
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 30 January 2023
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1080990
Gastric cancer is the fifth most common cancer worldwide, and the treatment of advanced gastric cancer has relatively little progress. With the continuous development of molecularly targeted therapy for tumors, it has been discovered that human epidermal growth factor receptor 2 (HER2) contributes to the poor prognosis and pathogenesis of various cancers. In order to treat HER2-positive advanced gastric cancer, Trastuzumab has emerged as the first first-line targeted medication used in conjunction with chemotherapy. The consequent trastuzumab resistance has become an important issue, and various new HER2-targeted gastric cancer drugs are emerging to address this challenge. This review’s primary concern is the drug mechanism of various HER2-positive gastric cancer targeted therapy and fresh techniques of detection.
Since 2010, China’s cancer incidence and fatality rates have increased, making cancer the leading cause of death and a major public health issue (1). Gastric cancer is the second most common cancer causing death in China, and its 5-year survival rate is very low because more than 80% of patients are diagnosed with advanced gastric cancer (AGC) (2). The 5-year survival rate for AGC is less than 10%, and even if novel chemotherapy protocols and biological therapy are being used, median overall survival (OS) is still less than 1 year (3). Gastric cancer remains important worldwide, causing over 1 million new cases and an estimated 769,000 deaths in 2020, incidence and mortality rank fifth and fourth in the world, respectively (4). Only in recent years has it been discovered that H. pylori infection can lead to antral/body gastric cancer. Additionally, H. pylori infection greatly increases the risk of developing gastric cancer. The risk of gastric cancer brought on by a chronic H. pylori infection may also be influenced by additional risk factors, such as smoking, excessive salt consumption, and drinking alcohol (5, 6).
Different perioperative treatment strategies (neo-adjuvant, adjuvant, or both) have improved survival for patients with locally AGC. For unresectable gastric cancer, In first-line therapy, the combination of platinum compounds with fluoropyrimidine-based chemotherapy is successful in extending survival, improving symptoms, and enhancing the quality of life (7). Adding a third drug, such as docetaxel, to the platinum-fluoropyrimidine combination increases toxicity but improves patient survival (8). With the application of various novel immunotherapy and molecular targeted therapy, anti-HER2 is the most widely used target because it significantly increases the survival of cancer patients.
Human epidermal growth factor receptor 2 (HER2), also known as Neu or ErbB2, Encoded by ERBB2 on chromosome 17, is a transmembrane tyrosine kinase (TK) receptor belonging to the epidermal growth factor receptor (EGFR) family, This family consists of four members (HER1 or EGFR, HER2, HER3, and HER4), all of which have an extracellular domain(ECD), a transmembrane domain, and an intracellular kinase domain (9, 10). (Figure 1.) The binding of different ligands to the ECD initiates a series of signal transduction pathways, which are crucial for the growth, apoptosis, adhesion, migration, and differentiation of tumor cells (11). The HER2 receptor, first discovered in 1984, is a 185kD transmembrane glycoprotein (12). HER2 lacks ligand-binding activity and requires heterodimerization with other family members (HER1 and/or HER3) to be activated (13). Among them, the HER2-HER3 heterodimer is the most active HER signaling dimer and plays a crucial role in HER2-driven tumor oncogenic transformation, HER-2 activates downstream pathways through heterodimerization and tyrosine kinase autophosphorylation mediated signal transduction, major signaling pathways include Ras/MAPK and PI3K/Akt (14). They are essential pathways regulating cell proliferation, differentiation and survival and are closely related to the pathogenesis of various tumors (15–17). HER2-specific antibodies interfere with HER2 signaling primarily by blocking heterodimerization between ErbB-2 and growth factor receptors (18). Use immunohistochemistry (IHC) to detect HER2 protein, fluorescence in situ hybridization (FISH) or chromogenic in situ hybridization (CISH) to detect gene amplification, HER2 overexpression can be classified as IHC0 (negative), IHC1+ (negative), IHC2+ (equivocal), or IHC3+ (positive), and samples with an IHC 2+ value should undergo another FISH or CISH test(Figure 2) (19, 20).
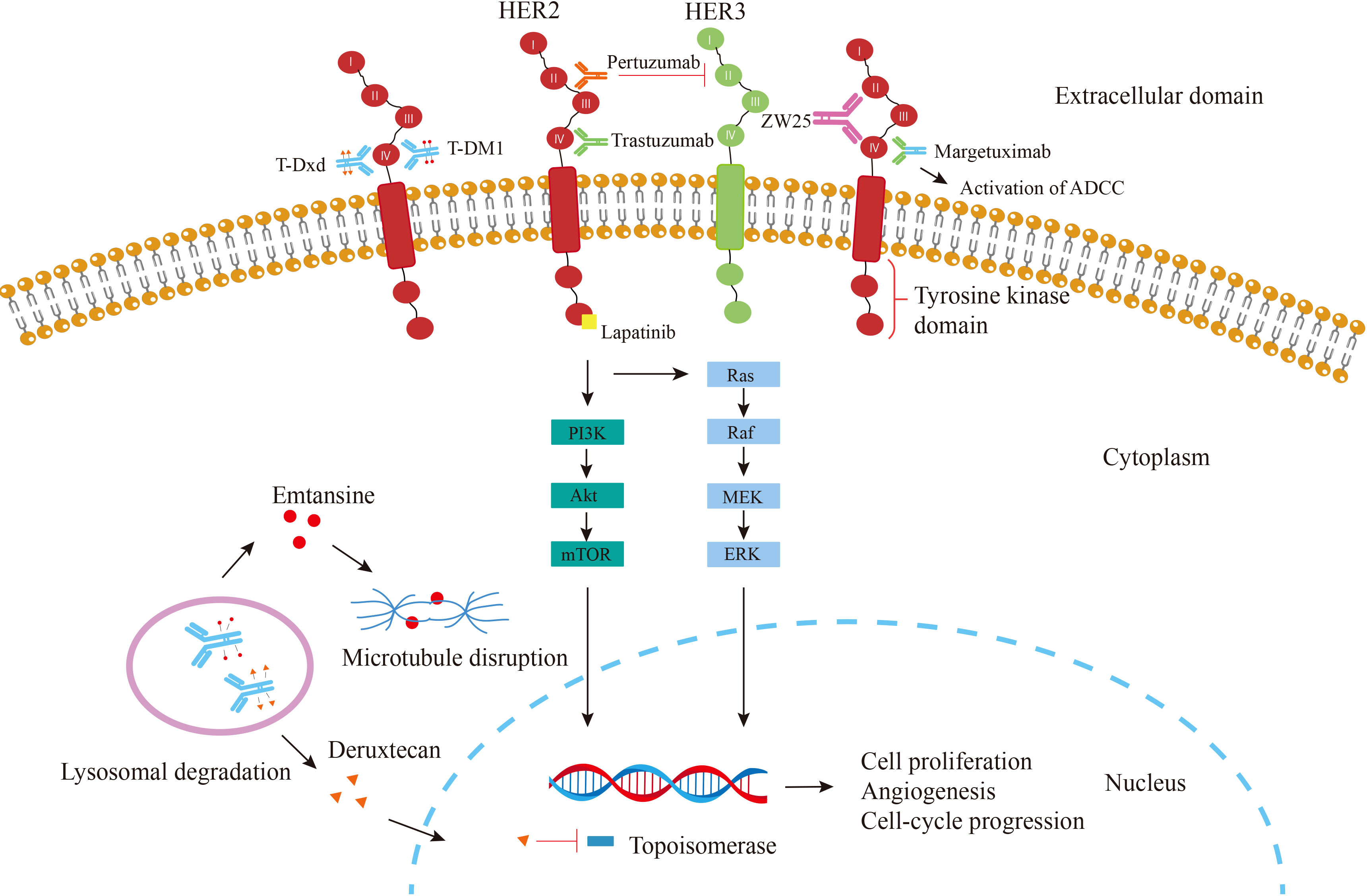
Figure 1 Mechanism of action of agents targeting HER2. Trastuzumab (green) binds to the ECD IV of HER2, thereby inhibiting the HER2 signaling pathway leading to cell cycle arrest; Pertuzumab (orange), which binds to the ECD II and Inhibits dimer formation; Margetuximab, which binds trastuzumab to an altered Fc-γ domain that involved in ADCC; Lapatinib (yellow) directly prevents the PI3K pathway from being activated by binding to the intracellular tyrosine kinase domain of HER2; T-DM1 (red circle)releases the emtansine moiety after the ADC is phagocytosed by lysosomes; T-Dxd (orange triangle), another ADC combining trastuzumab and deruxtecan, a potent topoisomerase I inhibitor; Bispecific antibody ZW25 (purple) binds to both extracellular domains II and IV of HER-2.
