
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 01 March 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1096714
 Xin Zhao1,2
Xin Zhao1,2 Feng-wei Gao1,2,3*
Feng-wei Gao1,2,3* Kang-yi Jiang1,2
Kang-yi Jiang1,2 Jie Yang1,2
Jie Yang1,2 Qing-yun Xie1,2
Qing-yun Xie1,2 Jie Gong1,2
Jie Gong1,2 Man-yu Yang3
Man-yu Yang3 Tian-yang Mao3
Tian-yang Mao3 Ze-hua Lei1,2*
Ze-hua Lei1,2*Background: Although laparoscopic hepatectomy has been widely used in the treatment of benign and malignant liver diseases, its applicability in intrahepatic cholangiocarcinoma (ICC) is controversial. We conducted a meta-analysis to compare the short-term and long-term outcomes of laparoscopic hepatectomy (Lap-ICC) and open hepatectomy (Open-ICC) in ICC patients.
Methods: The PubMed, Web of science, Cochrane Library, China National Knowledge Infrastructure and other databases were searched for the relevant literature. The research data were extracted according to the inclusion and exclusion criteria.
Results: Seventeen studies, including 3975 ICC patients, were selected for the meta-analysis. Compared to Open-ICC, Lap-ICC had lower rates of lymph node dissection (OR=0.44, P=0.01) and metastasis (OR=0.58, P=0.03), along with less intraoperative bleeding (MD=-128.43 ml, P<0.01) lower blood transfusion rate (OR=0.43, P<0.01), shorter hospital stay (MD=-2.75 day, P<0.01), higher R0 resection rate (OR=1.60, P<0.01), and lower tumor recurrence rate (OR=0.67, P=0.01). However, there was no difference between the two groups in terms of operation time, number of lymph node dissection, incision margin distance, overall complications rate, severe complications rate, and the 1-, 3- and 5-year DFS and OS rates.
Conclusion: Laparoscopic hepatectomy is partially superior to open hepatectomy in terms of less bleeding, shorter hospital stay and higher R0 resection rate, while the long-term efficacy of the two approaches is similar.
Intrahepatic cholangiocarcinoma (ICC) is the second most common malignancy of the liver, and its incidence is increasing on a yearly basis worldwide. According to the data reported in the United States, the incidence of ICC increased by about 5.9% annually from 2003 to 2009 (1–3). Recent advances in our knowledge of the mechanism of ICC have not translated to improved treatment strategies (4–6). Surgical resection and chemotherapy are the most common therapeutic modalities for ICC, and there have been some reports on immunotherapy as well (3, 7).
Hepatectomy, including the open, laparoscopic and robotic forms, is the most accurate curative option for ICC, especially for single lesions. Studies show that while the short-term outcomes of laparoscopic hepatectomy (Lap-ICC) are superior to that of open hepatectomy (Open-ICC), the long-term effects are similar (8–11). Unlike hepatocellular carcinoma (HCC), ICC often requires lymph node dissection. However, laparoscopic lymph node dissection is difficult, and beset with problems such as insufficient dissection and inaccurate tumor staging (12, 13). Therefore, it remains to be ascertained whether Lap-ICC is indeed better than Open-ICC.
Guerrini et al. conducted a meta-analysis of four studies, and found that the short-term efficacy of Lap-ICC was better than that of Open-ICC. In contrast, Wei et al. reported similar short-term and long-term outcomes of both approaches in a meta-analysis of six studies (14, 15). The meta-analyses conducted by Machairas et al., Ziogas et al. and Regmi on eight identical studies also differed in their results (16–18), which can be attributed to the limited number and quality of the included literature, as well as the lack of further subgroup analysis. To this end, we conducted a meta-analysis to compare the short-term and long-term outcomes of Lap-ICC and Open-ICC in a large cohort of ICC patients, and performed subgroup analysis to further assess the reliability of the results.
This study was conducted in accordance with the systematic review and meta-analysis (PRISMA) (19) and the guidelines for evaluating the methodological quality of systematic review (AMSTAR), and has been registered in PROPERO with the registration number CRD23457688. The PubMed, Web of science, EMbase, The Cochrane Library and CNKI databases were searched for randomized controlled trials (RCTs) and non-RCTs published till June 30, 2022 that compared the short-term and long-term efficacy of Lap-ICC and Open-ICC. The search keywords were “intrahepatic cholangiocarcinoma”, “laparoscopy”, “liver reservation”, “hepatectomy”, etc. The reference lists of the selected articles were manually searched for additional studies.
The inclusion criteria for the studies were as follows: (1) RCTs and well-designed non-RCTs, (2) comparison of Lap-ICC and Open-ICC groups, (3) outcome indicators such as operation time, intraoperative blood loss, blood transfusion rate, R0 resection rate, overall complication rate, postoperative hospital stay, tumor recurrence rate, and 1-, 3- and 5-year disease-free survival (DFS) and overall survival (OS) rates, (4) literature quality evaluated as medium low risk bias and NOS score ≥ 5, and (5) published in Chinese or English.
The exclusion criteria were as follows: (1) summaries, case reports, minutes of meeting and other articles, (2) lack of control group or absence of outcome indicators, and (3) highly biased according to The Cochrane Bias Risk Assessment Form and non-RCT with Ottawa Scale (NOS) scores below 5.
