
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 16 February 2023
Sec. Genitourinary Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1092734
Background: The effect of perioperative blood transfusion (PBT) on postoperative survival in RCC patients who underwent partial nephrectomy (PN) or radical nephrectomy (RN) remains controversial. Two meta-analyses in 2018 and 2019 reported the postoperative mortality of PBT patients with RCC, but they did not investigate the effect on the survival of patients. We performed a systematic review and meta-analysis of relevant literature to demonstrate whether PBT affected postoperative survival in RCC patients who received nephrectomy.
Methods: Pubmed, Web of Science, Cochrane, and Embase databases were searched. Studies comparing RCC patients with or without PBT following either RN or PN were included in this analysis. Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of the included literature, and hazard ratios (HRs) of overall survival (OS), recurrence-free survival (RFS), and cancer-specific survival (CSS), as well as 95% confidence intervals, were considered as effect sizes. All data were processed using Stata 15.1.
Results: Ten retrospective studies involving 19,240 patients were included in this analysis, with the publication dates ranging from 2014 to 2022. Evidence revealed that PBT was significantly associated with the decline of OS (HR, 2.62; 95%CI: 1,98-3.46), RFS (HR, 2.55; 95%CI: 1.74-3.75), and CSS (HR, 3.15; 95%CI: 2.3-4.31) values. There was high heterogeneity among the study results due to the retrospective nature and the low quality of the included studies. Subgroup analysis findings suggested that the heterogeneity of this study might be caused by different tumor stages in the included articles. Evidence implied that PBT had no significant influence on RFS and CSS with or without robotic assistance, but it was still linked to worse OS (combined HR; 2.54 95% CI: 1.18, 5.47). Furthermore, the subgroup analysis with intraoperative blood loss lower than 800 ML revealed that PBT had no substantial impact on OS and CSS of postoperative RCC patients, whereas it was correlated with poor RFS (1.42, 95% CI: 1.02-1.97).
Conclusions: RCC patients undergoing PBT after nephrectomy had poorer survival.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022363106.
Renal cell carcinoma (RCC) accounts for 2%-3% of adults with malignant tumors, second only to prostate cancer and bladder cancer while this disease has the highest mortality in the urinary system tumors (1). The incidence of RCC is surprisingly higher in developed countries, at approximately 3.8% in adults (2). A typical treatment strategy for RCC is the surgical resection of the primary tumor (3). Since RCC is characterized by significant angiogenic activity compared to other diseases, this procedure may result in a large volume of blood loss (4). Under the circumstances, renal cancer patients undergoing nephrectomy are prone to require allogeneic perioperative blood transfusion (PBT). The association has been reported between PBT and tumor recurrence, including colorectal, gastric, pancreatic, liver, prostate, and bladder cancers (5–13). Despite the fact that the underlying mechanism of this association remains unclear, researchers have hypothesized that blood transfusion may be responsible for the immunomodulatory effects and inflammatory responses (14). It is still controversial whether PBT results in poorer survival rates for RCC patients after nephrectomy (15–21). This controversy is probably due to research limitations such as a relatively small sample size, a short period of follow-up (20), insufficient data on potential confounders, the inclusion of heterogeneous RCC histological subtypes, and a lack of information on the timing or volume of blood transfusions (15, 21). Based on the literature published as of October 2022, two NRCT studies have evaluated the prognostic role of PBT in patients undergoing radical nephrectomy (RN) through systematic review and meta-analysis. Both have reported increased mortality in patients receiving PBT. However, such systematic reviews focused only on mortality as an outcome indicator instead of the survival rates of the patients.
The present work is the first systematic review and meta-analysis to comprehensively assess the effect of PBT on postoperative survival in RCC cases. It aims to assess clinicians’ decisions regarding the PBT management of RCC patients by evaluating relevant literature on this research topic, hoping to make some improvements in certain research directions.
We conducted a systematic review and meta-analysis following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). A relevant protocol has been registered on the Prospero website (https://www.crd.york.ac.uk/PROSPERO/) with the registration number: CRD42022363106.
Pubmed, Cochrane Library, Web of Science, and Embase databases were searched as of October 10, 2022. The literature search adopted subject headings combined with free words. The subject terms included renal cancer, renal cell carcinoma, perioperative blood transfusion, radical nephrectomy, and partial nephrectomy. The literature to be searched was limited to clinical trials and those written in the English language. Meanwhile, meta-analyses, conference abstracts, animal experiments, publications written in other languages other than English, and pathological reports were excluded. The participants were diagnosed with RCC and underwent nephrectomy, and the intervention was allogeneic PBT. Patients who received autologous blood or no transfusion and those who developed or had a history of metastatic disease or tumors other than kidney cancer were ineligible. References of the included studies and previous meta-analyses were also reviewed to identify possible eligible studies. Literature screening and retrieval were performed independently by two researchers, and dissents were resolved through discussion.
