- 1PET-CT Center, Chongqing University Three Gorges Hospital, Chongqing, China
- 2Department of radiology, Chongqing University Three Gorges Hospital, Chongqing, China
Purpose: The purpose of this study is to compare the application value of 68Ga-FAPI and 18F-FDG PET/CT in primary and metastatic lesions of abdominal and pelvic malignancies (APMs).
Materials: The search, limited to the earliest available date of indexing through 31 July 2022, was performed on PubMed, Embase, and Cochrane Library databases using a data-specific Boolean logic search strategy. We calculated the detection rate (DR) of 68Ga-FAPI and 18F-FDG PET/CT in the primary staging and recurrence of APMs, and pooled sensitivities/specificities based on lymph nodes or distant metastases.
Results: We analyzed 473 patients and 2775 lesions in the 13 studies. The DRs of 68Ga-FAPI and 18F-FDG PET/CT in evaluating the primary staging and recurrence of APMs were 0.98 (95% CI: 0.95-1.00), 0.76 (95% CI: 0.63-0.87), and 0.91(95% CI: 0.61-1.00), 0.56 (95% CI: 0.44-0.68), respectively. The DRs of 68Ga-FAPI and 18F-FDG PET/CT in primary gastric cancer and liver cancer were 0.99 (95% CI: 0.96-1.00), 0.97 (95% CI: 0.89-1.00) and 0.82 (95% CI: 0.59-0.97), 0.80 (95% CI: 0.52-0.98), respectively. The pooled sensitivities of 68Ga-FAPI and 18F-FDG PET/CT in lymph nodes or distant metastases were 0.717(95% CI: 0.698-0.735) and 0.525(95% CI: 0.505-0.546), and the pooled specificities were 0.891 (95% CI: 0.858-0.918) and 0.821(95% CI: 0.786-0.853), respectively.
Conclusions: This meta-analysis concluded that 68Ga-FAPI and 18F-FDG PET/CT had a high overall diagnostic performance in detecting the primary staging and lymph nodes or distant metastases of APMs, but the detection ability of 68Ga-FAPI was significantly higher than that of 18F-FDG. However, the ability of 68Ga-FAPI to diagnose lymph node metastasis is not very satisfactory, and is significantly lower than that of distant metastasis.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022332700.
1 Introduction
In recent years, the incidence and mortality of cancer have increased. An estimated 1,918,030 new cancer cases and 609,360 cancer deaths are expected in the United States, as published on January 12, 2022 (1). In the abdominal and pelvic malignancies (APMs), the proportions of liver cancer (LC), gastric cancer (GC), pancreatic cancer, colorectal cancer, and female reproductive system tumors are relatively high (1). Therefore, extensive clinical and basic research to strengthen the mission of health, extend life expectancy, and reduce the burden of disease and disability is crucial. Early diagnosis and accurate evaluation of treatment decisions and prognoses are of great significance (2). Given this fact, researchers have been stepping up their efforts to address these clinical issues (3).
The tumor microenvironment (TME) is a complex and dynamic framework that plays a key role in the survival, proliferation, spread and drug resistance of malignant cells through tumorigenic signaling pathways (4–6). Recently, the cancer-promoting role of the TME has become an the issues of interest to scientists (3). The tumor stroma is the main component of tumor lesions and has common components among various types of cancer (3). In addition to affecting tumor cells, the TME also affects a variety of nonmalignant cells (including immune cells, endothelial cells, epithelial cells, fibroblasts, and adipocytes), which are coordinated through complex, dynamic networks of different cytokines and chemokines (5, 7). A series of previous studies have led to a shift in the current research focus and direction of drug development from “tumor” to TME elements, which has aroused researchers’ interest in potential molecular imaging applications and therapies (8). Cancer-associated fibroblasts (CAFs) are an extremely heterogeneous and plastic cell population with different sources, functions and surface markers, which exist in various types of malignant solid tumors and are highly expressed, closely related to tumor progression, invasion and metastasis, and have become an attractive target for the TME (6, 9). However, when it is not expressed or is underexpressed in the stroma of normal tissues and benign tumors (10), it can usually be identified by fibroblast activating protein (FAP) as a marker (11). FAP, a type II membrane-bound glycoprotein with dipeptidyl peptidase and endopeptidase activities, is highly expressed in the membranes and stroma of CAFs, especially in approximately 90% of epithelial tumors (10), such as liver, colorectal, ovarian, and pancreatic cancers (2, 12, 13). In this case, using FAP as a CAF identifier and designing FAP-specific PET radiotracers and therapeutic radioligands are some of the results of efforts over the years (3). Therefore, FAP is an important and promising target for cancer therapy (14). In recent years, FAP inhibitors (FAPIs) have become a new targeted molecular probe in nuclear medicine and have attracted much attention in cancer diagnosis and treatment. Currently, dozens of radiopharmaceuticals targeting FAP have been developed, such as FAPI-01, FAPI-2, FAPI-04, FAPI-42, FAPI-46, and FAPI-74 (15–18). In a recent study, high-quality images were obtained using gallium-68-FAPI-04 positron emission tomography/computed tomography (68Ga-FAPI-04 PET/CT) showing good biodistribution properties and a high tumor background ratio in 28 tumors, including abdominal and pelvic tumors (19).
Recent years have seen an explosion in publications on 68Ga-FAPI, and FAPI imaging has opened a new chapter in molecular imaging for tumors and nontumor (6). Several studies have demonstrated 68Ga-DOTA-FAPI to be useful for diagnosing and differentiating primary tumors, detecting metastases, and performing image-guided intervention (20–24). However, its clinical effects and indications are not fully established (6). Fluorine-18-fluorodeoxyglucose (18F-FDG) PET/CT is an important imaging tool for preoperative systematic evaluation, tumor staging, and analysis of the efficacy of tumor treatment (25). However, 18F-FDG PET/CT imaging has certain limitations for some tumors, such as gastric mucinous adenocarcinoma, well-differentiated hepatocellular carcinoma, and renal cell carcinoma (25). Recently, 68Ga-FAPI and 18F-FDG imaging in various tumors has been studied to confirm the advantages and disadvantages of the two methods. FAPI is considered a promising molecular imaging agent because both of these studies confirm that 68Ga-FAPI can assess the primary tumor stage and detect lymph nodes in addition to distant metastases better than 18F-FDG (24, 26–30). Due to different sample sizes, uneven quality, and geographical influences, these results exhibit a high heterogeneity. The authors of a meta-analysis published in 2021 concluded that 68Ga-FAPI PET imaging was good at diagnosing primary and distant metastases in tumors and non-tumors (3). However, the study included only a few articles and did not include many valuable new papers published after March 2021. The results should also be interpreted cautiously because they are based on a heterogeneous set of studies.
Therefore, to further evaluate which of 68Ga-FAPI and 18F-FDG PET/CT were better in tumors, the aim of our study was to compare the application of 68Ga-FAPI and 18F-FDG PET/CT in primary and metastatic lesions of APMs.
2 Materials and methods
This meta-analysis was in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. This project was registered in the PROSPERO database (registration number: CRD42022332700).
2.1 Data sources and search strategy
We performed electronic literature searches of the PubMed, Embase, and Cochrane Library databases for English-language articles from the earliest available date of indexing through July 31, 2022. The search was performed using a data-specific Boolean logic search strategy using the following keywords: FAP, FAPI, fibroblasts, cancer-associated fibroblasts, CAF, PET, PET/CT, PET-CT, FDG, fluorodeoxyglucose, and positron emission tomography. To obtain more comprehensive search information, we also manually searched the reference lists of identified publications. The search process was performed independently by the two reviewers (XL and HTL).
2.2 Inclusion and exclusion criteria
Published articles that met the following conditions were included in the analysis.
(1) 68Ga-FAPI and 18F-FDG PET/CT were evaluated simultaneously as diagnostic methods for APMs (primary tumor, lymph node involvement, and distant metastasis). Abdominal and pelvic lesions refer to tumors of the liver, pancreas, gallbladder, spleen, gastrointestinal tract, urinary system, female reproductive system and adrenal glands.
(2) The lesions were confirmed by histopathology or combined clinical/imaging follow-up.
(3) Sufficient data were provided to calculate the number of positive cases with respect to the primary APM tumor, or true-positive, false-positive, false-negative, and true-negative of non-primary tumors (lymph nodes or distant metastases).
The exclusion criteria were as follows: (1) overlapping papers; (2) review articles, animal experiments, editorials or letters, comments, and conference proceedings; (3) a lack of access to the full text; and (4) a sample size of fewer than 10 patients or lesions.
2.3 Quality assessment
Two reviewers (XL and HTL) independently evaluated each eligible article’s methodological quality. Any disagreements were resolved through consultation or intervention by the third reviewer. The evaluation is based on the modified Quality Assessment of Diagnostic Accuracy Studies version 2 (QUADAS-2), as recommended by the Cochrane Collaboration (31). Each item was evaluated as “high”, “low”, or “unclear”.
2.4 Data extraction
Data extraction was carried out for the remaining articles that met the criteria. For each study, we extracted the following data: first author name, year of publication, country, study design (prospective, retrospective), type of APM, diagnostic criteria, imaging purpose, image interpretation, age, sex, sample size, PET/CT scan range, type of imaging agent, injection activity, interval between the FAPI and FDG scans, maximum standardized uptake value (SUVmax) and tumor-to-background ratio (TBR) of the primary lesion, type of image analysis (qualitative, quantitative or semiquantitative), adverse events of imaging agents.
We recorded or calculated the specificity (SEN), sensitivity (SPE), and accuracy per patient and per lesion. When literature evaluation included multiple malignancies such as those of the neck, chest and abdomen, we only extracted data from abdominal tumors. If the abdominal tumor had fewer than 10 patients or lesions, the article was abandoned. When both primary and non-primary tumors (metastases) were evaluated, these data were collected for subgroup analysis. The authors were not contacted to retrieve unpublished data. Data were cross-checked and any discrepancies were discussed to reach a consensus (XL, HTL and CLG).
