- 1Department of Thoracic Surgery, The First Hospital of China Medical University, Shenyang, Liaoning, China
- 2Department of Radiation Oncology, The First Hospital of China Medical University, Shenyang, Liaoning, China
Background: Previous studies have demonstrated that prophylactic cranial irradiation (PCI) could reduce the risk of brain metastases and prolong the overall survival (OS) of patients with small cell lung cancer (SCLC). However, it remains controversial whether the efficacy and safety of PCI would be subjected to the different characteristics of patients with extensive stage of SCLC. This meta-analysis aims to evaluate the efficacy and safety of PCI in patients with extensive stage SCLC.
Methods: PubMed, Embase, and the Cochrane Library were searched for relevant studies from inception to May, 2021. Hazard ratios (HRs) were used to measure the OS and progression-free survival (PFS), and relative risks (RRs) were employed to calculate the incidence of brain metastases, survival rate, and adverse events. Summary results were pooled using random-effect models.
Results: There were 1215 articles identified, and 15 trials were included, with a total of 1,623 participants. Patients who received PCI did not result in significantly improved OS [HR=0.87, 95%CI (0.70, 1.08) p=0.417] and PFS [HR=0.81, 95%CI (0.69, 0.95) p=0.001], compared with those who did not receive PCI, while patients who received PCI had a significantly decreased incidence of brain metastases [RR=0.57, 95%CI (0.45, 0.74), p<0.001]. PCI group showed no improvements in 2-year (RR=1.03, p=0.154), 3-year (RR=0.97, p=0.072), 4-year (RR=0.71, p=0.101) and 5-year survival rates (RR=0.32, p=0.307), compared with non-PCI group, whereas the overall RR indicated that PCI was associated with a higher 1-year survival rate [RR=1.46, 95%CI (1.08, 1.97), p=0.013]. In addition, PCI treatment was shown to be associated with increased incidence of adverse events, including fatigue, dermatitis, anorexia, nausea, vomiting, malaise, and cognitive impairment.
Conclusion: This meta-analysis suggests that PCI can reduce the incidence of brain metastases in extensive stage SCLC. Although PCI has no significant effect on the OS, it improves 1-year survival in patients with extensive stage SCLC. However, PCI does not significantly affect 2,3,4,5-year survival and may result in a significantly increased risk of adverse events.
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide, with approximately 1.38 million deaths reported annually (1). Small cell lung cancer (SCLC) accounts for nearly 14% of primary lung cancer, and over 70% of SCLC patients are diagnosed at extensive-stage (2, 3). Extensive stage SCLC (ES-SCLC) is characterized by a higher risk of metastasis, more rapid doubling time, and earlier dissemination (4). Four to six courses of cisplatin-containing chemotherapy are the primary regimen widely used for patients with extensive stage SCLC, with considerable clinical response of 60 to 70%, while the median survival of patients with extensive stage SCLC remains poor at 9 months (5–7). Further, brain metastases could compromise the quality of life and survival time in patients with extensive stage SCLC in that many of the patients died of intra-cerebral progression (8). MRI is one of the best diagnostic methods for early brain metastases.
Prophylactic cranial irradiation (PCI) is widely applied for the prevention of early cancer dissemination to the uninvolved brain, since not all systemic chemotherapeutic drugs can cross the blood-brain barrier (BBB). Existing BBB-crossing systemic therapies that are available is not first-line drugs or only applicable for tumors with specific mutations (9). Several studies have already demonstrated that PCI was associated with a lower risk of brain metastases and longer survival time in patients with limited stage SCLC (6, 10–14). Patients with extensive stage SCLC have a shorter median survival time and a higher risk of brain metastases after chemotherapy than those with limited stage SCLC. Therefore, PCI should be recommended for these patients to reduce the incidence of brain metastases. However, the data on the efficacy and safety of PCI for patients with extensive stage SCLC remains limited and inconclusive. In this study, we reviewed the available randomized controlled trials (RCTs) to evaluate the efficacy and safety of PCI in patients with extensive stage SCLC, and we further explored its effects in the treatment for patients with specific characteristics.
Methods
Search strategy
This systematic review and meta-analysis was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (15). PubMed, EMBASE, and the Cochrane Library databases were searched, from inception to May, 2021 for RCTs that conducted to evaluate the efficacy and safety of PCI for the treatment of extensive stage SCLC, without no language restrictions. Search items mainly included “small cell lung cancer” and “prophylactic cranial irradiation”. The reference lists of included studies were manually searched for potential eligible studies. Literature search was processed independently by 2 review authors. Any disagreement was settled by the primary author.
Inclusion criteria
Studies meeting the following criteria were included:
(1) Types of study: RCT-design;
(2) Types of intervention: comparison between PCI and non-PCI;
(3) Types of participants: patients with extensive stage SCLC (If both limited and extensive stage patients were included in trials, only the data of the latter were extracted separately);
(4) Types of outcome: overall survival (OS), progression free survival (PFS), incidence of brain metastases, and survival rate at different follow-up periods.
Non-randomized studies and studies that only involved patients with limited stage SCLC were excluded.
Quality assessment and data extraction
Quality assessment and data extraction were conducted by two review authors independently, and any disagreement was resolved by group discussion until a consensus was reached. The revised JADAD scale was applied to assess the quality of included studies, which is a comprehensive assessment tool and has been partially validated for evaluating the quality of RCTs in meta-analyses (16). The JADAD scale is based on 5 items: randomization (0-2), concealment of the treatment allocation (0-1), blinding (0-2), completeness of follow-up (0-1), and use of intention-to-treat analysis (0-1). Each item could be scored for 0 to 2, with a total score of 7. Study with a score of 4 or more would be regarded as high quality. Information was verified and adjudicated independently by a third author according to the original studies.
Data were extracted using a pre-designed form, containing name of the first author, publication date, country, sample size, characteristics of participants (mean age and gender distribution), follow-up duration, total dose/No. of fractions of PCI, OS, PFS, incidence of brain metastases, 1-, 2-, 3-, 4-, and 5-year survival rates, and any possible adverse events (all grades).
Statistical analysis
Hazard ratio (HR) was applied as pooled statistic for survival data including OS and PFS, with the 95% confidence intervals (CI) provided. Relative risk (RR) and the 95%CI were used for dichotomous data, including incidence of brain metastases, 1-, 2-, 3-, 4-, and 5-year survival rates, and any possible adverse events. Random-effect model were applied to pool the effects (17, 18). Heterogeneity test was performed using Cochrane’s Q test and I2 test. A p value less than 0.10 indicated statistical significance (19, 20). Sensitivity analysis was performed by removing each study one by one from the meta-analysis to assess its influence (21). Subgroup analysis was conducted for OS, PFS, incidence of brain metastases, 1-, 2-, 3-, 4-, and 5-year survival rates based on publication date (before vs after 2000), regions (Eastern vs Western), sample size (≥ 100 vs <100), mean age (≥ 60.0 vs <60.0), the proportion of males in the study (≥ 70.0% vs <70.0%), and quality of the studies (4-7 vs 1-4). The efficacy and safety of PCI according to the aforementioned factors were correlated with background treatment strategies, ethnicity, statistical power, patient characteristics, and strength of evidence. The p values between the subgroups were calculated using the Chi-square test and meta-regression (22). Publication bias was evaluated using the Egger`s test (23) and Begg`s test (24). All reported p values were two-sided and the values less than 0.05 were considered significant for all included trials. Statistical analyses were performed using STATA software (version 10.0 StataCorp, Texas, USA).
