
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 27 February 2023
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1098087
Background: Rectal cancer patients who received neoadjuvant chemoradiotherapy (CRT) may have a lower cancer stage and a better prognosis. Some patients may be able to avoid invasive surgery. It is critical to accurately assess lymph node metastases (LNM) after neoadjuvant chemoradiotherapy. The goal of this study is to identify clinical variables associated with LNM and to develop a nomogram for LNM prediction in rectal cancer patients following nCRT.
Methods: From 2010 to 2015, patients were drawn from the Surveillance, Epidemiology, and End Results (SEER) database. To identify clinical factors associated with LNM, the least absolute shrinkage and selection operator (LASSO) aggression and multivariate logistic regression analyses were used. To predict the likelihood of LNM, a nomogram based on multivariate logistic regression was created using decision curve analyses.
Reslut: The total number of patients included in this study was 6,388. The proportion of patients with pCR was 17.50% (n=1118), and the proportion of patients with primary tumor pCR was 20.84% (n = 1,331). The primary tumor was pCR in 16.00% (n=213) of the patients. Age, clinical T stage, clinical N stage, and histology were found to be significant independent clinical predictors of LNM using LASSO and multivariate logistic regression analysis. The nomogram was developed based on four clinical factors. The 5-year overall survival rate was 78.9 percent for those with ypN- and 66.3 percent for those with ypN+, respectively (P<0.001).
Conclusion: Patients over 60 years old, with clinical T1-2, clinical N0, and adenocarcinoma may be more likely to achieve ypN0. The watch-and-wait (WW) strategy may be considered. Patients who had ypN0 or pCR had a better prognosis.
In many nations, rectal cancer incidence rates are rising. It is difficult to treat early due to the lack of early clinical symptoms. Many rectal cancer patients are diagnosed at an advanced stage, with local or distant metastases. NCCN (1) and ESMO (2) recommend neoadjuvant chemoradiation (CRT) followed by total mesorectal excision (TME) surgery for locally advanced (T3/4 N0/+M0) rectal cancer. Currently, the lymph node status is crucial for staging, treatment, and prognosis, but the tumor regression grade (TRG) is also a useful marker to evaluate the response to neoadjuvant CRT (3). Many researchers have proposed using local excision or “watch and wait” treatment for rectal cancer patients who have clinically complete response after neoadjuvant CRT (4–6).
Clinical complete response (cCR) indicates that no rectal tumor was discovered during digital examination (DRE), endoscopy, or magnetic resonance imaging (MRI) (7). However, a cCR differs from no pathologic evidence of tumor (pathologic complete response [pCR]), and a pathological assessment can only be performed after TME (8). As a result, accurate assessment of lymph node status prior to surgery is critical for treatment design in rectal cancer patients receiving neoadjuvant CRT. The aim of the study is to investigate the risk factors for lymph node metastasis after neoadjuvant radiotherapy and chemotherapy for rectal cancer, and to develop a predictive model to serve as a guide for patients’ treatment options.
SEER*Stat(version 8.3.5) software was used to retrieve information on rectal cancer cases from the surveillance, epidemiology, and end results (SEER) public access database between 2010 and 2015. SEER*Stat is a SEER-provided online program for obtaining patient information. SEER is a representative sample of the US population, with patient-level data collected from 18 geographically diverse populations representing rural, urban, and regional populations (9). The 8th edition of the TNM classification was used to review and stage patients.The selection process is shown in Figure 1.
Patients who had received Neoadjuvant CRT after a radical resection were eligible. Patients with no clinical T, clinical N, or ypN stage information and no lymph node harvest were excluded.
The patient demographics (age, sex, race, year of diagnosis), tumor characteristics (differentiation, clinical T and N stage, ypN stage, tumor histology, tumor size, and pre-treatment CEA level) and survival data were obtained from the SEER database.
The R software (Version 3.6.3; https://www.R-project.org) was used for statistical analysis. Graphpad Prism (Version 6.0) was used to create forest maps and survival graphs.
First, we divided the data at random into training and validation sets in a 7:3 ratio. The least absolute shrinkage and selection operator (LASSO) (10, 11) method, was used to select the potential predictive features. Selected risk factors for lymph node metastasis (LNM) were subjected to multivariate logistic regression analysis (12). The variables with the P ≤ 0.05 were included in the model, whereas tumor size and race were excluded. A nomogram predictive model for LNM following neoadjuvant CRT was created using four variables. Plotting of calibration curves served to evaluate the nomogram’s calibration. The performance of the nomogram’s discrimination was measured using the ROC curve. By measuring the net benefits at various threshold probabilities, decision curve analysis (DCA) was used to assess the nomogram’s clinical applicability. The 5-year overall survival(OS) was estimated by Kaplan-Meier survival curves. Log-rank tests were conducted to assess statistical significance.
This study enrolled a total of 6388 rectal cancer patients. The proportion of patients with pCR was 17.50% (n=1118), with primary tumor pCR being 20.84% (n = 1,331). While the primary tumor was pCR, 16.00% (n=213) of the patients were N+. The training cohort consisted of 4504 patients, while the validation cohort consisted of 1884 patients. LNM was found in 27.4% (1232/4504) of the training cohort and 27.2% (513/1884) of the validation cohort. Preoperative clinical factors including age, race, sex, CEA, tumor size, clinical T stage, clinical N stage, Year of diagnosis, grade and histology were shown in Table 1. Six potential predictors were chosen from the training cohort’s ten clinicopathological factors.