The primary outcome was overall survival (OS), and the secondary outcomes included RFS and CSS. OS was defined as the duration of time from surgery to death due to any causes. RFS was defined as the duration of time from surgery to the recurrence of cancer. CSS was defined as the duration of time from surgery to death due to cancer recurrence or metastasis. Data were extracted and collected independently by two authors, and disagreements were resolved through discussion to reach a consensus.
The quality of the included studies was assessed using the Newcastle-Ottawa Scale (NOS) (https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp). The evaluation details are presented in the Supplementary Material; and the quality of the data extracted from each study was assessed based on case selection, comparability, and outcome reporting. Quality assessment was performed independently by two researchers, and disagreements were resolved through discussion.
Meta-analysis was carried out using the Stata 15.1 software (StataSE, USA). The hazard ratios (HRs) with 95% confidence intervals (CIs) for OS, RFS, and CSS were calculated. When both univariate and multivariate analyses were available, the HRs were extracted from multivariate analyses. Engauge Digitizer 4.1 and Adobe Photoshop software were applied for the HR extraction (22). Statistical heterogeneity was evaluated using I2 statistics; the value of I2 ≥ 50% (P ≤ 0.1) indicated a high level of heterogeneity, and a random effects model was employed; when the value of I2 < 50%, a fixed effects model was used (P > 0.1). A P < 0.05 was considered statistically significant (23). Subgroup and sensitivity analyses were performed to explore the source and degree of heterogeneity among studies, if necessary. Egger’s test and funnel plots were adopted to determine publication bias, and a P ≥ 0.05 indicated that publication bias had no statistical significance.
We initially screened a total of 1,295 potential articles, of which 565 were from PubMed, 37 from Cochrane Library, 25 from Embase, 668 from Web of Science, and 3 from other sources.
