- 1Cancer Center, Department of Medical Oncology, Zhejiang Provincial People's Hospital (Affiliated People's Hospital, Hangzhou Medical College), Hangzhou, Zhejiang, China
- 2Graduate School of Clinical Medicine, The Qingdao University Medical College, Qingdao, Shandong, China
- 3Graduate School of Clinical Medicine, Bengbu Medical College, Bengbu, Anhui, China
- 4Graduate School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
Background: Fruquintinib and regorafenib have been approved for the third-line therapy of metastatic colorectal cancer (mCRC) in China. However, at present, there is a lack of head-to-head clinical trials on the comparison of efficacy and safety between the two drugs.
Materials and methods: The data of patients with mCRC who were treated with fruquintinib or regorafenib after the standard chemotherapy in Zhejiang Provincial People’s Hospital from October 2018 to November 2021 were collected and analyzed. The primary endpoints were overall survival (OS), progression-free survival (PFS) and adverse events. The secondary endpoints were the appropriate sequence, objective remission rate (ORR) and disease control rate (DCR) of fruquintinib and regorafenib.
Results: A total of 105 patients were enrolled in this study. The ORR of fruquintinib group (n=55) and regorafenib group (n=50) were 6.1% and 2.0%; the DCR were 65.3% and 54.2%, respectively. There was no significant difference in median OS (mOS) and PFS (mPFS) between the two groups (mOS:14.2 vs12.0 months, p=0.057; mPFS:4.4 vs 3.5 months, p=0.150). Combined immunotherapy showed a synergistic effect. The mPFS and mOS of fruquintinib combined with anti-PD-1 therapy were longer than those of fruquintinib monotherapy (mPFS:5.9 vs 3.0 months, p=0.009; mOS:17.5 vs 11.3 months, p=0.008). The mOS of patients treated with regorafenib combined with anti-PD-1 therapy was 14.8 months higher than that of regorafenib monotherapy (p=0.045). When combined with anti-PD-1 therapy, the mPFS and mOS of fruquintinib was significantly longer than regorafenib (mPFS:5.9 vs 3.8 months, p=0.018; mOS:17.5 vs 14.8 months, p=0.044). In the treatment sequence, the OS of patients treated with regorafenib and then fruquintinib was significantly longer than that of the reverse treatment sequence (15.0 vs 8.3 months, p=0.019). The adverse reactions were generally similar, but the incidence of hand-foot syndrome of regorafenib was higher than that of fruquintinib, while fruquintinib was more prone to grade 3 hypertension.
Conclusion: Fruquintinib monotherapy showed better disease control rate and objective remission rate in the post-line therapy of metastasis colorectal cancer. Notably, the combination of PD-1 immunotherapy brought the additional effect, especially in the fruquintinib combined with anti-PD-1 therapy. Patients treated with regorafenib and then fruquintinib was significantly longer than that of the reverse treatment sequence. The toxicity of fruquintinib and regorafenib are similar.
Introduction
Colorectal cancer (CRC) is the third most common malignant tumor worldwide, and its mortality is second only to lung cancer (1). More than 20% of patients have metastasis at the time of initial diagnosis, most of them have unresectable tumors, and 25% of patients will develop metastasis after radical surgery (2). Standard treatment for these patients includes chemotherapy based on cytotoxic drugs (fluorouracil, oxaliplatin, irinotecan) and targeted therapy for vascular endothelial growth factor (VEGF; bevacizumab) and epidermal growth factor receptor (EGFR; cetuximab) (3). However, it is a pity that most patients with mCRC still have disease progression after first-or second-line standard treatment. At present, the choice of drugs for third-line or post-line treatment of mCRC is very limited.
Regorafenib is an oral multi-target kinase inhibitor, which inhibits tumorigenesis, tumor angiogenesis and tumor microenvironment by targeting VEGFR1/2/3, TIE-2, BRAF, KIT, RET, PDGFR and FGFR (4). The results of international randomized phase III CORRECT clinical trial show that regorafenib can prolong the survival time of chemotherapy-resistant patients with advanced CRC (5). The results of the subsequent phase III CONCUR clinical trial in Asia also show that the mOS and mPFS of patients with refractory mCRC were 8.8 months and 3.2 months respectively, significantly longer than those in the placebo group (6). Fruquintinib is a highly selective oral tyrosine kinase inhibitor targeting VEGFR1/2/3, which has a strong inhibitory effect on a variety of advanced tumors by inhibiting tumor angiogenesis (7, 8). The FRESCO study of phase III clinical study showed that the mOS in the fruquintinib group was significantly longer than that in the placebo group (9.3 vs 6.6 months, p<0.001), and the mPFS was significantly prolonged by 1.9 months (3.7 vs 1.8months, p<0.001) (9). Based on the above results, fruquintinib and regorafenib have been approved for third-line treatment of advanced CRC in China. However, there are still some questions about the application of these two multi-target kinase inhibitors.
Firstly, there is a lack of head-to-head clinical trials to confirm whether the efficacy and adverse reactions of the two drugs are different. A limited number of meta-analyses have indicated that the efficacy of fruquintinib and regorafenib is similar, but there is no consistent conclusion about the toxicity of the two drugs (10, 11). Second, as both drugs are approved and both are covered by health insurance in China, clinicians should prioritize which drug to use as a third-line treatment. Finally, the efficacy of fruquintinib or regorafenib alone is not significant. How to enhance the clinical efficacy of the two drugs or combine them with other treatment methods is still an urgent topic for clinical exploration. It is well known that tumors grow and evolve through continuous crosstalk with the surrounding microenvironment, and emerging evidence shows that angiogenesis and immunosuppression frequently occur simultaneously in response to this crosstalk. Accordingly, strategies combining anti-angiogenic therapy and immunotherapy seem to have the potential to tip the balance of the tumor microenvironment and improve treatment response (12).
Thus, we conducted this retrospective study to evaluate the efficacy and safety of fruquintinib and regorafenib in the real world and analyzed the rational use sequence of fruquintinib and regorafenib in patients with mCRC. Meanwhile, for the first time, we compared the continuing beneficial and adverse effects of long-term use of fruquintinib or regorafenib plus anti-PD-1 therapy.
Patients and methods
Patients
We collected information on patients with advanced CRC who were treated in Zhejiang Provincial people’s Hospital from October 2018 to November 2021 and conducted this real-world retrospective study. The follow-up period was from the first use of fruquintinib or regorafenib to death, loss of follow-up, or the end of the study. The main inclusion criteria of this study include: 1. mCRC confirmed by histopathology, computed tomography (CT) or magnetic resonance imaging (MRI). 2. Patients who had progressed or could not tolerate standard chemotherapy after second-line chemotherapy were treated with at least one cycle of fruquintinib or regorafenib on the third or posterior line. 3. The performance status of the eastern cooperative tumor group (ECOG-PS) was 0-2; 4. The age is between 18 and 78 years old. 5. At least one assessable lesion is included. The main exclusion criteria include: 1. Severe organ dysfunction; 2. Non-third-line or later-line use of regorafenib or fruquintinib. The deadline for data collection is November 1, 2021. This study was performed in line with the principles of the Declaration of Helsinki (as revised in Fortaleza, Brazil, in October 2013) and the international standard of good Clinical practice (GCP), and was approved by the Ethics Committee of Zhejiang Provincial people’s Hospital (approval No.: QT2022269).
Therapy schedule
The patients in the fruquintinib group were treated with fruquintinib 3-5mg, and the patients in the regorafenib group were treated with 80-160mg once a day for 21 days, 28 days as a treatment cycle. The dosage of the medicine was adjusted based the side effects and the tolerance of patients. Some patients accepted the combination of PD-1 inhibitors with fruquintinib or regorafenib, including: sintilimab (200mg, Q3W), camrelizumab (200mg, Q3W), tislelizumab (200mg, Q3W), pembrolizumab (200mg, Q3W), nivolumab (240mg, Q3W), toripalimab (240mg, Q3W). Treatment will no longer continue in the event of disease progression, intolerable toxicity or other reasons that require discontinuation of treatment.
Primary and secondary endpoints
The OS, PFS and adverse events (AEs) of fruquintinib and regorafenib were the main endpoints of this study, while the appropriate sequences, objective remission rate and disease control rate of fruquintinib and regorafenib were the secondary endpoints. The additional objective of this research is to compare the efficacy of fruquintinib and regorafenib combined immunotherapy.
