the disease control rate of distant metastases was noninferior in the RT-CT group. Improved response of primary rectal tumor facilitated PTR after radiotherapy. Histopathologically, no differences were noted between the group in terms of resection margin status, pCR rate, and TRG. However, tumor shrinkage was markedly higher in the RT-CT group than in the CT group. Furthermore, lymph nodes exhibited better response after pelvic irradiation since less ypN2 was obtained in the RT-CT group than in the CT group.
We observed satisfactory local control after concurrent radiotherapy and PTR. The addition of radiotherapy to the systemic chemotherapy regimen increased the rate of 24-month LRFS. It also prolonged (from 13.3 to 22.5 months) the PFS of patients with synchronous metastasis. Few studies have reported similar findings. Concurrent radiotherapy exerted no considerable positive effects on the OS of patients with stage IV LARC. The AEs associated with radiotherapy and systemic therapy were generally tolerable and easily manageable. However, clinicians must consider the risks of spontaneous rectal rupture and anastomotic insufficiency in patients with stage IV LARC receiving simultaneous radiotherapy and targeted therapy, particularly with bevacizumab.
Circulatory tumor cells (CTCs) accelerate micrometastases and are associated with disease progression and survival in breast cancer (25, 26). After preoperative chemoradiotherapy, the proportion of CTCs reportedly decrease in patients with rectal cancer, delaying disease progression (27). Sun et al. revealed considerably lower proportions of CTCs in patients with LARC receiving neoadjuvant CCRT, particularly the responders (28). As expected, we discovered that PFS improved after the addition of concurrent radiotherapy to the current multimodality treatment regimen for LARC with synchronous metastasis. This improvement may also be associated with changes in systemic inflammation and immune function. The neutrophil-to-lymphocyte ratio (NLR) is an indicator of systemic inflammation and may serve as a prognostic factor for various cancers, including rectal cancer (29). A strong correlation has been reported between tumor volume in rectal cancer and NLR (30); the high value of NLR observed in patients with rectal cancer after preoperative radiotherapy has been associated with poor pathological response and survival outcomes (31, 32).
Metastectomy is a key predictor of survival in patients with rectal cancer with metastasis; R0 resection of metastases confers the largest survival benefits (33, 34). In the present study, the improvement in PFS due to additional radiotherapy did not translate to long-term survival. The discrepancy between PFS and OS could be attributed to the low number of patients who underwent curative resection of metastases; in the RT-CT group, only 6 patients underwent metastectomy for liver or lung metastases, and 2 patients underwent RFA. Therefore, the major determinators of OS may depend on the control of distant metastasis. Hence, attempt should still be made for resection of distant metastases to prolong OS.
In patients with mCRC, the precise use of targeted therapy (on the basis of patients’ genetic profiles) and liver-directed therapy results in improved treatment outcomes. In this cohort, late LR become noteworthy, and radiotherapy is a reasonable option for reducing locoregional failure. However, the results in the literature are inconclusive. Kim et al. analyzed data on patients with stage IV rectal cancer with synchronous liver metastasis who underwent TME and liver-directed therapy; LR rate (LRR) was lower in patients receiving postoperative chemoradiotherapy than in those receiving only chemotherapy (35). Fossum et al. demonstrated that neoadjuvant radiotherapy markedly decreased LRR in patients with LARC with resectable liver and/or lung metastasis (36). Chang et al. revealed a trend toward relatively low LRR in patients who underwent PTR treated with postoperative CCRT (37). In their propensity score matching study, Lin et al. indicated improved survival in patients with stage IV rectal cancer when the patients had received CCRT before PTR (34). However, several other studies have reported contradictory findings. A study indicated poor treatment responses and reduced pathological downstaging rates after neoadjuvant radiotherapy in patients with stage IV rectal cancer compared with the findings observed in those with stage II or III disease (38). An et al. reported a nonsuperior LRR in patients who underwent TME and simultaneous metastectomy of limited liver metastases after additional radiotherapy than in those who underwent surgery after only systemic therapy (39). Lee et al. demonstrated that postoperative pelvic radiotherapy improved LRFS only in patients with pT4 disease with metastasis (33). Manyam et al. suggested that preoperative radiotherapy should be avoided in patients with metastatic rectal cancer because the pathological downstaging of rectal cancer for surgical resection is at the expense of increased postoperative complications (40). Consistent with the findings of our study, many studies have reported nonsignificant long-term survival benefits in patients with metastatic rectal cancer who received neoadjuvant or adjuvant radiotherapy, including those who exhibited improved local control (33, 35–41).