A total of 28 relevant articles were obtained after the initial screening. Based on further screening, ten clinical observational studies involving 19,240 patients were ultimately included. The specific literature screening process is shown in Figure 1. The ten included studies were conducted in different countries and published between 2014 and 2022. Two were from the United States, four were from South Korea, two were from Israel, one was from Austria, and the last was from France. The characteristics of the included studies are shown in Table 1. The median NOS for reporting OS was 7 in the studies, that of reporting RFS was 6, and that of CSS was 7.
Eight studies reported OS. Data on the occurrence time of events in all eight studies were available. Seven studies were adjusted for multivariate analysis. The adjusted data from three studies were directly extracted from the texts. However, the missing HR data of the remaining five studies were extracted using Engauge Digitizer 4.1 and Adobe Photoshop. One (20) study was excluded from multivariate analysis, and OS data were extracted only from univariate analysis.
A random-effects model was used for the meta-analysis of the eight articles (15, 17–20, 24–26), and the results implied that blood transfusion was significantly associated with decreased OS (HR, 2.62; 95% CI: 1,98-3.46; I2 = 90.3%, P < 0.0001; Figure 2A).
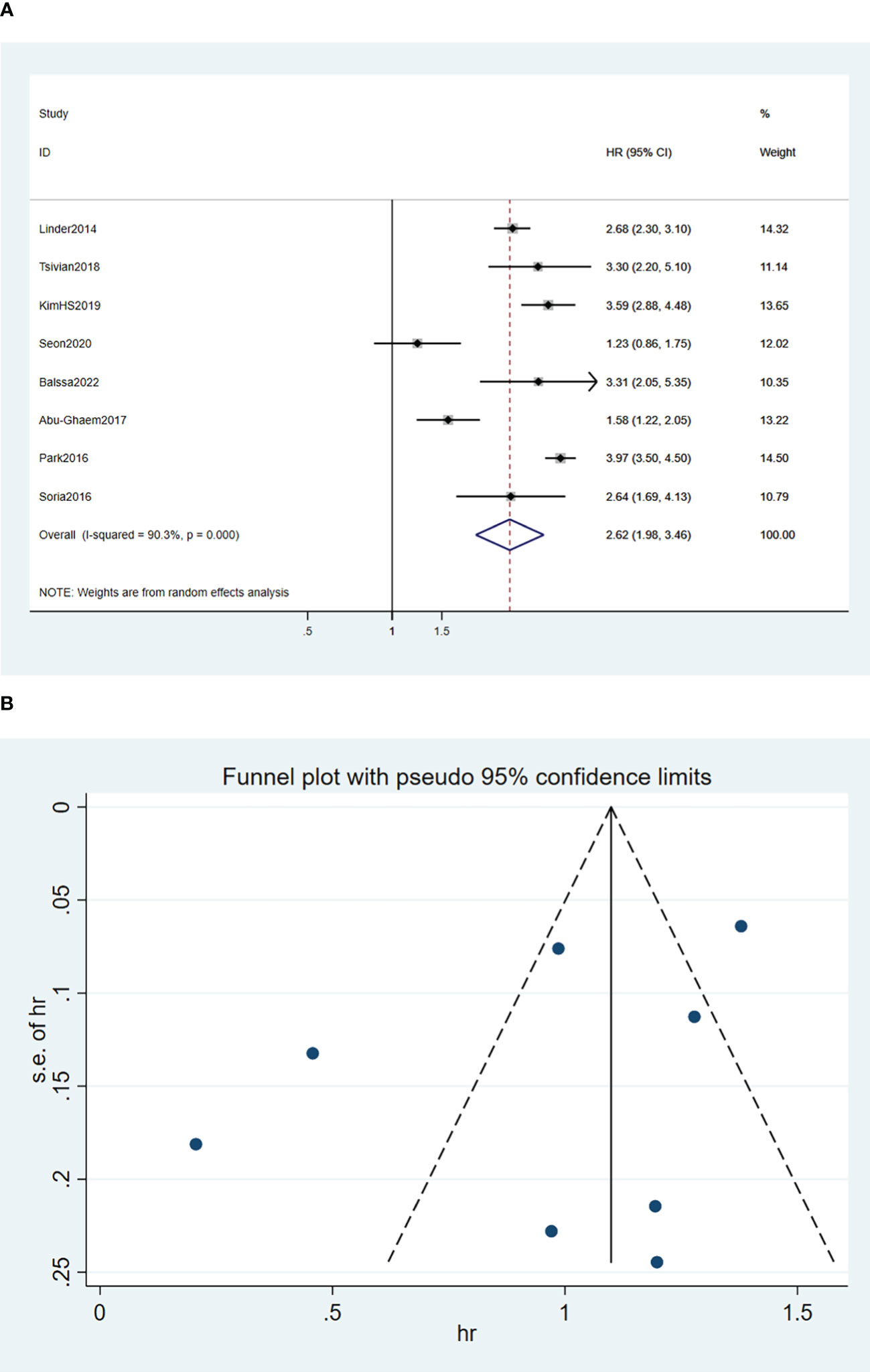
Figure 2 Forest plot (A) and funnel plot (B) depicting the association between PBT and overall survival. HR values greater than 1 indicate that the intervention is detrimental to survival, implying that PBT was associated with poorer OS.
Seven studies reported RFS. The data on the occurrence time of events were available in all seven studies, six of which were adjusted for multivariate analysis. The adjusted data of four studies were directly extracted from the texts, and the missing HR data of the rest three studies were extracted using Engauge Digitizer 4.1 and Adobe Photoshop. One study (20) was excluded from multivariate analysis and a single variable was adopted to analyze the data.
A random-effects model was used for the meta-analysis of the seven articles (18, 20, 24–28), and the results indicated that blood transfusion was significantly associated with reduced RFS (HR, 2.55; 95%CI: 1.74-3.75; I2 = 87.8%, P < 0.001; Figure 3A).
