- 1Minimally Invasive Tumor Therapies Center, Guangdong Second Provincial General Hospital, Guangzhou, China
- 2Department of Gastroenterology, Fogang County People’s Hospital, Fogang, China
- 3Center of Digestive Endoscopology, The Second People’s Hospital of Luoding City, Luoding, China
- 4Department of Acupuncture and Massage Rehabilitation, Integrated Hospital of Traditional Chinese Medicine, Southern Medical University, Guangzhou, China
Background: Early-onset colorectal cancer (EOCRC) has an alarmingly increasing trend and arouses increasing attention. Causes of death in EOCRC population remain unclear.
Methods: Data of EOCRC patients (1975–2018) were extracted from the Surveillance, Epidemiology, and End Results database. Distribution of death was calculated, and death risk of each cause was compared with the general population by calculating standard mortality ratios (SMRs) at different follow-up time. Univariate and multivariate Cox regression models were utilized to identify independent prognostic factors for overall survival (OS).
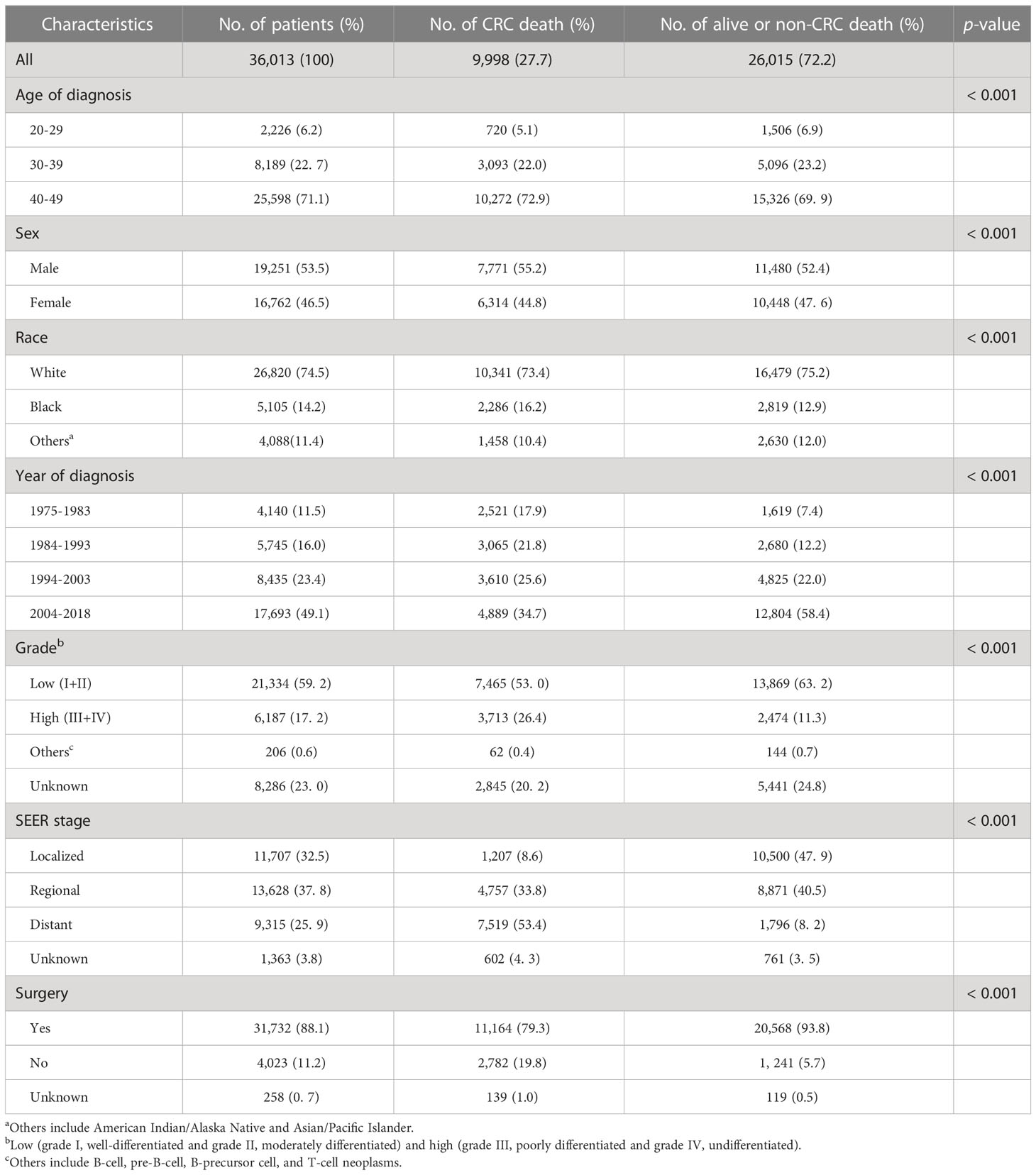
Results: The study included 36,013 patients, among whom 9,998 (27.7%) patients died of colorectal cancer (CRC) and 6,305 (17.5%) patients died of non-CRC causes. CRC death accounted for a high proportion of 74.8%–90.7% death cases within 10 years, while non-CRC death (especially cardiocerebrovascular disease death) was the major cause of death after 10 years. Non-cancer death had the highest SMR in EOCRC population within the first year after cancer diagnosis. Kidney disease [SMR = 2.10; 95% confidence interval (CI), 1.65–2.64] and infection (SMR = 1.92; 95% CI, 1.48–2.46) were two high-risk causes of death. Age at diagnosis, race, sex, year of diagnosis, grade, SEER stage, and surgery were independent prognostic factors for OS.
Conclusion: Most of EOCRC patients died of CRC within 10-year follow-up, while most of patients died of non-CRC causes after 10 years. Within the first year after cancer diagnosis, patients had high non-CRC death risk compared to the general population. Our findings help to guide risk monitoring and management for US EOCRC patients.