Assessment
Clinicians and researchers measured tumor lesions by CT or MRI every 2-3 treatment cycles, and evaluated tumor response according to The Response Evaluation Criteria in Solid Tumors (RECIST) Version 1.1. The observed drug-related adverse reactions were classified and graded according to The Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.
Statistical analysis
SPSS version 25.0 (IBM Corp., Armonk, N.Y., USA) and GraphPad Prism 5.0 (GraphPad Software, San Diego, California, USA) were used for statistical analysis. The continuous variables in the baseline characteristics were compared by independent sample T-test, and the categorical variables in the baseline characteristics and treatment responses were assessed using χ2 test, Fisher exact test and Mann Whitney U nonparametric test. The survival curve was drawn by Kaplan-Meier method and Log-rank test was carried out. Cox proportional regression model was used to analyze the survival and prognosis. The significant factors (p<0.1) determined by univariate Cox regression analysis were included in multivariate Cox regression analysis, and the hazard ratio (HR) and confidence interval (CI) were calculated. The difference was statistically significant with p<0.05.
Results
Patient characteristics
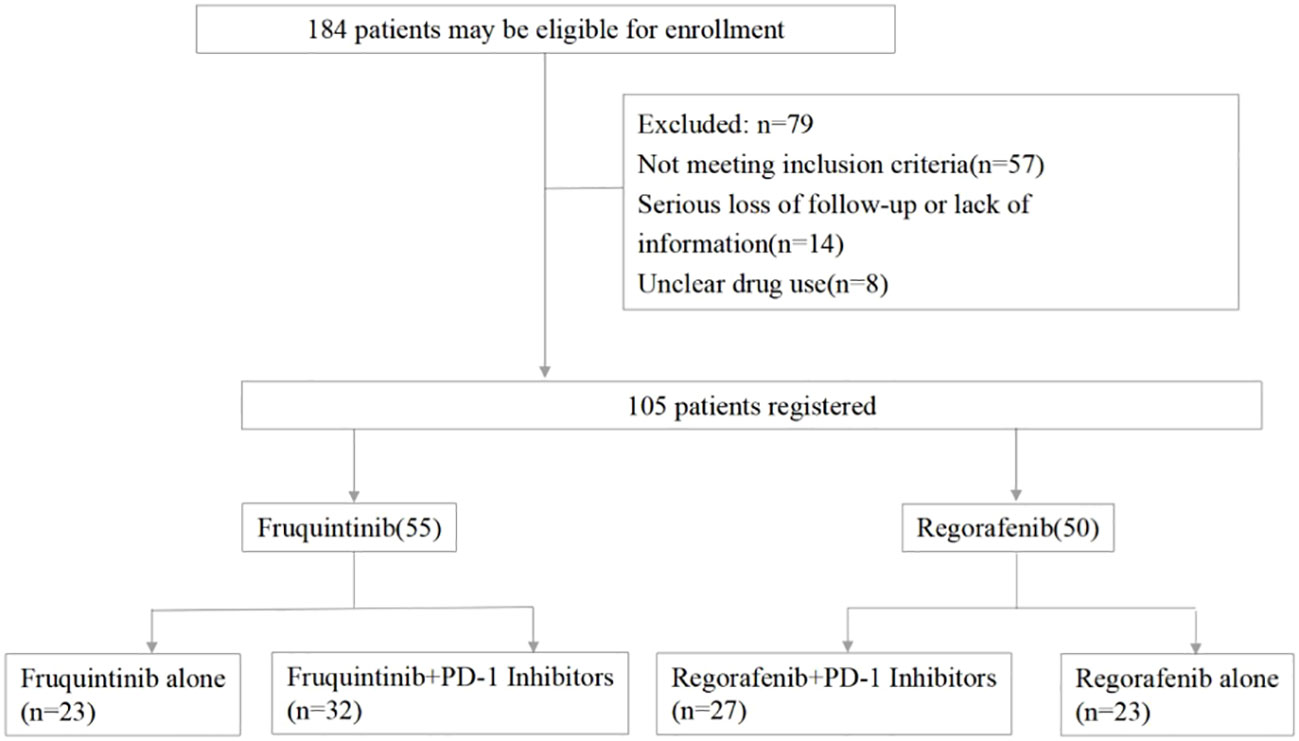
184 patients were screened altogether, and only 105 patients met all the criteria and were enrolled in this study, including 55 patients in the fruquintinib group and 50 patients in the regorafenib group, and 32 patients in the fruquintinib group and 27 patients in the regorafenib group were treated with PD-1 inhibitors (Figure 1).
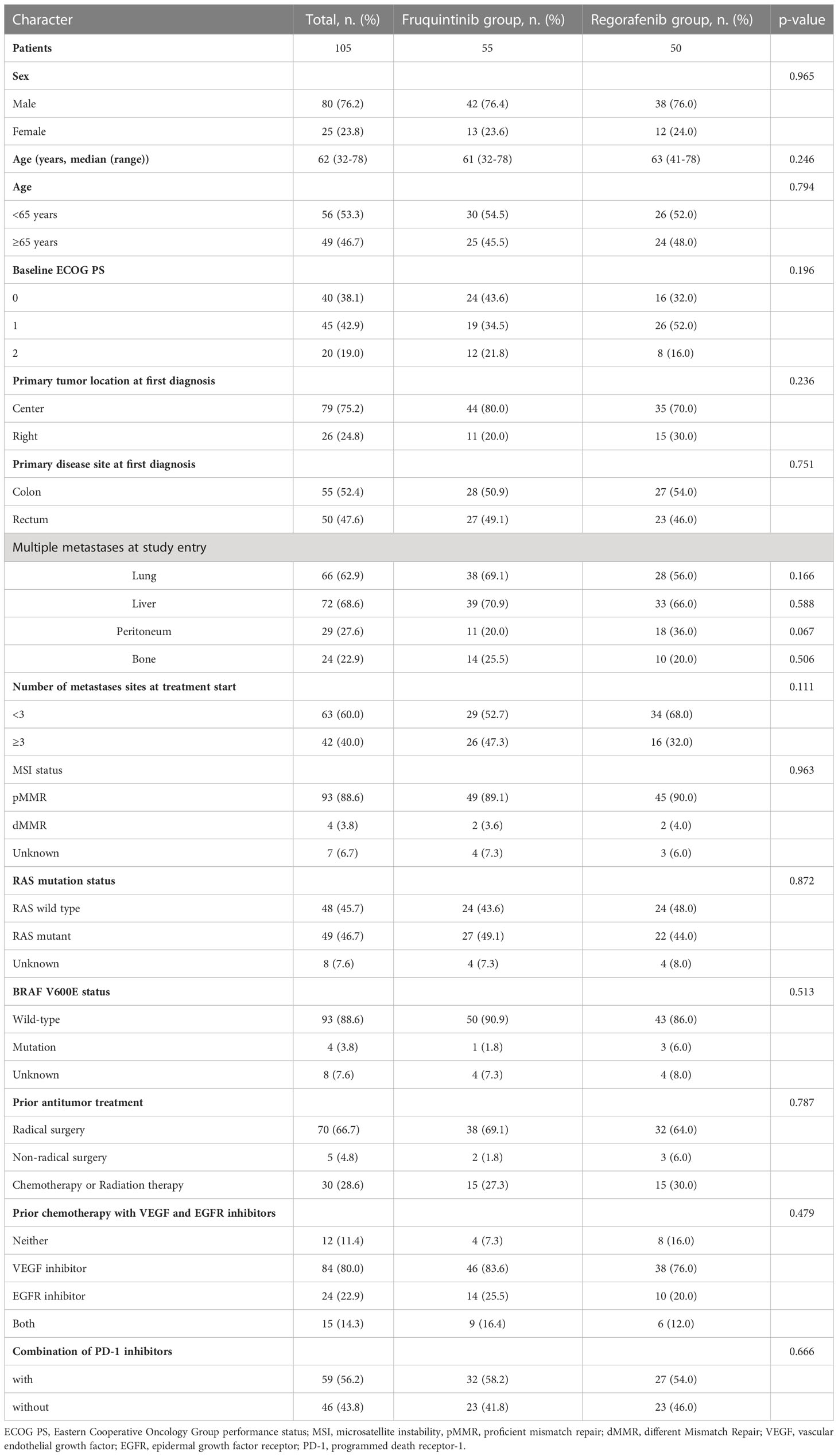
Table 1 showed the baseline characteristics of all enrolled patients, including 80 (76.2%) males, 25 (23.8%) females and a median age of 63 years (32-78). Most of the patients (81.0%) were in good condition, with an ECOG scores of 0-1. 79 (75.2%) patients had left-colon tumor and 26 (24.8%) patients had right-colon tumor. The main distant metastases were liver metastasis (68.6%), lung metastasis (62.9%), peritoneal metastasis (27.6%) and bone metastasis (22.9%). Among the primary tumor gene mutations, 49 (46.7%) patients had a RAS mutation, 48 (45.7%) patients were RAS wild-type, 4 (3.8%) patients had a BRAF mutation and 93 (88.6%) patients were BRAF wild type. In the previous anti-tumor targeted therapy, 83.6% and 76.0% of the patients in the fruquintinib group and regorafenib group received anti-VEGF therapy, and 25.5% and 20.0% of the patients received anti-EGFR therapy. There was no statistical difference in baseline characteristics between the two groups.