Results
Study selection and characteristics of included studies
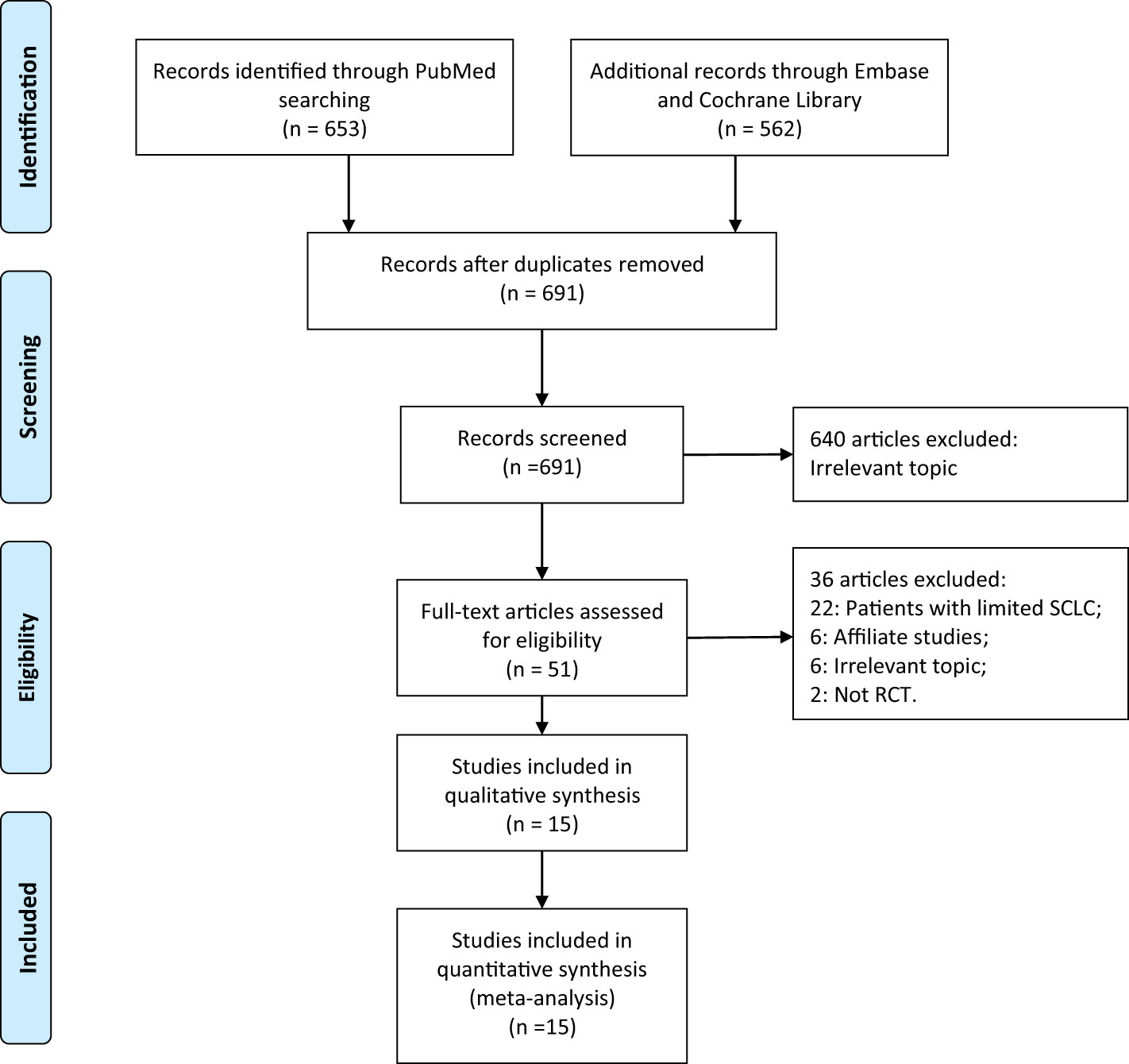
There were 1,215 articles identified, and 1,164 were removed after duplicate-checking and titles- and abstracts-reading. Full-texts of the remaining 51 articles were retrieved and read. We further excluded 22 studies that enrolled patients with limited stage SCLC, 6 studies reporting on the same populations, 2 non-RCT studies, and 6 studies focusing on other topics. After the detailed evaluation, 15 RCTs involving 1623 extensive stage SCLC patients were included (8, 9, 25–37). A manual search of the reference lists of these studies did not yield any additional eligible studies. The selection process of this study was presented in a PRISMA flowchart (Figure 1), and the baseline characteristics of included studies were shown in Table 1. Quality assessment was performed using the JADAD scale, with 1 study scoring for 5 (25), 8 studies scoring for 4 (8, 26, 27, 29, 33–36), and the remaining 6 for 3 (9, 28, 30–32, 37).