2.5 Statistical analysis
This study collected data for each eligible study. Descriptive statistics and frequency tables were used to summarize the data. The analysis was performed with subgroups of primary and non-primary tumors, and diagnostic pooled assessments of 68Ga-FAPI and 18F-FDG PET/CT were performed in the subgroups. On a patient-level basis, we evaluated the value of 68Ga-FAPI and 18F-FDG PET/CT in primary tumors, including primary staging and recurrence. Non-primary tumors, including lymph node, peritoneum, liver, bone and other metastases, were evaluated at the lesion-based level. The primary objective of this study was to evaluate the application value of 68Ga-FAPI and 18F-FDG PET/CT in the primary staging and recurrence of APMs using the detection rate (DR). In addition, we separately evaluated the detection value of 68Ga-FAPI and 18F-FDG PET/CT in the primary staging of GC and LC. DR was defined as the ratio between the number of patients or lesions with at least one suspected lesion detected by the imaging facility and the total number of abdomen-pelvic malignancy patients who underwent the scan. The secondary objective of this study was to evaluate the SEN, SPE, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) of 68Ga-FAPI and 18F-FDG PET/CT in metastatic lesions of APMs. A bivariate normal random-effects model for measures was used to analyze and pool the summary points for sensitivity and specificity and their 95% confidence intervals (CIs). The hierarchical summary receiver-operating characteristic (SROC) model was used performed to draw SROC curves and calculate the area under the curve (AUC). I2 and Cochran’s Q homogeneity tests were used to evaluate the consistency of the data (the higher the inconsistency, the greater the uncertainty of the meta-analysis results). According to Higgins JPT et al. (32) in 2003, heterogeneity was divided into low, medium and high levels, expressed with I2 as 25%, 50% and 75%, respectively. Multiple factors may lead to heterogeneity bias, and no single value is recommended for further analysis. We defined low/medium heterogeneity as acceptable (i.e., I2<50%). In the case of significant heterogeneity between studies, subgroup analysis or meta-regression was performed to analyze the data to determine the source of heterogeneity. As described by Deeks and colleagues (33), we examined the possibility of publication bias by using an effective sample size funnel plot and a regression test of asymmetry. Tests for significance were two-tailed, with a statistically significant P value threshold of 0.05. All statistical analyses were carried out using Stata version 16.0 software (StataCorp LP, College Station, TX, USA), Review Manager software (Cochrane Collaboration, version 5.3.5, London, United Kingdom) and MetaDiSc 1.4 (Clinical Biostatistics team of the Ramón y Cajal Hospital in Madrid, Spain).
3 Results
3.1 Literature search and study selection
A total of 452 articles were retrieved from the PubMed/MEDLINE, Embase and Cochrane Library databases. Two hundred and twenty-three duplicate articles were excluded. Titles and abstracts were screened according to the established inclusion and exclusion criteria, 208 articles were deleted, leaving 15 papers, and a full-text search was conducted. Full-text reading was conducted, and 13 articles were finally eligible for meta-analysis. The detailed process of literature screening is shown in Figure 1.
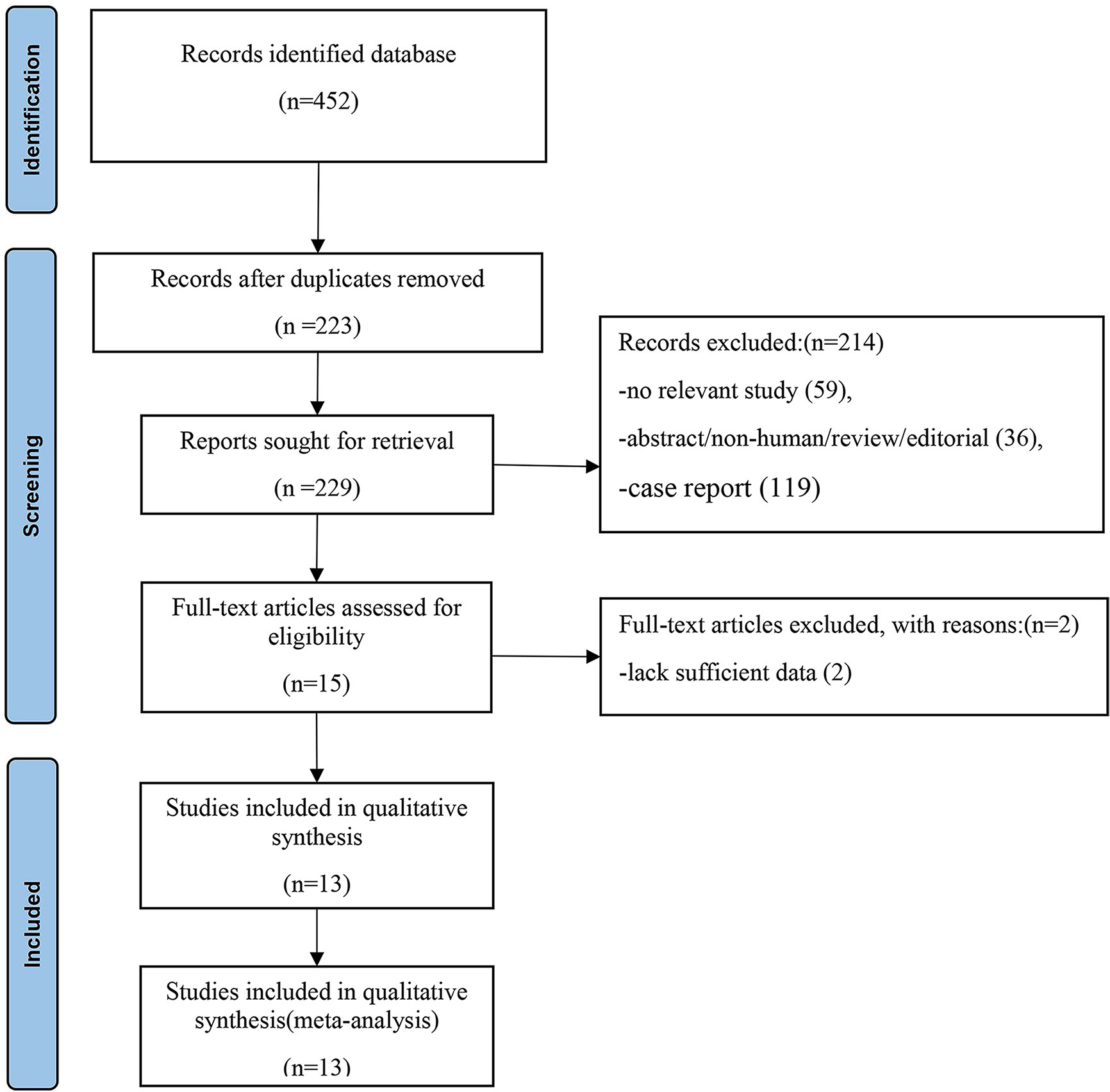
Figure 1 Flowchart of the search for eligible studies on 68Ga-PAFI and 18F-FDG PET/CT in patients of abdominal and pelvic malignancies. Thirteen articles were finally selected for this meta-analysis.