Treatment
All individuals received fruquintinib or regorafenib as the third- or later-line therapy after the failure of standard chemotherapy. In the fruquintinib group, the initial dose of fruquintinib was 3mg (n=31), 4mg (n=14) and 5mg (n=10) (Supplementary Figure 1A), and the final dose was 2mg (n=2), 3mg (n=21), 4mg (n=19) and 5mg (n=13) (Supplementary Figure 1B). In the fruquintinib group, the initial dose was 80mg (n=31), 120mg (n=11) and 160mg (n=8) (Supplementary Figure 1D), and the adjusted final dose was 40mg (n=1), 80mg (n=29), 120mg (n=13) and 160mg (n=7) (Supplementary Figure 1E). In terms of combined anti-PD-1 treatment, fruquintinib combined with PD-1 inhibitors (FP) accounted for 58.2% (32/55), while regorafenib combined with PD-1 inhibitor (RP) accounted for 54.0% (27/50). The most commonly used PD-1 inhibitors in the two groups were sintilimab, camrelizumab and tislelizumab, accounting for 56.25%, 25.00%, 9.38% and 44.44%, 29.63%, 7.41% respectively (Supplementary Figures 1C, F).
Clinical efficacy
By the end of last follow-up (September 1, 2022), a total of 105 patients, 97 patients had completed the efficacy evaluation, and 8 patients had not received PFS evaluation owing to serious adverse reactions or other disease progression, including 6 individuals in the fruquintinib group and 2 individuals in the regorafenib group. Finally, 81.1% of the patients in the fruquintinib group completed the PFS assessment, 67.3% (37/55) eventually died, and 32.7% (18/55) patients were still in follow-up, while 48 (96.0%) PFS events and 43 (86.0%) deaths were observed in the regorafenib group, and 7 (14.0%) patients were still followed up.
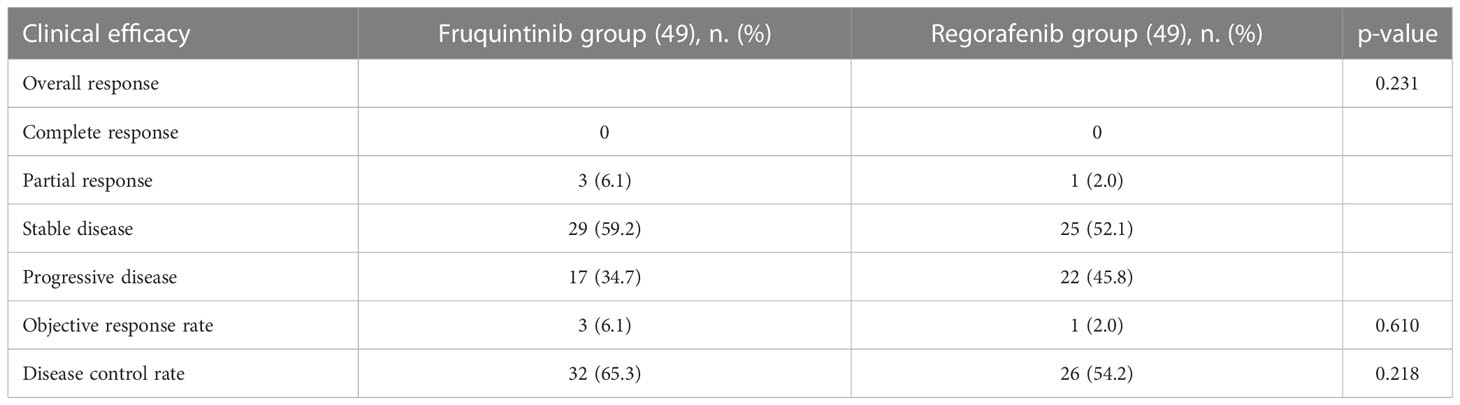
Table 2 summarized the clinical efficacy of each group. The best response achieved in both groups was partial remission (PR). There were 3 patients in the fruquintinib group, while only one in the regorafenib group achieved PR, 29 (59.2%) patients in the fruquintinib group achieved stable disease (SD), 17 (34.7%) patients received progressive disease (PD), while 25 (52.1%) patients in the regorafenib group achieved SD, and 22 (45.8%) patients experienced PD. Therefore, the ORR of the fruquintinib group was 6.1% (3/49), and the DCR was 65.3% (32/49), and the ORR and DCR of regorafenib group were 2.0% and 54.2%, respectively. Although the proportion of patients with ORR and DCR in fruquintinib group was higher than that in regorafenib group, the difference was not statistically significant.
In the subgroup analysis of fruquintinib and regorafenib monotherapy or combined immunotherapy, the DCR of fruquintinib monotherapy and regorafenib monotherapy were 54.5% and 47.6%, respectively, while the DCR and ORR of FP group and RP group were 74.1%, 7.4% and 59.3%, 3.7%, respectively. Although the DCR and ORR of fruquintinib were higher than those of regorafenib, whether alone or in combination with PD-1 inhibitor, no statistical significance was observed (Supplementary Table 1).
Survival analysis
The overall survival curve is shown in Figures 2, 3. The mPFS and mOS in fruquintinib group were 4.4 months and 14.2 months, and that of regorafenib group were 3.5 months and 12.0 months. There were no significant difference (p=0.150; p=0.057 Figures 2A, B). In the subgroup analysis of fruquintinib or regorafenib monotherapy, the median PFS of fruquintinib monotherapy and regorafenib monotherapy were 3.0 months and 2.4 months, respectively (p=0.527 Figure 3A), and the mOS was 11.3 months and 10 months respectively, and the difference was not statistically significant (p=0.687 Figure 3B). Subsequently, in the subgroup analysis of FP and RP, it was found that the median PFS of FP was significantly longer than that of RP (5.9 vs 3.8 months, p=0.018 Figure 3C). The median OS of FP and RP was 17.5 months and 14.8 months, respectively. Compared with the RP group, FP group has a longer OS (p=0.044 Figure 3D).
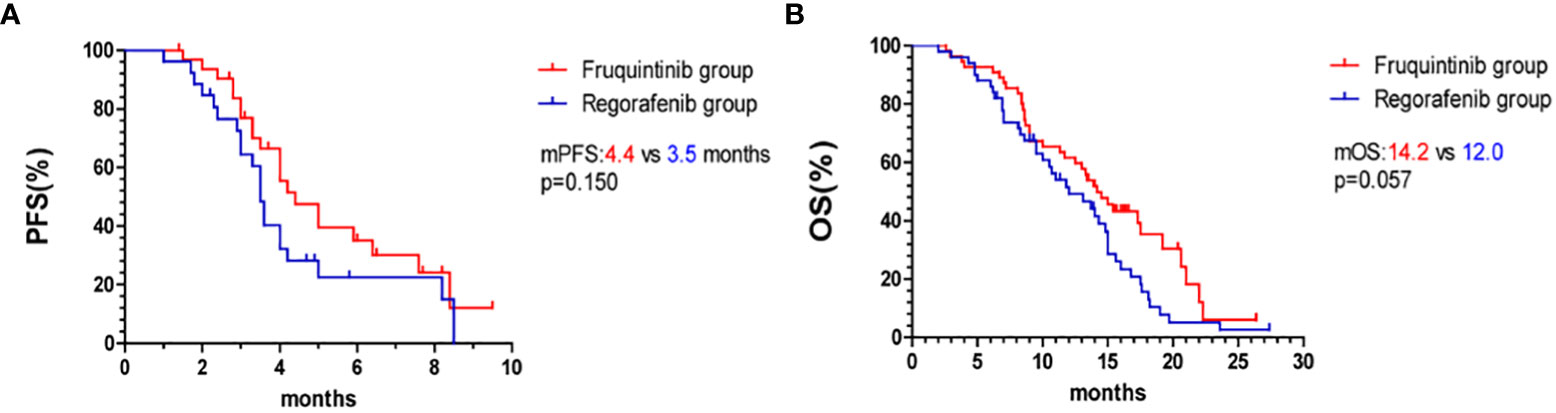
Figure 2 Kaplan–Meier curves. (A) PFS in the fruquintinib group and regorafenib group. (B) OS in the fruquintinib group and regorafenib group. mPFS, median progression-free survival. mOS, overall survival.