OS and PFS
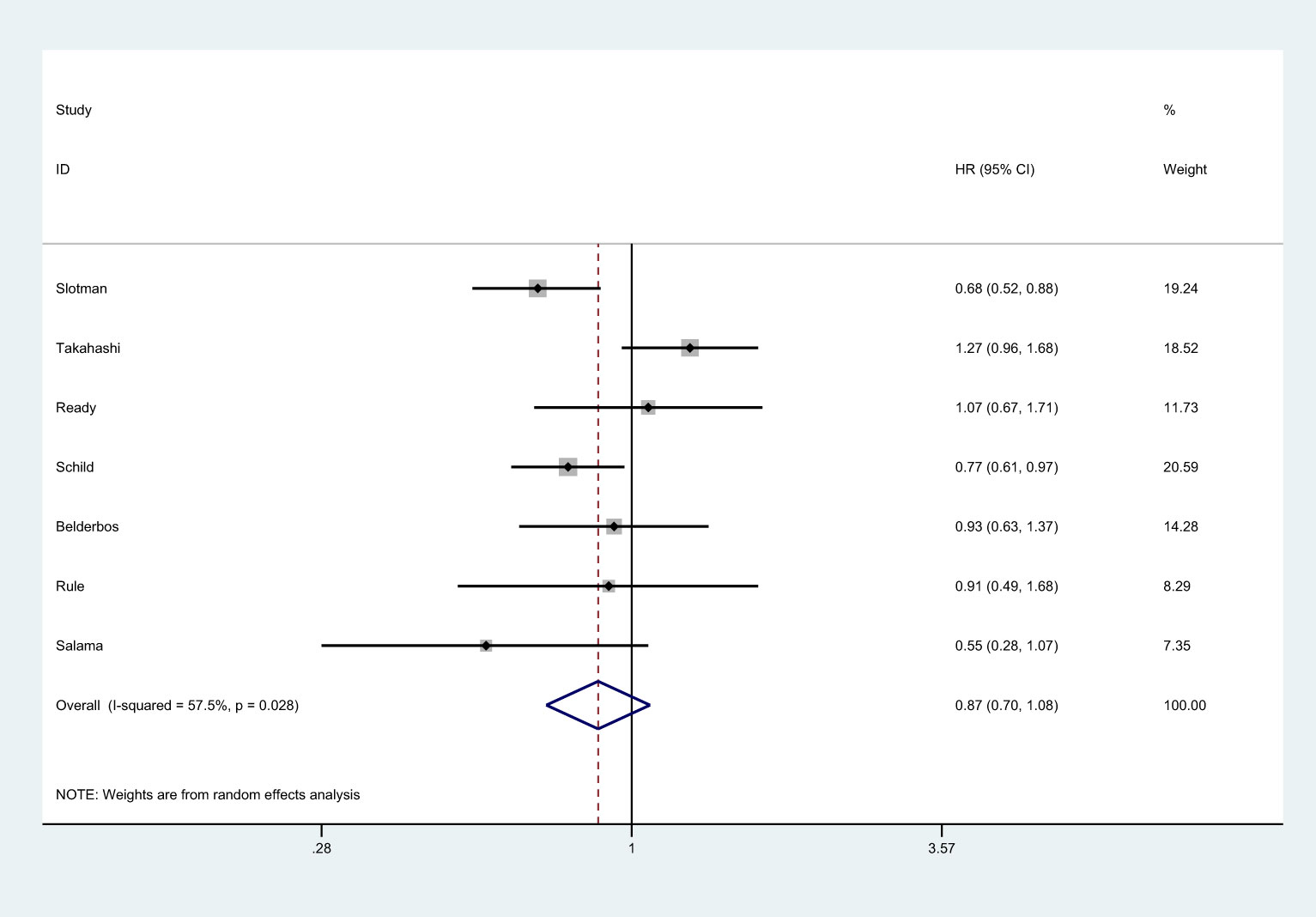
There were 7 studies that reported OS (8, 25–27, 36, 37). There was no significantly statistical difference in the improvement of OS between PCI group and non-PCI group [HR=0.87, 95%CI (0.70, 1.08), p=0.417] (Figure 2). There was significant heterogeneity among the studies (I2 = 57.5%; p=0.028). Sensitivity analysis showed that heterogeneity may come from Takahashi et al. (25) (Supplemental 1). Subgroup analysis indicated that PCI significantly improved OS in trials conducted in Western countries, studies with the proportion of males over 70.0%, and studies with low JADAD scores (Supplemental 2).
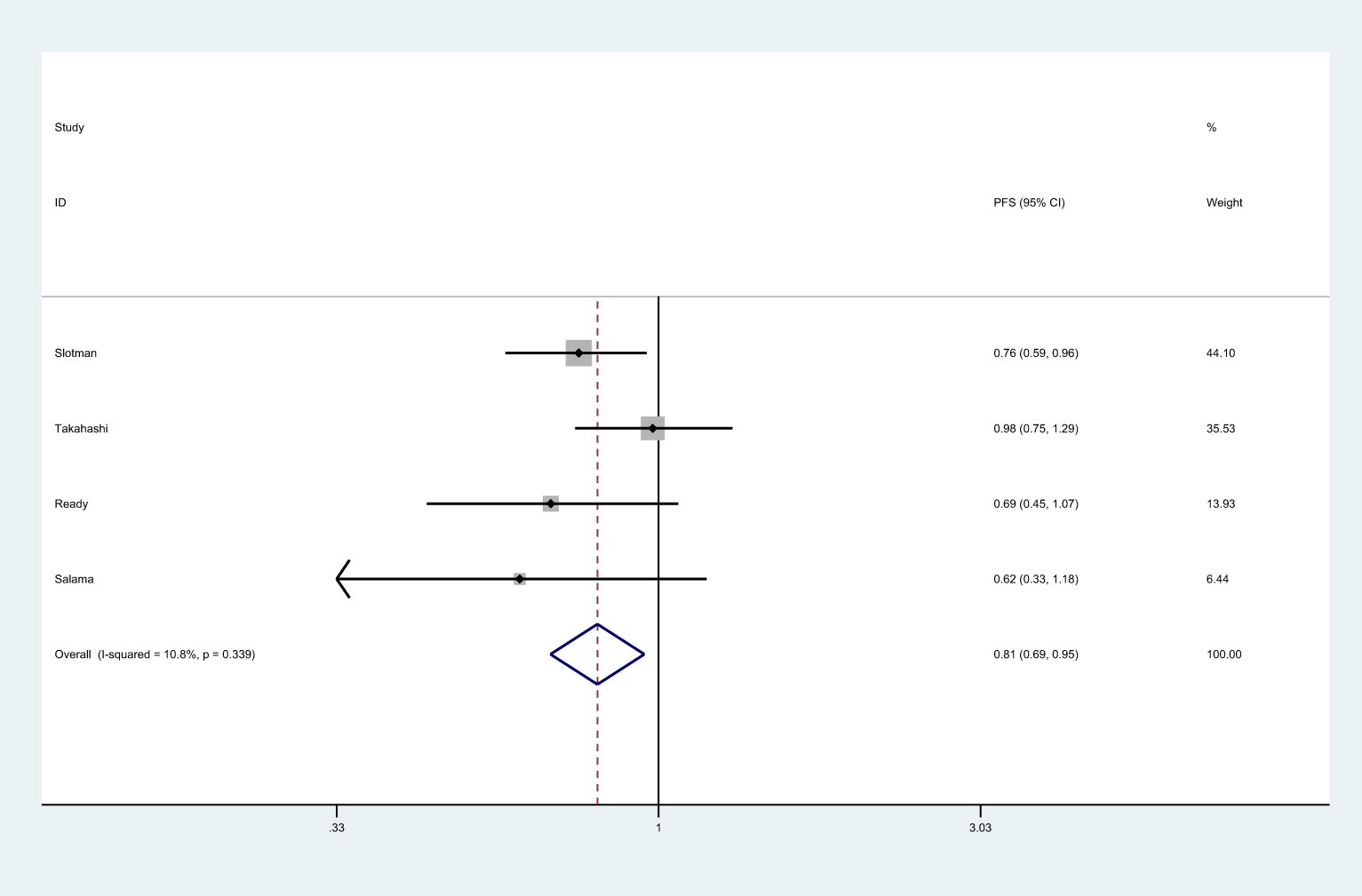
There were 4 studies that reported PFS (8, 25, 26, 37). Meta-analysis showed that patients in PCI group had longer PFS than those in non-PCI group [HR=0.81, 95%CI (0.69, 0.95), p=0.001] (Figure 3). No significant heterogeneity was observed among included trials (I2 = 10.08%, p=0.339). Sensitivity analysis showed that heterogeneity may be derived from Takahashi’s data, so we consider the removal of the study by Takahashi et al. (25) (Supplemental 1). Subgroup analysis indicated that PCI significantly improved PFS in studies conducted in Western countries or in those with the proportion of males over 70.0% (Supplemental 2).