3.2 Characteristics of the included studies
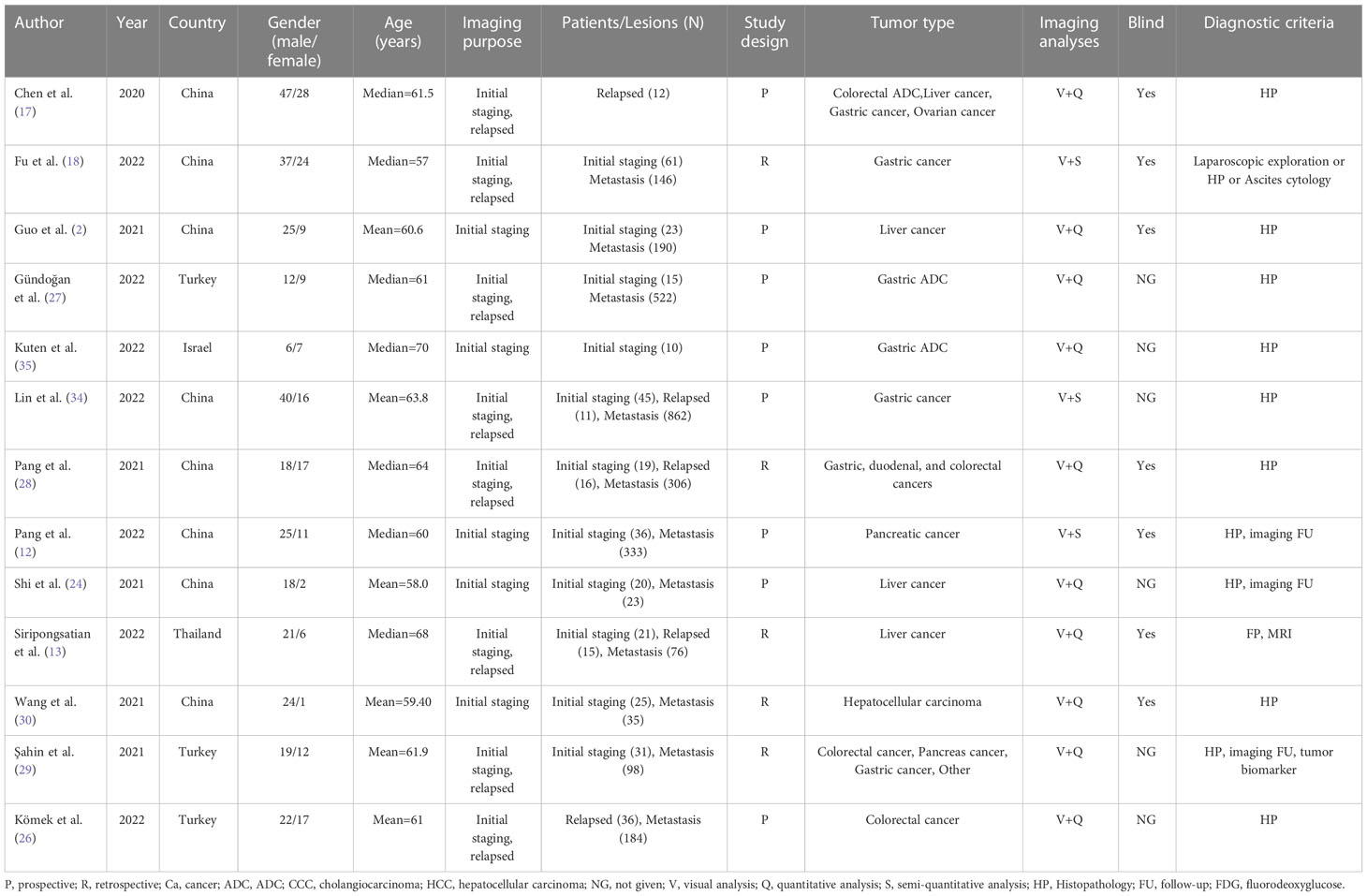
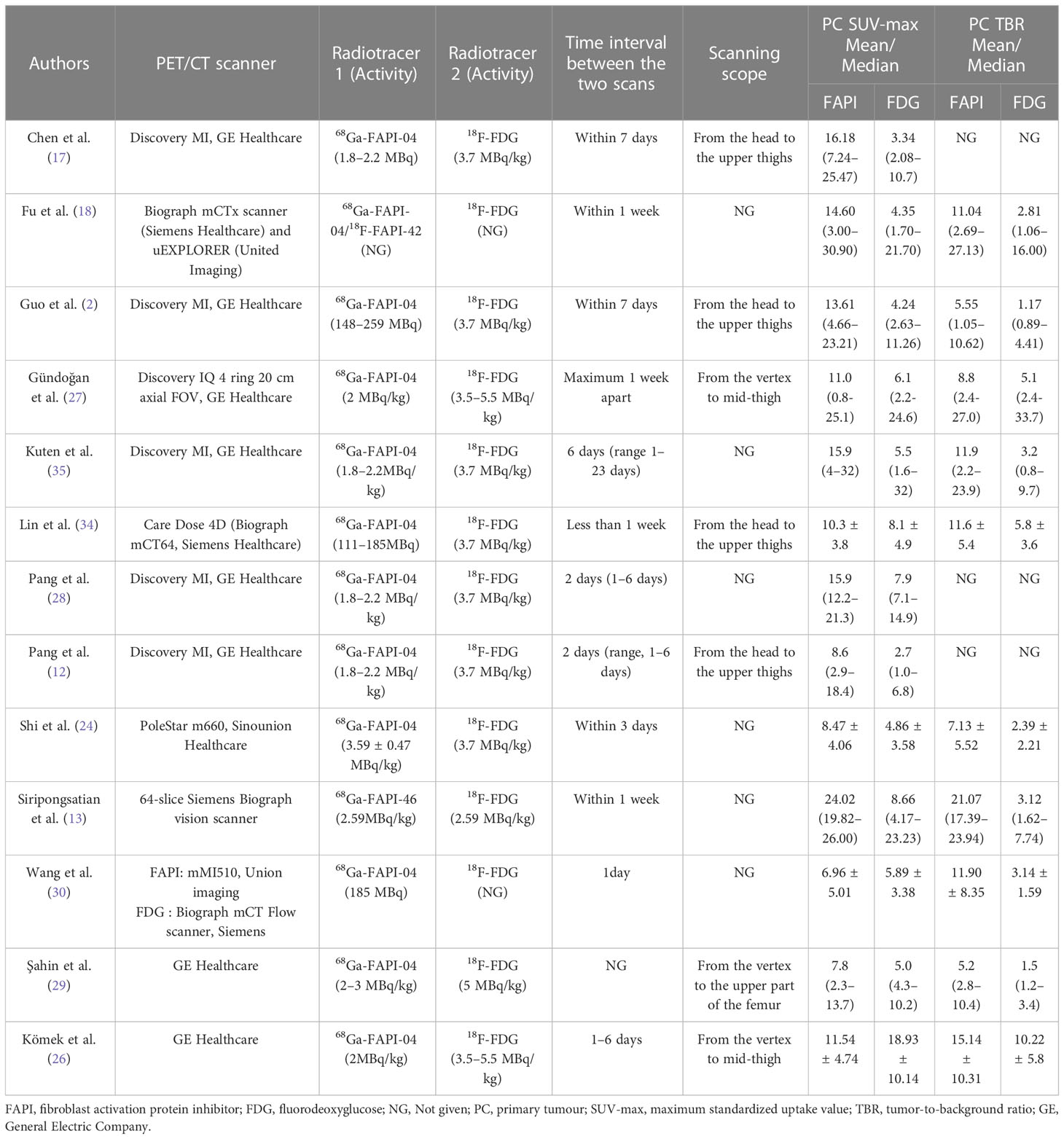
Overall, we analyzed 473 patients and 2775 lesions in the 13 studies (2, 12, 13, 17, 18, 24, 26–30, 34, 35). These studies were published between 2020 and 2022, among which 8 studies (2, 12, 17, 18, 24, 28, 30, 34) were from China, 3 studies (26, 27, 29) were from Turkey, and the others were from Thailand (13)and Israel (35). Eight studies (2, 12, 17, 24, 26, 27, 34, 35) had a prospective study design, and the remainder had a retrospective study design. Eight studies (13, 17, 18, 26–29, 34) assessed both primary staging and tumor recurrence, and five studies (2, 12, 24, 30, 35) assessed only primary staging. Although Chen et al. (17) and Komek et al. (26) evaluated the staging and recurrence of primary tumors in the original text, due to the limited sample size for the evaluation of primary tumors, we only extracted data on recurrence for analysis. One study (17) evaluated tumors in multiple parts of the body, including lung, esophageal, nasopharyngeal, colorectal, hepatic, gastric and ovarian cancer. However, we only extracted data from APM for analysis. All of the subjects included in this meta-analysis were APM, including 8 studies for gastrointestinal tumors (2, 17, 26–29, 34, 35), 4 for liver tumors (2, 13, 17, 24), 2 for pancreatic tumors (12, 29), and 1 for ovarian tumors (17). We found no other literature that simultaneously compared 68Ga-FAPI and 18F-FDG the female reproductive system (ovary, uterus, vagina), urinary system (kidney, prostate, bladder, ureter), adrenal gland, gallbladder, and spleen malignancies.
PET/CT was used as the imaging mode in all included studies. Seven studies (2, 12, 17, 26, 27, 29, 34) reported the PET/CT scanning scope, mostly from the head to the mid-upper thighs. The 68Ga-FAPI and 18F-FDG imaging scans were performed within a week of each other. 68Ga-FAPI-04 was employed in all studies except for that conducted by Siripongsatian et al. (13), who used the imaging agent 68Ga-FAPI-46. Fu et al. (18) used both imaging agents 68Ga-FDAPI-04 and 18F-DAPI-42 in their study.
All studies compared the SUVmax or TBR values of 68Ga-FAPI and 18F-FDG PET/CT in primary tumors, we found that FAPI-SUVmax was higher than FDG-SUVmax in most of them, and only the Komek et al. (26) study had a lower FAPI-SUVmax than FDG-SUVmax (mean: 11.54 vs. 18.93). All participants tolerated the 68Ga-FAPI PET/CT scan. No 68Ga-FAPI-related pharmacological effects or physiological responses occurred (12, 17, 28, 35). Furthermore, the authors of all the articles declared no conflicts of interest. The main characteristics of the 13 studies included in the meta-analysis are shown in Tables 1, 2.
3.3 Risk of bias and applicability
The risk of bias and applicability concerns for the included studies were assessed using QUADAS-2 (Figure 2). None of the studies were of low quality, and the overall quality of the studies was satisfactory.