Although non-small cell lung cancer (NSCLC), ovarian, colon, and pancreatic cancers overexpress HER2 protein and/or show gene amplification in varying proportions of cases, HER2 protein overexpression is most pronounced in breast and gastric cancers (21). In comparison to HER2-negative breast cancer, HER2-positive breast cancer is more well-studied and has greater mortality and risk of distant metastases. Following its approval in 1998, trastuzumab, one of the first molecularly targeted drugs to be developed, is now recommended for all patients with early-stage HER2-positive disease and has a better prognosis (22–24). According to the latest global report, the average HER2-positive rate of gastric cancer is 17.9%, of which the HER2-positive rate in Chinese gastric cancer patients was 8.8% (25, 26). HER2 overexpression was associated with tumor location, tumor differentiation, Bormann classification, Lauren’s classification, lymph node status, venous invasion, and lymphatic invasion in patients. Among gastric cancers with high expression of HER2 protein, gastroesophageal junction cancer(GEJ) is more common than gastric corpus cancer. Predominantly tubular adenocarcinoma (larger proportion of well/moderately differentiated carcinomas, lesser proportion of poorly differentiated adenocarcinomas), and HER2 positivity is more common in the Intestinal type than diffuse or mixed type. However, there is no correlation with gender, age, or clinical stage (27–30). However, there is controversy over the role of HER2 in predicting prognosis in gastric cancer, as opposed to the poor prognosis associated with HER2 positivity in breast cancer. Studies have revealed that the prognosis is independent of HER2 expression. Therefore, HER2 remains an uncertain predictor of gastric cancer prognosis, and more research is required (31–33).
Patients with HER2-positive gastric cancer have seen favorable clinical results after receiving anti-HER2 therapy. Trastuzumab is a humanized recombinant monoclonal antibody that selectively binds to HER2 ECD IV and reduces the expression of HER2 receptors, Thereby inhibiting angiogenesis, reducing DNA repair, and inducing apoptosis. On the other hand, trastuzumab contains an IgG1 Fc structure, which Can mediate antibody-dependent cellular cytotoxicity (ADCC) to attack target cells (34). Patients who underwent chemotherapy with cisplatin and fluorouracil in combination with trastuzumab had a better median OS than those who just got chemotherapy(16 months vs 11 months). This is mainly due to the survival advantage of patients with high expression of the HER2 protein (35, 36).
With the advancement of tumor immunotherapy, combined immune checkpoint inhibitors will emerge as a promising treatment. A phase II trial (NCT03409848) revealed that trastuzumab and PD-1 inhibitors nivolumab combined with first-line chemotherapy showed promising efficacy in HER2-positive GEJ Cancer (37). Meanwhile, pembrolizumab can also be safely used in combination with trastuzumab and chemotherapy (38). Pembrolizumab was added to trastuzumab and chemotherapy in a phase III trial (NCT03615326), which dramatically decreased tumor size and improved objective response rates (39).
The issue has gained attention as most patients develop resistance to trastuzumab. Trastuzumab resistance appears to be primarily mediated by tumor heterogeneity. Treatment failure with anti-HER2 therapy is also associated with changes in receptor tyrosine kinase-RAS-PI3K signaling. Additionally, Mucins, which are cell surface proteins, reduce the HER2 receptor’s interaction with trastuzumab. As a result, the drug’s inhibitory effect is blocked (31, 40). To overcome this problem, a variety of new drugs and treatments are emerging.
Pertuzumab is a recombinant humanized anti-HER2 antibody with different antitumor activity than trastuzumab. Pertuzumab binds to the ECD II of HER2. Therefore, pertuzumab can inhibit the dimerization of HER2 with other HER family members, especially effectively block HER2-HER3 heterodimerization, thereby preventing ligand-dependent HER2 signaling (41–43). Adding pertuzumab to trastuzumab and chemotherapy has not been found to increase survival in patients with HER2-positive metastatic gastric cancer, despite the fact that pertuzumab significantly prolongs the lives of patients with metastatic breast cancer. This may be a consequence of the different tumor biology exhibited by HER2-positive AGC and HER2-positive breast cancer (44, 45). The ongoing INNOVATION trial will individually evaluate the relative benefit of trastuzumab and pertuzumab in perioperative therapy (45).
Margetuximab is a HER2-targeted antibody with an engineered FCγ domain. Special modification of the Fc region increases its binding to the activating Fc receptor FcγRIIIA (CD16A) and reduces its binding to the inhibitory Fc receptor FcγRIIB (CD32B). Therefore, the response rate is increased (46). CD16A is an Fc receptor important in mediating ADCC effects, which renders margetuximab more potent and more cytotoxic than trastuzumab with the wild-type Fc domain. A phase Ib/II trial(NCT02689284) has shown that margetuximab combined with anti-PD-1 drugs such as pembrolizumab and retifanlimab have synergistic antitumor activity (47). At the same time, it has a stronger impact on people with tumors expressing low HER2 or those with CD16A low binding alleles (48). In addition, compared with trastuzumab, margetuximab can enhance the ADCC effect to produce a stronger killing effect on tumor cells (49). The current Phase II/III, randomized trial(MAHOGANY) is assessing margetuximab plus retifanlimab with/without chemotherapy and margetuximab plus tebotelimab with chemotherapy in HER2-Positive Gastric or GEJ Cancer (50).