Incidence of brain metastases
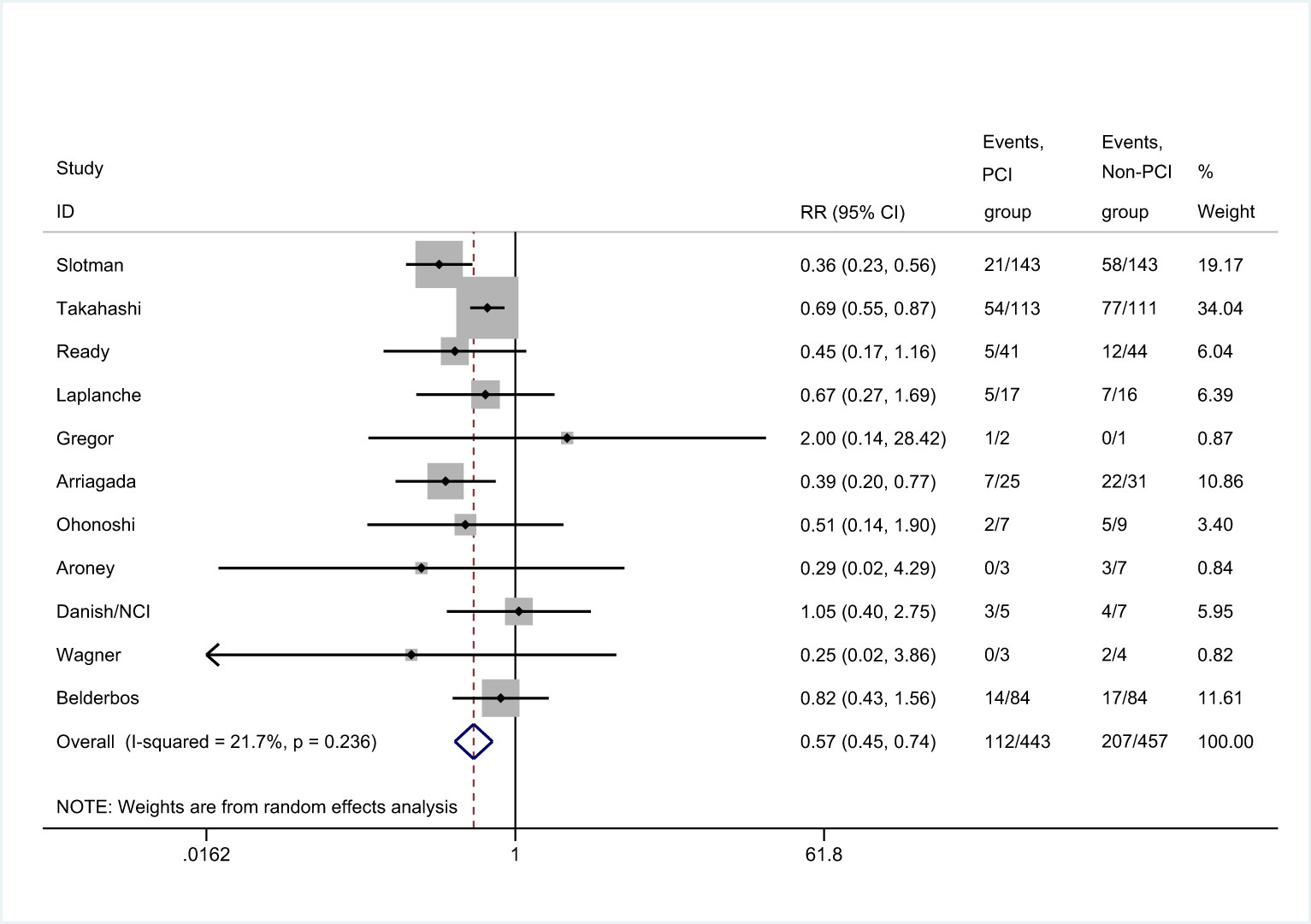
There were 11 trials that reported the incidence of brain metastases (8, 25, 26, 29–36). Meta-analysis showed that patients in PCI group had lower incidence of brain metastases than those in non-PCI group [RR=0.57, 95%CI (0.45, 0.74), p<0.001] (Figure 4). There was no significant heterogeneity considered among the studies. Sensitivity analysis showed that the conclusion was not affected by the exclusion of any of the study (Supplemental 1). Subgroup analysis found no significant difference in the incidence of brain metastases between PCI and non-PCI, when the mean age of included patients was less than 60.0 years-old (Supplemental 2).
Survival rate
There were 7 studies (8, 25–28, 37), 6 studies (25–28, 37), 4 studies (25, 27, 28,),6 studies (8, 27, 28, 31, 35,), and 3 studies (27–29) that reported 1-, 2-, 3-, 4-, and 5-year survival rate, respectively (Figure 5). Meta-analysis showed that PCI intervention did not improve the 2-year [RR=1.03, 95%CI (0.63, 1.69), p=0.154), 3-year [RR=0.97, 95%CI (0.42, 2.22), p=0.072], 4-year [RR=0.71, 95%CI (0.23, 2.19), p=0.101], and 5-year survival rate [RR=0.32, 95%CI (0.10, 1.08), p=0.307]. However, patients in PCI group had higher 1-year survival rate than those in non-PCI group [RR=1.46, 95%CI (1.08, 1.97), p=0.013]. The findings of the sensitivity analysis varied in 1-, 4-, and 5-year survival rates after excluding individual trials (Supplemental 1). Subgroup analysis showed that studies conducted in Western countries, studies with the proportion of male participants over 70.0%, and those with low-quality reported more increased 1-year survival rate. It also indicated no significant difference in 2- and 3-year survival rates between PCI and non-PCI group. Studies published before 2000, those conducted in Western countries, and those with lower JADAD scores reported lower 4-year survival rate. Finally, studies with more than 100 patients included or those with lower JADAD scores reported lower 5-year survival rate (Supplemental 2).