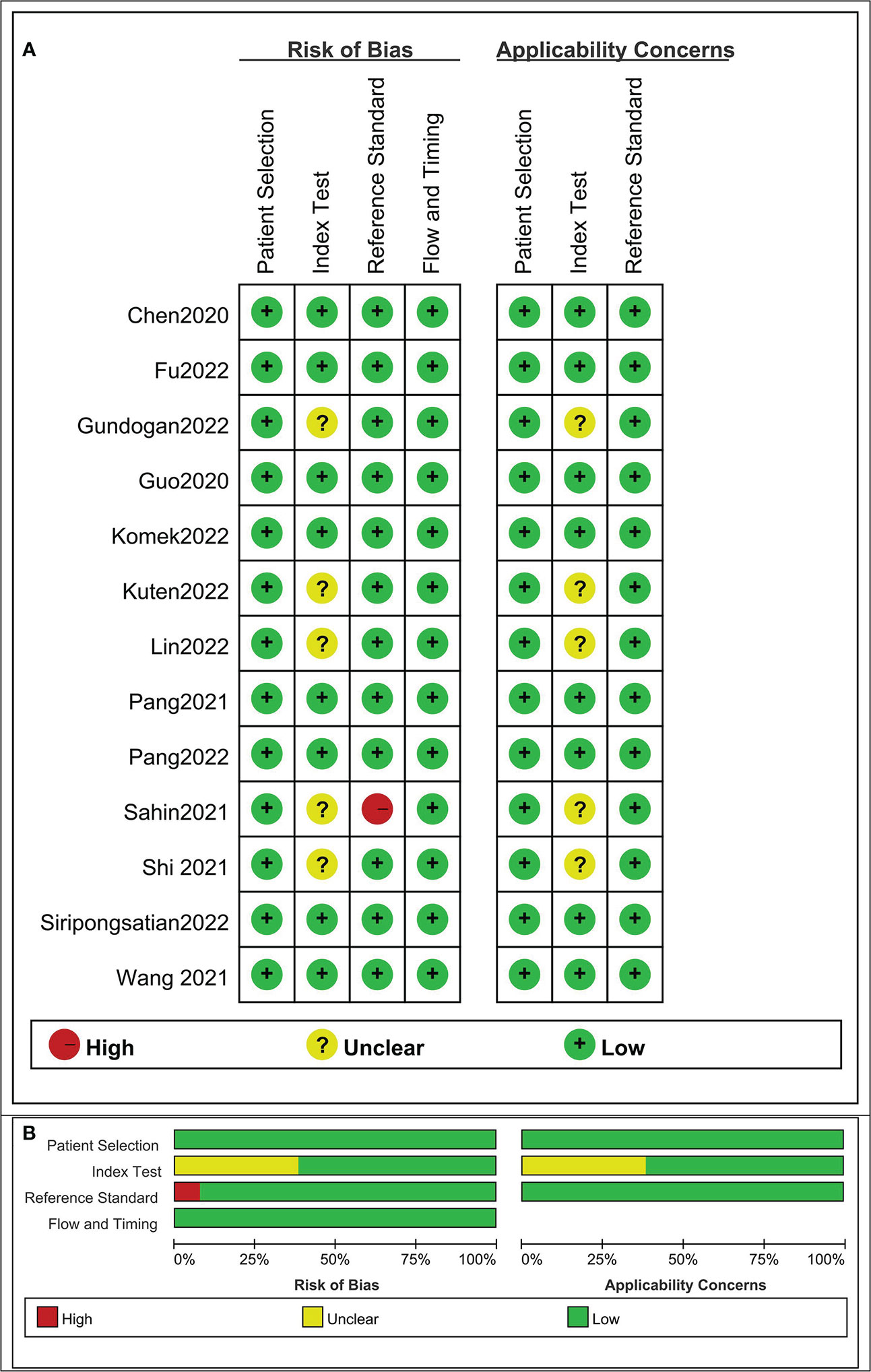
Figure 2 Risk of bias and applicability concerns the summary (A) and graph (B) of the studies included in the systematic review according to the QUADAS-2 tool. Overall quality of the included studies was deemed satisfactory.
3.4 Quantitative analysis (meta-analysis)
3.4.1 Based on primary tumor performance analysis
The DRs of 68Ga-FAPI and 18F-FDG PET/CT in evaluating the primary staging of APM were 0.98 (95% CI: 0.95-1.00; I2= 22.58%, p=0.23) and 0.76 (95% CI: 0.63-0.87; I2= 82.48%, p=0.00), respectively (Figure 3A). The difference between the two groups was statistically significant (P =0.00).
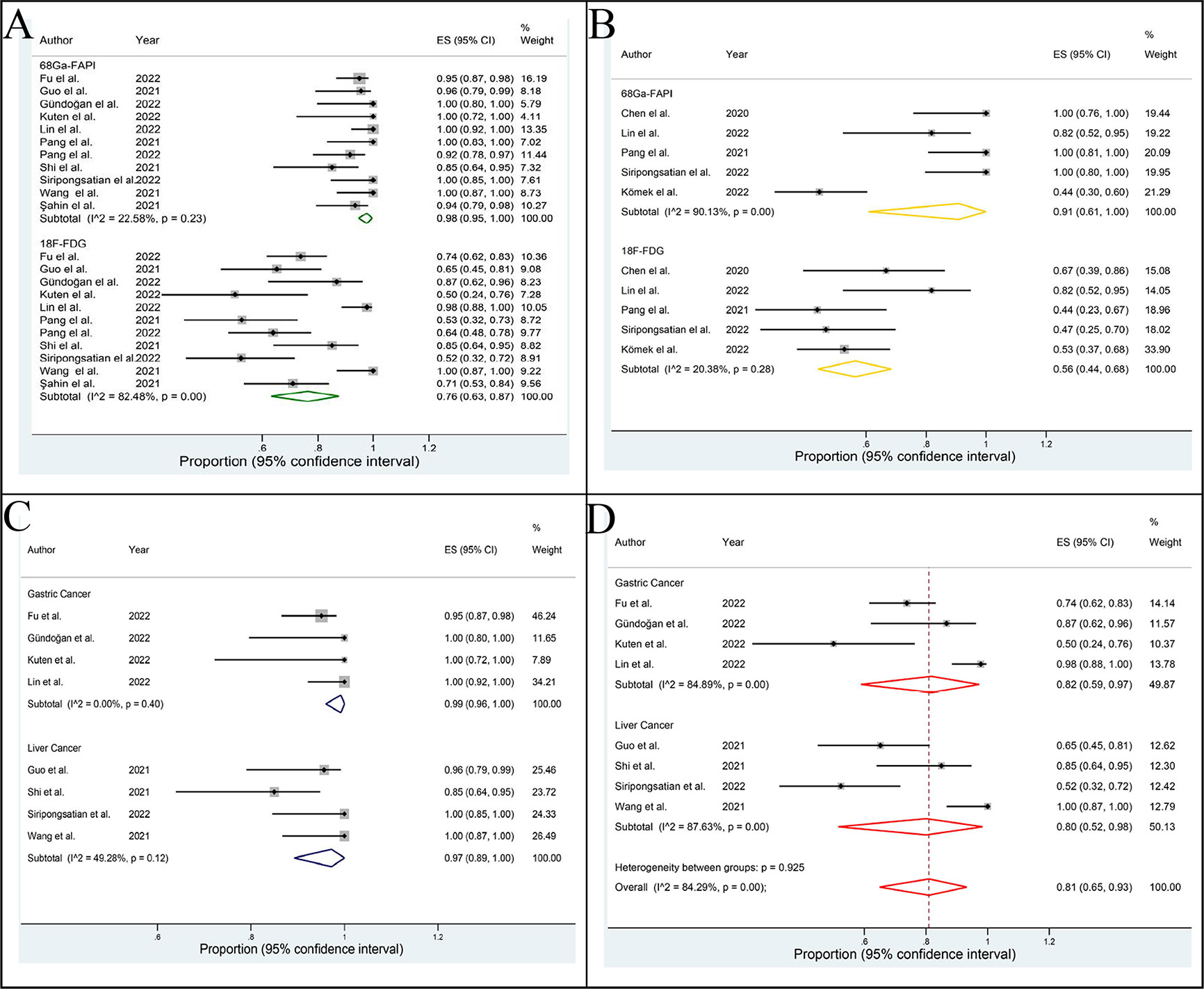
Figure 3 Forest plot of this meta-analysis. The detection rates of 68Ga-PAFI and 18F-FDG PET/CT in evaluating the primary staging (A) and recurrence (B) of abdominal and pelvic malignancy. The detection rates of 68Ga-FAPI (C) and 18F-FDG (D) PET/CT in evaluating the primary gastric cancer and liver cancer.
The DRs of 68Ga-FAPI and 18F-FDG PET/CT in identifying recurrence of APM were 0.91 (95% CI: 0.61-1.00; I2= 90.13%, p=0.00) and 0.56 (95% CI: 0.44 0.68; I2= 20.38%, p=0.28), respectively (Figure 3B). The difference between the two groups was statistically significant (p=0.04).
The DRs of 68Ga-FAPI and 18F-FDG PET/CT in primary GC and LC were 0.99 (95% CI: 0.96-1.00; I2= 0.00%, p=0.40), 0.97 (95% CI: 0.89-1.00; I2= 49.28%, p=0.12) and 0.82 (95% CI: 0.59-0.97; I2= 84.89%, p=0.00), 0.80 (95% CI: 0.52-0.98; I2= 87.63%, p=0.00), respectively (Figures 3C, D). Due to the limited sample size, we did not assess the DR of 68Ga-FAPI and 18F-FDG PET/CT in recurrence of GC and LC.
3.4.2 Based on non-primary tumor performance analysis
The pooled SENs of 68Ga-FAPI and 18F-FDG PET/CT in non-primary tumors were 0.717 (95% CI: 0.698-0.735; I2= 99.1%, p=0.000) and 0.525 (95% CI: 0.505-0.546; I2= 98.5%,p=0.000), and the pooled SPEs were 0.891 (95% CI: 0.858-0.918; I2= 83.0%, p=0.000) and 0.821 (95% CI: 0.786-0.853; I2= 64.4%, p=0.00), respectively. The AUCs were 0.946 and 0.841, respectively (Figure 4).