Two researchers extracted the data according to the preset form, and rechecked the data in case of any inconsistency. Any disputes were settled by discussing with a third researcher. The following data were extracted: (1) general information, including title, author, publication date, country, etc., (2) general data of subjects such as number of cases, male/female ratio, age, BMI, tumor diameter, etc., and (3) outcome indicators such as operation time, intraoperative blood loss, intraoperative lymph node dissection rate, blood transfusion rate, overall complication rate, serious complication rate, bile leakage rate, hospital stay, R0 resection rate, tumor recurrence rate, and the 1-, 3- and 5-year OS and DFS rates.
The quality of the non-RCTs was evaluated by two researchers using the NOS. The risk of bias in the RCTs was assessed as per the recommendations in Version 5.1.0 of the Cochrane System Evaluator’s Manual. In case of differences, a third researcher re-evaluated the studies.
The Revman 5.3 software of the Cochrane Center was used for statistical analysis. Odd ratio (OR) and 95% confidence interval (CI) were used as indicators for the counting data, and the mean difference (MD) and 95% confidence interval (CI) were used for measurement data. When only median and extreme values were reported in the study, the method proposed by Hozo et al. was used (20) to estimate the mean and standard deviation (SD). The heterogeneity among the studies was analyzed by Q test and I2 test. The fixed effect model was used to analyze studies with low heterogeneity (I2 < 50%), whereas the random effect model was used in case of high heterogeneity (I2≥ 50%). Subgroup analysis was performed as required. When the results of non-subgroup analyses were statistically consistent with the results of subgroup analyses, the former was considered. The PSM subgroups were used in case of any differences. The publication bias was evaluated by a funnel chart. All tests were two-sided and P < 0.05 was considered statistically significant.
A total of 365 articles were retrieved from the initial screening. Seventeen articles (21–37), including 15 that were published in English and 2 in Chinese, were incorporated into the meta-analysis. There were 8 propensity matching score (PSM) studies and 9 retrospective studies which unused propensity matching score, including a total of 3975 patients (1083 in the Lap-ICC group and 2892 in the Open-ICC group). The flow chart of study selection and the results of the meta-analysis are summarized in Figure 1. The basic characteristics and NOS scores of the included studies are summarized in Table 1.
The results of the meta-analysis are summarized in Table 2
Fourteen studies reported the operation time. There was a high degree of heterogeneity among the studies (I2 = 83%), which warranted the random effect model. As shown in Figure 2A, there was no significant difference in the operation time between the two groups (MD=10.30, 95%CI= -6.30~26.91, P=0.22).
Twelve studies reported the amount of intraoperative bleeding and showed significant heterogeneity (I2 = 80%). The random effect model analysis showed that intraoperative bleeding was significantly lower in the Lap-ICC group compared to that in the Open-ICC group (MD=-128.43, 95%CI= -116.74~-89.83, P<0.01) (Figure 2B).
Nine studies reported blood transfusion rates. The heterogeneity was insignificant (I2 = 0%) and the fixed effect model was used. As shown in Figure 2C, the rate of perioperative blood transfusion was significantly lower in the Lap-ICC group compared to that in Open-ICC group (OR=0.43, 95%CI= 0.31~0.58, P<0.01).
Ten studies reported lymph node dissection and there was a high degree of heterogeneity (I2 = 66%). Using the random effect model, we found that the rate of lymph node dissection was significantly lower in the Lap-ICC group compared to that in the Open-ICC group (OR=0.44, 95%CI= 0.23~0.82, P=0.01) (Figure 2D).