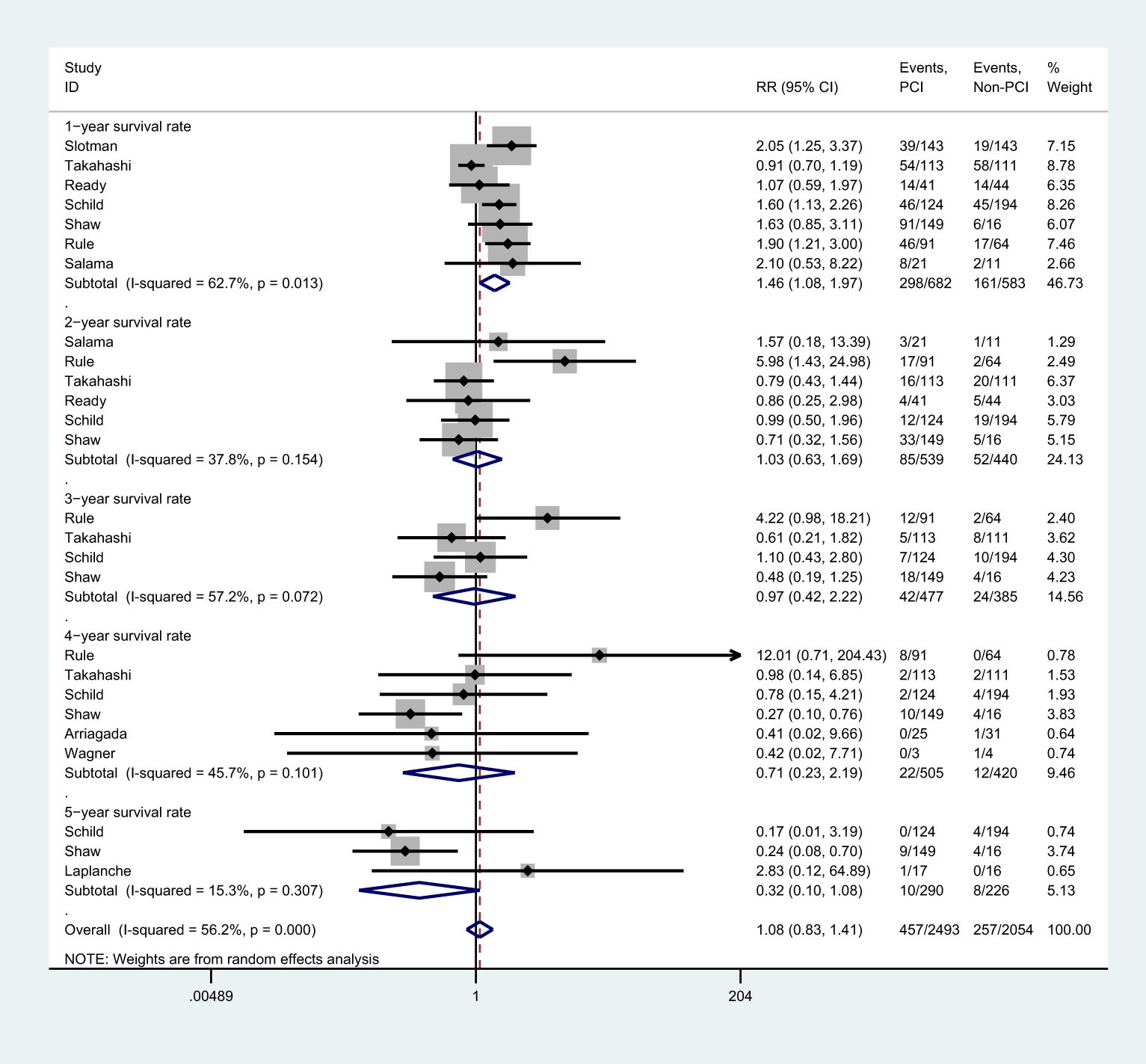
Figure 5 Effect of PCI on survival rates in different follow-up periods for patients with extensive SCLC.
Adverse events
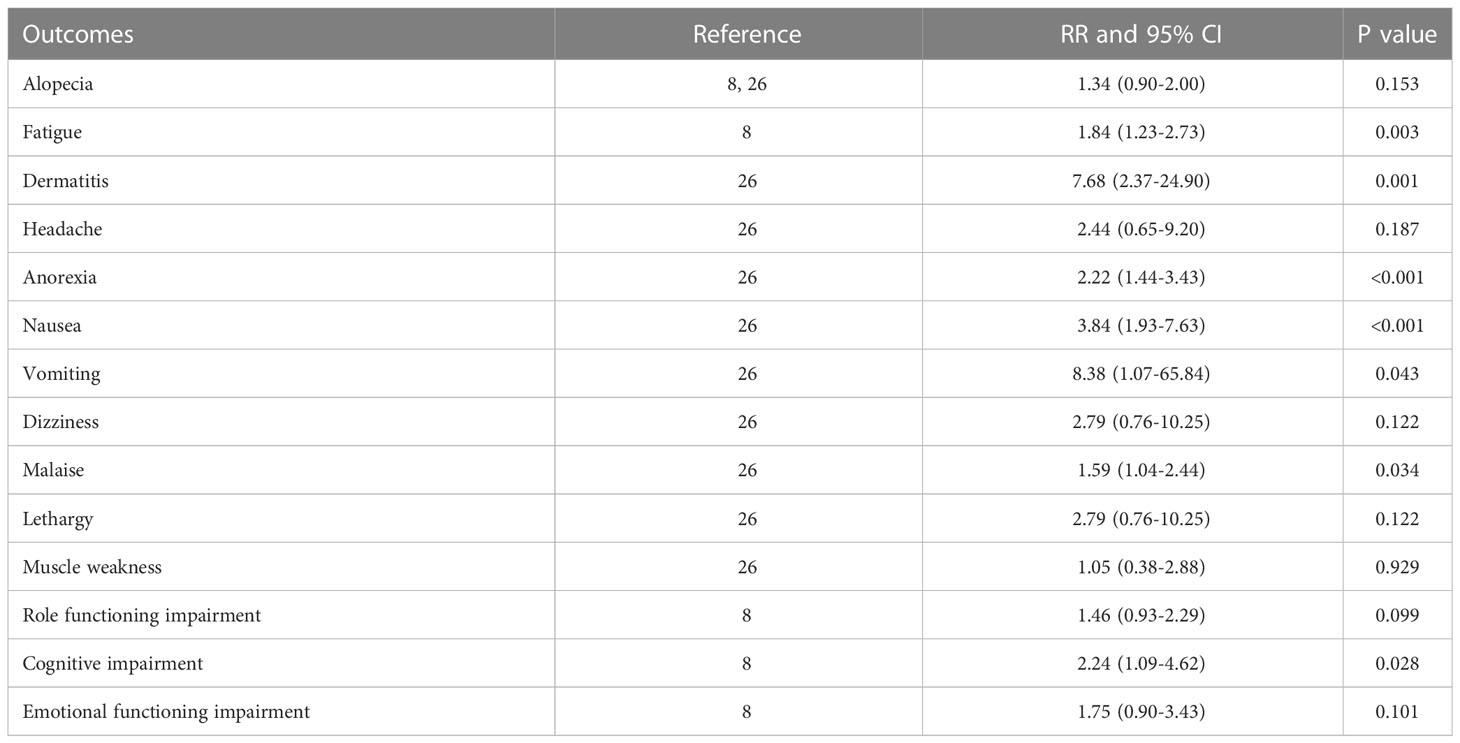
All reported adverse events are summarized in Table 2. Patients in PCI group had higher risk of fatigue [RR=1.84, 95%CI (1.23, 2.73) p=0.003], dermatitis [RR=7.68, 95%CI (2.37, 24.90), p=0.001], anorexia [RR=2.22, 95%CI (1.44, 3.43), p<0.001], nausea [RR=3.84, 95%CI (1.93, 7.63), p<0.001], vomiting [RR=8.38, 95%CI (1.07, 65.84), p=0.043], malaise [RR=1.59, 95%CI (1.04, 2.44), p=0.034], and cognitive impairment [RR=2.24, 95%CI (1.09,4.62), p=0.028], compared with those in non-PCI group. There was no significant difference in the risk of alopecia, headache, dizziness, lethargy, muscle weakness, impaired role functioning, and impaired emotional functioning between the two groups.