Based on nonzero coefficients in the LASSO logistic regression model, risk factors for LNM in rectal cancer patients receiving neoadjuvant CRT were identified using the LASSO method and multivariate logistic regression models (Figure 2). The six potential predictors were further evaluated using a multivariate logistic regression model to optimize the predictive model. Age (P<0.05), clinical T stage (P<0.001), clinical N stage (P<0.001), and histology (P=0.004) were found to be independent LNM risk factors for rectal cancer patients receiving neoadjuvant CRT by the multivariate logistic regression analysis (Figure 3).
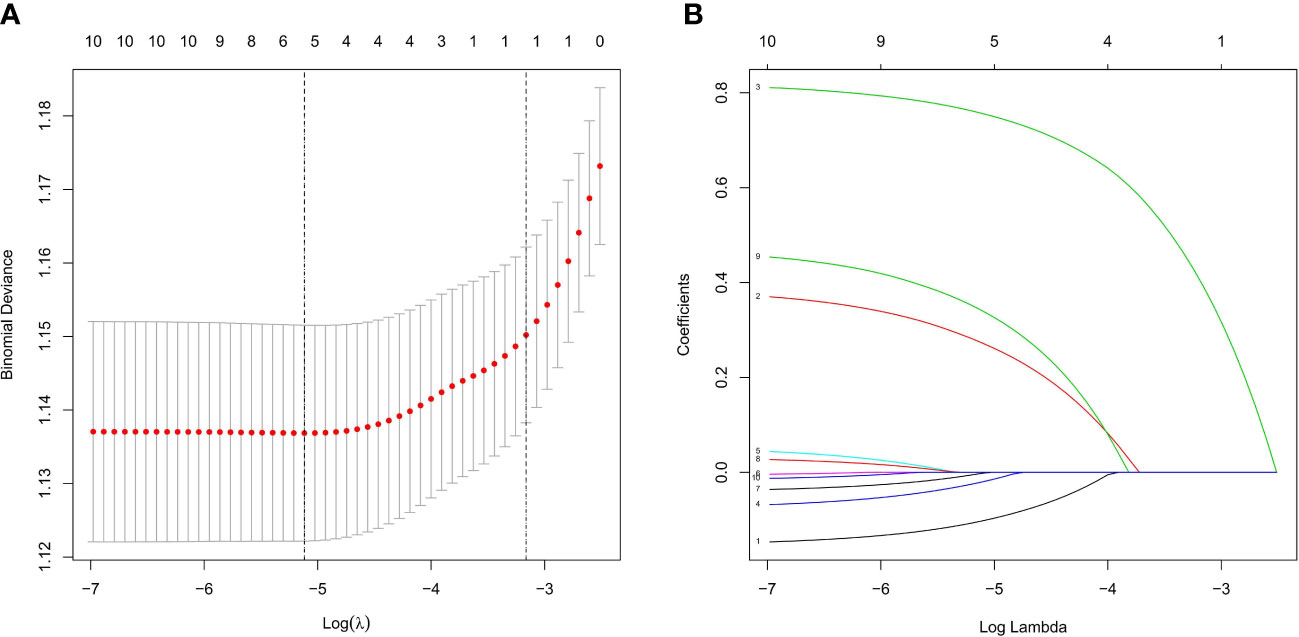
Figure 2 Feature selection using LASSO logistic regression. (A) Tuning parameter (l) selection in the LASSO logistic regression used 10-fold cross-validation via minimum criteria. The binomial deviance was plotted versus log (λ). The black vertical lines were plotted at the optimal λ based on the minimum criteria and λ standard error of the minimum criteria. (B) LASSO coefficient profiles of the 10 clinical factors. A coefficient profile plot was produced versus the log (λ).