Introduction
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer death worldwide, with more than 1.9 million new cases and 935,000 deaths in 2020 (1). The incidence and mortality rate of CRC have declined in the last several decades (2), whereas the incidence of CRC in population < 50 years (deemed early-onset colorectal cancer, EOCRC) is increasing alarmingly (3). It is reported that CRC is a leading cause of cancer incidence and death in those aged 20–49 in the United States (4).
Increasing incidence and mortality rate of EOCRC require more understanding and action to this disease. With the advancement of cancer treatment, CRC patients, especially those young, have longer survival expectancy. Analyzing distribution of causes of death helps to guide high-risk disease monitoring and prevention in EOCRC survivors, which plays an important role in improving overall survival. Some previous studies analyzed causes of death in CRC patients with all age (5–7) or old age (8, 9). However, to the best of our knowledge, few recent studies focus on causes of death in EOCRC population. Evidence from previous studies indicated that EOCRC is different from average-onset CRC (AOCRC) both in clinical characteristics and pathogenesis (10), and it should be distinct from traditional CRC (11). Compared to AOCRC patients, EOCRC patients have less comorbidities and better basic condition, but they receive more aggressive anti-cancer treatment (12, 13), which may result in increased long-term non-cancer death risk. Therefore, we hypothesize that distribution of causes of death in EOCRC population is different from that of AOCRC.
To identify high-risk diseases threatening EOCRC survivors’ life and guide surveillance, we analyzed causes of death in EOCRC population using a national cancer database with long-term follow-up. Furthermore, the risk of each cause of death is compared with the general population in the USA.
Methods
Data source
Data of the present study were obtained from the Surveillance, Epidemiology and End Results (SEER) database using SEER*Stat software (version 8.4.0) after submitting the data use agreement and getting permission. The SEER database, which covers approximately 34.6% population in the United States, is the authoritative program of the National Cancer Institute (NCI) (14). This national large-scale data with long-term follow-up information is an ideal source for causes of death analysis. Patients’ informed consents and Institutional Review Board (IRB) approval are not required, since these data are publicly available in the database.
Study design and participants
This is a national population-based retrospective study with long-term follow-up. To delineate the rough trends of EOCRC mortality in the last several decades, we first performed the joinpoint analysis in the US general population using data from the Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research (CDC WONDER, 1999–2019) (15). Distribution of causes of death in EOCRC population was analyzed at different follow-up times to primarily identify high-risk diseases. Standard mortality ratios (SMRs) and absolute excess risks (AERs) of each cause of death in the overall EOCRC population and subgroups were calculated to compare death risk with the US general population. Furthermore, univariate and multivariate analyses were conducted to identify independent prognostic factors correlated with patients’ survival. Patients who survived 0–1 months after EOCRC diagnosis were excluded for causes of death analysis due to very short survival time. The report of the present study follows the STROBE guidelines (16).
Patients in the SEER database who met the following eligibility criteria were included: (1) histological confirmation of colon or rectal cancer as the primary cancer, (2) age at diagnosis between 20 and 49 years old, and (3) diagnosed between 1975 and 2018. Patient exclusion criteria were as follows: (1) death certification or autopsy only, (2) unknown causes of death or race, and (3) without active follow-up.
Variables and outcomes
Patient variables include age, race, sex, year of diagnosis, grade, SEER stage, surgery, survival months, and cause of death. Patient age was categorized as 20–29, 30–39, and 40–49. Races included black, white, others, and unknown. Year of diagnosis was classified into four groups: 1975–1984, 1985–1994, 1995–2004, and 2005–2018. Tumor grade was categorized as grade I–IV, others, and unknown. SEER stage included localized, regional, distant, and unknown. Surgery was classified as yes, no, and unknown. Cause of death recode in the SEER database is based on the International Classification of Diseases (ICD) versions 8–10 since 1969 (17). Non-cancer causes of death in this study were further categorized as follows: cardiocerebrovascular diseases, infection, diabetes mellitus, Alzheimer’s disease, respiratory diseases, digestive diseases, kidney diseases, suicide, accidents and homicide, and other non-neoplastic diseases (Supplementary Table S1).
In this study, the primary end points for prognostic factor analysis were overall survival (OS), which was defined as the duration from cancer diagnosis to death from any causes. Patients who were still alive at the last follow-up time (31 December 2018) were viewed as censored events.
Statistical analysis
Chi-square test was used to compare patient baseline characteristics. Trends of crude mortality rate were analyzed using Joinpoint Regression Program, Version 4.9.1.0—April 2022 (Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute, USA) (https://surveillance.cancer.gov/joinpoint/). Microsoft Excel 2016 software (Microsoft, Redmond, WA, USA) was used to calculate distribution of causes of death and draw the histogram. SMR and AER were calculated by SEER*Stat software (version 8.4.0). SMR represents the observed-to-expected ratio. Observed deaths mean patient numbers die from a certain cause in the EOCRC population or a certain subgroup. Expected deaths were calculated using person-years multiplied by mortality rate of a certain cause in the general population provided by CDC WONDER. Person-years were calculated from the date of CRC diagnosis and ended at the date of death or the last follow-up, whichever came first. The AER was calculated as follows: (observed deaths − expected deaths)/10,000 person-years. The corresponding 95% confidence intervals (CIs) of the SMRs were calculated using Poisson regression models. Univariate and multivariate analyses were conducted utilizing Cox regression model. Hazard ratios (HRs) and the corresponding 95% CIs were reported. Two-tailed p < 0.05 was defined as statistically significant.
Results
Patient characteristics
Based on the above-mentioned criteria, 36,013 patients diagnosed with EOCRC in the SEER database were included in this study. The average follow-up time was 15.21 years (standard deviation, 0.08). Patient baseline characteristics are presented in Table 1. The majority of patients in this study aged at 40–49 years (71.1%), and were white (74.5%). Male patients were more than female patients (53.5% vs. 46.5%). Around half of the patients were diagnosed in 2004–2018 (49.1%). Most of the patients had low (I and II) grade tumor (59.2%), and most of the patients accepted surgery (88.1%) (Table 1). During follow-up time, 9,998 (27.7%) patients died of CRC, and 6,305 (17.5%) patients died of non-CRC causes.