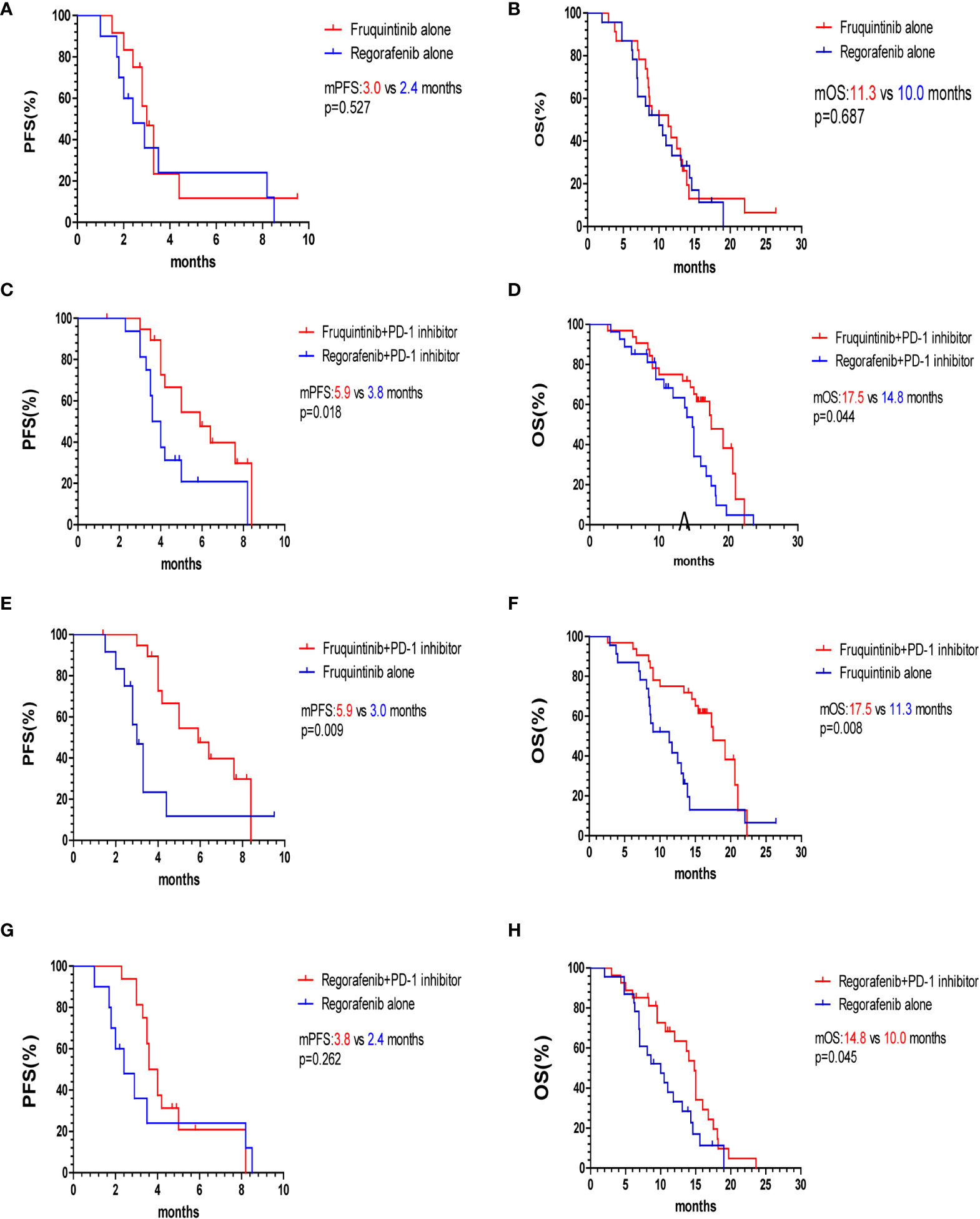
Figure 3 Kaplan–Meier survival curves of PFS and OS of two subgroups. (A, B) PFS and OS of fruquintinib and regorafenib monotherapy. (C, D) PFS and OS of FP and RP. (E, F) PFS and OS of fruquintinib with or without PD-1 inhibitors. (G, H) PFS and OS of regorafenib with or without PD-1 inhibitors. mPFS, median progression-free survival; mOS, overall survival; PD-1, programmed death receptor-1.
To further evaluate the survival benefits of immunotherapy, we compared the efficacy of fruquintinib or regorafenib monotherapy with that of combination with PD-1 inhibitors. The median PFS and OS of the FP group were significantly longer than that of the fruquintinib monotherapy group (p=0.009; p=0.008 Figures 3E, F). Compared with the regorafenib monotherapy group, the median OS of the RP group was prolonged (14.8 vs 10.0 months, p=0.045 Figure 3H). Although the PFS in the RP group showed an increasing trend compared with that in the regorafenib monotherapy group, there was no statistical significance (3.8 vs 2.4 months, p=0.262 Figure 3G).
Treatment sequence
In the fruquintinib group, 11 patients received fruquintinib after the progression of treatment with regorafenib, of which 8 (72.7%) achieved SD, while in the regorafenib group, 8 patients were treated with regorafenib after the progression of fruquintinib, of which only 3 (37.5%) controlled the progression of the disease, and the difference between the two was not statistically significant (p=0.181). However, the median OS of patients treated with fruquintinib after the progression of regorafenib treatment was significantly longer than that of the opposite treatment sequence (15.0 vs 8.3 months, p=0.019 Figure 4).
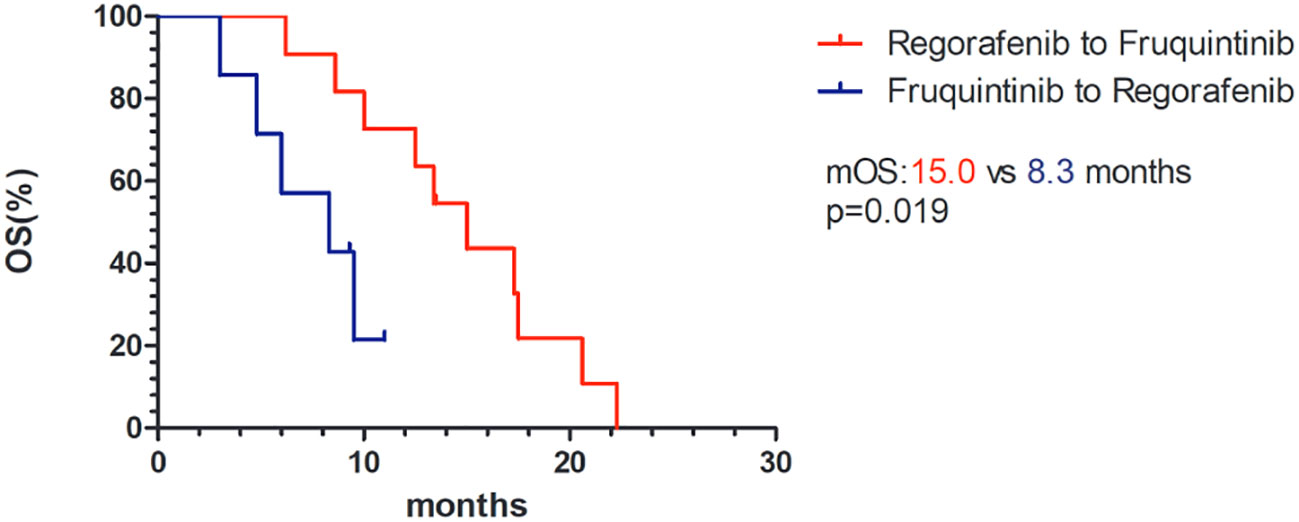
Figure 4 Kaplan–Meier survival curves of OS of different therapeutic sequences of fruquintinib and regorafenib. mOS, median overall survival.