Eleven studies reported overall complications, and no obvious heterogeneity was observed (I2 = 45%). The fixed effect model analysis showed that the Lap-ICC group had significantly lower overall complications than that in the Open-ICC group (OR=0.55, 95%CI= 0.41~0.75, P<0.01) (Figure 3A).
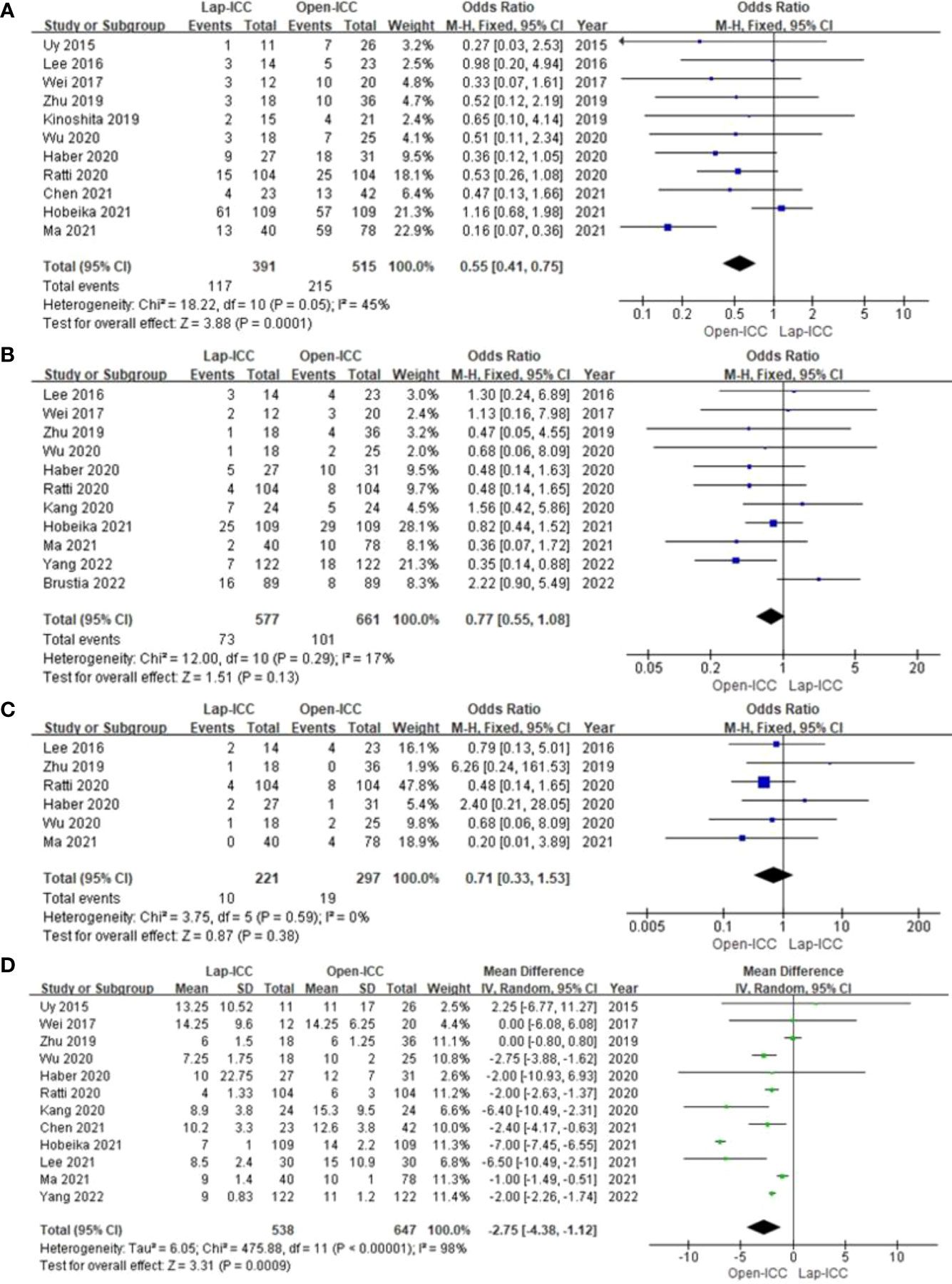
Figure 3 (A) overall complications; (B) severe complications; (C) biliary leakage; (D) hospital stay.
Eleven studies reported severe complications without any significant heterogeneity (I2 = 17%). Fixed effect model analysis did not show any significant difference between the two groups (OR=0.77, 95%CI= 0.55~1.08, P=0.13) (Figure 3B).
Six studies reported biliary leakage. The heterogeneity among the studies was low (I2 = 0%) and the fixed effect model did not reveal any significant differences (OR=0.71, 95%CI= 0.33~1.53, P=0.38) (Figure 3C).
Twelve studies reported the duration of hospital stay, and there was significant heterogeneity among the results (I2 = 98%). The random effect model analysis showed that Lap-ICC was associated with a significantly shorter hospital stay compared to Open-ICC (MD=-2.75, 95%CI= -4.38~-1.12, P<0.01) (Figure 3D).
Eleven studies reported the R0 resection rate, and the heterogeneity was low (I2 = 0%). Fixed effect model analysis showed that the rate of R0 in the Lap-ICC group was significantly higher than that in the Open-ICC group (OR=1.60, 95%CI= 1.28~1.99, P<0.01) (Figure 4A).
Six studies reported the number of lymph nodes (I2 = 98%). There was a high degree of heterogeneity among the studies, and the random effect model analysis showed that there was almost significant difference between the two groups (MD=-1.46, 95%CI= -2.94~0.02, P=0.05) (Figure 4B).
Five studies reported the resection margin with a high degree of heterogeneity (I2 = 71%). The random effect model analysis showed that there was no statistically significant difference between the two groups (MD=-1.53, 95%CI= -1.62~4.67, P=0.34) (Figure 4C).
Seven studies reported lymph node metastasis. The heterogeneity was low (I2 = 11%) and the fixed effect model showed that lymph node metastasis in the Lap-ICC group was significantly lower than that in the Open-ICC group (OR=0.58, 95%CI= -0.36~0.94, P=0.03) (Figure 4D).
Nine studies reported recurrence and the heterogeneity was low (I2 = 0%). Fixed effect model analysis revealed significantly lower recurrence rates in the Lap-ICC group compared to that in Open-ICC group (OR=0.67, 95%CI= 0.49~0.92, P=0.01) (Figure 5A). In addition, 5 studies reported intrahepatic (OR=0.68, 95%CI= 0.43~1.07, P=0.09) (Figure 5B) and extrahepatic recurrences (OR=0.69, 95%CI= 0.40~1.17, P=0.17), no significant differences were observed between the two groups (Figure 5C).
Eight studies reported 1-year DFS, 11 studies reported 3-year DFS and 4 studies reported 5-year DFS. The heterogeneity among studies reporting 1-year DFS and 3-year DFS was low (I2 = 0% and I2 = 20%), whereas high heterogeneity (I2 = 67%) was observed for studies reporting 5-year DFS. The respective models indicated that the 1-year DFS (OR=1.20, 95%CI= 0.89~1.62, P=0.22) (Figure 6A), 3-year DFS (OR=1.17, 95%CI= 0.87~1.56, P=0.3) (Figure 6B) and 5-year DFS (OR=0.79, 95%CI= 0.26~2.39, P=0.69) were similar in both groups (Figure 6C).
Ten studies reported 1-year OS, 13 studies reported 3-year OS and 6 studies reported 5-year OS. The heterogeneity was low for all categories (I2 = 0%), and the fixed effect model showed that 1-year OS (OR=0.92, 95%CI= 0.68~1.24, P=0.57) (Figure 7A), 3-year OS (OR=1.30, 95%CI= 1.00~1.68, P=0.05) (Figure 7B), 5-year OS (OR=1.38, 95%CI= 0.92~2.07, P=0.12) (Figure 7C), Which there no significant differences between the two groups.