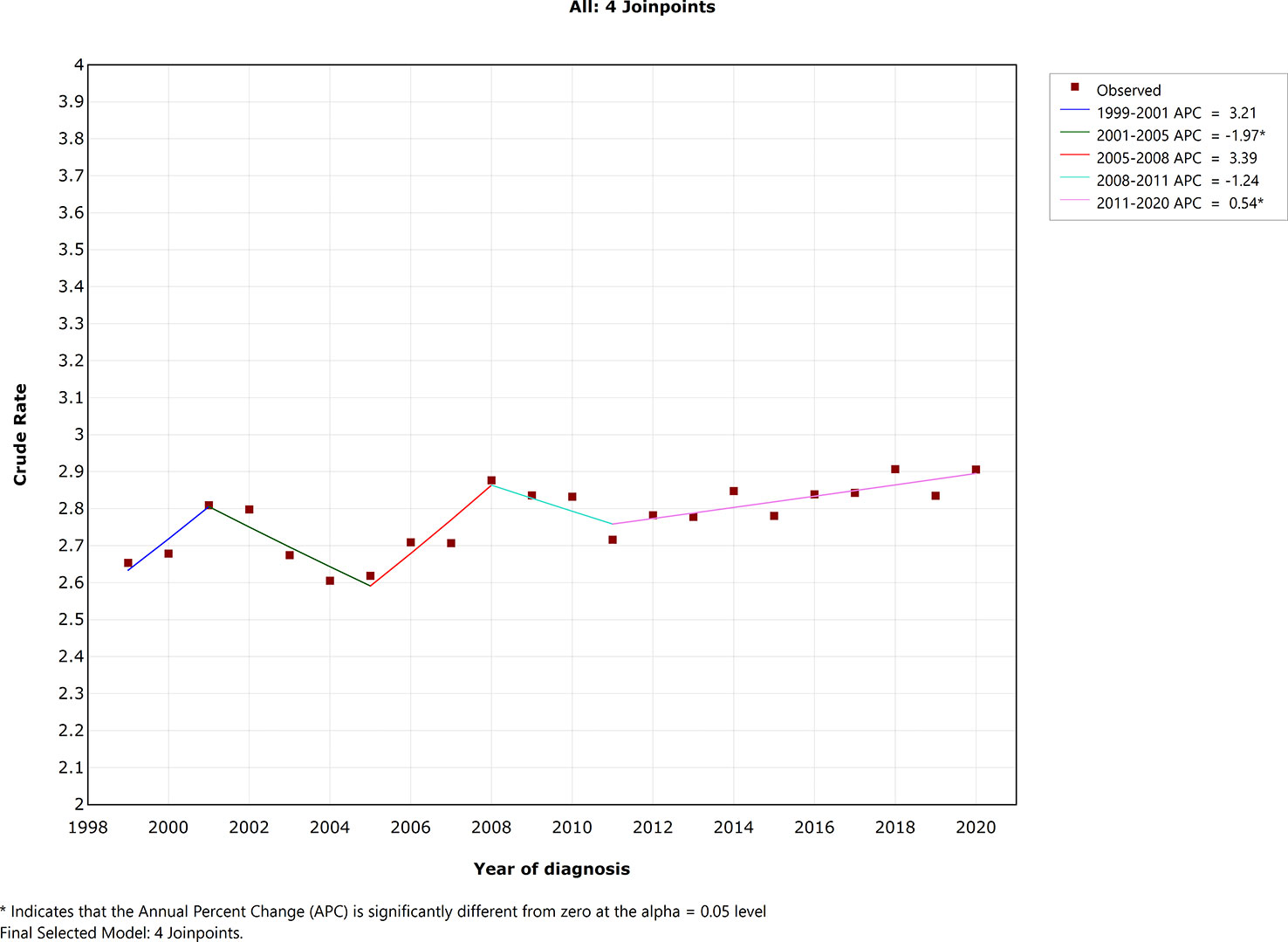
Mortality trends of EOCRC in the USA
As presented in Figure 1, crude mortality rate was not stable during the last two decades. There were four joinpoints in the final selected model. Crude mortality rate decreased significantly during 2001–2005 [annual percentage change (APC) = −1.97; 95% CI, −3.8 to −0.1; p =0.040], whereas crude mortality rate increased significantly during 2011–2020 (APC = 0.54; 95% CI, 0.2–0.9; p = 0.006). Crude mortality rate change was not statistically significant in the year 1999–2001 (APC = 3.21; 95% CI, −0.6 to 7.2; p = 0.092), 2005–2008 (APC = 3.39; 95% CI, −0.4 to 7.3; p = 0.074), 2008–2011 (APC = −1.24; 95% CI, −4.8 to 2.4, p = 0.454).
Distribution of causes of death at different follow-up time
The proportion of each death cause is presented in Figure 2 and Supplementary Table S2. CRC death was the major cause of death in 2–119 months after cancer diagnosis and accounted for a high proportion, with 87.08%, 90.69%, 87.24%, and 74.80% CRC-related death rate in 2–11, 12–35, 36–59, and 60–119 months, respectively (Figure 1 and Supplementary Table S2). However, the proportion of CRC-related death reduced dramatically after 120 months, with 35.58%, 19.54%, and 5.93% CRC-related death rate in 120–179, 180–239, and 240+ months, respectively (Figure 1 and Supplementary Table S2). Conversely, non-CRC death accounted for a high proportion of death after 120 months. Cardiocerebrovascular diseases (CVDs), other cancers, and respiratory diseases were the three most common non-CRC death causes. After 180 months, CVD exceeded CRC and became the leading cause of death in EOCRC population (Figure 2).