In patients with limited liver metastasis burden and satisfactory performance status, prolonged DFS and favorable OS may be achieved after combined liver and colorectal resection (2, 42); PTR with TME should be performed in patients exhibiting good prognosis. However, the optimal management strategy for mCRC with unresectable metastasis remains debatable because of various heterogeneities. The in-situ retention of primary tumors in patients with mCRC rarely results in life-threatening events unless complete obstruction, intractable bleeding, or potential tumor perforation is evident. Therefore, the efficacy of PTR in unresectable metastases remains controversial. In patients with asymptomatic mCRC with unresectable metastasis, PTR may be more effective than palliative chemotherapy alone in terms of the superiority of median OS (43). A propensity score matching analysis revealed a 2-year increase in the median OS of patients who underwent PTR (44). In a population-based cohort study including more than 37 000 patients with mCRC who did not undergo metastectomy, PTR in asymptomatic patients was associated with prolonged OS and cancer-specific survival (45).
Except for the low-lying tumor location, patients of better performance status and low metastatic burden appear to be highly likely to receive a multimodality treatment including concurrent radiotherapy and PTR. However, in the present study, the considerable differences in PFS between-group were unlikely solely due to the effects of unadjusted confounders. Unlike in other studies, all the patients included in our study received biologics as part of systemic therapy; this might have controlled metastasis and highlighted the positive effects of concurrent radiotherapy on PFS.
Our study has some limitations, such as the relatively small sample size and between-group heterogeneity in terms of metastatic tumor sites and load. Nevertheless, the finding that concurrent radiotherapy may delay disease progression may help improve the management of patients with LARC with synchronous metastasis.
The combination of concurrent radiotherapy and systemic therapy may increase primary tumors’ resectability and prolong LRFS in patients with LARC with de novo metastasis. Radiotherapy may also substantially improve PFS. However, the resection of distant metastases is recommended to improve OS. In the era of biologics, the combination of preoperative concurrent radiotherapy and subsequent PTR may be a promising multimodality treatment approach for patients with stage IV LARC.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board of Kaohsiung Medical University Hospital, Taiwan (approval number: KMUHIRB-E(II)-20220041). The patients/participants provided their written informed consent to participate in this study.
T-CY, being the first author of this manuscript, designed this study, analyzed the data, and wrote the manuscript. W-CS, P-JC, T-KC, Y-CC, C-CL, Y-SY, C-WH and C-MH made substantial contributions in terms of the data acquisition, interpretation and statistical analyses, in addition to assisting with the manuscript preparation. H-LT and J-YW, being the corresponding author for this manuscript, also participated in the study design and coordination, in addition to making critical revisions to the manuscript. All authors have reviewed and approved submission of the final version of the manuscript.
This work was supported by grants from the Ministry of Science and Technology (MOST109-2314-B-037-046-MY3, MOST 111-2314-B-037-070-MY3, and MOST 111-2314-B-037-049), Ministry of Health and Welfare (12D1-IVMOHW02), Health and Welfare Surcharge of Tobacco Products, Kaohsiung Municipal Tatung Hospital (KMTTH-104-023, KMTTH-111-007), Kaohsiung Medical University Hospital (KMUH111-1R31, KMUH111-1R32, KMUH111-1M28, KMUH111-1M29, KMUH111-1M31), and Kaohsiung Medical University. In addition, this study was supported by the Grant of Taiwan Precision Medicine Initiative and Taiwan Biobank, Academia Sinica, Taiwan, R.O.C.