N-PSM subgroup analysis showed differences in the results of lymph node metastasis and recurrence compared to that in non-subgroup analysis, whereas the other variables were consistent (Table 3). PSM subgroup analysis showed differences in the results of overall complications, while the other variables had similar results in both subgroup and non-subgroup analyses (Table 3). Therefore, the stability of our meta-analysis results is good (see Supplement Figures S1–S21). Results of previously published meta-analysis are summarized in supplement Table 4.
The results of operation time, blood loss, lymph node dissection, hospital stay, number of lymph node, retention margin, and 5-year DFS were highly heterogeneous. Exclusion of individual studies did not significantly affect the heterogeneity, and the results are consistent, indicating that our results are stable. After removing the study by Kinoshita et al. (35), the heterogeneity of 5-year DFS decreased significantly (I2 = 35%), although further analysis with the fixed effect model did not indicate any significant difference between the Lap-ICC and open-ICC groups (OR=1.49, 95%CI=0.84~2.62, P=0.17). Funnel maps were plotted based on transfusion (Figure 8A), overall complications (Figure 8B), R0 (Figure 8C), recurrence (Figure 8D), 3-year OS (Figure 8E) and 3-year DFS (Figure 8F), which showed a symmetrical distribution of scatter points on both sides of the funnel. This suggested the lack of any obvious publication bias in this meta-analysis.
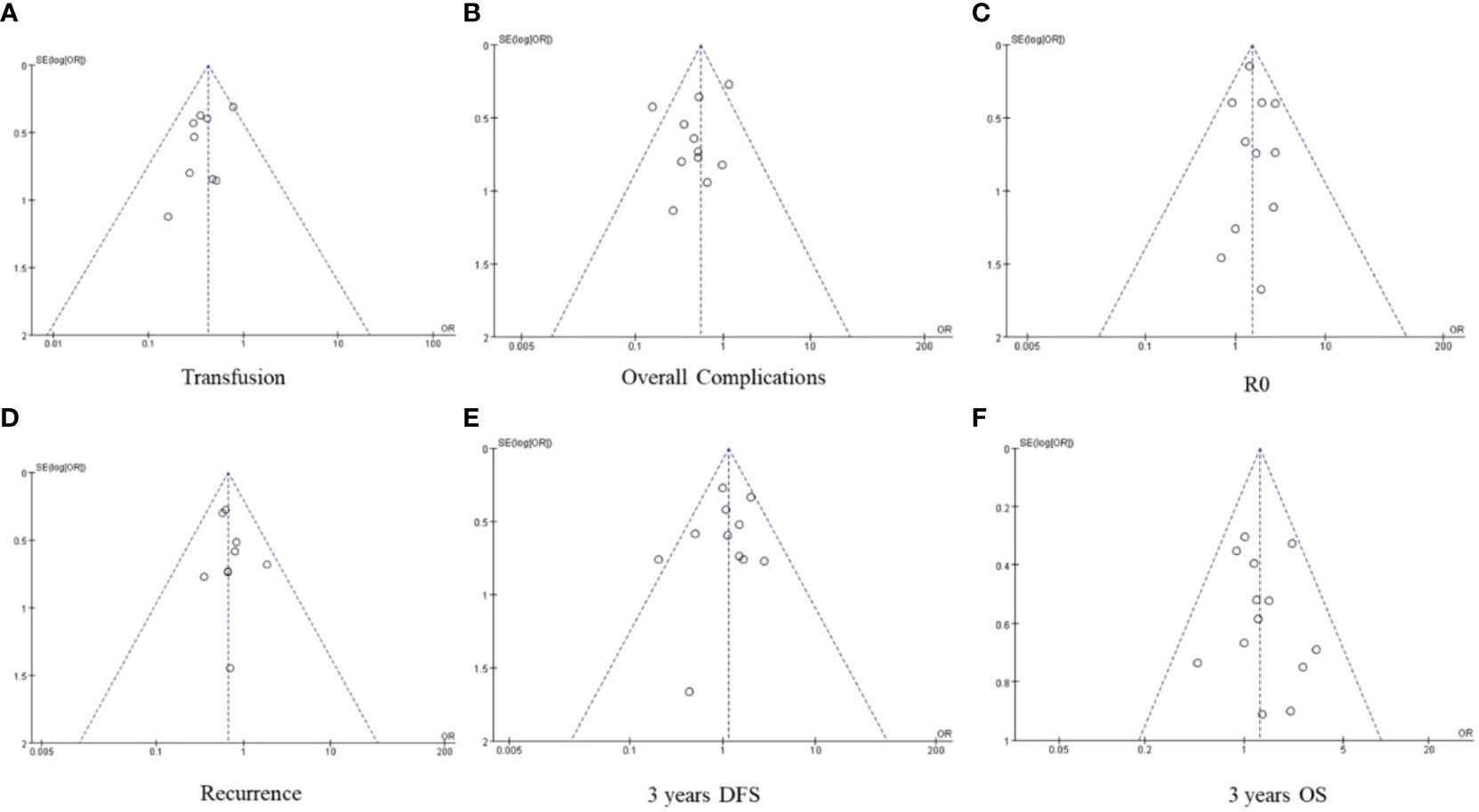
Figure 8 (A) transfusion (B) overall complication (C) R0 (D) recurrence (E) 3-year DFS (F) 3-year OS.