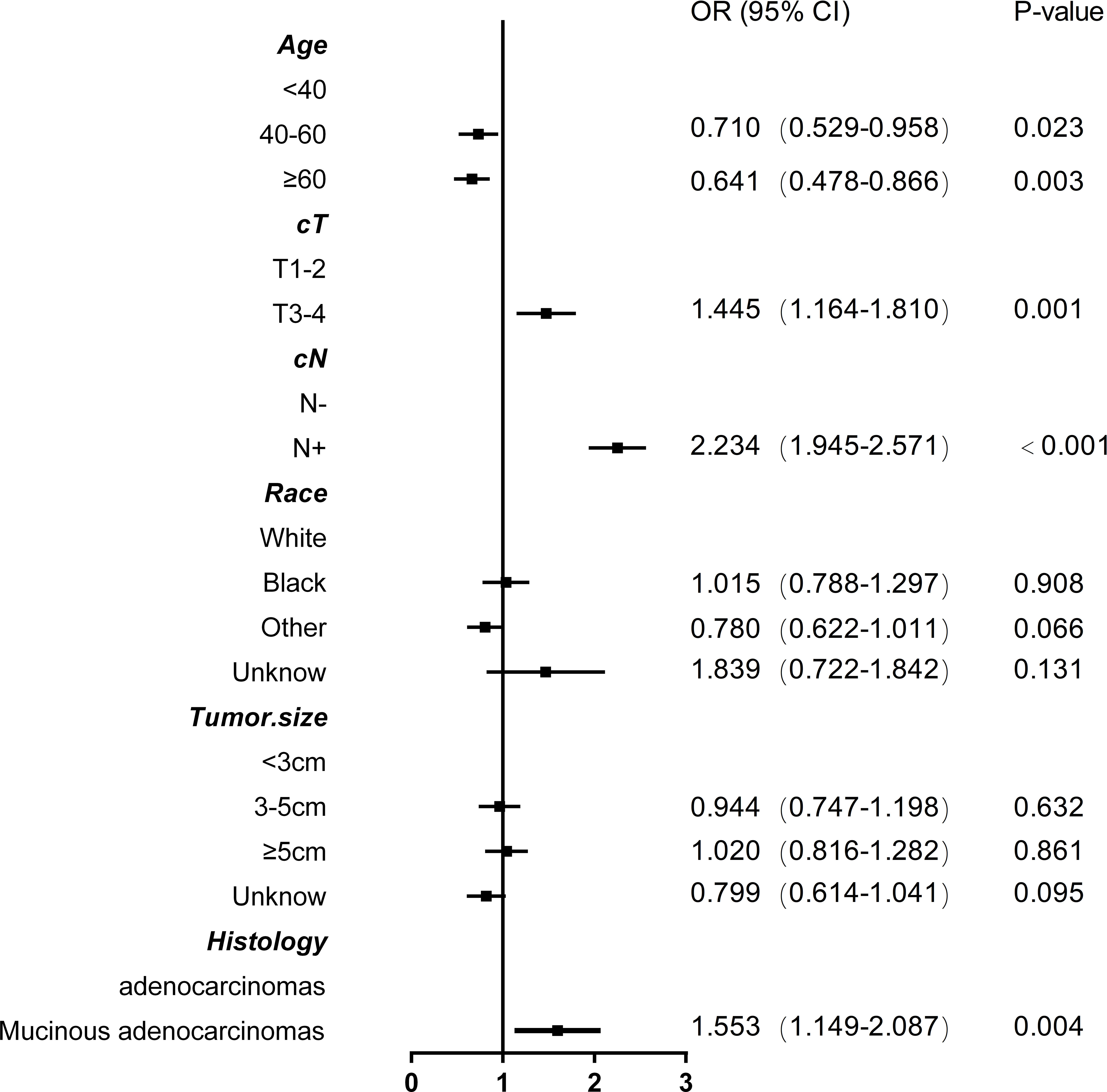
Figure 3 Forest plot with odds ratios base on multivariable logistic model associations with lymph node metastasis in rectal cancer patients after neoadjuvant chemoradiotherapy.
Based on the four independent risk factors (age, clinical T stage, clinical N stage, and histology), a nomogram was developed (Figure 4). A vertical line is drawn for each variable to see their respective score when using. The total score, which determines the risk probability of LNM, was calculated by adding each score together. For example, a 50- year-old patient (points= 12) with T3/T4 classification (points=46), with mucinous adenocarcinomas (points=55), with clinical N stage positive (points=100)would have a total score of 213, and a predicted LNM risk of 45%.
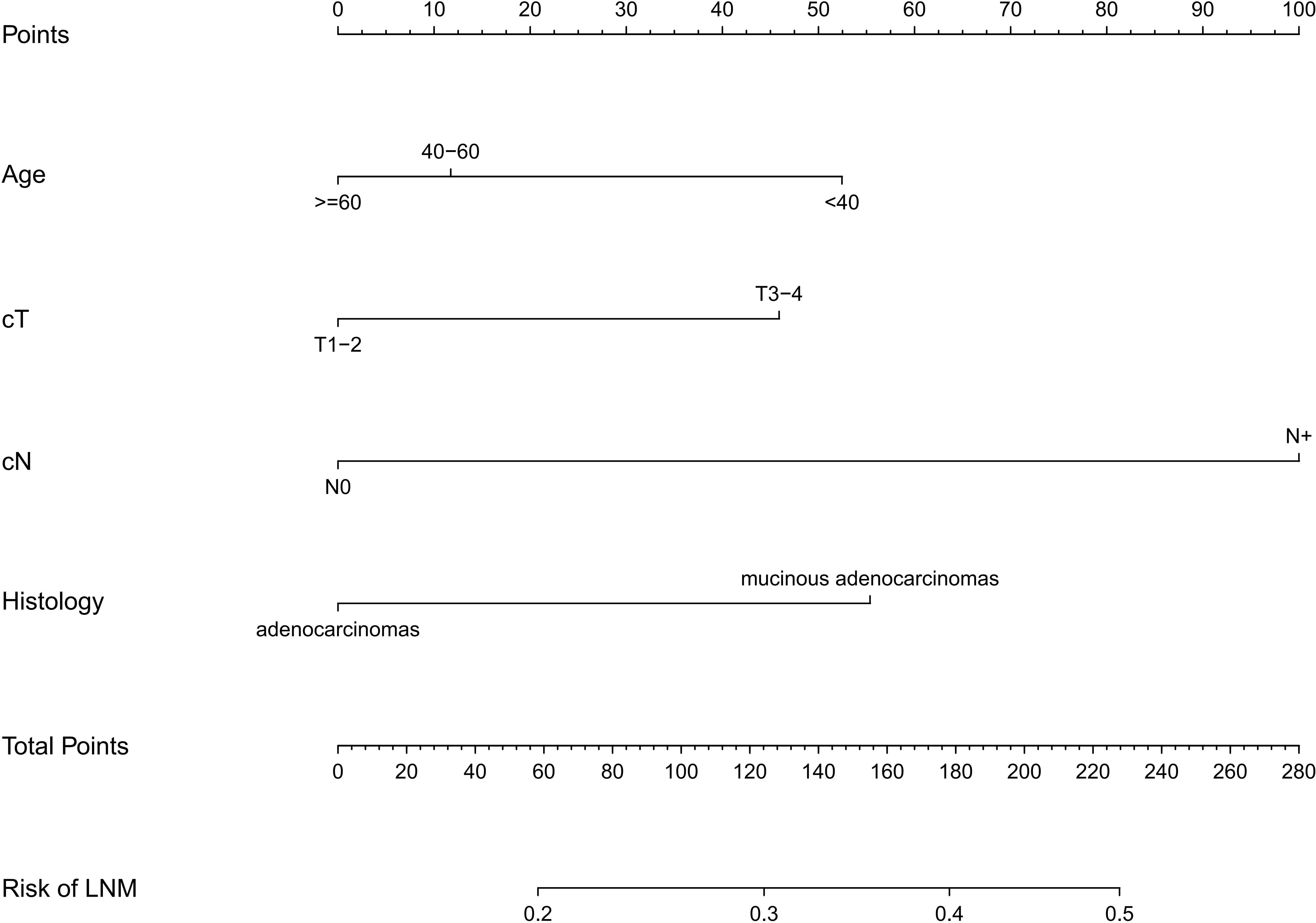
Figure 4 The nomogram for predicting lymph node metastasis in rectal cancer patients after neoadjuvant chemoradiotherapy. The nomogram was established in the training cohort using multivariable regression, consisting of age, cT(clinical T stage), cN(clinical T stage)and histology.
As shown in Figure 5, the calibration curve showed that the training and validation cohort curves were both close to the 45-degree line, indicating that the model can perfectly predict real events. The prediction model’s area under the ROC curve (AUC) for the training cohort was 0.635 (95% CI: 0.620-0.649).We used internal validation to test and verify the nomogram. The validation cohort’s calibration curve and ROC curve produced similar results to the training cohort. The area under the ROC curve (AUC) for the prediction model’s validation cohort was 0.624 (95% CI: 0.606-0.641).
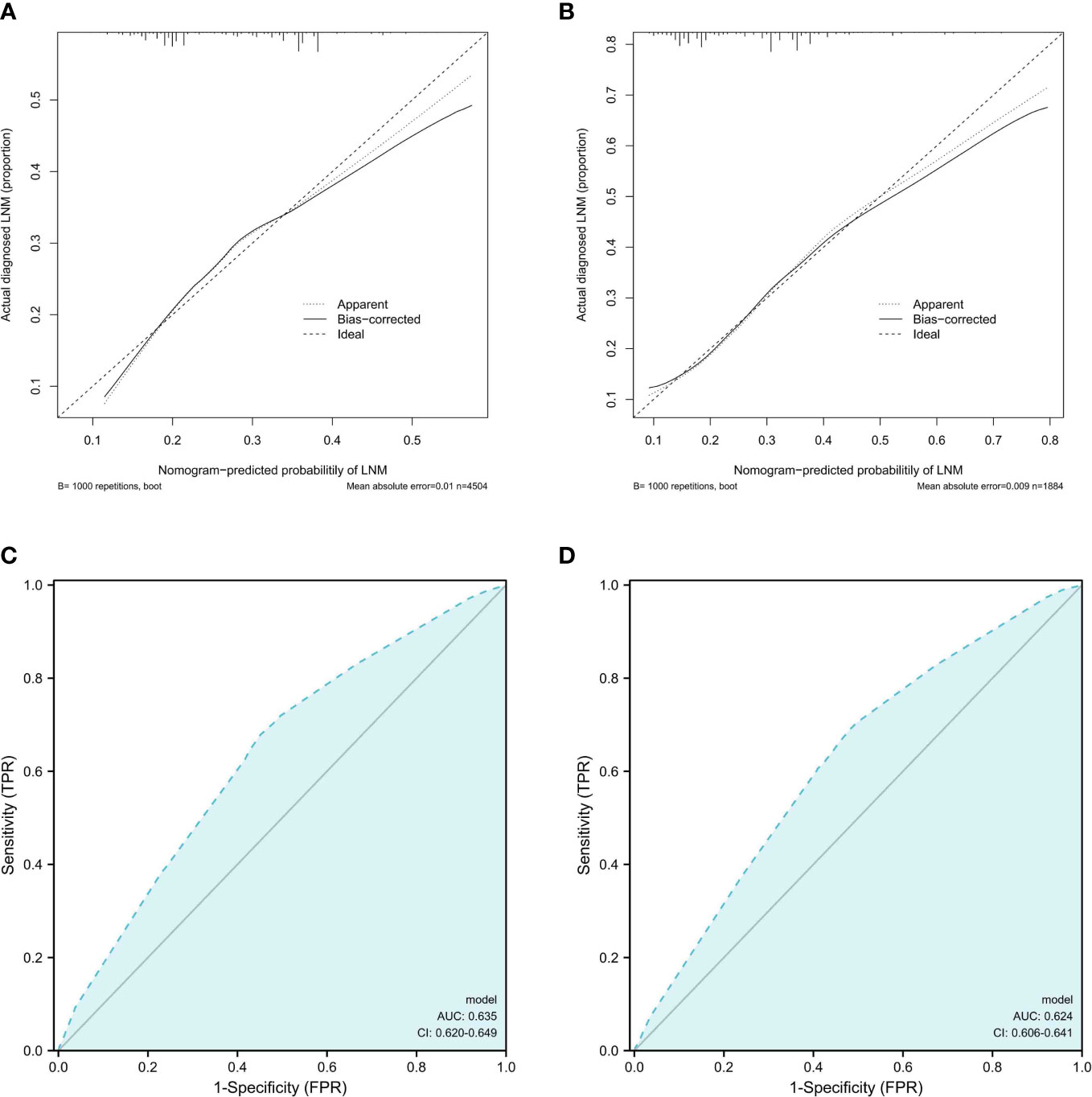
Figure 5 Calibration curves and ROC curves analysis. (A, B).Calibration curves for LNM rates predicted by nomogram for training and validation cohorts (C, D). Receiver operating characteristic (ROC) curves of the nomogram of LNM; The AUC values of the ROC curve predicted LNM rates for the nomogram in the training cohort, and validation cohort.
Figure 6 depicts the decision curve analysis for the nomogram. The decision curve analysis revealed that clinical decisions were superior to a scenario in which all patients or none were treated across a wide range of thresholds ranging from 0.10 to 0.46.
The 5-year overall survival of the N+ and N- groups is 66.3% and 78.9%, respectively (p<0.001), while the non-PCR and PCR groups are 75.2% and 85.7%, respectively (p<0.001) (Figure 7).
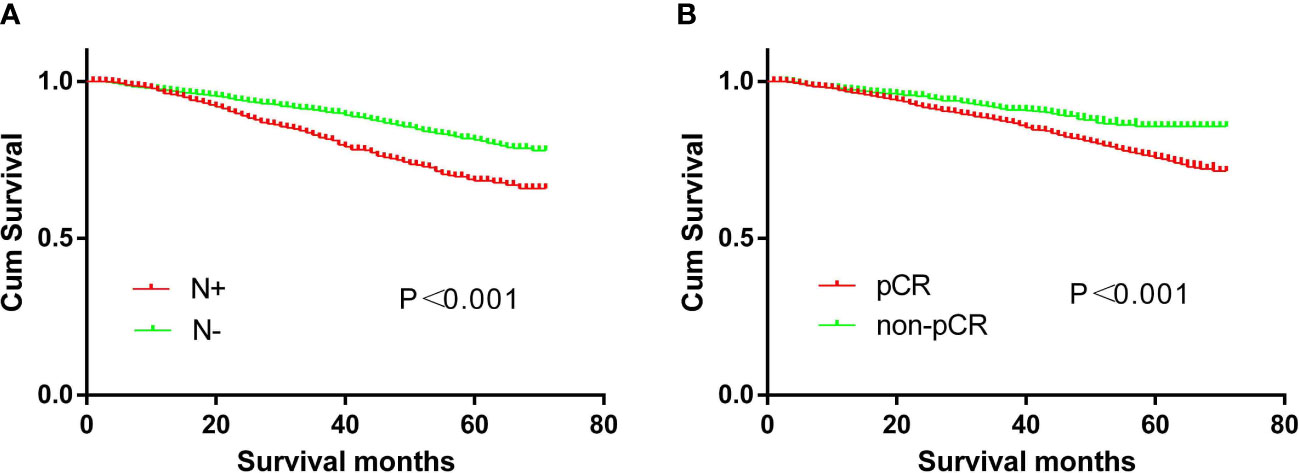
Figure 7 Survival curves. (A) The prognosis of CRC patients with or without N+ (p<0.01). (B) The prognosis of CRC patients with or without pCR (p<0.01).
Currently, neoadjuvant chemoradiotherapy is the pre-operative standard of care the for locally advanced rectal cancer. A promising non-operative management, the “watch and wait” approach, was first proposed by Dr. Angelita Habr-Gama for patients achieving clinical complete response following chemoradiotherapy (4). This approach avoids major surgical trauma, preserves anal function, and significantly improves the quality of life. Thus, the research interest has rapidly grown (8, 13, 14) and several clinical trials are ongoing (NCT03561142, NCT04246684). Julio Garcia-Aguilar and colleagues recently found that total neoadjuvant therapy (employing intensive schedules) may help half of the patients achieve organ preservation (14). Noteworthy, novel systemic agents are paving the way to futuristic non-operative management, also avoiding standard neoadjuvant treatments in selected clusters of patients (15). At present, there is currently no method for accurately predicting lymph node metastases after radiotherapy and chemotherapy. Residual metastatic lymph nodes may be the main reason for treatment failure in some patients. According to some studies, cCR does not equal pCR (16–18), and pCR of primary tumor does not equal complete lymph node remission. Patients with primary tumor pCR still have a 12.6-17.4% lymph node metastasis rate (17, 19).