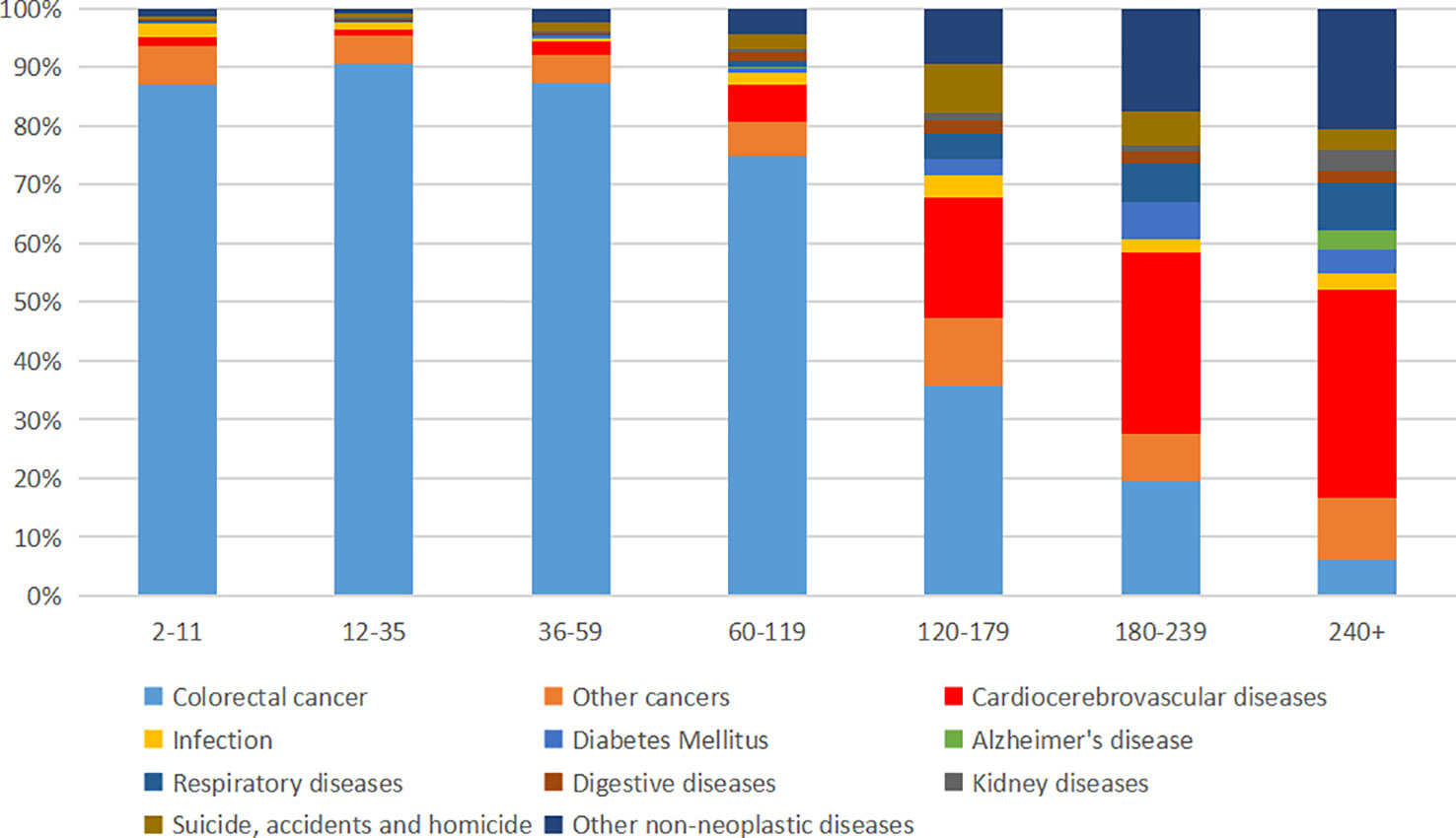
Figure 2 Proportion of death in early-onset colorectal cancer population at different follow-up times.
SMRs and AERs for non-cancer causes of death
Detailed SMRs and AERs for each non-cancer cause of death at different follow-up times are presented in Table 2. It indicated a trend that the death risk of various causes was significantly higher than the general population in the relatively short period of follow-up, and the death risk of these causes was gradually approaching that of the general population over time.
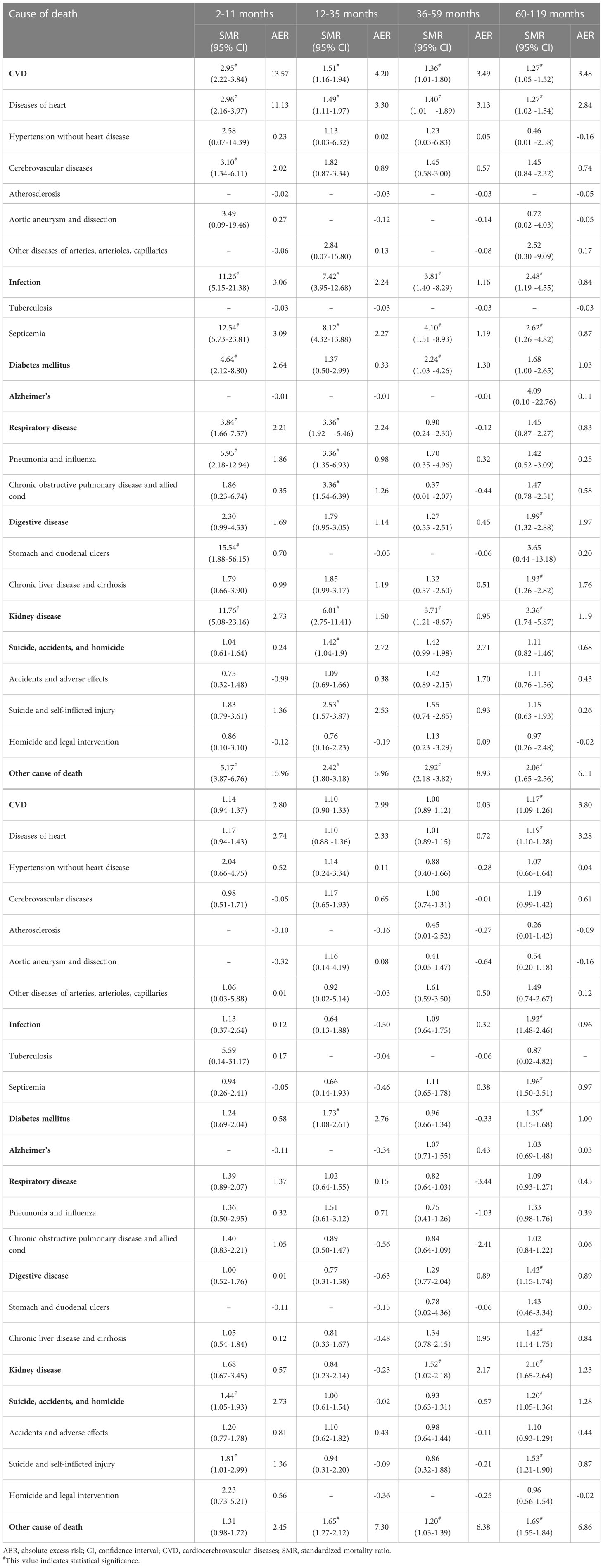
Table 2 Standardized mortality ratios and absolute excess risks for causes of death on different follow-up time in early-onset colorectal cancer population.