To the best of our knowledge, this is the latest meta-analysis and systematic review of laparoscopic and open hepatectomy outcomes in ICC patients with the largest sample size, the highest quality studies and the most comprehensive outcome indicators. Our findings indicate that Lap-ICC is superior to Open-ICC as far as some short-term outcomes are concerned, including less bleeding and shorter hospital stay. However, the rate of lymph node dissection was less in the Lap-ICC group. Furthermore, although laparoscopy was able to achieve a higher R0 resection rate and lower tumor recurrence, the two groups had similar DFS and OS rates at 1, 3 and 5 years.
The Liver Cancer Study Group of Japan recommends surgical resection for single tumors without regional lymph node metastasis (38). In addition, several guidelines also recommend palliative chemotherapy for bi- or multifocal ICC (39, 40). However, a recent cohort study reported similar long-term efficacy of surgical resection and chemotherapy for multifocal ICC, and a meta-analysis suggested that re-operation may partly improve the short-term and long-term outcomes of patients with recurrent ICC (41, 42). At present, open hepatectomy is the most common surgical method used for the treatment of ICC. It entails complete removal of the tumor while retaining sufficient liver volume, and requires regional lymph node dissection according to the latest guidelines of AJCC (43, 44). However, open hepatectomy is often traumatic and patients recover slowly. Although laparoscopy can reduce patient trauma and accelerate recovery to some extent, it is beset with challenges such as the difficulty in routine regional lymph node dissection, and increased risk of tumor implantation and metastasis in the pneumoperitoneum. Therefore, the choice of open versus laparoscopic hepatectomy is a controversial point in ICC.
Previous meta-analyses that compared open and laparoscopic hepatectomy for ICC (Table 4) differed in the conclusions compared to our study, which may be attributed to differences in tumor diameter, staging, lymph node dissection ratio, CA-199 level and other baseline characteristics. Therefore, we included 8 high-quality PSM studies for subgroup analysis to further ensure the reliability of our results. Laparoscopic hepatectomy was associated with less intraoperative bleeding and shorter hospital stay, which is consistent with the previous meta-analysis and also explains the lower blood transfusion rate in the Lap-ICC group. These observations are consistent with that of laparoscopic surgeries for colorectal and gastric tumors (45, 46). There was no difference in the operation time between the two groups. For centers that routinely perform laparoscopy, the duration of laparoscopy and laparotomy are similar (47, 48). Furthermore, lymph node dissection was rarely performed in the Lap-ICC group, which might have also reduced the operation time of laparoscopy comparable to that of open hepatectomy.
Laparoscopic lymph node dissection may increase the risk of postoperative complications, tumor implantation and metastasis. Therefore, the lower rate of lymph node dissection in the Lap-ICC group may have reduced the incidence of lymph node metastasis compared to that in the open-ICC group. Zhang et al. recently showed that lymph nodes metastasis is an independent predictor of long-term survival of ICC. The AJCC Eighth Edition Cancer Staging also stipulated the number of lymph nodes dissection, which should not be less than 6 (13, 49). However, there is no difference in the number of lymph node dissection between the two groups. Although there are reports that laparoscopic lymphadenectomy increases the incidence of perioperative complications, several meta-analyses have shown that the overall incidence of perioperative complications is lower in the laparoscopic group compared to the open hepatectomy group (12, 14–18). We also detected lower incidence of overall perioperative complications in the Lap-ICC group compared to the open-ICC group, whereas the incidence of severe complications and bile leakage were similar in the two groups. However, PSM subgroup analysis showed that there was no difference in the frequency of overall complications, severe complications and bile leakage between the two groups. Therefore, it is possible that laparoscopy may not be beneficial to ICC patients in terms of perioperative complications.
R0 resection rate is a significant predictor of tumor recurrence and long-term survival of cancer patients. A previous meta-analysis reported higher R0 resection rate for laparoscopy compared to laparotomy, which can be attributed to the smaller tumors and better accessibility in the laparoscopy group (16–18). However, the number of cases in the study of Martin et al. (24) was significantly higher than that in the other studies included in the meta-analysis. Therefore, after removing the data of this study, there was no significant difference in the R0 resection rate between the two groups. Likewise, we further analyzed the R0 resection rate of the five latest PSM studies, excluded the influence of tumor diameter and other factors on the results, and found that the R0 resection rate was higher in the Lap-ICC group (OR=1.60, P=0.01), which was in line with the lower tumor recurrence rate observed in this group (OR=0.67, P=0.01). However, the intrahepatic and extrahepatic recurrence rates were similar in both groups. Although the tumor recurrence rate was lower in the Lap-ICC group, the 1-year, 3-year and 5-year DFS of the two groups were similar, which may be due to the longer follow-up time of laparotomy or the difference of follow-up time between the two groups. In addition, there was no significant difference in 1-year OS, 3-year OS and 5-year OS between the two groups. It shows that the higher R0 resection rate and lower recurrence rate after laparoscopic surgery can not be transformed into survival benefits.
Our study has some limitations that ought to be considered. First, the included studies were non-RCTs. In addition, the selection criteria of minimally invasive approach is highly restrictive (small tumors, generally less than 5 cm, far away from the hepatic hilus, no need for biliary reconstruction). However, the data of large hepatectomy with or without relevant biliary or vascular reconstruction via minimally invasive approach are still very few. Although we conducted PSM subgroup analysis to control the interference of these factors as much as possible, some factors (such as small sample size, tumor location, adjuvant radiotherapy and chemotherapy) may affect the results. Third, the results of the survival analysis may have been statistically deficient since we used OR for comparison. Nevertheless, our aim was to assess the long-term survival trends. Our findings will have to be validated with an RCT on large sample size.