Publication bias
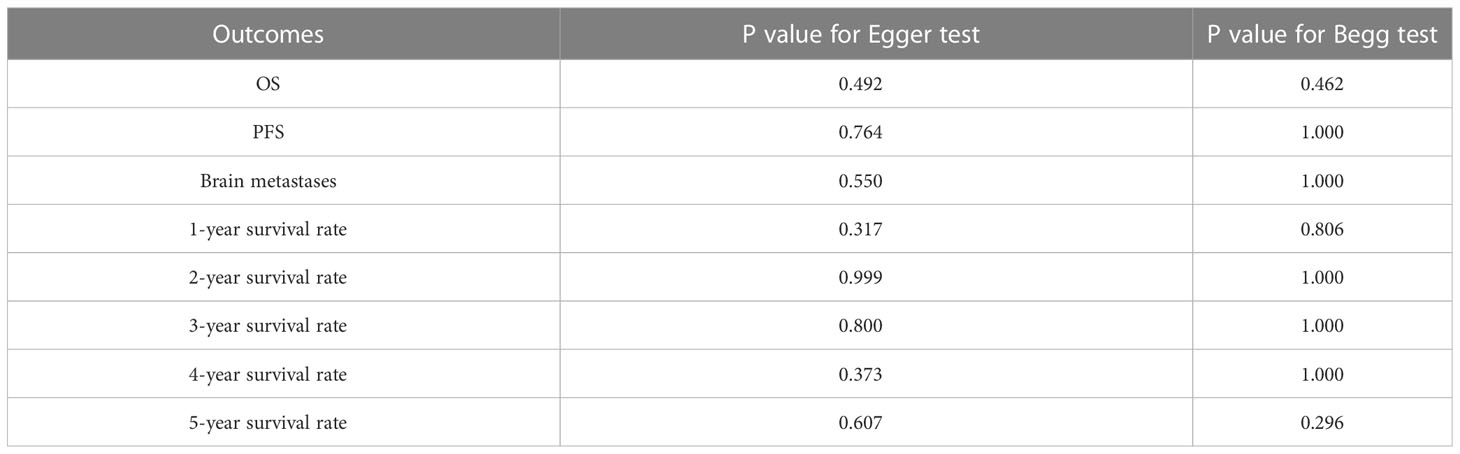
Egger`s test and Begg`s test showed no significant publication bias in OS, PFS, incidence of brain metastases, and 1-, 2-, 3-, 4-, and 5-year survival rates (Table 3). The results did not reverse after adjustment for publication bias using the trim-and-fill method (38).
Discussion
In this study, 15 RCTs that evaluated the efficacy and safety of PCI in the treatment for patients with extensive stage SCLC were included, with a total of 1623 patients, and the results showed that PCI did not contribute to longer OS or PFS in the patients, compared with non-PCI. However, PCI significantly reduced the incidence of brain metastases. PCI did not affect the 2-, 3-, 4- and 5-year survival rates, but reduced the 1-year survival rate. PCI also showed to be associated with higher risk of adverse events including fatigue, dermatitis, anorexia, nausea, vomiting, malaise, and cognitive impairment in patients with extensive SCLC. The therapeutic effect of PCI might be subjected to different countries, sample size, age, gender, and study quality.
A previous study suggested that PCI significantly improved the survival rate, disease-free survival, and incidence of brain metastases in SCLC patients with complete remission (13). Another important meta-analysis indicated that PCI could improve the survival and decrease the incidence of brain metastases in SCLC patients, while it only consisted of 5 RCTs involving both limited and extensive stage SCLC patients (14), and the therapeutic effects on extensive stage SCLC patients with specific characteristics were not analyzed (13, 14). Schild et al. conducted a qualitative review examining the evidence and provided recommendations for the role of PCI in extensive stage SCLS patients, but did not perform any quantitative analyses (39). Therefore, this meta-analysis was exclusively focused on extensive stage SCLC to evaluate the potential efficacy and safety of PCI.
There were no significant differences in the improvements of OS and PFS between PCI group and non-PCI group. However, the results were inconclusive and needed to be further validated by large-scale trials. Furthermore, several studies included in our meta-analysis reported inconsistent results. Slotman et al. indicated that PCI could reduce the risk of symptomatic brain metastases and improve OS and PFS. They illustrated a greater impact of PCI for patients with extensive stage SCLC than for those with limited stage SCLC (8). A study conducted by Schild et al. indicated that PCI could prolong the survival in patients with both limited and extensive stage SCLC. They also found that PCI resulted in higher clinical response of chemotherapy and thoracic radiation therapy (9). The inconsistent findings of this study with previous studies could be related to the differences in the proportion of patients receiving subsequent treatment strategies.
We found that PCI could reduce the risk of brain metastases, which was consistent with previous studies (13, 14). A Cochrane review indicated that the incidence of brain metastases was reduced by 77% in patients receiving PCI. However, it was not assessed whether this effect would be different among specific subpopulations (13). On the other hand, the study conducted by Zhang et al. only included two studies to assess the incidence of brain metastases so that the results might be varied (14). Several included studies also reported inconsistent results (26, 29, 30, 32–35). A possible reason could be that the sample size was too small to reveal the clinical benefit, especially if the event rates were lower than expected. This could lead to broad confidence intervals resulting in no significantly statistical differences.
The results showed that PCI improved 1-year survival, although it had no significant effect on 2-, 3-,4 or 5-year survival. This is consistent with other studies that 1-year survival was significantly higher in the PCI group than in the control group (37.1% vs. 27.1%; Human Resources:0.87; 95% confidence interval:0.80 0.95; P = 0.002) (12). It might be that the median survival time of most patients with extensive stage SCLC is less than 1 year (5), resulting in more 1-year survival events than in the other follow-up periods, and a significant difference that was easier to detect due to the higher statistical power. Additionally, more than half of the included participants died within 1 year after recruited in the study, which might result in no significant differences in the 2-, 3-,4 or 5-year survival rates between the two groups. Subgroup analysis showed that region, sample size, mean age, proportion of included male patients, and study quality could affect the treatment effect of PCI in extensive stage SCLC patients. However, these significant differences between PCI and non-PCI groups were not observed in investigated outcomes (except for the incidence of brain metastases) in Eastern countries (25), and the conclusion might vary since smaller cohorts were included in such subsets. Moreover, ongoing treatment strategies, old age, and mutated genes might affect the therapeutic effect of PCI in extensive stage SCLC patients (40).