We appreciate Kuan-Ting Lee’s effort to this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Venderbosch S, de Wilt JH, Teerenstra S, Loosveld OJ, van Bochove A, Sinnige HA, et al. Prognostic value of resection of primary tumor in patients with stage IV colorectal cancer: retrospective analysis of two randomized studies and a review of the literature. Ann Surg Oncol (2011) 18(12):3252–60. doi: 10.1245/s10434-011-1951-5
3. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med (2004) 351(17):1731–40. doi: 10.1056/NEJMoa040694
4. Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med (2006) 355(11):1114–23. doi: 10.1056/NEJMoa060829
5. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol (2012) 30(16):1926–33. doi: 10.1200/JCO.2011.40.1836
6. Guillem JG, Chessin DB, Cohen AM, Shia J, Mazumdar M, Enker W, et al. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg (2005) 241(5):829–36; discussion 36-8. doi: 10.1097/01.sla.0000161980.46459.96
7. Theodoropoulos G, Wise WE, Padmanabhan A, Kerner BA, Taylor CW, Aguilar PS, et al. T-Level downstaging and complete pathologic response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease-free survival. Dis Colon Rectum. (2002) 45(7):895–903. doi: 10.1007/s10350-004-6325-7
8. Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet (2009) 373(9666):811–20. doi: 10.1016/S0140-6736(09)60484-0
9. Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med (2001) 345(9):638–46. doi: 10.1056/NEJMoa010580
10. Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol (2011) 29(15):2011–9. doi: 10.1200/JCO.2010.33.5091
11. Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol (2009) 27(22):3677–83. doi: 10.1200/JCO.2008.20.5278
12. Tsai HL, Chen YC, Yin TC, Su WC, Chen PJ, Chang TK, et al. Comparison of UGT1A1 polymorphism as guidance of irinotecan dose escalation in RAS wild-type metastatic colorectal cancer patients treated with cetuximab or bevacizumab plus FOLFIRI as the first-line therapy. Oncol Res (2022) 29(1):47–61. doi: 10.3727/096504022X16451187313084
13. Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg (2008) 247(1):125–35. doi: 10.1097/SLA.0b013e31815aa2c2
14. Ardito F, Vellone M, Cassano A, De Rose AM, Pozzo C, Coppola A, et al. Chance of cure following liver resection for initially unresectable colorectal metastases: analysis of actual 5-year survival. J Gastrointest Surg (2013) 17(2):352–9. doi: 10.1007/s11605-012-2103-3
15. Poultsides GA, Servais EL, Saltz LB, Patil S, Kemeny NE, Guillem JG, et al. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol (2009) 27(20):3379–84. doi: 10.1200/JCO.2008.20.9817
16. Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg (2004) 239(6):818–25; discussion 25-7. doi: 10.1097/01.sla.0000128305.90650.71
17. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8th ed. New York, NY: Springer (2017) p. 252–4.
18. Li CC, Chang TK, Chen YC, Tsai HL, Huang CW, Su WC, et al. Clinical outcomes of patients with peritoneal metastasis-only colorectal cancer treated with first-line bevacizumab and FOLFIRI through irinotecan dose escalation according to UGT1A1 polymorphism: Compared to liver metastasis-only, and lung metastasis-only. Cancer Manag Res (2022) 14:1541–9. doi: 10.2147/CMAR.S355318
19. Huang CM, Huang MY, Tsai HL, Huang CW, Ma CJ, Yeh YS, et al. An observational study of extending FOLFOX chemotherapy, lengthening the interval between radiotherapy and surgery, and enhancing pathological complete response rates in rectal cancer patients following preoperative chemoradiotherapy. Ther Adv Gastroenterol (2016) 9(5):702–12. doi: 10.1177/1756283X16656690
20. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the united states, national cancer institute of Canada. J Natl Cancer Institute. (2000) 92(3):205–16. doi: 10.1093/jnci/92.3.205
21. Yin TC, Tsai HL, Yang PF, Su WC, Ma CJ, Huang CW, et al. Early closure of defunctioning stoma increases complications related to stoma closure after concurrent chemoradiotherapy and low anterior resection in patients with rectal cancer. World J Surg Oncol (2017) 15(1):80. doi: 10.1186/s12957-017-1149-9
22. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A 3rd. AJCC cancer staging manual. 7th ed. New York: Springer-Verlag (2010).