To sum up, laparoscopic hepatectomy has better short-term outcomes in ICC patients compared to open hepatectomy, such as less bleeding, shorter hospital stay and higher R0 resection rate, while the long-term efficacy of both is similar.
ZX, GFW and LZH conceived the study, had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. ZX and GFW designed the search strategy and discussed with YJ, JKY and XQY, GJ, YMY, YQ and MTY performed study selection, data extraction and synthesis. ZX and GFW drafted and led on the writing of the manuscript. All the other authors participated in the analysis and interpretation of the data, revised the manuscript critically for important intellectual content and re-drafted some of its section. All the authors read and approved the final version of the manuscript, and agreed to be accountable for all aspects of the work to ensure its accuracy and integrity.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1096714/full#supplementary-material
1. Van Dyke AL, Shiels MS, Jones GS, Pfeiffer RM, Petrick JL, Beebe-Dimmer JL, et al. Biliary tract cancer incidence and trends in the united states by demographic group, 1999-2013. Cancer (2019) 125(9):1489–98. doi: 10.1002/cncr.31942
2. Wu J, Yang S, Xu K, Ding C, Zhou Y, Fu X, et al. Patterns and trends of liver cancer incidence rates in Eastern and southeastern Asian countries (1983-2007) and predictions to 2030. Gastroenterology (2018) 154(6):1719–1728.e5. doi: 10.1053j/gastro.2018.01.033
3. Mazzaferro V, Gorgen A, Roayaie S, Droz Dit Busset M, Sapisochin G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J Hepatol (2020) 72(2):364–77. doi: 10.1016j/jhep.2019.11.020
4. Alvisi G, Termanini A, Soldani C, Portale F, Carriero R, Pilipow K, et al. Multimodal single-cell profiling of intrahepatic cholangiocarcinoma defines hyperactivated tregs as a potential therapeutic target. J Hepatol (2022) 77(5):1359–1372. doi: 10.1016j/jhep.2022.05.043
5. Lin Y, Peng L, Dong L, Liu D, Ma J, Lin J, et al. Geospatial immune heterogeneity reflects the diverse tumor-immune interactions in intrahepatic cholangiocarcinoma. Cancer Discovery (2022) 12(10):2350–2371. doi: 10.1158/2159-8290.CD-21-1640
6. Martin-Serrano MA, Kepecs B, Torres-Martin M, Bramel ER, Haber PK, Merritt E, et al. Novel microenvironment-based classification of intrahepatic cholangiocarcinoma with therapeutic implications. Gut (2022). doi: 10.1136/gutjnl-2021-326514
7. Yang XG, Sun YY, Li DS, Xu GH, Huang XQ. Efficacy and safety of drug-eluting beads transarterial chemoembolization combining immune checkpoint inhibitors in unresectable intrahepatic cholangiocarcinoma: A propensity score matching analysis. Front Immunol (2022) 13:940009. doi: 10.3389/fimmu.2022.940009
8. Han HS, Shehta A, Ahn S, Yoon YS, Cho JY, Choi Y. Laparoscopic versus open liver resection for hepatocellular carcinoma: Case-matched study with propensity score matching. J Hepatol (2015) 63(3):643–50. doi: 10.1016/j.jhep.2015.04.005
9. Yoon YI, Kim KH, Kang SH, Kim WJ, Shin MH, Lee SK, et al. Pure laparoscopic versus open right hepatectomy for hepatocellular carcinoma in patients with cirrhosis: A propensity score matched analysis. Ann Surg (2017) 265(5):856–63. doi: 10.1097/SLA.0000000000002072
10. Goh EL, Chidambaram S, Ma S. Laparoscopic vs open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: A meta-analysis of the long-term survival outcomes. Int J Surg (2018) 50:35–42. doi: 10.1016/j.ijsu.2017.12.021
11. Kabir T, Tan ZZ, Syn NL, Wu E, Lin JD, Zhao JJ, et al. Laparoscopic versus open resection of hepatocellular carcinoma in patients with cirrhosis: meta-analysis. Br J Surg (2021) 109(1):21–9. doi: 10.1093/bjs/znab376
12. Miyata T, Yamashita YI, Yamao T, Umezaki N, Tsukamoto M, Kitano Y, et al. Prognostic impacts of postoperative complications in patients with intrahepatic cholangiocarcinoma after curative operations. Int J Clin Oncol (2017) 22(3):526–32. doi: 10.1007/s10147-017-1099-9
13. Zhang XF, Xue F, Dong DH, Weiss M, Popescu I, Marques HP, et al. Number and station of lymph node metastasis after curative-intent resection of intrahepatic cholangiocarcinoma impact prognosis. Ann.Surg (2021) 274(6):e1187–95. doi: 10.1097/SLA.0000000000003788
14. Guerrini GP, Esposito G, Tarantino G, Serra V, Olivieri T, Catellani B, et al. Laparoscopic versus open liver resection for intrahepatic cholangiocarcinoma: the first meta-analysis. Langenbecks Arch Surg (2020) 405(3):265–75. doi: 10.1007/s00423-020-01877-0
15. Wei F, Wang G, Ding J, Dou C, Yu T, Zhang C. Is it time to consider laparoscopic hepatectomy for intrahepatic cholangiocarcinoma? A Meta-Anal J Gastrointest Surg (2020) 24(10):2244–50. doi: 10.1007/s11605-019-04404-9