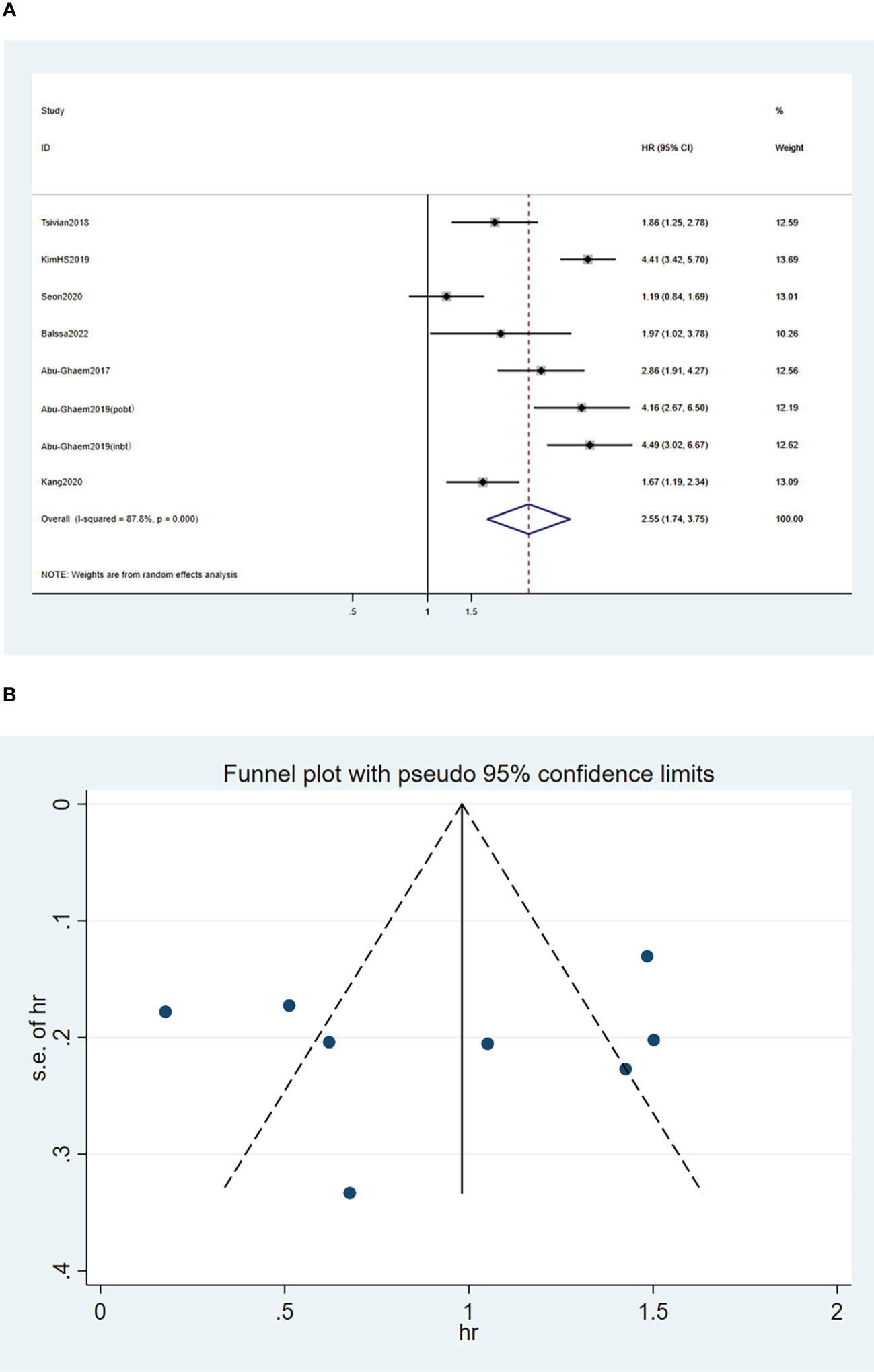
Figure 3 Forest plot (A) and funnel plot (B) depicting the association between PBT and recurrence-free survival. HR values greater than 1 indicate that the intervention is detrimental to survival, implying that PBT was associated with poorer RFS.
Nine studies reported CSS. The occurrence time of events was available in the nine studies, and the studies were adjusted for multivariate analysis. The adjusted data of three studies were directly extracted from the texts while the missing HR data of the rest six studies were extracted using Engauge Digitizer 4.1 and Adobe Photoshop software.
A random effects model was used for the meta-analysis of the nine articles (17–20, 24–28), and the results indicated that blood transfusion was positively associated with decreased CSS (HR, 3.15; 95%CI: 2.3-4.31; I2 = 81.5%; P < 0.001; Figure 4A).
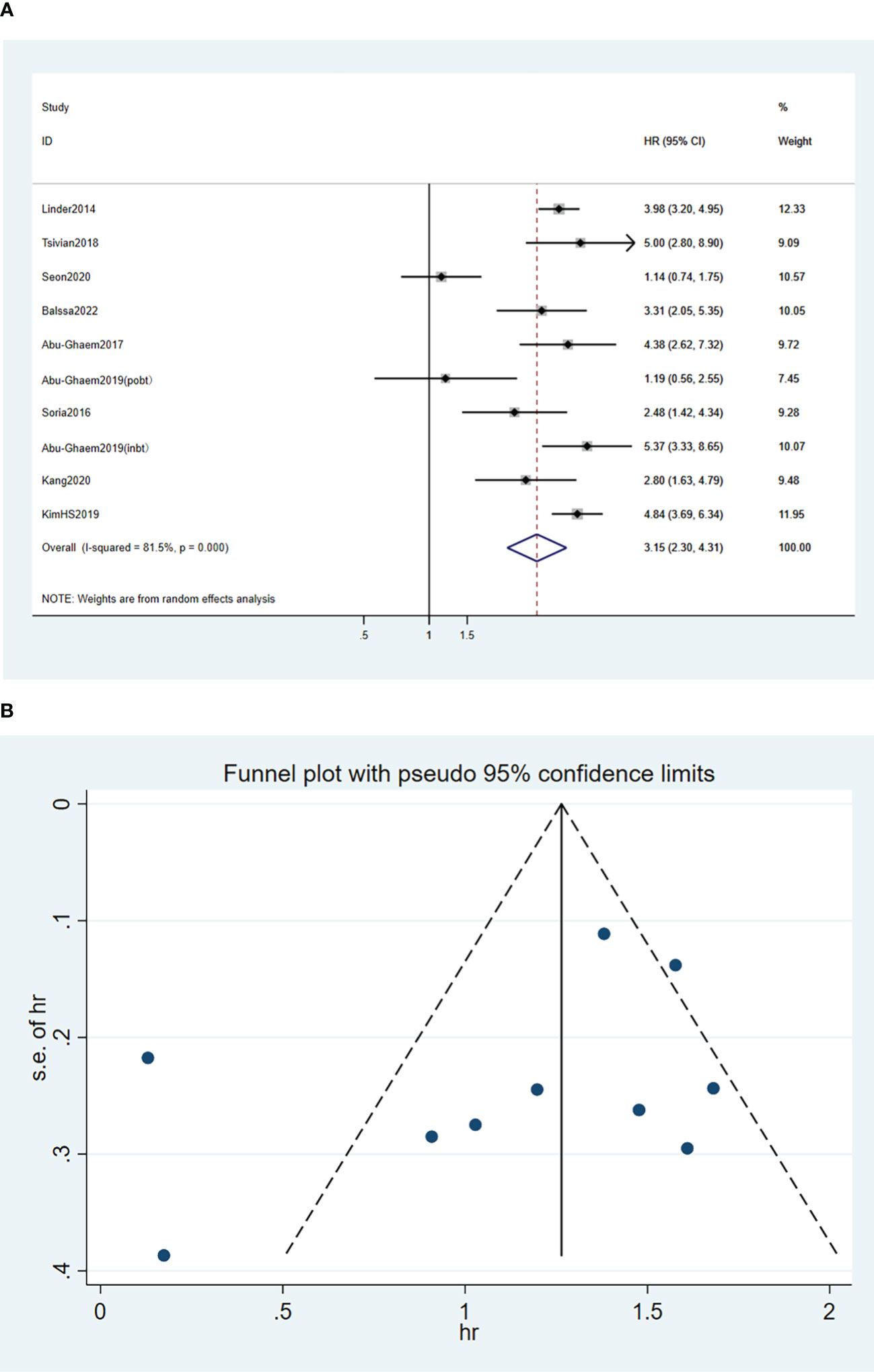
Figure 4 Forest plot (A) and funnel plot (B) depicting the association between PBT and cancer-specific survival. HR values greater than 1 indicate that the intervention is detrimental to survival, implying that PBT was associated with poorer CSS.
To determine the heterogeneity of this meta-analysis, subgroup analyses were performed according to the characteristics among the included studies (e. g. early T1-T2 tumor sample proportion, surgical pathway, blood transfusion volume, year of publication, blood loss volume, definitions of perioperative period, and the occurrence of distant metastasis of the enrolled patients). Tables 2–4 show the subgroup analyses of CSS, OS, and RFS, respectively. The subgroup analysis of RFS was conducted according to whether the patients at tumor stages 1-2 exceeded 80% of the total sample size. The results indicated that no RFS (HR, 1.53 95%CI: 1.18-1.99; I2 = 36%) was reported in the over 80% group, which was better than the below 80% group (HR, 3.37 95%CI: 2.43-4.67), I2 = 50.4%), and the heterogeneity of results was greatly reduced (Figure 5B). In contrast, the heterogeneity of the results of OS and CSS did not reduce (Figures 