As expected, higher risk of adverse events was observed in patients receiving PCI, which would counteract the clinical benefits of PCI. Increased adverse events could compromise the quality of life in those patients, while the data on quality of life were rarely available in these studies. However, one study indicated that PCI had a negative impact on selected health-related quality of life scales and recommend that PCI should be offered to all responding extensive stage SCLC patients (41). The significantly increased risk of fatigue, dermatitis, anorexia, nausea, vomiting, malaise, and cognitive impairment in patients receiving PCI might also lead to poor quality of life. Alopecia and lethargy are the common toxic effects of PCI treatment, while our meta-analysis found no significant difference in the incidence of alopecia and lethargy between PCI and non-PCI groups, which might be related to the chemotherapies these patients were receiving, and only smaller trials reporting on these outcomes (2 for alopecia (8, 25) and 1 for lethargy (25)). Lastly, given the current disagreement among the involved clinical practitioners (42), ongoing randomized controlled trials also recruiting patients with ES-SCLC could help clarify which is the best therapeutic approach between whole brain radiotherapy and stereotactic radiosurgery for the management of overt brain metastases in cases without prior PCI (43).
This meta-analysis had several limitations. Firstly, subsequent chemotherapeutic regimens were different between PCI and non-PCI groups, and these data were not available in most included trials. Secondly, subgroup analysis based on dose/fractionation was not conducted since the mean dose and fractionation varied and lacked a standard cutoff value. Thirdly, publication bias was inevitable due to unpublished literatures were not searched so that the negative results could not be included. Therefore, the therapeutic effect of PCI for extensive stage SCLC might be overestimated in this study. Lastly, the analysis used pooled data (individual data were not available), which restricted us from performing a more detailed analysis and obtaining more comprehensive results.
In conclusion, PCI can reduce the incidence of brain metastases in extensive stage SCLC, and although it has no significant effect on overall survival, it improves 1-year survival in patients with extensive stage SCLC. Further, higher risk of adverse events was observed in patients receiving PCI. Based on these findings, we may make corresponding adjustments to the clinical management of patients with ES-SCLC. Before applying PCI, we should re-evaluate the patients according to their actual conditions, such as physical conditions and economic conditions, rather than blindly applying it. We will pay more attention to the 1-year survival advantages brought by PCI as well as the practical benefits of reducing the economic and psychological burdens of some patients. At present, the follow-up chemotherapy strategies for ES-SCLC patients after PCI are still controversial. Future studies require more clinical trials to help us re-evaluate the combination strategy of PCI and subsequent chemotherapy regimen for patients with extensive stage SCLC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
ZW: Conceptualization and Writing of the first draft. LS, FC, LC, QY, XH, QF, WC: Review and Editing. PL and WL: Conceptualization and Review. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Natural Science Foundation of Liaoning Province (2020-BS-088 and 2023-MS-159). The sponsors were uninvolved in the study design, data collection, analysis, and manuscript submission.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1086290/full#supplementary-material
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin (2016) 66:7–30. doi: 10.3322/caac.21332
2. Gironés R, López P, Chulvi R, Caabate M, Dolores TM. Ten years of lung cancer in a single center: gender, histology, stage and survival. J Cancer Metastasis Treat (2015) 1:201–7. doi: 10.4103/2394-4722.166971
3. Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clin Proc (2008) 83:355–67. doi: 10.4065/83.3.355
4. Semenova A, Nagel R, Berns A. Origins, genetic landscape, and emerging therapies of small cell lung cancer. Genes Dev (2015) 29:1447–62. doi: 10.1101/gad.263145.115
5. Chen Y, Chen L, Zhong D. Comparing the adverse effects of platinum in combination with etoposide or irinotecan in previously untreated small-cell lung cancer patients with extensive disease: a network meta-analyses. Thorac Cancer (2017) 8:170–80. doi: 10.1111/1759-7714.12420
6. Auperin A, Arriagada R, Pignon JP, Le Péchoux C, Gregor A, Stephens RJ, et al. Prophylactic cranial irradiation for patients with small-cell-lung cancer in complete remission. N Engl J Med (1999) 341:476–84. doi: 10.1056/NEJM199908123410703
7. Guo S, Liang Y, Zhou Q. Complement and correction for meta-analysis of patients with extensive-stage small cell lung cancer managed with irinotecan/cisplatin versus etoposide/cisplatin as first-line chemotherapy. J Thorac Oncol (2011) 6:406–8. doi: 10.1097/JTO.0b013e3182061d8c
8. Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M, et al. Radiation Oncology Group and Lung Cancer Group. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med (2007) 357:664–72. doi: 10.1056/NEJMoa071780
9. Schild SE, Foster NR, Meyers JP, Ross HJ, Stella PJ, Garces YI, et al. Prophylactic cranial irradiation in small-cell lung cancer: findings from a north central cancer treatment group pooled analysis. Ann Oncol (2012) 23:2919–24. doi: 10.1093/annonc/mds123
10. Yang GY, Matthews RH. Prophylactic cranial irradiation in small-cell lung cancer. Oncologist (2000) 5:293–8. doi: 10.1634/theoncologist.5-4-293
11. Gregor A, Cull A, Stephens RJ, Kirkpatrick JA, Yarnold JR, Girling DJ, et al. Prophylactic cranial irradiation is indicated following complete response to induction therapy in small cell lung cancer: results of a multicentre randomised trial. united kingdom coordinating committee for cancer research (UKCCCR) and the European organization for research and treatment of cancer (EORTC). Eur J Cancer (1997) 33:1752–8. doi: 10.1016/S0959-8049(97)00135-4
12. Meert AP, Paesmans M, Berghmans T, Martin B, Mascaux C, Vallot F, et al. Prophylactic cranial irradiation in small cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer (2001) 1:5. doi: 10.1186/1471-2407-1-5
13. The Prophylactic Cranial Irradiation Overview Collaborative Group. Cranial irradiation for preventing brainmetastases of small cell lung cancer in patients in complete remission (Review). Cochrane Database Systematic Rev (2000) 4:CD002805. doi: 10.1002/14651858.CD002805.
14. Zhang W, Jiang W, Luan L, Wang L, Zheng X, Wang G. Prophylactic cranial irradiation for patients with small-cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer (2014) 14:793. doi: 10.1186/1471-2407-14-793
15. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
16. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
17. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
18. Ades AE, Lu G, Higgins JP. The interpretation of random-effects metaanalysis in decision models. Med Decis Making (2005) 25:646–54. doi: 10.1177/0272989X05282643
19. Deeks JJ, Higgins JPT, Altman DG. Analyzing data and undertaking meta-analyses. In: Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions 5.0.1. Oxford, UK: The Cochrane Collaboration (2008).
20. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
21. Tobias A. Assessing the influence of a single study in meta-analysis. Stata Tech Bull (1999) 47:15–7.
22. Deeks JJ, Altman DG, Bradburn MJ. Egger M, Davey Smith G, Altman DG, editors. Systematic reviews in health care: metaanalysis in context, 2nd ed. London: BMJ Books (2001). p. 285–312.
23. Egger M, Davey Smith G, Schneider M, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
24. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics (1994) 50:1088–101. doi: 10.2307/2533446
25. Takahashi T, Yamanaka T, Seto T, Harada H, Nokihara H, Saka H, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2017) 18:663–71. doi: 10.1016/S1470-2045(17)30230-9
26. Ready NE, Pang HH, Gu L, Otterson GA, Thomas SP, Miller AA, et al. Chemotherapy with or without maintenance sunitinib for untreated extensive stage small-cell lung cancer: a randomized, doubleblind, placebo-controlled phase II study-CALGB 30504 (Alliance). J Clin Oncol (2015) 33:1660–5. doi: 10.1200/JCO.2014.57.3105
27. Rule WG, Foster NR, Meyers JP, Ashman JB, Vora SA, Kozelsky TF, et al. Prophylactic cranial irradiation in elderly patients with small cell lung cancer: findings from a north central cancer treatment group pooled analysis. J Geriatr Oncol (2015) 6:119–26. doi: 10.1016/j.jgo.2014.11.002
28. Shaw EG, Eagan RT, Maksymiuk AW, Jett JR, Maksymiuk AW, Deigert FA. Prophylactic cranial irradiation in complete responders with small-cell lung cancer: analysis of the Mayo clinic and north central cancer treatment group data bases. J Clin Oncol (1994) 12:2327–32. doi: 10.1200/JCO.1994.12.11.2327
29. Laplanche A, Monnet I, Santos-Miranda JA, Bardet E, Le Péchoux C, Tarayre M, et al. Controlled clinical trial of prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Lung Cancer (1998) 21:193–201. doi: 10.1016/S0169-5002(98)00056-7
30. Gregor A, Cull A, Stephens RJ, Kirkpatrick JA, Yarnold JR, Girling DJ, et al. Prophylactic cranial irradiation is indicated following complete response to induction therapy in small cell lung cancer: results of a multicentre randomised trial. Eur J Cancer (1997) 33:1752–8. doi: 10.1016/S0959-8049(97)00135-4
31. Arriagada R, Le Chevalier T, Borie F, Rivière A, Chomy P, Monnet I, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst (1995) 87:183–90. doi: 10.1093/jnci/87.3.183
32. Ohonoshi T, Ueoka H, Kawahara S, Kiura K, Kamei H, Hiraki Y, et al. Comparative study of prophylactic cranial irradiation in patients with small cell lung cancer achieving a complete response: a long-term follow-up result. Lung Cancer (1993) 10:47–54. doi: 10.1016/0169-5002(93)90308-K
33. Aroney RS, Aisner J, Wesley MN, Le Péchoux C, Gregor A, Stephens RJ, et al. Value of prophylactic cranial irradiation given at complete remission in small cell lung carcinoma. Cancer Treat Rep (1983) 67:675–82.
35. Wagner H, Kim K, Turrisi A. A randomized phase III study of prophylactic cranial irradiation versus observation in patients with small cell lung cancer achieving a complete response: final report of an incomplete trial. Proc Am Soc Clin Oncol (1996) 15:376.
36. Belderbos JSA, Ruysscher DKM, De Jaeger K, Koppe F, Lambrecht MLF, Lievens YN, et al Phase 3 randomized trial of prophylactic cranial irradiation with or without hippocampus avoidance in SCLC (NCT01780675). J Thorac Oncol (2021) 16:840–9. doi: 10.1016/j.jtho.2020.12.024
37. Salama JK, Gu L, Wang X, Pang HH, Bogart JA, Crawford J, et al. Positive interaction between prophylactic cranial irradiation and maintenance sunitinib for untreated extensive-stage small cell lung cancer patients after standard chemotherapy: a secondary analysis of CALGB 30504 (ALLIANCE). J Thorac Oncol (2016) 11(3):361–9. doi: 10.1016/j.jtho.2015.11.001
38. Duvall S, Tweedie R. A nonparametric “trim and fill” method for assessing publication bias in meta-analysis. J Am Stat Assoc (2000) 95:89–98.
39. Schild SE, Sio TT, Daniels TB, Chun SG, Rades D. Prophylactic cranial irradiation for extensive small-cell lung cancer. J Oncol Pract (2017) 13:732–8. doi: 10.1200/JOP.2017.026765
40. Sundaresan V, Lin VT, Liang F, Kaye FJ, Kawabata-Iwakawa R, Shiraishi K, et al. Significantly mutated genes and regulatory pathways in SCLC-a meta-analysis. Cancer Genet (2017) 216-217:20–8. doi: 10.1016/j.cancergen.2017.05.003
41. Slotman BJ, Mauer ME, Bottomley A, Faivre-Finn C, Kramer GWOM, Rankin EM, et al. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: short-term health-related quality of life and patient reported symptoms–results of an international phase III randomized controlled trial by the EORTC radiation oncology and lung cancer groups. J Clin Oncol (2008) 27:78–84. doi: 10.1200/JCO.2008.17.0746
42. Pergolizzi S, Cacciola A, Parisi S, Lillo S, Tamburella C, Santacaterina A, et al. An Italian survey on "palliative intent" radiotherapy. Rep Pract Oncol Radiother (2022) 27(3):419–27. doi: 10.5603/RPOR.a2022.0052
43. Ferini G, Viola A, Valenti V. Whole brain irradiation or stereotactic RadioSurgery for five or more brain metastases (WHOBI-STER): a prospective comparative study of neurocognitive outcomes, level of autonomy in daily activities and quality of life. Clin Transl Radiat Oncol (2021) 32:52–8. doi: 10.1016/j.ctro.2021.11.008
Keywords: PCI for extensive stage SCLC prophylactic cranial irradiation, small cell lung cancer, overall survival, meta-analysis, brain metastases, survival rates
Citation: Wang Z, Chen L, Sun L, Cai F, Yang Q, Hu X, Fu Q, Chen W, Li P and Li W (2023) Prophylactic cranial irradiation for extensive stage small cell lung cancer: a meta-analysis of randomized controlled trials. Front. Oncol. 13:1086290. doi: 10.3389/fonc.2023.1086290
Received: 01 November 2022; Accepted: 14 April 2023;
Published: 17 May 2023.
Edited by:
Rongrong Zhou, Xiangya Hospital, Central South University, ChinaCopyright © 2023 Wang, Chen, Sun, Cai, Yang, Hu, Fu, Chen, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peiwei Li, bHB3Y211QDE2My5jb20=; Wenya Li, c2FpbnQ1Mjg4QGhvdG1haWwuY29t
 Ziyi Wang
Ziyi Wang Liang Chen1
Liang Chen1 Lu Sun
Lu Sun Qiwei Yang
Qiwei Yang Peiwei Li
Peiwei Li Wenya Li
Wenya Li