16. Machairas N, Kostakis ID, Schizas D, Kykalos S, Nikiteas N, Sotiropoulos GC. Meta-analysis of laparoscopic versus open liver resection for intrahepatic cholangiocarcinoma. Updates Surg (2021) 73(1):59–68. doi: 10.1007/s13304-020-00930-3
17. Regmi P, Hu HJ, Paudyal P, Liu F, Ma WJ, Yin CH, et al. Is laparoscopic liver resection safe for intrahepatic cholangiocarcinoma? a meta-analysis. Eur J Surg Oncol (2021) 47(5):979–89. doi: 10.1016/j.ejso.2020.11.310
18. Ziogas IA, Esagian SM, Giannis D, Hayat MH, Kosmidis D, Matsuoka LK, et al. Laparoscopic versus open hepatectomy for intrahepatic cholangiocarcinoma: An individual patient data survival meta-analysis. Am J Surg (2021) 222(4):731–8. doi: 10.1016/j.amjsurg.2021.03.052
19. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg (2021) 88(3):105906. doi: 10.1016/j.ijsu.2021.105918
20. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol (2005) 5:13. doi: 10.1186/1471-2288-5-13
21. Uy BJ, Han HS, Yoon YS, Cho JY. Laparoscopic liver resection for intrahepatic cholangiocarcinoma. J Laparoendosc Adv Surg Tech. A (2015) 25(4):272–7. doi: 10.1089/lap.2014.0233
22. Lee W, Park JH, Kim JY, Kwag SJ, Park T, Jeong SH, et al. Comparison of perioperative and oncologic outcomes between open and laparoscopic liver resection for intrahepatic cholangiocarcinoma. Surg Endosc (2016) 30(11):4835–40. doi: 10.1007/s00464-016-4817-x
23. Wei F, Lu C, Cai L, Yu H, Liang X, Cai X. Can laparoscopic liver resection provide a favorable option for patients with large or multiple intrahepatic cholangiocarcinomas? Surg Endosc (2017) 31(9):3646–55. doi: 10.1007/s00464-016-5399-3
24. Martin SP, Drake J, Wach MM, Ruff S, Diggs LP, Wan JY, et al. Laparoscopic approach to intrahepatic cholangiocarcinoma is associated with an exacerbatio/n of inadequate nodal staging. Ann Surg Oncol (2019) 26(6):1851–7. doi: 10.1245/s10434-019-07303-0
25. Zhu Y, Song J, Xu X, Tan Y, Yang J. Safety and feasibility of laparoscopic liver resection for patients with large or multiple intrahepatic cholangiocarcinomas: A propensity score based case-matched analysis from a single institute. Medicine (2019) 98(49):e18307. doi: 10.1097/MD.0000000000018307
26. Haber PK, Wabitsch S, Kästner A, Andreou A, Krenzien F, Schöning W, et al. Laparoscopic liver resection for intrahepatic cholangiocarcinoma: A single-center experience. J Laparoendosc Adv Surg Tech. A (2020) 30(12):1354–9. doi: 10.1089/lap.2020.0215
27. Kang SH, Choi Y, Lee W, Ahn S, Cho JY, Yoon YS, et al. Laparoscopic liver resection versus open liver resection for intrahepatic cholangiocarcinoma: 3-year outcomes of a cohort study with propensity score matching. Surg Oncol (2020) 33:63–9. doi: 10.1016/j.suronc.2020.01.001
28. Kinoshita M, Kanazawa A, Takemura S, Tanaka S, Kodai S, Shinkawa H, et al. Indications for laparoscopic liver resection of mass-forming intrahepatic cholangiocarcinoma. Asian J endoscopic Surg (2020) 13(3):46–58. doi: 10.1111/ases.12703
29. Ratti F, Rawashdeh A, Cipriani F, Primrose J, Fiorentini G, Abu Hilal M, et al. Intrahepatic cholangiocarcinoma as the new field of implementation of laparoscopic liver resection programs. a comparative propensity score-based analysis of open and laparoscopic liver resections. Surg Endosc. (2020) 35(4):1851–62. doi: 10.1007/s00464-020-07588-3
30. Wu J, Han J, Zhang Y, Liang L, Zhao J, Han F, et al. Safety and feasibility of laparoscopic versus open liver resection with associated lymphadenectomy for intrahepatic cholangiocarcinoma. Biosci Trends (2020) 14(5):376–83. doi: 10.5582/bst.2020.03293
31. Hobeika C, Cauchy F, Fuks D, Barbier L, Fabre JM, Boleslawski E, et al. Laparoscopic versus open resection of intrahepatic cholangiocarcinoma: nationwide analysis. Br J Surg (2021) 108(4):419–26. doi: 10.1093/bjs/znaa110
32. Jinhuan Y, Yi W, Yuanwen Z, Delin M, Xiaotian C, Yan W, et al. Laparoscopic versus open surgery for early-stage intrahepatic cholangiocarcinoma after mastering the learning curve: A multicenter data-based matched study. Front Oncol (2021) 11:742544. doi: 10.3389/fonc.2021.742544
33. Lee SJ, Kang SH, Choi Y, Lee B, Hong SK, Cho JY, et al. Long-term outcomes of laparoscopic versus open liver resection for intrahepatic combined hepatocellular-cholangiocarcinoma with propensity score matching. Ann Gastroenterol Surg (2022) 9;6(4):562–568. doi: 10.1002/ags3.12555
34. Chen TA, Yang FC, Li M, He Y, He L, Li JD, et al. Safety and efficacy of laparoscopic hepatectomy for intrahepatic cholangiocarcinoma. Chin J Hepatob Surg (2021) 27(7):485–8.