During 2- to 11-month follow-up, the EOCRC population had a high risk of death from kidney disease (SMR = 11.76; 95% CI, 5.08–23.16), infection (SMR = 11.26; 95% CI, 5.15–21.38), other causes of death (SMR = 5.17; 95% CI, 3.87–6.76), diabetes mellitus (SMR = 4.64; 95% CI, 2.12–8.80), respiratory disease (SMR = 3.84; 95% CI, 1.66–7.57), and CVD (SMR = 2.95; 95% CI, 2.22–3.84) (Table 2). Other causes of death and CVD had the highest AER (15.96 per 10,000 person-year and 13.57 per 10,000 person-year, respectively). SMRs and AERs of these causes declined within 10-year follow-up (Table 2). After 10 years, CVD, infection, and respiratory disease mortality risks were not significantly higher than the general population. During 120–179 months, suicide, accidents, and homicide risk was 1.44-fold higher than the general population (95% CI, 1.05–1.93). During 180–239 months, the mortality risk of diabetes mellitus was 1.73-fold higher than the general population (95% CI, 1.08–2.61) (Table 2).
For all follow-up time combination, patients had higher mortality risk of CVD (SMR = 1.17; 95% CI, 1.09–1.26), infection (SMR = 1.92; 95% CI, 1.48–2.46), diabetes mellitus (SMR = 1.39; 95% CI, 1.15–1.68), digestive disease (SMR = 1.42; 95% CI, 1.15–1.74), kidney disease (SMR = 2.10; 95% CI, 1.65–2.64), suicide, accidents, and homicide (SMR = 1.20; 95% CI, 1.05–1.36), and other cause of death (SMR = 1.69; 95% CI, 1.55–1.84) (Table 2). SMRs and AERs for each cause of death at different follow-up times in subgroups are presented in Supplementary Tables S3–S9.
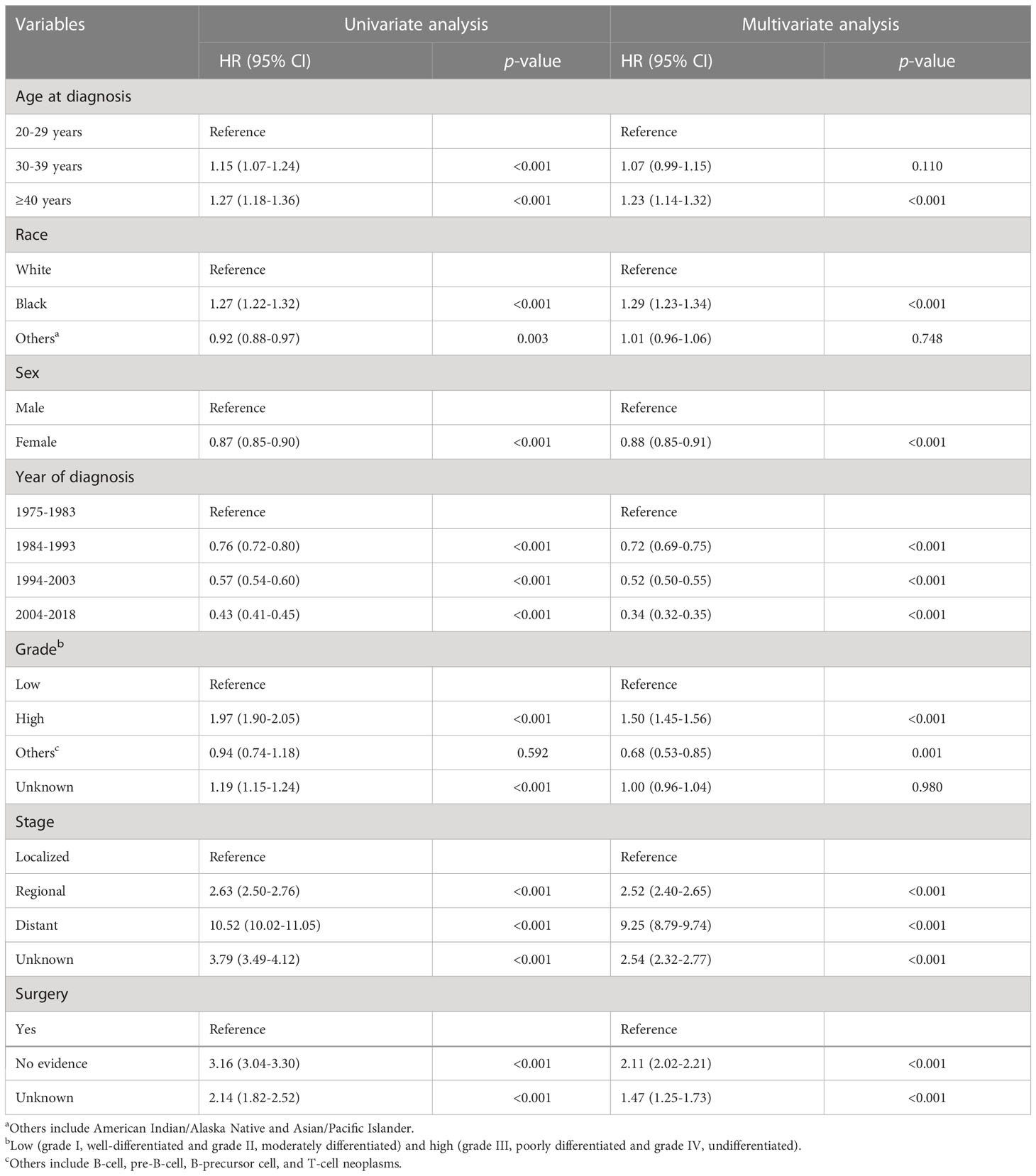
Prognostic factors for OS
As shown in Table 3, in univariate analysis, age at diagnosis, race, sex, year of diagnosis, grade, stage, and surgery were associated with overall survival. To avoid confounding factors, multivariate analysis was performed and further confirmed the above-mentioned factors as independent prognostic factors for OS. Compared with the younger patients, patients aged at 40–49 had higher death risk (HR = 1.23; 95% CI, 1.14–1.32, p < 0.001). Black patients had worse prognosis compared to white (HR = 1.29; 95% CI, 1.23–1.34, p <0.001). Female patients had better OS than male patients (HR = 0.88; 95% CI, 0.85–0.91, p <0.001) (Table 3). Diagnosed in more recent years indicated better OS. Patients with high-grade, regional or distant tumors, and no surgery evidence had worse OS.
Discussion
In the current long-term population-based study, we found that CRC death accounted for a high proportion of death within 10 years of cancer diagnosis, while non-CRC death, especially CVD death, was the major cause of death after 10 years. Compared to the general population, the EOCRC population had high risk of non-cancer death within 3 years after cancer diagnosis, but this disparity gradually diminished over time. Age at diagnosis, race, sex, year of diagnosis, grade, stage, and surgery were independent prognostic factors for OS. To the best of our knowledge, this is the first study focusing on cause of death in the EOCRC population.