5A, C). A robot-assisted surgical approach was reported in only three studies. Subgroup analysis revealed that the application of robot-assisted surgery was not a source of heterogeneity in the outcomes of the studies. Additionally, two studies reported intraoperative blood loss lower than 800 ML. Subgroup analysis of blood loss volume showed that the differences in blood loss volume between studies were also not the source of heterogeneity. One study reported RFS at different tumor stages (24), and only one study presented the postoperative survival of patients who underwent different surgical options (partial vs. total nephrectomy) (27). It is therefore not sufficient to perform a subgroup analysis of tumor stage and surgery. The remaining subgroup analyses also did not reduce the outcome heterogeneity. These subgroup analyses revealed an association between PBT and poor OS, RFS, and OS, implying that PBT was correlated with poor survival outcomes in RCC patients with the described characteristics.

Figure 5 Forest plot depicting the association between (A) tumor staging and OS; (B) tumor staging and RFS; (C) tumor staging and CSS. An HR value greater than 1 is detrimental to survival, implying that tumor staging did not affect PBT and poorer postoperative survival.
Funnel plots of the three outcome-Funnel plots of the three outcome (Figures 2B, 3B, 4B) indicators were asymmetry to the naked eye. Egger’s tests indicated no significant publication bias in OS (P = 0.248), RFS (P = 0.569), and CSS (P = 0.239). A P > 0.05 indicated that the publication bias was not significant. Sensitivity analyses revealed that the removal of any one article produced no significant influence on the results.
To our knowledge, the current study is the first meta-analysis to systematically evaluate the influence of PBT on survival outcomes in patients undergoing RCC surgery. Ten eligible studies were identified, involving more than 19,000 patients. The meta-analysis revealed that PBT was correlated with decreased OS (HR, 2.62; P < 0.01), RFS (HR, 2.55; P < 0.001), and CSS (HR, 3.15; P < 0.001) in RCC patients undergoing nephrectomy (i.e., non-metastatic and metastatic RCC).