35. Ma DL, Yang JH, Du G, Zhang TX, Wang JL, Qin GJ, et al. Comparative study of laparoscopic surgery versus open surgery in the treatment of intrahepatic cholangiocarcinoma. Chin J Hepatob Surg (2021) 27(9):645–51.
36. Brustia R, Laurent A, Goumard C, Langella S, Cherqui D, Kawai T, et al. Laparoscopic versus open liver resection for intrahepatic cholangiocarcinoma: Report of an international multicenter cohort study with propensity score matching. Surg (2022) 171(5):1290–302. doi: 10.1016/j.surg.2021.08.015
37. Salehi O, Kazakova V, Vega EA, Kutlu OC, Alarcon SV, Freeman R, et al. Selection criteria for minimally invasive resection of intrahepatic cholangiocarcinoma-a word of caution: a propensity score matched analysis using the national cancer database. Surg Endosc (2022) 36(7):5382–91. doi: 10.1007/s00464-021-08842-y
38. Kubo S, Shinkawa H, Asaoka Y, Ioka T, Igaki H, Izumi N, et al. Liver cancer study group of Japan clinical practice guidelines for intrahepatic cholangiocarcinoma. Liver Cancer (2022) 11(4):290–314. doi: 10.1159/000522403
39. Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol (2014) 60(6):1268–89. doi: 10.1016/j.jhep.2014.01.021
40. Kang SH, Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, et al. Prognostic comparison of the 7th and 8th editions of the American joint committee on cancer staging system for intrahepatic cholangiocarcinoma. J Hepatob Pancreat Sci (2018) 25(4):240–8. doi: 10.1002/jhbp.543
41. Franssen S, Soares KC, Jolissaint JS, Tsilimigras DI, Buettner S, Alexandrescu S, et al. Comparison of hepatic arterial infusion pump chemotherapy vs resection for patients with multifocal intrahepatic cholangiocarcinoma. JAMA Surg (2022) 157(4):590–6. doi: 10.1001/jamasurg.2022.1298
42. Ramouz A, Ali-Hasan-Al-Saegh S, Shafiei S, Fakour S, Khajeh E, Majlesara A, et al. Repeat liver resection for recurrent intrahepatic cholangiocarcinoma: meta-analysis. Br J Surg (2022) 109(7):580–7. doi: 10.1093/bjs/znac075
43. Bartsch F, Tripke V, Baumgart J, Hoppe-Lotichius M, Heinrich S, Lang H. Extended resection of intrahepatic cholangiocarcinoma: A retrospective single-center cohort study. Int J Surg (2019) 67:62–9. doi: 10.1016/j.ijsu.2019.05.006
44. Sa-Ngiamwibool P, Aphivatanasiri C, Sangkhamanon S, Intarawichian P, Kunprom W, Thanee M, et al. Modification of the AJCC/UICC 8th edition staging system for intrahepatic cholangiocarcinoma: proposal for an alternative staging system from cholangiocarcinoma-prevalent northeast Thailand. HPB (Oxford) (2022) 22:01504–0. doi: 10.1016/j.hpb.2022.06.004
45. Chan AKC, Jamdar S, Sheen AJ, Siriwardena AK. The OSLO-COMET randomized controlled trial of laparoscopic versus open resection for colorectal liver metastases. Ann Surg (2018) 268(6):e69. doi: 10.1097/SLA.0000000000002640
46. Lou S, Yin X, Wang Y, Zhang Y, Xue Y. Laparoscopic versus open gastrectomy for gastric cancer: A systematic review and meta-analysis of randomized controlled trials. Int J Surg (2022) 102:106678. doi: 10.1016/j.ijsu.2022.106678
47. Bailey MB, Davenport DL, Vargas HD, Evers BM, McKenzie SP. Longer operative time: deterioration of clinical outcomes of laparoscopic colectomy versus open colectomy. Dis Colon Rectum (2014) 57(5):616–22. doi: 10.1097/DCR.0000000000000114
48. Han ES, Lee KW, Suh KS, Yi NJ, Choi Y, Hong SK, et al. Shorter operation time and improved surgical outcomes in laparoscopic donor right hepatectomy compared with open donor right hepatectomy. Surg (2021) 170(6):1822–9. doi: 10.1016/j.surg.2021.06.005
49. Bagante F, Spolverato G, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, et al. Assessment of the lymph node status in patients undergoing liver resection for intrahepatic cholangiocarcinoma: the new eighth edition AJCC staging system. J Gastrointest Surg (2018) 22(1):52–9. doi: 10.1007/s11605-017-3426-x
Keywords: intrahepatic cholangiocarcinoma, laparotomy, laparoscopy, hepatectomy, meta-analysis
Citation: Zhao X, Gao F-w, Jiang K-y, Yang J, Xie Q-y, Gong J, Yang M-y, Mao T-y and Lei Z-h (2023) Laparoscopic or open liver resection for intrahepatic cholangiocarcinoma: A meta-analysis and systematic review. Front. Oncol. 13:1096714. doi: 10.3389/fonc.2023.1096714
Received: 12 November 2022; Accepted: 10 February 2023;
Published: 01 March 2023.
Edited by:
Andrea Belli, G. Pascale National Cancer Institute Foundation (IRCCS), ItalyReviewed by:
Robert Sutcliffe, University Hospitals Birmingham NHS Foundation Trust, United KingdomCopyright © 2023 Zhao, Gao, Jiang, Yang, Xie, Gong, Yang, Mao and Lei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng-wei Gao, NzA3ODUwMjU1QHFxLmNvbQ==; Ze-hua Lei, bGVpdHNlaHVhQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.