Nomograms have been widely used to visualize colorectal cancer risk factors and prognosis (20–22). The nomogram is a graphical computational scale, and the minimum absolute shrinkage and selection operator (LASSO) is a regression analysis method. The combination of these two approaches aids in the quantification of individual risks for specific outcomes in various cancers (23). Few studies have used nomogram to predict lymph node metastasis following neoadjuvant chemoradiotherapy.
Our study evaluated the risk factors for lymph node metastasis after neoadjuvant chemoradiotherapy for rectal cancer, and a nomogram was used to visualize which patients were more likely to develop lymph node metastasis. Ten risk factor candidates were chosen in our study to form a nomogram by narrowing down the regression coefficients using the lasso method, which has been recommended for variable selection (20, 24). The best predictors were then identified using a multivariate logistic regression model (25, 26). Finally, four potential predictors nomograms were established.
According to the findings, younger patients, particularly those under the age of 40, have a higher proportion of lymph node metastases after neoadjuvant chemoradiotherapy. According to the findings of a few non-neoadjuvant chemoradiotherapy studies, the risk factors for lymph node metastasis in colorectal cancer patients, the younger patients had a higher risk of lymph node metastasis (27–29). Other research has found that the number of lymph nodes detected in colon cancer is related to age, with younger patients having a higher number of lymph nodes harvested (30, 31). A Chinese study also found that the risk of lymph node metastasis increased with age (32). However, a Germany study found that age was not related to lymph node metastasis following rectal cancer radiotherapy and chemotherapy (33).
The study founded that the higher the T stage after neoadjuvant chemoradiotherapy for rectal cancer, the greater the risk of lymph node metastasis. This was consistent with our usual clinical perception. According to a meta-analysis, higher T stage was associated with a higher rate of lymph node metastasis (34). Wang et al. found that the risk of lymph node metastasis following neoadjuvant chemoradiotherapy was related to T stage, with the deeper the invasion, the greater the risk of lymph node metastasis (32).
MRI is the most commonly used imaging tool for assessing lymph node metastasis prior to treatment, and it has a high level of accuracy. It is obvious that clinical N stage is related to postoperative lymph node metastasis. This was consistent with our findings; a multi-center study in the Netherlands found that cN+ was associated with lymph node metastasis following neoadjuvant chemoradiotherapy (19). In the meantime, our study found that patients with rectal signet ring cell carcinoma had a higher risk of lymph node metastasis following neoadjuvant chemoradiotherapy. This was consistent with the findings of several studies. The more poorly differentiated patients in the Netherlands study also had higher lymph node metastasis after neoadjuvant chemoradiotherapy (19). Wang et al. found that the risk of lymph node metastasis after neoadjuvant chemoradiotherapy was related to differentiation (32).
In our study, patients with pCR had a 5-year overall survival rate of 85.7%. The survival was not poor, but the prognosis was poor when compared to other studies, in which the 5-year overall survival of pCR was 93% (35) and 94% (8), respectively. This could be due to the SEER database’s incomplete preoperative chemoradiotherapy. Furthermore, the data included ranged from 2010 to 2015, and chemoradiotherapy was inconsistent during this time period. Furthermore, the median patient age in both studies was less than 60 years, at 57 years (8) and 59 years (35), respectively.
The 5-year overall survival rate of patients with non-pCR in our study was 75.2%, and a study using National Cancer Database (NCDB) data showed a survival rate of 73% (35), which was similar to our findings. Meanwhile, a meta-analysis found that patients with ypN0 and ypN+ disease had 5-year overall survival rates of 83.2% and 63.4%, respectively (34). The rate was also comparable to our study, which was 78.9% and 66.3%, respectively.
However, there were some limitations. Firstly, because the study was conducted retrospectively, the level of evidence was low. Secondly, the study was based on data from the SEER database, and the dose and timing of radiotherapy and chemotherapy were unknown. Thirdly, the SEER database does not specify the examination method used for preoperative staging, which may cause some inaccurate staging. Finally, the study used internal verification rather than external data for verification.
In conclusion, we developed a four-risk nomogram for predicting LNM in CRC patients who received CRT. Lymph nodes are still more likely to be positive after CRT in young, pre-treatment cN+, cT3/4, or mucinous adenocarcinoma patients. Strategies such as radical surgery rather than local resection or “watch-and-wait” may be appropriate.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Shuguang Hospital. The patients/participants provided their written informed consent to participate in this study.
XL, LS, and CH contributed equally to this work. XL, FY, and XS contributed to the study design and literature search. XL, LS, CH, and XK collected and analyzed the data. XL, LS, and CH contributed to the literature search and the writing of the manuscript. FY, XS, and XT contributed to the assessment of literature quality, review and revise of the manuscript. FY, XS, and XT are the correspondent authors. All authors contributed to the article and approved the submitted version.