EOCRC death was the main cause of death within 10-year follow-up, while non-EOCRC death became the leading cause of death after 10 years. Our results are similar to previous studies indicating that cancer-related death accounted for a high proportion of death in a relatively short period after diagnosis, whereas non-cancer death increased overtime and exceeded cancer-related death in long-term period (18–20). In CRC patients over 65 years old, Wang et al. also found that the proportions of CRC death were over 70% and 60% within 3 and 9 years after CRC diagnosis, respectively (9). Our results reflect a higher proportion of CRC death in the young population, with approximately 90% and 75% CRC death within 3- and 10-year follow-up. This may be explained by the relatively higher prevalence of comorbidities in older patients, and they are more likely to die from other diseases. Clinical guidelines of the American Society of Clinical Oncology (ASCO) emphasize the high risk of recurrence in 2–4 years after CRC diagnosis (21). In EOCRC population, more attention should be paid to CRC recurrence and metastasis within a longer period (10 years).
Non-CRC death is also worth noting in the EOCRC population. We observed a trend that the EOCRC population had high risk of non-CRC death compared to the age-matched general population, and the SMRs decrease overtime. A similar trend was also reflected in studies of CRC (22) or other solid cancers (23). A previous study conducted in the entire age CRC population (age ≤49 accounted for 0.996%) also showed relatively high non-CRC death risk in 2–11months, and SMRs in 12–119 months were lower; however, SMRs increased after 120 months (24). This difference may be attributed to the high prevalence of comorbidities in the AOCRC population (25) and long-term treatment side effect of anti-cancer treatment, whereas the long-term effect did not add excess death risk to the EOCRC population who had low prevalence of comorbidities. High non-CRC death risk in the short follow-up time may be explained by the side effect of cancer treatment. Further analysis using SMRs highlighting that not only should we pay attention to CRC death but also to non-CRC death within a 10-year follow-up.
Our results indicate that the risk of kidney disease and infection death in the EORCR population was over 10-fold to the age-matched general population in 2–11 months after cancer diagnosis. It was reported that acute kidney injury was common after colorectal cancer surgery, and it was correlated with mortality (26). Furthermore, chemotherapy was confirmed to have nephrotoxicity (27). Infection after surgery is also a frequent problem (28). Our results demonstrated increased respiratory disease death risk within 1-year follow-up in younger cancer population, which was consistent with a previous study (29). This is mainly caused by pneumonia and influenza in the present study. Similar to a study in CRC population (6), we also found increased diabetes mellitus death risk. Interestingly, we found that CVD death risk was highest in 2–11 months after diagnosis, and it decreased within 10 years. Our results are in line with a study by Gaitanidis et al., which showed high CVD death risk within 1 year after CRC diagnosis (30). A previous study including 563,298 CRC patients also found that CRC population had higher cerebrovascular-specific mortality risk than the general population (31). Our results could be explained by cardiotoxicity of aggressive chemotherapy in the EOCRC population. After 10 years, CVD death risk was not statistically different from the general population in the EOCRC population, which was distinct from AOCRC (24) and other cancers (32). We speculated that anti-cancer therapy had acute toxicity, but long-term CVD effect was not so obvious in young patients. Preventing these high-risk diseases helps to improve patients’ survival.