Tumor recurrence and metastasis are associated with inflammation and immune regulation (29, 30). The allogeneic PBT may accelerate tumor progression via immune responses, the reaction of cytokine degradation of lipid membrane release, and other non-immunogenic mechanisms (31). Keown et al. have called the immune regulation mechanism caused by blood transfusion as transfusion-related immune regulation (TRIM). These immune regulation mechanisms include immunosuppression and proinflammatory effects mediated by residual leukocytes, apoptotic cells, and modified biological responses (e.g. cytokines, soluble mediators, and soluble HLA peptides) (31). It may produce adverse consequences such as CMV or HIV reactivation, red blood cell alloimmunization, and increased cancer recurrence (21, 32). This suggested that blood transfusion was independently associated with an increased risk of disease recurrence and compromised postoperative survival.
PBT has been reported to be associated with poor postoperative survival outcomes in some cancers (8, 33–36). It also produces a significant negative impact on long-term prognosis and increased short-term complications after colorectal cancer surgery (35). Annamaria Agnes et al. have uncovered a negative association between PBT and OS, DFS, and DSS in gastric cancer, and Moschini et al. have found that PBT was significantly correlated with adverse postoperative survival in urinary tumor patients undergoing radical bladder cancer surgery, and so does radical prostatectomy according to Kim et al. (8, 33, 34). Besides, ABU et al. have also revealed adverse effects of PBT on survival after nephrectomy (18). The results of this meta-analysis are consistent with the findings of previous literature, indicating the presence of an association between PBT and OS, RFS, and CSS. The final meta-analysis demonstrated a negative correlation between PBT and the survival indicators in RCC patients following nephrectomy.
Two previous meta-analyses (37, 38) have focused only on mortality as the outcome indicator, but our research is the first meta-analysis on survival, which fills the gap in this field and provides some references for clinical decision-making. However, there are still some limitations in the present research. First, despite a comprehensive search, the number of the included studies and samples was rather limited. Some of the included studies were from the same country and even the same unit, which might cause an overestimation of the effect of blood transfusion on survival rates. Meanwhile, all the included studies were designed retrospectively, and there were biases in reporting, selection, confirmation, and measurement. All this might cause high heterogeneity in our results. Being aware of the heterogeneity of the included literature, we conducted subgroup analyses to identify the source of heterogeneity among studies according to the proportion of early T1-T2 tumors, surgical pathway, blood loss volume, publication year, blood transfusion volume, definitions of perioperative periods, and the occurrence of distant metastasis in the included samples. However, due to limited data, subgroup analyses on tumor staging and surgical pathways could not be carried out. It is of great clinical significance, and thus more attention shall be paid to these data updated in the future. According to the subgroup analysis of the RFS, the proportion of early tumors might be the source of heterogeneity in the included studies.
Due to the heterogeneity caused by statistical methods among studies, confounding factors could not be excluded. Furthermore, various surgical techniques and patient case combinations might cause additional biases. Experiences of surgeons might be a confounding factor, and PBT decisions in the included literature were mostly made as per the experience of clinicians (surgeons and/or anesthesiologists), without a recognized transfusion criterion. Finally, the included articles had both single-center and multi-center data, whereas not all reported survival indicators. Engauge Digitizer 4.1 and Adobe Photoshop software were applied for HR extraction, which may have potential errors. Although the random-effects model took into account heterogeneity among studies, the conclusions should be interpreted with caution. In the current meta-analysis, funnel plots revealed the presence of reporting bias or an overestimation of the effect of PBT on survival outcomes.
In summary, this study confirmed that PBT had a significant negative correlation with survival rates in patients undergoing RCC nephrectomy, and further research is required to support and verify the findings.
PBT might be associated with reduced survival rates in RCC patients undergoing nephrectomy. Given the low quality of the extracted data and a high level of heterogeneity among the included studies, more prospective high-quality studies are needed to provide specific guidelines on PBT.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
YL, XD, and XY contributed to conception and design of the study. ZW and JH organized the database. YL and XD performed the statistical analysis. YL, XD, and XY wrote the first draft of the manuscript. ZW, JH, CW, and CC wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
We would like to thank the researchers and study participants for their contributions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1092734/full#supplementary-material
2. Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F. European association of urology guidelines on renal cell carcinoma: The 2019 update. Eur Urol (2019) 75(5):799–810.