Safety
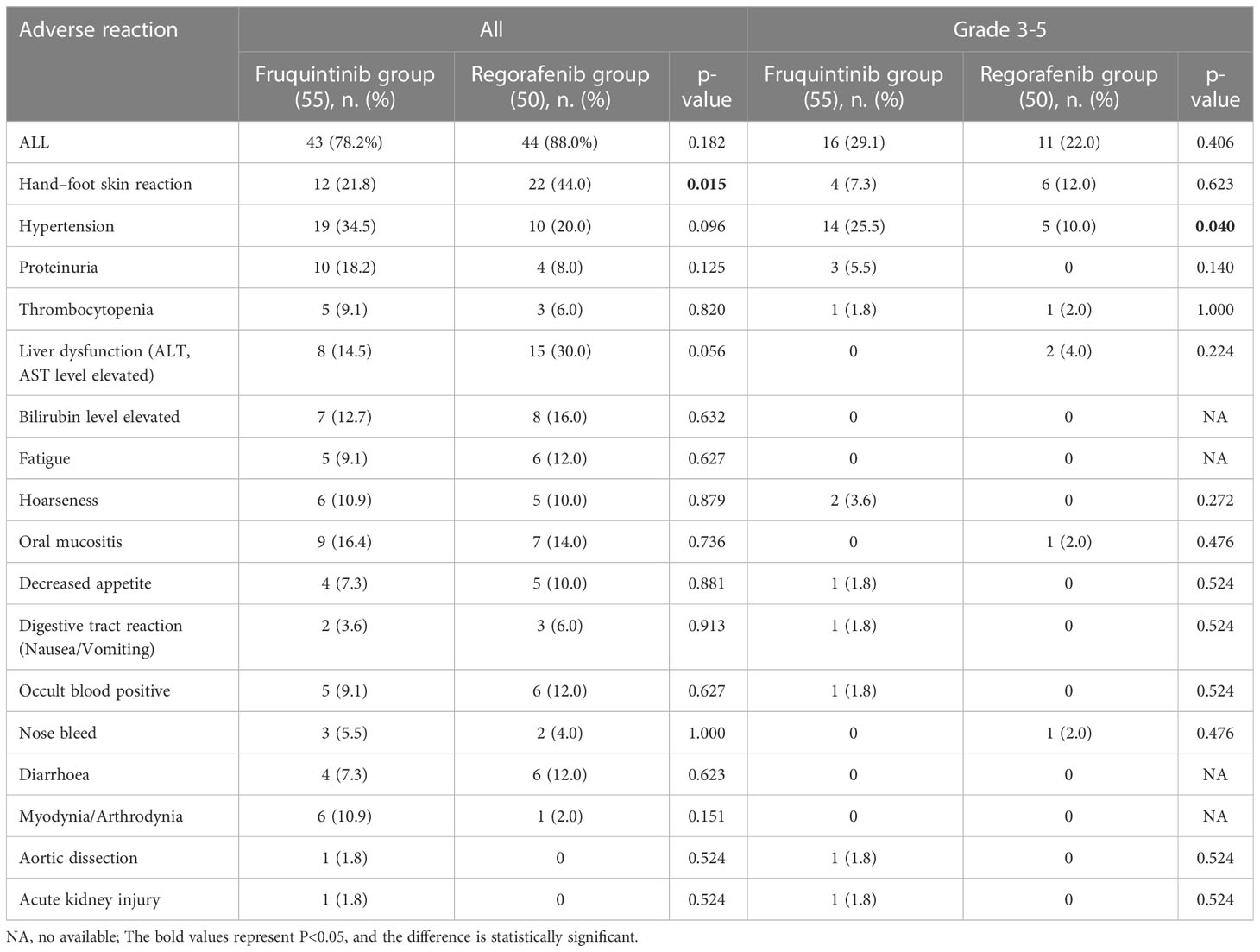
The adverse reactions (AEs) of fruquintinib and regorafenib are shown in Table 3. In general, 43 (78.2%) of the 55 suffers in the fruquintinib group experienced at least one AE, while 44 (88.0%) suffers in the regorafenib group experienced at least one AE, and there was no significant difference between the two groups (χ2 = 1.78, p=0.182). 16 (29.1%) patients had at least one grade 3 adverse event in the fruquintinib group, of which 14 (25.5%) had treatment-related withdrawal or reduction. 11 (22.0%) patients in the regorafenib group had at least one grade 3 adverse event, of which 8 (16%) were stopped or reduced owing to intolerable drug toxicity. There were no deaths due to AEs. The most common AEs in the fruquintinib group were hypertension (34.5%), hand-foot syndrome (21.8%) and proteinuria (18.2%), while in the regorafenib group, hand-foot syndrome (44.0%), abnormal liver function (30.0%) and hypertension (20.0%) were the most common AEs. The incidence of hand-foot syndrome in the regorafenib group was higher than that in the fruquintinib group (χ2 = 5.89, p=0.015), while the probability of hypertensive events with ≥ 3 grade in the fruquintinib group was higher than that in the regorafenib group (χ2 = 4.22, p=0.040). It is worth mentioning that in the fruquintinib group, we observed for the first time the occurrence of aortic dissection associated with fruquintinib.
Analysis of prognostic factors of combined immunotherapy with fruquintinib or regorafenib
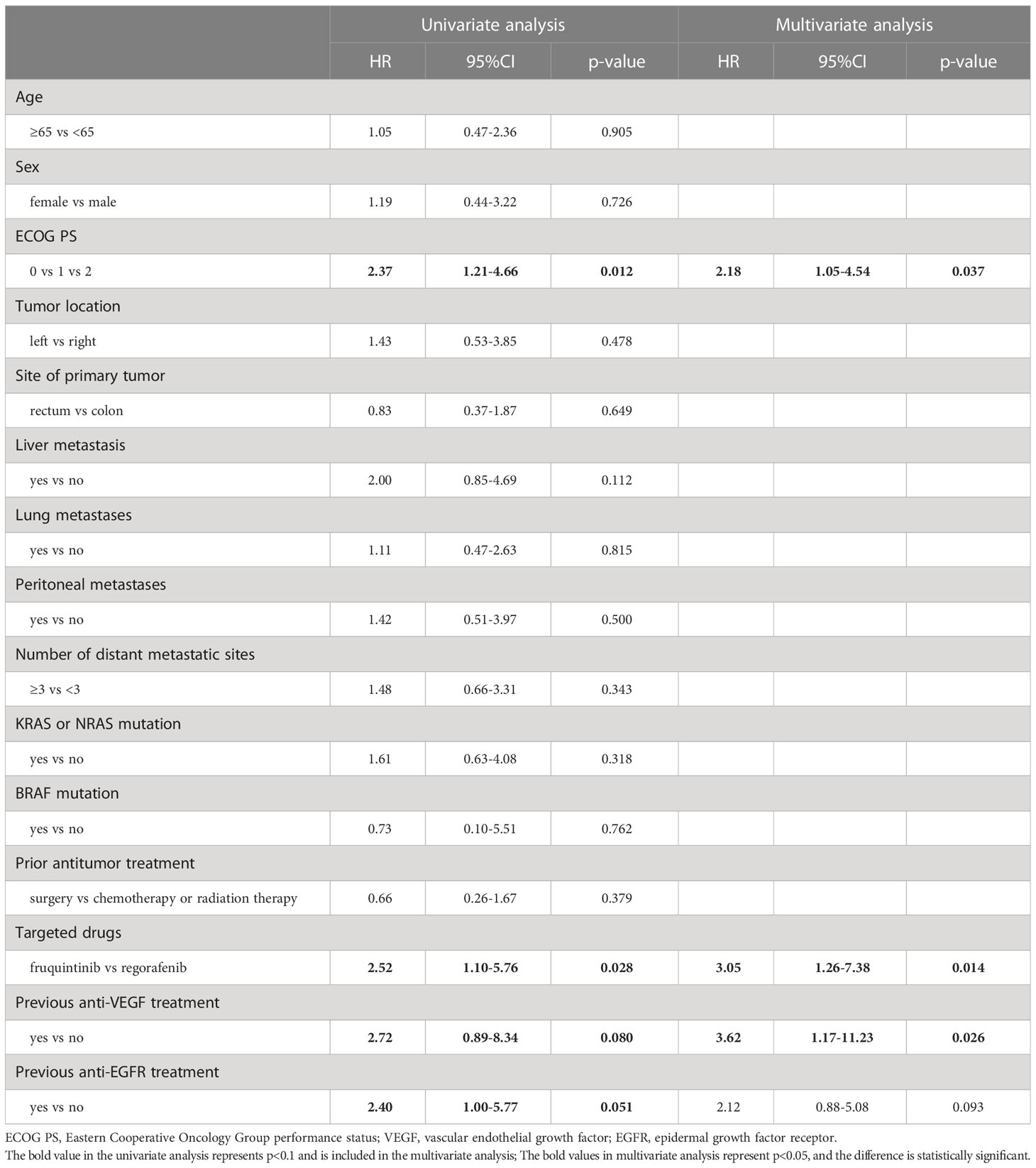
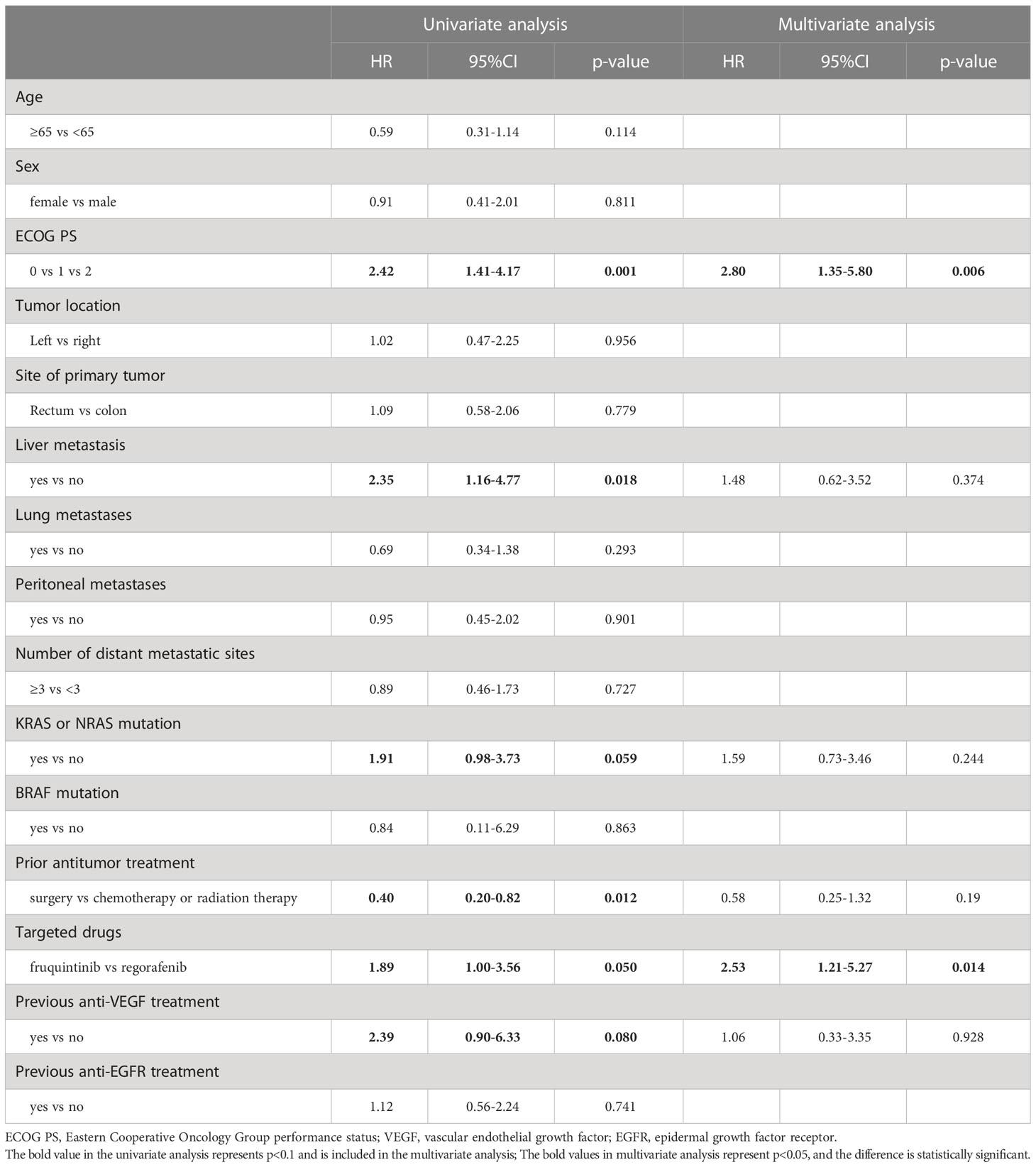
In the univariate analysis of 59 patients treated with combined immunotherapy, ECOG-PS score and fruquintinib or regorafenib targeted therapy were significantly correlated with PFS. Previous anti-VEGF therapy and anti-EGFR therapy were factors with p<0.1. In multivariate analysis, ECOG-PS score, previous anti-VEGF therapy and fruquintinib or regorafenib targeted therapy were significant independent predictors of PFS (Table 4). In the univariate analysis of OS, ECOG-PS score, liver metastasis and previous antineoplastic therapy were associated with OS. RAS mutation, fruquintinib or regorafenib targeted therapy and previous anti-VEGF therapy were factors with p<0.1. In multivariate analysis, ECOG-PS score and fruquintinib or regorafenib targeted therapy were independent predictors of OS (Table 5).
Discussion