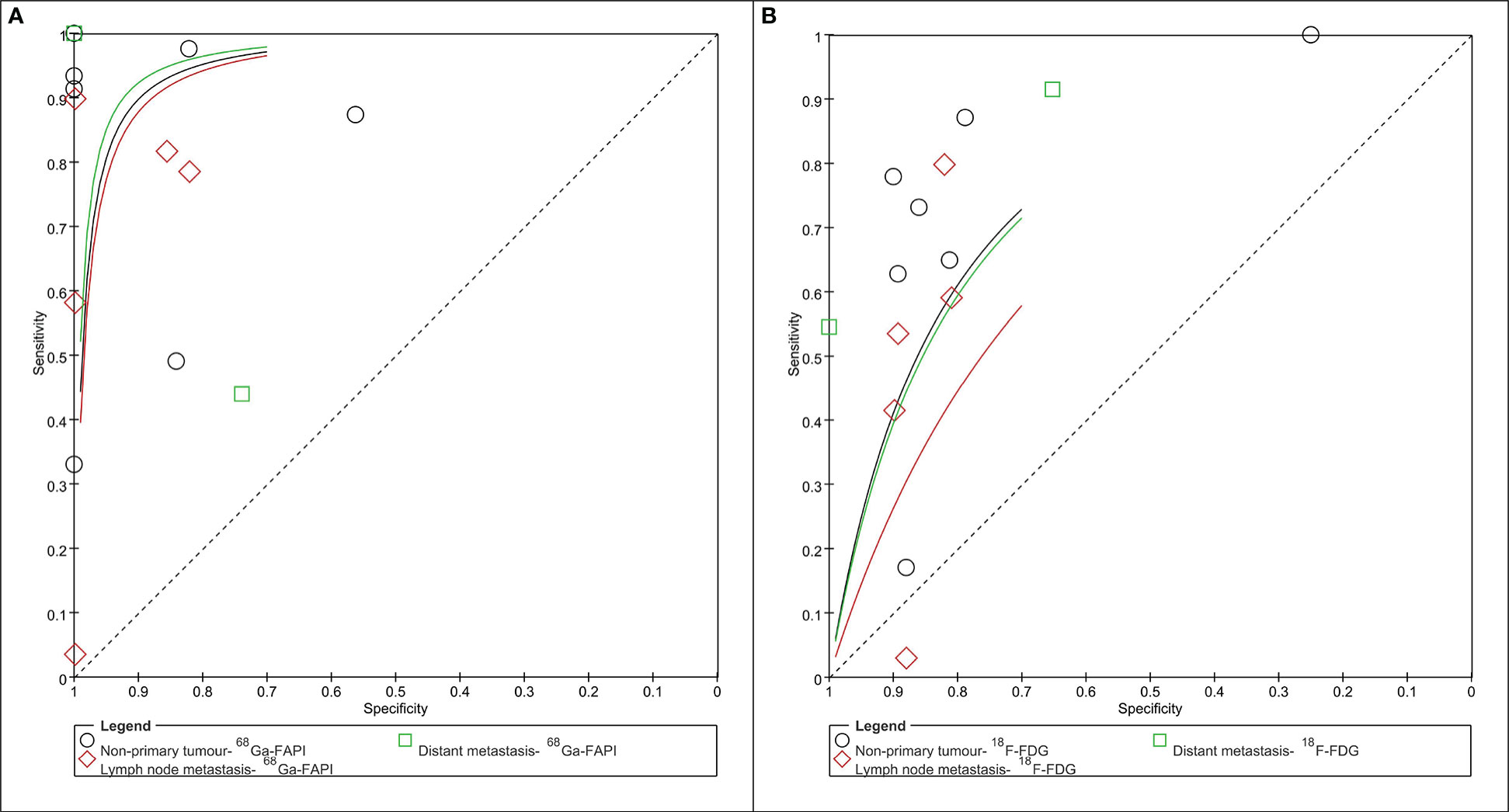
Figure 4 Summary receiver operator characteristic graph of 68Ga-PAFI (A) and 18F-FDG (B) PET/CT for non-primary tumor (lymph node and/or distant metastasis).
3.4.2.1 Based on lymph node metastasis performance analysis
The pooled SEN, SPE, and DOR of 68Ga-FAPI PET/CT in the assessment of lymph node metastases were 0.421 (95% CI: 0.389-0.453; I2= 99.4%, p=0.000), 0.908 (95% CI: 0.874-0.935; I2= 82.6%, p=0.000) and 35.860 (95% CI: 11.320-113.61; I2= 44.7%, p=0.093), respectively. The pooled SEN, SPE, and DOR of 18F-FDG PET/CT in the assessment of lymph node metastases were 0.235 (95% CI: 0.207-0.264; I2= 98.3%, p=0.000), 0.837 (95% CI: 0.799-0.870, I2= 0.0%, p=0.573) and 3.257 (95% CI: 0.656-16.176; I2= 90.1%, p=0.000), respectively. The AUC of 68Ga-FAPI and 18F-FDG PET/CT were 0.938 and 0.877, respectively (Figure 4).
3.4.2.2 Based on distant metastasis performance analysis
The pooled SEN, SPE, and DOR of 68Ga-FAPI PET/CT in the assessment of distant metastasis were 0.918 (95% CI:0.900-0.933; I2= 98.2%, p=0.000), 0.844 (95% CI: 0.729-0.924; I2= 52.6%, p=0.049) and 72.059 (95% CI:5.636-921.25; I2= 73.1%, p=0.001), respectively. The pooled SEN, SPE, and DOR of 18F-FDG PET/CT in the assessment of distant metastasis were 0.714 (95% CI:0.686-0.741; I2= 95.1%, p=0.000), 0.811 (95% CI: 0.691-0.900; I2= 62.0%, p=0.015), and 13.431 (95% CI: 5.759-31.322; I2= 0.0%, p=0.495), respectively. The AUC of 68Ga-FAPI and 18F-FDG PET/CT were 0.850 and 0.777, respectively (Figure 4).
3.5 Publication bias
Egger’s regression intercepts for DR pooling of 68Ga-FAPI and 18F-FDG PET/CT in primary tumor performance analysis were 0.317 (95% CI: -0.57 to 0.86, p=0.664) and 1.14 (95% CI: -3.68 to 1.47, p=0.358), respectively, indicating that publication bias was absent. Moreover, the funnel plots for both modalities were symmetric (Figures 5A, B). Analysis of non-primary tumors by 68Ga-FAPI and 18F-FDG PET/CT according to the linear regression detection method suggested regression coefficients of 3.52 (p=0.77) and 0.69 (p=0.92), respectively, indicating that there was no publication bias in the included studies (Figures 5C, D).
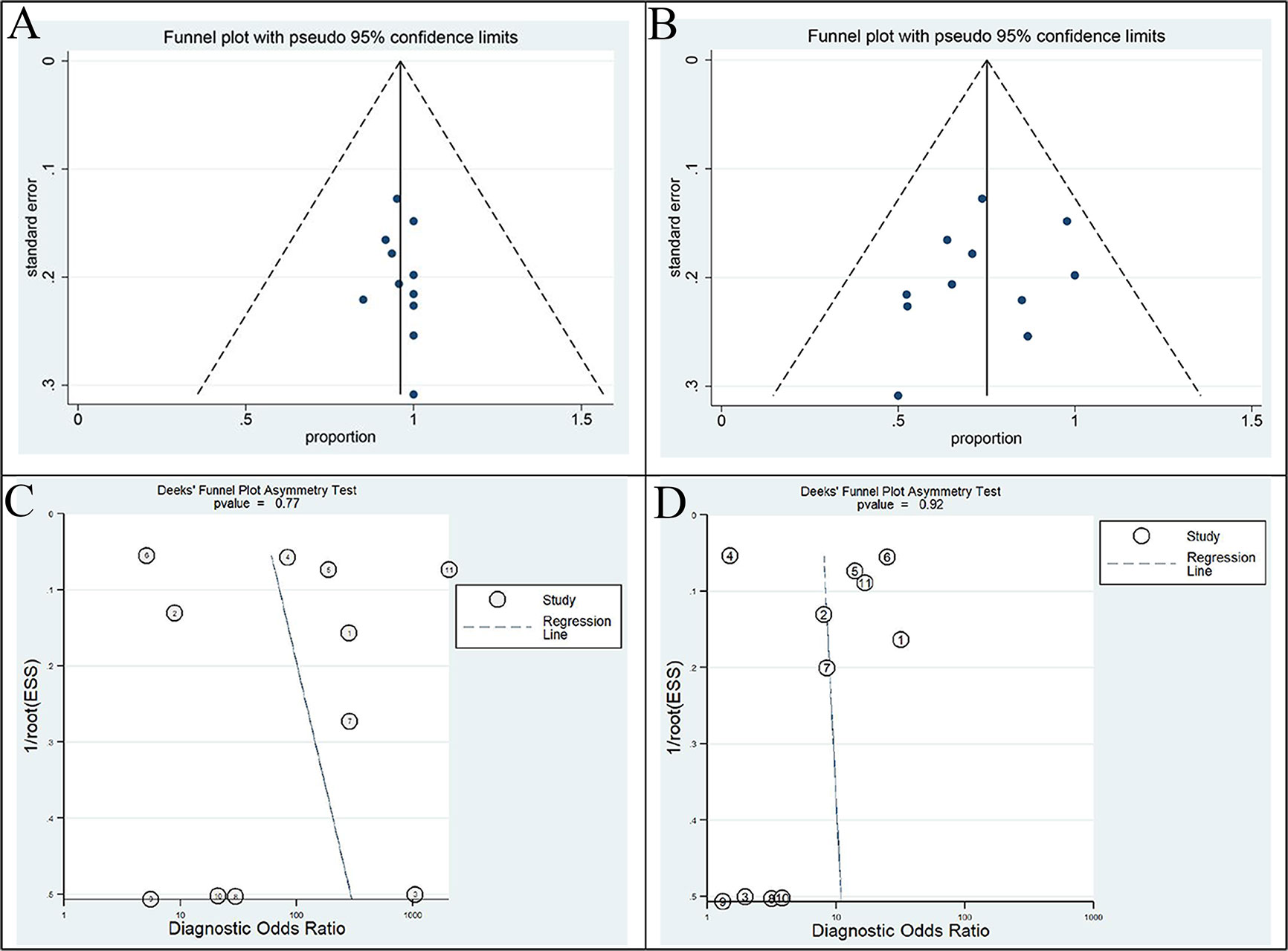
Figure 5 Funnel plot of this meta-analysis. Funnel plots with Egger’s test for 68Ga-FAPI (A) and 18F-FDG PET/CT (B) in primary tumor staging (all p>0.05). Funnel plots with Deek’s test for 68Ga-FAPI (C) and 18F-FDG PET/CT (D) in non-primary tumour (all p>0.05). All of these results indicate the absence of a publication bias.
4 Discussions
This is the first study to conduct a head-to-head comparison of 68Ga-FAPI with 18F-FDG PET/CT in APM using a meta-analysis. Based on our results, 68Ga-FAPl performed better than 18F-FDG in APM primary staging, with DRs of 98% and 70%, respectively. There was no interstudy heterogeneity, indicating that our results were stable and reliable. A previous meta-analysis included the assessment of various cancers, including glioblastoma, head and neck tumors, and nasopharyngeal carcinoma, and the researches believed that the sensitivity of 68Ga-FAPI in identifying primary tumors was 100% (95%CI: 98%-100%) (6). In addition, when they analyzed abdominal tumors as a subgroup, the sensitivity 68Ga-FAPI was 100% for primary tumors and 87% for non-primary tumors, indicating the high diagnostic efficacy of this molecule.