3. Nambirajan T, Jeschke S, Al-Zahrani H, Vrabec G, Leeb K, Janetschek G. Prospective, randomized controlled study: Transperitoneal laparoscopic versus retroperitoneoscopic radical nephrectomy. Urology (2004) 64(5):919–24. doi: 10.1016/j.urology.2004.06.057
4. Richstone L, Reggio E, Ost MC, Seideman C, Fossett LK, Okeke Z, et al. First prize (tie): Hemorrhage following percutaneous renal surgery: Characterization of angiographic findings. J Endourol (2008) 22(6):1129–35. doi: 10.1089/end.2008.0061
5. Wang YL, Jiang B, Yin FF, Shi HQ, Xu XD, Zheng SS, et al. Perioperative blood transfusion promotes worse outcomes of bladder cancer after radical cystectomy: A systematic review and meta-analysis. PLoS One (2015) 10(6):e0130122. doi: 10.1371/journal.pone.0130122
6. Pushan Z, Manbiao C, Sulai L, Jun L, Ruidong Z, Hanshen Y. The impact of perioperative blood transfusion on survival and recurrence after radical prostatectomy for prostate cancer: A systematic review and meta-analysis. J Cancer Res Ther (2018) 14(Supplement):S701–s7. doi: 10.4103/0973-1482.193115
7. Rivas JG, y Gregorio SA, Ledo JC, Gómez ÁT, Sebastián JD, de la Pena Barthel JJ, et al. The role of perioperative blood transfusion on postoperative outcomes and overall survival in patients after laparoscopic radical cystectomy. J Cancer Res Ther (2016) 12(1):146. doi: 10.4103/0973-1482.146125
8. Kim JK, Kim HS, Park J, Jeong CW, Ku JH, Kim HH, et al. Perioperative blood transfusion as a significant predictor of biochemical recurrence and survival after radical prostatectomy in patients with prostate cancer. PLoS One (2016) 11(5):e0154918. doi: 10.1371/journal.pone.0154918
9. Mavros MN, Xu L, Maqsood H, Gani F, Ejaz A, Spolverato G, et al. Perioperative blood transfusion and the prognosis of pancreatic cancer surgery: Systematic review and meta-analysis. Ann Surg Oncol (2015) 22(13):4382–91. doi: 10.1245/s10434-015-4823-6
10. Amato A, Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database systematic Rev (2006) 2006(1):Cd005033. doi: 10.1002/14651858.CD005033.pub2
11. Wang CC, Iyer SG, Low JK, Lin CY, Wang SH, Lu SN, et al. Perioperative factors affecting long-term outcomes of 473 consecutive patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg Oncol (2009) 16(7):1832–42. doi: 10.1245/s10434-009-0448-y
12. Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: A systematic review and meta-analysis. Ann Surg (2012) 256(2):235–44. doi: 10.1097/SLA.0b013e31825b35d5
13. Liu X, Ma M, Huang H, Wang Y. Effect of perioperative blood transfusion on prognosis of patients with gastric cancer: A retrospective analysis of a single center database. BMC cancer. (2018) 18(1):649. doi: 10.1186/s12885-018-4574-4
14. Lee JS, Kim HS, Jeong CW, Kwak C, Kim HH, Ku JH. The prognostic impact of perioperative blood transfusion on survival in patients with bladder urothelial carcinoma treated with radical cystectomy. Korean J Urol (2015) 56(4):295–304. doi: 10.4111/kju.2015.56.4.295
15. Park YH, Kim YJ, Kang SH, Kim HH, Byun SS, Lee JY, et al. Association between perioperative blood transfusion and oncologic outcomes after curative surgery for renal cell carcinoma. J Cancer. (2016) 7(8):965–72. doi: 10.7150/jca.15073
16. Jakobsen EB, Eickhoff JH, Andersen JP, Ottesen M. Perioperative blood transfusion does not affect survival after operation for renal cell cancer. Eur Urol (1994) 26(2):145–8. doi: 10.1159/000475365
17. Linder BJ, Thompson RH, Leibovich BC, Cheville JC, Lohse CM, Gastineau DA, et al. The impact of perioperative blood transfusion on survival after nephrectomy for non-metastatic renal cell carcinoma (RCC). BJU Int (2014) 114(3):368–74. doi: 10.1111/bju.12535
18. Abu-Ghanem Y, Zilberman DE, Dotan Z, Kaver I, Ramon J. Perioperative blood transfusion adversely affects prognosis after nephrectomy for renal cell carcinoma. Urologic Oncol (2018) 36(1):12.e5–.e20. doi: 10.1016/j.urolonc.2017.09.006
19. Soria F, de Martino M, Leitner CV, Moschini M, Shariat SF, Klatte T. Perioperative allogenic blood transfusion in renal cell carcinoma: Risk factors and effect on long-term outcomes. Clin Genitourin Cancer (2017) 15(3):e421–e7. doi: 10.1016/j.clgc.2016.12.002
20. Détrée P, Balssa L, Richard V, Francois C, Barkatz J, Bernardini S, et al. Impact of blood transfusion on survival after nephrectomy for localized or locally advanced renal cancer. Progres en urologie (2022) 32(8-9):577–84. doi: 10.1016/j.purol.2022.03.002
21. Keown PA, Descamps B. Improved renal allograft survival after blood transfusion: A nonspecific, erythrocyte-mediated immunoregulatory process? Lancet (London England) (1979) 1(8106):20–2. doi: 10.1016/S0140-6736(79)90458-6
22. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
23. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
24. Tsivian M, Abern MR, Tsivian E, Sze C, Jibara G, Rampersaud EN Jr., et al. Effect of blood transfusions on oncological outcomes of surgically treated localized renal cell carcinoma. Urologic Oncol (2018) 36(8):362.e1–.e7. doi: 10.1016/j.urolonc.2018.04.014
25. Kim H, Kim J, Yoon H, Lee H, Lee J, Bae J, et al. The impact of perioperative blood transfusion on oncologic outcomes in patients with non-metastatic renal cell carcinoma treated with surgery. Int J Clin Oncol (2019) 18(1):e2098–e9. doi: 10.1016/S1569-9056(19)31521-0
26. Seon DY, Kwak C, Kim HH, Ku JH, Kim HS. Impact of perioperative blood transfusion on oncologic outcomes in patients with nonmetastatic renal cell carcinoma treated with curative nephrectomy: A retrospective analysis of a large, single-institutional cohort. Invest Clin Urol (2020) 61(2):136–45. doi: 10.4111/icu.2020.61.2.136
27. Kang HW, Seo SP, Kim WT, Yun SJ, Lee SC, Kim WJ, et al. Intraoperative allogeneic blood transfusion is associated with adverse oncological outcomes in patients with surgically treated non-metastatic clear cell renal cell carcinoma. Int J Clin Oncol (2020) 25(8):1551–61. doi: 10.1038/s41598-018-37691-4
28. Abu-Ghanem Y, Dotan Z, Zilberman DE, Kaver I, Ramon J. Intraoperative but not postoperative blood transfusion adversely affect cancer recurrence and survival following nephrectomy for renal cell carcinoma. Sci Rep (2019) 9(1):1160. doi: 10.1007/s10147-020-01694-x
29. Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. New Engl J Med (2005) 353(25):2654–66. doi: 10.1056/NEJMoa051424
30. Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Sci (New York NY) (2006) 313(5795):1960–4. doi: 10.1126/science.1129139
31. Cata JP, Wang H, Gottumukkala V, Reuben J, Sessler DI. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J anaesthesia. (2013) 110(5):690–701. doi: 10.1093/bja/aet068
32. Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): An update. Blood Rev (2007) 21(6):327–48. doi: 10.1016/j.blre.2007.07.003
33. Moschini M, Dell' Oglio P, Capogrosso P, Cucchiara V, Luzzago S, Gandaglia G, et al. Effect of allogeneic intraoperative blood transfusion on survival in patients treated with radical cystectomy for nonmetastatic bladder cancer: Results from a single high-volume institution. Clin Genitourin Cancer (2015) 13(6):562–7. doi: 10.1016/j.clgc.2015.04.009
34. Moschini M, Bianchi M, Gandaglia G, Cucchiara V, Luzzago S, Pellucchi F, et al. The impact of perioperative blood transfusion on survival of bladder cancer patients submitted to radical cystectomy: Role of anemia status. Eur Urol Focus (2016) 2(1):86–91. doi: 10.1016/j.euf.2015.03.002
35. Pang QY, An R, Liu HL. Perioperative transfusion and the prognosis of colorectal cancer surgery: A systematic review and meta-analysis. World J Surg Oncol (2019) 17(1):7. doi: 10.1186/s12957-018-1551-y
36. Agnes A, Lirosi MC, Panunzi S, Santocchi P, Persiani R, D'Ugo D. The prognostic role of perioperative allogeneic blood transfusions in gastric cancer patients undergoing curative resection: A systematic review and meta-analysis of non-randomized, adjusted studies. Eur J Surg Oncol (2018) 44(4):404–19. doi: 10.1016/j.ejso.2018.01.006
37. Iwata T, Kimura S, Foerster B, Abufaraj M, Karakiewicz PI, Preisser F, et al. Perioperative blood transfusion affects oncologic outcomes after nephrectomy for renal cell carcinoma: A systematic review and meta-analysis. Urologic Oncol (2019) 37(4):273–81. doi: 10.1016/j.urolonc.2019.01.018
38. Arcaniolo D, Manfredi C, Cindolo L, Marchioni M, Zukovski EP, Mirone V, et al. Impact of perioperative blood transfusions on the outcomes of patients undergoing kidney cancer surgery: A systematic review and pooled analysis. Clin Genitourin Cancer (2019) 17(1):e72–e9. doi: 10.1016/j.clgc.2018.09.010
Keywords: blood transfusion, renal cell carcinoma, survival, systematic review, meta-analysis
Citation: Liu Y, Deng X, Wen Z, Huang J, Wang C, Chen C and Yang X (2023) The effect of perioperative blood transfusion on survival after renal cell carcinoma nephrectomy: A systematic review and meta-analysis. Front. Oncol. 13:1092734. doi: 10.3389/fonc.2023.1092734
Received: 08 November 2022; Accepted: 06 February 2023;
Published: 16 February 2023.
Edited by:
Aristotle Bamias, National and Kapodistrian University of Athens, GreeceReviewed by:
Tao Liu, Zhongnan Hospital, Wuhan University, ChinaCopyright © 2023 Liu, Deng, Wen, Huang, Wang, Chen and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuesong Yang, MzMwNDczODM1QHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.