Tyrosine kinase inhibitors (TKIs) compete with ATP for the ATP binding site of Protein tyrosine kinase(PTK), leading to a reduction in tyrosine kinase phosphorylation and blocking downstream signaling pathways, thereby inhibiting cancer cell proliferation (51, 52). Lapatinib, a small-molecule inhibitor of the EGFR and HER2 tyrosine kinase domains, was first approved for treating HER2-positive breast cancer (53). In treating HER2-positive gastric cancer, the median OS of capecitabine and oxaliplatin (CapeOx) combined with lapatinib and CapeOx plus placebo was 12.2 months and 10.5 months, respectively, and there was no significant difference. Median progression-free survival (PFS) was 6.0 months and 5.4 months, respectively (54). On the other hand, the median OS of lapatinib plus paclitaxel and paclitaxel alone was 11.0 months and 8.9 months, respectively (P=0.1044). There were also no significant differences in PFS (5.4 months vs. 4.4 months) and time to progression (TTP) (5.5 months vs. 4.4 months). Therefore, the addition of lapatinib to CapeOx’s first-line chemotherapy regimen and second-line regimens of lapatinib plus paclitaxel did not improve patients’ OS (55). Afatinib irreversibly inhibits the ErbB family, paclitaxel and afatinib are being tested in a trial (NCT01522768) for patients with HER2-positive, trastuzumab-refractory esophagogastric cancer.
Antibody-drug conjugates (ADC) is an emerging antibody bioconjugate, which is an immunoconjugate composed of a monoclonal antibody bound to a cytotoxic drug through a chemical linker, combining the antigen specificity of the antibody and the potency of the cytotoxic agent at the same time (56). Monoclonal antibodies are used as carriers to target cytotoxic drugs to specific cells. The antibodies bind to specific receptors on the surface of the target cells and are then degraded by lysosomes after endocytosis. Intracellular small-molecule cytotoxic drugs are released in large quantities, destroying DNA chains or microtubules, or exerting topoisomerase or RNA polymerase inhibitory effects, resulting in tumor cell death (57, 58). Trastuzumab emtansine (T-DM1) is a kind of ADC composed of trastuzumab linked to the tubulin inhibitor DM1 (a derivative of maytansine) through a stable linker. Catabolites containing cytotoxic emtansine are released intracellularly to induce mitotic arrest and apoptosis. However, AGC patients treated with T-DM1 did not have a clear advantage in OS compared with patients treated with taxanes (59, 60).
Trastuzumab deruxtecan (T-Dxd) is a novel ADC consisting of a humanized anti-HER2 antibody covalently linked to a topoisomerase I inhibitor (DXd) via a tetrapeptide-based cleavable linker (61). The ADC payload (ie, DXd) was delivered directly to HER2-expressing tumor cells, reducing damage to normal cells by cytotoxic agents (62). Unlike T-DM1, T-Dxd showed stronger antitumor activity against gastric or GEJ cancer with low HER2 expression (63). Compared with conventional chemotherapy, T-Dxd significantly improved patient response rate (RR) (51% vs 14%) and prolonged OS(12.5 months vs 8.4 months) (64). Other studies have shown that T-Dxd is not only effective against HER2 protein-positive tumor cells but also effective against HER2-negative tumor cells in the presence of HER2-positive cells. Due to the high membrane permeability of T-Dxd, this bystander-killing effect may be due to T-Dxd being internalized by HER2-positive cells, and DXd being released into the cytoplasm and then being transferred to adjacent HER2-negative cells (65, 66). Ongoing Phase II trials (NCT04014075) and (NCT04379596) will study safety and efficacy of T-Dxd drug alone or in combination with chemotherapy and/or immunotherapy.
ZW25 is a bispecific antibody that simultaneously binds two HER2 epitopes: ECD4 and ECD2. Compared with trastuzumab or pertuzumab, ZW25 has stronger antitumor activity, can effectively silence HER2 signaling, and also stimulate the immune system. ZW25 has been demonstrated to have single-agent action and to be well tolerated (67). A clinical trial recruiting 24 patients with HER2-positive cancer, 71% of whom had previously received trastuzumab, showed a median PFS of 6.2 months and a disease control rate of 82%. Diarrhea, infusion reactions, and nausea were the most common grade 1 or 2 side effects. Phase II trials will test the medication both by itself and when combined with chemotherapy (68). Additionally, a trial combining ZW25 with tislelizumab and chemotherapy is ongoing (NCT04276493).
Chimeric antigen receptor T (CAR-T) cell therapy is an adaptive cellular immunotherapy in which CAR-redirected T cells expressing engineered receptors for specific antigens are reinfused into patients, thereby triggering an effective antitumor immune response (69). Major histocompatibility complex (MHC)-independent tumor-associated antigen recognition is made feasible by CARs (70). CARs consist of an extracellular target antigen-binding domain, a hinge region transmembrane domain, and one or more intracellular signaling domains (71). Among them, chimeric antigen receptor-modified T cell therapy targeting CD19 is very effective in relapsed acute lymphoblastic leukemia (72). Due to the substantial heterogeneity of gastric cancer cells, there is not much research on the application of CAR-T cells in the treatment of gastric cancer. A study showed that expanded CAR-T cells efficiently eliminated HER2-positive gastric cancer cells from patients after being specifically triggered by the HER2 antigen (73). In HER2-positive xenograft tumors, CAR-T cells’ tumor suppressor and killing abilities were significantly enhanced compared with non-transduced T cells (74). HER2-targeted CAR-T cells for treating HER2-positive AGC is a promising therapeutic strategy, but additional study is required to determine its toxicity and immunogenicity. Patients with refractory HER2-positive solid tumors will participate in a phase I study(NCT04511871) to investigate the CAR-T’s safety and preliminary therapeutic efficacy.
Intratumor heterogeneity and genomic instability processes shape tumor evolution in space and time, and growing evidence suggests a link between assessment heterogeneity and poor prognosis. This explains the mismatch between the costs and benefits of some cancer treatments (75). Gastric cancer tumor cells have greater HER-2 heterogeneity (from 26 to 79% in IHC) compared to breast cancer (76). HER-2 heterogeneity may reduce the efficacy of trastuzumab. Two Japanese studies discovered it to be an independent predictor of poor prognosis (77). This may be the most important primary mechanism of anti-HER2 drug resistance.