23. Huang CW, Su WC, Yin TC, Chen PJ, Chang TK, Chen YC, et al. Time interval between the completion of radiotherapy and robotic-assisted surgery among patients with stage I-III rectal cancer undergoing preoperative chemoradiotherapy. PloS One (2020) 15(10):e0240742. doi: 10.1371/journal.pone.0240742
24. Chen YC, Tsai HL, Li CC, Huang CW, Chang TK, Su WC, et al. Critical reappraisal of neoadjuvant concurrent chemoradiotherapy for treatment of locally advanced colon cancer. PloS One (2021) 16(11):e0259460. doi: 10.1371/journal.pone.0259460
25. Wong NS, Kahn HJ, Zhang L, Oldfield S, Yang LY, Marks A, et al. Prognostic significance of circulating tumour cells enumerated after filtration enrichment in early and metastatic breast cancer patients. Breast Cancer Res Treat (2006) 99(1):63–9. doi: 10.1007/s10549-006-9181-4
26. Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, et al. Circulating tumor cells versus imaging–predicting overall survival in metastatic breast cancer. Clin Cancer Res an Off J Am Assoc Cancer Res (2006) 12(21):6403–9. doi: 10.1158/1078-0432.CCR-05-1769
27. Magni E, Botteri E, Ravenda PS, Cassatella MC, Bertani E, Chiappa A, et al. Detection of circulating tumor cells in patients with locally advanced rectal cancer undergoing neoadjuvant therapy followed by curative surgery. Int J Colorectal Dis (2014) 29(9):1053–9. doi: 10.1007/s00384-014-1958-z
28. Sun W, Li G, Wan J, Zhu J, Shen W, Zhang Z. Circulating tumor cells: A promising marker of predicting tumor response in rectal cancer patients receiving neoadjuvant chemo-radiation therapy. Oncotarget (2016) 7(43):69507–17. doi: 10.18632/oncotarget.10875
29. Huang CM, Huang MY, Tsai HL, Huang CW, Su WC, Chang TK, et al. Pretreatment neutrophil-to-Lymphocyte ratio associated with tumor recurrence and survival in patients achieving a pathological complete response following neoadjuvant chemoradiotherapy for rectal cancer. Cancers (Basel) (2021) 13(18):4589. doi: 10.3390/cancers13184589
30. Braun LH, Baumann D, Zwirner K, Eipper E, Hauth F, Peter A, et al. Neutrophil-to-Lymphocyte ratio in rectal cancer-novel biomarker of tumor immunogenicity during radiotherapy or confounding variable? Int J Mol Sci (2019) 20(10):2488. doi: 10.3390/ijms20102448
31. Ishikawa D, Nishi M, Takasu C, Kashihara H, Tokunaga T, Higashijima J, et al. The role of neutrophil-to-lymphocyte ratio on the effect of CRT for patients with rectal cancer. In Vivo. (2020) 34(2):863–8. doi: 10.21873/invivo.11850
32. Jeon BH, Shin US, Moon SM, Choi JI, Kim MS, Kim KH, et al. Neutrophil to lymphocyte ratio: A predictive marker for treatment outcomes in patients with rectal cancer who underwent neoadjuvant chemoradiation followed by surgery. Ann Coloproctol. (2019) 35(2):100–6. doi: 10.3393/ac.2018.10.01
33. Lee JH, Jo IY, Lee JH, Yoon SC, Kim YS, Choi BO, et al. The role of postoperative pelvic radiation in stage IV rectal cancer after resection of primary tumor. Radiat Oncol J (2012) 30(4):205–12. doi: 10.3857/roj.2012.30.4.205
34. Lin JK, Lee LK, Chen WS, Lin TC, Jiang JK, Yang SH, et al. Concurrent chemoradiotherapy followed by metastasectomy converts to survival benefit in stage IV rectum cancer. J Gastrointest Surg (2012) 16(10):1888–96. doi: 10.1007/s11605-012-1959-6
35. Kim JW, Kim YB, Kim NK, Min BS, Shin SJ, Ahn JB, et al. The role of adjuvant pelvic radiotherapy in rectal cancer with synchronous liver metastasis: a retrospective study. Radiat Oncol (2010) 5:75. doi: 10.1186/1748-717X-5-75