In addition, this meta-analysis also evaluated the application value of 68Ga-FAPI and 18F-FDG in disease recurrence in APM, and the combined DR was 91% and 56%, respectively. In a retrospective study, the authors analyzed 16 patients with recurrent gastrointestinal tumors (28). They found that the positive rates of 68Ga-FAPI-04, 18F-FDG PET/CT and conventional evaluation were 100%, 57.1%, and 33.3%, respectively. Siripongsatian et al. (13) reported that the uptake-positive rates in locally recurring and residual tumor lesions were 46.7% (7/15) on 18F-FDG PET/CT and 100% (15/15) on 68Ga-FAPI PET/CT. Compared with the 18F-FDG-based TNM staging system, the 68Ga-FAPI-based TNM staging system was upgraded in 6 patients (6/23, 26.1%), resulting in management changes in 2 patients (2/23, 8.7%) (12). Their results also indicated that 68Ga-FAPI PET/CT was superior to 18F-FDG in detecting primary and metastatic lesions. Researchers in Thailand (13) and China (28) showed that 68Ga-FAPI PET/CT was more sensitive than 18F-FDG in the identification of liver and gastrointestinal primary tumors (100% vs. 52% and 100% vs. 53%). It seems that all published studies thus far support the evidence that 68Ga-FAPI PET/CT has a higher detection value than 18F-FDG for primary tumors, and our meta-analysis results indicate the same. This finding is mainly attributed to the excellent biodistribution characteristics of FAPIs, which can provide a better TBR and yield detailed anatomical maps (34). In addition to the higher tracer uptake of 68Ga-FAPI, the superior performance of 68Ga-FAPI PET/CT includes its enhanced ability to detect small metastases (diameter<1.0 cm). Tumor lesions >1-2 mm require the formation of a supportive stroma, and since the stroma volume may be larger than the tumor volume, stromal targeted PET imaging may be more sensitive than glycolytic targeted PET imaging in detecting small lesions (2).
All studies compared SUVmax or TBR values in the primary tumor. We found that the vast majority of 68Ga-FAPI values were higher than those of 18F-FDG, and only the Komek et al. (26) study had a lower FAPI-SUVmax than FDG-SUVmax (mean: 11.54 vs. 18.93) but failed to demonstrate a significant difference in terms of TBR. The possible reason is that the researchers evaluated patients with colorectal cancer, and hemorrhoid lesions reaching the anal canal showed a higher 18F-FDG than 68Ga-FAPI uptake. Hemorrhoids may show increased radioactivity concentration on 18F-FDG PET/CT but their SUVmax is lower on 68Ga-FAPI PET/CT than on 18F-FDG PET/CT (26). The cause of abnormal FAPI concentrations in hemorrhoids may be associated with mild fibrous tissue hyperplasia due to inflammation of various veins and the anal canal (36). Whether the high sensitivity and specificity of FAPI for tumor stroma confer any clinical value beyond a numerical advantage in the TBR is still unknown (26).
Our results showed that the DRs of the imaging agent 68Ga-FAPI in GC and LC were 99% and 97%, respectively, which were higher than those of the imaging agent 18F-FDG (82% and 80%). In Israel’s (35) small cohort study, 68Ga-FAPI was superior to 18F-FDG in detecting primary GC, with a DR of 100%, while that of 18F-FDG was only 50%. This shows that the high DR of 68Ga-FAPI is mainly due to the degree GC of differentiation and the known limitations of 18F-FDG in examining several GC subtypes, such as mucinous adenocarcinoma, noninterstitial diffuse carcinoma, and signed-ring cell carcinoma, raising the possibility that 68Ga-FAPI can be used as a radiotracer of choice in the evaluation of GC. In addition, the physiological uptake of 18F-FDG by the gastric wall also further limits the application of 18F-FDG PET/CT in the detection of GC. The results of Pang et al. (28) showed that 68Ga-FAPI PET/CT can be used to analyze different types of GC and thus may play a complementary role in resolving the uncertain results of 18F-FDG PET/CT. Lin et al. (34) also suggested that the lesions of signet-ring cell carcinoma were positive for 68Ga-FAPI and negative for 18F-FDG. Studies have reported a low FDG uptake in signet-ring cell carcinoma and mucinous carcinoma than in conventional adenocarcinoma, which may be due to the low expression of glucose transporter 1 (37–39).
Similarly, 68Ga-FAPI PET/CT is superior to 18F-FDG PET/CT in identifying liver lesions, which may improve the staging and subsequent treatment of LC. Guo et al. (2) suggested that 68Ga-FAPI-04 PET/CT can detect 96% (22/23) of primary liver tumors, with good contrast between the tumor and background, comparable to the DR of contrast-enhanced CT (96%) and liver MRI (100%). In contrast, 18F-FDG detected only 65% (15/23) of primary liver tumors. The study of Siripongsatian et al. (13) reported that 100% (21/21) of intrahepatic tumors were detected by 68Ga-FAPI, whereas only 52% (11/21) were detected by 18F-FDG. These results may be due to the higher uptake of FAPI by primary tumors and the lower hepatic background activity of FAPI compared with 18F-FDG. For hepatocellular carcinoma (HCC) with low expression of glucose transporter-1 and high expression of glucose-6-phosphatase, 40% of such HCC lesions appeared nonavid on FDG PET images (40). The tumor-to-nontumour liver uptake ratio of the well-differentiated HCC was approximately 1.1, indicating that 18F-FDG PET imaging is difficult to distinguish between the uptake of well-differentiated HCC lesions and healthy liver tissues (41). In addition, investigators observed that 68Ga-FAPI-04 uptake was higher in most primary intrahepatic cholangiocarcinoma lesions than in HCC lesions (2). This finding may be attributed to the fact that intrahepatic cholangiocarcinoma is a particular type of fibroproliferative tumor and because the number of CAFs tends to significantly exceed that of actual cholangiocarcinoma cells (42). The severity of the corresponding pathological grade of the primary tumor was positively correlated with the 68Ga-FAPI-04 uptake activity of the lesion (2). Therefore, 68Ga-FAPI can be useful in assessing the extent of disease and differentiating benign from malignant lesions, especially when assessment is difficult with 18F-FDG or conventional imaging. In view of the above discussion and analysis, 68Ga-FAPI seems to be a promising imaging model that may replace 18F-FDG for evaluation of abdominal malignancies.
Although high uptake of 68Ga-FAPI helps to improve lesion identification, it may lead to a higher false-positive rate. Guo et al. (2) reported 3 false-positive cases caused by 68Ga-FAPI, including 1 pulmonary inflammatory granuloma, 1 pulmonary infection, and 1 thyroid adenoma, which also showed high uptake on 18F-FDG. In addition, there were 4 cases of high 68Ga-FAPI uptake due to postoperative infection, which was mistaken as an indication of tumor recurrence. Nonspecific fibrosis induced by inflammation may contribute to the positive uptake of 68Ga-FAPI-04 (28, 43, 44). False-positive uptake of 68Ga-FAPI has been observed in inflammatory diseases (e.g., uteritis and abscesses), granulomatous diseases (e.g., tuberculosis), and other diseases in which the fibrotic response is activated (e.g., myelofibrosis and cirrhosis) (28). Thus, 68Ga-FAPI PET/CT might be problematic when differentiating between residual and/or recurrent disease and postradiation and/or postoperative inflammatory reactions (43).
In addition to comparing 68Ga-FAPI and 18F-FDG in the primary staging and recurrence of APM, this study also evaluated their efficacy in non-primary tumors. In non-primary tumors, 68Ga-FAPI had a higher SEN, SPE, DOR, and AUC than 18F-FDG. However, the various effect indicators showed a high level of heterogeneity (all P <0.05). Consequently, lymph nodes and distant metastases were subgroup analyzed to improve performance and heterogeneity. From our combined results, 68Ga-FAPI outperforms 18F-FDG in all aspects. It is important to note, however, that the pooled SEN of these two types of imaging agents in evaluating lymph nodes is generally unsatisfactory, with effect sizes less than 50%. In the study by Fu et al. (18), the coincidence rates of 68Ga-FAPI and 18F-FDG in lymph node staging were 50% and 45.4%, respectively, compared with pathology. 68Ga-FAPI and 18F-FDG PET/CT had the same low SEN (58.3% vs. 41.7%) and moderate DOR (77.3% vs. 63.6%), although the SPE was high (100% vs. 90%) in their study. In comparison with 18F-FDG, 68Ga-FAPI-04 PET detected more suspicious lymph node lesions but did not improve lymph node staging clinically (18). Gundoğan et al. (27) found that the SEN and SPE of 68Ga-FAPI-04 PET/CT in detecting lymph node metastasis were 100% and 95.2%, respectively, while those of 18F-FDG PET/CT were 71.4% and 93.7%, respectively. In the study of Lin et al. (34), 68Ga-FAPI PET/CT found only 20 true positives in 625 resected lymph nodes, with a calculated SEN of 19.2%. Therefore, FAPI has a strong ability to exclude lymph node metastases but has an unstable and limited ability to detect lymph node metastases. Its expression has been associated with multiple factors, such as local tumor invasion, lymph node metastasis, and poor prognosis, including tumor invasion, metastasis, and angiogenesis (8). Therefore, the high variability of 68Ga-FAPI in lymph node stage assessment (pooled SEN 38.9%-45.3%) may be unexpected, especially given that the lymph nodes usually consist of mesh cell networks of the fiber layer (6). Its relatively low performance in detecting lymph node metastasis may be related to the biological characteristics of the cancer and the degree of lymph node cell enrichment (6). It has been suggested that reflective isotopes used for FAPI labeling may affect the image resolution and thus the detectability of smaller tumor aggregates in lymph nodes, because the 68Ga (3.5 mm) positrons have a larger average range in water than those of 18F (0.6 mm) (45).