HER2 loss is one of the primary causes of acquired resistance to trastuzumab in HER2-positive gastric cancer patients (78). In patients with HER2-positive gastric cancer receiving trastuzumab, 29.1%-64% of patients developed loss of HER2 expression during treatment (IHC score <3+ and absence of ISH amplification) and/or loss of HER2 overexpression (IHC “down scoring” from 2+/3+ to 0/1+), At the same time, the heterogeneity of HER2 gene expression increased. This phenomenon was found more frequent in tumors with an initial IHC score of 2+, suggesting that HER2 status needs to be reassessed before starting second-line anti-HER2 therapy (79, 80).
Src, a non-receptor tyrosine kinase of the Src family, plays a role in signaling and crosstalk between growth-promoting pathways (81). Studies have shown a relationship between the low clinical effect of trastuzumab and the changes of the PI3K/Akt pathway. Acquired resistance to trastuzumab in both HER2-overexpressing breast and gastric cancer are associated with sustained Src-mediated activation of the MAPK/ERK and PI3K/mTOR pathways (82, 83). Meanwhile, HER3 overexpression was observed in drug-resistant gastric cancer cell lines, which may be induced by HER2 blockade (84). Therefore, blocking these targets may improve drug resistance in patients.
Epithelial to Mesenchymal Transition (EMT) is a reversible cellular program that temporarily places epithelial cells in a quasi-mesenchymal state (85). EMT is essential for the invasion and metastasis of cancer cells as well as embryogenesis and the healing of wounds (86, 87). EMT and HER4 may be associated with resistance to HER2 therapy. HER4 and phosphorylated HER4 (p-HER4) expression was increased in trastuzumab-resistant cells while activating the downstream PI3K pathway through the HER4-YAP1 axis to promote the transformation of epithelial cells to mesenchymal cells to maintain the invasiveness of HER2-positive gastric cancer and escape the blockade of trastuzumab (88, 89).
To better assess patient outcomes, we need improved diagnostic, prognostic, and disease surveillance methods despite the availability of various treatments (90). Circulating tumor DNA (ctDNA) is released into the bloodstream by circulating tumor cells during tumor cell apoptosis or necrosis, a process that occurs before the tumor is detected by imaging means or clinical symptoms are not manifested. As a result, ctDNA is one of the most promising biomarkers for detecting cancer in its early stages (91, 92). In a study of ctDNA in early gastric cancer, HER2 amplification in tumor tissue and DNA samples matched at a rate of roughly 60% (93). With the application of modern technologies such as digital droplet PCR and next generation sequencing, the coincidence rate between ctDNA and her2 expression in tumor tissue has increased to about 90% (90, 94). Moreover, the detection of ctDNA can overcome the heterogeneity of some tumors, which suggests that the targeted HER2 population can be screened alternatively using ctDNA (95). Based on the advantages of non-invasive and dynamic monitoring of ctDNA, it can be used as a tool to evaluate and predict the effectiveness of anti-HER2 therapy (96).
Circulating tumor cells (CTCs) are tumor cells that shed into the blood and circulate throughout the body (97). The spread and migration of CTCs are important causes of distant metastasis of tumors (98). CTCs counting is a non-invasive method to monitor chemotherapy response and real-time progression, which is more valuable in predicting the sensitivity or prognosis of AGC patients to chemotherapy drugs (99–101). Recent research has found that HER2 amplification detected in CTCs is highly consistent with patient tissue. Therefore CTCs can serve as a non-invasive alternative to document gene amplification in GC patients (102–104).
89Zr-Trastuzumab PET/CT is an imaging test using radionuclide 89Zr-labeled trastuzumab as an imaging agent to determine HER2 expression heterogeneity in gastric cancers and the effect of HER2-targeted therapy. 89Zr-Trastuzumab has additional advantages over single-point biopsy, allowing simultaneous non-invasive assessment of changes in HER2 levels and target binding in the primary tumor and all metastatic sites (105–107). HER2 PET imaging will play an important role in improving diagnosis, staging (eg, when lesions cannot be biopsied), guiding individualized therapy, and the development of targeted drugs (108).
The role of HER-2 in gastric cancer has been demonstrated, and HER2-targeted therapy has dramatically improved the prognosis for patients with early- and late-stage HER2-positive AGC. But only trastuzumab prolongs OS and PFS and is approved as the first-line standard of care. Furthermore, gastric cancer’s intratumoral, intrapatient, and interpatient heterogeneity remains a crucial obstacle to developing targeted therapy drugs. At the same time, immune checkpoint inhibitor monotherapy is not effective against the majority of gastric cancer, hence a combination of immunotherapy and anti-HER2 monoclonal antibodies may be required. To overcome these challenges, novel HER2-targeted drugs have been developed, such as ADCs, TKIs, and bispecific antibodies. New screening methods, such as ctDNA and new imaging agents, allow real-time assessment and monitoring of anti-HER2 treatment in a less invasive manner. Overall, the outcomes for both present and future patients will be significantly improved by new research approaches that address the issues mentioned above.
CM: Writing- Original draft preparation, Investigation, and figure preparation. XW: data collection. JG: Investigation. BY: data collection. YL: Conceptualization, Methodology, Supervision. All authors contributed to the article and approved the submitted version.
This work was supported by the Project of Gansu Provincial Department of Education (2021jyjbgs-02); Project of Gansu Provincial Development and Reform Commission (2020-2022); Major Science and Technology Project of Gansu Province (20zd7fa003); and Project of Gansu Province Military Civilian Integration Development (2020-128).