As the choice of third-line therapy for mCRC, there are no clinical trials comparing the efficacy and safety of fruquintinib and regorafenib. Our research showed that fruquintinib and regorafenib have similar median overall survival (14.2 versus 12.0 months) and median progression-free survival (4.4 versus 3.5 months). Compared with FRESCO study and CONCUR study, our results are superior to these two key clinical studies, mainly due to the fact that in the real world, in addition to receiving fruquintinib or regorafenib, in order to control the progression of the disease, patients usually receive immunotherapy, local therapy or other forms of combination therapy, thus improving their survival prognosis.
Subsequently, we analyzed the different treatment sequence of fruquintinib and regorafenib, and the median OS of fruquintinib after the progression of treatment with regorafenib was significantly longer than that of the reverse treatment order. In terms of PFS, although the difference was not statistically significant, it may be related to the small sample size of this study. The results of the REVERCE study showed that the OS of mCRC patients who received sequential cetuximab treatment with regorafenib after completion of 6 cycles of chemotherapy was significantly longer than that of patients treated in the opposite order (mOS:17.4 vs 11.6 months, HR=0.61, p=0.029) (13). The work of Eraslan et al. has revealed that re-chemotherapy after the failure of regorafenib treatment can still prolong the survival time of patients with mCRC (14). These results showed that the efficacy of other treatments was cited after the progress of regorafenib treatment. Nevertheless, the mechanism of fruquintinib is different from that of cetuximab and chemotherapy. We surmise that: on the one hand, fruquintinib can maintain higher drug exposure. Due to the inhibition of multiple targets, the drug exposure at the maximum tolerance dose (MTD) is limited, resulting in poor inhibition and/or short duration of any target (especially VEGFR). Fruquintinib has high selectivity to VEGFR1/2/3, which can minimize miss toxicity and provide higher dose of drug exposure under MDT. On the other hand, fruquintinib had almost the same inhibitory effect on VEGFR2 and VEGFR3. VEGF-A/VEGFR1 mainly regulates angiogenesis, while VEGF-C/VEGFR3 regulates both vascular and lymphatic angiogenesis, promoting lymphatic vessel-mediated regional lymph node metastasis (15). Fruquintinib has similar activity to VEGFR2 and VEGFR3 and has higher selectivity than regorafenib, which can simultaneously block the blood vessels and lymphatic vessels affecting tumor growth and metastasis (16). Compared with Regorafenib, which mainly targets VEGF-A/VEGFR2, it can further inhibit tumor progression.