The study was supported by Special clinical research project of health industry of Shanghai Municipal Health Commission, 20224Y0075. "Shanghai specialist capacity building project (ZY(2018-2020)-ZYBZ-07)". “Medicine New Star” of Shanghai, China, 202265. “Deep blue” talent project, “sailing program” funding of PLA Naval Military Medical University, 202128, Project establishment and cultivation of teaching achievements of Chang-Hai Hospital, PLA Naval Military Medical University, CHPY2021B15, and Youth program of Nat ional Natural Science Foundation of China, 81802434.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. NCCN guidelines insights: Rectal cancer, version 6.2020. J Natl Compr Canc Netw (2020) 18(7):806–15. doi: 10.6004/jnccn.2020.0032
2. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2017) 28(suppl_4):iv22–40. doi: 10.1093/annonc/mdx224
3. Song C, Chung JH, Kang SB, Kim DW, Oh HK, Lee HS, et al. Impact of tumor regression grade as a major prognostic factor in locally advanced rectal cancer after neoadjuvant chemoradiotherapy: A proposal for a modified staging system. Cancers (Basel) (2018) 10(9):319. doi: 10.3390/cancers10090319
4. Habr-Gama A, Perez RO, Nadalin W, Sabbaga J Jr, Ribeiro U, Silva e Sousa AH Jr, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann Surg (2004) 240(4):711–7. doi: 10.1097/01.sla.0000141194.27992.32
5. Hupkens B, Breukink SO, Stoot J, Toebes RE, van der Sande ME, Melenhorst J, et al. Oncological outcomes and hospital costs of the treatment in patients with rectal cancer: Watch-and-Wait policy and standard surgical treatment. Dis Colon Rectum (2020) 63(5):598–605. doi: 10.1097/DCR.0000000000001594
6. Issa N, Murninkas A, Powsner E, Dreznick Z. Long-term outcome of local excision after complete pathological response to neoadjuvant chemoradiation therapy for rectal cancer. World J Surg (2012) 36(10):2481–7. doi: 10.1007/s00268-012-1697-7
7. Habr-Gama A, Perez RO, Wynn G, Marks J, Kessler H, Gama-Rodrigues J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum (2010) 53(12):1692–8. doi: 10.1007/DCR.0b013e3181f42b89
8. Smith JJ, Strombom P, Chow OS, Roxburgh CS, Lynn P, Eaton A, et al. Assessment of a watch-and-Wait strategy for rectal cancer in patients with a complete response after neoadjuvant therapy. JAMA Oncol (2019) 5(4):e185896. doi: 10.1001/jamaoncol.2018.5896
9. Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the united states elderly population. Med Care (2002) 40(8 Suppl):IV–3-18. doi: 10.1097/00005650-200208001-00002
10. Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med (2007) 26(30):5512–28. doi: 10.1002/sim.3148
11. Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Software (2010) 33(1):1–22. doi: 10.18637/jss.v033.i01
12. Heagerty PJ. Marginally specified logistic-normal models for longitudinal binary data. Biometrics (1999) 55(3):688–98. doi: 10.1111/j.0006-341X.1999.00688.x
13. van der Valk M, Hilling DE, Bastiaannet E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NL, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the international watch & wait database (IWWD): An international multicentre registry study. Lancet (2018) 391(10139):2537–45. doi: 10.1016/S0140-6736(18)31078-X
14. Garcia-Aguilar J, Patil S, Gollub MJ, Kim JK, Yuval JB, Thompson HM, et al. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J Clin Oncol (2022) 40(23):2546–56. doi: 10.1200/JCO.22.00032
15. Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med (2022) 386(25):2363–76. doi: 10.1056/NEJMoa2201445
16. Hiotis SP, Weber SM, Cohen AM, Minsky BD, Paty PB, Guillem JG, et al. Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer: an analysis of 488 patients. J Am Coll Surg (2002) 194(2):131–5. doi: 10.1016/S1072-7515(01)01159-0
17. Wan J, Liu K, Zhu J, Li G, Zhang Z. Implications for selecting local excision in locally advanced rectal cancer after preoperative chemoradiation. Oncotarget (2015) 6(13):11714–22. doi: 10.18632/oncotarget.3418
18. Zhang X, Fan J, Zhang L, Wang J, Wang M, Zhu J. Association between three-dimensional transrectal ultrasound findings and tumor response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: An observational study. Front Oncol (2021) 11:648839. doi: 10.3389/fonc.2021.648839
19. Bosch SL, Vermeer TA, West NP, Swellengrebel HA, Marijnen CA, Cats A, et al. Clinicopathological characteristics predict lymph node metastases in ypT0-2 rectal cancer after chemoradiotherapy. Histopathology (2016) 69(5):839–48. doi: 10.1111/his.13008
20. Huang YQ, Liang CH, He L, Tian J, Liang CS, Chen X, et al. Development and validation of a radiomics nomogram for preoperative prediction of lymph node metastasis in colorectal cancer. J Clin Oncol (2016) 34(18):2157–64. doi: 10.1200/JCO.2015.65.9128