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
2. Zong L, Abe M, Seto Y, Ji J. The challenge of screening for early gastric cancer in China. Lancet (2016) 388(10060):2606. doi: 10.1016/s0140-6736(16)32226-7
3. Price TJ, Shapiro JD, Segelov E, Karapetis CS, Pavlakis N, Van Cutsem E, et al. Management of advanced gastric cancer. Expert Rev Gastroenterol Hepatol (2012) 6(2):199–208. doi: 10.1586/egh.11.103
4. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
5. Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol (2013) 107(3):230–6. doi: 10.1002/jso.23262
6. Sano T. Gastric cancer: Asia and the world. Gastric Cancer (2017) 20(Suppl 1):1–2. doi: 10.1007/s10120-017-0694-9
7. Obermannova R, Lordick F. Insights into next developments in advanced gastric cancer. Curr Opin Oncol (2016) 28(4):367–75. doi: 10.1097/CCO.0000000000000289
8. Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase iii study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 study group. J Clin Oncol (2006) 24(31):4991–7. doi: 10.1200/JCO.2006.06.8429
9. Tebbutt N, Pedersen MW, Johns TG. Targeting the erbb family in cancer: Couples therapy. Nat Rev Cancer (2013) 13(9):663–73. doi: 10.1038/nrc3559
10. Olayioye MA. Update on her-2 as a target for cancer therapy: Intracellular signaling pathways of Erbb2/Her-2 and family members. Breast Cancer Res BCR (2001) 3(6):385–9. doi: 10.1186/bcr327
11. Gravalos C, Jimeno A. Her2 in gastric cancer: A new prognostic factor and a novel therapeutic target. Ann Oncol (2008) 19(9):1523–9. doi: 10.1093/annonc/mdn169
12. Schechter AL, Stern DF, Vaidyanathan L, Decker SJ, Drebin JA, Greene MI, et al. The neu oncogene: An erb-B-Related gene encoding a 185,000-Mr tumour antigen. Nature (1984) 312(5994):513–6. doi: 10.1038/312513a0
13. Moasser MM. The oncogene Her2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene (2007) 26(45):6469–87. doi: 10.1038/sj.onc.1210477
14. Zhu Y, Zhu X, Wei X, Tang C, Zhang W. Her2-targeted therapies in gastric cancer. Biochim Biophys Acta Rev Cancer (2021) 1876(1):188549. doi: 10.1016/j.bbcan.2021.188549
15. Rezatabar S, Karimian A, Rameshknia V, Parsian H, Majidinia M, Kopi TA, et al. Ras/Mapk signaling functions in oxidative stress, DNA damage response and cancer progression. J Cell Physiol (2019) 234(9):14951-965. doi: 10.1002/jcp.28334
16. Yuan J, Dong X, Yap J, Hu J. The mapk and ampk signalings: Interplay and implication in targeted cancer therapy. J Hematol Oncol (2020) 13(1):113. doi: 10.1186/s13045-020-00949-4
17. Noorolyai S, Shajari N, Baghbani E, Sadreddini S, Baradaran B. The relation between Pi3k/Akt signalling pathway and cancer. Gene (2019) 698:120–8. doi: 10.1016/j.gene.2019.02.076
18. Klapper LN, Vaisman N, Hurwitz E, Pinkas-Kramarski R, Yarden Y, Sela M. A subclass of tumor-inhibitory monoclonal antibodies to erbb-2/Her2 blocks crosstalk with growth factor receptors. Oncogene (1997) 14(17):2099–109. doi: 10.1038/sj.onc.1201029
19. Hanna WM, Kwok K. Chromogenic in-situ hybridization: A viable alternative to fluorescence in-situ hybridization in the Her2 testing algorithm. Mod Pathol (2006) 19(4):481–7. doi: 10.1038/modpathol.3800555
20. Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, et al. Assessment of a Her2 scoring system for gastric cancer: Results from a validation study. Histopathology (2008) 52(7):797–805. doi: 10.1111/j.1365-2559.2008.03028.x
21. Yan M, Schwaederle M, Arguello D, Millis SZ, Gatalica Z, Kurzrock R. Her2 expression status in diverse cancers: Review of results from 37,992 patients. Cancer Metastasis Rev (2015) 34(1):157–64. doi: 10.1007/s10555-015-9552-6
22. Choong GM, Cullen GD, O'Sullivan CC. Evolving standards of care and new challenges in the management of Her2-positive breast cancer. CA Cancer J Clin (2020) 70(5):355–74. doi: 10.3322/caac.21634
23. Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by Her2 status and trastuzumab treatment: An institutional-based review. J Clin Oncol (2010) 28(1):92–8. doi: 10.1200/JCO.2008.19.9844
24. Figueroa-Magalhaes MC, Jelovac D, Connolly R, Wolff AC. Treatment of Her2-positive breast cancer. Breast (2014) 23(2):128–36. doi: 10.1016/j.breast.2013.11.011
25. Abrahao-Machado LF, Scapulatempo-Neto C. Her2 testing in gastric cancer: An update. World J Gastroenterol (2016) 22(19):4619–25. doi: 10.3748/wjg.v22.i19.4619
26. Dan H, Zengshan Li, Xiangshan F, Hongmei Wu, Jianping L, Wenyong S, et al. HER2 status in gastric adenocarcinoma of Chinese: a multicenter study of 40,842 patients. Chin J Pathol (2018) 47(11):822–6. doi: 10.3760/cma.j.issn.0529-5807.2018.11.002
27. Liang JW, Zhang JJ, Zhang T, Zheng ZC. Clinicopathological and prognostic significance of Her2 overexpression in gastric cancer: A meta-analysis of the literature. Tumour Biol (2014) 35(5):4849–58. doi: 10.1007/s13277-014-1636-3
28. Takehana T, Kunitomo K, Kono K, Kitahara F, Iizuka H, Matsumoto Y, et al. Status of c-Erbb-2 in gastric adenocarcinoma: A comparative study of immunohistochemistry, fluorescence in situ hybridization and enzyme-linked immuno-sorbent assay. Int J Cancer (2002) 98(6):833–7. doi: 10.1002/ijc.10257
29. Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, et al. Her2 screening data from toga: Targeting Her2 in gastric and gastroesophageal junction cancer. Gastric Cancer (2015) 18(3):476–84. doi: 10.1007/s10120-014-0402-y
30. Lauren P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta pathologica microbiologica Scandinavica (1965) 64:31–49. doi: 10.1111/apm.1965.64.1.31
31. Pazo Cid RA, Anton A. Advanced Her2-positive gastric cancer: Current and future targeted therapies. Crit Rev Oncol Hematol (2013) 85(3):350–62. doi: 10.1016/j.critrevonc.2012.08.008
32. Jorgensen JT, Hersom M. Her2 as a prognostic marker in gastric cancer - a systematic analysis of data from the literature. J Cancer (2012) 3:137–44. doi: 10.7150/jca.4090
33. Allgayer H, Babic R, Gruetzner KU, Tarabichi A, Schildberg FW, Heiss MM. C-Erbb-2 is of independent prognostic relevance in gastric cancer and is associated with the expression of tumor-associated protease systems. J Clin Oncol (2000) 18(11):2201–9. doi: 10.1200/jco.2000.18.11.2201
34. Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol (2009) 27(34):5838–47. doi: 10.1200/JCO.2009.22.1507
35. Smyth EC, Nilsson M, Grabsch HI, van Grieken NCT, Lordick F. Gastric cancer. Lancet (2020) 396(10251):635–48. doi: 10.1016/s0140-6736(20)31288-5
36. Bang Y-J, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of Her2-positive advanced gastric or gastro-oesophageal junction cancer (Toga): A phase 3, open-label, randomised controlled trial. Lancet (2010) 376(9742):687–97. doi: 10.1016/s0140-6736(10)61121-x
37. Stein A, Paschold L, Tintelnot J, Goekkurt E, Henkes SS, Simnica D, et al. Efficacy of ipilimumab vs folfox in combination with nivolumab and trastuzumab in patients with previously untreated Erbb2-positive esophagogastric adenocarcinoma: The aio intega randomized clinical trial. JAMA Oncol (2022) 8(8):1150–8. doi: 10.1001/jamaoncol.2022.2228
38. Janjigian YY, Maron SB, Chatila WK, Millang B, Chavan SS, Alterman C, et al. First-line pembrolizumab and trastuzumab in Her2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: An open-label, single-arm, phase 2 trial. Lancet Oncol (2020) 21(6):821–31. doi: 10.1016/s1470-2045(20)30169-8
39. Janjigian YY, Kawazoe A, Yanez P, Li N, Lonardi S, Kolesnik O, et al. The keynote-811 trial of dual pd-1 and Her2 blockade in Her2-positive gastric cancer. Nature (2021) 600(7890):727–30. doi: 10.1038/s41586-021-04161-3
40. Price-Schiavi SA, Jepson S, Li P, Arango M, Rudland PS, Yee L, et al. Rat Muc4 (Sialomucin complex) reduces binding of anti-Erbb2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int J Cancer (2002) 99(6):783–91. doi: 10.1002/ijc.10410
41. Metzger-Filho O, Winer EP, Krop I. Pertuzumab: Optimizing Her2 blockade. Clin Cancer Res (2013) 19(20):5552–6. doi: 10.1158/1078-0432.Ccr-13-0518
42. Yamashita-Kashima Y, Iijima S, Yorozu K, Furugaki K, Kurasawa M, Ohta M, et al. Pertuzumab in combination with trastuzumab shows significantly enhanced antitumor activity in Her2-positive human gastric cancer xenograft models. Clin Cancer Res (2011) 17(15):5060–70. doi: 10.1158/1078-0432.Ccr-10-2927
43. Oh DY, Bang YJ. Pertuzumab in gastrointestinal cancer. Expert Opin Biol Ther (2016) 16(2):243–53. doi: 10.1517/14712598.2016.1126578
44. Tabernero J, Hoff PM, Shen L, Ohtsu A, Shah MA, Cheng K, et al. Pertuzumab plus trastuzumab and chemotherapy for Her2-positive metastatic gastric or gastro-oesophageal junction cancer (Jacob): Final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol (2018) 19(10):1372–84. doi: 10.1016/s1470-2045(18)30481-9
45. Wagner AD, Grabsch HI, Mauer M, Marreaud S, Caballero C, Thuss-Patience P, et al. Eortc-1203-Gitcg - the "Innovation"-trial: Effect of chemotherapy alone versus chemotherapy plus trastuzumab, versus chemotherapy plus trastuzumab plus pertuzumab, in the perioperative treatment of Her2 positive, gastric and gastroesophageal junction adenocarcinoma on pathologic response rate: A randomized phase ii-intergroup trial of the eortc-gastrointestinal tract cancer group, Korean cancer study group and Dutch upper gi-cancer group. BMC Cancer (2019) 19(1):494. doi: 10.1186/s12885-019-5675-4
46. Markham A. Margetuximab: First approval. Drugs (2021) 81(5):599–604. doi: 10.1007/s40265-021-01485-2
47. Catenacci DVT, Kang Y-K, Park H, Uronis HE, Lee K-W, Ng MCH, et al. Margetuximab plus pembrolizumab in patients with previously treated, Her2-positive gastro-oesophageal adenocarcinoma (Cp-Mgah22–05): A single-arm, phase 1b–2 trial. Lancet Oncol (2020) 21(8):1066–76. doi: 10.1016/s1470-2045(20)30326-0
48. Nordstrom JL, Gorlatov S, Zhang W, Yang Y, Huang L, Burke S, et al. Anti-tumor activity and toxicokinetics analysis of Mgah22, an anti-Her2 monoclonal antibody with enhanced fcγ receptor binding properties. Breast Cancer Res BCR (2011) 13(6):R123. doi: 10.1186/bcr3069
49. Bang YJ, Giaccone G, Im SA, Oh DY, Bauer TM, Nordstrom JL, et al. First-in-Human phase 1 study of margetuximab (Mgah22), an fc-modified chimeric monoclonal antibody, in patients with Her2-positive advanced solid tumors. Ann Oncol (2017) 28(4):855–61. doi: 10.1093/annonc/mdx002
50. Catenacci DV, Rosales M, Chung HC, HY H, Shen L, Moehler M, et al. Mahogany: Margetuximab combination in Her2+ Unresectable/Metastatic Gastric/Gastroesophageal junction adenocarcinoma. Future Oncol (London England) (2021) 17(10):1155–64. doi: 10.2217/fon-2020-1007
51. Levitzki A. Tyrosine kinase inhibitors: Views of selectivity, sensitivity, and clinical performance. Annu Rev Pharmacol Toxicol (2013) 53:161–85. doi: 10.1146/annurev-pharmtox-011112-140341
52. Jiao Q, Bi L, Ren Y, Song S, Wang Q, Wang YS. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol Cancer (2018) 17(1):36. doi: 10.1186/s12943-018-0801-5
53. Nahta R, Yuan LX, Du Y, Esteva FJ. Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: Effects on insulin-like growth factor I signaling. Mol Cancer Ther (2007) 6(2):667–74. doi: 10.1158/1535-7163.MCT-06-0423
54. Hecht JR, Bang YJ, Qin SK, Chung HC, Xu JM, Park JO, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: Trio-013/Logic–a randomized phase iii trial. J Clin Oncol (2016) 34(5):443–51. doi: 10.1200/JCO.2015.62.6598
55. Satoh T, Xu RH, Chung HC, Sun GP, Doi T, Xu JM, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of Her2-amplified advanced gastric cancer in Asian populations: Tytan–a randomized, phase iii study. J Clin Oncol (2014) 32(19):2039–49. doi: 10.1200/JCO.2013.53.6136
56. Hafeez U, Parakh S, Gan HK, Scott AM. Antibody-drug conjugates for cancer therapy. Molecules (2020) 25(20):4764. doi: 10.3390/molecules25204764
57. Lu G, Nishio N, van den Berg NS, Martin BA, Fakurnejad S, van Keulen S, et al. Co-Administered antibody improves penetration of antibody-dye conjugate into human cancers with implications for antibody-drug conjugates. Nat Commun (2020) 11(1):5667. doi: 10.1038/s41467-020-19498-y
58. Tsuchikama K, An Z. Antibody-drug conjugates: Recent advances in conjugation and linker chemistries. Protein Cell (2018) 9(1):33–46. doi: 10.1007/s13238-016-0323-0