36. Fossum CC, Alabbad JY, Romak LB, Hallemeier CL, Haddock MG, Huebner M, et al. The role of neoadjuvant radiotherapy for locally-advanced rectal cancer with resectable synchronous metastasis. J Gastrointest Oncol (2017) 8(4):650–8. doi: 10.21037/jgo.2017.06.07
37. Chang CY, Kim HC, Park YS, Park JO, Choi DH, Park HC, et al. The effect of postoperative pelvic irradiation after complete resection of metastatic rectal cancer. J Surg Oncol (2012) 105(3):244–8. doi: 10.1002/jso.22109
38. Kim SH, Kim JH, Jung SH. Comparison of oncologic outcomes of metastatic rectal cancer patients with or without neoadjuvant chemoradiotherapy. Int J Colorectal Dis (2015) 30(9):1193–9. doi: 10.1007/s00384-015-2272-0
39. An HJ, Yu CS, Yun SC, Kang BW, Hong YS, Lee JL, et al. Adjuvant chemotherapy with or without pelvic radiotherapy after simultaneous surgical resection of rectal cancer with liver metastases: analysis of prognosis and patterns of recurrence. Int J Radiat Oncol Biol Phys (2012) 84(1):73–80. doi: 10.1016/j.ijrobp.2011.10.070
40. Manyam BV, Mallick IH, Abdel-Wahab MM, Reddy CA, Remzi FH, Kalady MF, et al. The impact of preoperative radiation therapy on locoregional recurrence in patients with stage IV rectal cancer treated with definitive surgical resection and contemporary chemotherapy. J Gastrointest Surg (2015) 19(9):1676–83. doi: 10.1007/s11605-015-2861-9
41. Huh JW, Kim HC, Park HC, Choi DH, Park JO, Park YS, et al. Is chemoradiotherapy beneficial for stage IV rectal cancer? Oncology (2015) 89(1):14–22. doi: 10.1159/000371390
42. Martin R, Paty P, Fong Y, Grace A, Cohen A, DeMatteo R, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg (2003) 197(2):233–41; discussion 41-2. doi: 10.1016/S1072-7515(03)00390-9
43. Huang L, Wei G, Chen N, Liu J, Wang Z, Yu Y, et al. Impact of upfront chemotherapy on the effect of primary tumour resection for asymptomatic synchronous colorectal cancer with unresectable metastases: A propensity-Score-Matched cohort analysis. Clin Med Insights Oncol (2022) 16:11795549221085054. doi: 10.1177/11795549221085054
44. Chen X, Hu W, Huang C, Liang W, Zhang J, Wu D, et al. Survival outcome of palliative primary tumor resection for colorectal cancer patients with synchronous liver and/or lung metastases: A retrospective cohort study in the SEER database by propensity score matching analysis. Int J Surg (2020) 80:135–52. doi: 10.1016/j.ijsu.2020.06.024
45. Tarantino I, Warschkow R, Worni M, Cerny T, Ulrich A, Schmied BM, et al. Prognostic relevance of palliative primary tumor removal in 37,793 metastatic colorectal cancer patients: A population-based, propensity score-adjusted trend analysis. Ann Surg (2015) 262(1):112–20. doi: 10.1097/SLA.0000000000000860
Keywords: metastatic rectal cancer, locally advanced rectal cancer, concurrent radiotherapy, primary tumor resection (PTR), systemic chemotherapy, systemic targeted therapy
Citation: Yin T-C, Chen P-J, Yeh Y-S, Li C-C, Chen Y-C, Su W-C, Chang T-K, Huang C-W, Huang C-M, Tsai H-L and Wang J-Y (2023) Efficacy of concurrent radiotherapy in patients with locally advanced rectal cancer and synchronous metastasis receiving systemic therapy. Front. Oncol. 13:1099168. doi: 10.3389/fonc.2023.1099168
Received: 13 December 2022; Accepted: 21 March 2023;
Published: 30 March 2023.
Edited by:
Marco Rengo, Sapienza University of Rome, ItalyReviewed by:
Simone Vicini, Sapienza University of Rome, ItalyCopyright © 2023 Yin, Chen, Yeh, Li, Chen, Su, Chang, Huang, Huang, Tsai and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jaw-Yuan Wang, Y3k2MTQxMTJAbXMxNC5oaW5ldC5uZXQ=; amF3eXVhbndhbmdAZ21haWwuY29t; Hsiang-Lin Tsai, Y2h1bnBpbjg3MDEzMkB5YWhvby5jb20udHc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.