In the subgroup analysis of fruquintinib and regorafenib monotherapy or combined immunotherapy, the mPFS, ORR and DCR of fruquintinib and regorafenib monotherapy were 3.0 months, 4.5%, 54.5% and 2.4 months, 0, 47.6%, respectively. The results of the study were lower than those of the FRESCO study or CONCUR study. This may be related to the fact that the overall condition of the patients included in the clinical trial is good and that the dose of fruquintinib or regorafenib in most patients in this study did not reach the standard dose of the clinical trial (fruquintinib 5mg/d, regorafenib 160mg/d). Studies have shown that, first of all, antiangiogenic small molecule tyrosine kinase inhibitor can target VEGFR, normalize tumor vessels, thus improve tumor cell hypoxia and promote effective T cell infiltration into tumor tissue (17). Secondly, antiangiogenic drugs can inhibit the production of Tregs, TAM and MDSC in tumor sites and down-regulate the expression of immunosuppressive factors, which leads to the reprogramming of immunosuppressive microenvironment to immunostimulatory microenvironment (18, 19). Finally, antiangiogenic drugs can enhance the efficacy of immunotherapy by promoting antigen presentation and activating cytotoxic CD8+T cells (20). The crosstalk between angiogenesis and immune cells provides a theoretical basis for immunotherapy combined with antiangiogenic targeted therapy. In our study, the mOS and mPFS of FP were 17.5months and 5.9months, respectively. Compared with fruquintinib monotherapy, immunotherapy played a synergistic effect. At present, the data on the efficacy of fruquintinib combined with immunotherapy in real-world practice is limited. Phase I clinical trials show that the mPFS of fruquintinib combined with immunotherapy is 5.45-6.8 months (21, 22), which is similar to our results. In the RP group, the mOS was 14.8 months, and the mPFS was 3.8 months. The results of the Ib phase REGONIVO study conducted in Japan showed that the mPFS and ORR were 6.3 months and 40%, respectively (23). Our study did not achieve the surprising results of the REGONIVO study. This is related to the different baseline characteristics of the patients. In the phase Ib/II REGOTORI study conducted in China, the baseline characteristics of the patients included were similar to our research. 39 patients who received regorafenib combined with toripalimab, with a median PFS of 2.6 months, a median OS of 15.5 months, and an ORR of 15.2% (24). Another multicenter retrospective study of regorafenib combined with immune checkpoint inhibitors included 84 mCRC patients, with an ORR of 5% and a median PFS of 3.1 months (25). Obviously, our results are similar to those of the appeal study in China. Compared with the regorafenib monotherapy, the median PFS of the combination immunotherapy was longer, but the difference was not statistically significant, which may be related to the small number of patients enrolled in this study. The results of the multi-center retrospective study conducted by xu et al (26) revealed that compared with the monotherapy group, the combination immunotherapy of regorafenib could prolong the median PFS (3.5 vs 2.2 months, p=0.043). In the comparative analysis of FP and RP, the PFS and OS of the FP group were significantly better than those of the RP group. On the one hand, fruquintinib is a highly selective tyrosinase inhibitor, which has a stronger inhibitory effect on VEGFR1/2/3, while regorafenib is a multi-target inhibitor, which has less inhibitory effect on VEGF-VEGFR pathway than fruquintinib, which may also mean that the immunosuppressive effect of regorafenib in reversing VEGF is not as good as that of fruquintinib. On the other hand, the tolerance of fruquintinib is better than that of regorafenib. In our study, most patients took regorafenib from a low dose and then, based on the results of the ReDOS study (27), increased the dose weekly to the standard dose of 160mg/d. Fruquintinib was used to increase the dose up to 5mg/d based on a similar principle. Although the adverse reactions of fruquintinib and regorafenib were similar, fewer patients in the fruquintinib group had adverse reactions and more patients achieved dose increments.
With the wide application of immunotherapy in clinic, it is particularly important to select the dominant population of immunotherapy. In this study, the baseline characteristics of patients in the FP and RP group were analyzed for efficacy predictors. The results showed that ECOG-PS score, fruquintinib or regorafenib targeted therapy and previous anti-VEGF therapy were independent prognostic factors affecting PFS, and patients who did not receive anti-VEGF therapy in previous anti-tumor therapy may have longer PFS than those who received anti-VEGF therapy. In the FRESCO study, a subgroup analysis of previous anti-VEGF or anti-EGFR targeted therapy found that the mOS and mPFS of patients who had not received anti-VEGF therapy were significantly longer than those who had received anti-VEGF (9). Another retrospective study also showed that the mPFS of patients previously treated with anti-VEGFR drugs was shorter than that of patients without anti-VEGFR drugs (1.9 vs 3.7 month, p=0.006), but there was no significant difference in mOS between the two groups (9.0 vs 8.5 months, p=0.992) (28). ECOG-PS score, targeted therapy with fruquintinib or regorafenib were independent predictors of OS in patients. It should be pointed out that the physical status (PS) score of patients not only affects the total survival time of patients, but also affects the progression-free survival time of patients with drug treatment, and the patients with higher PS score are related to poor prognosis.
With regard to the adverse events (AEs) of the two drugs, meta-analysis of 1380 patients included by Jing et al. showed that fruquintinib was less toxic than regorafenib (10). However, Chen et al. included a meta-analysis of 2604 mCRC patients in five randomized controlled clinical trials that found that fruquintinib significantly increased the risk of serious adverse events (SAEs) compared with regorafenib (11). In the present study, although the toxicity of the two drugs was generally similar, the proportion of patients taking fruquintinib had fewer AEs than that of regorafenib, and more patients increased the dose. It seemed that fruquintinib was better tolerated than regorafenib. Nevertheless, SAEs accounted for 27.1% (29/107) of any grade of AEs in the fruquintinib group, while only 18% (16/89) in the regorafenib group. It seems that fruquintinib would increase the risk of SAEs, which may be related to the high selectivity of fruquintinib to the target.
In this study, without directly comparing the randomized controlled trials of regorafenib and fruquintinib, we evaluated the similar efficacy and safety of regorafenib and fruquintinib in the real world, explored the rational use sequence of regorafenib followed by fruquintinib and summarized the relevant molecular mechanisms. Meanwhile, we also compared for the first time the degree of sustained beneficial and adverse effects of long-term administration of fruquintinib or regorafenib combined with anti-PD-1 treatment. However, this research still has some limitations. Firstly, this study is a single-center retrospective study with a small sample size and a certain selection deviation. Prospective randomized clinical trials are still needed to verify our results in the future. Secondly, although the type and proportion of PD-1 inhibitors used in combination with fruquintinib or regorafenib are similar in this study, it will inevitably affect the consistency of the treatment process. Third, MSS status is not available in a small number of patients, but only a small number of patients with MSI-H, this restriction may not cause much deviation. Fourth, the PD-L1 CPS and TMB status of the included patients is unknown, resulting in the inability to determine the advantage of immunotherapy. Finally, this study is a real-world study, and not all patients have been tested for RAS and BRAF genes, which may limit the analysis of the efficacy of drug therapy.
Conclusion
In summary, the efficacy and toxicity of fruquintinib and regorafenib in the treatment of advanced CRC are similar, but the incidence of hand-foot syndrome of regorafenib is higher than that of regorafenib, and fruquintinib is more prone to grade 3 hypertension. Fruquintinib can still prolong the survival time of patients after the progression of regorafenib treatment. Combined immunotherapy has a synergistic effect, which is more obvious in fruquintinib.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by The Ethics Committee of Zhejiang Provincial people’s Hospital. The ethics committee waived the requirement of written informed consent for participation. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LY and Z-LC made contribution to conception and design. Y-YD analyzed and interpreted the data. Y-YD, X-YZ, P-FZ, H-RL, QL, and S-YP performed the data collection. Y-YD and XZ prepared the final draft. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Key Medical and Health Science and Technology Plan of Zhejiang Province (No. 2023552922).