59. Thuss-Patience PC, Shah MA, Ohtsu A, Van Cutsem E, Ajani JA, Castro H, et al. Trastuzumab emtansine versus taxane use for previously treated Her2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (Gatsby): An international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol (2017) 18(5):640–53. doi: 10.1016/s1470-2045(17)30111-0
60. LoRusso PM, Weiss D, Guardino E, Girish S, Sliwkowski MX. Trastuzumab emtansine: A unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancer. Clin Cancer Res (2011) 17(20):6437–47. doi: 10.1158/1078-0432.CCR-11-0762
61. Takegawa N, Nonagase Y, Yonesaka K, Sakai K, Maenishi O, Ogitani Y, et al. Ds-8201a, a new Her2-targeting antibody-drug conjugate incorporating a novel DNA topoisomerase I inhibitor, overcomes Her2-positive gastric cancer T-Dm1 resistance. Int J Cancer (2017) 141(8):1682–9. doi: 10.1002/ijc.30870
62. Keam SJ. Trastuzumab deruxtecan: First approval. Drugs (2020) 80(5):501–8. doi: 10.1007/s40265-020-01281-4
63. Yamaguchi K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, et al. 1422mo trastuzumab deruxtecan (T-dxd; ds-8201) in patients with Her2-low, advanced gastric or gastroesophageal junction (Gej) adenocarcinoma: Results of the exploratory cohorts in the phase ii, multicenter, open-label destiny-Gastric01 study. Ann Oncol (2020) 31:S899–900. doi: 10.1016/j.annonc.2020.08.1928
64. Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, et al. Trastuzumab deruxtecan in previously treated Her2-positive gastric cancer. N Engl J Med (2020) 382(25):2419–30. doi: 10.1056/NEJMoa2004413
65. Takegawa N, Tsurutani J, Kawakami H, Yonesaka K, Kato R, Haratani K, et al. [Fam-] trastuzumab deruxtecan, antitumor activity is dependent on Her2 expression level rather than on Her2 amplification. Int J Cancer (2019) 145(12):3414–24. doi: 10.1002/ijc.32408
66. Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of ds-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci (2016) 107(7):1039–46. doi: 10.1111/cas.12966
67. Meric-Bernstam F, Beeram M, Mayordomo JI, Hanna DL, Ajani JA, Murphy MAB, et al. Single agent activity of Zw25, a Her2-targeted bispecific antibody, in heavily pretreated Her2-expressing cancers. J Clin Oncol (2018) 36(15_suppl):2500–. doi: 10.1200/JCO.2018.36.15_suppl.2500
68. Zw25 effective in Her2-positive cancers. Cancer Discovery (2019) 9(1):8. doi: 10.1158/2159-8290.CD-NB2018-162
69. Huang R, Li X, He Y, Zhu W, Gao L, Liu Y, et al. Recent advances in car-T cell engineering. J Hematol Oncol (2020) 13(1):86. doi: 10.1186/s13045-020-00910-5
70. Hong M, Clubb JD, Chen YY. Engineering car-T cells for next-generation cancer therapy. Cancer Cell (2020) 38(4):473–88. doi: 10.1016/j.ccell.2020.07.005
71. Sterner RC, Sterner RM. Car-T cell therapy: Current limitations and potential strategies. Blood Cancer J (2021) 11(4):69. doi: 10.1038/s41408-021-00459-7
72. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med (2014) 371(16):1507–17. doi: 10.1056/NEJMoa1407222
73. Song Y, Tong C, Wang Y, Gao Y, Dai H, Guo Y, et al. Effective and persistent antitumor activity of Her2-directed car-T cells against gastric cancer cells in vitro and xenotransplanted tumors in vivo. Protein Cell (2018) 9(10):867–78. doi: 10.1007/s13238-017-0384-8
74. Forsberg EMV, Lindberg MF, Jespersen H, Alsen S, Bagge RO, Donia M, et al. Her2 car-T cells eradicate uveal melanoma and T-cell therapy-resistant human melanoma in Il2 transgenic Nod/Scid Il2 receptor knockout mice. Cancer Res (2019) 79(5):899–904. doi: 10.1158/0008-5472.CAN-18-3158
75. McGranahan N, Swanton C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell (2015) 27(1):15–26. doi: 10.1016/j.ccell.2014.12.001
76. Palle J, Rochand A, Pernot S, Gallois C, Taieb J, Zaanan A. Human epidermal growth factor receptor 2 (Her2) in advanced gastric cancer: Current knowledge and future perspectives. Drugs (2020) 80(4):401–15. doi: 10.1007/s40265-020-01272-5
77. Kaito A, Kuwata T, Tokunaga M, Shitara K, Sato R, Akimoto T, et al. Her2 heterogeneity is a poor prognosticator for Her2-positive gastric cancer. World J Clin cases (2019) 7(15):1964–77. doi: 10.12998/wjcc.v7.i15.1964
78. Saeki H, Oki E, Kashiwada T, Arigami T, Makiyama A, Iwatsuki M, et al. Re-evaluation of Her2 status in patients with Her2-positive advanced or recurrent gastric cancer refractory to trastuzumab (Kscc1604). Eur J Cancer (2018) 105:41–9. doi: 10.1016/j.ejca.2018.09.024
79. Pietrantonio F, Caporale M, Morano F, Scartozzi M, Gloghini A, De Vita F, et al. Her2 loss in Her2-positive gastric or gastroesophageal cancer after trastuzumab therapy: Implication for further clinical research. Int J Cancer (2016) 139(12):2859–64. doi: 10.1002/ijc.30408
80. Seo S, Ryu MH, Park YS, Ahn JY, Park Y, Park SR, et al. Loss of Her2 positivity after anti-Her2 chemotherapy in Her2-positive gastric cancer patients: Results of the gastric cancer Her2 reassessment study 3 (Gasther3). Gastric Cancer (2019) 22(3):527–35. doi: 10.1007/s10120-018-0891-1
81. Roskoski R Jr. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol Res (2015) 94:9–25. doi: 10.1016/j.phrs.2015.01.003
82. Sampera A, Sanchez-Martin FJ, Arpi O, Visa L, Iglesias M, Menendez S, et al. Her-family ligands promote acquired resistance to trastuzumab in gastric cancer. Mol Cancer Ther (2019) 18(11):2135–45. doi: 10.1158/1535-7163.MCT-19-0455
83. Peiro G, Ortiz-Martinez F, Gallardo A, Perez-Balaguer A, Sanchez-Paya J, Ponce JJ, et al. Src, a potential target for overcoming trastuzumab resistance in Her2-positive breast carcinoma. Br J Cancer (2014) 111(4):689–95. doi: 10.1038/bjc.2014.327
84. Garrett JT, Olivares MG, Rinehart C, Granja-Ingram ND, Sanchez V, Chakrabarty A, et al. Transcriptional and posttranslational up-regulation of Her3 (Erbb3) compensates for inhibition of the Her2 tyrosine kinase. Proc Natl Acad Sci U.S.A. (2011) 108(12):5021–6. doi: 10.1073/pnas.1016140108
85. Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol (2019) 20(2):69–84. doi: 10.1038/s41580-018-0080-4