We confirmed that age at diagnosis, race, sex, year of diagnosis, grade, stage, and surgery were independent prognostic factors for OS in the EOCRC population, which was supported by previous studies (33–36). Worse prognosis in black population could be explained by poverty, less access to medical resource, and unfavorable tumor characteristics (34). Gender disparities in OS may be caused by the differences in life behaviors, genetics, and hormonal factors between men and women (37). With the advances of cancer detection and treatment, patients’ OS was significantly improved during the last four decades. Patients who had high stage or grade tumor had worse prognosis, and surgery was an effective treatment. These independent prognostic factors assist to identify high-risk patients.
The current study has some limitations. First, this is a retrospective study, and some unknown information may cause bias. Second, the population in the study span a long time and disease classification, treatment, and survival care change during this period, which may also affect the results. Third, limited data in the SEER database do not allow us to further analyze reasons behind each cause of death. However, the main purpose of the study is to describe causes of death in the EOCRC population. Fourth, the newest histological and molecular factors including tumor budding, microsatellite instability, epithelial–mesenchymal transition, and molecular classification also had prognostic impact (38). However, data from the SEER database lack this information, which limited us to further analyze the prognostic effect of the newest histological and molecular factors. Finally, our results should be interpreted with caution when they are extrapolated to population outside the US because these results are based on the US population. Despite these limitations, the present study is the first to focus on causes of death in a large-scale EOCRC population with long-term follow-up.
Conclusions
In conclusion, we found that death from CRC accounted for a high proportion within a 10-year follow-up, and non-CRC death exceeded it after 10 years. Compared to the general population, patients had high risk of non-cancer death, especially within the first year after cancer diagnosis. Non-CRC death cannot be ignored after EOCEC diagnosis. Monitoring and preventing these high risk causes help to improve patients’ survival in the US.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Patients’ informed consents and Institutional Review Board (IRB) approval are not required, since these data are publicly available in the database.
Author contributions
YRC: conceptualization, data curation, roles/writing—original draft, investigation, and software. LH: data curation, writing—original draft, resources, and validation. XL: data curation, visualization, and investigation. YT: formal analysis, methodology, and software; GL: writing—original draft and validation. YJC: writing—data curation and visualization. CW: software and data curation. QL: conceptualization, project administration, supervision, and writing—review and editing. XX: conceptualization, funding acquisition, writing—review and editing, and supervision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the President Foundation of Integrated Hospital of Traditional Chinese Medicine, Southern Medical University, No. 1202102004.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1094493/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin (2017) 67(3):177–93. doi: 10.3322/caac.21395
3. Siegel RL, Torre LA, Soerjomataram I, Hayes RB, Bray F, Weber TK, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut (2019) 68(12):2179–85. doi: 10.1136/gutjnl-2019-319511
4. Bhandari A, Woodhouse M, Gupta S. Colorectal cancer is a leading cause of cancer incidence and mortality among adults younger than 50 years in the USA: a SEER-based analysis with comparison to other young-onset cancers. J Investig Med (2017) 65(2):311–5. doi: 10.1136/jim-2016-000229
5. Tong L, Ahn C, Symanski E, Lai D, Du XL. Temporal trends in the leading causes of death among a large national cohort of patients with colorectal cancer from 1975 to 2009 in the united states. Ann Epidemiol (2014) 24(6):411–7. doi: 10.1016/j.annepidem.2014.01.005
6. Chen J, Zheng Y, Wang H, Zhang D, Zhao L, Yu D, et al. Cause of death among patients with colorectal cancer: a population-based study in the united states. Aging (Albany NY) (2020) 12(22):22927–48. doi: 10.18632/aging.104022
7. Afifi AM, Elmehrath AO, Ruhban IA, Saad AM, Gad MM, Al-Husseini MJ, et al. Causes of death following nonmetastatic colorectal cancer diagnosis in the U.S.: A population-based analysis. Oncologist (2021) 26(9):733–9. doi: 10.1002/onco.13854
8. Tong L, Ahn C, Symanski E, Lai D, Du XL. Effects of newly developed chemotherapy regimens, comorbidities, chemotherapy-related toxicities on the changing patterns of the leading causes of death in elderly patients with colorectal cancer. Ann Oncol (2014) 25(6):1234–42. doi: 10.1093/annonc/mdu131
9. Wang R, Han L, Dai W, Mo S, Xiang W, Li Q, et al. Cause of death for elders with colorectal cancer: a real-world data analysis. J Gastrointest Oncol (2020) 11(2):269–76. doi: 10.21037/jgo.2020.03.04
10. Stoffel EM, Murphy CC. Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology (2020) 158(2):341–53. doi: 10.1053/j.gastro.2019.07.055
11. Yeo H, Betel D, Abelson JS, Zheng XE, Yantiss R, Shah MA, et al. Early-onset colorectal cancer is distinct from traditional colorectal cancer. Clin Colorectal Cancer (2017) 16(4):293–299.e6. doi: 10.1016/j.clcc.2017.06.002
12. Murphy CC, Harlan LC, Lund JL, Lynch CF, Geiger AM. Patterns of colorectal cancer care in the united states: 1990-2010. J Natl Cancer Inst (2015) 107(10). doi: 10.1093/jnci/djv198
13. Kneuertz PJ, Chang GJ, Hu CY, Rodriguez-Bigas MA, Eng C, Vilar E, et al. Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA Surg (2015) 150(5):402–9. doi: 10.1001/jamasurg.2014.3572
14. Institute NC. Surveillance, epidemiology, and end results program. Available at: https://seer.cancer.gov/about/overview.html.