Acknowledgments
We thank the patients who participated and all clinical staff who assisted. And we also sincerely thank the editors and reviewers for their careful review and valuable opinions that helped to greatly improve the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1097911/full#supplementary-material
Supplementary Figure 1 | Initial and final doses of fruquintinib and regorafenib and types of combined PD-1 inhibitors. (A, B) Initial and final dose of fruquintinib. (C) Types and proportion of fruquintinib combined with PD-1 inhibitors. (D, E) Initial and final dose of regorafenib. (F) Types and proportion of regorafenib combined with PD-1 inhibitors.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA: Cancer J Clin (2017) 67(3):177–93. doi: 10.3322/caac.21395
3. Modest DP, Pant S, Sartore-Bianchi A. Treatment sequencing in metastatic colorectal cancer. Eur J Cancer (Oxford Engl 1990) (2019) 109:(70–83). doi: 10.1016/j.ejca.2018.12.019
4. Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer (2011) 129(1):245–55. doi: 10.1002/ijc.25864
5. Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet (London England) (2013) 381(9863):303–12. doi: 10.1016/s0140-6736(12)61900-x
6. Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2015) 16(6):619–29. doi: 10.1016/s1470-2045(15)70156-7
7. Lu S, Chen G, Sun Y, Sun S, Chang J, Yao Y, et al. A phase III, randomized, double-blind, placebo-controlled, multicenter study of fruquintinib in Chinese patients with advanced nonsquamous non-small-cell lung cancer - the FALUCA study. Lung Cancer (Amsterdam Netherlands) (2020) 146:(252–262). doi: 10.1016/j.lungcan.2020.06.016
8. Cao J, Zhang J, Peng W, Chen Z, Fan S, Su W, et al. A phase I study of safety and pharmacokinetics of fruquintinib, a novel selective inhibitor of vascular endothelial growth factor receptor-1, -2, and -3 tyrosine kinases in Chinese patients with advanced solid tumors. Cancer chemother Pharmacol (2016) 78(2):259–69. doi: 10.1007/s00280-016-3069-8
9. Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: The FRESCO randomized clinical trial. Jama (2018) 319(24):2486–96. doi: 10.1001/jama.2018.7855
10. Jing Z, Rui Z, Binglan Z. A comparison of regorafenib and fruquintinib for metastatic colorectal cancer: a systematic review and network meta-analysis. J Cancer Res Clin Oncol (2019) 145(9):2313–23. doi: 10.1007/s00432-019-02964-6
11. Chen J, Wang J, Lin H, Peng Y. Comparison of regorafenib, fruquintinib, and TAS-102 in previously treated patients with metastatic colorectal cancer: A systematic review and network meta-analysis of five clinical trials. Med Sci monitor Int Med J Exp Clin Res (2019) 25:(9179–9191). doi: 10.12659/msm.918411
12. Ribatti D, Solimando AG, Pezzella F. The anti-VEGF(R) drug discovery legacy: Improving attrition rates by breaking the vicious cycle of angiogenesis in cancer. Cancers (2021) 13(14):3433. doi: 10.3390/cancers13143433
13. Shitara K, Yamanaka T, Denda T, Tsuji Y, Shinozaki K, Komatsu Y, et al. REVERCE: a randomized phase II study of regorafenib followed by cetuximab versus the reverse sequence for previously treated metastatic colorectal cancer patients. Ann Oncol (2019) 30(2):259–65. doi: 10.1093/annonc/mdy526
14. Eraslan E, Doğan M, Yildiz F, İlhan A, Öksüzoğlu Ö B. Treatment options after regorafenib failure in metastatic colorectal cancer. Eur Rev Med Pharmacol Sci (2021) 25(9):3470–7. doi: 10.26355/eurrev_202105_25828
15. Heinolainen K, Karaman S, D'Amico G, Tammela T, Sormunen R, Eklund L, et al. VEGFR3 modulates vascular permeability by controlling VEGF/VEGFR2 signaling. Circ Res (2017) 120(9):1414–25. doi: 10.1161/circresaha.116.310477
16. Sun Q, Zhou J, Zhang Z, Guo M, Liang J, Zhou F, et al. Discovery of fruquintinib, a potent and highly selective small molecule inhibitor of VEGFR 1, 2, 3 tyrosine kinases for cancer therapy. Cancer Biol Ther (2014) 15(12):1635–45. doi: 10.4161/15384047.2014.964087
17. Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev (2011) 91(3):1071–121. doi: 10.1152/physrev.00038.2010
18. Elovic AE, Ohyama H, Sauty A, McBride J, Tsuji T, Nagai M, et al. IL-4-dependent regulation of TGF-alpha and TGF-beta1 expression in human eosinophils. J Immunol (Baltimore Md. 1950) (1998) 160(12):6121–7. doi: 10.4049/jimmunol.160.12.6121
19. Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol (2018) 52(Pt 2):117–24. doi: 10.1016/j.semcancer.2017.12.002
20. Furukawa K, Nagano T, Tachihara M, Yamamoto M, Nishimura Y. Interaction between immunotherapy and antiangiogenic therapy for cancer. Molecules (Basel Switzerland) (2020) 25(17):3900. doi: 10.3390/molecules25173900
21. Guo Y, Zhang W, Ying J, Zhang Y, Pan Y, Qiu W, et al. Preliminary results of a phase 1b study of fruquintinib plus sintilimab in advanced colorectal cancer. J Clin Oncol (2021) 39(15_suppl):2514–4. doi: 10.1200/JCO.2021.39.15_suppl.2514
22. Bai Y, Xu N, An S, Chen W, Gao C, Zhang D. A phase ib trial of assessing the safety and preliminary efficacy of a combination therapy of geptanolimab (GB 226) plus fruquintinib in patients with metastatic colorectal cancer (mCRC). J Clin Oncol (2021) 39(15_suppl):e15551–1. doi: 10.1200/JCO.2021.39.15_suppl.e15551
23. Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: An open-label, dose-escalation, and dose-expansion phase ib trial (REGONIVO, EPOC1603). J Clin Oncol (2020) 38(18):2053–61. doi: 10.1200/jco.19.03296
24. Wang F, He MM, Yao YC, Zhao X, Wang ZQ, Jin Y, et al. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: a phase Ib/II clinical trial and gut microbiome analysis. Cell Rep Med (2021) 2(9):100383. doi: 10.1016/j.xcrm.2021.100383
25. Yang K, Han L, Wu S, Qu X, Li Q, Zhao C, et al. Real-world outcomes of regorafenib combined with immune checkpoint inhibitors in patients with advanced or metastatic microsatellite stable colorectal cancer: A multicenter study. Cancer immunol immunother CII (2022) 71(6):1443–51. doi: 10.1007/s00262-021-03083-3
26. Xu D, Liu Y, Tang W, Xu L, Liu T, Jiang Y, et al. Regorafenib in refractory metastatic colorectal cancer: A multi-center retrospective study. Front Oncol (2022) 12:838870. doi: 10.3389/fonc.2022.838870
27. Bekaii-Saab TS, Ou F-S, Anderson DM, Ahn DH, Boland PM, Ciombor KK, et al. Regorafenib dose optimization study (ReDOS): Randomized phase II trial to evaluate dosing strategies for regorafenib in refractory metastatic colorectal cancer (mCRC)—An ACCRU network study. J Clin Oncol (2018) 36(4_suppl):611–1. doi: 10.1200/JCO.2018.36.4_suppl.611
Keywords: fruquintinib, regorafenib, immunotherapy, advanced colorectal cancer, real-world study
Citation: Deng Y-Y, Zhang X-Y, Zhu P-F, Lu H-R, Liu Q, Pan S-Y, Chen Z-L and Yang L (2023) Comparison of the efficacy and safety of fruquintinib and regorafenib in the treatment of metastatic colorectal cancer: A real-world study. Front. Oncol. 13:1097911. doi: 10.3389/fonc.2023.1097911
Received: 13 December 2022; Accepted: 10 February 2023;
Published: 03 March 2023.
Edited by:
Fabiana Conciatori, Hospital Physiotherapy Institutes (IRCCS), ItalyReviewed by:
Antonella Argentiero, National Cancer Institute Foundation (IRCCS), ItalyEleonora Lai, University of Cagliari, Italy
Copyright © 2023 Deng, Zhang, Zhu, Lu, Liu, Pan, Chen and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liu Yang, eWFuZ2xpdUBobWMuZWR1LmNu; Zhe-Ling Chen, Y2hlbnpoZWxpbmdAaG1jLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Ya-Ya Deng1,2†
Ya-Ya Deng1,2† Peng-Fei Zhu
Peng-Fei Zhu Shuang-Yue Pan
Shuang-Yue Pan Zhe-Ling Chen
Zhe-Ling Chen Liu Yang
Liu Yang