Distant metastases of APMs occur in the liver, bone, lung, peritoneum and adrenal gland. The results of this study showed that 68Ga-FAPI was also better than 18F-FDG in the assessment of distant metastasis. Peritoneal metastases are common in APMs and can cause uncontrolled disease and even death (2). The uptake of 68Ga-FAPI-04 by peritoneal metastatic lesions is so avid that FAPI-04 clearly delineates and sensitively detects lesions (18). In a single-center retrospective study (18), the rate of positive detection of peritoneal metastases with 68Ga-FAPI was 93.2%, significantly higher than that with 18F-FDG (53.8%). Their results are similar to ours. In addition, the researchers reported that 68Ga-FAPI-04 PET/CT accurately detected advanced peritoneal lesions with a peritoneal cancer index ≥20 in 12 patients, all of whom were underestimated by 18F-FDG PET/CT (12/26 vs. 0/26, P < 0.001). It has been reported that a peritoneal cancer index score of 20 or more usually indicates a poor prognosis and the need for more aggressive treatment (46). This finding may be attributed to the invasion of peritoneal tissue by the tumor, which triggers a fibrotic response that leads to severe fibrosis (2). Thus, the advantage of FAPI in detecting peritoneal metastases of tumors may have a positive impact on patient management (47) and may be a promising tool for the assessment of peritoneal carcinomas (2, 18). FAPI PET/CT also shows strong potential for detecting liver, bone, and other metastases. The SUVmax and TBR of bone metastases in 68Ga-FAPI were significantly higher than those in 18F-FDG (p<0.001) (26). In the study of Fu et al. (18), 68Ga-FAPI and 18F-FDG showed similar abilities to detect bone metastases (108 vs. 104) and had complementary roles.
Heterogeneity across studies may be a potential source of bias in meta-analyses (48). The diversity of patient characteristics, methodological differences and overall quality of the study may all be sources of heterogeneity (48). Our results showed that 68Ga-FAPI had no heterogeneity in studies assessing primary tumor staging (I2 = 22.58%, p=0.23), but 18F-FDG had heterogeneity (I2 = 82.48%, p=0.00). Therefore, we performed a subgroup analysis of gastric and liver cancers in the primary tumor group and found improved heterogeneity of FAPI, while FDG remained, perhaps because more studies on FDG in tumors were not included. In the evaluation of non-primary tumors, the I2 value of the consistency test for all statistical indicators (SEN, SPE, DOR) was greater than 50%, so random effect models were used to combine effect sizes. Publication bias is a major concern in all meta-analyses, as studies reporting significantly positive results are more likely to be published than studies reporting negative results (49). In our meta-analysis, we used Deek funnel plots and Egger’s test to assess publication bias. Regardless of whether primary tumor staging or non-primary tumor metastases were detected, the funnel plots showed symmetry, indicating that there was no publication bias.
Our meta-analysis is innovative, and it is the first head-to-head comparison of the application of 68Ga-FAPI and 18F-FDG PET/CT to APMs. We evaluated not only the primary staging of 68Ga-FAPI and 18F-FDG PET/CT in the primary tumors but also the application of lymph nodes and distant metastases. We assessed the quality of the included studies using the QUADAS-2 tool; and no study was considered of low quality, and the overall quality of the studies was satisfactory. Certainly, our meta-analysis has some limitations. First, the number of published articles in the field was relatively small, which may be a source of bias. Second, heterogeneity among studies may affect the performance of pooled results. This may be because the subjects included in our study had tumors in different regions of the abdominal and pelvic cavities, with many types of diseases, but this was remedied after subgroup analysis. Third, there were many differences in the sample size and study design of the included studies, which may affect the reliability of the results. The high quality evidence provided by this meta-analysis may pave the way for opening the discussion on a change in the current diagnostic paradigm for solid gastrointestinal tumours. FAPI-imaging may be soon the standard of care in these tumours, given its advantages over FDG-imaging in this setting (50). However, generation of high-quality evidence is still warranted.
5 Conclusions
Our meta-analysis showed that 68Ga-FAPI and 18F-FDG PET/CT had a high overall diagnostic performance in detecting the primary staging and non-primary tumor metastasis of APMs, but the detection ability of 68Ga-FAPI was significantly higher than that of 18F-FDG. However, the ability of 68Ga-FAPI to diagnose lymph node metastasis is not very satisfactory, and is significantly lower than that of distant metastasis. In the future, 68Ga-FAPI will be a promising imaging model that may replace 18F-FDG for APM, but this still needs to be further confirmed by multicenter, large-sample, and prospective studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
XL, HL and CG contributed to conception and design of the study. XL and HL organized the database. CG performed the statistical analysis. XL wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was supported by Chongqing Regional Key Disciplines (Medical Imaging) (zdxk202116) and 2022 Hospital-level Scientific Research Project of Chongqing University Three Gorges Hospital (2022YJKYXM-021).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1093861/full#supplementary-material
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72:7–33. doi: 10.3322/caac.21708
2. Guo W, Pang Y, Yao L, Zhao L, Fan C, Ke J, et al. Imaging fibroblast activation protein in liver cancer: A single-center post hoc retrospective analysis to compare [(68)Ga]Ga-FAPI-04 PET/CT versus MRI and [(18)F]-FDG PET/CT. Eur J Nucl Med Mol Imaging (2021) 48:1604–17. doi: 10.1007/s00259-020-05095-0
3. Roustaei H, Kiamanesh Z, Askari E, Sadeghi R, Aryana K, Treglia G. Could fibroblast activation protein (FAP)-specific radioligands be considered as pan-tumor agents. Contrast Media Mol Imaging. (2022) 2022:3948873. doi: 10.1155/2022/3948873
4. Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci (2012) 125:5591–6. doi: 10.1242/jcs.116392
5. Du B, Shim JS. Targeting epithelial-mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules (2016) 21:965. doi: 10.3390/molecules21070965
6. Sollini M, Kirienko M, Gelardi F, Fiz F, Gozzi N, Chiti A. State-of-the-art of FAPI-PET imaging: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. (2021) 48:4396–414. doi: 10.1007/s00259-021-05475-0
7. Busek P, Mateu R, Zubal M, Kotackova L, Sedo A. Targeting fibroblast activation protein in cancer - prospects and caveats. Front Biosci (Landmark Ed). (2018) 23:1933–68. doi: 10.2741/4682
8. Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, et al. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal (2020) 18:59. doi: 10.1186/s12964-020-0530-4
9. Nurmik M, Ullmann P, Rodriguez F, Haan S, Letellier E. In search of definitions: Cancer-associated fibroblasts and their markers. Int J Cancer. (2020) 146:895–905. doi: 10.1002/ijc.32193
10. Calais J. FAP: The next billion dollar nuclear theranostics target. J Nucl Med (2020) 61:163–5. doi: 10.2967/jnumed.119.241232
11. Zhou X, Wang S, Xu X, Meng X, Zhang H, Zhang A, et al. Higher accuracy of [(68) Ga]Ga-DOTA-FAPI-04 PET/CT comparing with 2-[(18)F]FDG PET/CT in clinical staging of NSCLC. Eur J Nucl Med Mol Imaging. (2022) 49:2983–93. doi: 10.1007/s00259-022-05818-5
12. Pang Y, Zhao L, Shang Q, Meng T, Zhao L, Feng L, et al. Positron emission tomography and computed tomography with [(68)Ga]Ga-fibroblast activation protein inhibitors improves tumor detection and staging in patients with pancreatic cancer. Eur J Nucl Med Mol Imaging. (2022) 49:1322–37. doi: 10.1007/s00259-021-05576-w
13. Siripongsatian D, Promteangtrong C, Kunawudhi A, Kiatkittikul P, Boonkawin N, Chinnanthachai C, et al. Comparisons of quantitative parameters of Ga-68-Labelled fibroblast activating protein inhibitor (FAPI) PET/CT and [(18)F]F-FDG PET/CT in patients with liver malignancies. Mol Imaging Biol (2022) 24:818–29. doi: 10.1007/s11307-022-01732-2
14. Koczorowska MM, Tholen S, Bucher F, Lutz L, Kizhakkedathu JN, De Wever O, et al. Fibroblast activation protein-α, a stromal cell surface protease, shapes key features of cancer associated fibroblasts through proteome and degradome alterations. Mol Oncol (2016) 10:40–58. doi: 10.1016/j.molonc.2015.08.001