86. Bure IV, Nemtsova MV, Zaletaev DV. Roles of e-cadherin and noncoding rnas in the epithelial-mesenchymal transition and progression in gastric cancer. Int J Mol Sci (2019) 20(12):2870. doi: 10.3390/ijms20122870
87. Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol (2018) 13:395–412. doi: 10.1146/annurev-pathol-020117-043854
88. Shi J, Li F, Yao X, Mou T, Xu Z, Han Z, et al. The Her4-Yap1 axis promotes trastuzumab resistance in Her2-positive gastric cancer by inducing epithelial and mesenchymal transition. Oncogene (2018) 37(22):3022–38. doi: 10.1038/s41388-018-0204-5
89. Canfield K, Li J, Wilkins OM, Morrison MM, Ung M, Wells W, et al. Receptor tyrosine kinase Erbb4 mediates acquired resistance to Erbb2 inhibitors in breast cancer cells. Cell Cycle (2015) 14(4):648–55. doi: 10.4161/15384101.2014.994966
90. Roviello G, Catalano M, Iannone LF, Marano L, Brugia M, Rossi G, et al. Current status and future perspectives in Her2 positive advanced gastric cancer. Clin Transl Oncol (2022) 24(6):981–96. doi: 10.1007/s12094-021-02760-0
91. Campos-Carrillo A, Weitzel JN, Sahoo P, Rockne R, Mokhnatkin JV, Murtaza M, et al. Circulating tumor DNA as an early cancer detection tool. Pharmacol Ther (2020) 207:107458. doi: 10.1016/j.pharmthera.2019.107458
92. Cheng ML, Pectasides E, Hanna GJ, Parsons HA, Choudhury AD, Oxnard GR. Circulating tumor DNA in advanced solid tumors: Clinical relevance and future directions. CA Cancer J Clin (2021) 71(2):176–90. doi: 10.3322/caac.21650
93. Kinugasa H, Nouso K, Tanaka T, Miyahara K, Morimoto Y, Dohi C, et al. Droplet digital pcr measurement of Her2 in patients with gastric cancer. Br J Cancer (2015) 112(10):1652–5. doi: 10.1038/bjc.2015.129
94. Maron SB, Chase LM, Lomnicki S, Kochanny S, Moore KL, Joshi SS, et al. Circulating tumor DNA sequencing analysis of gastroesophageal adenocarcinoma. Clin Cancer Res (2019) 25(23):7098–112. doi: 10.1158/1078-0432.CCR-19-1704
95. Gao J, Wang H, Zang W, Li B, Rao G, Li L, et al. Circulating tumor DNA functions as an alternative for tissue to overcome tumor heterogeneity in advanced gastric cancer. Cancer Sci (2017) 108(9):1881–7. doi: 10.1111/cas.13314
96. Wang H, Li B, Liu Z, Gong J, Shao L, Ren J, et al. Her2 copy number of circulating tumour DNA functions as a biomarker to predict and monitor trastuzumab efficacy in advanced gastric cancer. Eur J Cancer (2018) 88:92–100. doi: 10.1016/j.ejca.2017.10.032
97. Lin D, Shen L, Luo M, Zhang K, Li J, Yang Q, et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct Target Ther (2021) 6(1):404. doi: 10.1038/s41392-021-00817-8
98. Yap TA, Lorente D, Omlin A, Olmos D, de Bono JS. Circulating tumor cells: A multifunctional biomarker. Clin Cancer Res (2014) 20(10):2553–68. doi: 10.1158/1078-0432.CCR-13-2664
99. Liu Y, Ling Y, Qi Q, Lan F, Zhu M, Zhang Y, et al. Prognostic value of circulating tumor cells in advanced gastric cancer patients receiving chemotherapy. Mol Clin Oncol (2017) 6(2):235–42. doi: 10.3892/mco.2017.1125
100. Tsujiura M, Ichikawa D, Konishi H, Komatsu S, Shiozaki A, Otsuji E. Liquid biopsy of gastric cancer patients: Circulating tumor cells and cell-free nucleic acids. World J Gastroenterol (2014) 20(12):3265–86. doi: 10.3748/wjg.v20.i12.3265
101. Thanh Huong P, Gurshaney S, Thanh Binh N, Gia Pham A, Hoang Nguyen H, Thanh Nguyen X, et al. Emerging role of circulating tumor cells in gastric cancer. Cancers (Basel) (2020) 12(3):695. doi: 10.3390/cancers12030695
102. Nevisi F, Yaghmaie M, Pashaiefar H, Alimoghaddam K, Iravani M, Javadi G, et al. Correlation of Her2, Mdm2, c-myc, c-met, and Tp53 copy number alterations in circulating tumor cells with tissue in gastric cancer patients: A pilot study. Iranian Biomed J (2020) 24(1):47–53. doi: 10.29252/ibj.24.1.47
103. Mishima Y, Matsusaka S, Chin K, Mikuniya M, Minowa S, Takayama T, et al. Detection of Her2 amplification in circulating tumor cells of Her2-negative gastric cancer patients. Target Oncol (2017) 12(3):341–51. doi: 10.1007/s11523-017-0493-6
104. Nanou A, Zeune LL, Bidard FC, Pierga JY, Terstappen L. Her2 expression on tumor-derived extracellular vesicles and circulating tumor cells in metastatic breast cancer. Breast Cancer Res BCR (2020) 22(1):86. doi: 10.1186/s13058-020-01323-5
105. O'Donoghue JA, Lewis JS, Pandit-Taskar N, Fleming SE, Schoder H, Larson SM, et al. Pharmacokinetics, biodistribution, and radiation dosimetry for (89)Zr-trastuzumab in patients with esophagogastric cancer. J Nucl Med (2018) 59(1):161–6. doi: 10.2967/jnumed.117.194555
106. Janjigian YY, Viola-Villegas N, Holland JP, Divilov V, Carlin SD, Gomes-DaGama EM, et al. Monitoring afatinib treatment in Her2-positive gastric cancer with 18f-fdg and 89zr-trastuzumab pet. J Nucl Med (2013) 54(6):936–43. doi: 10.2967/jnumed.112.110239
107. Ulaner GA, Hyman DM, Lyashchenko SK, Lewis JS, Carrasquillo JA. 89zr-trastuzumab Pet/Ct for detection of human epidermal growth factor receptor 2-positive metastases in patients with human epidermal growth factor receptor 2-negative primary breast cancer. Clin Nucl Med (2017) 42(12):912–7. doi: 10.1097/RLU.0000000000001820
108. Bensch F, Brouwers AH, Lub-de Hooge MN, de Jong JR, van der Vegt B, Sleijfer S, et al. 89Zr-trastuzumab pet supports clinical decision making in breast cancer patients, when Her2 status cannot be determined by standard work up. Eur J Nucl Med Mol Imaging (2018) 45(13):2300–6. doi: 10.1007/s00259-018-4099-8
Keywords: gastric cancer, receptor ErbB-2, HER2, Her2 resistance, trastuzumab
Citation: Ma C, Wang X, Guo J, Yang B and Li Y (2023) Challenges and future of HER2-positive gastric cancer therapy. Front. Oncol. 13:1080990. doi: 10.3389/fonc.2023.1080990
Received: 26 October 2022; Accepted: 09 January 2023;
Published: 30 January 2023.
Edited by:
Yibo Fan, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Yoontaek Lee, Ewha Womans Medical Center, Republic of KoreaCopyright © 2023 Ma, Wang, Guo, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yumin Li, bGl5bUBsenUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.