15. Centers for Disease Control and Prevention. CDC Wonder (2022). Available at: http://wondercdcgov/.
16. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg (2014) 12(12):1495–9. doi: 10.1016/j.ijsu.2014.07.013
17. SEER. Cause of death recode 1969 (2022). Available at: https://seer.cancer.gov/codrecode/1969_d03012018/index.html.
18. Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res (2011) 13(3):R64. doi: 10.1186/bcr2901
19. Afifi AM, Saad AM, Al-Husseini MJ, Elmehrath AO, Northfelt DW, Sonbol MB. Causes of death after breast cancer diagnosis: A US population-based analysis. Cancer (2020) 126(7):1559–67. doi: 10.1002/cncr.32648
20. Horn SR, Stoltzfus KC, Mackley HB, Lehrer EJ, Zhou S, Dandekar SC, et al. Long-term causes of death among pediatric patients with cancer. Cancer (2020) 126(13):3102–13. doi: 10.1002/cncr.32885
21. Meyerhardt JA, Mangu PB, Flynn PJ, Korde L, Loprinzi CL, Minsky BD, et al. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American society of clinical oncology clinical practice guideline endorsement. J Clin Oncol (2013) 31(35):4465–70. doi: 10.1200/JCO.2013.50.7442
22. Feng Y, Jin H, Guo K, Wasan HS, Ruan S, Chen C. Causes of death after colorectal cancer diagnosis: A population-based study. Front Oncol (2021) 11:647179. doi: 10.3389/fonc.2021.647179
23. Zaorsky NG, Churilla TM, Egleston BL, Fisher SG, Ridge JA, Horwitz EM, et al. Causes of death among cancer patients. Ann Oncol (2017) 28(2):400–7. doi: 10.1093/annonc/mdw604
24. Lu L, Ma L, Zhang X, Susanne Mullins C, Linnebacher M. Analyzing non-cancer causes of death of colorectal carcinoma patients in the US population for the years 2000-2016. Cancer Med (2021) 10(8):2740–51. doi: 10.1002/cam4.3673
25. Cuthbert CA, Hemmelgarn BR, Xu Y, Cheung WY. The effect of comorbidities on outcomes in colorectal cancer survivors: a population-based cohort study. J Cancer Surviv (2018) 12(6):733–43. doi: 10.1007/s11764-018-0710-z
26. Slagelse C, Gammelager H, Iversen LH, Sørensen HT, Christiansen CF. Acute kidney injury and 1-year mortality after colorectal cancer surgery: a population-based cohort study. BMJ Open (2019) 9(3):e024817. doi: 10.1136/bmjopen-2018-024817
27. Jagieła J, Bartnicki P, Rysz J. Nephrotoxicity as a complication of chemotherapy and immunotherapy in the treatment of colorectal cancer, melanoma and non-small cell lung cancer. Int J Mol Sci (2021) 22(9). doi: 10.3390/ijms22094618
28. Konishi T, Watanabe T, Kishimoto J, Nagawa H. Elective colon and rectal surgery differ in risk factors for wound infection: results of prospective surveillance. Ann Surg (2006) 244(5):758–63. doi: 10.1097/01.sla.0000219017.78611.49
29. Ye Y, Otahal P, Marwick TH, Wills KE, Neil AL, Venn AJ. Cardiovascular and other competing causes of death among patients with cancer from 2006 to 2015: An Australian population-based study. Cancer (2019) 125(3):442–52. doi: 10.1002/cncr.31806
30. Gaitanidis A, Spathakis M, Tsalikidis C, Alevizakos M, Tsaroucha A, Pitiakoudis M. Risk factors for cardiovascular mortality in patients with colorectal cancer: a population-based study. Int J Clin Oncol (2019) 24(5):501–7. doi: 10.1007/s10147-018-01382-x
31. Dai ZH, Tang M, Chen YL, Zhang TL, Li J, Lv GH, et al. Incidence and risk factors for cerebrovascular-specific mortality in patients with colorectal cancer: A registry-based cohort study involving 563,298 patients. Cancers (Basel) (2022) 14(9). doi: 10.3390/cancers14092053
32. Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J (2019) 40(48):3889–97. doi: 10.1093/eurheartj/ehz766
33. Li Y, Liu W, Zhou Z, Ge H, Zhao L, Liu H, et al. Development and validation of prognostic nomograms for early-onset locally advanced colon cancer. Aging (Albany NY) (2020) 13(1):477–92. doi: 10.18632/aging.202157
34. Kamath SD, Torrejon N, Wei W, Tullio K, Nair KG, Liska D, et al. Racial disparities negatively impact outcomes in early-onset colorectal cancer independent of socioeconomic status. Cancer Med (2021) 10(21):7542–50. doi: 10.1002/cam4.4276
35. Yu C, Zhang Y. Development and validation of a prognostic nomogram for early-onset colon cancer. Biosci Rep (2019) 39(6). doi: 10.1042/BSR20181781
36. Wu J, Lu L, Chen H, Lin Y, Zhang H, Chen E, et al. Prognostic nomogram to predict the overall survival of patients with early-onset colorectal cancer: a population-based analysis. Int J Colorectal Dis (2021) 36(9):1981–93. doi: 10.1007/s00384-021-03992-w
37. Samawi HH, Yin Y, Speers CH, Cheung WY. Sex disparities in outcomes of early stage colorectal cancer: A population-based study. Clin Colorectal Cancer (2018) 17(4):e711–7. doi: 10.1016/j.clcc.2018.07.006
Keywords: early-onset colorectal cancer, cause of death, prognostic factor, surveillance, survivorship
Citation: Chen Y, He L, Lu X, Tang Y, Luo G, Chen Y, Wu C, Liang Q and Xu X (2023) Causes of death among early-onset colorectal cancer population in the United States: a large population-based study. Front. Oncol. 13:1094493. doi: 10.3389/fonc.2023.1094493
Received: 22 November 2022; Accepted: 30 March 2023;
Published: 24 April 2023.
Edited by:
Meritxell Rovira, Institut d’Investigacio Biomedica de Bellvitge (IDIBELL), SpainReviewed by:
Umamaheswaran Gurusamy, Nationwide Children’s Hospital, United StatesSimona Gurzu, George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of Târgu Mureş, Romania
Copyright © 2023 Chen, He, Lu, Tang, Luo, Chen, Wu, Liang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qihua Liang, MzA5NzQ1MjY2QHFxLmNvbQ==; Xiuhong Xu, ZWlsZWVueHVhZEAxNjMuY29t
 Yuerong Chen
Yuerong Chen Lanping He2
Lanping He2 Xiuhong Xu
Xiuhong Xu