15. Glatting FM, Hoppner J, Liew DP, van Genabith A, Spektor AM, Steinbach L, et al. Repetitive early FAPI-PET acquisition comparing FAPI-02, FAPI-46 and FAPI-74: methodological and diagnostic implications for malignant, inflammatory and degenerative lesions. J Nucl Med (2022) 63:1844–51. doi: 10.2967/jnumed.122.264069
16. Imlimthan S, Moon ES, Rathke H, Afshar-Oromieh A, Rösch F, Rominger A, et al. New frontiers in cancer imaging and therapy based on radiolabeled fibroblast activation protein inhibitors: A rational review and current progress. Pharm (Basel). (2021) 14:1023. doi: 10.3390/ph14101023
17. Chen H, Pang Y, Wu J, Zhao L, Hao B, Wu J, et al. Comparison of [(68)Ga]Ga-DOTA-FAPI-04 and [(18)F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging (2020) 47:1820–32. doi: 10.1007/s00259-020-04769-z
18. Fu L, Huang S, Wu H, Dong Y, Xie F, Wu R, et al. Superiority of [(68)Ga]Ga-FAPI-04/[(18)F]FAPI-42 PET/CT to [(18)F]FDG PET/CT in delineating the primary tumor and peritoneal metastasis in initial gastric cancer. Eur Radiol (2022) 32:6281–90. doi: 10.1007/s00330-022-08743-1
19. Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. (68)Ga-FAPI PET/CT: Tracer uptake in 28 different kinds of cancer. J Nucl Med (2019) 60:801–5. doi: 10.2967/jnumed.119.227967
20. Çermik TF, Ergül N, Yılmaz B, Mercanoğlu G. Tumor imaging with 68Ga-DOTA-FAPI-04 PET/CT: Comparison with 18F-FDG PET/CT in 22 different cancer types. Clin Nucl Med (2022) 47:e333–39. doi: 10.1097/RLU.0000000000004073
21. Dendl K, Koerber SA, Finck R, Mokoala K, Staudinger F, Schillings L, et al. (68)Ga-FAPI-PET/CT in patients with various gynecological malignancies. Eur J Nucl Med Mol Imaging. (2021) 48:4089–100. doi: 10.1007/s00259-021-05378-0
22. Elboga U, Sahin E, Kus T, Cayirli YB, Aktas G, Okuyan M, et al. Comparison of (68)Ga-FAPI PET/CT and (18)FDG PET/CT modalities in gastrointestinal system malignancies with peritoneal involvement. Mol Imaging Biol (2022) 24:789–97. doi: 10.1007/s11307-022-01729-x
23. Novruzov E, Dendl K, Ndlovu H, Choyke PL, Dabir M, Beu M, et al. Head-to-head intra-individual comparison of [(68)Ga]-FAPI and [(18)F]-FDG PET/CT in patients with bladder cancer. Mol Imaging Biol (2022) 48:4377–4385. doi: 10.1007/s11307-022-01715-3
24. Shi X, Xing H, Yang X, Li F, Yao S, Congwei J, et al. Comparison of PET imaging of activated fibroblasts and (18)F-FDG for diagnosis of primary hepatic tumours: a prospective pilot study. Eur J Nucl Med Mol Imaging (2021) 48:1593–603. doi: 10.1007/s00259-020-05070-9
25. Yang T, Ma L, Hou H, Gao F, Tao W. FAPI PET/CT in the diagnosis of abdominal and pelvic tumors. Front Oncol (2021) 11:797960. doi: 10.3389/fonc.2021.797960
26. Kömek H, Can C, Kaplan İ, Gündoğan C, Kepenek F, Karaoglan H, et al. Comparison of [(68) Ga]Ga-DOTA-FAPI-04 PET/CT and [(18)F]FDG PET/CT in colorectal cancer. Eur J Nucl Med Mol Imaging (2022) 49:3898–909. doi: 10.1007/s00259-022-05839-0
27. Gündoğan C, Kömek H, Can C, Yildirim ÖA, Kaplan İ, Erdur E, et al. Comparison of 18F-FDG PET/CT and 68Ga-FAPI-04 PET/CT in the staging and restaging of gastric adenocarcinoma. Nucl Med Commun (2022) 43:64–72. doi: 10.1097/MNM.0000000000001489
28. Pang Y, Zhao L, Luo Z, Hao B, Wu H, Lin Q, et al. Comparison of (68)Ga-FAPI and (18)F-FDG uptake in gastric, duodenal, and colorectal cancers. Radiology (2021) 298:393–402. doi: 10.1148/radiol.2020203275
29. Şahin E, Elboğa U, Çelen YZ, Sever ÖN, Çayırlı YB, Çimen U. Comparison of (68)Ga-DOTA-FAPI and (18)FDG PET/CT imaging modalities in the detection of liver metastases in patients with gastrointestinal system cancer. Eur J Radiol (2021) 142:109867. doi: 10.1016/j.ejrad.2021.109867
30. Wang H, Zhu W, Ren S, Kong Y, Huang Q, Zhao J, et al. (68)Ga-FAPI-04 versus (18)F-FDG PET/CT in the detection of hepatocellular carcinoma. Front Oncol (2021) 11:693640. doi: 10.3389/fonc.2021.693640
31. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
32. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
33. Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. (2005) 58:882–93. doi: 10.1016/j.jclinepi.2005.01.016
34. Lin R, Lin Z, Chen Z, Zheng S, Zhang J, Zang J, et al. [(68)Ga]Ga-DOTA-FAPI-04 PET/CT in the evaluation of gastric cancer: Comparison with [(18)F]FDG PET/CT. Eur J Nucl Med Mol Imaging. (2022) 49:2960–71. doi: 10.1007/s00259-022-05799-5
35. Kuten J, Levine C, Shamni O, Pelles S, Wolf I, Lahat G, et al. Head-to-head comparison of [(68)Ga]Ga-FAPI-04 and [(18)F]-FDG PET/CT in evaluating the extent of disease in gastric adenocarcinoma. Eur J Nucl Med Mol Imaging. (2022) 49:743–50. doi: 10.1007/s00259-021-05494-x
36. Caparelli ML, Batey JC, Tailor A, Braverman T, Barrat C. Internal hemorrhoid harboring adenocarcinoma: A case report. World J Gastrointest Oncol (2021) 13:87–91. doi: 10.4251/wjgo.v13.i1.87
37. Mochiki E, Kuwano H, Katoh H, Asao T, Oriuchi N, Endo K. Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg (2004) 28:247–53. doi: 10.1007/s00268-003-7191-5
38. De Potter T, Flamen P, Van Cutsem E, Penninckx F, Filez L, Bormans G, et al. Whole-body PET with FDG for the diagnosis of recurrent gastric cancer. Eur J Nucl Med Mol Imaging. (2002) 29:525–9. doi: 10.1007/s00259-001-0743-8
39. Kawamura T, Kusakabe T, Sugino T, Watanabe K, Fukuda T, Nashimoto A, et al. Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival. Cancer (2001) 92:634–41. doi: 10.1002/1097-0142(20010801)92:3<634::aid-cncr1364<3.0.co;2-x
40. Torizuka T, Tamaki N, Inokuma T, Magata Y, Sasayama S, Yonekura Y, et al. In vivo assessment of glucose metabolism in hepatocellular carcinoma with FDG-PET. J Nucl Med (1995) 36:1811–7.
41. Ho CL, Yu SC, Yeung DW. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses. J Nucl Med (2003) 44:213–21.
42. Mertens JC, Rizvi S, Gores GJ. Targeting cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis (2018) 1864:1454–60. doi: 10.1016/j.bbadis.2017.08.027
43. Chen H, Zhao L, Ruan D, Pang Y, Hao B, Dai Y, et al. Usefulness of [(68)Ga]Ga-DOTA-FAPI-04 PET/CT in patients presenting with inconclusive [(18)F]FDG PET/CT findings. Eur J Nucl Med Mol Imaging. (2021) 48:73–86. doi: 10.1007/s00259-020-04940-6
44. Luo Y, Pan Q, Zhang W, Li F. Intense FAPI uptake in inflammation may mask the tumor activity of pancreatic cancer in 68Ga-FAPI PET/CT. Clin Nucl Med (2020) 45:310–1. doi: 10.1097/RLU.0000000000002914
45. Conti M, Eriksson L. Physics of pure and non-pure positron emitters for PET: A review and a discussion. EJNMMI Phys (2016) 3:8. doi: 10.1186/s40658-016-0144-5
46. Mo S, Cai G. Multidisciplinary treatment for colorectal peritoneal metastases: Review of the literature. Gastroenterol Res Pract (2016) 2016:1516259. doi: 10.1155/2016/1516259
47. Zhao L, Pang Y, Luo Z, Fu K, Yang T, Zhao L, et al. Role of [(68)Ga]Ga-DOTA-FAPI-04 PET/CT in the evaluation of peritoneal carcinomatosis and comparison with [(18)F]-FDG PET/CT. Eur J Nucl Med Mol Imaging. (2021) 48:1944–55. doi: 10.1007/s00259-020-05146-6
48. Liu X, Jiang T, Gao C, Liu H, Sun Y, Zou Q, et al. Detection rate of fluorine-18 prostate-specific membrane antigen-1007 PET/CT for prostate cancer in primary staging and biochemical recurrence with different serum PSA levels: A systematic review and meta-analysis. Front Oncol (2022) 12:911146. doi: 10.3389/fonc.2022.911146
49. Mlinarić A, Horvat M, Šupak Smolčić V. Dealing with the positive publication bias: Why you should really publish your negative results. Biochem Med (Zagreb). (2017) 27:30201. doi: 10.11613/BM.2017.030201
50. Delgado Bolton RC, Calapaquí Terán AK, Herrmann K, Fanti S, Giammarile F. Are we approaching a change in paradigm in PET/CT imaging of solid gastrointestinal (or digestive) tract tumours with the clinical application of FAPI-imaging? Clin Nucl Med (2023) 12:854658. doi: 10.1097/RLU.0000000000004602
Keywords: 68Ga-FAPI, 18F-FDG, fibroblast activating protein, abdominal and pelvic malignancy, meta-analysis, PET/CT
Citation: Liu X, Liu H, Gao C and Zeng W (2023) Comparison of 68Ga-FAPI and 18F-FDG PET/CT for the diagnosis of primary and metastatic lesions in abdominal and pelvic malignancies: A systematic review and meta-analysis. Front. Oncol. 13:1093861. doi: 10.3389/fonc.2023.1093861
Received: 09 November 2022; Accepted: 09 January 2023;
Published: 17 February 2023.
Edited by:
Abhishek Mahajan, The Clatterbridge Cancer Centre, United KingdomReviewed by:
Roberto C. Delgado Bolton, Hospital San Pedro, SpainHua Zhu, Beijing Cancer Hospital, China
Copyright © 2023 Liu, Liu, Gao and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cailiang Gao, MTgzMTUwMjUzODZAMTYzLmNvbQ==; Wenbing Zeng, NDIyODE3NTkzQHFxLmNvbQ==
 Xue Liu
Xue Liu Huiting Liu1
Huiting Liu1