21. Wada Y, Shimada M, Murano T, Takamaru H, Morine Y, Ikemoto T, et al. A liquid biopsy assay for noninvasive identification of lymph node metastases in T1 colorectal cancer. Gastroenterology (2021) 161(1):151–162.e1. doi: 10.1053/j.gastro.2021.03.062
22. Ding X, Yang X, Wu D, Huang Y, Dai Y, Li J, et al. Nomogram predicting the cancer-specific survival of early-onset colorectal cancer patients with synchronous liver metastasis: a population-based study. Int J Colorectal Dis (2022) 37(6):1309–19. doi: 10.1007/s00384-022-04175-x
23. Kawai K, Sunami E, Yamaguchi H, Ishihara S, Kazama S, Nozawa H, et al. Nomograms for colorectal cancer: A systematic review. World J Gastroenterol (2015) 21(41):11877–86. doi: 10.3748/wjg.v21.i41.11877
24. Liang C, Huang Y, He L, Chen X, Ma Z, Dong D, et al. The development and validation of a CT-based radiomics signature for the preoperative discrimination of stage I-II and stage III-IV colorectal cancer. Oncotarget (2016) 7(21):31401–12. doi: 10.18632/oncotarget.8919
25. Lee TF, Chao PJ, Chang L, Ting HM, Huang YJ. Developing multivariable normal tissue complication probability model to predict the incidence of symptomatic radiation pneumonitis among breast cancer patients. PloS One (2015) 10(7):e0131736. doi: 10.1371/journal.pone.0131736
26. Lee TF, Chao PJ, Ting HM, Chang L, Huang YJ, Wu JM, et al. Using multivariate regression model with least absolute shrinkage and selection operator (LASSO) to predict the incidence of xerostomia after intensity-modulated radiotherapy for head and neck cancer. PloS One (2014) 9(2):e89700. doi: 10.1371/journal.pone.0089700
27. Kudo SE, Ichimasa K, Villard B, Mori Y, Misawa M, Saito S, et al. Artificial intelligence system to determine risk of T1 colorectal cancer metastasis to lymph node. Gastroenterology (2021) 160(4):1075–1084.e2. doi: 10.1053/j.gastro.2020.09.027
28. Zhang QW, Sun LC, Tang CT, Liang Q, Zhou YY, Chen HM, et al. Inverse association of age with risk of lymph node metastasis in superficial colorectal cancer: A Large population-based study. Oncologist (2020) 25(6):e920–7. doi: 10.1634/theoncologist.2019-0815
29. Ye H, Zheng B, Zheng Q, Chen P. Influence of old age on risk of lymph node metastasis and survival in patients with T1 colorectal cancer: A population-based analysis. Front Oncol (2021) 11:706488. doi: 10.3389/fonc.2021.706488
30. Samdani T, Schultheis M, Stadler Z, Shia J, Fancher T, Misholy J, et al. Lymph node yield after colectomy for cancer: Is absence of mismatch repair a factor. Dis Colon Rectum (2015) 58(3):288–93. doi: 10.1097/DCR.0000000000000262
31. Ahmadi O, Stringer MD, Black MA, McCall JL. Influence of age and site of disease on lymph node yield in colorectal cancer. N Z Med J (2014) 127(1395):31–40.
32. Zhao Q, Shi X, Fu C, Yu E, Zhang W, Meng R, et al. [Assessment of the risk factors relating to lymph node metastasis in rectal cancer after neoadjuvant chemoradiotherapy and the clinical significance]. Zhonghua Wei Chang Wai Ke Za Zhi (2016) 19(9):1040–3. doi: 10.3760/cma.j.issn.1671-0274.2016.09.017
33. Kreis ME, Maurer CA, Ruppert R, Ptok H, Strassburg J, Junginger T, et al. [Lymph node dissection after primary surgery and neoadjuvant radiochemotherapy of rectal cancer. Interim analysis of a multicenter prospective observational study (OCUM)]. Chirurg (2015) 86(12):1132–7. doi: 10.1007/s00104-015-0062-4
34. Haak HE, Beets GL, Peeters K, Nelemans PJ, Valentini V, Rödel C, et al. Prevalence of nodal involvement in rectal cancer after chemoradiotherapy. Br J Surg (2021) 108(10):1251–8. doi: 10.1093/bjs/znab194
35. Hasan S, Renz P, Wegner RE, Finley G, Raj M, Monga D, et al. Microsatellite instability (MSI) as an independent predictor of pathologic complete response (PCR) in locally advanced rectal cancer: A national cancer database (NCDB) analysis. Ann Surg (2020) 271(4):716–23. doi: 10.1097/SLA.0000000000003051
Keywords: rectal cancer (RC), lymph node metastasis, neoadjuvant chemoradiotherapy (NACRT), nomogram, prediction model, SEER
Citation: Liu X, Sha L, Huang C, Kong X, Yan F, Shi X and Tang X (2023) A nomogram prediction model for lymph node metastasis risk after neoadjuvant chemoradiotherapy in rectal cancer patients based on SEER database. Front. Oncol. 13:1098087. doi: 10.3389/fonc.2023.1098087
Received: 14 November 2022; Accepted: 14 February 2023;
Published: 27 February 2023.
Edited by:
Francesca De Felice, Sapienza University of Rome, ItalyReviewed by:
Mikhail Danilov, A.S. Loginov Moscow Clinical Scientific Centre, RussiaCopyright © 2023 Liu, Sha, Huang, Kong, Yan, Shi and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feihu Yan, ZmVpaHVfMTk4OUAxNjMuY29t; Xiaohui Shi, c2hpeGlhb2h1aTAwMjFAMTYzLmNvbQ==; Xuefeng Tang, Z2cxMzM3MDE3OTgzNUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.